Abstract
Purpose
Flavopiridol, a cyclin-dependent kinase inhibitor, has promising clinical activity when combined with chemotherapy. Preclinical data indicate that flavopiridol enhances oxaliplatin (OX)- and fluorouracil (5FU)-induced apoptosis in a sequence-dependent manner.
Experimental Design
We conducted a phase I trial of flavopiridol + FOLFOX (folinic acid, 5FU, and OX) for advanced solid tumors. Flavopiridol was administered every two weeks with OX before 5FU, based on sequence-dependent growth inhibition. Flavopiridol pharmacokinetics and p53 status were evaluated.
Results
Forty-eight patients were treated on study. With dose escalation of OX (85 mg/m2) and 5FU (2400 mg/m2), dose-limiting toxicities (DLT) included hyponatremia, thrombocytopenia, and neutropenia. 5FU was subsequently reduced to allow for dose escalation of flavopiridol. DLTs with escalation of flavopiridol were nausea, vomiting, and neutropenia. The maximum tolerated dose (MTD) was flavopiridol 70 mg/m2, oxaliplatin 85 mg/m2, and 5FU 1800 mg/m2 continuous infusion over 48 hours. Clinical activity was noted in platinum-refractory germ cell tumors (GCTs): 3 out of 9 (33%) evaluable patients demonstrated a partial response on imaging, and 7 out of 10 (70%) had a decline in serum tumor markers. Responses were also observed in pancreatic, gastric, and sweat gland tumors. Flavopiridol pharmacokinetics had significant interpatient variability. At the MTD, tumor samples were p53 mutant (>30% positive cells) for responders and p53 wild-type for non-responders.
Conclusions
Flavopiridol with FOLFOX is a safe and tolerable regimen. Promising clinical activity was seen across tumor types. Encouraging results in the platinum-refractory GCT population has prompted a phase II trial which is currently open for accrual.
Keywords: flavopiridol, FOLFOX, germ cell tumor, solid tumor, refractory
Introduction
Flavopiridol is a pan-cyclin-dependent kinase inhibitor that promotes cell cycle arrest at nanomolar concentrations and has been associated with the selective induction of apoptosis in DNA-damaged tumor cells. (1, 2) In the laboratory, flavopiridol has been shown to potently enhance the effects of a wide range of chemotherapeutic agents, including SN38 and taxane derivatives, in a time- and sequence-dependent manner. (3-5) This has been translated into a series of phase I trials in advanced solid tumors with encouraging clinical results, a reasonable safety profile, and pharmacologic levels of the drug that are sufficient to potentiate the effect of chemotherapy in vivo.(6– 8)
Oxaliplatin, a platinum-based agent, has demonstrated antiproliferative activity equivalent to or higher than that of cisplatin in a wide range of experimental tumor models. In vitro and in vivo, oxaliplatin has exhibited enhanced cytotoxic properties when combined with fluoropyrimidines (fluorouracil [5FU] and gemcitabine), thymidylate synthase inhibitors (AG337), topoisomerase I inhibitors (CPT-11 and SN38), microtubule inhibitors (paclitaxel), and DNA-modifying agents (cisplatin and cyclophosphamide). (9, 10) In the clinic, oxaliplatin has demonstrated antitumor activity as a single agent in a variety of solid tumors, and also in combination with leucovorin (folinic acid [FOL]) and 5FU as part of the FOLFOX regimen for the treatment of metastatic colon cancer. (11)
Similar to preclinical data on the effects of flavopiridol with mitomycin-C, paclitaxel, and SN38, flavopiridol enhances the effect of oxaliplatin in a sequence-dependent manner. However, in HCT-116 colon cancer cells, flavopiridol exhibits its most potent effects when administered concomitantly with oxaliplatin, rather than sequentially (GK Schwartz, unpublished). This effect is similar to that reported for flavopiridol in combination with cisplatin. (12) Therefore, based on our preclinical observations, we elected to add flavopiridol to the FOLFOX regimen for the treatment of patients with advanced solid tumors. Every other week flavopiridol was administered concurrently with oxaliplatin and leucovorin as a 1-hour bolus infusion, followed by 5FU to maximize the treatment effect.
During the course of this study, the 5FU continuous infusion was de-escalated from 2400 mg/m2 over 48 hours to 1800 mg/m2 over 48 hours, in order to facilitate dose-escalation of the flavopiridol. At the recommended phase II dose, additional patients were treated to better define the toxicity profile of the combination. Since we had previously reported that the expression of wild-type p53 status at baseline appeared to be predictive of clinical benefit from flavopiridol when combined with irinotecan, (7) pretherapy tumor samples were examined for p53 status. Classical pharmacokinetic (PK) analysis with flavopiridol plasma levels was performed at all dose levels.
Patients and Methods
Eligibility
Patients >18 years of age with advanced solid tumors refractory to standard therapy, or for which there was no standard therapy, were eligible. Patients had a Karnofsky performance status ≥70% and adequate organ function. Prior chemotherapy, immunotherapy, hormonal therapy, or radiotherapy was allowed, but only if 4 weeks had elapsed between the last dose and study entry. The protocol was approved by the institutional review board of Memorial Sloan-Kettering Cancer Center, and all patients signed informed consent forms.
Study Design
This was a phase I open-label, nonrandomized, dose-escalation study. A minimum of 3 patients were followed for at least one complete cycle (3 treatments in 6 weeks) before dose escalation. If one instance of dose-limiting toxicity (DLT) was observed, an additional 3 patients were treated at that dose level. The maximum tolerated dose (MTD) was defined as the dose one level below the dose at which 2 or more patients within a cohort experienced DLT.
Toxicity was graded in accordance with the National Cancer Institute (Bethesda, MD) Common Toxicity Criteria (NCI-CTC, version 3.0). DLT was defined in cycle 1 as the occurrence of any of the following during the first cycle of therapy: grade 4 hematologic toxicity, grade 3 or 4 nonhematologic toxicity including diarrhea despite prophylaxis, or any delay in treatment resulting in fewer than 3 treatments in 6 weeks. If a DLT was observed in the first cohort, the patient would be removed from the study without further dose attenuation. At the discretion of the investigator, patients who experienced toxicity in subsequent cycles could continue to receive study treatment after recovery with appropriate dose modifications defined by protocol. All treatments were administered in the outpatient setting.
Treatment Plan
Groups of 3 to 6 patients were treated sequentially with flavopiridol (starting dose 40 mg/m2 over 60 minutes), concomitant oxaliplatin (starting dose 60 mg/m2 over 120 minutes) and leucovorin (fixed dose 400 mg/m2 over 120 minutes). This was immediately followed by a bolus of 5FU (fixed dose 400 mg/m2) and continuous 5FU (starting dose 1800 mg/m2 over 48 hours) (Fig. 1). This regimen was administered intravenously every 2 weeks. Due to toxicity prior to flavopiridol escalation with 5FU at 2400 mg/m2 over 48 hours, 5FU was de-escalated to the starting dose of 1800 mg/m2 over 48 hours. Dose escalation with flavopiridol was then pursued in 10 mg/m2 intervals up to a 80 mg/m2. The MTD of 70 mg/m2 was then expanded to additional patients.
Fig 1.
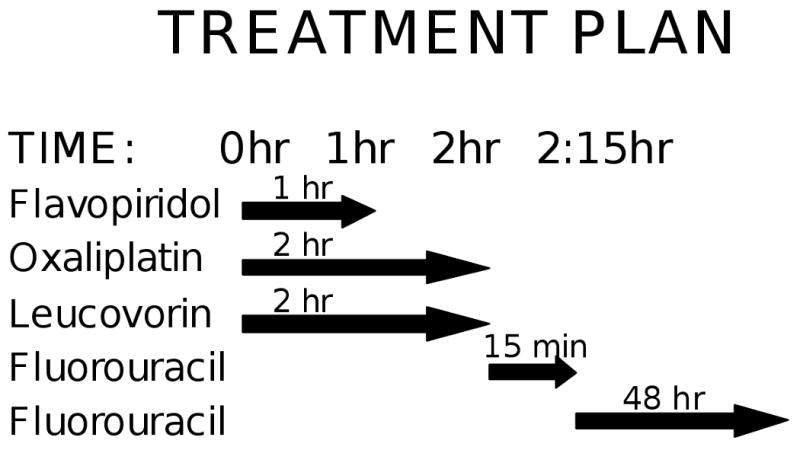
Patients received flavopiridol as a 1-hour infusion (t=0 hr), concurrently with oxaliplatin at a starting dose of 60 mg/m2, and leucovorin at 400 mg/m2 administered over two hours (t=0 hr). This was followed by 5-fluorouracil given as a bolus infusion starting at 400 mg/m2 (t=2 hr), and then a 48-hour continuous infusion at either 1800 or 2400 mg/m2 over 48 hours (t=2:15 hr).
Treatment Assessments
Patients were evaluated by a physician biweekly at the time of treatment for the first 2 cycles (12 weeks) to document toxicities. Following the second cycle, these evaluations were performed at the initiation of each cycle, or more frequently if necessary. Treatment responses were evaluated after every two cycles.Standard Response Evaluation Criteria in Solid Tumors (RECIST) was used for response assessment and was performed by an independent protocol radiologist. (13)
Drug Supply
Flavopiridol (HMR 1275) was provided by Sanofi-aventis and distributed by the National Cancer Institute in 10 mg and 50 mg sterile vials, as previously reported. (6) Flavopiridol was reconstituted in 250 mL of 0.9% sodium chloride injection, USP, or 5% dextrose for injection, USP, so that the final concentration recommended by the company ranged from 0.09 to 1 mg/mL to decrease the risk of thrombotic complications.
Statistical Design
The main objective of this study was to determine the MTD of biweekly flavopiridol when administered in combination with FOLFOX to patients with advanced solid tumors. The incidence of hematologic and nonhematologic toxicities was summarized separately, by cycle and by flavopiridol cohort. Secondary analyses included a PK analysis of flavopiridol.
Pharmacokinetics
PK studies of flavopiridol were performed for each patient during week 1 and compared with historical controls. Blood samples were collected via an indwelling peripheral catheter or via peripheral venipuncture into heparinized coated tubes: prior to treatment (0 hr), completion of flavopiridol (1:00 hr), oxaliplatin (2:00 hr), 5FU bolus (2:15 hr), and 5FU continuous infusion (50:15 hr). Frozen plasma samples were thawed at ambient temperature. The liquid-liquid phase extraction was done in a solvent mixture of acetonitrile and methanol (4/1, v/v). The supernatant was injected onto a C18 column. High-performance liquid chromatography/tandem mass spectrometry (Sciex API 4000, Applied Biosystems, Foster City, CA) analysis using an electrospray ionization method in the positive ion mode was used to separate the compound from any potential interference and measured by the MS/MS detection method. Calibration curves were determined for the compound (402 [M+H]+) to permit conversion of peak areas to compound amounts against external reference standards. The tandem MS/MS detector also permitted verification of peak identity as well as a quantitative assessment of the compounds in the samples. The limit of quantitation for flavopiridol was less than 0.01 nM.
Biological Assays
Pretreatment tumor samples of patients enrolled in the expanded cohort at the MTD were evaluated for p53 status. The biopsy specimen was fixed in formalin and embedded in paraffin. Five-micrometer sections were cut for hematoxylin and eosin (H&E) and immunohistochemistry staining. Monoclonal antibody for p53 (PAb1801, Calbiochem Immunochemicals, EMD Biosciences North America) were used at a concentration of 0.2 μg/mL. Both positive and negative controls were run at the time of each experiment. Nuclear staining was considered specific reactivity for p53 and percent of positive tumor cells was estimated by examining different fields throughout the entire tissue section. The staining was reviewed by a pathologist. Mutant (or positive) p53 staining was considered if >20% of the nuclei stained positive.
Results
Patient Characteristics
Between March 2007 and October 2008, 52 patients with advanced solid tumors were registered to the study. Of the 52 patients enrolled, 4 were not treated, and an additional 11 patients did not complete a full cycle of treatment (6 weeks). These patients came off study early due to personal choice (1), intolerability of or hypersensitivity to oxaliplatin (2), hypersensitivity to flavopiridol (1), progression based on early imaging (4), or progression based on symptoms of disease (3). Baseline characteristics for the 48 patients who received at least one treatment with flavopiridol and FOLFOX are outlined in Table 1.
Table 1.
Patient characteristics
| Characteristic | No. patients | |
|---|---|---|
| Total | 48 | |
| Male | 31 | |
| Female | 17 | |
| Age, y | ||
| Median | 51 | |
| Range | 19 to 77 | |
| KPS, % | ||
| Median | 90 | |
| Range | 70 to 90 | |
| Prior chemotherapy | 47 | |
| No. prior regimens | ||
| Median | 3 | |
| Range | 0 to 10 | |
| Prior platinum | 33 | |
| Prior oxaliplatin | 16 | |
| Primary sites of disease | ||
| Germ cell tumor | 10 | |
| Colon | 8 | |
| Pancreatic | 6 | |
| Ampullary | 3 | |
| Melanoma | 3 | |
| Stomach | 3 | |
| Gastric | 2 | |
| Rectal | 2 | |
| Anal | 1 | |
| Breast | 1 | |
| Desmoplastic small round cell tumor | 1 | |
| Esophageal | 1 | |
| Head and neck | 1 | |
| Liposarcoma | 1 | |
| Lung | 1 | |
| Neuroendocrine carcinoma | 1 | |
| Small bowel | 1 | |
| Sweat gland carcinoma | 1 | |
| Wilms tumor | 1 |
The median age was 51 (range, 19–77) and the Karnofsky performance status was 90% (range, 70–90). All but 1 patient with metastatic gastric cancer had received prior chemotherapy. The median number of prior treatment regimens was 3 (range, 0– 10); 33 patients (69%) had previously received a platinum agent, of which 16 (33%) had received oxaliplatin. All germ cell tumor (GCT)patients had received prior cisplatin; 1 had also received oxaliplatin.
Dose-Limiting Toxicity
Table 2 lists the dose levels and most common cumulative toxicities (grade 2 to 4) for the 48 patients treated on study. In total, there were 6 DLTs noted, including thrombocytopenia in cohort 1, syncope attributed to hyponatremia and neutropenia in cohort 3 (prompting 5FU de-escalation in cohorts 4a through 7a in order to continue flavopiridol escalation), and febrile neutropenia, nausea and vomiting, and failure to complete 3 cycles of therapy within 6 weeks in cohort 7a. As a result, the MTD was determined to be cohort 6a with flavopiridol 70 mg/m2, oxaliplatin 85 mg/m2, and 5FU 1800 mg/m2 continuous infusion over 48 hours. There were no observed DLTs in the expanded MTD cohort.
Table 2.
Cycle 1 nonhematologic and hematologic toxicity (n=48)
| Fatigue | Diarrhea | Nausea | Vomiting | Febrile Neut | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort (patients) | O | F | 5FU | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | |
| Cohort 1 (9) | 60 | 40 | 1800 | 2 | 2 | 1 | 1 | ||||||||||||
| Cohort 2 (3) | 85 | 40 | 1800 | 1 | 1 | 1 | 1 | ||||||||||||
| Cohort 3 (6) | 85 | 40 | 2400 | 4 | 2 | 1 | |||||||||||||
| Cohort 4a (4) | 85 | 50 | 1800 | 1 | |||||||||||||||
| Cohort 5a (3) | 85 | 60 | 1800 | ||||||||||||||||
| Cohort 6a (17) | 85 | 70 | 1800 | 4 | |||||||||||||||
| Cohort 7a (6) | 85 | 80 | 1800 | 1 | 1* | 1* | 1 | ||||||||||||
| ANC | Platelets | Leukocytes | Lymphopenia | Hyponatremia | |||||||||||||||
| Cohort (patients) | O | F | 5FU | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | |
| Cohort 1 (9) | 60 | 40 | 1800 | 1 | 1 | 1 | 1 | 2 | |||||||||||
| Cohort 2 (3) | 85 | 40 | 1800 | 1 | 2 | 1 | |||||||||||||
| Cohort 3 (6) | 85 | 40 | 2400 | 2 | 1 | 1 | 2 | 4 | 1 | ||||||||||
| Cohort 4a (4) | 85 | 50 | 1800 | 3 | 1 | 2 | 2 | ||||||||||||
| Cohort 5a (3) | 85 | 60 | 1800 | 1 | |||||||||||||||
| Cohort 6a (17) | 85 | 70 | 1800 | 1 | 2 | 2 | 2 | ||||||||||||
| Cohort 7a (6) | 85 | 80 | 1800 | 1 | 4** | 1 | 2 | 1 | 3 | 3 | 2 | ||||||||
NOTE: DLTs are in boldface and underlined. Grade 2 to 4 toxicities for possibly, probably, or definitely attributable to therapy.
Abbreviations: O, oxaliplatin; F, flavopiridol; 5FU, fluorouracil; ANC, absolute neutrophil count.
Patient experienced grade 3 nausea and vomiting, as well as grade 3 hypophosphatemia.
Patient experienced treatment delays due to grade 3 ANC (×2); unable to complete 3 treatments in 6 weeks.
Hematologic and Nonhematologic Toxicity
As shown, the most common ≥grade 3 toxicities were hematologic (leukopenia, lymphopenia, neutropenia). All grade 4 toxicities were hematologic including neutropenia (2 patients) and thrombocytopenia (1 patient). As would be expected with FOLFOX chemotherapy, nonhematologic toxicities occurring <20% of the time included fatigue, diarrhea, nausea and vomiting, electrolyte abnormalities, sensory neuropathy, and febrile neutropenia.
Pharmacokinetics
Blood samples for PK analysis were obtained from 30 patients. Table 3 summarizes the maximum observed plasma concentration (Cmax) across all subjects in a cohort. Flavopiridol PK demonstrated significant interpatient variability. When evaluated by higher (70 mg/m2 to 80 mg/m2) and lower (40 mg/m2) dose levels, flavopiridol Cmax appeared to increase with dose (1.95μM ± 0.56 vs 1.23μM ± 0.56; paired samples t-test, P = 0.002). In the last cohort (7a), the 3 patients who experienced a DLT had a higher flavopiridol Cmax than the other patients in the cohort (2.26μM ± 0.03 vs 1.45μM ± 0.44; paired samples t-test, P = 0.07).
Table 3.
Dose levels, dose-limiting toxicities, and pharmacokinetics (PK)
| Cohort | Evaluable for DLT* N=37 | Evaluable for PK N=30 | Flavo (mg/m2) | OX (mg/m2) | LV/5FU (mg/m2) | 5FU CI/48hr | DLT | Mean Cmax (μM) |
|---|---|---|---|---|---|---|---|---|
| 1 | 6 | 6 | 40 | 60 | 400/400 | 1800 | Yes | 1.51 ± 0.45 |
| 2 | 3 | 3 | 40 | 85 | 400/400 | 1800 | No | 1.52 ± 0.38 |
| 3 | 6 | 6 | 40 | 85 | 400/400 | 2400 | Yes | 0.66 ± 0.34 |
| 4a | 3 | 3 | 50 | 85 | 400/400 | 1800 | No | 1.80 ± 0.40 |
| 5a | 3 | 3 | 60 | 85 | 400/400 | 1800 | No | 0.94 ± 0.24 |
| 6a | 12 | 5 | 70 | 85 | 400/400 | 1800 | No | 2.23 ± 0.51 |
| 7a | 4 | 4 | 80 | 85 | 400/400 | 1800 | Yes | 1.72 ± 0.54 |
Abbreviations: Flavo, flavopiridol; OX, oxaliplatin; LV, leucovorin; 5FU, fluorouracil; DLT, dose-limiting toxicity.
48 patients were treated on study; 37 were evaluable for DLT. The additional 11 patients did not complete 1 cycle of therapy due to a hypersensitivity reaction (n=3), disease progression (n=7), or elective withdrawal of consent (n=1).
Antitumor Activity
In total, 42 out of 48 treated patients were evaluable for antitumor response. Twenty-two of these patients had progression of disease based on imaging or symptoms as their best response. Table 4 outlines the 20 patients who had stable disease (SD), a partial response (PR), or a complete response (CR) to the treatment combination. A CR was seen in 1 patient with pancreatic cancer (2%) who had previously progressed on treatment with gemcitabine. A PR was seen in 6 patients (14%): 3 with GCTs, 2 with gastric, and 1 with sweat gland carcinoma. An additional 13 patients (31%) demonstrated SD. The median time on study was 20 weeks (range, 8– 39).
Table 4.
Clinical activity by tumor type (n=20)*
| Tumor Type | Response | Duration (wk) | Prior Platinum |
|---|---|---|---|
| Anal | SD | 29 | Cisplatin |
| Ampullary | SD | 33 | No |
| SD | 14 | No | |
| Breast | SD | 19 | No |
| Colon | SD | 31 | No |
| SD | 29 | Oxaliplatin | |
| SD | 14 | Oxaliplatin | |
| SD | 12 | Oxaliplatin | |
| Esophageal | SD | 19 | Cisplatin, Oxaliplatin |
| Gastric | PR | 27 | Cisplatin |
| PR | 22 | No | |
| Germ cell tumor | PR | 39 | Cisplatin, Carboplatin |
| PR | 36 | Cisplatin, Carboplatin | |
| PR | 17 | Cisplatin, Carboplatin | |
| SD | 20 | Cisplatin | |
| SD | 12 | Cisplatin, Carboplatin | |
| SD | 11 | Cisplatin | |
| Neuroendocrine carcinoma | SD | 9 | Oxaliplatin |
| Pancreatic | CR | 34 | No |
| Sweat gland carcinoma | PR | 25 | No |
Abbreviations: SD, stable disease; PR, partial response.
42 patients were evaluable for antitumor response. An additional 22 patients had progression of disease as their best response.
Of the 10 patients with platinum-refractory GCTs enrolled on study, 1 patient who had progressed on prior oxaliplatin had a hypersensitivity reaction to oxaliplatin (although the patient's serum alpha-fetoprotein [AFP] declined by 40%) and was inevaluable for response. Examples of tumor response are shown in Fig.2. Of the 9 evaluable patients, 3 achieved a PR (33%) and 3 demonstrated SD. Notably, of the 3 patients who progressed, 1 developed new brain metastases despite a 65% reduction in his serum AFP, and the other 2 patients demonstrated disease progression after only 1 week of treatment prompting removal from study. Overall, 7 of 10 (70%) patients with GCTs who received at least one cycle of treatment demonstrated a decline in tumor markers.
Fig 2.
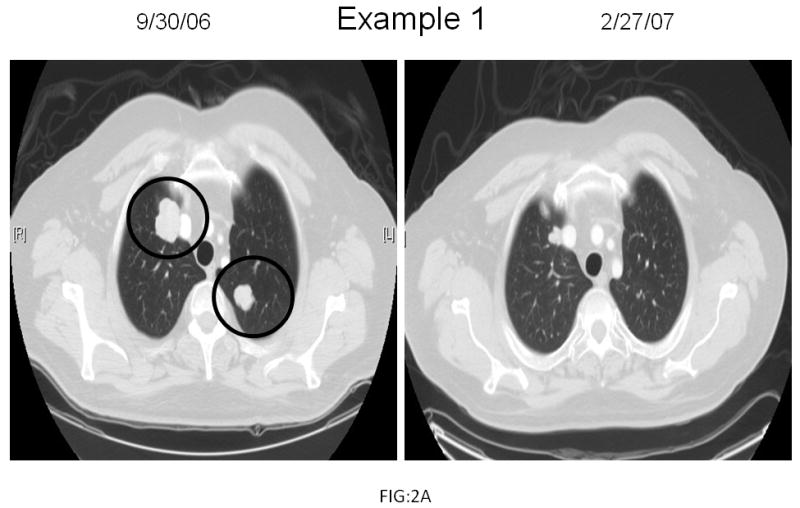
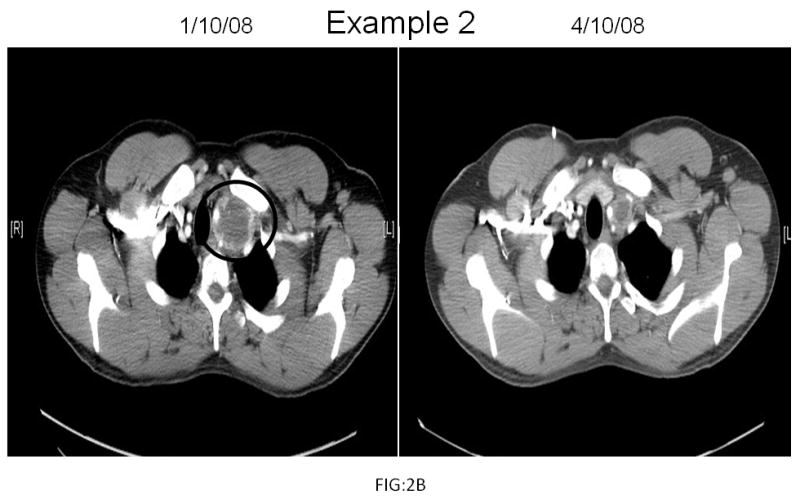
Radiographic examples of germ cell tumor response. Two cisplatin-refractory germ cell tumor patients demonstrated areas of disease pre-treatment (circled in black) which decreased by 65% (Example 1) and 43% (Example 2) post-treatment.
Correlative Studies
All 9 patients enrolled in the expanded MTD cohort (6a) were eligible for and underwent computed tomography-guided biopsy of their tumor to assess pretreatment p53 status. All samples showed tumor on H&E staining and were adequate for subsequent immunohistochemical analysis for p53. Based on preclinical studies indicating that flavopiridol enhanced the effect of the DNA-damaging agent irinotecan in a p53-dependent manner, we hypothesized that patients with pretreatment wild-type p53 positivity would also respond better than patients who were negative. (14) However, this was not borne out in our immunohistochemical analysis for p53. In fact, the 2 patients (GCT, sweat gland) who achieved a PR at the MTD were p53 mutant (≥30% p53 positive tumor cells), and the 4 patients (1 gastric, 3 GCT) with SD and 3 patients (GCT, lung, pancreas) with disease progression were p53 wild-type (0%–15% p53 positive tumor cells).
Discussion
Based on the success of oxaliplatin as part of the FOLFOX regimen in colorectal cancer and the preclinical evidence that flavopiridol enhances the cytotoxicity of oxaliplatin, we conducted a phase I trial of flavopiridol plus FOLFOX in patients with advanced solid tumors. The primary endpoint of the trial was to establish the MTD of the drugs used in this combination; additional endpoints focused on antitumor activity and biological correlates.
Forty-eight patients were treated on this trial, including 16 who had received prior oxaliplatin. Notably, 11 patients did not complete a full cycle of therapy (3 treatments in 6 weeks). Although hypersensitivity reactions and patient choice played a role in early withdrawal from the study, 7 patients had disease progression based on imaging or symptoms which prompted discontinuation of flavopiridol and FOLFOX (F-FOLFOX) after only 1 or 2 treatments. Given the advanced stage and refractory nature of the tumors treated on this study, the early progression rate of 15% (7 of 48 treated patients) appears to be a reasonable expectation and further underscores the need for safe and effective therapies in this population of heavily pretreated patients.
Overall, treatment with F-FOLFOX was well tolerated in the majority of patients despite a median of 3 prior chemotherapy regimens (range, 0–10). DLTs included neutropenia, thrombocytopenia, nausea and vomiting, and electrolyte abnormalities (hyponatremia, hyperphosphatemia). De-escalation of the 5FU continuous infusion from 2400 mg/m2 to 1800 mg/m2 took place in favor of dose-escalating the flavopiridol. The MTD was established as flavopiridol 70 mg/m2, oxaliplatin 85 mg/m2, leucovorin 400 mg/m2, 5FU bolus 400 mg/m2, and 5FU continuous infusion over 48 hours at a dose of 1800 mg/m2. In 12 patients who were treated at this dose level, no DLTs occurred.
Previous studies of flavopiridol alone, and in combination with chemotherapy, have confirmed an MTD of 70 mg/m2 when administered as a 1-hour infusion, with a similar DLT profile consisting of neutropenia, diarrhea, and fatigue. (6) At this dose level, PK during cycle 1 appeared to be consistent with other chemotherapy combinations (Cmax 2.23 ± 0.51 μmol/L combined with FOLFOX vs Cmax 2.76 ± 0.54 μmol/L combined with irinotecan). (5) However, in contrast to prior studies combining flavopiridol with chemotherapy, p53 wild-type status did not correlate with increased sensitivity. In fact the patients who had the major tumor regressions were p53 mutant. This may be related to different mechanisms for a DNA damage response between irinotecan and oxaliplatin, such that only irinotecan is p53 dependent.
Antitumor activity was seen across a variety of tumor types in this phase I study, independent of prior treatment with platinum agents. Seven of 42 evaluable patients (17%) experienced either a CR or PR, including 4 patients who had previously received platinum-based therapy. Although the results documented with F-FOLFOX in our trial are consistent with data from the GERCOR V308 and EFC4584 trials, which reported overall response rates of 10% and 15% with second-line FOLFOX alone for advanced colon cancer, (16–18) promising activity was noted in the subset of patients with platinum-refractory GCT treated on this study.
Ten patients with platinum-refractory GCT were enrolled on the F-FOLFOX trial, 5 of whom had either a mediastinal primary tumor or late relapse, two features that predict a lack of response to salvage treatments. (19) Of the 9 patients evaluable for radiographic response by RECIST criteria, 3 had PR, 3 had SD, and 1 patient had progression in the brain despite a 68% reduction in the tumor marker AFP. The 2 additional GCT patients who demonstrated disease progression on study did so after only 1 treatment. Another patient who had previously progressed on oxaliplatin 130 mg/m2, experienced a 40% decline in his AFP after 1 cycle of F-FOLFOX but came off study due to an oxaliplatin-related hypersensitivity reaction.
For patients with relapsed or refractory GCTs, the optimal treatment regimen has not yet been established. Up to 40% of patients will not be cured after treatment with high-dose therapy in the second-line setting, and specific subgroups of patients, such as those with mediastinal nonseminomatous GCT or primary refractory GCT, have been identified as being particularly unlikely to benefit from this approach. (20) Oxaliplatin has previously been studied as a single agent for patients with cisplatin-refractory GCT using two different dosing schedules. (21) An initial group of patients was treated with 60 mg/m2 weekly on days 1, 8, and 15 every 28 days with an overall response rate of 6%. A second cohort was treated with 130 mg/m2 every 2 weeks with an objective response rate of 19%.
Overall the combination of FOLFOX plus flavopiridol was well-tolerated with activity observed across a range of solid tumors. Taking into account the published response rates to single agent oxaliplatin, the extent of prior treatment, and the high-risk refractory nature of the GCTs treated on this study, the response in the GCT population is particularly encouraging. A phase II trial of F-FOLFOX for patients with refractory GCTs is currently open to accrual and will continue to explore anti-tumor activity and the role of p53 relative to treatment response.
Acknowledgments
Supported by NCI R01CA67819
Footnotes
The material in this work has previously been presented in abstract format at the 2008 American Society of Clinical Oncology Annual symposium.
Translational Relevance: Flavopiridol is a potent cyclin-dependent kinase inhibitor that promotes cell cycle arrest and has been associated with apoptosis in DNA-damaged tumor cells. Based on preclinical data showing that flavopiridol enhances the effect of oxaliplatin in a time and sequence-dependent manner, we conducted a phase I trial of bi-weekly flavopiridol administered concurrently with oxaliplatin and leucovorin as a 1-hour bolus infusion, followed by fluorouracil (FOLFOX). This trial in patients with advanced solid tumors demonstrated a tolerable safety profile and pharmacologic levels of flavopiridol sufficient to potentiate the effects of chemotherapy in vivo. In contrast to prior studies combining flavopiridol with irinotecan, p53 wild-type status did not correlate with increased sensitivity. Encouraging clinical results were seen, particularly in patients with platinum-refractory germ cell tumors prompting a phase II trial for this patient population which will continue to explore the role of p53 and apoptotic markers relative to treatment response.
References
- 1.Kaur G, Stelter-Stevenson M, Sebers S, et al. Growth inhibition with reversible cell cycle arrest of carcinoma cells by flavone L86-8275. J Natl Cancer Inst. 1992;84:1736–40. doi: 10.1093/jnci/84.22.1736. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz GK, Shah MA. Targeting the cell cycle: a new approach to cancer therapy. J Clin Oncol. 2005;23:9408–21. doi: 10.1200/JCO.2005.01.5594. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz GK, Farsi K, Maslak P, Kelsen DP, Spriggs D. Potentiation of apoptosis by flavopiridol in mitomycin-C-treated gastric and breast cancer cells. Clin Cancer Res. 1997;5:1467–72. [PubMed] [Google Scholar]
- 4.Motwani M, Delorhery TM, Schwartz GK. Sequential dependent enhancement of caspase activation and apoptosis by flavopiridol on paclitaxel-treated human gastric and breast cancer cells. Clin Cancer Res. 1999;7:1876–83. [PubMed] [Google Scholar]
- 5.Motwani M, Jung C, Sirotnak FM, et al. Augmentation of apoptosis and tumor regression by flavopiridol in the presence of CPT-11 in HCT-116 colon cancer monolayers and xenografts. J Clin Onc. 2001;7:4209–19. [PubMed] [Google Scholar]
- 6.Schwartz GK, O'Reilly E, Ilson D, et al. Phase I study of the cyclin-dependent kinase inhibitor flavopiridol in combination with paclitaxel in patients with advanced solid tumors. J Clin Onc. 2002;20:2157–70. doi: 10.1200/JCO.2002.08.080. [DOI] [PubMed] [Google Scholar]
- 7.Shah MA, Kortmansky J, Motwani M, et al. A phase I clinical trial of the sequential combination of irinotecan followed by flavopiridol. Clin Cancer Res. 2005;11:3836–45. doi: 10.1158/1078-0432.CCR-04-2651. [DOI] [PubMed] [Google Scholar]
- 8.Fornier MN, Rathkopf D, Shah M, et al. Phase I dose-finding study of weekly docetaxel followed by flavopiridol for patients with advanced solid tumors. Clin Cancer Res. 2007;13:5841–6. doi: 10.1158/1078-0432.CCR-07-1218. [DOI] [PubMed] [Google Scholar]
- 9.Fukuda M, Ohe Y, Kanzawa F, Oka M, Hara K, Saijo N. Evaluation of novel platinum complexes, inhibitors of topoisomerase I and II in non-small cell lung cancer (NSCLC) sublines resistant to cisplatin. Anticancer Res. 1995;15:393–8. [PubMed] [Google Scholar]
- 10.Raymond E, Buquet-Fagot C, Djelloul S, et al. Antitumor activity of oxaliplatin in combination with 5-fluorouracil and the thymidylate synthase inhibitor AG337 in human colon, breast and ovarian cancers. Anticancer Drugs. 1997;8:876–85. doi: 10.1097/00001813-199710000-00009. [DOI] [PubMed] [Google Scholar]
- 11.de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–47. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 12.Bible KC, Kaufmann SH. Flavopiridol: a cytotoxic flavone that induces cell death in noncycling A549 human lung carcinoma cells. Cancer Res. 1996;56:4856–61. [PubMed] [Google Scholar]
- 13.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 14.Ambrosini G, Seelman SL, Qin LX, Schwartz GK. The cyclin-dependent kinase inhibitor flavopiridol potentiates the effects of topoisomerase I poisons by suppressing Rad51 expression in a p53-dependent manner. Cancer Res. 2008;68:2312–20. doi: 10.1158/0008-5472.CAN-07-2395. [DOI] [PubMed] [Google Scholar]
- 15.Senderowicz AM, Headlee D, Stinson S, et al. Phase I trial of continuous infusion flavopiridol, a novel cyclic-dependent kinase inhibitor, in patients with refractory neoplasms. J Clin Oncol. 1998;16:2986–99. doi: 10.1200/JCO.1998.16.9.2986. [DOI] [PubMed] [Google Scholar]
- 16.Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–37. doi: 10.1200/JCO.2004.05.113. [DOI] [PubMed] [Google Scholar]
- 17.Rothenberg ML, Oza AM, Bigelow RH, et al. Superiority of oxaliplatin and fluorouracil-leucovorin compared with either therapy alone in patients with progressive colorectal cancer after irinotecan and fluorouracil-leucovorin: interim results of a phase III trial. J Clin Oncol. 2003;21:2059–69. doi: 10.1200/JCO.2003.11.126. [DOI] [PubMed] [Google Scholar]
- 18.Rothenberg ML, Oza AM, Burger B, et al. Final results of a phase III trial of 5-FU/leucovorin versus oxaliplatin versus the combination in patients with metastatic colorectal cancer following irinotecan, 5-FU, and leucovorin. J Clin Oncol. 2003;22 Abstr 1011. [Google Scholar]
- 19.Motzer RJ, Geller NL, Tan CC, et al. Salvage chemotherapy for patients with germ cell tumors. The Memorial Sloan-Kettering Cancer Center experience (1979-1989) Cancer. 1991;67:1305–10. doi: 10.1002/1097-0142(19910301)67:5<1305::aid-cncr2820670506>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 20.Feldman DR, Bosl GJ, Sheinfeld J, Motzer RJ. Medical treatment of advanced testicular cancer. JAMA. 2008;299:672–84. doi: 10.1001/jama.299.6.672. Review. [DOI] [PubMed] [Google Scholar]
- 21.Kollmannsberger C, Rick O, Derigs HG, et al. Activity of oxaliplatin in patients with relapsed or cisplatin-refractory germ cell cancer: a study of the German Testicular Cancer Study Group. J Clin Oncol. 2002;20:2031–7. doi: 10.1200/JCO.2002.08.050. [DOI] [PubMed] [Google Scholar]


