Abstract
Influenza virus-like particles (VLPs) were produced in Sf9 insect cells by co-expressing the matrix protein M1 and the surface glycoproteins hemagglutinin (HA) and neuraminidase (NA) using the recombinant baculovirus expression system. The VLPs were morphologically similar to influenza virions. Both HA and NA proteins were incorporated into VLPs and these proteins retained their functional activities. Further, influenza VLPs but not inactivated influenza viruses (IIV) stimulated secretion of inflammatory cytokines from mouse bone marrow-derived dendritic cells (BMDC). Immunogenicity of influenza VLPs and their protective efficacies against lethal influenza virus challenge were evaluated in young and aged mice. Immunization with influenza VLPs induced strong antibody responses against HA that inhibited hemagglutination by influenza virus, similar to IIV vaccines. Compared to young mice, antibody responses in aged mice immunized with a low dose of either influenza VLPs or IIV vaccines exhibited markedly reduced avidity for HA. However, immunization of aged mice with a high dose of influenza VLPs induced antibody responses with high avidity similar to those in young mice. Furthermore, all vaccinated animals survived a lethal challenge by a mouse-adapted influenza virus (A/PR/8/34), indicating that influenza VLPs are highly efficacious for protection against influenza virus infection in both young and aged mice.
Keywords: Influenza, virus-like particle, aging, antibody response, avidity
1. INTRODUCTION
Influenza virus is a highly contagious airborne pathogen that enters through infection of the respiratory tract. Seasonal epidemics of influenza virus infection are estimated to cause about 36,000 deaths and over 200,000 hospitalizations annually in the United States alone (Bridges et al., 2003). In particular, elderly humans over 65 years old are at great risk and constitute up to 90% of all mortality associated with influenza virus infection (Thompson et al., 2003; Webster, 2000). Vaccination represents a highly effective approach to reduce the rate of influenza virus infection and the associated social and economical burdens (Szucs, 1999). Currently there are two types of human influenza vaccines, inactivated influenza virus (IIV) vaccines and cold-adapted influenza virus (CAIV) vaccines (Cox et al., 2004). However, although IIV vaccines provide effective protection in healthy young adults with up to 90% efficacy, protection of elderly adults is substantially lower even with a good match between the vaccine and the circulating influenza virus (de Bruijn et al., 1999, Govaert et al., 1994; Gross et al., 1995; Stepanova et al., 2002). On the other hand, CAIV vaccines are currently only approved for healthy people between age 2 to 49 (Fiore et al., 2008). The high fatality rate of influenza virus infection and the low vaccine efficacy in the elderly underscore the need to evaluate new vaccine strategies for this high risk population.
Influenza virus is an enveloped virus with a segmented negative strand RNA genome. It has two surface glycoproteins, the hemagglutinin (HA) and neuraminidase (NA) that are anchored to the viral lipid membranes. Early studies have shown that immune protection against influenza virus infection is primarily mediated by antibody responses against the HA, the major viral surface glycoprotein that mediates virus attachment to cellular receptors and fusion between viral and cellular membranes during infection (Ada and Jones, 1986; Carroll and Paulson, 1985; Daniels et al., 1987). In humans, an antibody response that inhibits hemagglutination by influenza virus at a titer of 1:40 or higher is found to correlate with protection against influenza virus infection and pathogenicity (de Jong et al., 2003). A number of new influenza vaccine strategies, including DNA vaccines, influenza virosomes and immunostimulatory complexes, cell-culture derived inactivated virus vaccines, as well as recombinant HA subunit vaccines are being explored and evaluated (Kemble and Greenberg, 2003; Nicholson et al., 2003; Stephenson et al., 2004). Like many other viruses, co-expression of influenza virus structural proteins including the matrix protein M1 and the surface glycoproteins HA and NA leads to assembly and release of virus-like particles (VLPs) from the cells (Chen et al., 2007; Gomez-Puertas et al., 1999; Latham and Galarza, 2001; Neumann et al., 2000). Recent studies have shown that immunization by influenza VLPs can induce protective immune responses against lethal influenza virus challenge (Bright et al., 2007; Galarza et al., 2005; Latham and Galarza, 2001; Mahmood et al., 2008; Pushko et al., 2005, 2007; Quan et al., 2007, 2008).
The development of these new strategies opens new avenues for the attainment of a more effective influenza vaccine. However, these new generations of influenza vaccines need to be evaluated for their immunogenicity and efficacy in aged populations which are at high-risk of influenza virus infection. While the efficacy of influenza VLPs has been demonstrated in several animal species (Cox, 2008), their immunogenicity and protective efficacy in aged animals against influenza virus infection have not been examined. The aged mice provide a useful model for studies of age-related decline in immune responses and protection against influenza virus infection and preclinical evaluation of influenza vaccines in aged animals (Ben-Yehuda et al., 1993; Bender et al., 1991, 1998; Bender and Small, 1993; Guebre-Xabier et al., 2004; Katz et al., 2000; Powers and Belshe, 1993; Yager et al., 2008; Zheng et al., 2007). In this study, we evaluated the immunogenicity of influenza VLPs and their protective efficacy against influenza virus challenge in aged mice as well as young mice for comparison. Our results show that immunization with influenza VLPs induced strong immune responses that conferred effective protection against lethal influenza virus challenge in both young and aged mice.
2. MATERIALS AND METHODS
2.1. Animals, influenza virus, IIV vaccines, and purified HA proteins
Young adult female Balb/c mice (8 week-old) were purchased from the Charles River Laboratory, and aged female Balb/c mice (18 month-old) were purchased from the National Institute of Aging. All mice were housed in isolated chambers at the animal facility of the Emory University and all animal studies were carried out in accordance with guidelines of the Institutional Animal Care and Use Committee (IACUC) at Emory University.
Influenza virus A/PR/8/34 has been maintained in our group over 10 years (Sha and Compans, 2000). Mouse-adapted influenza virus A/PR/8/34 (H1N1) was prepared as lung homogenates from intranasally infected naïve mice and stored at −80 °C in aliquots until use. The titer of the challenge virus stock was first determined in chicken eggs to calculate the 50% egg-infectious dose (EID50) and then the 50% lethal infectious dose (LD50) was determined in young naïve mice (Balb/c, 8 weeks old). It was calculated that the LD50 of the challenge virus stock was approximately 104 EID50. For IIV vaccine preparation, influenza virus A/PR/8/34 was grown in chicken eggs and purified by centrifugation through a sucrose gradient. The purified virus was then inactivated by formalin as described previously (Sha and Compans, 2000). Protein concentration of the inactivated virus preparation (IIV vaccines) was determined by a BCA protein assay (Pierce Biotechnology, Rockford, IL), and then resuspended in PBS at 1 mg/ml and stored at −80 °C until use. The genes HA, NA, and M1 proteins were cloned by RT-PCR amplification of viral genomic RNA from purified influenza virus A/PR/8/34 following established protocols (Zhu et al., 2008).
A recombinant vaccinia virus expressing an HA-his fusion protein (The ecto-domain of influenza virus A/PR/8/34 HA was fused with a histag segment HHHHHH at its C-terminus) was generated and used to infect Hela cells. At 48 hr post-infection, supernatant was harvested and HA–his protein released into the medium was purified using Ni-NTA agarose beads (QIAGEN, Valencia, CA). Purified HA-his proteins were characterized by Coomassie Blue staining and Western blot.
2.2. Production and characterization of influenza VLPs
Influenza VLPs were produced in Sf9 insect cells by co-expression of influenza virus M1, HA, and NA proteins using the recombinant baculovirus expression system. The genes for influenza virus A/PR/8/34 (H1N1) HA and NA proteins were cloned into the plasmid vector pFastBacDual (Invitrogen, Carlsbad, California) under the Pphol and P10 promoters respectively. The gene for the M1 protein was cloned into the plasmid vector pFastBacI under the Pphol promoter. Recombinant baculoviruses were generated using the Bac-to-Bac Bacmid system (Invitrogen, Carlsbad, California) following the manufacturer’s protocol, and designated as rBV-HA/NA and rBV-M1 respectively. For VLP production, Sf9 cells (2 × 106/ml) were co-infected with rBV-HA/NA and rBV-M1 at MOIs (multiplicity of infection) of 5 and 2 respectively, and VLPs released into the medium were collected at 60 hr post infection. After clarification of cell debris, VLPs were concentrated by ultra-centrifugation and further purified through a discontinuous sucrose gradient (10–50%). Purified VLPs were then concentrated by ultra-centrifugation and re-suspended in PBS. Protein concentration of VLPs was determined using a BCA assay kit and the VLP preparations were adjusted by PBS to a final protein concentration of 1 mg/ml. Purified VLPs were characterized by Coomassie blue as well as Western blot analysis for the presence of HA, NA, and M1 proteins and the amount of incorporated HA proteins was analyzed by densitometry analysis using FluoroChem FC2 Imaging Illuminator coupled with AlphaEaseFC software (Alpha Innotech, San Leandro, CA). The influenza VLPs were further examined by electron microscopy using a Hitachi-H7500 transmission electron microscope (Hitachi. Ltd., Tokyo, Japan) by negative staining with 1% uranyl acetate, following established protocols (Ye et al., 2006). Rift valley fever virus (RVFV) VLPs were produced using recombinant baculovirus expressing the RVFV glycoprotein GP and purified following the same procedure, and were used as control VLPs in this study as indicated.
The hemagglutination activity of influenza VLPs or IIV was determined by incubating serial dilutions of VLPs with 0.5% chicken red blood cells for 45 min at room temperature following standard procedures. Neuraminidase activity of VLPs and purified influenza viruses was determined using an Amplex red neuraminidase assay kit (Molecular Probes, Invitrogen, Carlsbad, California). Briefly, VLPs or purified influenza viruses were serially diluted in 50 ul of 1x reaction buffer followed by addition of 50 ul of 2x working solution. After incubation for 30 min at 37 °C under dark, fluorescence activity was measured by a Synergy 2 bioreader (BioTek, Winooski, VT) using 530/590 nm (excitation/emission) wavelengths and the values were used to indicate relative NA activities.
2.3. Bone marrow-derived dendritic cell (BMDC) preparation and stimulation
Mouse BMDCs were generated following procedures as reported by Lutz et al. (Lutz et al., 1999). Briefly, femurs were obtained from sacrificed mice and the marrows were flushed out with complete medium (RPM1640 plus 10% fetal calf serum) and then filtered through a cell strainer. After lysis of red blood cells, 2×106 bone marrow cells were cultured in a 100 mm petri dish in 10 ml culture medium (complete medium supplemented with 20 ng/ml of recombinant mouse GM-CSF, Sigma-Aldrich, St. Louis, MO), and half of the medium was replaced with fresh culture medium on days 3 and 6. BMDCs were collected on day 7 and over 70% of the cells were CD11c positive based on flow cytometry analysis. For DC-stimulation, 105 BMDCs were seeded in each well in a 96-well plate and incubated with influenza VLPs (10 μg/ml), IIV (10 μg/ml), as well as LPS (10 ng/ml, positive control, Sigma-Aldrich, St. Louis, MO) or culture media only (negative control) in triplicates. In parallel, the samples were heated at 100 °C for 30 min before being added to the DCs. Supernatants were harvested after 24 hr incubation at 37 °C, and cytokine levels in the supernatants were measured by ELISA in duplicate using commercially available kits (eBioscience, San Diego, CA) for IL-6 and TNF-alpha.
2.4. Immunization and challenge of mice
Groups of young (8 weeks old) and aged mice (18 months old) were immunized by intramuscular injection (IM) twice at 4-week intervals with 10 or 1 μg of influenza VLPs or 1 ug of IIV vaccines dissolved in 100 ul PBS as indicated for each group. At two weeks after the second immunization, serum samples were collected by retro-orbital bleeding, heat-inactivated ay 55 °C for 30 min, and stored in −80 °C until analysis. At four weeks after the second immunization, mice were lightly anesthetized by isofluorane and challenged by intranasal instillation of 10x LD50 (approximately 105 EID50) of the homologous mouse-adapted influenza virus A/PR/8/34 (diluted in in 50 μl PBS). Mice were monitored daily for weight changes and signs of disease for 14 days, and mice that exhibited over 25% body weight loss were sacrificed in accordance with IACUC guidelines.
2.5. ELISA
Influenza HA-specific antibodies were measured in individual mouse sera by ELISA following established protocols (Sun et al., 2009). Purified HA-his proteins were used as coating antigens and a standard curve was constructed by coating each ELISA plate with serial 2-fold dilutions of purified mouse IgG with known concentrations (eBioscience, San Diego, CA). The concentrations of HA-specific antibodies in serum samples were calculated using the obtained standard curves and expressed as the amount of HA-specific antibodies in 1 ml of serum samples (ng/ml).
The amount of HA proteins incorporated in VLPs or IIV vaccines was further determined by a quantitative ELISA. Briefly, microtiter plate was first coated with goat antibodies against H1N1 influenza virus (US Biological, Swampscott, MA) followed by addition of serial dilutions of influenza VLPs or IIV vaccines. HA proteins bound to coated wells were detected by ELISA using mouse antibodies against HA (prepared from mice immunized with A/PR/8/34 HA DNA vaccines) and then HRP-conjugated goat-anti-mouse IgG antibodies following standard procedures. A standard curve was constructed by using serial dilutions of purified HA proteins to calculate the amount of HA in the samples, which was expressed as the amount of HA in 1 μg of samples (ng/μg).
2.6. Antibody avidity
The avidity of mouse antibody responses against HA was determined using sodium thiocyanate (NaSCN) displacement ELISA as developed by Luxton and Thompson (1990) with slight modifications. Briefly, serum samples were diluted in PBS to a concentration that would give an O.D. value of about 1.0 in ELISA based on obtained results. Microtiter plate was coated with purified HA followed by blocking and then addition of 100 μl diluted serum samples for 2 hr binding at room temperature. After washing, 100 μl of different concentrations of NaSCN (a chaotic agent diluted in PBS) was added to each well and incubated for 30 min at room temperature. The amount of antibodies bound to HA was determined by addition of HRP-conjugated goat-anti-mouse IgG antibodies. The antibody avidity was expressed as the percentage of remaining HA-binding antibodies for each serum sample after incubation with different concentrations of NaSCN compared to incubation with PBS only (set as 100%).
2.7. Hemagglutination inhibition (HAI) assay
The HAI assay was performed following established protocols (Wang et al., 2008). Briefly, mouse sera was heat-inactivated at 56 °C for 1 h and then treated with receptor destroying enzyme (Denka Seiken, Tokyo, Japan) at 37 °C overnight according to the manufacturer’s instruction. After treatment, 25-μl aliquots of 2-fold serially diluted serum samples were incubated with 25-μl virus containing 4 HA units of influenza virus A/PR/8/34 at 37 °C for 1 h, followed by incubation with 50 μl of 0.5% chicken red blood cells (LAMPIRE Biological Laboratories, Pipersville, PA) at 25 °C for 45 min. The HAI titer was defined as the reciprocal of the highest serum dilution that inhibited hemagglutination.
2.8. Flow cytometry analysis of CD8 T cell responses
Mice that survived the challenge were sacrificed on day 15 post challenge to prepare splenocytes following established protocols (Ye et al., 2004). Lymphocytes were stimulated with a peptide corresponding to a CD8 T cell epitope for the influenza virus A/PR/8/34 HA or NP protein (IYSTVASSL and TYQRTRALV respectively, synthesized at the Emory Microchemical Facility, Atlanta, GA) or an irrelevant peptide (AMQMLKETI, corresponding to a segment in the HIV Gag protein) at 10 μg/ml for 6 h in presence of 10 μg/ml Brefeldin A (Sigma-Aldrich, St. Louis, MO). After stimulation, the cells were washed twice with PBS containing 3% fetal calf serum and then stained with PerCP-conjugated rat anti-mouse CD4 and phycoerythrin (PE)-conjugated rat anti-mouse CD8 antibodies (BD Pharmingen, San Diego, CA). Cells were then fixed and permeabilized with Fix&Perm buffers and stained for intracellular interferon gamma (IFN-γ) with allophycocyanin (APC)-conjugated rat anti-mouse IFN-γ antibody (BD Pharmingen, San Diego, CA). The levels of CD8 T cell responses were determined by flow cytometry on a BD FACS-Calibre Station (BD Immunocytometry Systems, San Jose, CA) and data was analyzed with the Flowjo 4.2 software (Tree Star, Ashland, OR).
2.9. Statistical Analysis
The average value and standard deviation for the level of immune responses within each group were calculated for comparison and the significance of the differences between the results from different groups was determined by a student t test using the Excel program (Microsoft, Redmond, WA).
3. RESULTS
3.1. Characterization of influenza VLPs
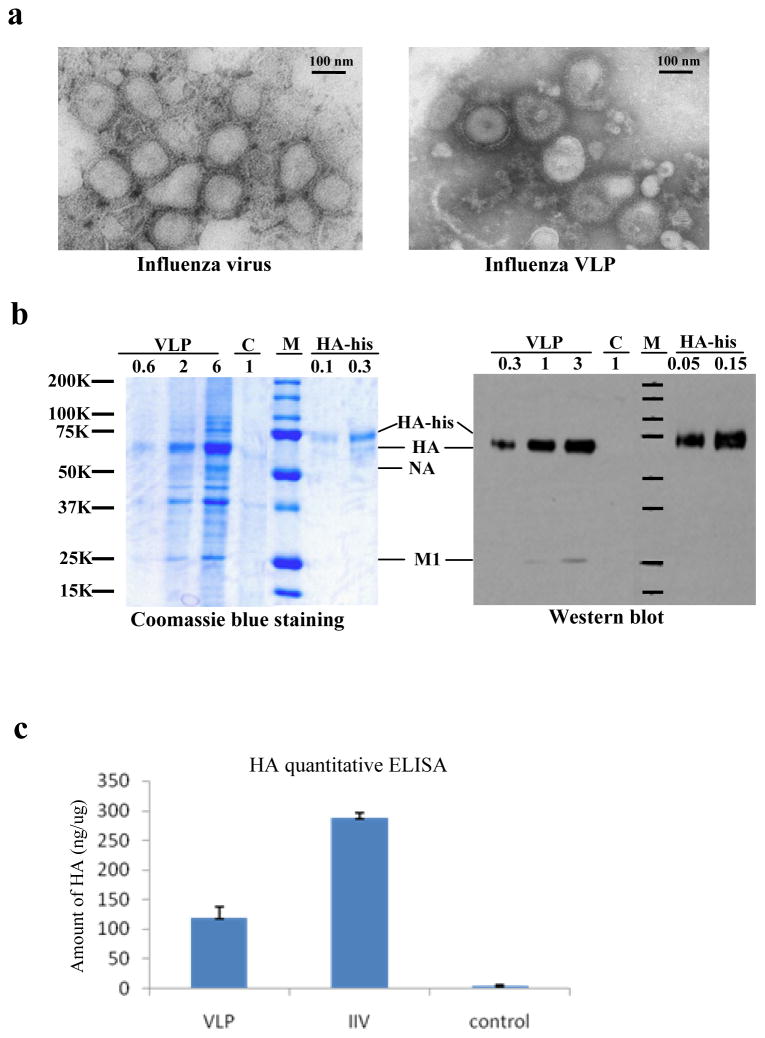
Influenza VLPs were produced by co-expression of viral M1, HA, and NA proteins in Sf9 insect cells using the recombinant baculovirus expression system and purified as described in Materials and Methods. As shown in Figure 1a, influenza VLPs exhibit a spherical morphology that is similar in size to purified influenza virus when examined under an electron microscope. Protein profiles of influenza VLPs were analyzed by Coomassie blue staining and Western blot analysis. As shown in Figure 1b, the HA was readily detected in influenza VLPs by both Coomassie blue staining and Western blot analysis. Densitometry analysis showed that the amount of HA incorporated in VLPs represents approximately 15% of total protein (2 μg of VLPs contained about 0.35 μg HA proteins as determined from Coomassie blue staining, whereas 1 μg of VLPs contained about 0.12 μg HA proteins as determined from Western blot analysis). The M1 and NA proteins were also readily detected in VLPs by Coomassie blue staining. However, the NA protein was not detected by Western blot, probably due to relatively lower level of NA-specific antibodies in detecting sera. The amount of HA proteins in influenza VLPs was also determined by quantitative ELISA. As shown in Figure 1c, 1 μg of influenza VLPs contained about 120 ng HA protein, which is in agreement with the results from Western blot analysis and Coomassie blue staining. In comparison, 1 μg of purified influenza virus contained approximately 280 ng HA proteins.
Figure 1. Characterization of influenza VLPs.
(a). Electron microscopy of influenza VLPsPurified influenza VLPs and influenza virus were stained with 1% uranyl acetate followed by examination under a transmission electron microscope. (b). Characterization of influenza VLPs by Coomassie blue staining and Western blot analysis. The VLPs, purified influenza viruses, and purified HA proteins were analyzed by SDS-PAGE followed by Coomassie blue staining or Western blot. VLP, influenza VLPs; C, control RVFV VLPs produced in Sf9 insect cells; M, molecular weight markers; HA-his, purified HA proteins. The numbers on top of each lane represent the amount of total proteins (μg) in the samples. (c). Comparison of the amount of HA in influenza VLPs and IIV vaccines. The amount of HA protein in influenza VLPs and IIV vaccine preparations were determined by a quantitative ELISA as described in Materials and Methods. A standard curve was constructed using purified HA-his proteins to calculate the amount of HA in 1 μg of influenza VLPs or IIV vaccines (ng/μg). VLP, purified influenza VLPs; IIV, inactivated influenza virus; Control, RVFV VLPs. Error bars represent standard deviations of results from 3 independent assays.
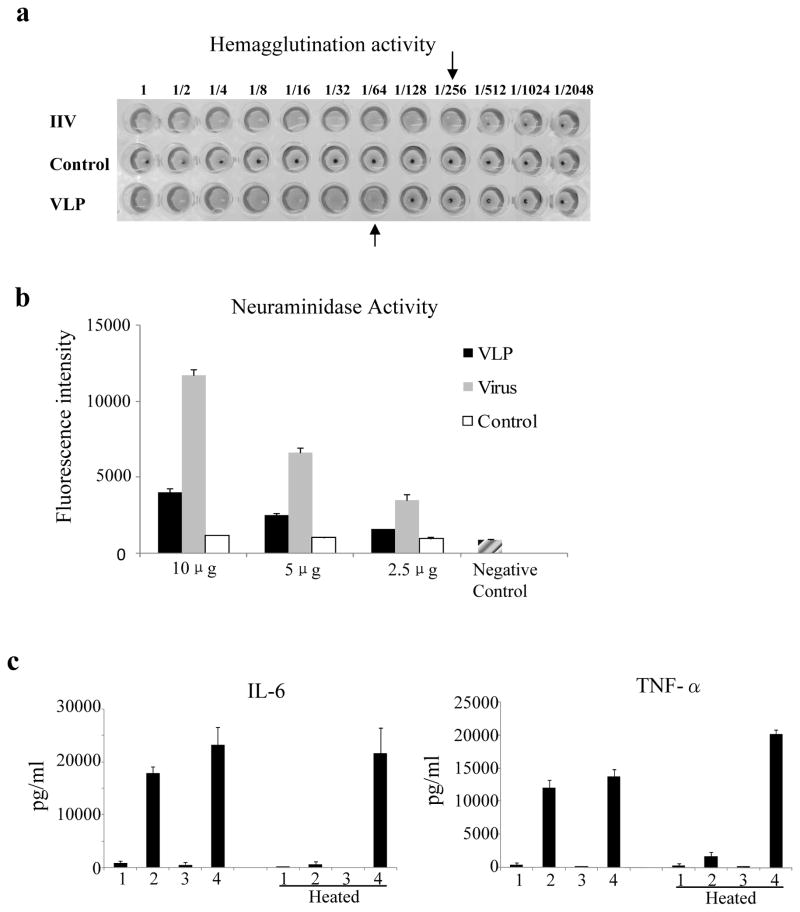
We further characterized the functional activity of influenza VLPs. The hemagglutination (HA) and neuraminidase (NA) activities of influenza VLPs were determined in comparison with purified influenza virus. As shown in Figure 2, both HA activity and NA activities were readily detected in influenza VLPs, However, it was also noted that the HA and NA activities in influenza VLPs were relatively lower compared to purified influenza viruses. As shown in Figure 2a, 1 μg of VLPs contained approximately 64 HA units whereas 1 μg of purified virus contained about 256 HA units. More detailed analysis showed that HA units in 1 μg of VLPs (80±20) was about 4-fold lower compared to the HA units in 1 μg of purified virus (300±20). Further, as shown in Figure 2b, the level of NA activity detected in 10 μg VLPs was similar to those detected in 2.5 μg purified virus. The lower HA and NA activities in influenza VLPs compared to purified influenza virus were probably due to the relatively lower amount of protein incorporation as shown for the HA in Figure 1c. In addition, the HA proteins in insect cell-produced influenza VLPs migrated slightly faster in SDS-PAGE compared to mammalian cell-produced purified HA (Figure 1b), probably due to the differences in glycosylation between glycoproteins synthesized in insect cells and mammalian cells (Mellquist-Reimenschneider et al., 2003). Such differences in glycosylation may also affect HA and NA activities. Nonetheless, the detection of both HA and NA activities in influenza VLPs indicated that the viral HA and NA proteins incorporated in influenza VLPs produced in Sf9 insect cells retained their functional activities. We also investigated whether influenza VLPs exhibit DC-stimulating activities as observed for Ebola VLPs in our previous studies (Ye et al., 2006). As shown in Figure 2c, incubation of DCs with influenza VLPs but not IIV stimulated secretion of IL-6 and TNF-alpha at high levels. Further, heating VLPs at 100 °C for 30 min eliminated their cytokine-stimulating activity while heating LPS at 100 °C for 30 min did not affect its activity, indicating that the DC-stimulating activity by VLPs is not due to endotoxin contamination.
Figure 2. Functional analysis of influenza VLPs.
(a). Hemagglutination activity of influenza VLPsSerial dilutions of influenza VLPs, inactivated influenza viruses, or control rift valley fever virus VLPs in 50 μl of PBS were added to each wells of a v-bottom plate, followed by addition of 50 μl chicken red blood cells (0.5%). Hemagglutination of chicken red blood cells was recorded after 45 min incubation at room temperature. VLP, influenza VLPs; Control, control rift valley fever virus VLPs; IIV, inactivated influenza virus. (b). Neuraminidase (NA) activity in influenza VLPs. Different amounts of influenza VLPs, control rift valley fever virus VLPs, or purified influenza viruses were incubated with a substrate solution (Amplex Red/HRP/galactose oxidase/fetuin) for 30 min at 37 °C. The levels of NA activities were determined by measuring the fluorescence activity at 530/590 nm (excitation/emission) wavelengths. Error bars represent standard deviations for each sample. VLP, influenza VLPs; Control, RVFV VLPs; Virus, purified influenza virus A/PR/8/34; Negative control, supplied with the assay kit. (c). Stimulation of mouse bone marrow-derived dendritic cells (BMDCs) by influenza VLPs. BMDCs were prepared and stimulated with purified influenza VLPs or IIV vaccines as described in Materials and Methods. DCs incubated with culture medium only or LPS (10 ng/ml) were used as negative and positive controls respectively. To ensure that stimulation of DC by VLPs or purified viruses was not due to contamination by endotoxin, the DCs were also incubated with the same amount of VLPs or IIV vaccines that had been heat-treated at 100 °C for 30 min. Cell-free supernatants were harvested 24 hr after incubation at 37 °C in 5% CO2 and assayed for the levels (pg/ml) of IL-6 and TNF-alpha by ELISA. Error bars represent standard deviations of the triplicate wells for each sample and the results shown represent typical results obtained in two different stimulation experiments. 1, Medium (negative control); 2, influenza VLPs (10 μg/ml); 3, IIV (10 μg/ml); 4, LPS (10 ng/ml, positive control).
3.2. Immunization with influenza VLPs confers effective protection against lethal influenza virus challenge in both young and aged mice
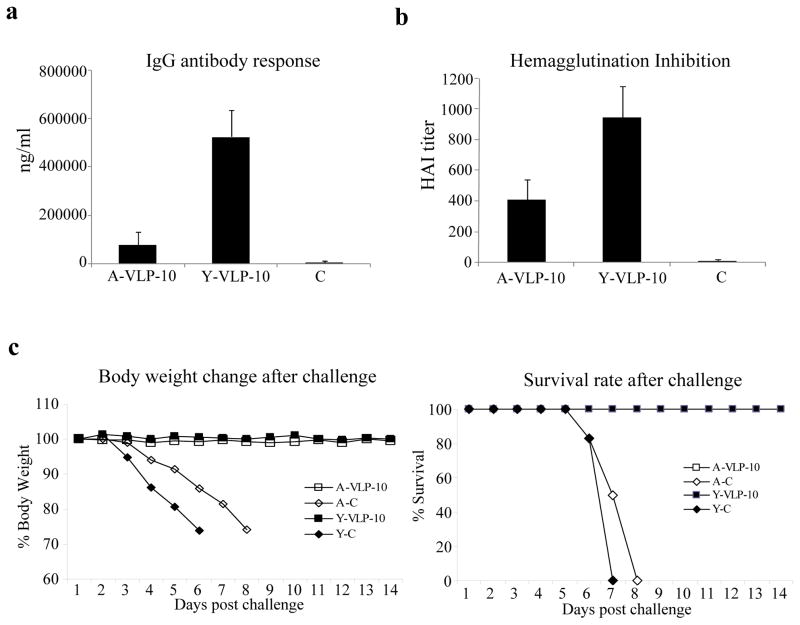
After characterization, the immunogenicity of influenza VLPs was investigated in both young and aged mice for comparison. Groups of young (8 weeks old) and aged mice (18 months old) were immunized with 10 μg of influenza VLPs twice at 4-week intervals and blood samples were collected at two weeks after the second immunization for analysis of antibody responses. As shown in Figure 3a, the levels of IgG antibodies against HA induced by immunization with influenza VLPs reached approximately 110 000 ng and 500 000 ng per ml in aged and young mice respectively, and the levels of antibody response induced in young mice were significantly higher than those induced in aged mice (p<0.01). The functional activities of the antibody responses were determined by an HAI assay and as shown in Figure 3b, the HAI titers of sera from vaccinated mice were about 1:400 in aged mice and were significantly higher in young mice at about 1:900 (p<0.05).
Figure 3. Immunization with influenza VLPs induced strong antibody responses and conferred effective protection against lethal influenza virus challenge in both young and aged mice.
Groups of young and aged mice (6 per group, 8-weeks old and 18-months old respectively) were immunized twice at 4-week intervals by IM injection of 10 μg influenza VLPs. The control groups received PBS only. Blood samples were collected at 2 weeks after the second immunization and analyzed for antibody responses against influenza virus. (a). IgG antibody responses against the influenza virus HA. The levels of antibody responses against HA were determined by ELISA using purified HA proteins as coating antigens, and expressed as the amount of HA-specific antibodies in 1 ml of serum samples (ng/ml). Error bars indicate standard deviations for each group. A-VLP-10, aged mice immunized with 10 μg VLPs; Y-VLP-10, young mice immunized with 10 μg VLPs; control, sera from young and aged control group mice. (b). Inhibition of hemagglutination by sera from immunized mice. The ability of sera to inhibit hemagglutination by influenza virus A/PR/8/34 were determined and expressed as the highest dilution that resulted in complete inhibition of hemagglutination (HAI titer). Data are presented as the mean ± standard deviation. (c). Mouse survival rate and body weight change after lethal influenza virus challenge. At 4 weeks after the second immunization, mice were challenged by intranasal instillation of 10x LD50 of mouse-adapted influenza virus A/PR/8/34. Mice were monitored and weighed daily after challenge, and were sacrificed when found to display severe signs of illness or loss more than 25% body weight in accordance with IACUC guidelines. A-VLP-10, aged mice immunized with 10 μg VLPs; Y-VLP-10, young mice immunized with 10 μg VLPs; Y-C, control group of young mice; A-C, control group of aged mice.
To investigate whether these mice would be protected against lethal influenza virus challenge, mice were challenged with 10x LD50 of mouse-adapted influenza virus A/PR/8/34 at 4 weeks after the second immunization. As shown in Figure 3c, all control mice (including both young and aged) succumbed to the challenge by day 8 post infection, whereas all vaccinated mice survived. Further, no weight loss was observed after challenge in either young or aged mice that were immunized with influenza VLPs. These results show that immunization with influenza VLPs induced strong antibody responses that provided highly effective protection against lethal influenza virus challenge in both young and aged mice.
3.3. Protection of young and aged mice against lethal influenza virus challenge by immunization with a low dose of influenza VLPs
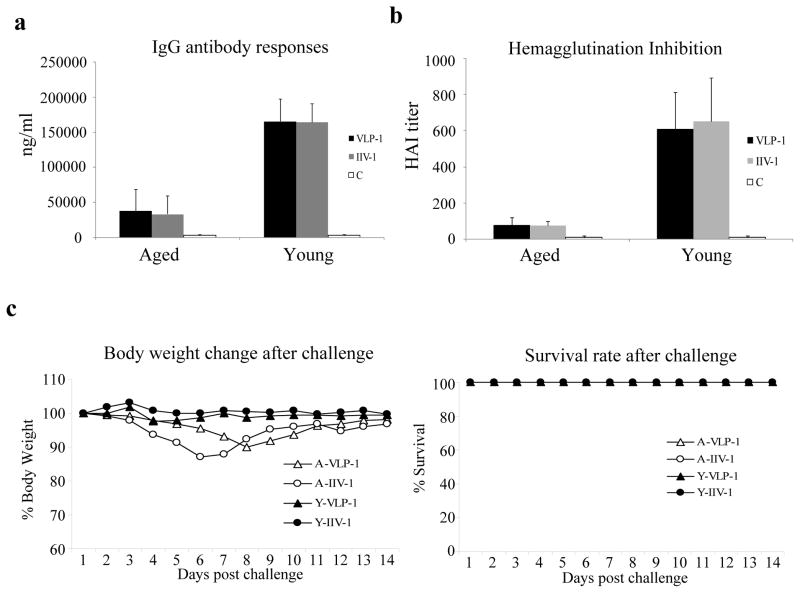
We further investigated whether protection of young and aged mice against influenza virus infection can be achieved by immunization with a reduced dose of influenza VLPs. Mice (8-weeks old for young adult mice and 18-months old for aged mice respectively) were immunized twice by IM injection of 1 μg influenza VLPs at 4-week intervals. In parallel, we also carried out immunization studies using 1 μg of IIV vaccines to determine whether there might be a difference between the immunogenicity of influenza VLPs and IIV vaccines in young and/or aged mice. At two weeks after the second immunization, blood samples were collected and analyzed for antibodies against influenza HA and hemagglutination inhibition (HAI) activities. As shown in Figure 4a, similar to immunization with a high dose (10 ug) of influenza VLPs, immunization with a low dose (1 ug) of influenza VLPs or IIV vaccines induced significantly higher levels of antibodies against HA in young mice than in aged mice (p<0.01), at approximately 160 000 ng per ml in young mice and at about 33 000 ng per ml in aged mice respectively. Similarly, the serum HAI titers induced by immunization with a low dose of influenza VLPs or IIV vaccines were also significantly higher in young mice than in aged mice (p<0.01) at 1:600 and 1:80 respectively (Figure 4b). On the other hand, it was also observed that immunization with influenza VLPs or IIV vaccines induced similar levels of antibody responses and serum HAI titers in young mice as well as in aged mice, indicating that the influenza VLPs exhibit similar immunogenicity to IIV vaccines in both young and aged mice. At 4 weeks after the second immunization, mice immunized with the low dose of VLPs or IIV vaccines were challenged with 10x LD50 of mouse-adapted influenza virus A/PR/8/34. As shown in Figure 4c, all mice survived the challenge. However, while no weight loss was observed in young mice, aged mice immunized by a low dose of either VLPs or IIV vaccines experienced 10 to 12% weight loss after challenge.
Figure 4. Protective efficacy in young and aged mice against lethal influenza virus challenge by immunization with a low dose of influenza VLPs or IIV vaccines.
Groups of young and aged mice (6 per group, 8-weeks old and 18-months old respectively) were immunized twice at 4-week intervals by IM injection of 1 μg influenza VLPs or IIV vaccines. Blood samples were collected at 2 weeks after the second immunization and analyzed for antibody responses against influenza virus. (a). IgG antibody responses against the influenza virus HA. The levels of antibody responses against HA were determined by ELISA using purified HA proteins as coating antigens, and expressed as the amount of HA-specific antibodies in 1 ml of serum samples (ng/ml). Error bars indicate standard deviations for each group. VLP-1, mice immunized with 1 μg VLPs; IIV-1, aged mice immunized with 1 μg IIV vaccines; C, control group mice; (b). Inhibition of hemagglutination by sera from immunized mice. The ability of sera to inhibit hemagglutination by influenza virus A/PR/8/34 were determined and expressed as the highest dilution that resulted in complete inhibition of hemagglutination (HAI titer). Data are presented as the mean ± standard deviation. (c). Mouse survival rate and body weight change after lethal influenza virus challenge. At 4 weeks after the second immunization, mice were challenged by intranasal instillation of 10x LD50 of mouse-adapted influenza virus A/PR/8/34 and mice were monitored and weighed daily after challenge. A-VLP-1, aged mice immunized with 1 μg VLPs; A-IIV-1, aged mice immunized with 1 μg IIV vaccines; Y-VLP-1, young mice immunized with 1 μg VLPs; Y-IIV-1, young mice immunized with 1 μg IIV vaccines.
3.4. Vaccine dose reduction affects the avidity of antibody responses induced in aged mice but not in young mice
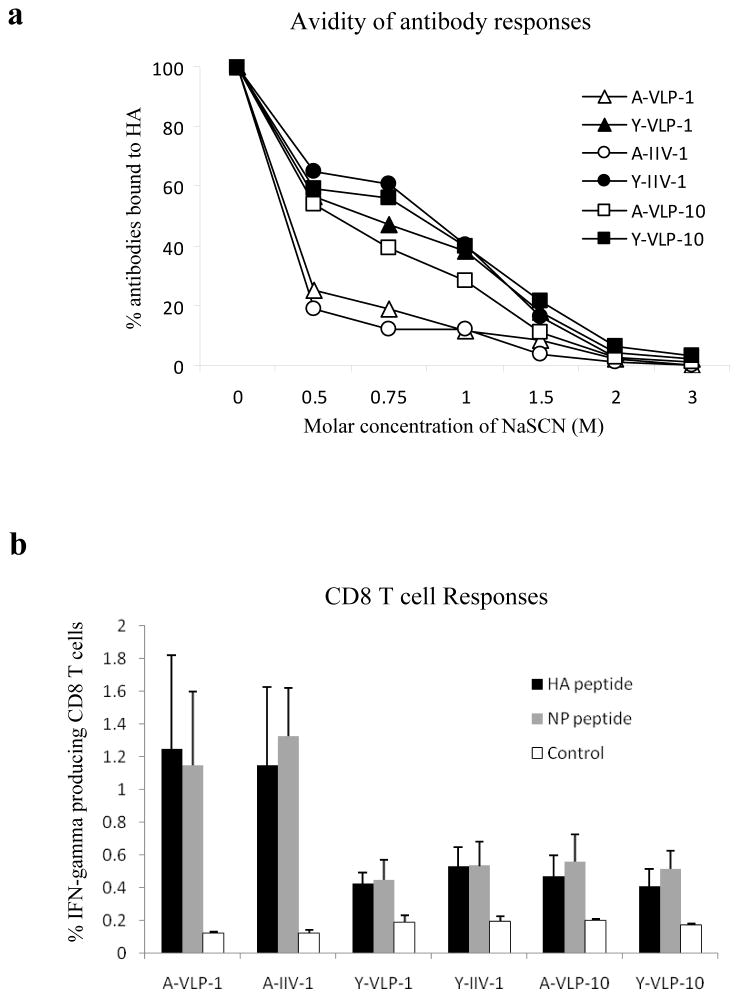
The significant weight loss observed in aged mice immunized with low dose VLPs or IIV vaccines indicates that the reduced vaccine doses exert a more profound effect on the protective efficacy in aged mice than in young mice. Further, it was noted that the HAI titers of sera from aged mice immunized with a low dose (1 μg) of VLPs or IIV vaccines were significantly reduced (p<0.01) by about 5-fold compared to those immunized with a high dose (10 μg) of VLPs (HAI titers at about 1:80 and 1:400 respectively). On the other hand, the HAI titers of sera from young mice immunized with a low dose of VLPs or IIV vaccines were only slightly reduced (p>0.05) compared to those immunized with a high dose of VLPs (HAI titers at about 1:600 and 1:900 respectively). To gain further understanding of the differences in protective efficacy between young and aged mice, we compared the avidity of vaccine-induced antibody responses using sodium thiocyanate (NaSCN) displacement ELISA as developed by Luxton and Thompson (1990). In this assay, antibody avidity is measured by determining the relative levels of antibody-binding in presence of different concentrations of NaSCN and antibodies with lower avidity are more easily displaced from their cognate antigens by using NaSCN at low concentrations. As shown in Figure 5a, the addition of 0.5M NaSCN drastically reduced (by almost 80%) binding of HA by antibodies from aged mice immunized with a low dose of VLPs or IIV vaccines. In contrast, a similar reduction in HA binding by antibodies from young mice immunized with a low dose of VLPs or IIV vaccines required the addition of 1.5M NaSCN, a 3-fold increase. The displacement of HA-binding antibodies in sera from aged mice immunized with a low dose of influenza VLPs or IIV vaccines by low concentrations of NaSCN showed that these antibodies are of lower avidity to HA as compared to sera from vaccinated young mice. Interestingly, antibodies in both young and aged mice immunized with a high dose of VLPs exhibited similar avidities to HA that were comparable to antibodies in young mice immunized with a low dose of VLPs or IIV vaccines.
Figure 5.
(a). Comparison of the avidity of antibody responses induced in young and aged mice. Sera collected from vaccinated mice were tested for their avidity to purified HA as described in Materials and Methods. The antibody avidity was expressed as the percentage of remaining HA-binding antibodies for each serum sample after incubation with different concentrations of NaSCN compared to incubation with PBS only (set as 100%). A-VLP-1, aged mice immunized with 1 μg VLPs; A-IIV-1, aged mice immunized with 1 μg IIV vaccines; Y-VLP-1, young mice immunized with 1 μg VLPs; Y-IIV-1, young mice immunized with 1 μg IIV vaccines; A-VLP-10, aged mice immunized with 10 μg VLPs; Y-VLP-10, young mice immunized with 10 μg VLPs. (b). Comparison of CD8 T cell responses induced in mice after challenge. At 2 weeks after challenge, mice were sacrificed and splenocytes were prepared and stimulated with peptides corresponding to known CD8 T cell epitopes in HA or NP or a Control peptide. The cells were then stained for cell surface CD8 as well as intracellular IFN-γ protein followed by flow cytometry analysis. HA peptide, a peptide corresponding to a CD8 T cell epitope in HA; NP peptide, a peptide corresponding to a CD8 T cell epitope in NP; Control, a peptide corresponding to a CD8 T cell epitope in the HIV Gag. The percentages of IFN-γ producing CD8 T cells stimulated by different peptides are shown. Data are presented as the mean ± standard deviation for each group.
Mice that survived the challenge were sacrificed on day 15 post infection and splenocytes were prepared and analyzed for CD8 T cell responses against known epitopes in the influenza virus HA and nucleoprotein (NP). As shown in Figure 5b, IFN-γ-producing CD8 T cells against epitopes in the influenza virus HA or NP were induced in both young and aged mice after challenge. The levels of IFN-γ-producing CD8 T cells against epitopes in the influenza virus HA or NP in young mice immunized with a low dose of VLPs or IIV vaccines as well as in aged mice immunized with a high dose of VLPs were about 2-fold higher than background levels that were stimulated by an irrelevant peptide (p<0.05). On the other hand, the levels of IFN-γ-producing CD8 T cells against epitopes in the influenza virus HA or NP in aged mice immunized with low dose VLPs or IIV vaccines were more than 5-fold higher than background levels and were significantly higher than those induced in young mice or in aged mice vaccinated with a high dose of influenza VLPs (p<0.05).
4. DISCUSSION
In this study, we investigated the efficacy of insect cell-produced influenza VLPs to protect against lethal influenza virus challenge in both young and aged mice. Characterization of these VLPs showed that the incorporated HA and NA proteins retained their functional activities and the influenza VLPs but not inactivated influenza viruses were found to stimulate secretion of inflammatory cytokines from mouse BMDCs. In agreement with previous studies (Bright et al., 2007; Galarza et al., 2005; Latham and Galarza, 2001; Mahmood et al., 2008; Pushko et al., 2005, 2007; Quan et al., 2007, 2008), immunization of young mice with influenza VLPs induced high levels of antibody responses and conferred highly effective protection against lethal influenza virus challenge. We further extended the evaluation of influenza VLPs in aged mice and our results showed that immunization with influenza VLPs also protected aged mice against lethal influenza virus challenge, despite induction of relatively lower levels of antibody responses compared to the responses induced in young mice. Moreover, immunization with a higher dose of influenza VLPs conferred highly effective protection of aged mice against lethal influenza virus challenge with no visible signs of disease.
Immunosenescence is a hallmark of the aging process, which is manifested by reduced immune responses to vaccination and increased susceptibility to virus infection as observed in human clinical studies (Aw et al., 2007; Kovaiou et al., 2007). While the underlying mechanism remains to be elucidated, cumulative evidence from both clinical studies and studies using animal models indicates that immunosenescence is associated with a decline in many aspects of both innate and adaptive immune responses (Aw et al., 2007; Kumar and Burns, 2008). In this study, we observed that IgG antibody responses induced by both influenza VLPs and IIV vaccines were significantly ower (by about 5-fold, p<0.01) in aged mice than in young mice. This is similar to the results from several early studies that investigated the immunogenicity of different influenza vaccine approaches in aged mice (Bender et al., 1998; Guebre-Xabier et al., 2004; Katz et al., 2000; Sambhara et al., 1998). Moreover, in addition to their reduced levels, the antibody responses in aged mice immunized with a low dose of influenza VLPs or IIV vaccines also exhibit markedly reduced avidity for HA compared to the antibody responses induced in young mice. It has long been established that upon antigen stimulation, activated B cells undergo class switching as well as somatic hypermutation in germinal centers to produce antibodies with increased avidity (Stavnezer, 1996). Thus, the differences in the avidity of antibody responses in young and aged mice suggest that the process of B cell activation may be impaired in aged mice. This profound reduction in both the levels and avidity of the antibody responses in aged mice may result from a dysfunctional T cell responses (Haynes and Eaton, 2005), which are important for both B cell class switching (Zheng et al., 1997) and antibody maturation (Romagnani, 1997; Paul and Benacerraf, 1977). Interestingly, immunization with a higher dose of influenza VLPs effectively increased the avidity of the antibody responses in aged mice to a level that is comparable to those in young mice. These results indicate that the deficiency in B cell activation and antibody maturation in aged mice can be overcome by a higher vaccine dose. It is possible that a higher vaccine dose may exert a more potent stimulation of T cell responses in aged mice that leads to more effective B cell activation. Further elucidation of the underlying mechanism for inducing antibody responses of high avidities in aged mice as well as in elderly humans is needed for the development of new vaccine strategies to improve protection of the aged population against influenza virus infection.
In assessing the efficacy of protection against influenza virus infection, aged mice immunized by a high dose of influenza VLPs were effectively protected from lethal influenza virus challenge with no weight loss, similar to their younger counterparts. On the other hand, significant weight loss was observed in aged mice but not in young mice that were immunized with a low dose of influenza VLPs or IIV vaccines. Further, we also observed that significantly higher levels of CD8 T cell responses were induced in aged mice immunized with a low dose of influenza VLPs or IIV vaccines after challenge compared to young mice (p<0.05). As CD8 T cell responses are more effectively induced by virus infection, the induction of higher levels of CD8 T cell responses after challenge indicate more extended virus replication, which is in agreement with the observed weight loss in aged mice immunized with a low dose of VLPs or IIV vaccines. The less effective protection seen in aged mice immunized with a low dose of influenza vaccines may result from reduced levels and lower avidity of induced antibody responses. Of note, sera from aged mice immunized by a low dose of influenza vaccines had HAI titers at about 1:80 on average before challenge, ranging between 1:40 and 1:160. In contrast, we observed in recent studies that young mice which received a single immunization by IIV vaccines had serum HAI titers at about 1:40 and these mice were completely protected against influenza virus challenge with no significant weight loss (Zhu et al., 2009). Thus, in addition to the reduced immunogenicity of influenza vaccines in aged mice, these results also indicate that effective protection of aged mice against influenza infection may require a higher level of antibody responses as compared to young mice. The different requirements for protection of young and aged mice against lethal influenza virus challenge underscore the need to develop new vaccine strategies with enhanced protective efficacy in the aged population, which is more susceptible to influenza virus infection and associated illness.
It has been reported that antibody responses induced by immunization with influenza VLPs in young mice exhibited broader and higher levels of hemagglutination inhibition activity than immunization with IIV vaccines containing the same amount of HA (Bright et al., 2007). In this study, we show that the levels of antibody responses induced by influenza VLPs were comparable to those induced by IIV vaccines in both young and aged mice when vaccines were given at the same dose of total protein, despite the lower amount of HA proteins contained in influenza VLPs compared to IIV vaccines. Our results confirm previous observations from a different angle and further demonstrate the potency of influenza VLPs compared to IIV vaccines. Of interest, the present study of influenza VLPs showed that they also stimulated secretion of inflammatory cytokines by mouse BMDCs. In contrast, IIV vaccines did not exhibit DC-stimulating activities. A similar observation had also been made in earlier studies on Ebola VLPs and inactivated virions, which showed that VLPs but not inactivated virions of Ebola virus stimulated cytokine secretion from DCs (Bosio et al., 2004; Warfield et al., 2003). Thus, it is possible that the DC-stimulating activity of influenza VLPs may augment the induction of antibody responses. In support of this, it has been shown that induction of antibody responses by influenza vaccines can be augmented through providing co-stimulation of DCs during immunization by using a co-polymer adjuvant (Katz et al., 2000), an immunostimulant patch containing bacterial heat-liable enterotoxin (Guebre-Xabier et al., 2004), a modified vaccinia virus expressing co-stimulatory molecules CD80 and CD86 (Sambhara et al., 2001), or immunostimulating complexes (Sambhara et al., 1998). As immunosenescence in the aged population is also manifested by reduced antigen presenting activity of DCs (Plowden et al., 2004; Sambhara et al., 2001), the DC-stimulating activity of influenza VLPs makes it particularly attractive for vaccination of elderly humans against influenza virus infection.
The development of influenza VLPs as a candidate vaccine strategy offers several potential advantages. Production of VLPs in cell culture may eliminate several drawbacks associated with the egg-based system for production of influenza vaccines such as possible disruption of vaccine supplies due to shortage of fertilized chicken embryos or potential low-yield when producing highly pathogenic avian influenza viruses. Also, VLPs are devoid of viral genomic RNAs and they are thus non-infectious and safe for broad applications. Moreover, influenza VLPs have also been reported to elicit broadly protective immune responses against virus infection (Bright et al., 2007; Mahmood et al., 2008; Wang et al., 2008). In addition, production of influenza VLPs in insect cells using the recombinant baculovirus expression system offers high yields, producing 5 to 10 mg purified VLPs in 1 liter insect cell culture that is similar in range to the amount of purified influenza virus obtained from 1 liter egg allantoic fluid. As insect cells are cultured in suspension, this process can be easily scaled up using large bioreactors for production under GMP (Good Manufacturing Practice) conditions. The ability of influenza VLPs to activate DCs and to induce protective immune responses in both young and aged mice further demonstrates their potential as a new vaccine strategy for achieving more effective protection against influenza virus infection in the aged population.
Acknowledgments
This work was supported in part by the National Institutes of Health grant AI06652 and funding from the Emory University Global Health Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ada GL, Jones PD. The immune response to influenza infection. Curr Top Microbiol Immunol. 1986;128:1–54. doi: 10.1007/978-3-642-71272-2_1. [DOI] [PubMed] [Google Scholar]
- Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology. 2007;120:435–446. doi: 10.1111/j.1365-2567.2007.02555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Yehuda A, Ehleiter D, Hu AR, Weksler ME. Recombinant vaccinia virus expressing the PR/8 influenza hemagglutinin gene overcomes the impaired immune response and increased susceptibility of old mice to influenza infection. J Infect Dis. 1993;168:352–357. doi: 10.1093/infdis/168.2.352. [DOI] [PubMed] [Google Scholar]
- Bender BS, Johnson MP, Small PA. Influenza in senescent mice: impaired cytotoxic T-lymphocyte activity is correlated with prolonged infection. Immunology. 1991;72:514–519. [PMC free article] [PubMed] [Google Scholar]
- Bender BS, Small PA., Jr Heterotypic immune mice lose protection against influenza virus infection with senescence. J Infect Dis. 1993;168:873–880. doi: 10.1093/infdis/168.4.873. [DOI] [PubMed] [Google Scholar]
- Bender BS, Ulmer JB, DeWitt CM, Cottey R, Taylor SF, Ward AM, Friedman A, Liu MA, Donnelly JJ. Immunogenicity and efficacy of DNA vaccines encoding influenza A proteins in aged mice. Vaccine. 1998;16:1748–1755. doi: 10.1016/s0264-410x(98)00135-2. [DOI] [PubMed] [Google Scholar]
- Bosio CM, Moore BD, Warfield KL, Ruthel G, Mohamadzadeh M, Aman MJ, Bavari S. Ebola and Marburg virus-like particles activate human myeloid dendritic cells. Virology. 2004;326:280–287. doi: 10.1016/j.virol.2004.05.025. [DOI] [PubMed] [Google Scholar]
- Bridges CB, Harper SA, Fukuda K, Uyeki TM, Cox NJ, Singleton JA. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2003;52(RR8):1–34. [PubMed] [Google Scholar]
- Bright RA, Carter DM, Daniluk S, Toapanta FR, Ahmad A, Gavrilov V, Massare M, Pushko P, Mytle N, Rowe T, Smith G, Ross TM. Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin. Vaccine. 2007;25:3871–3878. doi: 10.1016/j.vaccine.2007.01.106. [DOI] [PubMed] [Google Scholar]
- Carroll SM, Paulson JC. Differential infection of receptor-modified host cells by receptor-specific influenza viruses. Virus Res. 1985;3:165–179. doi: 10.1016/0168-1702(85)90006-1. [DOI] [PubMed] [Google Scholar]
- Chen BJ, Leser GP, Morita E, Lamb RA. Influenza virus hemagglutinin and neuraminidase, but not the matrix protein, are required for assembly and budding of plasmid-derived virus-like particles. J Virol. 2007;81:7111–7123. doi: 10.1128/JVI.00361-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox MM. Progress on baculovirus-derived influenza vaccines. Curr Opin Mol Ther. 2008;10:56–61. [PubMed] [Google Scholar]
- Cox RJ, Brokstad KA, Ogra P. Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand J Immunol. 2004;59:1–15. doi: 10.1111/j.0300-9475.2004.01382.x. [DOI] [PubMed] [Google Scholar]
- Daniels PS, Jeffries S, Yates P, Schild GC, Rogers GN, Paulson JC, Wharton SA, Douglas AR, Skehel JJ, Wiley DC. The receptor-binding and membrane-fusion properties of influenza virus variants selected using anti-haemagglutinin monoclonal antibodies. EMBO J. 1987;6:1459–1465. doi: 10.1002/j.1460-2075.1987.tb02387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn IA, Remarque EJ, Jol-van der Zijde CM, van Tol MJ, Westendorp RG, Knook DL. Quality and quantity of the humoral immune response in healthy elderly and young subjects after annually repeated influenza vaccination. J Infect Dis. 1999;179:31–36. doi: 10.1086/314540. [DOI] [PubMed] [Google Scholar]
- de Jong JC, Palache AM, Beyer WE, Rimmelzwaan GF, Boon AC, Osterhaus AD. Haemagglutination-inhibiting antibody to influenza virus. Dev Biol (Basel) 2003;115:63–73. [PubMed] [Google Scholar]
- Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G, Bresee JS, Cox NS. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep. 2008;57:1–60. [PubMed] [Google Scholar]
- Galarza JM, Latham T, Cupo A. Virus-like particle (VLP) vaccine conferred complete protection against a lethal influenza virus challenge. Viral Immunol. 2005;18:244–251. doi: 10.1089/vim.2005.18.244. [DOI] [PubMed] [Google Scholar]
- Gomez-Puertas P, Mena I, Castillo M, Vivo A, Perez-Pastrana E, Portela A. Efficient formation of influenza virus-like particles: dependence on the expression levels of viral proteins. J Gen Virol. 1999;80:1635–1645. doi: 10.1099/0022-1317-80-7-1635. [DOI] [PubMed] [Google Scholar]
- Govaert TM, Sprenger MJ, Dinant GJ, Aretz K, Masurel N, Knottnerus JA. Immune response to influenza vaccination of elderly people. A randomized double-blind placebo-controlled trial. Vaccine. 1994;12:1185–1189. doi: 10.1016/0264-410x(94)90241-0. [DOI] [PubMed] [Google Scholar]
- Gross PA, Hermogenes AW, Sacks HS, Lau J, Levandowski RA. The efficacy of influenza vaccine in elderly persons. A meta-analysis and review of the literature. Ann Intern Med. 1995;123:518–527. doi: 10.7326/0003-4819-123-7-199510010-00008. [DOI] [PubMed] [Google Scholar]
- Guebre-Xabier M, Hammond SA, Ellingsworth LR, Glenn GM. Immunostimulant patch enhances immune responses to influenza virus vaccine in aged mice. J Virol. 2004;78:7610–7618. doi: 10.1128/JVI.78.14.7610-7618.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes L, Eaton SM. The effect of age on the cognate function of CD4+ T cells. Immunol Rev. 2005;205:220–228. doi: 10.1111/j.0105-2896.2005.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JM, Lu X, Todd CW, Newman MJ. A nonionic block co-polymer adjuvant (CRL1005) enhances the immunogenicity and protective efficacy of inactivated influenza vaccine in young and aged mice. Vaccine. 2000;18:2177–2187. doi: 10.1016/s0264-410x(00)00022-0. [DOI] [PubMed] [Google Scholar]
- Kemble G, Greenberg H. Novel generations of influenza vaccines. Vaccine. 2003;21:1789–1795. doi: 10.1016/s0264-410x(03)00074-4. [DOI] [PubMed] [Google Scholar]
- Kovaiou RD, Herndler-Brandstetter D, Grubeck-Loebenstein B. Age-related changes in immunity: implications for vaccination in the elderly. Expert Rev Mol Med. 2007;9:1–17. doi: 10.1017/S1462399407000221. [DOI] [PubMed] [Google Scholar]
- Kumar R, Burns EA. Age-related decline in immunity: implications for vaccine responsiveness. Expert Rev Vaccines. 2008;7:467–479. doi: 10.1586/14760584.7.4.467. [DOI] [PubMed] [Google Scholar]
- Latham T, Galarza JM. Formation of wild-type and chimeric influenza virus-like particles following simultaneous expression of only four structural proteins. J Virol. 2001;75:6154–6165. doi: 10.1128/JVI.75.13.6154-6165.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- Luxton RW, Thompson EJ. Affinity distributions of antigen-specific IgG in patients with multiple sclerosis and in patients with viral encephalitis. J Immunol Methods. 1990;131:277–282. doi: 10.1016/0022-1759(90)90199-6. [DOI] [PubMed] [Google Scholar]
- Mahmood K, Bright RA, Mytle N, Carter DM, Crevar CJ, Achenbach JE, Heaton PM, Tumpey TM, Ross TM. H5N1 VLP vaccine induced protection in ferrets against lethal challenge with highly pathogenic H5N1 influenza viruses. Vaccine. 2008;26:5393–5399. doi: 10.1016/j.vaccine.2008.07.084. [DOI] [PubMed] [Google Scholar]
- Mellquist-Riemenschneider JL, Garrison AR, Geisbert JB, Saikh KU, Heidebrink KD, Jahrling PB, Ulrich RG, Schmaljohn CS. Comparison of the protective efficacy of DNA and baculovirus-derived protein vaccines for EBOLA virus in guinea pigs. Virus Res. 2003;92:187–193. doi: 10.1016/s0168-1702(02)00338-6. [DOI] [PubMed] [Google Scholar]
- Neumann G, Watanabe T, Kawaoka Y. Plasmid-driven formation of influenza virus-like particles. J Virol. 2000;74:547–551. doi: 10.1128/jvi.74.1.547-551.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson KG, Wood JM, Zambon M. Influenza. Lancet. 2003;362:1733–1745. doi: 10.1016/S0140-6736(03)14854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul WE, Benacerraf B. Functional specificity of thymus-dependent lymphocytes. Science. 1977;195:1293–1300. doi: 10.1126/science.320663. [DOI] [PubMed] [Google Scholar]
- Plowden J, Renshaw-Hoelscher M, Engleman C, Katz J, Sambhara S. Innate immunity in aging: impact on macrophage function. Aging Cell. 2004;3:161–167. doi: 10.1111/j.1474-9728.2004.00102.x. [DOI] [PubMed] [Google Scholar]
- Powers DC, Belshe RB. Effect of age on cytotoxic T lymphocyte memory as well as serum and local antibody responses elicited by inactivated influenza virus vaccine. J Infect Dis. 1993;167:584–592. doi: 10.1093/infdis/167.3.584. [DOI] [PubMed] [Google Scholar]
- Pushko P, Tumpey TM, Bu F, Knell J, Robinson R, Smith G. Influenza virus-like particles comprised of the HA, NA, and M1 proteins of H9N2 influenza virus induce protective immune responses in BALB/c mice. Vaccine. 2005;23:5751–5759. doi: 10.1016/j.vaccine.2005.07.098. [DOI] [PubMed] [Google Scholar]
- Pushko P, Tumpey TM, Van Hoeven N, Belser JA, Robinson R, Nathan M, Smith G, Wright DC, Bright RA. Evaluation of influenza virus-like particles and Novasome adjuvant as candidate vaccine for avian influenza. Vaccine. 2007;25:4283–4290. doi: 10.1016/j.vaccine.2007.02.059. [DOI] [PubMed] [Google Scholar]
- Quan FS, Huang C, Compans RW, Kang SM. Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J Virol. 2007;81:3514–3524. doi: 10.1128/JVI.02052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan FS, Steinhauer D, Huang C, Ross TM, Compans RW, Kang SM. A bivalent influenza VLP vaccine confers complete inhibition of virus replication in lungs. Vaccine. 2008;26:3352–3361. doi: 10.1016/j.vaccine.2008.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani S. The Th1/Th2 paradigm. Immunol Today. 1997;18:263–266. doi: 10.1016/s0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- Sambhara S, Kurichh A, Miranda R, James O, Underdown B, Klein M, Tartaglia J, Burt D. Severe impairment of primary but not memory responses to influenza viral antigens in aged mice: costimulation in vivo partially reverses impaired primary immune responses. Cell Immunol. 2001;210:1–4. doi: 10.1006/cimm.2001.1799. [DOI] [PubMed] [Google Scholar]
- Sambhara S, Kurichh A, Miranda R, Tamane A, Arpino R, James O, McGuinness U, Kandil A, Underdown B, Klein M, Burt D. Enhanced immune responses and resistance against infection in aged mice conferred by Flu-ISCOMs vaccine correlate with up-regulation of costimulatory molecule CD86. Vaccine. 1998;16:1698–1704. doi: 10.1016/s0264-410x(98)00130-3. [DOI] [PubMed] [Google Scholar]
- Sha Z, Compans RW. Induction of CD4(+) T-cell-independent immunoglobulin responses by inactivated influenza virus. J Virol. 2000;74:4999–5005. doi: 10.1128/jvi.74.11.4999-5005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavnezer J. Antibody class switching. Adv Immunol. 1996;61:79–146. doi: 10.1016/s0065-2776(08)60866-4. [DOI] [PubMed] [Google Scholar]
- Stepanova L, Naykhin A, Kolmskog C, Jonson G, Barantceva I, Bichurina M, Kubar O, Linde A. The humoral response to live and inactivated influenza vaccines administered alone and in combination to young adults and elderly. J Clin Virol. 2002;24:193–201. doi: 10.1016/s1386-6532(01)00246-3. [DOI] [PubMed] [Google Scholar]
- Stephenson I, Nicholson KG, Wood JM, Zambon MC, Katz JM. Confronting the avian influenza threat: vaccine development for a potential pandemic. Lancet Infect Dis. 2004;4:499–509. doi: 10.1016/S1473-3099(04)01105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Carrion R, Jr, Ye L, Wen Z, Ro YT, Brasky K, Ticer AE, Schwegler EE, Patterson JL, Compans RW, Yang C. Protection against lethal challenge by Ebola virus-like particles produced in insect cells. Virology. 2009;383:12–21. doi: 10.1016/j.virol.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szucs T. The socio-economic burden of influenza. J Antimicrob Chemother. 1999;44(Suppl B):11–15. doi: 10.1093/jac/44.suppl_2.11. [DOI] [PubMed] [Google Scholar]
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- Wang BZ, Quan FS, Kang SM, Bozja J, Skountzou I, Compans RW. Incorporation of membrane-anchored flagellin into influenza virus-like particles enhances the breadth of immune responses. J Virol. 2008;82:11813–11823. doi: 10.1128/JVI.01076-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warfield KL, Bosio CM, Welcher BC, Deal EM, Mohamadzadeh M, Schmaljohn A, Aman MJ, Bavari S. Ebola virus-like particles protect from lethal Ebola virus infection. Proc Natl Acad Sci USA. 2003;100:15889–15894. doi: 10.1073/pnas.2237038100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster RG. Immunity to influenza in the elderly. Vaccine. 2000;18:1686–1689. doi: 10.1016/s0264-410x(99)00507-1. [DOI] [PubMed] [Google Scholar]
- Yager EJ, Ahmed M, Lanzer K, Randall TD, Woodland DL, Blackman MA. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J Exp Med. 2008;205:711–723. doi: 10.1084/jem.20071140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Bu Z, Vzorov A, Taylor D, Compans RW, Yang C. Surface stability and immunogenicity of the human immunodeficiency virus envelope glycoprotein: role of the cytoplasmic domain. J Virol. 2004;78:13409–13419. doi: 10.1128/JVI.78.24.13409-13419.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Lin J, Sun Y, Bennouna S, Lo M, Wu Q, Bu Z, Pulendran B, Compans RW, Yang C. Ebola virus-like particles produced in insect cells exhibit dendritic cell stimulating activity and induce neutralizing antibodies. Virology. 2006;351:260–270. doi: 10.1016/j.virol.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Zheng B, Han S, Takahashi Y, Kelsoe G. Immunosenescence and germinal center reaction. Immunol Rev. 1997;160:63–77. doi: 10.1111/j.1600-065x.1997.tb01028.x. [DOI] [PubMed] [Google Scholar]
- Zheng B, Zhang Y, He H, Marinova E, Switzer K, Wansley D, Mbawuike I, Han S. Rectification of age-associated deficiency in cytotoxic T cell response to influenza A virus by immunization with immune complexes. J Immunol. 2007;179:6153–6159. doi: 10.4049/jimmunol.179.9.6153. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Yang H, Chen W, Cao W, Zhong G, Jiao P, Deng G, Yu K, Yang C, Bu Z, Kawaoka Y, Chen H. A naturally occurring deletion in its NS gene contributes to the attenuation of an H5N1 swine influenza virus in chickens. J Virol. 2008;82:220–228. doi: 10.1128/JVI.00978-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Zarnitsyn VG, Ye L, Wen Z, Gao Y, Pan L, Skountzou I, Gill HS, Prausnitz MR, Yang C, Compans RW. Immunization by vaccine-coated microneedle arrays protects against lethal influenza virus challenge. Proc Natl Acad Sci USA. 2009;106:7968–7973. doi: 10.1073/pnas.0812652106. [DOI] [PMC free article] [PubMed] [Google Scholar]