Abstract
Aldose reductase (AR), that catalyzes the rate limiting step of the polyol pathway of glucose metabolism, besides reducing glucose to sorbitol, reduces a number of lipid peroxidation –derived aldehydes and their glutathione conjugates. Recent studies suggest that apart from its involvement in diabetic complications, AR’s catalytic activity plays a key role in a number of inflammatory diseases such as atherosclerosis, sepsis, asthma, uveitis, and colon cancer. Furthermore, AR is overexpressed in human cancers such as liver, colon, breast, cervical and ovarian. Since AR inhibitors have already undergone up to phase-iii clinical trials for diabetic complications, they could be safe anti-inflammatory drugs. Therefore the future use of AR inhibitors in down-regulating major inflammatory pathologies such as cancer and cardiovascular diseases could relieve some of the major health concerns of worldwide.
Keywords: Aldose reductase, inflammation, oxidative stress, NF-kB, aldehydes
1. Introduction
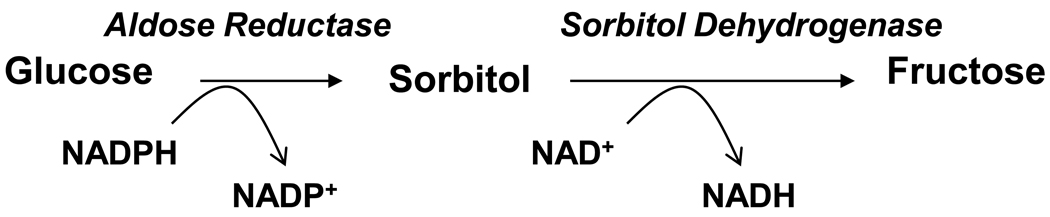
AR (EC: 1.1.1.21) is an enzyme responsible for conversion of glucose to sorbitol in the polyol pathway of glucose metabolism (Fig.1). Extensive studies during past several decades indicate the involvement of AR in the pathophysiology of diabetic complications as this enzyme plays a pivotal role in glucose metabolism (Srivastava et al, 2005). However, our recent studies indicate that the physiological function of AR is unlikely to be restricted to metabolism of glucose, since AR’s major function is not only metabolizing glucose, but also in catalyzing the reduction of a wide array of substances including lipid aldehydes generated during lipid peroxidation and their glutathione (GSH)-conjugates, phospholipids, atherogenic aldehydes, and steroids (Srivastava et al, 2005). Further, our studies have also demonstrated a novel of role of AR in mediation of cell signals initiated by cytokines, growth factors and chemokines.
Figure-1.
Polyol pathway of glucose metabolism. In polyol pathway aldose reductase (AR) catalyzes the reduction of glucose to sorbitol. Sorbitol is converted to fructose by sorbitol dehydrogenase. Both the steps require NADPH and NAD+ as co-factors.
2. Structure
AR, a cytosolic protein belongs to the superfamily of aldo-keto reductases (AKR) which comprise of several enzymes that catalyze oxidation and reduction reactions involved in various cellular processes such as biosynthesis, detoxification and metabolism (Barski et al, 2008). Human AR, a monomeric protein of 315 amino acids, (Molecular wt 36 kDa) is encoded by AKR1B1 gene which is mapped at chromosome region 7q35. AR, like other AKR family of proteins, adopts a (βα)8 or TIM-barrel motif (triosphosphate isomerase), which represents a dense yet flexible scaffolding with structural variations essential for binding to a number of chemically distinct variety of carbonyl compounds. The active site of AR is located at the C-terminal face of the barrel which is best suited for efficient interaction with NADPH, a cofactor required for AR’s reduction reactions. The AR-mediated catalytic reaction involves transfer of a hydride ion from NADPH to the carbonyl substrate and addition of a proton from the solvent to reduce the carbonyl substrate to an alcohol product. The binding of specific substrates depends up on the active site amino acid residues. The Cys298, present near the AR active site, has been shown to control the catalytic activity and inhibitor sensitivity of the enzyme (Petrash et al, 1992). A recent study by Fournier et al, 2009 suggests that the substrate recognition and binding efficiency are directly related to the charge distributions of the ligand and of the AR active site. Several structural and kinetic studies suggest that AR’s active site is lined with several hydrophobic amino acids that prefer hydrophobic aldehyde substrates rather than hydrophilic aldoses (Srivastava et al, 1999; 2005).
3. Function
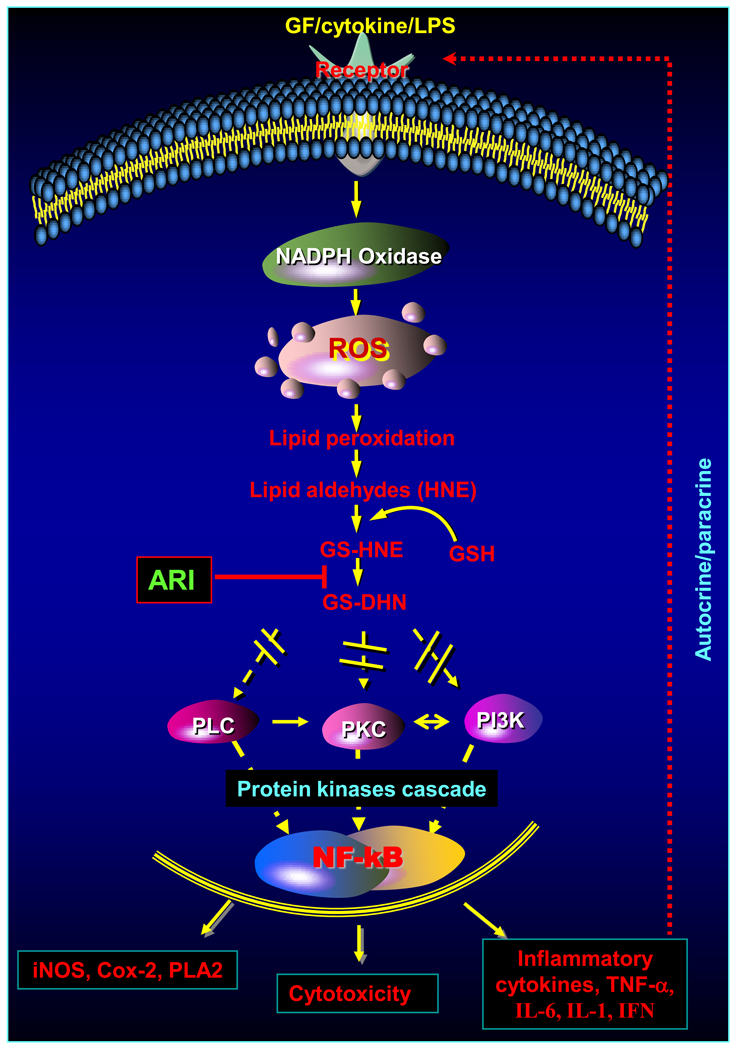
AR was first identified as an enzyme that provides energy source to sperm cells by converting blood glucose to fructose in the seminal vesicle (Hers, 1956). Four years after its identification, van Heyningen, 1959 showed accumulation of high concentrations of galactitol and sorbitol in the rat lens during sugar-induced cataractogenesis. Based on these observations, Kinoshita et al., 1981 proposed that the accumulation of polyols in the lens would lead to an increase in intracellular osmotic pressure, resulting in excessive hydration, gain of Na+, and loss of K+ ions. Since in the lens, as well as in other tissues, AR is the only enzyme that catalyzes the reduction of aldoses to polyols, this enzyme was identified as the chief facilitator of hyperglycemic injury. Indeed many AR inhibitors have been identified, synthesized and used to prevent secondary complications of diabetes in experimental animals (Alexiou et al, 2009; Oates, 2008). Therefore, by inference, it was assumed that the normal function of this enzyme was to catalyze the reduction of aldose (hence the name aldose reductase). However, the homogenous enzyme displayed poor affinity for glucose, with Km for glucose to be 50–100 mM. Kinetic properties of AR are unlike those of other glucose-metabolizing enzymes. These anomalies came into sharp focus when the X-ray structure of the enzyme showed that its active site is unlike that of other carbohydrate-binding proteins. The high hydrophobicity of the putative substrate-binding domain essentially precludes efficient carbohydrate reduction, and suggests that hydrophobic aldehydes are likely to be the preferred physiological substrates. The most obvious endogenous source of hydrophobic aldehydes is lipid peroxidation. It is well known that during free radical-mediated peroxidation of lipids, toxic and abundant aldehydes such as 4-hydroxynonenal (HNE) is produced in large amounts. Our and others observations show that AR may represent an important metabolic route for the detoxification of lipid aldehydes with Km in micro molar range. Further studies have also shown that GSH-conjugates of aldehydes such as GS-HNE and GS-acrolein are excellent substrates of AR (Ramana et al, 2000). Our site directed mutagenesis as well as molecular modeling studies have indicated the presence of GSH-binding site at the active site of AR (Ramana et al, 2000). Indeed, we have solved the crystal structure of AR-NADPH-GSH-analogue bound ternary complex of the enzyme. The crystallographic analysis indicates that AR has a specific GSH-aldehyde conjugates binding site distinct from the aldehyde binding site (Singh et al, 2006). Several investigations have also shown that AR catalyzes the reduction of lipid aldehydes and their GSH metabolites both in vitro and in vivo (Srivastava et al 2005). Inhibition of AR exacerbates the toxicity of aldehydes for the ocular lens, isolated cardiac myocytes and smooth muscle cells. These studies suggest that AR is required for the detoxification of a wide range of aldehydes and GS-aldehyde adducts generated during lipid peroxidation. In addition to reducing lipid peroxidation-derived aldehydes, AR has been shown to reduce phospholipid-aldehydes, steroids, base-propenals and 2-oxoaldehydes (Srivastava et al, 2005). An antioxidative role for AR is further supported by the observation that exposure of vascular smooth muscle cells (VSMC) to HNE up-regulates AR (Srivastava et al, 2005). Moreover, the presence of binding site for redox-regulated transcription factor NF-κB at the AR gene’s promoter site further supports the view that AR may be a significant component of antioxidant defenses involved in redox cell signaling. Indeed, recent studies indicate that AR is an oxidant-response protein which is highly expressed upon exposure to oxidative stress, growth factors and cytokines (Srivastava et al, 2005). Further, our recent studies show that inhibition of AR prevents cytokines- and hyperglycemia–induced proliferation of VSMC indicating AR’s role in mitogenicity (Srivastava et al, 2005). Our studies indicate that AR inhibition prevents NF-κB-dependent inflammatory signals induced by cytokines, growth factors and endotoxin which suggest that AR may be involved in inflammation (Fig.2). Interestingly, we have shown that reduced form of GS-HNE, GS-DHN catalyzed by AR mediates oxidative stress-induced NF-κB-dependent cytotoxic signals in VSMC and macrophages suggesting an unanticipated role of GS-HNE in inflammatory signaling (Ramana et al, 2006a).
Figure-2.
Role of aldose reductase in mediation of inflammatory signals. Cytokines, growth factors (GF), and lipopolysaccharide (LPS) cause oxidative stress via generation of ROS which forms toxic lipid aldehydes such as HNE by lipid peroxidation. HNE being highly electrophilic conjugates with cellular glutathione (GSH) spontaneously or catalyzed by GST to form GS-HNE. The reduced products of GS-aldehydes, GS-DHN, transduce inflammatory signaling via cascade of protein kinases leading to activation of NF-κB. Activation of NF-κB transcribes genes responsible for various inflammatory pathologies.
4. Clinical Implications
Based upon extensive experimental evidence that the inhibition of AR prevents or delays hyperglycemic injury in several experimental models of diabetes, it has been suggested that AR is involved in such secondary diabetic complications as cataractogenesis, retinopathy, neuropathy, nephropathy, and microangiopathy (Alexiou et al, 2009; Oates, 2008; Srivastava et al, 2005). Increased flux of glucose via AR could cause osmotic and oxidative stress, which in turn, could trigger a sequence of metabolic changes resulting in gross tissue dysfunction, altered intracellular signaling, and extensive cell death. Based on this rationale, extensive research efforts have been directed towards understanding the structure and function of AR and for developing effective anti-AR interventions for the clinical management of secondary diabetic complications (Alexiou et al, 2009). It has also been demonstrated that high glucose in diabetes leads to up-regulation of AR in several tissues, and that treatment with ARIs prevents hyperglycemia-induced hyperplasia and hyperproliferation of VSMC (Srivastava et al, 2005). Based on these studies, several ARIs are currently in clinical trials in the United States, whereas in other countries such as Japan an AR inhibitor epalrestat is already in clinical use. Nonetheless, the mechanistic reasons how inhibition of AR prevents diabetic complications continue to be elusive. Accumulation of sorbitol due to increased AR activity during hyperglycemia has been hypothesized. However, in several tissues the intracellular accumulation of sorbitol is not high enough to cause significant osmotic stress, especially in human tissues; sorbitol concentration never reaches to a level which could cause significant osmotic changes that would cause diabetic complications (Srivastava et al, 2005). Moreover, the high efficacy of antioxidants in preventing cataractogenesis in rodent models, without preventing sorbitol accumulation, suggests that oxidative stress may be an important feature of hyperglycemic injury. This is evident by the recent reports from our lab and others that AR catalyzes the reduction of lipid aldehydes and their GSH conjugates with high efficiency indicating that this enzyme may act as an antioxidant, protect DNA damage by reducing base propanals, and mediate the cytokine and growth hormone signals (Alexiou et al, 2009; Oates, 2008). Since NF-κB transcribes a number of inflammatory cytokines, growth factors and chemokines responsible for inflammatory response and a number of such inflammatory markers are highly elevated during inflammation, we examined the effect of AR inhibition on NF-κB–dependent inflammatory signals and found that inhibition of AR prevents cytokine-, growth factor-, hyperglycemia- and LPS–induced cytotoxic signals as well as the expression of inflammatory markers in various cultured cells (Ramana et al, 2002, 2003 and 2006b). These results indicated that AR inhibition could be anti-inflammatory. Therefore, we investigated the role of AR in prevention of colon carcinogenesis. Colon cancer is one of the leading causes of cancer related death in the world, and inflammation is being considered as a major culprit. Indeed, patients with inflammatory diseases such as inflammatory bowel disease, ulcerative colitis and Crohn's disease are at increased risk of developing colorectal cancer. We have shown that inhibition of AR prevents colon cancer cell growth in vitro as well as in nude mice xenografts (Tammali et al, 2006). Further, in a chemically–induced colon cancer model in mice, we have shown that inhibition or genetic ablation of AR (AR knockout mice) prevents azoxymethane–induced aberrant crypt foci formation (Tammali et al, 2009). These studies indicate that AR inhibitors could be chemopreventive. Several investigators have shown AR is over-expressed in a number of human cancers. Further, we have also shown that inhibition of AR prevents Gram-negative bacterial endotoxin, lipopolysaccharide–induced inflammatory signals in murine macrophages indicating that AR inhibition could prevent sepsis. Sepsis is an uncontrolled inflammatory response due to severe bacterial infections which could cause tissue dysfunction and damage leading to destructive inflammation throughout the body, often leading to multi-organ failure and death. We have shown that inhibition of AR could prevent endotoxin-induced cardiac dysfunction as well as cardiomyopathy in mice (Ramana et al, 2006c). Further, AR inhibition decreases the lethality associated with endotoximia in mice. Recently using a clinically more relevant cecal ligation and puncture model, we have shown that inhibition of AR prevents polymicrobial sepsis in mice. Further, we have also shown that AR inhibition prevents airway inflammation in an allergen-induced mouse of model of asthma (Yadav et al, 2009). These studies further affirm the anti-inflammatory role of AR inhibitors. The anti-inflammatory role of AR has also been examined in ocular inflammatory disease, uveitis in rats. By using endotoxin-induced uveitis in rats, we have shown that AR inhibition prevents inflammatory complications of the eye suggesting that AR inhibitors could be useful for preventing uveitis and its associated complications (Yadav et al, 2007). Other investigators have also shown that inhibition of AR could prevent atherosclerosis, restenosis and diabetic cardiomyopathy in animal models (Alexiou et al, 2009; Oates, 2008). These studies indicate the involvement of AR in various inflammatory pathologies and suggest the development of AR inhibitors as anti-inflammatory agents. Since these inhibitors have already gone through FDA approved clinical trials for diabetic complications their clinical use in diseases other than diabetes could be beneficial for preventing inflammatory diseases including asthma, colon cancer and sepsis in a relatively short time.
Acknowledgements
This work was supported in parts by National Institute of Health grants DK36118 and CA129383 (S.K.S), and GM 71036 and EY018591 (K.V.R).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- Alexiou P, Pegklidou K, Chatzopoulou M, Nicolaou I, Demopoulos VJ. Aldose reductase enzyme and its implication to major health problems of the 21(st) century. Curr Med Chem. 2009;16:734–752. doi: 10.2174/092986709787458362. [DOI] [PubMed] [Google Scholar]
- Barski OA, Tipparaju SM, Bhatnagar A. The aldo-keto reductase superfamily and its role in drug metabolism and detoxification. Drug Metab Rev. 2008;40:553–624. doi: 10.1080/03602530802431439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier B, Bendeif EE, Guillot B, Podjarny A, Lecomte C, Jelsch C. Charge Density and Electrostatic Interactions of Fidarestat, an Inhibitor of Human Aldose Reductase. J. Am. Chem. Soc. 2009;131:10929–10941. doi: 10.1021/ja8095015. [DOI] [PubMed] [Google Scholar]
- Hers HG. The mechanism of the transformation of glucose in fructose in the seminal vesicles. Biochim Biophys Acta. 1956;22:202–203. doi: 10.1016/0006-3002(56)90247-5. [DOI] [PubMed] [Google Scholar]
- Kinoshita JH, Kador P, Catiles M. Aldose reductase in diabetic cataracts. JAMA. 1981;246:257–261. [PubMed] [Google Scholar]
- Oates PJ. Aldose reductase, still a compelling target for diabetic neuropathy. Curr Drug Targets. 2008;9:14–36. doi: 10.2174/138945008783431781. [DOI] [PubMed] [Google Scholar]
- Petrash JM, Harter TM, Devine CS, Olins PO, Bhatnagar A, Liu S, Srivastava SK. Involvement of cysteine residues in catalysis and inhibition of human aldose reductase. Site-directed mutagenesis of Cys-80, -298, and -303. J Biol Chem. 1992;267:24833–24840. [PubMed] [Google Scholar]
- Ramana KV, Bhatnagar A, Srivastava S, Yadav UC, Awasthi S, Awasthi YC, Srivastava SK. Mitogenic responses of vascular smooth muscle cells to lipid peroxidation-derived aldehyde 4-hydroxy-trans-2-nonenal (HNE): role of aldose reductase-catalyzed reduction of the HNE-glutathione conjugates in regulating cell growth. J Biol Chem. 2006a;281:17652–17660. doi: 10.1074/jbc.M600270200. [DOI] [PubMed] [Google Scholar]
- Ramana KV, Chandra D, Srivastava S, Bhatnagar A, Aggarwal BB, Srivastava SK. Aldose reductase mediates mitogenic signaling in vascular smooth muscle cells. J Biol Chem. 2002;277:32063–32070. doi: 10.1074/jbc.M202126200. [DOI] [PubMed] [Google Scholar]
- Ramana KV, Dixit BL, Srivastava S, Balendiran GK, Srivastava SK, Bhatnagar A. Selective recognition of glutathiolated aldehydes by aldose reductase. Biochemistry. 2000;39:12172–12180. doi: 10.1021/bi000796e. [DOI] [PubMed] [Google Scholar]
- Ramana KV, Fadl AA, Tammali R, Reddy AB, Chopra AK, Srivastava SK. Aldose reductase mediates the lipopolysaccharide-induced release of inflammatory mediators in RAW264.7 murine macrophages. J Biol Chem. 2006b;281:33019–33029. doi: 10.1074/jbc.M603819200. [DOI] [PubMed] [Google Scholar]
- Ramana KV, Friedrich B, Bhatnagar A, Srivastava SK. Aldose reductase mediates cytotoxic signals of hyperglycemia and TNF-alpha in human lens epithelial cells. FASEB J. 2003;17:315–317. doi: 10.1096/fj.02-0568fje. [DOI] [PubMed] [Google Scholar]
- Ramana KV, Willis MS, White MD, Horton JW, DiMaio JM, Srivastava D, Bhatnagar A, Srivastava SK. Endotoxin-induced cardiomyopathy and systemic inflammation in mice is prevented by aldose reductase inhibition. Circulation. 2006c;114:1838–1846. doi: 10.1161/CIRCULATIONAHA.106.630830. [DOI] [PubMed] [Google Scholar]
- Singh R, White MA, Ramana KV, Petrash JM, Watowich SJ, Bhatnagar A, Srivastava SK. Structure of a glutathione conjugate bound to the active site of aldose reductase. Proteins. 2006;64:101–110. doi: 10.1002/prot.20988. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Watowich SJ, Petrash JM, Srivastava SK, Bhatnagar A. Structural and kinetic determinants of aldehyde reduction by aldose reductase. Biochemistry. 1999;38:42–54. doi: 10.1021/bi981794l. [DOI] [PubMed] [Google Scholar]
- Srivastava SK, Ramana KV, Bhatnagar A. Role of aldose reductase and oxidative damage in diabetes and the consequent potential for therapeutic options. Endocr Rev. 2005;26:380–392. doi: 10.1210/er.2004-0028. [DOI] [PubMed] [Google Scholar]
- Tammali R, Ramana KV, Singhal SS, Awasthi S, Srivastava SK. Aldose reductase regulates growth factor-induced cyclooxygenase-2 expression and prostaglandin E2 production in human colon cancer cells. Cancer Res. 2006;66:9705–9713. doi: 10.1158/0008-5472.CAN-06-2105. [DOI] [PubMed] [Google Scholar]
- Tammali R, Reddy AB, Ramana KV, Petrash JM, Srivastava SK. Aldose reductase deficiency in mice prevents azoxymethane-induced colonic preneoplastic aberrant crypt foci formation. Carcinogenesis. 2009;30:799–807. doi: 10.1093/carcin/bgn246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heyningen R. Formation of polyols by the lens of the rat with ‘sugar’ cataract. Nature. 1959;468:194–195. [Google Scholar]
- Yadav UC, Ramana KV, Aguilera-Aguirre L, Boldogh I, Boulares HA, Srivastava SK. Inhibition of aldose reductase prevents experimental allergic airway inflammation in mice. PLoS One. 2009;4:e6535. doi: 10.1371/journal.pone.0006535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav UC, Srivastava SK, Ramana KV. Aldose reductase inhibition prevents endotoxin-induced uveitis in rats. Invest Ophthalmol Vis Sci. 2007;48:4634–4642. doi: 10.1167/iovs.07-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]