Abstract
The peptides galanin (GAL) and orexin (OX) share common features with the opioid enkephalin (ENK) in their relationship to ingestive behavior, stimulating consumption of a fat-rich diet and ethanol when injected into the hypothalamus. Since receptors for GAL and OX are dense in areas where ENK-expressing neurons are concentrated, these non-opioid peptides may exert their effects, in part, through the stimulation of endogenous ENK. This study was conducted to determine whether injection of GAL or OX affects the expression of ENK in hypothalamic and mesolimbic nuclei involved in consummatory behavior. Rats were injected with GAL (1 μg), OX-A (1 μg), or saline vehicle just dorsal to the hypothalamic paraventricular nucleus (PVN). They were sacrificed one hour later for analysis of ENK mRNA levels in the PVN, ventral tegmental area (VTA), central nucleus of the amygdala (CeA), and nucleus accumbens (NAc). Both GAL and OX had similar effects, significantly increasing ENK mRNA expression in each of these areas, except for the NAc. This enhanced ENK expression in the PVN, VTA and CeA was demonstrated with real-time quantitative polymerase chain reaction and confirmed in separate groups using radiolabeled and digoxigenin-labeled in situ hybridization. These findings demonstrate that the non-opioid peptides, GAL or OX, which have similar effects on consummatory behavior, are also similar in their effect on endogenous ENK. In light of published findings showing an opioid antagonist to block GAL- and OX-induced feeding, these results provide additional evidence that ENK is involved in mediating the common behavioral effects of these peptides.
Keywords: orexin, galanin, enkephalin, rat, mRNA
1. Introduction
Certain peptides expressed in the hypothalamus share common features in their relationship with ingestive behavior. For example, injection of galanin (GAL) into the hypothalamic paraventricular nucleus (PVN) is found to stimulate the consumption of ethanol [66, 75] as well as ingestion of a diet rich in fat [85]. Similar effects have been described with injection of orexin (OX) into the PVN [21, 75]. This raises the possibility that GAL and OX may act through some common mechanism to produce these ingestive behaviors. Evidence suggests that this mechanism may involve the opioid peptide enkephalin (ENK). As with GAL and ORX, PVN injection of ENK analogues causes an increase in the consumption of ethanol [5] as well as a high-fat diet [56, 79]. Furthermore, the feeding response induced by central injection of GAL or OX is greatly attenuated by administration of an opioid receptor antagonist [20, 24, 83-84]. Thus, the non-opioid peptides GAL and OX may affect consummatory behavior by stimulating the opioid ENK, perhaps within the PVN where it has an important role in ingestive behavior [87] but also in mesolimbic nuclei where it is involved in reward [19, 42].
Neuroanatomical evidence suggests that GAL and OX may exert their effects through these different brain areas. Neurons expressing GAL, which are heavily concentrated in the PVN as well as other areas of the brain [30], send projections locally and to mesolimbic nuclei, such as the ventral tegmental area (VTA), central nucleus of the amygdala (CeA), and nucleus accumbens (NAc), where its receptors (GALR1 and GALR2) are expressed at moderate to high levels [33, 59]. Similarly, neurons expressing OX, found exclusively in the perifornical and lateral area (PFLH) of the hypothalamus [16, 73], project to the PVN and mesolimbic areas where the OX receptors (OX1R and OX2R) are densely expressed [48, 51]. In these different areas, ENK-expressing neurons are known to be densely concentrated [6, 15, 25, 72], providing the neuroanatomical basis for an interaction between the non-opioid and opioid peptides. Thus, both GAL and OX have the potential for far-reaching and possibly similar effects on ENK throughout the brain, in nuclei where this opioid may be involved in mediating different behavioral functions related to consummatory behavior.
This study was designed to test this possibility by examining the effects of GAL and OX injection on the expression of the opioid peptide ENK in different brain areas. Animals were injected with GAL, OX, or vehicle just dorsal to the PVN, and their effects on mRNA expression of ENK were examined in the PVN, VTA, CeA, and NAc. The results revealed a stimulatory effect of these non-opioid peptides, GAL and OX, on the opioid peptide ENK in these different brain areas.
2. Materials and methods
2.1. Subjects
Adult, male Sprague–Dawley rats (200-250 g, Charles River Laboratories International, Inc., Wilmington, MA) were housed individually, on a 12-hour reversed light/dark cycle. All animals were allowed 1 week to acclimate to their individual housing conditions, during which time they received ad libitum access to standard rodent chow (LabDiet Rodent Chow 5001, St. Louis, MO) and water. Separate groups of animals were used for each experiment. The housing facility was fully accredited by AAALAC, and the behavioral protocols were approved by the Rockefeller University Animal Care Committee.
2.2. Drugs
The drugs injected were orexin-A (human, rat, mouse) (1 μg) and galanin (rat) (1 μg), both from Sigma-Aldrich Inc. (St. Louis, MO, USA). They were dissolved in saline and injected in a volume of 1 μl. Saline vehicle was used as a control injection, also injected in a volume of 1 μl.
2.3. Surgery
Subjects were anesthetized with ketamine (80 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.), supplemented with ketamine as needed. Guide shafts, made of 21-gauge stainless steel, 12 mm in length, were implanted perpendicularly and unilaterally just dorsal to the PVN. The coordinates were A-P -1.8 (relative to bregma), L 0.4 (relative to midsaggital sinus), and D-V 5.0 (relative to level skull surface), with half on the left side and half on the right side. Injectors protruded 3 mm beyond the guide shafts. One week of recovery was provided after surgery before testing. Between procedures, stainless steel obdurators were left in the guide shafts to prevent occlusion.
2.4. Test procedures
For each experiment, food was removed 90 minutes prior to injection. Injections were given 2 hours prior to the end of the light cycle. The OX, GAL, or saline vehicle (n=5/group) was delivered through concentric microinjectors made of 26-gauge stainless steel outside and fused-silica tubing inside (74 μm ID, 154 μm OD, Polymicro Technologies, Phoenix AZ) that protruded 3 mm beyond the guide shaft to reach just dorsal to the PVN (V 8.0). A volume of 1.0 μl was delivered during 1 min, and the microinjector remained in place for another 1 min to allow diffusion into the injection site. Animals were then sacrificed by rapid decapitation 1 hour after injection.
In Experiment 1, the PVN, VTA, CeA, and NAc were microdissected for measurements of ENK mRNA using quantitative real-time polymerase chain reaction (qRT-PCR). In Experiment 2, the whole brain was removed and placed in a 4% paraformaldehyde solution for ENK measurements using radiolabeled in situ hybridization histochemistry (ISH). In Experiment 3, the whole brain was removed and placed in a 4% paraformaldehyde solution for ENK measurements using digoxigenin-labeled in situ hybridization (DIG). This procedure of injecting and analyzing gene expression in the same hypothalamic nucleus has recently been used with leptin injection in the ventromedial hypothalamus [2].
2.5. Brain dissection
Immediately after sacrifice, the brain was placed in a matrix slicing guide with the ventral surface facing up. A total of three coronal cuts, yielding two slices, were made rostrally. The first cut was made in the anterior middle optic chiasm (Bregma −0.8 mm), according to the atlas of Paxinos and Watson [63]. The second cut was 1.5 mm rostral to this (Bregma −0.8 to 0.7 mm), yielding a first slice, which was discarded. Then, one 1.0 mm cut was made (Bregma 0.7 to 1.7 mm) rostral to this first slice, yielding a slice that was used for microdissection of the NAc (Bregma 0.7 to 1.7 mm). Caudal to the original slice, two additional 1.0 mm slices (Bregma −0.8 to −2.8 mm) were made, with the first used for microdissection of the PVN (Bregma −0.8 to −1.8 mm) and the second for the CeA (Bregma −1.8 to −2.8 mm). Further caudally, one 0.5 mm slice was made by cutting between the caudal boundary of the mamillary bodies and the rostral boundary of the pons, which was used for microdissection of the VTA (Bregma −5.6 to −6.1 mm).
These sections were placed on a glass slide and rapidly dissected under a microscope. The NAc was dissected bilaterally in an egg shape, with the dorsal tip beginning at the lateral ventricle, the medial aspect at the semilunar nucleus, the lateral aspect medial to the lateral stripe of the striatum, and the ventral edge along the ventral pallidum. The PVN was dissected as a reversed isosceles triangle, 1.0 mm bilateral to the third ventricle and between the fornix structures [11]. The CeA was dissected bilaterally as a circle, immediately dorsomedial to the basolateral amygdala and 0.2 mm dorsolateral to the optic tract. The VTA was also dissected bilaterally as a circle, lateral to the interfascicular nuleus, medial to the medial lemniscus, ventral to the red nucleus and dorsal to the paranigral nucleus.
2.6. Histology
Cannula placement was visually confirmed during brain dissection. Also, a cannulated rat not sacrificed for analysis of ENK mRNA was examined for microscopic verification of probe placement. This rat was injected with 0.25 μl methylene blue dye (Sigma, St. Louis, MO) and its brain kept in formalin for one week prior to slicing. The brain was then cut in 40 μm sections on a freezing microtome and slide-mounted for microscopic examination.
2.7. Quantitative real-time PCR
As previously described [11], total RNA from pooled microdissected samples was extracted with TRIzol reagent. RNA was treated with RNase-free DNase I before RT. For qRT-PCR, cDNA and minus RT were synthesized using an oligo-dT primer with or without SuperScript II Reverse Transcriptase. The SYBR Green PCR core reagents kit (Applied Biosystems, Foster City, CA) was used, with cyclophilin as an endogenous control. qRT-PCR was performed in MicroAmp Optic 96-well Reaction Plates (Applied Biosystems). This was done on an ABI PRISM 7900 Sequence Detection system (Applied Biosystems), under the condition of 2 minutes at 50°C, 10 minutes at 95°C, and 40 cycles of 15 seconds at 95°C and 1 minute at 60°C. Each study consisted of 4 independent runs of qRT-PCR in triplicate, and each run included a standard curve, a nontemplate control, and a negative RT control. The levels of target gene expression were quantified relative to the level of cyclophilin, using the comparative Ct method. The primers, designed with ABI Primer Express V.1.5a software from published sequences, were: (1) cyclophilin: 5′- GTGTTCTTCGACATCACGGCT -3′ (forward) and 5′- CTGTCTTTGGAACTTTGTCTGCA -3′ (reverse); and (2) E N K: 5′-GGACTGCGCTAAATGCAGCTA-3′ (forward) and 5′-GTGTGCATGCCAGGAAGTTG-3′ (reverse). The concentrations of primers were 100 nM. All reagents, unless indicated, were from Invitrogen (Carlsbad, CA).
2.8. Radiolabeled in situ hybridization histochemistry
Besides qRT-PCR, mRNA levels of ENK were measured with radiolabeled ISH. This technique allows for more anatomically precise measurements of gene expression than qRT-PCR. Antisense and sense RNA probes were labeled with 35S-UTP (Amersham Biosciences, Piscataway, NJ), as previously described [7, 49]. Alternate free-floating coronal sections were consecutively processed as follows: 10 minutes in 0.001% proteinase K, 5 minutes in 4% paraformaldehyde, and 10 minutes each in 0.2 N HCl and acetylation solution, with a 10-minute wash in PB between each step. After the wash, the sections were hybridized with a 35S-labeled probe (103 cpm/mL) at 55°C for 18 hours. Following hybridization, the sections were washed in 5X sodium chloride and sodium citrate (SSC), and the nonspecifically bound probe was removed by RNase (Sigma) treatment for 30 minutes at 37°C. Sections were then run through further stringency washes with 0.1 M dithiothreitol (Sigma) in 2X SSC and 1X SSC and 0.1X SSC at 55°C. Sections were finally mounted, air-dried, and exposed to a Kodak BioMax MR film for 18 to 24 hours at −80°C, when films were developed and microscopically analyzed. The sense probe control was performed in the same tissue, and no signal was found.
Computer-assisted microdensitometry of autoradiographic images was determined as described [49, 70] on the MCID image analysis system (Image Research Inc., St. Catherines, ON, Canada). Microscale 14C standards (Amersham Biosciences) were exposed on the same Kodak film with the sections and digitized. Gray-level/optical density calibrations were performed with a calibrated film-strip ladder (Imaging Research Inc.) for optical density. This was plotted as a function of microscale calibration values. All subsequent optical density values of digitized autoradiographic images fell within the linear range of the function. The values obtained represent the average of measurements taken from 10 sections per animal. Within each section, the optical density for the nucleus was recorded, from which the background optical density from a same-size area in the corpus callosum was subtracted. The mean value of the ethanol-drinking group in each experiment was reported as a percentage of the water-drinking control group.
2.9. Digoxigenin-labeled in situ hybridization histochemistry
In situ hybridization histochemistry with digoxigenin-labeled probes was used to quantify ENK gene expression. This technique measures specifically the density of neurons expressing the peptide gene above threshold levels but not the level of mRNA expressed per cell. Brains were cut into 30 μm thick sections with a cryostat. DIG-labeled cRNA probes of ENK were synthesized by in vitro transcription as previously described [7, 49]. Free-floating coronal sections were processed for DIG-ISH as for radiolabeled ISH until the high stringency wash with the exception of replacement with the DIG-labeled probe. After the high stringency wash, the sections were blocked and incubated in AP-conjugated sheep anti-digoxigenin antibody (Sheep Anti-DIG, Fab fragments, 1:1000; Boehringer Mannheim) overnight. After washing in Tris buffer (0.1 M, pH 9.5), the signal was revealed with NBT/BCIP. Then, the sections were mounted, dehydrated, and coverslipped as previously described [7].
Gene expression level was measured by semiquantification with Image-Pro Plus software (Version 4.5, Media Cybernetics Inc., Silver Spring, MD) as previously described [45]. It is expressed as the density of mRNA-containing cells, “cells/mm2”.
2.10. Data analysis
The data in the figures and table are expressed as mean ± SEM. Statistical analyses of these data were performed using unpaired, two-tailed t-tests.
3. Results
3.1. Effect of GAL and OX injection on ENK mRNA measured by qRT-PCR
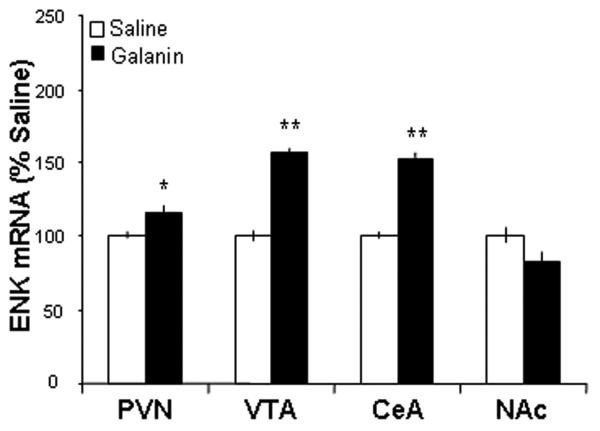
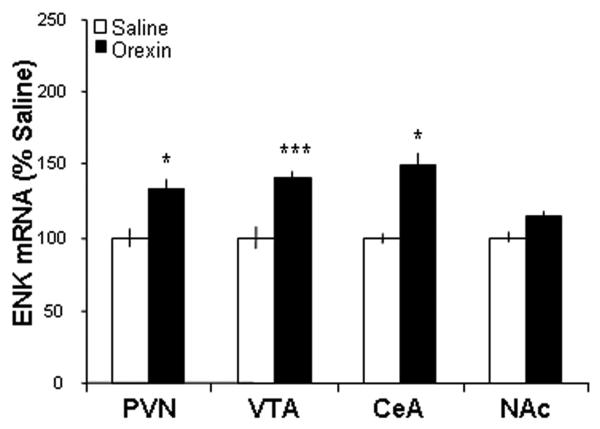
This experiment used qRT-PCR to determine whether hypothalamic injection of the non-opioid peptides can modulate ENK gene expression in different brain sites. Rats with implanted cannulae aimed just dorsal to the PVN were given a single injection of GAL (n = 5), OX (n = 5), or saline vehicle (n = 5) and were examined for the expression of ENK mRNA, both locally and in different mesolimbic structures. Compared to saline vehicle, injection of GAL produced a significant increase in the expression of ENK mRNA in the PVN (p < 0.05), just below the area of the injection (Fig. 1). A similar effect was also detected in the VTA (p < 0.01) and CeA (p < 0.01), but not in the NAc (ns) (Fig. 1). Interestingly, a very similar change in ENK gene expression was observed with PVN injection of OX. As with GAL, this peptide increased ENK mRNA levels in the PVN (p < 0.05), VTA (p < 0.001) and CeA (p < 0.05), but not the NAc (ns) (Fig. 2). Thus, as shown by qRT-PCR, PVN injections of GAL and OX produce the same pattern of enhanced ENK expression in the different hypothalamic and mesolimbic nuclei examined, with the exception of the NAc where ENK was unresponsive to these peptides.
Fig. 1.
Injection of galanin into the hypothalamic paraventricular nucleus (PVN) enhances enkephalin mRNA expression in the PVN, ventral tegmental area (VTA), and central nucleus of the amygdala (CeA), but not in the nucleus accumbens (NAc) as assessed by quantitative real-time polymerase chain reaction (n = 5/group). Values are mean ± S.E.M., **p < 0.01, *p < 0.05 vs. saline injection.
Fig. 2.
Injection of orexin into the hypothalamic paraventricular nucleus (PVN) enhances enkephalin mRNA expression in the PVN, ventral tegmental area (VTA), and central nucleus of the amygdala (CeA), but not in the nucleus accumbens (NAc) as assessed by quantitative real-time polymerase chain reaction (n = 5/group). Values are mean ± S.E.M., ***p < 0.001, *p < 0.05 vs. saline injection.
3.2. Effect of GAL and OX injection on ENK mRNA measured radiolabeled ISH
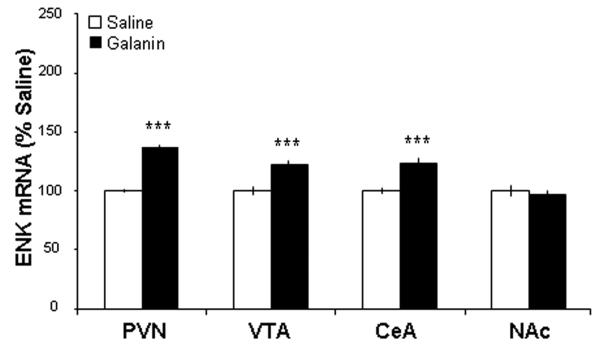
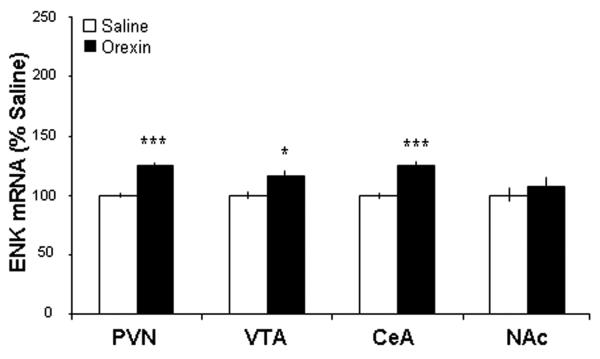
This experiment was conducted to confirm, with a different technique, the changes in gene expression observed in Experiment 1. The animals were, once again, implanted with cannulae aimed just dorsal to the PVN, injected with GAL (n = 5), OX (n = 5), or saline vehicle (n = 5), and were then examined for ENK mRNA levels using radiolabeled ISH. The results obtained in the PVN and extra-hypothalamic areas revealed a similar pattern to that shown with qRT-PCR. Compared to rats injected with saline vehicle, GAL injection again increased ENK mRNA levels in the PVN (p < 0.001), VTA (p < 0.001) and CeA (p < 0.001) but not the NAc (ns) (Fig. 3). The same pattern, of enhanced ENK mRNA in the PVN (p < 0.001), VTA (p < 0.05) and CeA (p < 0.001), but not the NAc (ns), was also produced by injection of OX (Fig. 4). These results obtained with radiolabeled ISH are illustrated in photomicrographs showing ENK expression in the PVN and CeA of rats injected with GAL compared to saline (Fig. 5). Thus, the stimulatory effect of PVN injection of GAL or OX on ENK mRNA in different brain areas is robust and confirmed using ISH.
Fig. 3.
Injection of galanin into the hypothalamic paraventricular nucleus (PVN) enhances enkephalin mRNA expression in the PVN, ventral tegmental area (VTA), and central nucleus of the amygdala (CeA), but not in the nucleus accumbens (NAc) as assessed by radiolabeled in situ hybridization histochemistry (n = 5/group). Values are mean ± S.E.M., ***p < 0.001vs. saline injection.
Fig. 4.
Injection of orexin into the hypothalamic paraventricular nucleus (PVN) enhances enkephalin mRNA expression in the PVN, ventral tegmental area (VTA), and central nucleus of the amygdala (CeA), but not in the nucleus accumbens (NAc) as assessed by radiolabeled in situ hybridization histochemistry (n = 5/group). Values are mean ± S.E.M., ***p < 0.001, *p < 0.05 vs. saline injection.
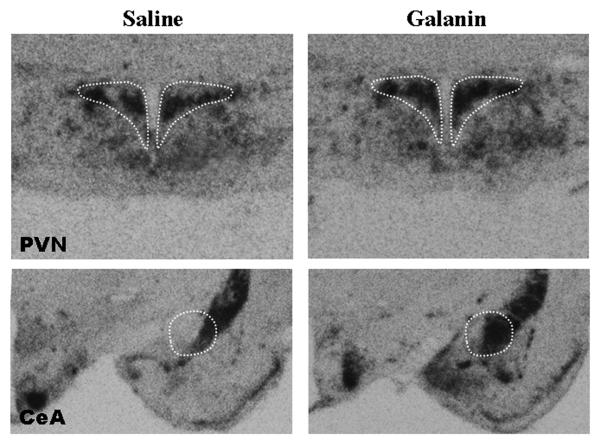
Fig. 5.
Photomicrographs showing radiolabeled in situ hybridization histochemistry analysis of enkephalin (ENK) mRNA in the paraventricular nucleus (PVN) and central nucleus of the amygdala (CeA) of rats injected in the PVN with galanin or saline vehicle (4X magnification). The anatomical pattern of expression was the same in response to injection of orexin. The ventral tegmental area is not included here due to its low levels of ENK expression.
3.3. Effect of GAL and OX injection on ENK cell density measured by DIG
To provide a better visualization of the ENK-expressing neurons affected by GAL or OX injection, this experiment, using the same test paradigm, employed DIG, which allows ENK-expressing neurons to be individually counted. With a cannula aimed just dorsal to the PVN, rats were injected with GAL (n = 5), OX (n = 5), or saline vehicle (n = 5). In this experiment, the NAc was not analyzed, as it failed in both Experiments 1 and 2 to exhibit any change in response to GAL or OX. Examination of the saline-injected animals revealed ENK-expressing neurons in the different areas under study. In the PVN, a dense concentration was detected in the medial parvicellular area at all anterior-posterior levels and in the lateral magnocellular area of the posterior PVN. In the VTA, ENK-expressing cells were scattered throughout the nucleus, and in the CeA, they were densely expressed in the lateral and capsular parts and moderately or sparsely expressed in the medial part. Consistent with Experiments 1 and 2, the injection of GAL or OX in the PVN was found to have a stimulatory effect on these different populations of ENK neurons. Compared to rats injected with saline vehicle, GAL and OX both increased the density of ENK-expressing neurons in the PVN (p < 0.001), VTA (p < 0.001), and CeA (GAL: p < 0.001, OX: p < 0.01) (Table 1). These results obtained with DIG are illustrated in photomicrographs of the PVN and CeA of rats injected with GAL compared to saline (Fig. 6). Thus, the finding that PVN peptide injections stimulate ENK gene expression throughout different brain regions was once again confirmed.
Table 1.
Cell density of hypothalamic and extra-hypothalamic enkephalin is elevated in rats injected in the paraventricular nucleus (PVN) with galanin (n = 5) or orexin (n = 5) versus saline (n = 5) as assessed by digoxigenin-labeled in situ hybridization. Values are mean ± S.E.M.
| PVN cells/μm × 10 −4 |
VTA cells/μm × 10 −5 |
CeA cells/μm × 10 −3 |
|
|---|---|---|---|
| Saline | 2.47 ± 0.18 | 1.32 ± 0.04 | 0.97 ± 0.02 |
| Galanin | 3.41 ± 0.06 *** | 1.78 ± 0.02*** | 1.40 ± 0.04*** |
| Orexin | 3.37 ± 0.03*** | 1.76 ± 0.02*** | 1.28 ± 0.11** |
p < 0.001
p < 0.01 vs. saline.
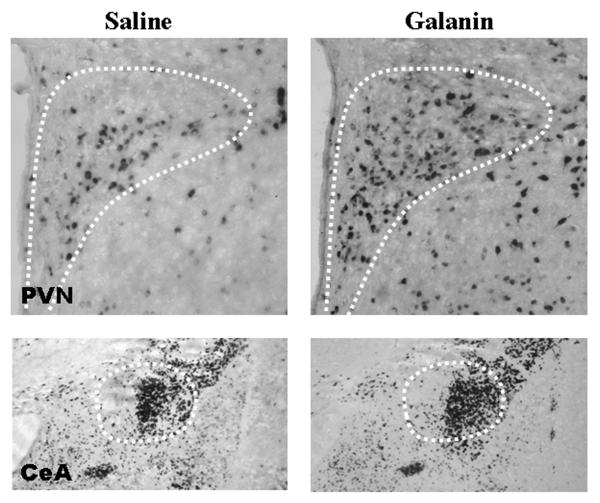
Fig. 6.
Photomicrographs showing digoxigenin-labeled in situ hybridization histochemistry analysis of cells expressing enkephalin (ENK) in the paraventricular nucleus (PVN) (10X magnification) and central nucleus of the amygdala (CeA) (4X magnification) of rats injected in the PVN with galanin or saline vehicle. The anatomical pattern of expression was the same in response to injection of orexin. The ventral tegmental area is not included here due to its low levels of ENK expression.
3.4. Histology
Based on visual inspection at the time of brain dissection and on microscopic verification, injections sites were found to be located just dorsal to the PVN. No injector tip was more than 0.7 mm above the dorsal aspect of the PVN, nor were any observed within the borders of the PVN itself. The location of the injections is illustrated in Fig. 7.
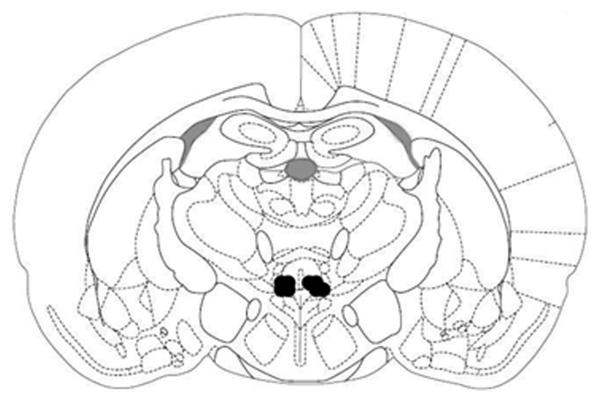
Fig. 7.
Histological verification of injector sites. Black dots indicate injector tip placement. Injections were unilateral and counterbalanced, left and right. Coronal section is −1.8 mm caudal to Bregma. Adapted from The Rat Brain, compact 3rd edition, G. Paxinos and C. Watson, Copyright 1997, with permission from Elsevier.
4. Discussion
Building on the shared features of GAL, OX and ENK with respect to food and ethanol consumption as described in Section 1, we wanted to determine whether injection of the non-opioid peptides have effects on ENK mRNA expression, within or outside the hypothalamus, that may contribute to their common, stimulatory effect on consummatory behavior. The results obtained with qRT-PCR demonstrated a similar effect of GAL and OX injection on ENK expression in the PVN, VTA and CeA, with no change with either peptide observed in the NAc. With a single injection just dorsal to the PVN, both of these non-opioid peptides increased ENK mRNA levels by up to 50%. These findings were confirmed with the more anatomically precise, radiolabeled ISH technique, which revealed a 20-40% increase in mRNA expression, and also with DIG, which showed a 40% increase in the density of ENK-expressing neurons in these 3 areas. This is the first study showing hypothalamic injection of an orexigenic peptide, such as GAL and OX, to influence mRNA expression of an opioid peptide that is known to promote consummatory behavior.
Injection of GAL or OX immediately dorsal to the PVN may alter gene expression by acting on different receptor subtypes and peptide systems within the PVN. The distribution of GAL and OX terminals within this nucleus is found to be similar [16, 74]. Whereas there are no anatomical studies showing the existence of GAL and OX receptors specifically on ENK-expressing neurons, the PVN is known to have moderate-to-high levels of the GAL receptors, GALR1 and GALR2 [29, 53-54], and the OX receptors, OX1R and OX2R [48, 96], and these receptors are found to be located on PVN neurons that express other peptides, e.g., vasopressin, oxytocin, or corticotrophin-releasing factor [3, 29]. Electrophysiological studies demonstrate that the GALR2 and both OX receptors are neuronally excitatory [18, 90], although GALR1 is inhibitory [90]. Thus, cellular mechanisms exist within the PVN that may underlie the similar behavioral and neurochemical effects produced by injection of GAL or OX.
The finding that these non-opioid peptides have a local stimulatory effect on ENK expression suggests that this opioid in the PVN may be involved in mediating their behavioral effects. This is consistent with evidence that administration of ENK in the PVN acts similarly to GAL and OX, in stimulating the ingestion of food [52, 91], particularly a high-fat diet [56, 79], and of ethanol [5]. Also, PVN ENK mRNA is similar to GAL and OX mRNA in being increased by the ingestion of a high-fat diet [10] or ethanol [8, 61] and it is similar to OX mRNA in being stimulated by nicotine [38, 47]. Direct support for the idea that ENK mediates the actions of GAL or OX in promoting consummatory behavior is provided by the finding that an opioid antagonist blocks the feeding response induced by injection of these non-opioid peptides [12, 20]. Recent evidence showing that PVN injection of an ENK analogue can stimulate the release of dopamine (DA) in the NAc [67], similar to PVN injection of GAL [68], suggests the additional involvement of this catecholamine, which is known to mediate the motivational aspects of feeding behavior [62].
The finding that ENK mRNA in the VTA, similar to the PVN, is stimulated by hypothalamic injection of GAL and OX suggests a role for this area in mediating the behavioral effects induced by these non-opioid, orexigenic peptides. The PVN and VTA are connected via a bi-directional, mu-receptor mediated pathway [65], and the PVN sends projections to the VTA that contain corticotrophin-releasing factor [71]. The VTA is known to have a predominant role in reward function. Neurons containing DA are concentrated in the VTA [82], and injection studies in this area show enhanced activity of DA cells in response to GAL [22] and drug-seeking in response to OX [31]. A function in reward specifically for ENK in the VTA is supported by evidence obtained with local administration of opioid agonists and antagonists. Injections of ENK analogues into this region are found to stimulate food intake [50, 55, 58] and establish place preferences [4, 64], while opioid antagonists suppress food intake [69] and ethanol consumption [37]. Moreover, ENK mRNA in the VTA, like the PVN, is found to be stimulated by chronic consumption of ethanol [9]. Therefore, the enhanced ENK expression in the VTA induced by hypothalamic GAL or OX injection may contribute to the stimulation of food and ethanol intake based on its rewarding characteristics.
Like the PVN and VTA, the CeA responds to injection of GAL and ORX with a significant increase in ENK expression. Whereas the PVN does not appear to project directly to the CeA, it may communicate indirectly via the VTA, which sends DA projections to the CeA [32, 60], and the CeA, in turn, may communicate with the PVN via mu-mediated projections [27] and GABA projections [88-89] that return back to the PVN. The CeA has been implicated in reward and addiction [76] and may also play a role in states of anxiety [43]. Thus, the increased expression of ENK in the CeA suggests a possible role for this opioid in mediating both ingestive behavior and anxiety. Food intake is stimulated by injection of GAL into the amygdala [13, 44, 80] or ENK analogues in the CeA [28, 41, 46], and opioid antagonists in the CeA suppress operant responding for ethanol [23, 35]. Further, endogenous ENK expression in the CeA is enhanced by intake of ethanol [14] as well as nicotine [47]. This enhanced expression of CeA ENK may reflect an increase in anxiety, as ENK levels in the amygdala are stimulated by immobilization stress [36] or predator odor [34] and reduced by adrenalectomy [1]. This opioid in the CeA is suggested to provide a coping mechanism, since mice with high anxiety, when faced with a predator odor, show a blunted elevation of ENK compared to those with low anxiety [34]. Thus, the increase in ENK mRNA in this nucleus produced by hypothalamic GAL or OX injection may have a function in attenuating the impact of stressors or enhancing processes involved in addiction.
Of the four areas examined in the rats injected with GAL and OX, the NAc was the only one that failed to show any change in ENK mRNA. The basis for this is unclear, as ENK analogues injected into this nucleus, particularly in the border between the shell and core subregions, produce similar effects to those seen in the PVN, VTA and CeA, namely, an increase in consumption of food [55], fat-rich diet [94], and ethanol [95]. Also, endogenous ENK in the NAc, similar to the PVN, is enhanced by consumption of ethanol [17]. However, there are clear differences between the NAc and PVN that should be noted. In the NAc, GAL or OX injection fails to enhance ethanol intake [75], in contrast to the stimulatory effect in the PVN [75], and ENK mRNA in the NAc is suppressed by morphine [26, 86] and hedonic foods, such as sucrose [81] and chocolate Ensure [40], once again in contrast to the PVN [10]. It is noteworthy that these investigations, as in the present study, did not separately examine the shell and core of this nucleus, subregions with differential functions, respectively, in motivational valence or goal-directed behavior from conditioned learning [39, 78]. Thus, the lack of effect in the present report and divergent effects observed in the literature may reflect the degree to which these subregions of the NAc were involved.
Behavioral studies examining the relationship between GAL and ENK or between OX and ENK generally suggest that these peptides may interact positively. This is demonstrated by studies of food intake, showing GAL-induced feeding to be blocked by pretreatment with icv injection of the general opioid antagonist naloxone [20] or co-injection of a mu-receptor antagonist [84]. Thus, endogenous ENK, when stimulated by GAL, may contribute to the overconsumption of fat or ethanol [8, 10]. Studies of avoidance behavior also suggest a positive relationship between GAL and ENK, with the GAL-induced decrease in active behavioral avoidance blocked by peripheral administration of naloxone [77]. Further, the finding that peripheral administration of a GAL agonist diminishes morphine withdrawal [92] suggests that GAL can substitute for morphine, although other evidence suggests that it may block morphine-induced place preference [93]. Similar to GAL, there is evidence that OX may also interact positively with ENK. The increase in food intake induced by central OX injection is blocked by pretreatment with naloxone [24, 83], suggesting that OX exerts its effects through endogenous ENK. The reverse possibility, that ENK acts through enhanced functioning of OX, is supported by the findings that feeding induced by the ENK-analogue, DAMGO, in the NAc is blocked by an OX antagonist in the VTA [97] and that morphine-induced place preference is diminished by VTA injection of an OX antagonist and is lost in mice lacking the gene for prepro-orexin [57]. Together, these behavioral findings support the possibility that both GAL and OX exert at least some of their effects by enhancing the functioning of the ENK system.
4.2. Conclusions
In summary, injection of GAL and OX into the PVN leads to a similar pattern of ENK expression in hypothalamic and extra-hypothalamic nuclei. These results suggest that these non-opioid peptides acting within the hypothalamus may have similar behavioral effects, in part, through their common, stimulatory effect on opioid expression specifically in the PVN, VTA and CeA. Through this positive relationship, GAL and OX may function together with ENK to promote excess consummatory behavior.
Acknowledgements
This research was supported by USPHS Grants AA12882 and DA21518. We extend many thanks to Valerie Weed and Si-Yi Chang for their technical assistance.
References
- 1.Ahima RS, Garcia MM, Harlan RE. Glucocorticoid regulation of preproenkephalin gene expression in the rat forebrain. Brain Res Mol Brain Res. 1992;16:119–27. doi: 10.1016/0169-328x(92)90201-l. [DOI] [PubMed] [Google Scholar]
- 2.Ambati S, Duan J, Choi YH, Hartzell DL, Della-Fera MA, Baile CA. ICV vs. VMH injection of leptin: comparative effects on hypothalamic gene expression. Behav Brain Res. 2009;196:279–85. doi: 10.1016/j.bbr.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Backberg M, Hervieu G, Wilson S, Meister B. Orexin receptor-1 (OX-R1) immunoreactivity in chemically identified neurons of the hypothalamus: focus on orexin targets involved in control of food and water intake. Eur J Neurosci. 2002;15:315–28. doi: 10.1046/j.0953-816x.2001.01859.x. [DOI] [PubMed] [Google Scholar]
- 4.Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–95. [PubMed] [Google Scholar]
- 5.Barson JR, Carr A, Soun JE, Sobhani NC, Rada P, Leibowitz SF, et al. Opioids in the hypothalamic paraventricular nucleus stimulate ethanol intake. 2009. Unpublished Results. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cassell MD, Gray TS, Kiss JZ. Neuronal architecture in the rat central nucleus of the amygdala: a cytological, hodological, and immunocytochemical study. J Comp Neurol. 1986;246:478–99. doi: 10.1002/cne.902460406. [DOI] [PubMed] [Google Scholar]
- 7.Chang G-Q, Gaysinskaya V, Karatayev O, Leibowitz SF. Maternal high-fat diet and fetal programming: increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. J Neurosci. 2008;28:12107–19. doi: 10.1523/JNEUROSCI.2642-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang G-Q, Karatayev O, Ahsan R, Avena NM, Lee C, Lewis MJ, et al. Effect of ethanol on hypothalamic opioid peptides, enkephalin, and dynorphin: relationship with circulating triglycerides. Alcohol Clin Exp Res. 2007;31:249–59. doi: 10.1111/j.1530-0277.2006.00312.x. [DOI] [PubMed] [Google Scholar]
- 9.Chang GQ, Barson JR, Karatayev O, Chang SY, Chan YW, Leibowitz SF. Effect of chronic ethanol on enkephalin in the hypothalamus and extra-hypothalamic areas. 2009. Unpublished Results. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang GQ, Karatayev O, Ahsan R, Gaysinskaya V, Marwil Z, Leibowitz SF. Dietary fat stimulates endogenous enkephalin and dynorphin in the paraventricular nucleus: role of circulating triglycerides. Am J Physiol Endocrinol Metab. 2007;292:E561–70. doi: 10.1152/ajpendo.00087.2006. [DOI] [PubMed] [Google Scholar]
- 11.Chang GQ, Karatayev O, Davydova Z, Leibowitz SF. Circulating triglycerides impact on orexigenic peptides and neuronal activity in hypothalamus. Endocrinology. 2004;145:3904–12. doi: 10.1210/en.2003-1582. [DOI] [PubMed] [Google Scholar]
- 12.Clegg DJ, Air EL, Woods SC, Seeley RJ. Eating elicited by orexin-a, but not melanin-concentrating hormone, is opioid mediated. Endocrinology. 2002;143:2995–3000. doi: 10.1210/endo.143.8.8977. [DOI] [PubMed] [Google Scholar]
- 13.Corwin RL, Robinson JK, Crawley JN. Galanin antagonists block galanin-induced feeding in the hypothalamus and amygdala of the rat. EurJNeurosci. 1993;5:1528–33. doi: 10.1111/j.1460-9568.1993.tb00221.x. [DOI] [PubMed] [Google Scholar]
- 14.Cowen MS, Lawrence AJ. Alterations in central preproenkephalin mRNA expression after chronic free-choice ethanol consumption by fawn-hooded rats. Alcohol Clin Exp Res. 2001;25:1126–33. [PubMed] [Google Scholar]
- 15.Curran EJ, Watson SJ., Jr Dopamine receptor mRNA expression patterns by opioid peptide cells in the nucleus accumbens of the rat: a double in situ hybridization study. JComp Neurol. 1995;361:57–76. doi: 10.1002/cne.903610106. [DOI] [PubMed] [Google Scholar]
- 16.Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, et al. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. ProcNatlAcadSciUSA. 1999;96:748–53. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Gortari P, Mendez M, Rodriguez-Keller I, Perez-Martinez L, Joseph-Bravob P. Acute ethanol administration induces changes in TRH and proenkephalin expression in hypothalamic and limbic regions of rat brain. Neurochem Int. 2000;37:483–96. doi: 10.1016/s0197-0186(00)00059-0. [DOI] [PubMed] [Google Scholar]
- 18.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. ProcNatlAcadSciUSA. 1998;95:322–7. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Chiara G. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res. 2002;137:75–114. doi: 10.1016/s0166-4328(02)00286-3. [DOI] [PubMed] [Google Scholar]
- 20.Dube MG, Horvath TL, Leranth C, Kalra PS, Kalra SP. Naloxone reduces the feeding evoked by intracerebroventricular galanin injection. Physiol Behav. 1994;56:811–3. doi: 10.1016/0031-9384(94)90247-x. [DOI] [PubMed] [Google Scholar]
- 21.Dube MG, Kalra SP, Kalra PS. Food intake elicited by central administration of orexins/hypocretins: identification of hypothalamic sites of action. Brain Res. 1999;842:473–7. doi: 10.1016/s0006-8993(99)01824-7. [DOI] [PubMed] [Google Scholar]
- 22.Ericson E, Ahlenius S. Suggestive evidence for inhibitory effects of galanin on mesolimbic dopaminergic neurotransmission. Brain Res. 1999;822:200–9. doi: 10.1016/s0006-8993(99)01144-0. [DOI] [PubMed] [Google Scholar]
- 23.Foster KL, McKay PF, Seyoum R, Milbourne D, Yin W, Sarma PV, et al. GABA(A) and opioid receptors of the central nucleus of the amygdala selectively regulate ethanol-maintained behaviors. Neuropsychopharmacology. 2004;29:269–84. doi: 10.1038/sj.npp.1300306. [DOI] [PubMed] [Google Scholar]
- 24.Furudono Y, Ando C, Yamamoto C, Kobashi M, Yamamoto T. Involvement of specific orexigenic neuropeptides in sweetener-induced overconsumption in rats. Behav Brain Res. 2006;175:241–8. doi: 10.1016/j.bbr.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 25.Garzon M, Pickel VM. Ultrastructural localization of enkephalin and mu-opioid receptors in the rat ventral tegmental area. Neuroscience. 2002;114:461–74. doi: 10.1016/s0306-4522(02)00249-x. [DOI] [PubMed] [Google Scholar]
- 26.Georges F, Stinus L, Bloch B, Le Moine C. Chronic morphine exposure and spontaneous withdrawal are associated with modifications of dopamine receptor and neuropeptide gene expression in the rat striatum. Eur J Neurosci. 1999;11:481–90. doi: 10.1046/j.1460-9568.1999.00462.x. [DOI] [PubMed] [Google Scholar]
- 27.Giraudo SQ, Billington CJ, Levine AS. Effects of the opioid antagonist naltrexone on feeding induced by DAMGO in the central nucleus of the amygdala and in the paraventricular nucleus in the rat. Brain Res. 1998;782:18–23. doi: 10.1016/s0006-8993(97)01140-2. [DOI] [PubMed] [Google Scholar]
- 28.Gosnell BA. Involvement of mu opioid receptors in the amygdala in the control of feeding. Neuropharmacology. 1988;27:319–26. doi: 10.1016/0028-3908(88)90050-0. [DOI] [PubMed] [Google Scholar]
- 29.Gundlach AL, Burazin TC, Larm JA. Distribution, regulation and role of hypothalamic galanin systems: renewed interest in a pleiotropic peptide family. Clin Exp Pharmacol Physiol. 2001;28:100–5. doi: 10.1046/j.1440-1681.2001.03411.x. [DOI] [PubMed] [Google Scholar]
- 30.Gundlach AL, Wisden W, Morris BJ, Hunt SP. Localization of preprogalanin mRNA in rat brain: in situ hybridization study with a synthetic oligonucleotide probe. Neuroscience Letters. 1990;114:241–7. doi: 10.1016/0304-3940(90)90570-y. [DOI] [PubMed] [Google Scholar]
- 31.Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–7. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Hasue RH, Shammah-Lagnado SJ. Origin of the dopaminergic innervation of the central extended amygdala and accumbens shell: a combined retrograde tracing and immunohistochemical study in the rat. J Comp Neurol. 2002;454:15–33. doi: 10.1002/cne.10420. [DOI] [PubMed] [Google Scholar]
- 33.Hawes JJ, Picciotto MR. Characterization of GalR1, GalR2, and GalR3 immunoreactivity in catecholaminergic nuclei of the mouse brain. JComp Neurol. 2004;479:410–23. doi: 10.1002/cne.20329. [DOI] [PubMed] [Google Scholar]
- 34.Hebb AL, Zacharko RM, Gauthier M, Trudel F, Laforest S, Drolet G. Brief exposure to predator odor and resultant anxiety enhances mesocorticolimbic activity and enkephalin expression in CD-1 mice. Eur J Neurosci. 2004;20:2415–29. doi: 10.1111/j.1460-9568.2004.03704.x. [DOI] [PubMed] [Google Scholar]
- 35.Heyser CJ, Roberts AJ, Schulteis G, Koob GF. Central administration of an opiate antagonist decreases oral ethanol self-administration in rats. Alcohol Clin Exp Res. 1999;23:1468–76. [PubMed] [Google Scholar]
- 36.Honkaniemi J. Colocalization of peptide- and tyrosine hydroxylase-like immunoreactivities with Fos-immunoreactive neurons in rat central amygdaloid nucleus after immobilization stress. Brain Res. 1992;598:107–13. doi: 10.1016/0006-8993(92)90173-7. [DOI] [PubMed] [Google Scholar]
- 37.June HL, Cummings R, Eiler WJ, 2nd, Foster KL, McKay PF, Seyoum R, et al. Central opioid receptors differentially regulate the nalmefene-induced suppression of ethanol- and saccharin-reinforced behaviors in alcohol-preferring (P) rats. Neuropsychopharmacology. 2004;29:285–99. doi: 10.1038/sj.npp.1300338. [DOI] [PubMed] [Google Scholar]
- 38.Kane JK, Parker SL, Matta SG, Fu Y, Sharp BM, Li MD. Nicotine up-regulates expression of orexin and its receptors in rat brain. Endocrinology. 2000;141:3623–9. doi: 10.1210/endo.141.10.7707. [DOI] [PubMed] [Google Scholar]
- 39.Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86:773–95. doi: 10.1016/j.physbeh.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 40.Kelley AE, Will MJ, Steininger TL, Zhang M, Haber SN. Restricted daily consumption of a highly palatable food (chocolate Ensure(R)) alters striatal enkephalin gene expression. EurJNeurosci. 2003;18:2592–8. doi: 10.1046/j.1460-9568.2003.02991.x. [DOI] [PubMed] [Google Scholar]
- 41.Kim EM, Quinn JG, Levine AS, O'Hare E. A bi-directional mu-opioid-opioid connection between the nucleus of the accumbens shell and the central nucleus of the amygdala in the rat. Brain Res. 2004;1029:135–9. doi: 10.1016/j.brainres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 42.Koob GF. Dynamics of neuronal circuits in addiction: reward, antireward, and emotional memory. Pharmacopsychiatry. 2009;42(Suppl 1):S32–41. doi: 10.1055/s-0029-1216356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koob GF, Le Moal M. Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3113–23. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kyrkouli SE, Stanley BG, Seirafi RD, Leibowitz SF. Stimulation of feeding by galanin: anatomical localization and behavioral specificity of this peptide's effects in the brain. Peptides. 1990;11:995–1001. doi: 10.1016/0196-9781(90)90023-x. [DOI] [PubMed] [Google Scholar]
- 45.Leibowitz SF, Akabayashi A, Wang J, Alexander JT, Dourmashkin JT, Chang GQ. Increased caloric intake on a fat-rich diet: role of ovarian steroids and galanin in the medial preoptic and paraventricular nuclei and anterior pituitary of female rats. J Neuroendocrinol. 2007;19:753–66. doi: 10.1111/j.1365-2826.2007.01584.x. [DOI] [PubMed] [Google Scholar]
- 46.Levine AS, Olszewski PK, Mullett MA, Pomonis JD, Grace MK, Kotz CM, et al. Intra-amygdalar injection of DAMGO: effects on c-Fos levels in brain sites associated with feeding behavior. Brain Res. 2004;1015:9–14. doi: 10.1016/j.brainres.2004.04.039. [DOI] [PubMed] [Google Scholar]
- 47.Loughlin SE, Islas MI, Cheng MY, Lee AG, Villegier AS, Leslie FM. Nicotine modulation of stress-related peptide neurons. J Comp Neurol. 2006;497:575–88. doi: 10.1002/cne.20999. [DOI] [PubMed] [Google Scholar]
- 48.Lu XY, Bagnol D, Burke S, Akil H, Watson SJ. Differential distribution and regulation of OX1 and OX2 orexin/hypocretin receptor messenger RNA in the brain upon fasting. HormBehav. 2000;37:335–44. doi: 10.1006/hbeh.2000.1584. [DOI] [PubMed] [Google Scholar]
- 49.Lucas LR, Pompei P, Ono J, McEwen BS. Effects of adrenal steroids on basal ganglia neuropeptide mRNA and tyrosine hydroxylase radioimmunoreactive levels in the adrenalectomized rat. JNeurochem. 1998;71:833–43. doi: 10.1046/j.1471-4159.1998.71020833.x. [DOI] [PubMed] [Google Scholar]
- 50.MacDonald AF, Billington CJ, Levine AS. Alterations in food intake by opioid and dopamine signaling pathways between the ventral tegmental area and the shell of the nucleus accumbens. Brain Res. 2004;1018:78–85. doi: 10.1016/j.brainres.2004.05.043. [DOI] [PubMed] [Google Scholar]
- 51.Martin G, Fabre V, Siggins GR, de Lecea L. Interaction of the hypocretins with neurotransmitters in the nucleus accumbens. Regul Pept. 2002;104:111–7. doi: 10.1016/s0167-0115(01)00354-8. [DOI] [PubMed] [Google Scholar]
- 52.McLean S, Hoebel BG. Feeding induced by opiates injected into the paraventricular hypothalamus. Peptides. 1983;4:287–92. doi: 10.1016/0196-9781(83)90134-1. [DOI] [PubMed] [Google Scholar]
- 53.Mitchell V, Bouret S, Howard AD, Beauvillain JC. Expression of the galanin receptor subtype Gal-R2 mRNA in the rat hypothalamus. J Chem Neuroanat. 1999;16:265–77. doi: 10.1016/s0891-0618(99)00011-3. [DOI] [PubMed] [Google Scholar]
- 54.Mitchell V, Habert-Ortoli E, Epelbaum J, Aubert JP, Beauvillain JC. Semiquantitative distribution of galanin-receptor (GAL-R1) mRNA-containing cells in the male rat hypothalamus. Neuroendocrinology. 1997;66:160–72. doi: 10.1159/000127234. [DOI] [PubMed] [Google Scholar]
- 55.Mucha RF, Iversen SD. Increased food intake after opioid microinjections into nucleus accumbens and ventral tegmental area of rat. Brain Res. 1986;397:214–24. doi: 10.1016/0006-8993(86)90622-0. [DOI] [PubMed] [Google Scholar]
- 56.Naleid AM, Grace MK, Chimukangara M, Billington CJ, Levine AS. Paraventricular opioids alter intake of high-fat but not high-sucrose diet depending on diet preference in a binge model of feeding. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2007;293:R99–R105. doi: 10.1152/ajpregu.00675.2006. [DOI] [PubMed] [Google Scholar]
- 57.Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake M, et al. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26:398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Noel MB, Wise RA. Ventral tegmental injections of a selective mu or delta opioid enhance feeding in food-deprived rats. Brain Res. 1995;673:304–12. doi: 10.1016/0006-8993(94)01442-k. [DOI] [PubMed] [Google Scholar]
- 59.O'Donnell D, Ahmad S, Wahlestedt C, Walker P. Expression of the novel galanin receptor subtype GALR2 in the adult rat CNS: distinct distribution from GALR1. J Comp Neurol. 1999;409:469–81. [PubMed] [Google Scholar]
- 60.Oades RD, Halliday GM. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res. 1987;434:117–65. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- 61.Oliva JM, Manzanares J. Gene transcription alterations associated with decrease of ethanol intake induced by naltrexone in the brain of Wistar rats. Neuropsychopharmacology. 2007;32:1358–69. doi: 10.1038/sj.npp.1301237. [DOI] [PubMed] [Google Scholar]
- 62.Palmiter RD. Is dopamine a physiologically relevant mediator of feeding behavior? Trends Neurosci. 2007;30:375–81. doi: 10.1016/j.tins.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 63.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1998. [Google Scholar]
- 64.Phillips AG, LePiane FG. Reward produced by microinjection of (D-Ala2),Met5-enkephalinamide into the ventral tegmental area. Behav Brain Res. 1982;5:225–9. doi: 10.1016/0166-4328(82)90057-2. [DOI] [PubMed] [Google Scholar]
- 65.Quinn JG, O'Hare E, Levine AS, Kim EM. Evidence for a mu-opioid-opioid connection between the paraventricular nucleus and ventral tegmental area in the rat. Brain Res. 2003;991:206–11. doi: 10.1016/j.brainres.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 66.Rada P, Avena NM, Leibowitz SF, Hoebel BG. Ethanol Intake is Increased by PVN Galanin Injection and Reduced by a GAL Antagonist. Alcohol. 2004;33:91–7. doi: 10.1016/j.alcohol.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 67.Rada P, Barson JR, Leibowitz SF, Hoebel BG. Opioids in the hypothalamus control dopamine and acetylcholine levels in the nucleus accumbens. 2009. Unpublished Results. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rada P, Mark GP, Hoebel BG. Galanin in the hypothalamus raises dopamine and lowers acetylcholine release in the nucleus accumbens: a possible mechanism for hypothalamic initiation of feeding behavior. Brain Res. 1998;798:1–6. doi: 10.1016/s0006-8993(98)00315-1. [DOI] [PubMed] [Google Scholar]
- 69.Ragnauth A, Ruegg H, Bodnar RJ. Evaluation of opioid receptor subtype antagonist effects in the ventral tegmental area upon food intake under deprivation, glucoprivic and palatable conditions. Brain Res. 1997;767:8–16. doi: 10.1016/s0006-8993(97)00539-8. [DOI] [PubMed] [Google Scholar]
- 70.Reagan LP, Rosell DR, Wood GE, Spedding M, Munoz C, Rothstein J, et al. Chronic restraint stress up-regulates GLT-1 mRNA and protein expression in the rat hippocampus: reversal by tianeptine. ProcNatlAcadSciUSA. 2004;101:2179–84. doi: 10.1073/pnas.0307294101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rodaros D, Caruana DA, Amir S, Stewart J. Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience. 2007;150:8–13. doi: 10.1016/j.neuroscience.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 72.Sakanaka M, Magari S, Shibasaki T, Inoue N. Co-localization of corticotropin-releasing factor- and enkephalin-like immunoreactivities in nerve cells of the rat hypothalamus and adjacent areas. Brain Res. 1989;487:357–62. doi: 10.1016/0006-8993(89)90840-8. [DOI] [PubMed] [Google Scholar]
- 73.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–85. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 74.Sawchenko PE, Pfeiffer SW. Ultrastructural localization of neuropeptide Y and galanin immunoreactivity in the paraventricular nucleus of the hypothalamus in the rat. Brain Res. 1988;474:231–45. doi: 10.1016/0006-8993(88)90438-6. [DOI] [PubMed] [Google Scholar]
- 75.Schneider ER, Rada P, Darby RD, Leibowitz SF, Hoebel BG. Orexigenic peptides and alcohol intake: Differential effects of orexin, galanin, and ghrelin. Alcoholism-Clinical and Experimental Research. 2007;31:1858–65. doi: 10.1111/j.1530-0277.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- 76.See RE. Neural substrates of cocaine-cue associations that trigger relapse. Eur J Pharmacol. 2005;526:140–6. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 77.Shandra AA, Mazarati AM, Servetskii KL. Influence of the neuropeptide galanin on active avoidance in rats. Neurosci Behav Physiol. 1994;24:429–32. doi: 10.1007/BF02359796. [DOI] [PubMed] [Google Scholar]
- 78.Shirayama Y, Chaki S. Neurochemistry of the nucleus accumbens and its relevance to depression and antidepressant action in rodents. Curr Neuropharmacol. 2006;4:277–91. doi: 10.2174/157015906778520773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shor-Posner G, Azar AP, Filart R, Tempel D, Leibowitz SF. Morphine-stimulated feeding: analysis of macronutrient selection and paraventricular nucleus lesions. Pharmacology, Biochemistry & Behavior. 1986;24:931–9. doi: 10.1016/0091-3057(86)90439-9. [DOI] [PubMed] [Google Scholar]
- 80.Smith BK, York DA, Bray GA. Effects of dietary preference and galanin administration in the paraventricular or amygdaloid nucleus on diet self-selection. Brain Res Bull. 1996;39:149–54. doi: 10.1016/0361-9230(95)02086-1. [DOI] [PubMed] [Google Scholar]
- 81.Spangler R, Wittkowski KM, Goddard NL, Avena NM, Hoebel BG, Leibowitz SF. Opiate-like effects of sugar on gene expression in reward areas of the rat brain. Brain Res MolBrain Res. 2004;124:134–42. doi: 10.1016/j.molbrainres.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 82.Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–53. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- 83.Sweet DC, Levine AS, Kotz CM. Functional opioid pathways are necessary for hypocretin-1 (orexin-A)-induced feeding. Peptides. 2004;25:307–14. doi: 10.1016/j.peptides.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 84.Tachibana T, Mori M, Khan MS, Ueda H, Sugahara K, Hiramatsu K. Central administration of galanin stimulates feeding behavior in chicks. Comp Biochem Physiol A Mol Integr Physiol. 2008 doi: 10.1016/j.cbpa.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 85.Tempel DL, Leibowitz KJ, Leibowitz SF. Effects of PVN galanin on macronutrient selection. Peptides. 1988;9:309–14. doi: 10.1016/0196-9781(88)90265-3. [DOI] [PubMed] [Google Scholar]
- 86.Uhl GR, Ryan JP, Schwartz JP. Morphine alters preproenkephalin gene expression. Brain Res. 1988;459:391–7. doi: 10.1016/0006-8993(88)90658-0. [DOI] [PubMed] [Google Scholar]
- 87.Valassi E, Scacchi M, Cavagnini F. Neuroendocrine control of food intake. Nutr Metab Cardiovasc Dis. 2008;18:158–68. doi: 10.1016/j.numecd.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 88.Wallace DM, Magnuson DJ, Gray TS. The amygdalo-brainstem pathway: selective innervation of dopaminergic, noradrenergic and adrenergic cells in the rat. Neurosci Lett. 1989;97:252–8. doi: 10.1016/0304-3940(89)90606-x. [DOI] [PubMed] [Google Scholar]
- 89.Wallace DM, Magnuson DJ, Gray TS. Organization of amygdaloid projections to brainstem dopaminergic, noradrenergic, and adrenergic cell groups in the rat. Brain Res Bull. 1992;28:447–54. doi: 10.1016/0361-9230(92)90046-z. [DOI] [PubMed] [Google Scholar]
- 90.Wang S, Gustafson EL. Galanin receptor subtypes. Drug News Perspect. 1998;11:458–68. [PubMed] [Google Scholar]
- 91.Woods JS, Leibowitz SF. Hypothalamic sites sensitive to morphine and naloxone: effects on feeding behavior. Pharmacol Biochem Behav. 1985;23:431–8. doi: 10.1016/0091-3057(85)90017-6. [DOI] [PubMed] [Google Scholar]
- 92.Zachariou V, Brunzell DH, Hawes J, Stedman DR, Bartfai T, Steiner RA, et al. The neuropeptide galanin modulates behavioral and neurochemical signs of opiate withdrawal. Proc Natl Acad Sci U S A. 2003;100:9028–33. doi: 10.1073/pnas.1533224100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zachariou V, Parikh K, Picciotto MR. Centrally administered galanin blocks morphine place preference in the mouse. Brain Res. 1999;831:33–42. doi: 10.1016/s0006-8993(99)01476-6. [DOI] [PubMed] [Google Scholar]
- 94.Zhang M, Gosnell BA, Kelley AE. Intake of high-fat food is selectively enhanced by mu opioid receptor stimulation within the nucleus accumbens. J Pharmacol Exp Ther. 1998;285:908–14. [PubMed] [Google Scholar]
- 95.Zhang M, Kelley AE. Intake of saccharin, salt, and ethanol solutions is increased by infusion of a mu opioid agonist into the nucleus accumbens. Psychopharmacology (Berl) 2002;159:415–23. doi: 10.1007/s00213-001-0932-y. [DOI] [PubMed] [Google Scholar]
- 96.Zhang S, Blache D, Vercoe PE, Adam CL, Blackberry MA, Findlay PA, et al. Expression of orexin receptors in the brain and peripheral tissues of the male sheep. Regul Pept. 2005;124:81–7. doi: 10.1016/j.regpep.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 97.Zheng H, Patterson LM, Berthoud HR. Orexin signaling in the ventral tegmental area is required for high-fat appetite induced by opioid stimulation of the nucleus accumbens. J Neurosci. 2007;27:11075–82. doi: 10.1523/JNEUROSCI.3542-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]