Abstract
Background
The prevalence of chronic kidney disease is growing most rapidly among older adults, but determinants of impaired kidney function in this population are not well understood. Obesity assessed in mid-life has been associated with chronic kidney disease.
Study design
Cohort study.
Setting & Participants
4,295 participants in the community-based Cardiovascular Health Study, ages >=65 years.
Predictors
Body mass index, waist circumference, and fat mass measured using bioelectrical impedance.
Outcome
Change in glomerular filtration rate (GFR) over 7 years of follow-up.
Measurements
Longitudinal estimates of GFR calculated with the 4-variable Modification of Diet in Renal Disease Study equation.
Results
Estimated GFR declined by an average of 0.4 +/− 3.6 mL/min/1.73m2/year, and rapid GFR loss (> 3 mL/min/1.73m2/year) occurred in 693 participants (16%). Baseline body mass index, waist circumference, and fat mass were each associated with increased risk of rapid GFR loss: odds ratios (95% confidence intervals) 1.19 (1.09, 1.30) per 5 kg/m2, 1.25 (1.16, 1.36) per 12 cm, and 1.14 (1.05, 1.24) per 10 kg, respectively, after adjustment for age, sex, race, and smoking. Magnitude of increased risk was larger for participants with estimated GFR < 60 mL/min/1.73m2 at baseline (p for interaction < 0.05). Associations were substantially attenuated by further adjustment for diabetes, hypertension, and C-reactive protein. Obesity measurements were not associated with change in GFR estimated using serum cystatin C.
Limitations
Few participants with advanced chronic kidney disease at baseline, no direct GFR measurements.
Conclusion
Obesity may be a modifiable risk factor for the development and progression of kidney disease among older adults.
Introduction
The prevalence of chronic kidney disease (CKD) is highest and growing most rapidly among older adults.1 Even mild impairment of kidney disease is associated with increased risk for cardiovascular disease and mortality in this population.2, 3 However, determinants of impaired kidney function and progression of kidney disease in the elderly are not well described.
In middle age, obesity is associated with increased risk of developing CKD.4–11 Renal plasma flow, renin-angiotensin-aldosterone system activity, and intraglomerular pressure are each elevated in the setting of obesity and may contribute to kidney damage.12 Adipose tissue has been associated with impaired kidney function, especially when present in a central distribution.13 Obesity also increases the risk of diabetes and hypertension, the most common causes of kidney disease.
However, the relation of obesity with kidney function has not been described among older adults. Understanding this relationship is particularly important because associations of body composition measures with health outcomes vary with age.14 Specifically, muscle mass declines as part of the aging process and as a consequence of diseases that are common in older adults, so measurements that encompass both muscle and fat mass, including body mass index (BMI), may not accurately reflect adiposity and its associated health risks. This may explain why the association of higher BMI with mortality risk is attenuated with older age.15–17 In contrast, higher waist circumference, a more specific measure of central adiposity, is associated with adverse cardiovascular outcomes among adults of all ages.18, 19
We examined associations of obesity with longitudinal change in estimated glomerular filtration rate (GFR) among older adults who participated in the community-based Cardiovascular Health Study. We hypothesized that measures of obesity, particularly waist circumference and fat mass, would be associated with more rapid loss of estimated GFR.
Methods
Study Population
The Cardiovascular Health Study (CHS) is a cohort study of risk factors for development and progression of cardiovascular disease in people aged 65 years and older. 5,888 participants were recruited from four communities in the United States: Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; and Pittsburgh, Pennsylvania. Eligible participants, sampled from Medicare eligibility lists, were not institutionalized and were expected to remain in the area for at least three years. Persons who were wheelchair-bound in the home or receiving hospice treatment, radiation therapy, or chemotherapy for cancer were excluded. The original CHS cohort of 5,201 participants were enrolled in 1989–90, with an additional 687 predominantly African American participants enrolled in 1992–93. Participants were followed through 1999 with scheduled visits and telephone interviews. The current study includes 4,295 CHS participants (73%) who (1) completed anthropometric and bioelectrical impedance measurements at baseline and (2) underwent measurement of serum creatinine and cystatin C on at least two occasions. Excluded were 42 participants without anthropometric data, 142 participants without bioelectrical impedance, and 1,452 participants with fewer than 2 creatinine/cystatin C measurements.
Obesity measures
Anthropometric measurements and bioelectrical impedance analysis were completed at each participant’s baseline visit. These methods technically measure adiposity but are referred to as obesity measures for simplicity in this presentation. Body mass index (BMI) was calculated as weight (kg) divided by height (m2) and classified according to World Health Organization (WHO) guidelines.20 Waist circumference was measured at the level of the umbilicus and categorized by quartiles within each sex. For analysis as a continuous variable, BMI was assessed per 5 kg/m2, the difference between WHO BMI categories and approximately 1 standard deviation. To facilitate comparisons, waist circumference and bioelectrical impedance variables were also scaled to 1 standard deviation for continuous analyses.
Body composition was assessed by bioelectrical impedance as previously described.21 Adhesive electrodes were placed in standard distal positions on the right hand and foot. Resistance was measured at 50 kHz with a TVI-50 Body Composition Analyzer (Danninger Medical Technology Inc, Columbus, OH). Fat-free mass was derived using a formula developed in a population with similar age and body size: fat-free mass (kg) = 6710 × ht2/R + 3.1 × S + 3.9, where ht2 is body height in meters squared, R is resistance in ohms, and S is sex (0 = women, 1 = men).22 Body fat was calculated as weight minus fat-free mass. Reproducibility of resistance measurements within healthy individuals is excellent, with a reliability coefficient for a single R measurement over 5 days of 0.99.23
Estimated GFR
Change in kidney function was estimated using longitudinal measurements of serum creatinine and cystatin C. Measurements were collected at three CHS visits, in 1989–90, 1992–93, and 1996–97. Serum creatinine was measured using the Kodak Ektachem 700 Analyzer (Eastman Kodak, Rochester, NY), which employs a colorimetric method. Mean coefficient of variation was 1.94% (range 1.16–3.60).24 Serum creatinine was calibrated to Cleveland Clinic using indirect calibration to the NHANES III (Third National Health and Nutrition Examination Survey) data and then used to estimate GFR using the 4-variable MDRD Study equation.25, 26 Serum cystatin C was measured using a BNII nephelometer (N Latex Cystatin C; Dade Behring, Deerfield, IL).27 The 1992–93 measurements were conducted in 2003, and the 1989–90 and 1996–97 measures in 2006. All measurements used the same equipment in the same laboratory. Coefficients of variation range from 2.0 – 2.8% (intra-assay) and 2.3 – 3.1% (inter-assay). Serum cystatin C is stable to multiple freeze-thaw cycles,28 though effects of long-term storage at −70°C are note known. GFR was estimated from cystatin C using the equation: 76.7 * [cystatin C]−1.18, where [cystatin C] is the concentration of cystatin C.29 Results were unchanged using the equation: 127.7 * [cystatin C] −1.17 * age−0.13 * (0.91 if female) * (1.06 if African American).29
Covariates
Race (white or African American) and smoking status were defined by self-report. Diabetes was defined as fasting glucose ≥ 126 mg/dL or use of insulin or oral diabetes medications. Hypertension was defined as use of antihypertensive medications, systolic blood pressure ≥ 140 mmHg, or diastolic blood pressure ≥ 90 mmHg. C-reactive protein was measured with an enzyme-linked immunosorbent assay developed in the CHS laboratory.30
Statistical analysis
Pearson correlation was used to assess relationships of obesity measurements with estimated GFR at baseline. For each participant, annualized change in estimated GFR was calculated using linear regression. Associations of baseline obesity measurements (independent variables) with annualized change in estimated GFR (continuous outcome) and rapid decline in kidney function (dichotomous outcome) were assessed using linear and logistic regression, respectively. Annualized change in estimated GFR < −3 mL/min/1.73m2/year was used to define rapid decline in kidney function, because this threshold has been associated with an increased risk of mortality in this population.31 Serial models were adjusted for potential confounding variables (age, sex, race, and smoking) and adjusting for potential confounding and/or mediating variables (causal pathway model, additionally includes diabetes, hypertension, and C-reactive protein). P-values for association were calculated from models using continuous exposure variables. In parallel interaction models, we included as independent variables the products of obesity measurements (continuous variables) with sex or baseline estimated GFR (categorical variables), testing the statistical significance of interaction using the likelihood ratio test. S-Plus (release 8.0, Insightful Inc, Seattle, WA) and SPSS statistical software (release 15.0.1, SPSS Inc, Chicago, IL) were used for analyses.
Results
Baseline characteristics
CHS participants excluded from the current analyses because they had < 2 estimated GFR measurements were older (mean age 75 versus 72 years) and more likely to be men (48% versus 41%), African American (23% versus 13%), and to have diabetes (24% versus 14%). However, there were no substantial differences in BMI (26.7 versus 26.7 kg/m2), waist circumference (96 versus 94 cm), fat mass (41 versus 40 kg), or fat-free mass (33 versus 33 kg).
At baseline, among participants included in the current analyses, mean age was 72 years, 59% were women, and 13% were African American (Table 1). 43% of participants were overweight (BMI 25–29 kg/m2), and 19% of participants were obese (BMI ≥ 30 kg/m2). On average, larger participants were slightly younger, more likely to be African American, more likely to have diabetes and/or hypertension, less likely to smoke, and more likely to have unfavorable HDL cholesterol and triglyceride concentrations. Correlation coefficients of BMI with waist circumference and fat mass were 0.79 and 0.86, respectively, and correlation coefficient of waist circumference with fat mass was 0.71 (each p<0.001).
Table 1.
Participant characteristics at baseline.
| BMI (kg/m2) | |||
|---|---|---|---|
| < 25 n=1633 |
25–29 n=1830 |
≥ 30 n=832 |
|
| Demographic and clinical data | |||
| Age (years) | 73 (5) | 72 (5) | 71 (4) |
| Sex (Men) | 617 (38%) | 865 (47%) | 264 (32%) |
| Race (African American) | 134 (8%) | 249 (14%) | 180 (22%) |
| Diabetes (n,%) | 140 (9%) | 249 (14%) | 215 (26%) |
| Hypertension (n,%) | 782 (48%) | 1051 (58%) | 587 (71%) |
| Active smoking (n,%) | 238 (15%) | 167 (9%) | 51 (6%) |
| Physical measurements | |||
| Body mass (kg) | 62 (9) | 75 (9) | 90 (12) |
| Body mass index (kg/m2) | 22.5 (1.8) | 27.2 (1.4) | 33.7 (3.5) |
| Waist circumference (cm) | 84 (9) | 96 (8) | 109 (11) |
| Fat mass (kg) | 25 (6) | 34 (6) | 48 (10) |
| Fat-free mass (kg) | 37 (9) | 41 (10) | 42 (10) |
| Systolic blood pressure (mmHg) | 134 (22) | 135 (21) | 138 (21) |
| Diastolic blood pressure (mmHg) | 69 (11) | 71 (11) | 72 (11) |
| Laboratory data | |||
| Total cholesterol (mg/dL) | 210 (38) | 213 (39) | 212 (38) |
| LDL cholesterol (mg/dL) | 127 (35) | 132 (35) | 131 (34) |
| HDL cholesterol (mg/dL) | 59 (17) | 53 (15) | 50 (13) |
| Triglycerides* (mg/dL) | 106 [84, 144] | 125 [96, 171] | 138 [104, 188] |
| C-reactive protein* (mg/dL) | 1.77 [0.85, 3.20] | 2.46 [1.35, 4.19] | 3.55 [2.26, 7.62] |
| Creatinine (mg/dL) | 0.91 (0.29) | 0.96 (0.28) | 0.93 (0.35) |
| MDRD eGFR (mL/min/1.73m2) | 81 (23) | 78 (21) | 80 (23) |
| Cystatin C (mg/dL) | 0.99 (0.24) | 1.02 (0.23) | 1.07 (0.30) |
| Cystatin C eGFR (mL/min/1.73m2) | 82 (19) | 78 (19) | 75 (18) |
Note: Data are mean (standard deviation) or n (%).
Abbreviations and definition: MDRD eGFR, estimated glomerular filtration rate calculated using the MDRD (Modification of Diet in Renal Disease) Study equation; eGFR, estimated glomerular filtration rate; HDL, high density lipoprotein; LDL, low density lipoprotein.
Triglycerides and C-reactive protein are presented as median [quartile (Q) 1, Q3].
Baseline GFR estimated by the MDRD Study equation was < 60 mL/min/1.73m2 for 841 participants (20%; median estimated GFR in this subgroup 53 mL/min/1.73m2, quartile [Q] 1-Q3, 48–58 mL/min/1.73m2). At baseline, each measure of obesity was negatively correlated with GFR estimated from serum cystatin C, but only waist circumference was correlated with MDRD Study estimated GFR, and this correlation was weak (Table 2). Each GFR estimate was negatively correlated with fat-free mass.
Table 2.
Correlations of obesity and body composition measurements with eGFR at baseline.
| MDRD eGFR | Cystatin C eGFR | |
|---|---|---|
| Body mass index | −0.03 | −0.15* |
| Waist circumference | −0.05* | −0.20* |
| Fat mass | 0.005 | −0.11* |
| Fat-free mass | −0.12* | −0.13* |
Data presented are Pearson correlation coefficients.
p<0.01 (all other p-values > 0.05). MDRD eGFR = estimated glomerular filtration rate calculated using the 4-variable MDRD (Modification of Diet in Renal Disease) Study equation. Cystatin C GFR was calculated using the equation: 76.7 * [cystatin C]−1.18.
Change in estimated GFR
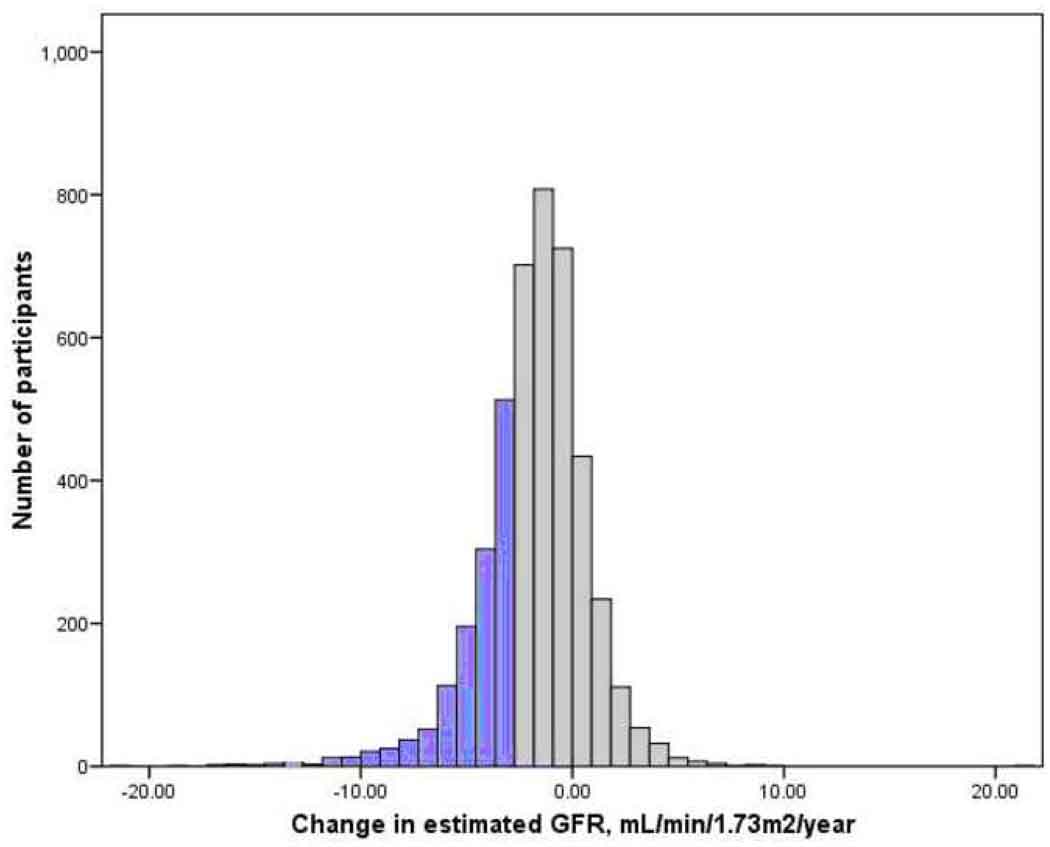
Three longitudinal estimates of GFR were available for 2726 participants (64%) and two estimates for the remainder. Duration of follow-up was seven years for 67% of participants, four years for 9%, and three years for 24%. Mean annualized change in MDRD Study estimated GFR over follow-up was −0.4 ± 3.6 mL/min/1.73m2/year, and 693 participants (16%) lost GFR at a rate greater than 3 mL/min/1.73m2/year (Figure 1).
Figure 1.
Distribution of change in kidney function over follow-up. Change in glomrular filtration rate (GFR) is calculated from longitudinal measurements of serum creatinine using the 4-variable MDRD (Modification of Diet in Renal Disease) Study equation. Rapid renal function decline, defined as loss of estimated glomerular filtration rate exceeding 3 mL/min/1.73m2/year, occurred in 16% of participants and is shown with black bars.
Obesity and change in estimated GFR
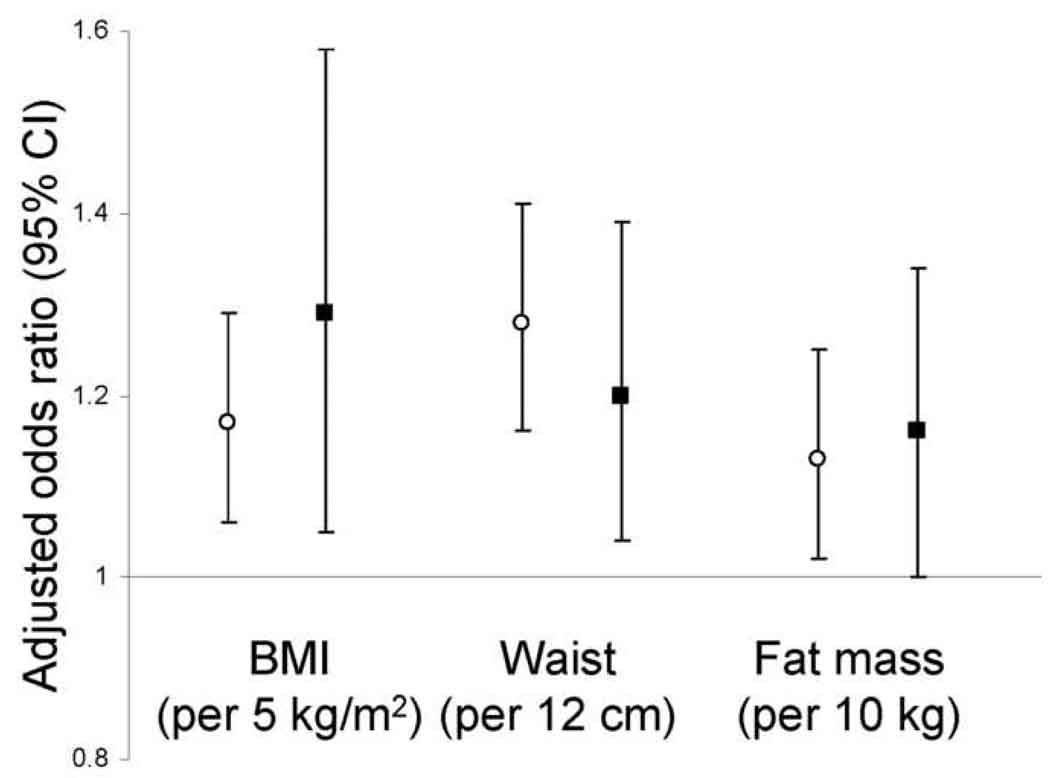
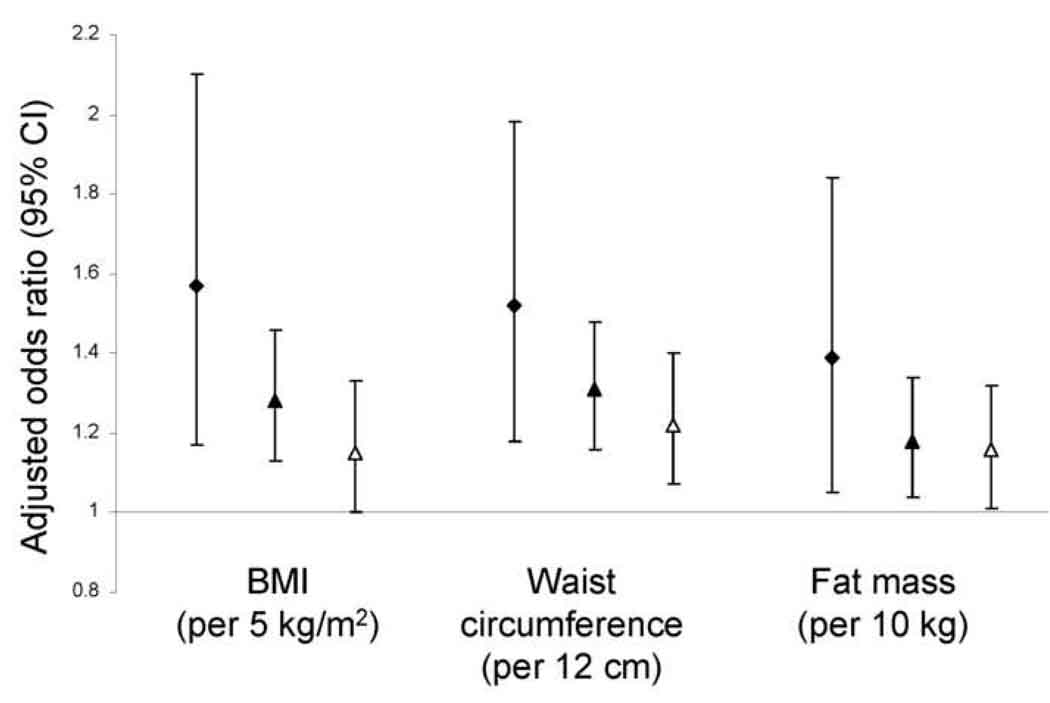
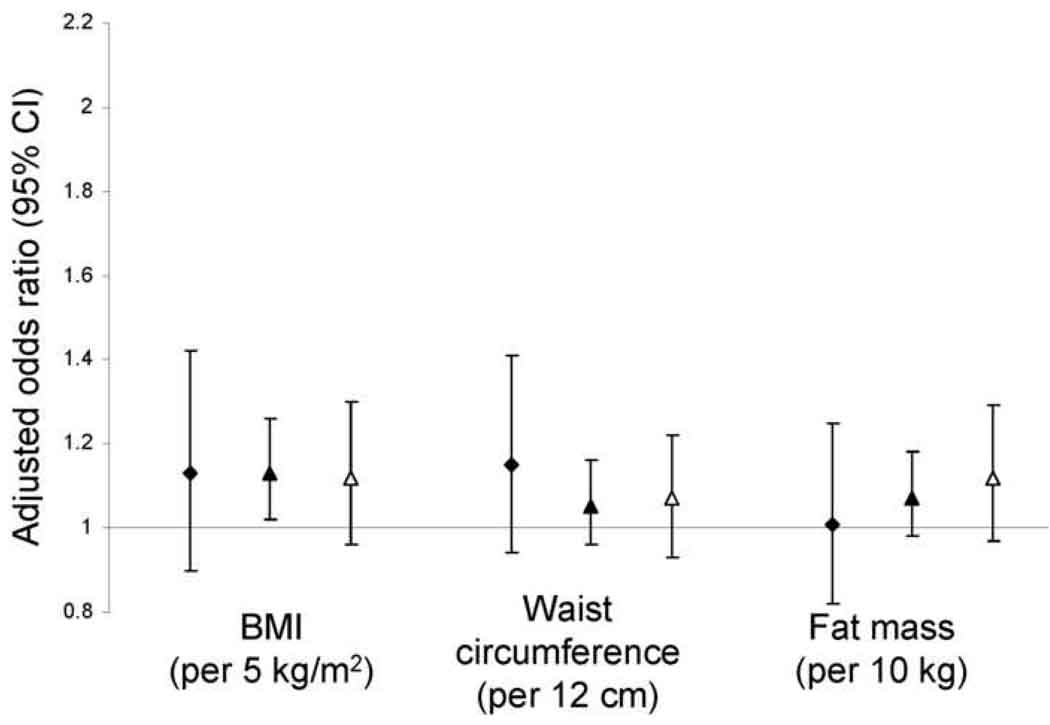
Higher BMI, waist circumference, and fat mass were each associated with loss of MDRD Study estimated GFR over follow-up in unadjusted analyses and in analyses adjusted for age, sex, race, and smoking (Table 3). Results were consistent in each sex (Figure 2). Obesity was associated with a greater magnitude of increased risk when estimated GFR was lower at baseline (Figure 3, p values for interaction by estimated GFR 0.04 for BMI, 0.04 for waist circumference, and 0.4 for fat mass). With further adjustment for diabetes, hypertension, and C-reactive protein, associations of obesity measurements with GFR loss were substantially attenuated and remained statistically significant only for waist circumference (Table 3, causal pathway model). Evaluated individually, diabetes, hypertension, and C-reactive protein each attenuated associations of obesity with GFR loss to a similar degree, with adjustment for diabetes having a nominally larger effect. Including additional variables for impaired fasting glucose, systolic and diastolic blood pressure, and/or antihypertensive medications did not further affect these associations. When the population was restricted to participants without diabetes or hypertension (n = 1,706, 40%), associations of obesity measures with risk of rapid GFR loss were reduced: odds ratios (95% confidence intervals) of 1.10 (0.92, 1.32) per 5 kg/m2 BMI and 1.14 (0.97, 1.34) per 12 cm waist circumference. Fat-free mass was not associated with loss of MDRD Study estimated GFR (data not shown). BMI, waist circumference, and fat mass were not associated with loss of GFR calculated from serum cystatin C, regardless of baseline estimated GFR (Table 4 and Figure 4). All results were similar when analyses were restricted to participants who maintained body weight within 5% of baseline over follow-up and did not vary by number of follow-up GFR estimates available for analysis. Baseline BMI was inversely correlated with change in BMI over follow-up (ρ=−0.13).
Table 3.
Associations of obesity and body composition with change in GFR estimated using the MDRD Study equation
| Annualized change in eGFR | Rapid decline in eGFR* (dichotomous outcome) |
|||
|---|---|---|---|---|
| Measurement | Adjusted** difference ± SD |
Adjusted† difference ± SD |
Adjusted** odds ratio (95% CI) |
Adjusted† odds ratio (95% CI) |
| Body mass index (kg/m2) | ||||
| < 25.0 | 0.00 (Ref) | 0.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| 25.0–29.9 | −0.27 ± 5.22 | −0.12 ± 5.22 | 1.21 (1.00, 1.46) | 1.11 (0.92, 1.35) |
| ≥ 30.0 | −0.39 ± 4.38 | −0.08 ± 4.53 | 1.56 (1.25, 1.95) | 1.22 (0.97, 1.54) |
| Per 5 kg/m2† | −0.16 ± 4.00 | −0.03 ± 4.13 | 1.19 (1.09, 1.30) | 1.08 (0.99, 1.18) |
| P† | 0.01 | 0.5 | 0.001 | 0.2 |
| Waist circumference (cm) | ||||
| <82 (w); <92 (m) | 0.00 (Ref) | 0.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| 82–91 (w); 92–96 (m) | −0.55 ± 5.02 | −0.47 ± 5.02 | 1.38 (1.08, 1.76) | 1.29 (1.01, 1.65) |
| 92–100 (w); 97–103 (m) | −0.34 ± 5.00 | −0.22 ± 5.00 | 1.28 (1.00 (1.64) | 1.16 (0.90, 1.49) |
| ≥101 (w); ≥103 (m) | −0.64 ± 5.01 | −0.36 ± 5.14 | 1.82 (1.44, 2.31) | 1.46 (1.14, 1.86) |
| Per 12 cm† | −0.20 ± 3.53 | −0.09 ± 3.67 | 1.25 (1.16, 1.36) | 1.15 (1.06, 1.25) |
| P† | <0.001 | 0.09 | 0.01 | 0.02 |
| Fat mass (kg) | ||||
| <28 (w); <45 (m) | 0.00 (Ref) | 0.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| 28–33 (w); 25–30 (m) | 0.03 ± 5.05 | 0.09 ± 5.01 | 0.98 (0.77, 1.25) | 0.93 (0.73, 1.19) |
| 34–41 (w); 31–36 (m) | −0.39 ± 5.08 | −0.28 ± 5.08 | 1.35 (1.07, 1.71) | 1.23 (0.97, 1.56) |
| >41 (w); >36 (m) | −0.23 ± 5.08 | 0.05 ± 5.21 | 1.36 (1.08, 1.72) | 1.09 (0.86, 1.40) |
| Per 10 kg† | −0.11 ± 3.60 | −0.01 ± 3.67 | 1.14 (1.05, 1.24) | 1.02 (0.98, 1.06) |
| P† | 0.04 | 0.7 | 0.003 | 0.3 |
Rapid decline in eGFR defined as loss exceeding 3 mL/min/1.73m2/year.
Adjusted for age, sex, race, and smoking.
Additionally adjusted for diabetes, hypertension and C-reactive protein. Estimated glomerular filtration rate (eGFR) is expressed in mL/min/1.73 m^2 and is calculated from serum creatinine using the 4-variable MDRD (Modification of Diet in Renal Disease) Study equation. SD = standard deviation. CI = confidence interval. Evaluated as a continuous, linear exposure.
Abbreviation: ref, reference.
Figure 2.
Risk of rapid renal function decline, stratified by sex (women, open circles; men, filled squares) and adjusted for age, race, and smoking. Change in kidney function is calculated from longitudinal measurements of serum creatinine using the 4-variable MDRD (Modification of Diet in Renal Disease) Study equation. Rapid renal function decline is defined as loss of estimated glomerular filtration rate exceeding 3 mL/min/1.73m2/year.
Figure 3.
Risk of rapid renal function decline, stratified by baseline estimated glomerular filtration rate (<60 mL/min/1.73m2, filled diamonds; 60–89 mL/min/1.73m2, filled triangles; ≥ 90 mL/min/1.73m2, open triangles) and adjusted for age, race, and smoking. Change in kidney function is calculated from longitudinal measurements of serum creatinine using the 4-variable MDRD (Modification of Diet in Renal Disease) Study equation. Rapid renal function decline is defined as loss of estimated glomerular filtration rate exceeding 3 mL/min/1.73m2/year.
Table 4.
Associations of obesity and body composition with change in GFR estimated from serum cystatin C.
| Annualized change in eGFR | Rapid decline in eGFR* (dichotomous outcome) |
|||
|---|---|---|---|---|
| Measurement | Adjusted** difference ± SD |
Adjusted† difference ± SD |
Adjusted** odds ratio (95% CI) |
Adjusted† odds ratio (95% CI) |
| Body mass index (kg/m2) | ||||
| < 25.0 | 0.00 (Ref) | 0.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| 25.0–29.9 | −0.14 ± 3.81 | −0.07 ± 3.81 | 1.07 (0.92, 1.26) | 1.01 (0.86, 1.18) |
| ≥ 30.0 | −0.15 ± 3.20 | 0.06 ± 3.32 | 1.00 (0.82, 1.22) | 0.84 (0.69, 1.04) |
| Per 5 kg/m2 † | −0.09 ± 2.88 | −0.01 ± 2.95 | 1.02 (0.94, 1.10) | 0.95 (0.87, 1.03) |
| P† | 0.1 | 0.8 | 0.6 | 0.2 |
| Waist circumference (cm) | ||||
| <82 (w); <92 (m) | 0.00 (Ref) | 0.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| 82–91 (w); 92–96 (m) | −0.26 ± 3.68 | −0.20 ± 3.68 | 1.12 (0.92, 1.36) | 1.07 (0.88, 1.30) |
| 92–100 (w); 97–103 (m) | −0.11 ± 3.66 | −0.03 ± 3.66 | 1.01 (0.82, 1.23) | 0.94 (0.77, 1.15) |
| ≥101 (w); ≥103 (m) | −0.18 ± 3.67 | 0.01 ± 3.77 | 1.00 (0.82, 1.22) | 0.85 (0.69, 1.04) |
| Per 12 cm† | −0.03 ± 1.05 | −0.00 ± 1.05 | 1.00 (0.97, 1.03) | 0.97 (0.95, 1.01) |
| P† | 0.3 | 0.6 | 0.6 | 0.1 |
| Fat mass (kg) | ||||
| <28 (w); <45 (m) | 0.00 (Ref) | 0.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| 28–33 (w); 25–30 (m) | −0.10 ± 3.67 | −0.06 ± 3.67 | 0.99 (0.81, 1.20) | 0.95 (0.78, 1.15) |
| 34–41 (w); 31–36 (m) | −0.14 ± 3.70 | −0.06 ± 3.70 | 1.01 (0.83, 1.23) | 0.93 (0.76, 1.14) |
| >41 (w); >36 (m) | −0.06 ± 3.70 | 0.13 ± 3.80 | 0.89 (0.72, 1.08) | 0.75 (0.61 0.93) |
| Per 10 kg† | −0.02 ± 1.25 | 0.01 ± 1.31 | 1.00 (0.96, 1.03) | 0.97 (0.94, 1.01) |
| P† | 0.6 | 0.3 | 0.6 | 0.1 |
Rapid decline in eGFR defined as loss exceeding 3 mL/min/1.73m2/year.
Adjusted for age, sex, race, and smoking.
Additionally adjusted for diabetes, hypertension and C-reactive protein. Estimated glomerular filtration rate (eGFR) is expressed in mL/min/1.73s m^2 and is calculated from serum cystatin C using the equation: 76.7 * [cystatin C]−1.18. SD = standard deviation. CI = confidence interval. Evaluated as a continuous, linear exposure.
Abbreviation: ref, reference.
Figure 4.
Risk of rapid renal function decline calculated from longitudinal measurements of serum cystatin C. Analyses are stratified by baseline estimated glomerular filtration rate (<60 mL/min/1.73m2, filled diamonds; 60–89 mL/min/1.73m2, filled triangles; ≥ 90 mL/min/1.73m2, open triangles) and adjusted for age, race, and smoking. Rapid renal function decline is defined as loss of estimated glomerular filtration rate exceeding 3 mL/min/1.73m2/year.
Discussion
Obesity was associated with a decline in GFR estimated by the MDRD Study equation over 7 years of follow-up in a large, community-based population of older adults. Results were consistent within each sex and were observed whether obesity was measured as BMI, waist circumference, or fat mass using bioelectrical impedance. Associations of obesity with GFR loss were stronger among participants with impaired GFR at baseline and were substantially attenuated by adjustment for diabetes, hypertension, and C-reactive protein.
These results are consistent with observations in younger cohorts, which have often but not always observed associations of obesity with impaired kidney function.4–11, 32 Our focus on older adults is particularly important because: (1) the prevalence of CKD is highest and increasing most rapidly among older adults; (2) risk factors for kidney disease in older adults are poorly understood; (3) associations of obesity with non-renal health outcomes have not been consistently found in older populations; and, (4) kidney disease appears to confer a particularly poor prognosis for cardiovascular and mortality outcomes among older adults.1–3, 14 Our results suggest that obesity may be a modifiable risk factor for the development and progression of kidney disease among older adults.
In contrast to results assessing MDRD Study estimated GFR as an outcome, obesity measurements were not associated with change in GFR estimated using serum cystatin C. Reasons for this discrepancy are not clear and require further investigation. However, our data raise the possibility that, among participants who were more obese at baseline, loss of renal cystatin C elimination over follow-up was balanced by simultaneous loss of cystatin C production (which derives in part from adipose tissue), resulting in no observed net change in steady-state serum cystatin C concentration. This hypothesis is supported by the observed inverse correlations of obesity measurements both with baseline GFR estimated from cystatin C (cross-sectional correlations, Table 2) and with change in BMI over follow-up. In contrast, serum creatinine is not produced by adipose tissue, so that changes in serum creatinine caused by loss of renal function are less likely to be obscured by simultaneous loss of adipose tissue. Supporting this concept is the published observation that the relationship of change in MDRD Study estimated GFR with change in iothalamate GFR was not biased by baseline BMI in the MDRD Study, though the MDRD Study contained relatively few obese individuals.33 Thus, production of endogenous filtration markers is an important consideration when assessing the relationship of obesity with kidney function,34–36 and this may be a unique circumstance in which creatinine-based GFR estimates have advantage over GFR estimates based on serum cystatin C.
Associations of obesity with loss of MDRD Study estimated GFR were strongest when baseline GFR was less than 60 mL/min/1.73m2. It is possible that obesity places a physiologic strain on the kidney that is most detrimental in the setting of prior kidney damage and limited renal reserve. In this setting, obesity may act as a “second hit,” accelerating the progression of CKD. Alternatively, the detrimental effects of obesity on GFR loss may be equally strong in persons with normal baseline GFR, but the strength of association in this subgroup may be underestimated due to imprecise GFR estimation in the normal range.
Associations of obesity with GFR loss were substantially attenuated by adjustment for diabetes, hypertension, and C-reactive protein (causal pathway model). This is consistent with prior studies and suggests that obesity may adversely affect kidney function in large part through established mechanistic pathways: impaired glucose metabolism, blood pressure, and inflammation. Alternatively, diabetes, hypertension, and elevated C-reactive protein may reflect underlying poor health which leads to both obesity and GFR loss, thus confounding the association of obesity with GFR loss.
Strengths of this study include its focus on older adults, multiple measurements of obesity and adiposity, and longitudinal measurement of estimated GFR to assess change over time. In addition, we avoided use of a threshold level of GFR to define impaired kidney function. Use of thresholds may introduce bias when evaluating obesity as a risk factor because the same estimated GFR may reflect higher true GFR for people with greater body size due to increased creatinine/cystatin production.9, 37 There are also potential limitations to this study. First, results are susceptible to survival bias, as with all studies requiring repeated measurements, because participants with fewer than two kidney function measurements were excluded. Fortunately, excluded patients did not differ substantially from those included in analyses with regard to baseline obesity measurements. Second, direct measurements of GFR were not available. In particular, use of GFR estimates may have underestimated true loss of GFR in the cohort,33 and differences comparing GFR estimates based on creatinine versus cystatin cannot be conclusively explained. Third, results may not apply to younger populations, or to populations with more severe CKD.
In conclusion, obesity is associated with increased risk of GFR loss in older adults, when GFR is estimated using serum creatinine. This relationship appears to be strongest among persons with impaired GFR at baseline and to be largely the result of higher prevalences of diabetes, hypertension, and inflammation among more obese individuals.
Acknowledgements
A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm. Data contained in this manuscript were presented, in part, at the American Society of Nephrology Renal Week, 2008, Philadelphia, PA.
Support: The research reported in this article was supported by contract numbers N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, grant number U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support came from grant 1KL2RR025015-01 from the National Center for Research Resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
N section:
Because the Editor-in-Chief and Deputy Editor recused themselves from consideration of this manuscript, the peer-review and decision-making processes were handled entirely by a Co-Editor (Bertrand L. Jaber, MD, MS, Caritas St. Elizabeth's Medical Center) who served as Acting Editor-in-Chief. Details of the journal’s procedures for potential editor conflicts are given in the Editorial Policies section of the AJKD website.
Financial Disclosure
None.
Contributor Information
Ian H. de Boer, University of Washington, Seattle, WA.
Ronit Katz, University of Washington, Seattle, WA.
Linda F. Fried, VA Pittsburgh Healthcare System, Pittsburgh, PA.
Joachim H. Ix, University of California, San Diego, San Diego, CA.
Jose Luchsinger, Columbia University, New York, NY.
Mark J. Sarnak, Tufts Medical Center, Boston, MA.
Michael G. Shlipak, University of California, San Francisco, San Francisco, CA.
David S. Siscovick, University of Washington, Seattle, WA.
Bryan Kestenbaum, University of Washington, Seattle, WA.
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. Jama. 2007;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman-Breen C. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352(20):2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 3.Hallan S, Astor B, Romundstad S, Aasarod K, Kvenild K, Coresh J. Association of Kidney Function and Albuminuria With Cardiovascular Mortality in Older vs Younger Individuals: The HUNT II Study. Arch Intern Med. 2007;167(22):2490–2496. doi: 10.1001/archinte.167.22.2490. [DOI] [PubMed] [Google Scholar]
- 4.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. Jama. 2004;291(7):844–850. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 5.Gelber RP, Kurth T, Kausz AT, Manson JE, Buring JE, Levey AS, Gaziano JM. Association between body mass index and CKD in apparently healthy men. Am J Kidney Dis. 2005;46(5):871–880. doi: 10.1053/j.ajkd.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Kramer H, Luke A, Bidani A, Cao G, Cooper R, McGee D. Obesity and prevalent and incident CKD: the Hypertension Detection and Follow-Up Program. Am J Kidney Dis. 2005;46(4):587–594. doi: 10.1053/j.ajkd.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Iseki K, Ikemiya Y, Kinjo K, Inoue T, Iseki C, Takishita S. Body mass index and the risk of development of end-stage renal disease in a screened cohort. Kidney Int. 2004;65(5):1870–1876. doi: 10.1111/j.1523-1755.2004.00582.x. [DOI] [PubMed] [Google Scholar]
- 8.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144(1):21–28. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen S, Hsu CY. Excess weight as a risk factor for kidney failure. Current opinion in nephrology and hypertension. 2007;16(2):71–76. doi: 10.1097/MNH.0b013e32802ef4b6. [DOI] [PubMed] [Google Scholar]
- 10.Elsayed EF, Sarnak MJ, Tighiouart H, Griffith JL, Kurth T, Salem DN, Levey AS, Weiner DE. Waist-to-hip ratio, body mass index, and subsequent kidney disease and death. Am J Kidney Dis. 2008;52(1):29–38. doi: 10.1053/j.ajkd.2008.02.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster MC, Hwang SJ, Larson MG, Lichtman JH, Parikh NI, Vasan RS, Levy D, Fox CS. Overweight, obesity, and the development of stage 3 CKD: the Framingham Heart Study. Am J Kidney Dis. 2008;52(1):39–48. doi: 10.1053/j.ajkd.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Praga M, Morales E. Obesity, proteinuria and progression of renal failure. Current opinion in nephrology and hypertension. 2006;15(5):481–486. doi: 10.1097/01.mnh.0000242172.06459.7c. [DOI] [PubMed] [Google Scholar]
- 13.Pinto-Sietsma SJ, Navis G, Janssen WM, de Zeeuw D, Gans RO, de Jong PE. A central body fat distribution is related to renal function impairment, even in lean subjects. Am J Kidney Dis. 2003;41(4):733–741. doi: 10.1016/s0272-6386(03)00020-9. [DOI] [PubMed] [Google Scholar]
- 14.Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr. 2005;82(5):923–934. doi: 10.1093/ajcn/82.5.923. [DOI] [PubMed] [Google Scholar]
- 15.Manson JE, Colditz GA, Stampfer MJ, Willett WC, Rosner B, Monson RR, Speizer FE, Hennekens CH. A prospective study of obesity and risk of coronary heart disease in women. N Engl J Med. 1990;322(13):882–889. doi: 10.1056/NEJM199003293221303. [DOI] [PubMed] [Google Scholar]
- 16.Stevens J, Cai J, Pamuk ER, Williamson DF, Thun MJ, Wood JL. The effect of age on the association between body-mass index and mortality. N Engl J Med. 1998;338(1):1–7. doi: 10.1056/NEJM199801013380101. [DOI] [PubMed] [Google Scholar]
- 17.Diehr P, Bild DE, Harris TB, Duxbury A, Siscovick D, Rossi M. Body mass index and mortality in nonsmoking older adults: the Cardiovascular Health Study. American journal of public health. 1998;88(4):623–629. doi: 10.2105/ajph.88.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 19.Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr. 2004;79(3):379–384. doi: 10.1093/ajcn/79.3.379. [DOI] [PubMed] [Google Scholar]
- 20.Obesity: preventing and managing the global epidemic: World Health Organization. 2000 [PubMed] [Google Scholar]
- 21.Visser M, Langlois J, Guralnik JM, Cauley JA, Kronmal RA, Robbins J, Williamson JD, Harris TB. High body fatness, but not low fat-free mass, predicts disability in older men and women: the Cardiovascular Health Study. Am J Clin Nutr. 1998;68(3):584–590. doi: 10.1093/ajcn/68.3.584. [DOI] [PubMed] [Google Scholar]
- 22.Deurenberg P, van der Kooij K, Evers P, Hulshof T. Assessment of body composition by bioelectrical impedance in a population aged greater than 60 y. Am J Clin Nutr. 1990;51(1):3–6. doi: 10.1093/ajcn/51.1.3. [DOI] [PubMed] [Google Scholar]
- 23.Lukaski HC, Johnson PE, Bolonchuk WW, Lykken GI. Assessment of fat-free mass using bioelectrical impedance measurements of the human body. Am J Clin Nutr. 1985;41(4):810–817. doi: 10.1093/ajcn/41.4.810. [DOI] [PubMed] [Google Scholar]
- 24.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995;41(2):264–270. [PubMed] [Google Scholar]
- 25.Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15(5):1307–1315. doi: 10.1097/01.asn.0000123691.46138.e2. [DOI] [PubMed] [Google Scholar]
- 26.Levey AS, Greene T, Kusek JW, Beck GJ. A simplified equation to predict glomerular filtration rate from serum creatinine [Abstract] J Am Soc Nephrol. 2000;11:A0828. [Google Scholar]
- 27.Erlandsen EJ, Randers E, Kristensen JH. Evaluation of the Dade Behring N Latex Cystatin C assay on the Dade Behring Nephelometer II System. Scand J Clin Lab Invest. 1999;59(1):1–8. doi: 10.1080/00365519950185940. [DOI] [PubMed] [Google Scholar]
- 28.Kyhse-Andersen J, Schmidt C, Nordin G, Andersson B, Nilsson-Ehle P, Lindstrom V, Grubb A. Serum cystatin C, determined by a rapid, automated particle-enhanced turbidimetric method, is a better marker than serum creatinine for glomerular filtration rate. Clin Chem. 1994;40(10):1921–1926. [PubMed] [Google Scholar]
- 29.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, Greene T, Levey AS. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51(3):395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Macy EM, Hayes TE, Tracy RP. Variability in the measurement of C-reactive protein in healthy subjects: implications for reference intervals and epidemiological applications. Clin Chem. 1997;43(1):52–58. [PubMed] [Google Scholar]
- 31.Rifkin DE, Shlipak MG, Katz R, Fried LF, Siscovick D, Chonchol M, Newman AB, Sarnak MJ. Rapid kidney function decline and mortality risk in older adults. Arch Intern Med. 2008;168(20):2212–2218. doi: 10.1001/archinte.168.20.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Boer IH, Sibley SD, Kestenbaum B, Sampson JN, Young B, Cleary PA, Steffes MW, Weiss NS, Brunzell JD. Central obesity, incident microalbuminuria, and change in creatinine clearance in the epidemiology of diabetes interventions and complications study. J Am Soc Nephrol. 2007;18(1):235–243. doi: 10.1681/ASN.2006040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie D, Joffe MM, Brunelli SM, Beck G, Chertow GM, Fink JC, Greene T, Hsu CY, Kusek JW, Landis R, Lash J, Levey AS, O'Conner A, Ojo A, Rahman M, Townsend RR, Wang H, Feldman HI. A comparison of change in measured and estimated glomerular filtration rate in patients with nondiabetic kidney disease. Clin J Am Soc Nephrol. 2008;3(5):1332–1338. doi: 10.2215/CJN.05631207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knight EL, Verhave JC, Spiegelman D, Hillege HL, de Zeeuw D, Curhan GC, de Jong PE. Factors influencing serum cystatin C levels other than renal function and the impact on renal function measurement. Kidney Int. 2004;65(4):1416–1421. doi: 10.1111/j.1523-1755.2004.00517.x. [DOI] [PubMed] [Google Scholar]
- 35.Macdonald J, Marcora S, Jibani M, Roberts G, Kumwenda M, Glover R, Barron J, Lemmey A. GFR estimation using cystatin C is not independent of body composition. Am J Kidney Dis. 2006;48(5):712–719. doi: 10.1053/j.ajkd.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Stevens LA, Schmid CH, Greene T, Li L, Beck GJ, Joffe MM, Froissart M, Kusek JW, Zhang YL, Coresh J, Levey AS. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75(6):652–660. doi: 10.1038/ki.2008.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delanaye P, Radermecker RP, Rorive M, Depas G, Krzesinski JM. Indexing glomerular filtration rate for body surface area in obese patients is misleading: concept and example. Nephrol Dial Transplant. 2005;20(10):2024–2028. doi: 10.1093/ndt/gfh983. [DOI] [PubMed] [Google Scholar]