Abstract
Proteins use multiple routes for transport from endosomes to the Golgi complex. Shiga and cholera toxins and TGN38/46 are routed from early and recycling endosomes, while mannose 6-phosphate receptors are routed from late endosomes. The identification of distinct molecular requirements for each of these pathways makes it clear that mammalian cells have evolved more complex targeting mechanisms and routes than previously anticipated.
Keywords: endosome, Golgi, Rab GTPase, mannose 6-phosphate receptors, Shiga and cholera toxins
Introduction
Retrograde transport of proteins from endosomes to the trans Golgi network (TGN) is a specialized pathway not taken by most cell surface glycoproteins. Duncan and Kornfeld [1] used modification of galactose-terminating oligosaccharide chains to show that most cell surface glycoproteins fail to reach TGN-localized sialyltransferase, but mannose 6-phosphate receptors (MPRs) are efficiently sialylated. In some cell types, transferrin receptors are also resialylated [2]. Thus, the major cargoes studied in endosome to Golgi transport are the MPRs, but additional cargoes include a protein named TGN46 (same as TGN38), which is a TGN-localized protein of unknown function that cycles between endosomes and the TGN, the protein processing enzyme furin, and toxins that are transported in a retrograde direction from endosomes through the Golgi and back to the ER [3,4].
Using light microscopy, Mallet and Maxfield [5] showed that chimeric forms of furin and TGN38 use different routes to get to the TGN. A Tac-TGN38 hybrid was transported from early endosomes via recycling endosomes to the TGN, while a Tac-furin construct moved to the TGN via late endosomes. Tac-furin transport was sensitive to nocodazole and wortmannin while Tac-TGN38 was only slowly blocked by wortmannin. Thus, the existence of distinct routes to the TGN for those proteins that traverse this pathway was established over ten years ago. Despite some confusion in the literature, as described below, numerous lines of experimentation and molecular distinctions show that MPRs are also transported to the TGN via late endosomes.
Mallard et al. [6] showed early on that slowing Shiga toxin retrograde transport by low temperature resulted in significant ultrastructural co-localization of the toxin with transferrin receptor-containing tubules that include recycling endosomes. That recycling endosomes are important intermediates comes from the requirement for the recycling endosome-specific, Rab11 GTPase [7], EHD3 protein [8], as well as the proposed roles for Rabs21 and 22 [9]. Moreover, overexpression of the putative GAP protein, TBC1D14, leads to the accumulation of this toxin in recycling endosomes [9]. This phenotype was obtained whether or not TBC1D14 carried a mutation that should block its potential GAP activity. Its inhibition of Shiga toxin transport is therefore likely due to titration of a limiting binding partner of exogenous TBC1D14--perhaps Rab11.
Mannose 6-phosphate receptor transport
Mannose 6-phosphate receptors carry newly made lysosomal enzymes from the Golgi to early endosomes, and then return to the TGN to retrieve additional cargo [10]. Live cell video microscopy has shown that MPRs depart the Golgi in clathrin and GGA-coated, vesicular/tubular structures [11,12] that contain Rab31 protein and are delivered to early endosomes [13,14].
Because depletion of a number of proteins leads to the accumulation of MPRs in early endosomes (for example, AP1 [15] and retromer [16,17]), several labs concluded that MPRs can be transported from that compartment directly to the Golgi complex. However, MPRs cannot release their bound ligands upon arrival in early endosomes; they require a lower pH than typical endocytic receptors for efficient ligand release [18], and must pass through a compartment of pH ≤5.5 prior to release bound ligands. In normal cells, the majority of MPRs reside in more acidic late endosomes at steady state [19]. The finding that the late endosomal Rab9 GTPase is required for MPR recycling [20–22] supports a model in which MPRs travel from early endosomes to late endosomes before arrival at the TGN. More recent work demonstrating the requirement for the late endosomal Rab7 GTPase in retromer recruitment to membranes [23,24] (see below) confirms the importance of the late endosome compartment for MPR retrograde transport. Live cell video microscopy has also detected the direct transfer of vesicles from Rab9-positive, late endosomes to distinctly labeled Golgi complexes, marked with GFP-galactosyltransferase [25]. Thus, it is likely that MPRs travel from early endosomes to late endosomes en route to the Golgi complex.
Could MPRs be transported from early endosomes directly to the TGN? If MPRs could traverse directly from early endosomes to the Golgi, loss of the late endosomal Rab7 or Rab9 should be inconsequential for MPR recycling since late endosomes could be bypassed. Yet we have shown that loss of Rab9 strongly destabilizes MPRs and triggers their mis-sorting to lysosomes [22,26]. Loss of Rab7 interferes with MPR transport [23,24]. As discussed below, the same is true in cells lacking the Rab9 effectors, TIP47 [22,27], GCC185 [28], and RhoBTB3 [29]--MPRs cannot bypass a requirement for these Rab9 effectors by rerouting through early endosomes to the Golgi. Thus, early microscopy and more recent molecular distinctions between early and recycling endosome to Golgi transport and late endosome to Golgi transport (discussed below) confirm the existence of distinct pathways for receptor transport (Figure 1).
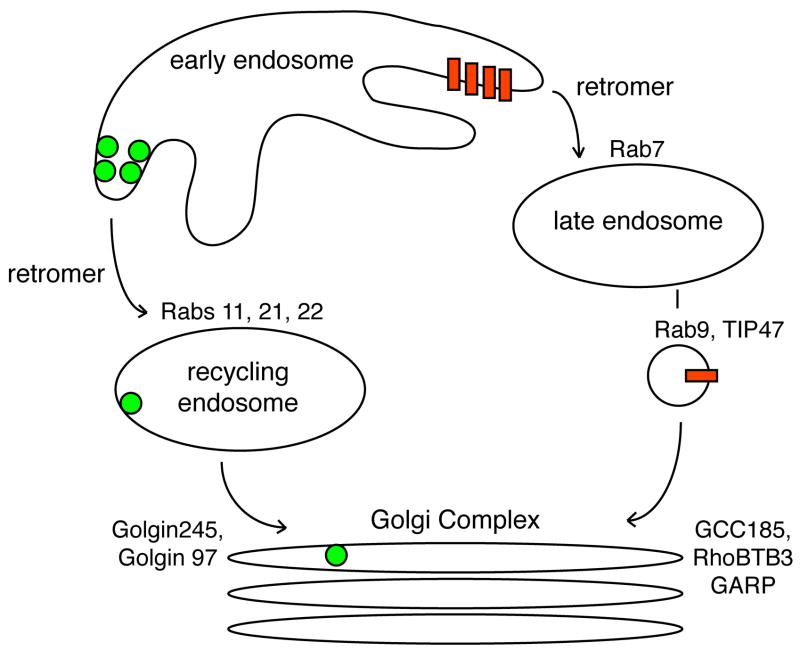
Figure 1.
Sorting of retrograde cargoes in endosomes. MPRs (red bars) and toxins (green spheres) are present in early endosomes and are sorted in that compartment prior to delivery to the Golgi complex. The toxins are directed towards Rab11-containing recycling endosomes prior to arrival at the Golgi. Rabs 21 and 22 may also play a role at this location [9]. MPRs are sorted towards Rab7 and Rab9-compartments. Both cargoes use Retromer as they leave early endosomes. Because toxins use recycling endosomes, and retromer depletion accumulates toxins in early endosomes, the presumed step is at early endosome exit. Similarly, for MPRs, exit from early endosomes is the presumed site of retromer function. Distinct Golgins and SNARE complexes at the TGN participate in vesicle receipt. This includes the GARP complex [65]. See text for additional details.
Retromer is clearly important for MPR recycling, as are Rab7 and Rab9, two late endosome specific Rab GTPases. Why then do MPRs accumulate in early endosomes when retromer is depleted, and show a more rapid rate of degradation, if retromer functions in exit from early endosomes (Fig. 1)? One possibility is that retromer-deficient early endosomes are direct targets for lysosomal fusion rather than the normal progression via late endosomes for degradation. Indeed, perhaps the role of Rab7 interaction with retromer is to guide early to late endosome trafficking. It will be important to learn whether other protein partners are concomitantly lost in cells depleted of retromer subunits to better understand this phenotype. For the time being, Figure 1 presents a working model that is consistent with existing data.
Molecular requirements distinguish multiple retrograde transport routes
An excellent review highlighting a long and growing list of distinct molecular requirements for toxins, MPRs and other retrograde cargoes was recently published [3]. Of particular note is the distinct requirement for Syntaxin 10 (STX10) for MPR recycling to the TGN [26] where it functions as part of a STX16, Vti1A and VAMP3-containing SNARE complex [26,30,31]. Shiga toxin, cholera toxin and ricin utilize a different SNARE complex containing STX6, STX16, Vti1a and the v-SNAREs, VAMP3 or VAMP4, as well as the Rab6A GTPase [32] but not Rab9 GTPase [33,34]. While TGN46 and cholera toxin require STX6, MPR recycling does not [26,30,31]. The putative tethering proteins, Golgin-97 [35] and Golgin-245 [36] are both important for Shiga toxin retrograde transport. In contrast, MPRs (but not cholera toxin) use GCC185 [28], and TGN38 (but not Shiga toxin) uses the GCC88 protein [37].
The distinction between STX10/Rab9 and GCC185-independent, early/recycling endosome to Golgi transport of TGN38/46 and Shiga and cholera toxin, versus STX10/Rab9 and GCC185-dependent transport of MPRs (Fig. 1) provides molecular proof of the distinction between these two transport routes. Why cells have evolved such complex machineries is not entirely clear.
It should be noted that STX10 blocks MPR export from early endosomes despite the fact that STX10 is primarily a Golgi localized t-SNARE [23]. From this, we have concluded that STX10 may function at both early to late endosome transport as well as late endosome to TGN transport [23]. Nevertheless, it was not needed for cholera toxin transport, confirming a molecular distinction between toxin transport and MPR transport and their distinct transport routes between endosomes and the Golgi complex.
Despite many differences, the pathways share a number of components including retromer components and cytoplasmic dynein. This suggests either a common, early step prior to sorting, or the use of common components after an initial sorting process. Future work will be needed to address this in greater detail. Here, I will focus on the components that facilitate MPR transport from late endosomes to the TGN.
Rab9 effectors in MPR retrograde transport
Rab9-dependent transport from late endosomes to the Golgi requires the Rab9 effectors, p40 [38] and TIP47 [27], a protein that recognizes the cytoplasmic domains of the two types of MPRs and packages them into nascent transport vesicles [39]. MPR recycling also utilizes a TGN-localized, coiled-coil protein named GCC185 that is also a Rab9 effector [28,40]. GCC185 is a putative transport vesicle tether that associates with the TGN by interaction of its C-terminus with both Rab6 and Arl1 GTPases. GCC185 has numerous, additional Rab binding sites across its entire length [41]. Also at the TGN is the RhoBTB3 ATPase that has been proposed to participate in the uncoating of transport vesicles upon their arrival at the TGN [29].
TIP47 in MPR trafficking
We discovered TIP47 in a yeast two hybrid screen, looking for proteins that bound to the cytoplasmic domains of both the cation-independent and cation-dependent MPRs [27]. Purified, recombinant TIP47 binds both MPR cytoplasmic domains directly, and although the majority of TIP47 is cytosolic, a fraction co-localizes in cells with perinuclear MPRs and co-fractionates with endosomal markers upon sucrose gradient flotation. Depletion of TIP47 using antisense oligonucleotides [27] or siRNA [22] strongly destabilizes MPRs in living cells, and antibody-depletion of TIP47 from cytosol leads to a complete loss of cytosol activity in terms of its ability to support in vitro transport of MPRs from endosomes to the TGN [27]. The association of cytosolic TIP47 with endosome-enriched membranes can be blocked by anti-MPR cytoplasmic domain antibodies, and binding requires specific residues in each of the MPRs: a FW sequence in the CD-MPR and a proline-rich sequence in the CI-MPR [42]. Binding is somewhat stronger to the CI-MPR than to the CD-MPR (1μM versus 3μM [43]) and no binding is seen with TGN38 or LDL-receptors. Importantly, TIP47 expression actually stimulates MPR transport to the TGN [39,44]. These data strongly support a model in which TIP47 acts as a cargo selection device for MPR retrograde transport.
TIP47 also binds the late endosomal Rab9 GTPase [39]. Importantly, Rab9 binding increases the affinity with which TIP47 binds CI-MPR cytoplasmic domains: the Kd improves from 0.9μM to 0.3μM. Moreover, the interaction of TIP47 with Rab9 is needed for its ability to stimulate the transport of MPRs from late endosomes to the TGN [39,45], and expression of a mutant TIP47 protein that cannot bind Rab9 changes the morphology of late endosomes [25]. These data indicate that TIP47 is recruited onto late endosomes by Rab9 GTPase, which participates in cargo collection by TIP47 protein.
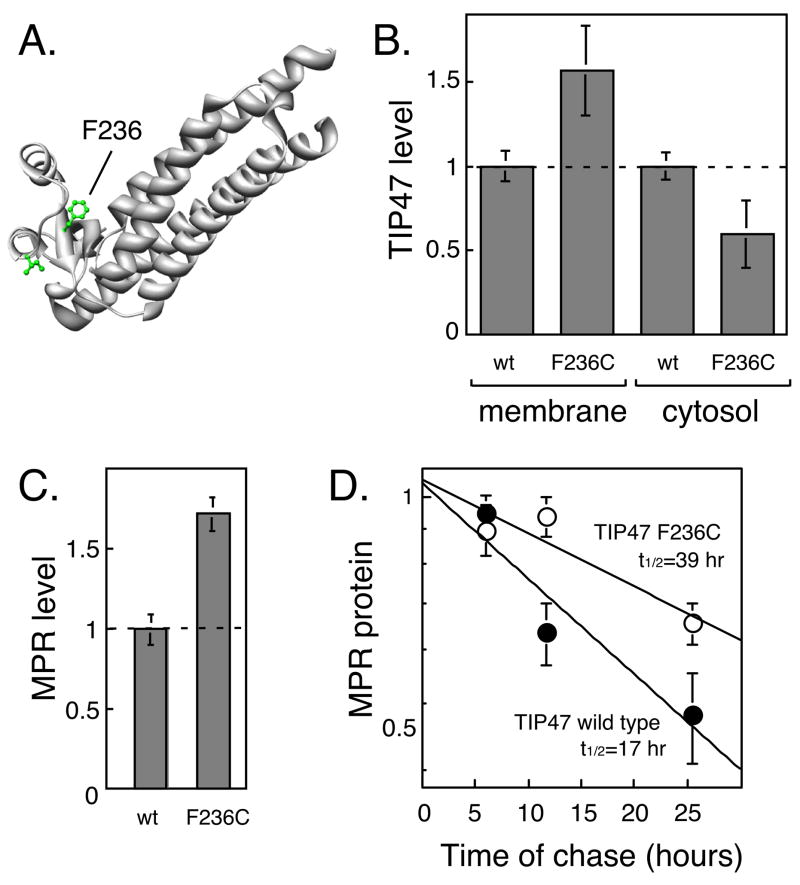
We used mis-incorporator tRNAs to generate a large number of TIP47 mutant proteins and combined this with affinity chromatography to identify two TIP47 variants with increased (F236C) or decreased (I214C) affinity for MPR cytoplasmic domains [46]. Localization of these amino acid residues on the three dimensional structure of TIP47 [47] revealed a binding pocket that can accommodate the MPR cytoplasmic domains (Fig. 2A). A TIP47 F236C mutant protein binds MPR cytoplasmic domains with a Kd of 0.2μM, which may reflect the high affinity state observed for wild type MPR binding to TIP47 in the presence of Rab9 GTPase. The ability of these mutant proteins to bind MPRs correlates with their relative membrane/cytosol distributions in transfected cells ([48]; Fig. 2B). Also, TIP47 F236C stabilizes MPRs from degradation in transfected cells (Fig. 2C,D) under conditions in which Rab9 turnover is unchanged [48]. These findings demonstrate that TIP47 interacts with MPRs in living cells and influences their fate.
Figure 2.
TIP47 binds MPRs in living cells and stabilizes them. (A) F236 was shown to be important for MPR binding because mutation of this residue to a cysteine increased TIP47 binding 5 fold. The structure shown is from [47] (PDB 1SZI). (B) TIP47 F236C with enhanced affinity for MPR cytoplasmic domains increases the amount of membrane-associated TIP47 protein in trasnfected cells. Shown are the relative ratios of membrane and cytosol-associated TIP47 protein comparing wild type and mutant protein-expressing cells. (C) Cells expressing F236C TIP47 show a higher steady state level of MPRs, presumably due to stabilization by direct binding. (D) Expression of TIP47 F236C stabilizes MPRs as seen when the protein half life is compared with cells in which TIP47 wild type protein is expressed. Data were presented in [48] and the figure was redrawn.
The importance of TIP47 in Rab9 function is further highlighted by our surprising finding that depletion of TIP47 destabilized Rab9 protein [22]. Importantly, the total level of Rab9 does not change, but its rates of degradation and new synthesis do. Intracellular interaction of TIP47 with Rab9’s hypervariable domain was shown definitively by relocalization of a chimeric Rab5/Rab9 protein [49]. The Rab5/Rab9 chimera binds both Rab5 effectors and less abundant Rab9 effectors. Simply introducing more TIP47 into cells moved the Rab5/9 protein from early endosomes to late endosomes. Thus, TIP47 binds both Rab9 and MPRs in live cells, and is important for the stability of both of these proteins, via precise residues that comprise the distinct Rab9 and MPR binding sites on TIP47.
Most TIP47 protein is cytosolic. In some cell types and under several physiological conditions, TIP47 is also present on lipid droplets, and its role there is also currently an area of active investigation [see 50 for review]. Recently, Bulankina et al. [51] challenged some of our previous work on TIP47 as a Rab9 effector and MPR modulator. There are trivial reasons why these workers may have obtained different results. The C-terminus of Rab9 is essential for TIP47 binding [49] and was very likely not present in their Rab preparations because wild type Rab9 loses its 23 C-terminal most residues upon expression in bacteria [20, 52]. As mentioned above, most TIP47 is cytosolic so to see it on late endosomes, one must permeabilize cells under conditions that permit slow release of cytosol (liquid nitrogen freeze thaw or digitonin before fixation) [see 27]. This may explain Bulankina et al.’s failure to detect TIP47 on the structures that we readily detect: they used Saponin permeabilization and very little TIP47 staining was seen in their images.
Rab9 and TIP47 are key for the production of several viruses
Rab9 and TIP47 are important for the production of infectious HIV particles [53–55]; indeed, Ebola virus, Marburg virus, and measles virus all require Rab9 GTPase function and certain Rab9 effectors [56] for their biogenesis. Vaccinia virus p37 protein also interacts with TIP47 during the process of intracellular mature virus production [57]. Finally, cellular intoxication by Pseudomonas exotoxin appears to use a Rab9 dependent pathway, unlike cholera and Shiga toxins [58].
Rab6 anchors the GCC185 tethering protein for MPR recycling
Rab6 is an important player in the retrograde transport of all cargoes. For MPRs, it plays an unexpected role in the anchoring of the GCC185 tether at the TGN [59]. A Rab6 binding site is located immediately upstream of a C-terminal, Arl binding GRIP domain in GCC185 (Fig. 3). Isothermal titration calorimetry revealed that Rab6 binds to this site with an affinity of 2.3 μM. We identified a mutant protein (I1588A/L1595A) that was significantly diminished in its ability to bind Rab6. These residues lie at the interface between Rab6 and GCC185. In the context of full length GCC185 protein, mutation of these residues generates a protein that is not Golgi-localized, demonstrating their importance for Golgi localization [59]. Consistent with this finding, siRNA depletion of Rab6 decreased the ability of the GCC185 C-terminus to bind to the Golgi. To our surprise, however, Arl1 depletion also blocked Golgi association of the GCC185 C-terminus. One possible explanation would be that both Arl1 and Rab6 contributed to localization. To test this, we measured directly the affinity of GCC185 for Arl1. The affinity is 7μM but improves two fold such that at certain concentrations, the concomitant presence of Rab6 and Arl1 led to a seven fold increase in GCC185 binding [59]. Thus, careful biochemistry revealed complex modes of interaction that could not have been detected by immunofluorescence assays. It therefore appears that Rab6 enhances the ability of Arl1 to bind the GCC185 GRIP domain to localize this protein at the TGN [59].
Figure 3.
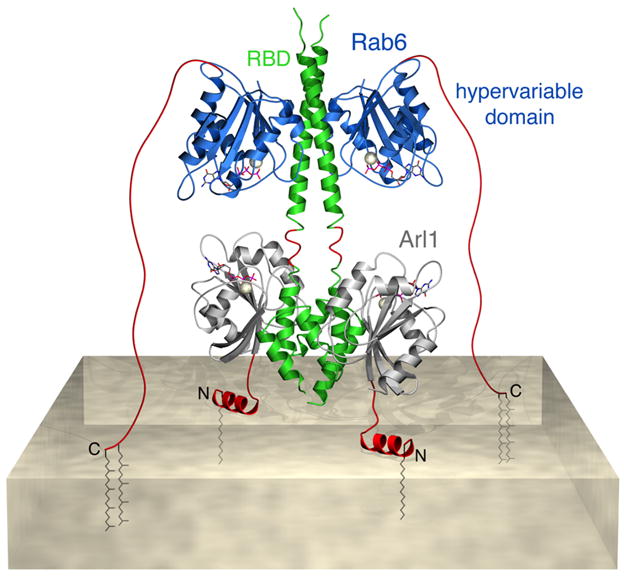
A model for anchoring of the C-terminus of the GCC185 Golgin (green) with the trans Golgi network (marbled platform). Shown are Rab6 GTPase (blue) with its C-terminal, unstructured hypervariable domain (red), anchored to the membrane by two, C-terminal, geranylgeranyl moieties, and Arl1 GTPase (gray) anchored to the membrane by a myristoylated, hydrophobic N-terminus (red). The structure of the GCC185 Rab binding domain (RBD) bound to Rab6 GTPase (PDB 1D 3BBP) was from [59]; the structure of the Arl1 GTPase bound to the GRIP domain (PDB 1D 1UPT) was modeled from [66]. Although GCC185 binds as many as 14 other GTPases across its length [41], only the Rab6:GCC185 interface shown here is essential for GCC185’s TGN association.
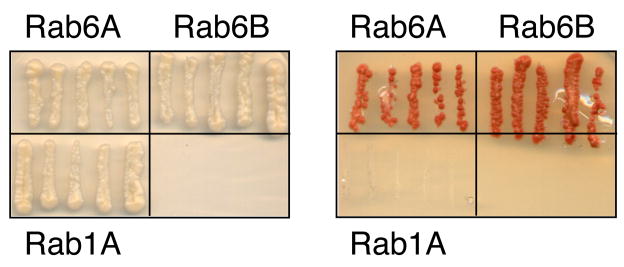
Recently, Houghton et al. [60] questioned our model. We welcome any alternative model that can also explain all the data in the literature and most importantly, can be tested experimentally; unfortunately, Houghton et al. did not provide one. Our siRNA experiments absolutely required very efficient depletion of Arl1 and Rab6. Houghton et al. [60] report only 80% depletion by the appropriate immunoblot assay, which would not have been adequate to see an effect. Houghton et al. [50] were concerned that most cellular GCC185 does not co-localize with Rab6. We know, for example, that Rab6 plays an important role on post-Golgi transport vesicles [61] but most does not localize there. Lack of majority co-localization does not in any way rule out essential roles for protein-protein interactions. Houghton et al. noted that it is easier to displace Golgin 245 and Golgin 97 in Arl1 depletions. We agree completely and this is easily explained--those proteins bind Arl1 more tightly (2μM), and do not appear to also require Rab6. Houghton et al. also found that the C-terminal 200 residues of GCC185 are more efficiently localized to the Golgi than the C-terminal most, 82 residues. That fits our data and our model but it should also be noted that the C-terminal, 82 residue fragment includes part of the Rab6 binding site and also part of the Rab9 binding site. Finally, Houghton et al. fail to detect Rab6 binding to the C-terminal 110 residues by yeast two hybrid. Negative yeast two hybrid should never be used as a final assessment of “in vivo” protein:protein interactions since there are many reasons that negative results can be obtained. Indeed, we tested this precise interaction and in this lab, Rab6A and 6B binding to the 110 C-terminal residues of GCC185 can be detected by yeast two hybrid (Figure 4). In summary, careful biochemistry combined with quantitative light microscopy support a role for Rab6 and Arl1 GTPases in GCC185 localization. Arl1 is already known to be important for the localization of Golgin-97, and Golgin-245. Other Rab6 effectors play critical roles for Shiga toxin transport [3] and include TMF/ARA160 [62]. The full list of Rab6 effectors that participate in retrograde transport pathways will be important to obtain.
Figure 4.
Two Hybrid detection of the interaction of GCC185 C-terminal 110 residues with Rab6A and Rab6B, but not Rab 1A. This experiment was carried out as described in [41] with Rab6A, 6B, and Rab1A in a Gal4BD fusion (pGBT9) and GCC185 C110 in a Gal4AD fusion (pACT2). Non-selective growth condition is shown at left; selective growth of the same yeast strains to reveal interaction is shown at right.
The Rab9 effector, RhoBTB3, is needed for MPR retrieval
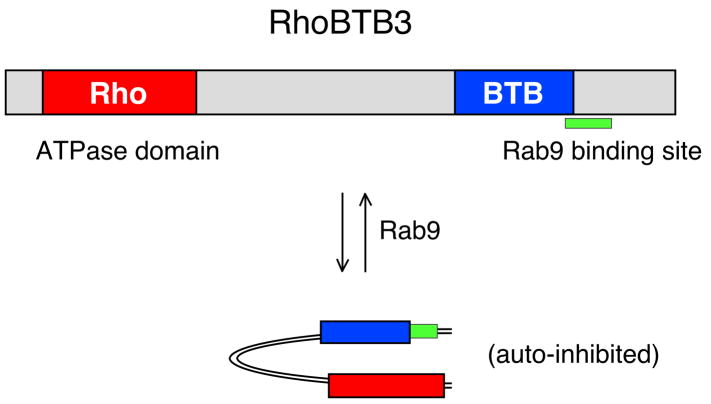
Rho BTB3 was discovered in a yeast two hybrid screen as a protein that binds active Rab9 GTPase and is required for MPR recycling to the Golgi complex [29]. The protein has an N-terminal, Rho GTPase-related domain and a C-terminal BTB domain that is likely a dimerization module (Figure 5). The Rab9 binding site lies just downstream of the BTB domain.
Figure 5.
Domain analysis of RhoBTB3. RhoBTB3 has an N-terminal Rho GTPase-related ATPase domain and a C-terminal Rab9 binding site. A BTB domain that may be responsible for dimerization is also shown. Binding of Rab9 activates the RhoBTB3 ATPase by release of autoinhibition of this enzyme. See [29] for more details.
Point mutations in either RhoBTB3’s Rab9 binding site or the nucleotide binding pocket of RhoBTB3 were used to demonstrate the importance of both of these domains of RhoBTB3 in siRNA depletion and plasmid rescue experiments [29]. To our surprise, the Rho GTPase domain encodes an ATPase with kinetic properties of a molecular chaperone. Moreover, we detected interaction of RhoBTB3 with TIP47 on membranes. One possibility that will require future work is that the Golgi-localized RhoBTB3 uncoats incoming transport vesicles carrying MPRs. Satisfyingly, Rab9 binding relieves autoinhibition of RhoBTB3’s ATPase activity [29]. Rab9 GTPase is present on the surface of inbound transport vesicles [25], and would be in the right place at the right time to turn on a vesicle-uncoating enzyme. The full range of RhoBTB3 substrates will be important to determine in the future.
Endosome maturation in retrograde sorting
The importance of organelle maturation as part of membrane traffic has been underscored by the recent demonstration of late endosome maturation from early endosomes by Rab conversion [63]. In this case, loss of the early endosomal Rab5 is coordinated with acquisition of the late endosomal Rab7; in this manner a given membrane changes its molecular functionality. Segregation of proteins into distinct domains on a given membrane may be sufficient in some cases to alter their routing. Specifically, if Rab11/Rab21/Rab22 and their effectors encounter recycling cargoes in early endosomes or in recycling endosomes, the fate of those cargoes may be the same. The only difference might be whether a motor protein has facilitated the dispersal of a Rab11 domain from a Rab5 domain on the early endosome. This subtlety may have caused some confusion for those analyzing endosome to Golgi transport--the specific molecular requirements provide a more precise description of pathways taken route than broader, endosome names. Within the Golgi, Rab conversion also takes place [64] and may account for maturation of cisternae in yeast Golgi and for maturation of mammalian cell Golgi carrying larger than normal cargo proteins.
Because Rab GTPases define function-specifying membrane microdomains, their participation or lack thereof is essential for our understanding of trafficking routes. As mentioned earlier, the toxins do not require Rab9 but late endosome-derived cargoes do.
Summary and Future Perspectives
All of the above data suggest that the earliest sorting events take place in early endosomes to segregate toxins from other, late endosome-bound, retrograde cargoes. How this sorting takes place and why it is important for cells are fundamental questions of broad importance. Why do cargoes use multiple tethering factors for successful delivery to the TGN? Perhaps this redundancy ensures successful transport. Yet individual depletions show strong phenotypes, suggesting that the functions of TGN tethers are not entirely overlapping. Functional tethering studies will soon reveal the mechanisms by which multiple transport carrier types are able to identify their TGN targets and fuse with that compartment. Elucidation of the full repertoire of Rab effectors mediating retrogade transport should provide an enormous amount of molecular detail and clues to the mechanisms of protein sorting and transport from endosomes to the Golgi of mammalian cells.
Acknowledgments
Work in this laboratory was supported by grants from the U.S. National Institutes of Health (DK37332 and GM79322). I heartily thank Frank Brown of this laboratory for the two hybrid experiment presented in Figure 4.
Abbreviations
- MPR
mannose 6-phosphate receptor
- TGN
trans Golgi network
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Duncan JR, Kornfeld S. Intracellular movement of two mannose 6-phosphate receptors: return to the Golgi apparatus. J Cell Biol. 1988;106:617–28. doi: 10.1083/jcb.106.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Snider MD, Rogers OC. Intracellular movement of cell surface receptors after endocytosis: resialylation of asialo-transferrin receptor in human erythroleukemia cells. J Cell Biol. 1985;100:826–34. doi: 10.1083/jcb.100.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johannes L, Popoff V. Tracing the retrograde route in protein trafficking. Cell. 2008;1357:1175–87. doi: 10.1016/j.cell.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Bonifacino JS, Rojas R. Retrograde transport from endosomes to the trans-Golgi network. Nat Rev Mol Cell Biol. 2006;7:568–579. doi: 10.1038/nrm1985. [DOI] [PubMed] [Google Scholar]
- 5.Mallet WG, Maxfield FR. Chimeric forms of furin and TGN38 are transported with the plasma membrane in the trans-Golgi network via distinct endosomal pathways. J Cell Biol. 1999;146:345–59. doi: 10.1083/jcb.146.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mallard F, Antony C, Tenza D, Salamero J, Goud B, Johannes L. Direct pathway from early/recycling endosomes to the Golgi apparatus revealed through the study of shiga toxin B-fragment transport. J Cell Biol. 1998;143:973–90. doi: 10.1083/jcb.143.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilcke M, Johannes L, Galli T, Mayau V, Goud B, Salamero J. Rab11 regulates the compartmentalization of early endosomes required for efficient transport from early endosomes to the trans-golgi network. J Cell Biol. 2000;151:1207–1220. doi: 10.1083/jcb.151.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naslavsky N, McKenzie J, Altan-Bonnet N, Sheff D, Caplan S. EHD3 regulates early-endosome-to-Golgi transport and preserves Golgi morphology. J Cell Sci. 2009;122:389–400. doi: 10.1242/jcs.037051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuchs E, Haas AK, Spooner RA, Yoshimura S, Lord JM, Barr FA. Specific Rab GTPase-activating proteins define the Shiga toxin and epidermal growth factor uptake pathways. J Cell Biol. 2007;177:1133–43. doi: 10.1083/jcb.200612068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh P, Dahms NM, Kornfeld S. Mannose 6-phosphate receptors: new twists in the t10le. Nat Rev Mol Cell Biol. 2003;4:202–212. doi: 10.1038/nrm1050. [DOI] [PubMed] [Google Scholar]
- 11.Doray B, Ghosh P, Griffith J, Geuze HJ, Kornfeld S. Cooperation of GGAs and AP-1 in packaging MPRs at the trans-Golgi network. Science. 2002;297:1700–3. doi: 10.1126/science.1075327. [DOI] [PubMed] [Google Scholar]
- 12.Puertollano R, van der Wel NN, Greene LE, Eisenberg E, Peters PJ, Bonifacino JS. Morphology and dynamics of clathrin/GGA1-coated carriers budding from the trans-Golgi network. Mol Biol Cell. 2003;14:1545–57. doi: 10.1091/mbc.02-07-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waguri S, Dewitte F, Le Borgne R, Rouille Y, Uchiyama Y, Dubremetz JF, Hoflack B. Visualization of TGN to endosome trafficking through fluorescently labeled MPR and AP-1 in living cells. Mol Biol Cell. 2003;14:142–155. doi: 10.1091/mbc.E02-06-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez-Gabin AG, Yin X, Si Q, Larocca JN. Transport of mannose-6-phosphate receptors from the trans-Golgi network to endosomes requires Rab31. Exp Cell Res. 2009;315:2215–30. doi: 10.1016/j.yexcr.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer C, Zizioli D, Lausmann S, Eskelinen EL, Hamann J, Saftig P, von Figura K, Schu P. mu1A-adaptin-deficient mice: lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors. EMBO J. 2000;19:2193–2203. doi: 10.1093/emboj/19.10.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arighi CN, Hartnell LM, Aguilar RC, Haft CR, Bonifacino JS. Role of the mammalian Retromer in sorting of the cation-independent mannose 6-phosphate receptor. J Cell Biol. 2004;165:123–33. doi: 10.1083/jcb.200312055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seaman MN. Cargo-selective endosomal sorting for retrieval to the Golgi requires retromer. J Cell Biol. 2004;165:111–22. doi: 10.1083/jcb.200312034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tong PY, Kornfeld S. Ligand interactions of the cation-dependent Mannose 6-phosphate receptor. Comparison with the cation-independent Mannose 6-phosphate receptor. J Biol Chem. 1989;264:7970–75. [PubMed] [Google Scholar]
- 19.Griffiths G, Hoflack B, Simons K, Mellman I, Kornfeld S. The mannose 6-phosphate receptor and the biogenesis of lysosomes. Cell. 1988;12:329–41. doi: 10.1016/s0092-8674(88)80026-6. [DOI] [PubMed] [Google Scholar]
- 20.Lombardi D, Soldati T, Riederer MA, Goda Y, Zerial M, Pfeffer SR. Rab9 functions in transport between late endosomes and the trans Golgi network. EMBO J. 1993;12:677–82. doi: 10.1002/j.1460-2075.1993.tb05701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riederer MA, Soldati T, Shapiro AD, Lin J, Pfeffer SR. Lysosome biogenesis requires Rab9 function and receptor recycling from endosomes to the trans-Golgi network. J Cell Biol. 1994;125:573–82. doi: 10.1083/jcb.125.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ganley IG, Carroll K, Bittova L, Pfeffer S. Rab9 GTPase regulates late endosome size and requires effector interaction for its stability. Mol Biol Cell. 2004;15:5420–30. doi: 10.1091/mbc.E04-08-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rojas R, van Vlijmen T, Mardones GA, Prabhu Y, Rojas AL, Mohammed S, Heck AJ, Raposo G, van der Sluijs P, Bonifacino JS. Regulation of retromer recruitment to endosomes by sequential action of Rab5 and Rab7. J Cell Biol. 2008;183:513–26. doi: 10.1083/jcb.200804048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seaman MN, Harbour ME, Tattersall D, Read E, Bright N. Membrane recruitment of the cargo-selective retromer subcomplex is catalysed by the small GTPase Rab7 and inhibited by the Rab-GAP TBC1D5. J Cell Sci. 2009;122:2371–82. doi: 10.1242/jcs.048686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbero P, Bittova L, Pfeffer SR. Visualization of Rab9-mediated vesicle transport from endosomes to the trans-Golgi in living cells. J Cell Biol. 2002;156:511–18. doi: 10.1083/jcb.200109030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganley IG, Espinosa E, Pfeffer SR. A syntaxin 10-SNARE complex distinguishes two distinct transport routes from endosomes to the trans-Golgi in human cells. J Cell Biol. 2008;180:159–72. doi: 10.1083/jcb.200707136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diaz E, Pfeffer SR. TIP47: a cargo selection device for mannose 6-phosphate receptor trafficking. Cell. 1998;93:433–43. doi: 10.1016/s0092-8674(00)81171-x. [DOI] [PubMed] [Google Scholar]
- 28.Reddy JV, Burguete AS, Sridevi K, Ganley IG, Nottingham RM, Pfeffer SR. A functional role for the GCC185 golgin in mannose 6-phosphate receptor recycling. Mol Biol Cell. 2006;17:4353–63. doi: 10.1091/mbc.E06-02-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Espinosa EJ, Calero M, Sridevi K, Pfeffer SR. RhoBTB3: a Rho GTPase-family ATPase required for endosome to Golgi transport. Cell. 2009;137:938–48. doi: 10.1016/j.cell.2009.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saint-Pol A, Yelamos B, Amessou M, Mills IG, Dugast M, Tenza D, Schu P, Antony C, McMahon HT, Lamaze C, Johannes L. Clathrin adaptor epsinR is required for retrograde sorting on early endosomal membranes. Dev Cell. 2004;6:525–38. doi: 10.1016/s1534-5807(04)00100-5. [DOI] [PubMed] [Google Scholar]; Seaman MN. Cargo-selective endosomal sorting for retrieval to the Golgi requires Retromer. J Cell Biol. 2004;165:111–22. doi: 10.1083/jcb.200312034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amessou M, Fradagrada A, Falguieres T, Lord JM, Smith DC, Roberts LM, Lamaze C, Johannes L. Syntaxin 16 and syntaxin 5 are required for efficient retrograde transport of several exogenous and endogenous cargo proteins. J Cell Sci. 2007;120:1457–68. doi: 10.1242/jcs.03436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mallard F, Tang BL, Galli T, Tenza D, Saint-Pol A, Yue X, Antony C, Hong W, Goud B, Johannes L. Early/recycling endosomes-to-TGN transport involves two SNARE complexes and a Rab6 isoform. J Cell Biol. 2002;156:653–64. doi: 10.1083/jcb.200110081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iversen TG, Skretting G, Llorente A, Nicoziani P, van Deurs B, Sandvig K. Endosome to Golgi transport of ricin is independent of clathrin and of the Rab9- and Rab11-GTPases. Mol Biol Cell. 2001;12:2099–107. doi: 10.1091/mbc.12.7.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith DC, Spooner RA, Watson PD, Murray JL, Hodge TW, Amessou M, Johannes L, Lord JM, Roberts LM. Internalized Pseudomonas exotoxin A can exploit multiple pathways to reach the endoplasmic reticulum. Traffic. 2006;7:379–93. doi: 10.1111/j.1600-0854.2006.00391.x. [DOI] [PubMed] [Google Scholar]
- 35.Lu L, Tai G, Hong W. Autoantigen Golgin-97, an effector of Arl1 GTPase, participates in traffic from the endosome to the trans-golgi network. Mol Biol Cell. 2004;15:4426–43. doi: 10.1091/mbc.E03-12-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshino A, Setty SR, Poynton C, Whiteman EL, Saint-Pol A, Burd CG, Johannes L, Holzbaur EL, Koval M, McCaffery JM, Marks MS. tGolgin-1 (p230, golgin-245) modulates Shiga-toxin transport to the Golgi and Golgi motility towards the microtubule-organizing centre. J Cell Sci. 2005;118:2279–93. doi: 10.1242/jcs.02358. [DOI] [PubMed] [Google Scholar]
- 37.Lieu ZZ, Derby MC, Teasdale RD, Hart C, Gunn P, Gleeson PA. The golgin GCC88 is required for efficient retrograde transport of cargo from the early endosomes to the trans-Golgi network. Mol Biol Cell. 2007;18:4979–91. doi: 10.1091/mbc.E07-06-0622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diaz E, Schimmoller F, Pfeffer SR. A novel Rab9 effector required for endosome-to-TGN transport. J Cell Biol. 1997;138:283–90. doi: 10.1083/jcb.138.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carroll KS, Hanna J, Simon I, Krise J, Barbero P, Pfeffer SR. Role of Rab9 GTPase in facilitating receptor recruitment by TIP47. Science. 2001;292:1373–76. doi: 10.1126/science.1056791. [DOI] [PubMed] [Google Scholar]
- 40.Derby MC, Lieu ZZ, Brown D, Stow JL, Goud B, Gleeson PA. The trans-Golgi network golgin, GCC185, is required for endosome-to-Golgi transport and maintenance of Golgi structure. Traffic. 2007;8:758–73. doi: 10.1111/j.1600-0854.2007.00563.x. [DOI] [PubMed] [Google Scholar]
- 41.Hayes GL, Brown FC, Haas AK, Nottingham RM, Barr FA, Pfeffer SR. Multiple Rab GTPase binding sites in GCC185 suggest a model for vesicle tethering at the trans-Golgi. Mol Biol Cell. 2009;20:209–17. doi: 10.1091/mbc.E08-07-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orsel JG, Sincock PM, Krise JP, Pfeffer SR. Recognition of the 300K mannose 6-phosphate receptor cytoplasmic domain by TIP47. Proc Natl Acad Sci USA. 2000;97:9047–51. doi: 10.1073/pnas.160251397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krise JP, Sincock PM, Orsel JG, Pfeffer SR. Quantitative Analysis of TIP47 (Tail-interacting protein of 47kD)-receptor cytoplasmic domain interactions: implications for endosome-to-trans Golgi network trafficking. J Biol Chem. 2000;275:25188–93. doi: 10.1074/jbc.M001138200. [DOI] [PubMed] [Google Scholar]
- 44.Sincock PM, Ganley IG, Krise J, Diederichs S, Sivars U, O’Connor B, Ding L, Pfeffer SR. Self-assembly is important for TIP47 function in mannose 6-phosphate receptor transport. Traffic. 2003;4:18–25. doi: 10.1034/j.1600-0854.2003.40104.x. [DOI] [PubMed] [Google Scholar]
- 45.Hanna J, Carroll K, Pfeffer SR. Identification of residues in TIP47 essential for Rab9 binding. Proc Natl Acad Sci USA. 2002;99:7450–54. doi: 10.1073/pnas.112198799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burguete AS, Harbury PB, Pfeffer SR. In vitro selection and prediction of TIP47 protein-interaction interfaces. Nature Methods. 2004;1:55–60. doi: 10.1038/nmeth702. [DOI] [PubMed] [Google Scholar]
- 47.Hickenbottom SJ, Kimmel AR, Londos C, Hurley JH. Structure of a lipid droplet protein; the PAT family member TIP47. Structure. 2004;12:1199–207. doi: 10.1016/j.str.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 48.Burguete AS, Sivars U, Pfeffer S. Purification and analysis of TIP47 function in Rab9-dependent mannose 6-phosphate receptor trafficking. Meth Enzymol. 2005;403:357–366. doi: 10.1016/S0076-6879(05)03031-4. [DOI] [PubMed] [Google Scholar]
- 49.Aivazian D, Serrano RL, Pfeffer S. TIP47 is a key effector for Rab9 localization. J Cell Biol. 2006;173:917–926. doi: 10.1083/jcb.200510010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bickel PE, Tansey JT, Welte MA. PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim Biophys Acta. 2009;1791:419–40. doi: 10.1016/j.bbalip.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bulankina AV, Deggerich A, Wenzel D, Mutenda K, Wittmann JG, Rudolph MG, Burger KN, Höning S. TIP47 functions in the biogenesis of lipid droplets. J Cell Biol. 2009;185:641–55. doi: 10.1083/jcb.200812042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riederer MA, Soldati T, Pfeffer SR. Expression, purification and in vitro isoprenylation of rab9 protein produced in E. coli. Meth Enzymol. 1995;257:15–21. doi: 10.1016/s0076-6879(95)57005-5. [DOI] [PubMed] [Google Scholar]
- 53.Blot G, Janvier K, Le Panse S, Benarous R, Berlioz-Torrent C. Targeting of the human immunodeficiency virus type 1 envelope to the trans-Golgi network through binding to TIP47 is required for env incorporation into virions and infectivity. J Virol. 2003;77:6931–45. doi: 10.1128/JVI.77.12.6931-6945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lopez-Vergès S, Camus G, Blot G, Beauvoir R, Benarous R, Berlioz-Torrent C. Tail-interacting protein TIP47 is a connector between Gag and Env and is required for Env incorporation into HIV-1 virions. Proc Natl Acad Sci U S A. 2006;103:14947–52. doi: 10.1073/pnas.0602941103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brass AL, Dykxhoorn DM, Benita Y, Yan N, Engelman A, Xavier RJ, Lieberman J, Elledge SJ. Identification of host proteins required for HIV infection through a functional genomic screen. Science. 2008;319:921–926. doi: 10.1126/science.1152725. [DOI] [PubMed] [Google Scholar]
- 56.Murray JL, Mavrakis M, McDonald NJ, Yilla M, Sheng J, Bellini WJ, Zhao L, Le Doux JM, Shaw MW, Luo CC, Lippincott-Schwartz J, Sanchez A, Rubin DH, Hodge TW. Rab9 GTPase is required for replication of human immunodeficiency virus type 1, filoviruses, and measles virus. J Virol. 2005;79:11742–51. doi: 10.1128/JVI.79.18.11742-11751.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Y, Honeychurch KM, Yang G, Byrd CM, Harver C, Hruby DE, Jordan R. Vaccinia virus p37 interacts with host proteins associated with LE-derived transport vesicle biogenesis. Virol J. 2009;6:44. doi: 10.1186/1743-422X-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith DC, Spooner RA, Watson PD, Murray JL, Hodge TW, Amessou M, Johannes L, Lord JM, Roberts LM. Internalized Pseudomonas exotoxin A can exploit multiple pathways to reach the endoplasmic reticulum. Traffic. 2006;7:379–93. doi: 10.1111/j.1600-0854.2006.00391.x. [DOI] [PubMed] [Google Scholar]
- 59.Burguete AS, Fenn TD, Brunger AT, Pfeffer SR. Rab and Arl GTPase family members cooperate in the localization of the golgin GCC185. Cell. 2008;132:286–98. doi: 10.1016/j.cell.2007.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Houghton FJ, Chew PL, Lodeho S, Goud B, Gleeson PA. The localization of the golgin, GCC185 is independent of Rab6A/A′ and Arl1. Cell. 2009;138:787–94. doi: 10.1016/j.cell.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 61.Grigoriev I, Splinter D, Keijzer N, Wulf PS, Demmers J, Ohtsuka T, Modesti M, Maly IV, Grosveld F, Hoogenraad CC, Akhmanova A. Rab6 regulates transport and targeting of exocytotic carriers. Dev Cell. 2007;13:305–14. doi: 10.1016/j.devcel.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 62.Yamane J, Kubo A, Nakayama K, Yuba-Kubo A, Katsuno T, Tsukita S, Tsukita S. Functional involvement of TMF/ARA160 in Rab6-dependent retrograde membrane traffic. Exp Cell Res. 2007;313:3472–85. doi: 10.1016/j.yexcr.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 63.Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122:735–49. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 64.Rivera-Molina FE, Novick PJ. A Rab GAP cascade defines the boundary between two Rab GTPases on the secretory pathway. Proc Natl Acad Sci USA. 2009 Aug 4; doi: 10.1073/pnas.0906536106. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pérez-Victoria FJ, Mardones GA, Bonifacino JS. Requirement of the human GARP complex for mannose 6-phosphate-receptor-dependent sorting of cathepsin D to lysosomes. Mol Biol Cell. 2008;19:2350–62. doi: 10.1091/mbc.E07-11-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Panic B, Perisic O, Veprintsev DB, Williams RL, Munro S. Structural basis for Arl1-dependent targeting of homodimeric GRIP domains to the Golgi apparatus. Mol Cell. 2003;12:863–74. doi: 10.1016/s1097-2765(03)00356-3. [DOI] [PubMed] [Google Scholar]