Abstract
Objective
Borderline left heart disease is characterized by left heart obstructive lesions (coarctation, aortic and mitral stenosis, left ventricular (LV) hypoplasia) and endocardial fibroelastosis (EFE). The multilevel obstruction and impaired LV systolic and diastolic function contribute to failure of biventricular circulation. We studied the effects of LV rehabilitation - EFE resection with mitral and/or aortic valvuloplasty - on LV function and clinical outcomes.
Methods
All patients with borderline left heart structures and EFE who underwent primary LV rehabilitation procedure were retrospectively analyzed to determine operative mortality, reintervention rates, and hemodynamic status. Left heart dimensions and hemodynamics were recorded from pre- and post-operative echocardiogram and cardiac catheterization. Postoperative left atrial pressure was obtained from the intracardiac line early after LV rehabilitation. Pre- and post-operative values were compared by paired t-test.
Results
Between 1999 and 2008, 9 patients with EFE and borderline left heart underwent LV rehabilitation at a median age of 5.6 months (range 1–38 months). There was no operative mortality, and at a median follow up of 25 months (6 months to 10 years), there was one death from non cardiac causes, and two patients required reoperations. Significant increase in ejection fraction and LV end diastolic volume were observed, whereas left atrial pressure and RV/LV pressure ratios decreased postoperatively.
Conclusion
In patients with borderline left hearts, primary LV rehabilitation with EFE resection and mitral and aortic valvuloplasty results in improved LV systolic and diastolic performance and decreased RV pressures. This approach may provide an alternative to single ventricle management in this difficult patient group.
Background
Hypoplastic left heart disease occurs as a spectrum, with variable hypoplasia and dysfunction of one or more left-sided structures. Patients with more severe disease are managed with univentricular palliation or transplant, while patients at the milder end of the spectrum (e.g., neonatal aortic stenosis with normal left ventricular size and mild mitral hypoplasia) undergo attempts at biventricular repair. Despite a number of studies investigating factors associated with successful biventricular management, there is a population of patients with moderate hypoplastic left heart disease in whom it is difficult to determine whether a biventricular circulation is sustainable.(1–3) Such patients are often referred to as having a borderline left heart disease. Infants with borderline hypoplastic left heart structures present a unique challenge to the clinician. The constellation of aortic and mitral valve stenosis, small left ventricular (LV) cavity volume, and ventricular restriction due to the presence of endocardial fibroelastosis (EFE) impedes biventricular repair. Treatment of patients with extremely small LV is single ventricle palliation, whreas treatment of borderline left heart disease is dichotomous – single ventricle palliation or biventricular repair. Interventions commonly performed to promote initial biventricular circulation consist of relief of inflow and outflow tract obstructions, either by catheter or surgical maneuvers.(4, 5) However the presence of endocardial fibroelastosis, which impedes both systolic and diastolic myocardial function, is a risk factor for biventricular repair and may necessitate eventual pursuit of single ventricle palliation.(3)
A surgical strategy consisting of primary relief of LV inflow and outflow tract obstruction by aortic and mitral valvuloplasty, coarctation repair, and resection of EFE has been applied to a subgroup of patients with borderline hypoplastic left heart structures and EFE. The goal of this strategy, which we have referred to as primary LV rehabilitation, is to recruit the left heart into a biventricular circulation. Since 1999, we have employed the LV rehabilitation strategy in select patients with borderline hypoplastic left heart structures. The primary goal of this study is to report the clinical outcomes of the LV rehabilitation strategy.
Methods
Study design
All infants who underwent primary LV rehabilitation at Children's Hospital Boston between 1999 and 2008 were reviewed. Primary LV rehabilitation refers to mitral valvuloplasty, relief of LV outflow tract obstruction, and EFE resection as a means of maintaining biventricular circulation. Left heart structures were termed “borderline” hypoplastic if the dimension Z score was less than −2.0). Patients were selected for this strategy if they demonstrated borderline hypoplasia of one or more left heart structures, LV EFE, and clinical deterioration despite initial attempts at maintaining biventricular circulation. Most patients had critical aortic valve stenosis and coarctation, and had required catheter-based balloon dilation of the aortic valve or surgical coarctation repair, but demonstrated systolic and diastolic ventricular dysfunction despite these interventions. Clinical deterioration in these patients was associated with elevated left-sided filling pressures on cardiac catheterization.
Patients were excluded if they had aortic or mitral atresia, ventricular septal defect, heterotaxy syndrome, or atrio-ventricular or ventriculo-arterial discordance. The study was approved by the Children's Hospital Boston Institutional Review Board. Interventions performed prior to LV rehabilitation (fetal or postnatal balloon dilation of the aortic valve, surgical coarctation repair), details of the operative procedure, and reinterventions following LV rehabilitation were recorded from hospital records. Echocardiographic data and hemodynamic measurements from cardiac catheterization were recorded pre- and post-operatively. The Univentricular Suvival Advantage Prediction tool (UVR-SA), a regression model that calculates the predicted survival advantage of single ventricle repair over biventricular repair in critical left ventricular outflow tract (LVOT) obstruction, was determined from the online calculator available on the Congenital Heart Surgeons' Society website (www.chss.org). The primary outcome measures of this study were survival, hemodynamics, and sizes of left heart structures.
LV Rehabilitation Procedure
The primary LV rehabilitation strategy employed a combination of techniques to relieve inflow and outflow tract obstruction and resect EFE. The procedure was performed through a median sternotomy with cardiopulmonary bypass and moderate hypothermia. The mitral valve was approached transeptally, and inspected to determine the mechanisms of mitral stenosis or regurgitation. Commonly employed techniques to relieve inflow obstruction include division of secondary or accessory chordae, separation (splitting) of fused papillary muscles and abnormal attachments of papillary muscles to septum or LV free wall, chordal elongation, commissurotomy, and débridement of thickened leaflet tissue. EFE resection involved removal of this non-compliant endocardial material by sharp dissection, either with a surgical scalpel or tonotomy scissors. Resection was performed through the mitral valve orifice or the LV outflow tract.
The mechanisms of aortic stenosis or regurgitation were assessed by preoperative echocardiogram or MRI and intraoperative inspection. Techniques employed for obstruction at the valvar level included commissurotomy, débridement of thickened aortic valve leaflets, and augmentation of deficient leaflets with pericardium. When subvalvar obstruction was present, resection of the subvalvar membrane, muscle bar or accessory chordae between mitral valve and LV outflow tract was performed. Fenestrated closure of the atrial septal defect was performed either by partial primary reapproximation of the rim of the atrial septal defect or by fenestrated pericardial patch closure (4 mm fenestration) as a means of allowing decompression of the LV. The duration of cardiopulmonary bypass, cross clamp and fibrillatory arrest were recorded.
Hospital Course
The intensive care unit and hospital lengths of stay, duration of mechanical ventilation, and duration of inotropic support were recorded. The left atrial pressure, measured by the surgically placed intracardiac line placed at the time of surgery, was recorded prior to removal of the line.
Echocardiographic, MRI, and hemodynamic measurements
All echocardiograms were reviewed by an independent reviewer to determine the ejection fraction, LV mass to volume ratio, and dimensions of left heart structures prior to and following primary LV rehabilitation. On MRI, EFE manifested at the endocardial surface as a rim of hyperintense signal in the myocardial delayed-enhancement sequences (Figure 1). The degree of endocardial fibroelastosis was graded according to previously published methodology (0 = none; 1 = involvement of papillary muscles only; 2 = papillary muscle with some endocardial surface involvement; 3 = extensive endocardial surface involvement). The sizes of left heart structures were recorded from echocardiograms obtained postnatally, prior to surgical intervention, and at most recent follow-up. All dimensional measurements were compared to normative plot according to body surface area and expressed as a Z score value. Right ventricular pressure was estimated from the velocity of the tricuspid regurgitation jet (when present) and used as a surrogate for pulmonary arterial pressures. Hemodynamic data (left atrial, LV end diastolic, and right ventricular pressures) were obtained from cardiac catheterization performed prior to and following LV rehabilitation.
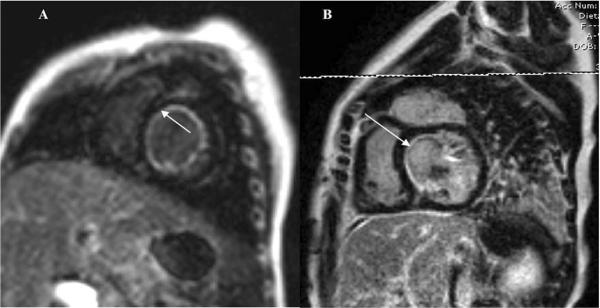
Figure 1.
MRI images depicting circumferential EFE prior to LV rehabilitation (A) and residual EFE along the interventricular septum on follow-up MRI (B).
Statistical analysis
Comparison of preoperative and postoperative LH dimensions and hemodynamics were analyzed by paired t-test. Descriptive data are expressed as mean (± standard deviation) or median (range). P values of less than 0.05 are considered significant.
Results
Patient characteristics
Between January, 1999 and December, 2008, 9 patients (3 female) with borderline left heart and EFE underwent primary LV rehabilitation as a means of maintaining biventricular circulation. Attempts to maintain biventricular circulation prior to LV rehabilitation included postnatal balloon dilation of the aortic valve (n=7) and coarctation repair (n=3). Prenatal balloon dilation of the aortic valve was performed in five patients (Table 1). None were dependent upon prostaglandin infusion beyond the neonatal period. In all patients, the indication for surgical evaluation was development of symptoms of congestive heart failure and evidence of left atrial hypertension. Dimensions of left heart structures and hemodynamics measured on the preoperative echocardiogram and cardiac catheterization are shown in Table 2. Eight patients had grade 3 EFE, and one patient had grade 2 EFE. The median UVR-SA score for all patients was 6.1 (range −15.5 to 23.6). Six of the 9 patients had positive values for the UVR-SA score, suggesting survival benefit for single ventricle palliation over biventricular repair in these patients.
Table 1.
Preoperative Echocardiographic and Hemodynamic Characteristics
| Preoperative interventions | Age at LV rehabilitation (months) | Details of LV rehabilitation | Reintervention | |
|---|---|---|---|---|
| 1 | Fetal balloon dilation | 4 | Mitral valvuloplasty, EFE resection | none |
| 2 | Postnatal balloon dilation of aortic valve, coarctation repair through thoracotomy | 21 | Mitral valvuloplasty, EFE resection | none |
| 3 | Left thoracotomy for coarctation repair, postnatal balloon dilation of aortic and mitral valves | 7 | Mitral valvuloplasty, EFE resection | Two reoperations for mitral replacement |
| 4 | Postnatal balloon dilation of aortic valve | 38 | Mitral valvuloplasty, EFE resection, aortic valvuloplasty | none |
| 5 | Left thoracotomy for coarctation repair | 12 | Mitral valvuloplasty, EFE resection, resection of subaortic obstruction | none |
| 6 | Fetal and postnatal balloon dilations of aortic valve | 1 | Mitral valvuloplasty, EFE resection, aortic valvuloplasty, fenestrated ASD closure | Aortic and mitral repair |
| 7 | Fetal and postnatal balloon dilations of aortic valve | 2 | Mitral valvuloplasty, EFE resection, aortic valvuloplasty | none |
| 8 | Fetal and postnatal balloon dilations of aortic valve | 1 | Mitral valvuloplasty, EFE resection, resection of subaortic obstruction, fenestrated ASD closure | none |
| 9 | Fetal and postnatal balloon dilations of aortic valve, left thoracotomy for coarctation repair | 6 | Mitral valvuloplasty, EFE resection, aortic valvuloplasty, fenestrated ASD closure | none |
Table 2.
Preoperative Echocardiographic and Hemodynamic Characteristics
| Echocardiogram - derived | |
| LV end-diastolic volume Z score | −0.18 ± 0.03 |
| Aortic valve Z score | −1.6 ± 0.4 |
| Mitral valve Z score | 0.5 ± 0.6 |
| Cardiac Catheterization - derived | |
| LV end-diastolic pressure (mmHg) | 22 ± 2.4 |
| Left atrial pressure (mmHg) | 28 ± 6.3 |
| Right ventricular pressure (mmHg) | 70 ± 18 |
LV = left ventricle
Operative Procedure
Median age at operation was 5.6 months (19 days to 3 years). Details of the primary LV rehabilitation procedure are provided in Table 3. Mitral valvuloplasty and EFE resection were performed in all patients, whereas LVOT procedures were performed in 6 patients. Mitral valvuloplasty entailed separation of fused papillary muscles in all 9 patients, thinning of thickened leaflets in 5 patients, division of accessory or secondary chordae in 7 patients, and commissurotomy in 3 patients. Median durations of cardiopulmonary bypass, fibrillatory arrest, and cross clamp times are shown in Table 3. Seven patients had a period of fibrillatory arrest to allow examination of intracardiac structures prior to application of the cross clamp.
Table 3.
Techniques Employed During LV Rehabilitation Procedures
| MV repair | N=9 |
| EFE resection | N=9 |
| Aortic valve repair | N=4 |
| Subaortic resection | N=2 |
| Fenestrated ASD closure | N=3 |
| Total pump time (min) | 106 ± 6 |
| Aortic cross clamp time (min) | 59 ± 7 |
| Fibrillatory Arrest time (min) | 19 ± 4 |
LV = left ventricle; MV = mitral valve; EFE = endocardial fibroelastosis
Hospital Course
Inotropic support with dopamine (3 to 10 mcg/kg/min) was maintained in all patients for a median of 4 days (1 to 12 days). The median duration of mechanical ventilatory support was 7 days (9 hours to 22 days). Eight patients required milrinone for a median of 5 days (3 to 7 days), and three patients required additional inotropic support with epinephrine. Temporary dual chamber pacing was required in one patient who developed transient second degree heart block postoperatively. Postoperative intensive care unit and hospital length of stays were 17 days (1 to 45 days) and 27 days (5 to 64 days), respectively.
Clinical Outcomes
At median follow up of 25 months (6 months to 10 years), there was one death (11%) due to non-cardiac causes (motor vehicle accident). Two patients underwent reinterventions. One patient had mitral valve replacement for severe mitral regurgitation and subsequent re-replacement due to development of thrombosis of the mechanical prosthesis. Another patient underwent surgical reintervention for aortic and mitral valve repair. Three of nine patients had evidence of recurrent or persistent endocardial fibroelastosis on the interventricular septal surface of the LV on echocardiogram or MRI (Figure 1). Three patients had a right bundle branch block or hemifascicular block with mild prolongation of the QRS complex on electrocardiography, but none have required permanent pacing. Aortic regurgitation was moderate in one patient and mild or none on the rest.
Left heart dimensions and hemodynamics at postoperative follow up
Post-operative left atrial pressures measured by the intracardiac catheter placed during the operation were recorded. The median LA pressure prior to removal of the catheter was 11±2.4 mmHg, significantly lower than at preoperative catheterization (see Table 4). Left heart dimensions and estimated RV pressure on the most recent echocardiogram were compared to preoperative values, as summarized in Table 4. Postoperative cardiac catheterization was performed in 5 patients at a median of 4 months after surgery. The mean left atrial and LV end-diastolic pressures in these 5 patients were 16±1.1 and 12±2 mmHg, respectively, which were significantly lower than preoperative pressures measured at cardiac catheterization (P<0.05).
Table 4.
Comparison of preoperative and postoperative echocardiographic and hemodynamic parameters
| Preoperative | Recent follow up | P value | |
|---|---|---|---|
| Echocardiogram | |||
| Ejection fraction (%) | 36 ± 12 | 58 ± 10 | <0.01 |
| LVEDV Z score | −0.2 ± 1.7 | 2.7 ± 1.8 | <0.05 |
| LV mass Z score | 0.63±2.2 | 2.5±0.39 | 0.04 |
| LV mass:volume ratio Z score | 0.6 ± 1.2 | 0.9 ± 2.1 | NS |
| Aortic valve gradient (mmHg) | 39±22 | 28±19 | NS |
| Mitral valve gradient (mmHg) | 7±3 | 5±2 | NS |
| RV:LV systolic pressure ratio | 0.78±0.36 | 0.32 ± 0.11 | <0.05 |
| Cardiac catheterization or intracardiac line | |||
| LA pressure (mmHg) | 28±6.3 | 11 ± 2.4* | <0.01 |
LVEDV = Left ventricular end diastolic volume; LV = Left ventricle; RV = Right ventricle; LA = Left atrium
Obtained prior to removal of the intracardiac line
Discussion
This retrospective study reports our early experience with primary LV rehabilitation for elimination of LV inflow and outflow tract obstructions and resection of endocardial fibroelastosis, in a group of patients with borderline hypoplastic left heart who were considered to be failing biventricular physiology. The LV rehabilitation procedure was associated with low operative mortality, immediate improvement in left atrial and right ventricular pressures, and maintenance of biventricular circulation at mid-term follow up.
Risk factors that have been associated with poor outcome (death or conversion to SVP) following biventricular repair include size and multiplicity of the left-sided obstructive lesions, and the presence of endocardial fibroelastosis.(2, 3, 6) Higher grade of endocardial fibroelastosis (moderate or severe) has been shown to be a strong predictor of mortality following biventricular repair.(6–8) The poor prognosis in patients with circumferential EFE may be due to impairment of both systolic and diastolic ventricular performance.(9) Previous reports of biventricular repair in patients with borderline hypoplastic left heart disease have demonstrated the importance of relieving inflow and outflow tract obstructions, but have not addressed the endocardial fibroelastosis that contributes to both diastolic and systolic dysfunction of the LV.(5, 10) Hanley et al. successfully performed EFE resection combined with the Ross-Konno procedure in a group of patients with borderline left heart disease, and observed increase in LV cavity volume with this procedure. However, there have been no published reports describing the efficacy or intermediate-term results of EFE resection.
Candidates for primary LV rehabilitation include patients with borderline left heart structures and severe EFE who have failed attempts at biventricular repair, or are considered high risk for biventricular repair due to elevated LV end-diastolic pressure and pulmonary hypertension. Most of the patients in this series underwent catheter-based intervention on the aortic valve or coarctation repair in the newborn period in attempts to maintain a biventricular circulation, thus permitting delay of LV rehabilitation until several months of age; only two patients had repair prior to one month of age. LV rehabilitation during the neonatal period can be challenging due to the difficulty of EFE resection and mitral valve repair through a small mitral valve orifice. Thus LV rehabilitation procedure must be incorporated into a management strategy consisting of catheter-based intervention and relief of LV outflow tract obstruction to allow optimal timing of repair.
Ejection fraction and left atrial pressure were measured as crude surrogates for systolic and diastolic LV performance. Although ejection fraction improved in patients undergoing LV rehabilitation, it was unclear whether this improvement was secondary to relief of LVOT obstruction, resection of EFE, or alteration in loading conditions. Significant impairment of systolic function is associated with severe circumferential EFE, and its removal may have contributed to the improved ejection fraction. Left atrial pressure was significantly reduced following LV rehabilitation; however, this pressure is affected by multiple factors including intravascular volume, mitral valve stenosis or regurgitation, and ventricular compliance. More sensitive measures of systolic and diastolic function are required to accurately characterize changes in ventricular performance following LV rehabilitation. Another limitation of this study is the lack of long-term follow-up cardiac catheterization in all patients. Comparison of the intracardiac pressures to preoperative hemodynamics measured at cardiac catheterization is confounded by inherent differences between the two methods of measurement.
The study was unable to determine the relative importance of individual components of the LV rehabilitation procedure to the hemodynamic and clinical outcomes. The low mean LV outflow tract gradient preoperatively in several patients undergoing the procedure suggests that restriction of blood flow through the LV was primarily due to the combination of mitral stenosis and endocardial restriction. Residual LV inflow and outflow gradients were encountered in many patients despite surgical intervention, either due to increase in cardiac output or recalcitrant lesions. Improvement in ventricular performance despite persistence of inflow and outflow obstruction may suggest a dominant role of EFE resection in the favorable outcome. Although aortic and mitral valve repairs without EFE resection may result in some hemodynamic improvement, EFE resection likely provides the additional hemodynamic benefit necessary to maintain biventricular circulation.
EFE is commonly associated with left heart obstructive lesions (secondary EFE), although it may also occur in structurally normal hearts (primary EFE).(11, 12) Mechanisms underlying the development of secondary EFE are unknown, although endocardial ischemia and decreased ventricular blood flow in-utero have been proposed. In fetal animal models, LV unloading, but not LV outflow tract obstruction, leads to development of EFE.(13, 14) Once EFE has formed, spontaneous regression is unlikely to occur, and surgical resection is the only means of relieving the endocardial restriction.
Recurrent or residual EFE was detected postoperatively by MRI in three patients. In these patients, the location of EFE was the LV surface of the interventricular septum. With a trans-mitral approach, access to the interventricular septum, particularly the basal aspect, is impaired by the anterior leaflet of the mitral valve. Similarly, visualization of this region through the aortic valve is limited by the small annular dimension and presence of subaortic obstruction. The impact of incomplete EFE resection in this region upon ventricular systolic and diastolic function or long term outcome is unclear. None of the patients in this series had recurrence of EFE within previous resection fields, suggesting that EFE does not redevelop in the postnatal myocardium. However, our experience with EFE resection in a different patient population (patients undergoing single ventricle palliation and staged LV rehabilitation) has demonstrated that the fibrosis that occurs in the EFE resection bed is qualitatively different from true EFE(15).
The mitral valve in patients with borderline left heart disease tends to share some common morphological features. At the valvular level, mild annular hypoplasia, thickened leaflets, and commissural fusion may be present, but it is the subvalvar pathology that results in the most significant stenosis and abnormal flow dynamics. Fusion of the papillary muscles to the ventricular wall, foreshortened primary chordae, and hypertrophic accessory and secondary chordae limit excursion of the mitral leaflets and result in posteriorly-oriented mitral orifice. By addressing the pathology at both the valvar and subvalvar levels, mitral valve rehabilitation promotes leaflet excursion and thereby redirects the inflow jet towards the apex rather than the posterior LV free wall. Development of mitral regurgitation in one patient early in this series necessitated mitral valve replacement, but significant mitral regurgitation has not occurred in our more recent experience with these techniques.
MRI has become our preferred imaging modality in patients with borderline left heart structures who are considered candidates for LV rehabilitation. MRI allows quantification of LV blood flow, which is useful in patients with an atrial septal defect and left-to-right atrial shunting. It is also more sensitive than echocardiography for the detection of EFE, although its sensitivity for recurrent EFE is unknown.(16, 17) Three dimensional echocardiography allows surgical planning and has improved our understanding of the mechanisms underlying the aortic and mitral valve pathology in this population.
Primary LV rehabilitation procedure, when applied to patients with borderline left heart structures and severe EFE, allows maintenance of biventricular circulation with low operative mortality. Further follow-up is needed to establish whether the hemodynamic improvements will translate into long-term survival and improvement in quality of life.
Ultramini Abstract.
Primary left ventricular rehabilitation, consisting of aortic and mitral valvuloplasty and resection of endocardial fibroelastosis, improves systolic and diastolic function in infants with borderline left heart structures and severe endocardial fibroelastosis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rhodes LA, Colan SD, Perry SB, Jonas RA, Sanders SP. Predictors of survival in neonates with critical aortic stenosis. Circulation. 1991 Dec;84(6):2325–35. doi: 10.1161/01.cir.84.6.2325. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz ML, Gauvreau K, Geva T. Predictors of outcome of biventricular repair in infants with multiple left heart obstructive lesions. Circulation. 2001 Aug 7;104(6):682–7. doi: 10.1161/hc3101.093904. [DOI] [PubMed] [Google Scholar]
- 3.Lofland GK, McCrindle BW, Williams WG, Blackstone EH, Tchervenkov CI, Sittiwangkul R, et al. Critical aortic stenosis in the neonate: a multi-institutional study of management, outcomes, and risk factors. Congenital Heart Surgeons Society. The Journal of thoracic and cardiovascular surgery. 2001 Jan;121(1):10–27. doi: 10.1067/mtc.2001.111207. [DOI] [PubMed] [Google Scholar]
- 4.Corno AF. Borderline left ventricle. Eur J Cardiothorac Surg. 2005 Jan;27(1):67–73. doi: 10.1016/j.ejcts.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 5.Tchervenkov CI, Tahta SA, Jutras LC, Beland MJ. Biventricular repair in neonates with hypoplastic left heart complex. The Annals of thoracic surgery. 1998 Oct;66(4):1350–7. doi: 10.1016/s0003-4975(98)00803-0. [DOI] [PubMed] [Google Scholar]
- 6.Hickey EJ, Caldarone CA, Blackstone EH, Lofland GK, Yeh T, Jr., Pizarro C, et al. Critical left ventricular outflow tract obstruction: The disproportionate impact of biventricular repair in borderline cases. The Journal of thoracic and cardiovascular surgery. 2007 Dec;134(6):1429–36. doi: 10.1016/j.jtcvs.2007.07.052. discussion 36–7. [DOI] [PubMed] [Google Scholar]
- 7.Han RK, Gurofsky RC, Lee KJ, Dipchand AI, Williams WG, Smallhorn JF, et al. Outcome and growth potential of left heart structures after neonatal intervention for aortic valve stenosis. Journal of the American College of Cardiology. 2007 Dec 18;50(25):2406–14. doi: 10.1016/j.jacc.2007.07.082. [DOI] [PubMed] [Google Scholar]
- 8.Miyamoto T, Sinzobahamvya N, Wetter J, Kallenberg R, Brecher AM, Asfour B, et al. Twenty years experience of surgical aortic valvotomy for critical aortic stenosis in early infancy. Eur J Cardiothorac Surg. 2006 Jul;30(1):35–40. doi: 10.1016/j.ejcts.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 9.Jalil JE, Janicki JS, Pick R, Abrahams C, Weber KT. Fibrosis-induced reduction of endomyocardium in the rat after isoproterenol treatment. Circulation research. 1989 Aug;65(2):258–64. doi: 10.1161/01.res.65.2.258. [DOI] [PubMed] [Google Scholar]
- 10.Serraf A, Piot JD, Bonnet N, Lacour-Gayet F, Touchot A, Bruniaux J, et al. Biventricular repair approach in ducto-dependent neonates with hypoplastic but morphologically normal left ventricle. Journal of the American College of Cardiology. 1999 Mar;33(3):827–34. doi: 10.1016/s0735-1097(98)00636-6. [DOI] [PubMed] [Google Scholar]
- 11.Angelov A, Kulova A, Gurdevsky M. Endocardial fibroelastosis. Clinico-pathological study of 38 cases. Pathology, research and practice. 1984 Mar;178(4):384–8. doi: 10.1016/S0344-0338(84)80031-X. [DOI] [PubMed] [Google Scholar]
- 12.Sellers FJ, Keith JD, Manning JA. The Diagnosis of Primary Endocardial Fibroelastosis. Circulation. 1964 Jan;29:49–59. doi: 10.1161/01.cir.29.1.49. [DOI] [PubMed] [Google Scholar]
- 13.Eghtesady P, Michelfelder E, Altaye M, Ballard E, Hirsh R, Beekman RH., 3rd Revisiting animal models of aortic stenosis in the early gestation fetus. The Annals of thoracic surgery. 2007 Feb;83(2):631–9. doi: 10.1016/j.athoracsur.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 14.Melnychenko I, Vasilyev NV, Scherr E, Freihs I, Poutias D, del Nido PJ. Hemodynamic Mechanisms of Endocardial Fibroelastosis (EFE) in the Developing Rat Heart. Circulation. 2008;118(18)(Supplement 2):S908. (Abstract) [Google Scholar]
- 15.Emani SM, Tworetzky W, McElhinney DB, Schroeder B, Zurakowski D, Bacha E, et al. Staged Left Ventricular Recruitment in Patients with Hypoplastic Left Heart Disease and Borderline Left Ventricle (Abstract) Circulation. 2008;2008:S750–S1. [Google Scholar]
- 16.Tworetzky W, del Nido PJ, Powell AJ, Marshall AC, Lock JE, Geva T. Usefulness of magnetic resonance imaging of left ventricular endocardial fibroelastosis in infants after fetal intervention for aortic valve stenosis. The American journal of cardiology. 2005 Dec 1;96(11):1568–70. doi: 10.1016/j.amjcard.2005.07.066. [DOI] [PubMed] [Google Scholar]
- 17.Stranzinger E, Ensing GJ, Hernandez RJ. MR findings of endocardial fibroelastosis in children. Pediatric radiology. 2008 Mar;38(3):292–6. doi: 10.1007/s00247-007-0707-7. [DOI] [PubMed] [Google Scholar]