Abstract
A network of cortical brain regions including the insula and anterior cingulate cortex (ACC) has been proposed as the critical and sole substrate for interoceptive awareness. Combining lesion and pharmacological approaches in humans, we found the insula and ACC are not critical for awareness of heartbeat sensations. Rather, both somatosensory afferents from the skin and a network including the insula and ACC mediate it. Together these pathways enable the core human experience of the cardiovascular state of the body.
Recent functional neuroimaging studies have highlighted a network of brain regions including the insula and the ACC as important for representing the visceral state of the body, since they are activated when we feel interoceptive stimuli such as the heartbeat1–3 and gastrointestinal sensations4. The insula has been proposed as the sole and critical cortical substrate for interoceptive awareness5,6, while the ACC has been suggested to play a visceromotor role2,5. It has been further proposed that the anterior insula “instantiates all subjective feelings from the body and feelings of emotion”5,6. Under this view, in the complete absence of the insula, interoceptive awareness would be fully absent.
However, the same studies supporting a role for the insula and ACC also show that interoceptive stimuli robustly activate somatosensory cortices1–4. The experience of our skin blushing during embarrassment, or the feeling of our heart pounding in the chest, and of blood vessels pulsating in the throat during anxiety and fear, intuitively suggests that perhaps the skin and its somatosensory afferent projections may also critically contribute to interoceptive awareness and emotion7–9. Under this view, the insula (and ACC) would not be necessary for interoceptive awareness, and, in addition to “interoceptive” pathways projecting to the insula, other somatosensory pathways would play a role10.
We thus considered two possible interoceptive awareness “pathways”7,9: one involving visceral afferents projecting to the insula, and another involving skin afferents projecting to somatosensory cortex (see Fig. 1 for specific alternative hypotheses). We examined the contributions of these two pathways to interoception in a patient (Roger) with virtually complete bilateral insula and ACC damage, but intact bilateral primary somatosensory cortex11 (Fig. 2). Roger's neurological presentation is extremely rare and represented a unique opportunity to test, in humans, predictions about these pathways.
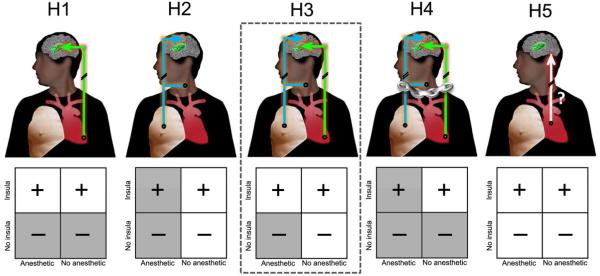
Figure 1.
Schematic representing possible pathways of interoceptive awareness. Two possible interoceptive awareness “pathways” are envisioned: one involving visceral afferents projecting to the insula (green arrow), and another involving somatosensory skin afferents (cyan arrow). Several alternative hypotheses can be proposed: interoceptive awareness is only mediated by the insula pathway (H1; standard hypothesis); interoceptive awareness is only mediated by the somatosensory pathway (H2); interoceptive awareness is independently mediated by each pathway (H3); interoceptive awareness is dependent on the simultaneous action of both pathways (H4); finally, interoceptive awareness could be dependent on other pathways (H5). If H1 is true, then only bilateral damage to the insula pathway should abolish interoceptive awareness. If H2 is true, then only disrupting the somatosensory pathway should abolish interoceptive awareness. If H3 is true, then disruption of either pathway should not abolish interoceptive awareness but disruption of both should. If H4 is true, then disrupting either pathway should abolish interoceptive awareness. If H5 is true, disruption of both pathways should not abolish interoceptive awareness. Results are in support of H3 (dashed outline). + indicates interoceptive awareness present, − indicates interoceptive awareness absent. See text for details.
Figure 2.
Brain damage in patient Roger. Top: extent of damage (black) on MRI views of lateral (upper left and right), ventral (middle) and mesial (lower left and right) cerebrum. Bottom: Axial (a–d) and saggital (1–4) slices (cf top lines). Ins = insula.
We assessed moment-to-moment awareness of cardiovascular sensations in response to bolus administration of isoproterenol, a sympathetic (beta adrenergic) agonist similar to adrenaline, as a marker of interoceptive awareness8 (Supplementary Methods). After each bolus participants turned a dial to track their moment-to-moment experience of the intensity of heartbeat sensations. All participants provided informed written consent as approved by the General Clinical Research Center Advisory Committee and the Institutional Review Board of the University of Iowa prior to participation.
As expected, Roger demonstrated dose-dependent heart rate increases indistinguishable from healthy comparison participants (Fig. 3a and Supplementary Table 1). However, contrary to what would be predicted based on H1 or H4 (Fig. 1), Roger demonstrated dose-dependent changes in interoceptive awareness comparable to healthy comparison participants, albeit, somewhat delayed in time (Fig. 3b and Supplementary Fig. 1b). Verbal responses recorded during the experimental session also suggested that Roger perceived qualitative changes in cardiovascular sensation (Supplementary Table 2). This suggested that the insula and ACC were not necessary for interoceptive awareness and that H2 or H3 should be true.
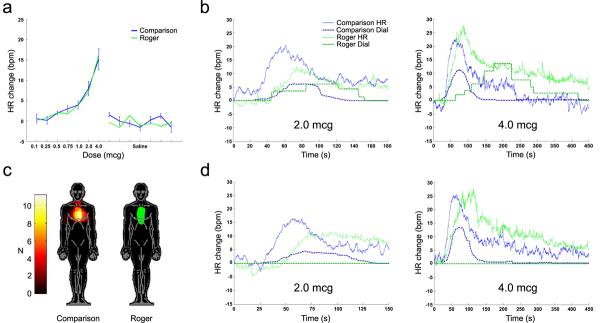
Figure 3.
Heart rate response and online subjective dial ratings of interoceptive awareness changes induced by isoproterenol. (a) Roger and eleven healthy age-matched male comparison participants exhibited equivalent dose-dependent heart rate increases. (b) Time course of heart rate response and dial ratings. Roger and the healthy participants appropriately demonstrated dose-dependent changes in interoceptive awareness. Bolus infusions occurred at time zero. (c) Overlap map showing the region of maximal heartbeat sensation, corresponding to the area of topical anesthetic application. (d) Time course of heart rate response and dial ratings after anesthetic application. Roger no longer demonstrated appropriate changes in interoceptive awareness, even at the two highest doses. Comparisons' interoceptive awareness was unaffected. All comparison data depict means. Error bars = SE. See supplementary material for additional results.
To further assess these two remaining hypotheses, we applied a topical lidocaine anesthetic to the skin covering each participant's region of maximal heartbeat sensation, as reported during the previous isoproterenol challenge (Fig. 3c). We then repeated a new challenge with the highest doses (details in Supplementary Methods). Roger again demonstrated heart rate increases identical to healthy comparison participants (Supplementary Fig. 2a). However, under anesthetic he no longer reported any changes in cardiac sensation (Fig. 3d). Verbal responses further suggested that Roger failed to experience any qualitative changes in awareness (Supplementary Table 3). On the other hand, sensation in healthy comparison participants was unaffected by anesthetic. Quantitative sensory testing demonstrated a satisfactory anesthetic effect in all participants.
Taken together, these results support the hypothesis (H3) that both neural structures innervating the skin, presumably involving primary and secondary somatosensory cortices, and the network of regions damaged in our patient, including the insula and ACC, independently mediate the ability to feel the heartbeat. These results also suggest that the insula is not the sole necessary substrate for interoceptive awareness. This study represents the first empirical demonstration of this concept7,9,12,13. Although it would have served to further test these hypotheses, it was not possible to include a patient with complete bilateral somatosensory cortex damage. This type of damage is highly implausible, and we have never encountered such an individual.
The precise contribution of the two “pathways” remains to be determined, not only for their link to interoceptive awareness but also in regard to their role in the experience of emotion5,7,9,14,15. It is possible that each pathway contributes to different aspects of interoception. For instance, the observed delay in Roger's ratings is compatible with a role for the insula in the online, instantaneous representation of the state of the body in time6. It is important to note that beyond the insula Roger also has bilateral damage to the ACC, orbitofrontal cortices, basal forebrain, hippocampus, amygdala, and temporal poles11. While the insula is typically considered as the key structure relevant for visceral sensation, the ACC is thought to play a visceral motor role, and therefore, is not expected to be critical for awareness2,6. The remaining structures are less often implicated in interoceptive awareness. We cannot rule out however that the damage incurred to these regions played a significant role in the present findings.
This lesion study challenges classic definitions of what constitutes interoception, validates functional neuroimaging findings that implicate both brain regions (insula and ACC) and somatosensory cortices in interoceptive awareness1–4, and demonstrates the set of pathways that enable the core human experience of the cardiovascular state of the body. Our findings provide empirical support for a comprehensive redefinition of interoception involving “afferent information that arises from anywhere and everywhere within the body”14, including through the skin via pathways usually considered to support exteroception. Such redefinition focuses on the source of stimulation within the body, and not on the intrinsic nature of the sensory pathway5,7,9,14. Additional investigations in the same patient are underway to determine whether these findings generalize to different interoceptive stimuli, and will be the subject of future reports.
Supplementary Material
Acknowledgements
Joel Bruss for figure assistance, Sonia Schubert and Michael Bosch for isoproterenol administration, Chirag Sandesara, Erik St. Louis, Brian Olshansky and HyungSub Shim for medical supervision, Thomas Grabowski for neurological expertise, and James Martins for safety monitoring. Supported by US National Center for Complementary & Alternative Medicine grant F31-AT003061, US National Institute on Drug Abuse grant R01-DA022549, and US National Center for Research Resources, General Clinical Research Center Program M01-RR-59.
References
- 1.Cameron OG, Minoshima S. Psychosom Med. 2002;64:851–61. doi: 10.1097/01.psy.0000038939.33335.32. [DOI] [PubMed] [Google Scholar]
- 2.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Nat Neurosci. 2004;7:189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 3.Pollatos O, Schandry R, Auer DP, Kaufmann C. Brain Res. 2007;1141:178–87. doi: 10.1016/j.brainres.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 4.Van Oudenhove L, Demyttenaere K, Tack J, Aziz Q. Best Pract Res Clin Gastroenterol. 2004;18:663–80. doi: 10.1016/j.bpg.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 5.Craig AD. Nat Rev Neurosci. 2002;3:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 6.Craig AD. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 7.Rudrauf D, et al. Int J Psychophysiol. 2008;72:13–23. doi: 10.1016/j.ijpsycho.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Khalsa SS, Rudrauf D, Sandesara C, Olshansky B, Tranel D. Int J Psychophysiol. 2009;72:34–45. doi: 10.1016/j.ijpsycho.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khalsa S, Rudrauf D, Tranel D. Psychophysiol. (in press) [Google Scholar]
- 10.Bechara A, Naqvi N. Nat Neurosci. 2004;7:102–3. doi: 10.1038/nn0204-102. [DOI] [PubMed] [Google Scholar]
- 11.Feinstein JS, et al. J Clin Exp Neuropsy. (in press) [Google Scholar]
- 12.Dworkin BR. Interoception. In: Cacciopo JT, Tassinary LG, Bernston GG, editors. Handbook of psychophysiology. Cambridge Univ. Press; 2007. pp. 482–506. [Google Scholar]
- 13.Jones GE. Perception of visceral sensations: a review of recent findings, methodologies, and future directions. In: Jennings JR, Ackels PK, editors. Advances in Psychophysiology. Vol 5. Jessica Kingsley Publishers; London: 1994. [Google Scholar]
- 14.Cameron OG. Psychosom Med. 2001;63:697–710. doi: 10.1097/00006842-200109000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Damasio AR. The Feeling of What Happens: Body and Emotion in the Making of Consciousness. Harcourt Brace; New York: 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.