Abstract
The vast majority of serine/threonine protein kinases have a strong preference for ATP over GTP as a phosphate donor. CK2 (Casein kinase 2) is an exception to this rule and in this study we investigate whether calcium/calmodulin-dependent protein kinase II (CaMKII) has the same extended nucleotide range. Using the Drosophila enzyme, we have shown that CaMKII uses Mg2+GTP with a higher Km and Vmax compared to Mg2+ATP. Substitution of Mn2+ for Mg2+ resulted in a much lower Km for GTP, while nearly abolishing the ability of CaMKII to use ATP. These similar results were obtained with rat αCaMKII, showing the ability to use GTP to be a general property of CaMKII. The Vmax difference between Mg2+ATP and Mg2+GTP was found to be due to the fact that ADP is a potent inhibitor of phosphorylation, while GDP has modest effects. There were no differences found between sites autophosphorylated by ATP and GTP, either by partial proteolysis or mass spectrometry. Phosphorylation of fly head extract revealed that similar proteins are substrates for CaMKII whether using Mg2+ATP or Mg2+GTP. This new information confirms that CaMKII can use both ATP and GTP, and opens new avenues for the study of regulation of this kinase.
Introduction
In the world of synaptic plasticity, one of the major molecular players is calcium/calmodulin-dependent protein kinase II (CaMKII). This serine/threonine kinase is highly enriched in the brain, and may constitute as much as 1% of the total protein in the rat cortex [1]. It is known to phosphorylate a wide variety of substrates, including ion channels and other synaptic proteins. CaMKII also autophosphorylates at T286 (T287 in Drosophila) in conditions of high calcium and continues to be active after calcium levels drop [2; 3]. CaMKII can also, after autophosphorylation in the CaM-binding domain, become calcium-insensitive, creating a pool of inert kinase that can be reactivated through actions of a phosphatase [4; 5; 6]. These properties have led to CaMKII being associated with “molecular memory” [7]. The kinase is also known to bind to synaptic proteins including the NMDA channel in mammals [8] and the ether-à-go-go channel in Drosophila [9]. This binding can regulate kinase activity, making the kinase active and calcium-independent, and also anchors it at the synapse [10].
While a wide range of phosphate acceptors for this kinase have been well studied, variation in phosphate donors has been unexplored since the assumption has been that ATP is the only biologically relevant donor. In this study we examine the ability of both Drosophila and rat αCaMKII to transfer phosphate from GTP, a nucleotide found in high concentration in cells [11]. This ability is exceptional since very few serine/threonine kinases are known to use GTP, and very few kinases are able to use both ATP and GTP.
Structurally, the active sites of kinases are usually not able to accommodate both ATP and GTP. An exception to this is CK2 (formerly known as casein kinase 2), a multifunctional and widely expressed protein kinase. It has been shown that this kinase can use both ATP and GTP [12], although there were differences in kinetics and in divalent metal ion requirements[13]. CK2 had a lower Km with ATP than GTP; Mn2+ and Co2+ could both substitute for Mg2+ (although both required lower molarity for optimal function of the kinase) and with 20–30% of the maximal activity seen with Mg2+. One interesting finding was that in the presence of Mn2+, Km was lower for GTP than for ATP. The structural basis of this broad substrate specificity is the ability of water, especially in the presence of Mn2+, to allow interaction of GTP in the active site of CK2 [14]. The accommodation of either ATP or GTP in the binding pocket also requires a freedom of orientation of the planar purine moiety that is not seen in most serine/threonine kinases [15]. In this study, we show that CaMKII is similar to CK2 in its ability to use GTP and Mn2+.
We also examined the question of whether GTP could be used for autophosphorylation of CaMKII and whether there were sites specific for particular nucleotide donors. One other serine/threonine kinase, protein kinase Cδ, preferentially uses GTP as a phosphate donor for autophosphorylation and autophosphorylates different sites with GTP than with ATP [16]. A new site for CaMKII that was GTP-specific would add yet another potential mechanism for activity regulation. We determined that CaMKII autophosphorylates identically using either ATP or GTP. We did, however, identify several previously unreported sites on the C-terminal end of CaMKII which are phosphorylated basally in kinase purified from baculovirus-infected beetle cells.
Methods
Proteins
Drosophila CaMKII was purified as detailed by Sun [9]. The R3 isoform, which is most similar to rat α [17], was used in all experiments. Recombinant rat αCaMKII was a gift from Neal Waxham (University of Texas Medical School). Calmodulin was purchased from CalBiochem.
Peptide assay for CaMKII activity
Calcium-stimulated activity (“+“) was measured in an assay reaction of 50 µl containing 50 mM PIPES (pH 7.0), 15 mM MgC12 or 5 mM MnCl2, 1 mM CaCl2, 1 mg/ml bovine serum albumin, 50 µM autocamtide-3 peptide substrate, 10 µg/ml CaM, and indicated amounts of [γ-32P]-ATP or [γ-32P]-GTP (Perkin-Elmer). Assays involving inhibition added ADP or GDP (Sigma) on ice before starting the reaction. In minus calcium reactions (“−“), 0.5 mM EGTA was used and CaCl2 and CaM were omitted. Reactions were started by adding radioactively labeled ATP or GTP and run at 30°C for 1 min. Reactions were stopped by adding 50 µl of 10% trichloroacetic acid. The samples were centrifuged for 5 min after stop solution was added, and again for 3 min immediately before spotting. One quarter of the supernatant was spotted onto a strip of phosphocellulose paper. The paper strips were washed for 15–30 min with water and dried, and the amount of radioactivity was determined by measuring Cerenkov radiation in a Beckman LS6500 scintillation counter. Within an experiment all reactions were carried out in triplicate.
Phosphorylation of fly head extract
Extract phosphorylation was done under standard assay conditions described for peptide assay but without bovine serum albumin or peptide substrate, substituting 10 µl of a 2 mg/ml fly head extract in RIPA buffer. The amount of CaMKII added to the samples was increased tenfold. Samples missing one or more components had an equal volume of empty vehicle substituted in the reaction (i.e. fly head extract/RIPA buffer). After addition of the radioactive ATP or GTP, samples were incubated for 2 min and one half volume of 3X SDS-PAGE sample buffer (150 mM Tris Cl pH 6.8, 6% SDS, 0.3% Bromphenol blue, 30% glycerol, 3% βME ) was added to the reaction mixture and heated to 100°C for 3 min. Samples were separated by SDS-PAGE on 8%, 10% and 12% gels on successive days, samples being frozen in between gels. Gels were stained with Coommassie blue, washed with Destain I (50% MeOH, 10% HAc) for one hour, washed with Destain II (5% MeOH, 7% HAc) for one hour, washed with dH2O for 30 min and dried. Radioactive gels were exposed to a phosphor storage screen (GE) and read with a GE Storm phosphoimager after several days of exposure.
Kinase reactions for autophosphorylation
Reactions were carried out as described previously omitting peptide substrate and bovine serum albumin. For partial proteolysis experiments, ATP and GTP were used at 1 Ci/mmol.
Partial Proteolysis by SDS-PAGE
Radioactive protein bands identified by exposure to film, excised from dried 9% gels and rehydrated in 0.125 M Tris, pH 6.8 with 0.1% SDS and 5% β-mercaptoethanol. Protease was diluted in protease buffer consisting of 0.125 M Tris pH 6.8, 0.1% SDS, 1 mM EDTA, 15% glycerol, with trace amounts of bromphenol blue. Protease stock was kept at −20°C at 1 mg/mL in 10 mM Tris pH 6.8.
Excised gel pieces were heated to 100°C for 2 min then placed in 1.5 mm wide loading wells of a second 15% gel and covered with 20 µl of protease buffer with either 1 or 2 ug total protease per well. Samples were run at 30V for approximately 4 hours and power shut off for 30 minutes after samples had migrated into gel. Gel was then run to complete separation of peptide fragments, stained, dried and exposed to film.
Mass Spectrotrometry
Autophosphorylation reactions were prepared as described using cold Mg2+ATP and Mg2+GTP. After incubation at 30°, samples were separated on a 9% SDS-PAGE gel. The gel was stained and CaMKII bands were located by Coomassie staining and excised. Mass spectrometry was performed by Taplin Mass Spec Facility, Harvard Medical School, Boston, Massachusetts.
Results
CaMKII can use GTP as a phosphate donor for substrate phosphorylation
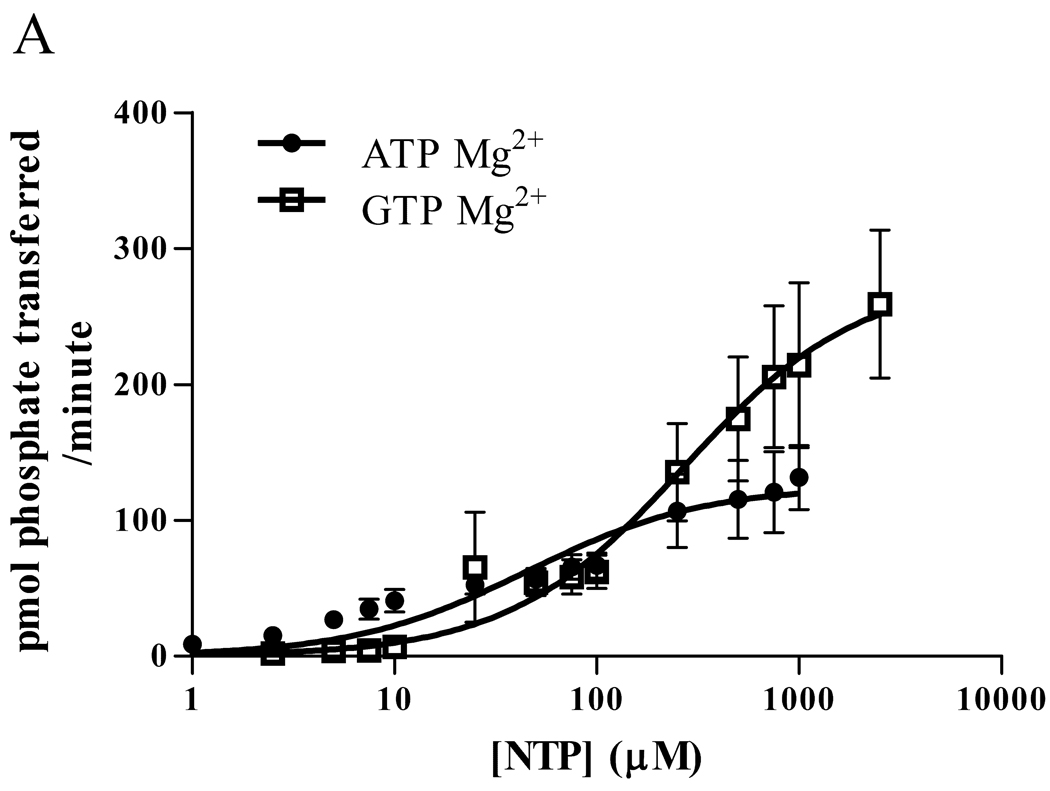
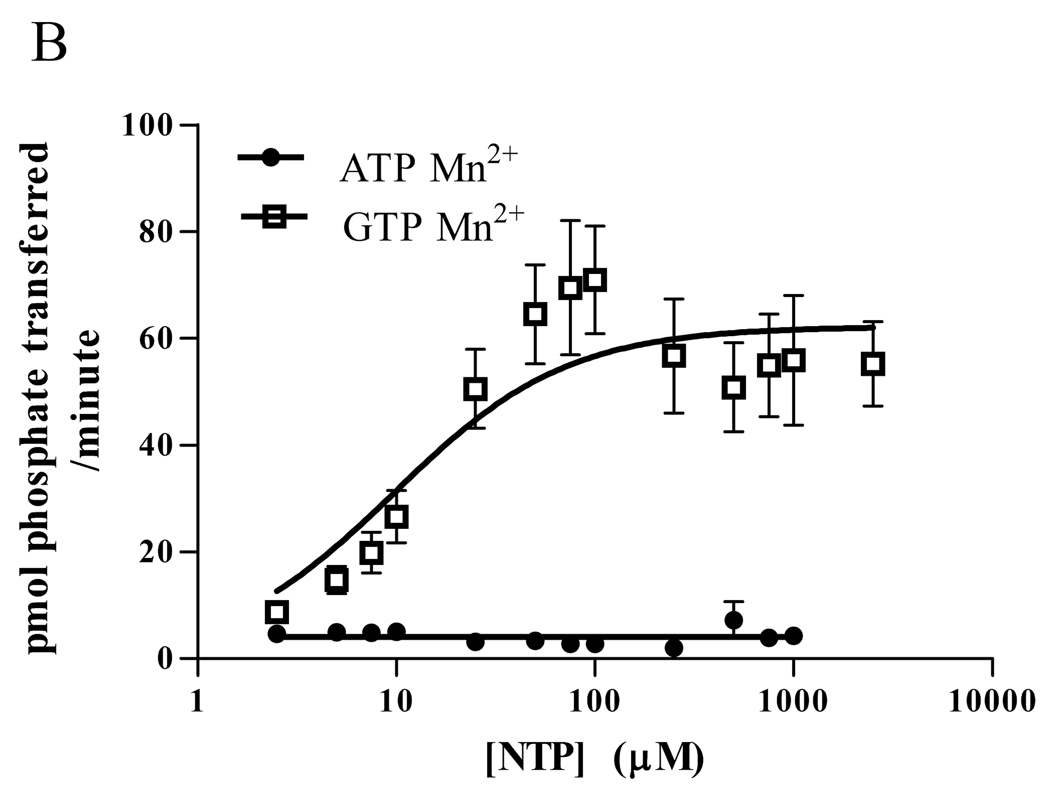
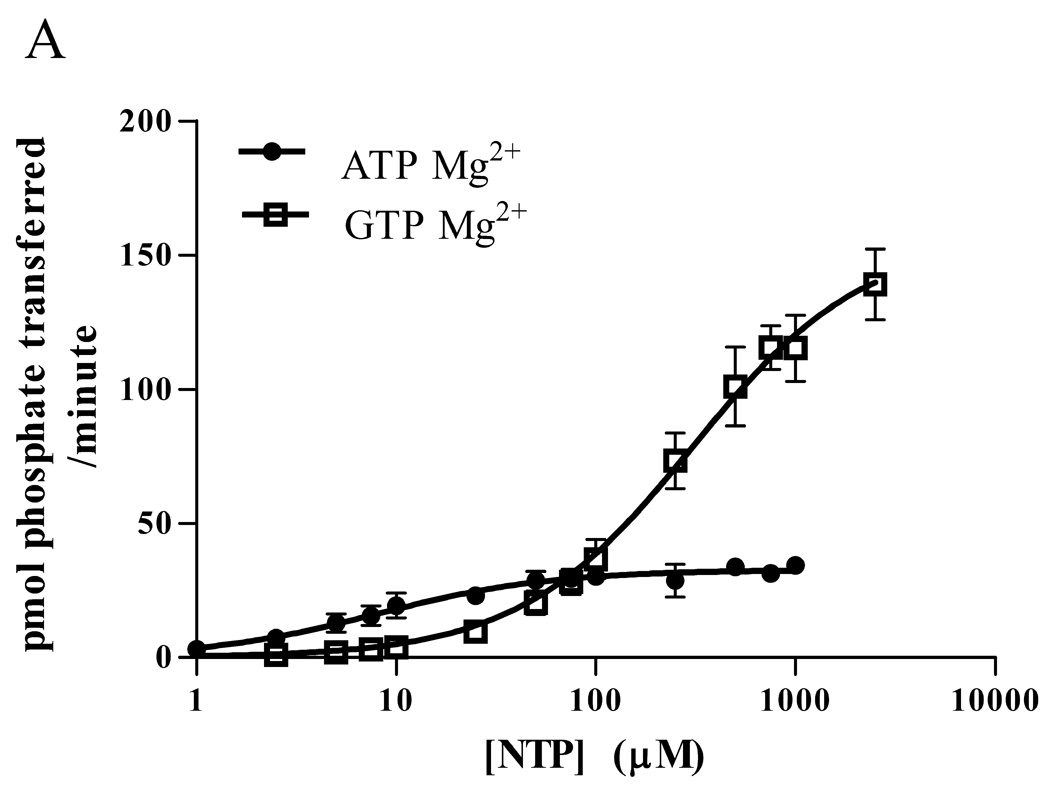
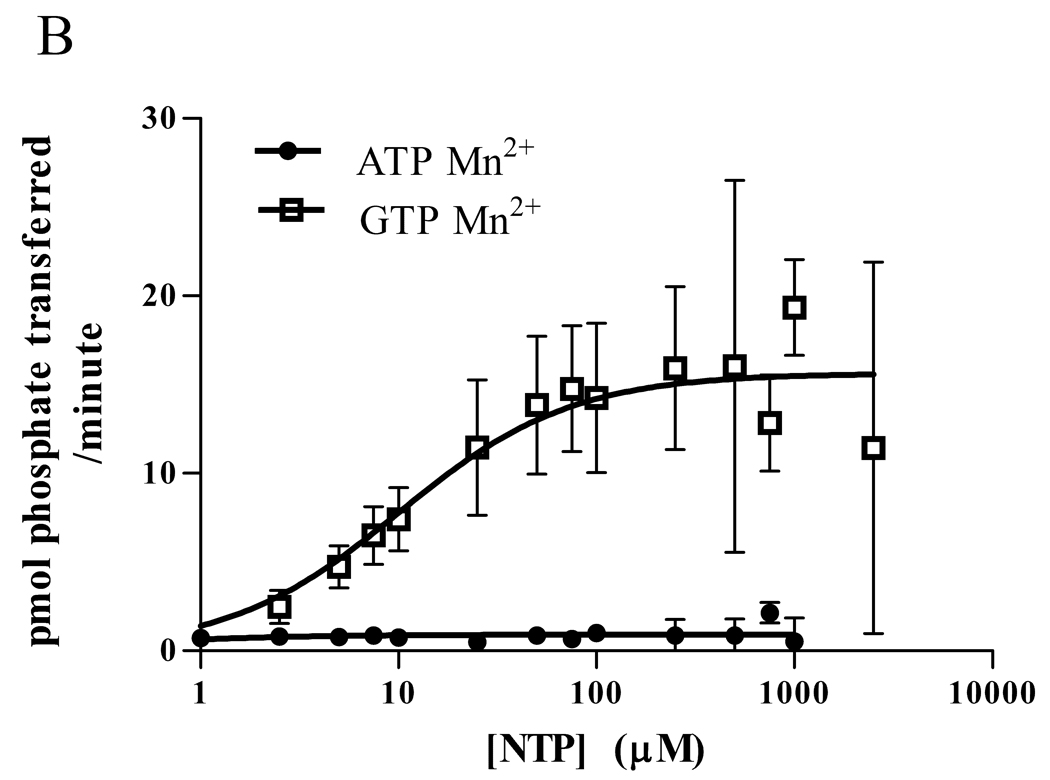
To determine the extent to which CaMKII can use GTP and the kinetics of the phosphorylation, we measured Drosophila CaMKII activity using a synthetic substrate, autocamtide-3, and compared the ability of γ-32P ATP and GTP to be utilized. (Figure 1A) When Mg2+ was used as the nucleotide ligand, CaMKII used ATP as a phosphate donor with a Km of approximately 45 µM and a Vmax near 125 pmol/min. In contrast, the Km with GTP was approximately 270 µM, but the Vmax was much greater, nearing 280 pmol/min. (Table 1).
Figure 1.
Phosphorylation of substrate by Drosophila CaMKII (A,B) or rat α-CaMKII (C,D) in 15 mM Mg2+ (A,C) or 5 mM Mn2+ (B,D). A synthetic peptide, autocamtide-3 (AC3), was phosphorylated by Drosophila CaMKII or rat α-CaMKII in the presence of γ-32P ATP or GTP. After 1 minute, the phosphorylation was stopped and the AC3 was adhered to P81 paper and washed. The bound radioactivity was counted and kinase velocity was calculated using the specific activity of the nuclide. Data are expressed as mean ± SEM. N = 4 (A,B), N=3 (C,D).
Table 1.
Kinetic parameters for Drosophila and rat CaMKII with ATP and GTP. 95% confidence intervals for Vmax and Km for Figure 1 and Figure 2. Values were calculated from non-linear regression. Columns with GTP are shaded.
| CaMKII | ATP Mg2+ | GTP Mg2+ | ATP Mn2+ | GTP Mn2+ |
|---|---|---|---|---|
| Drosophila | ||||
| Km | 45.41 ± 26.63 | 269.20 ± 103.80 | N/A | 9.77 ± 7.40 |
| Vmax | 125.20 ± 18.30 | 278.70 ± 34.20 | 4.09 ± 1.14 | 62.21 ± 8.64 |
| Rat α | ||||
| Km | 8.08 ± 1.78 | 304.0 ± 41.9 | 0.43 ± 1.86 | 10.12 ± 6.42 |
| Vmax | 32.63 ± 1.39 | 156.9 ± 7.10 | 0.92 ± 0.31 | 15.64 ± 1.82 |
Previous work with CK2 has shown that the divalent metal ion chelated by the nucleotide can have a significant effect on the kinetics of phosphorylation [13]. With this in mind, we repeated the experiment, this time using 5 mM MnCl2 in place of 15 mM MgCl2. (Figure 1B) The results were similar to those obtained with CK2, in that Mn2+ dramatically changed the kinetics. With Mn2+, even 1 mM ATP induced very little phosphorylation, such that a Km could not be calculated. The Vmax for Mn2+ ATP was only 4 pmol/min. As with CK2, however, the GTP Km dropped to near 10 µM, with a Vmax of approximately 62 pmol/min. (Table 1)
Although the Drosophila CaMKII has high homology to mammalian CaMKII, its ability to use GTP as a phosphate donor might be a unique feature. To test the generality of GTP use, we did the same experiments with rat αCaMKII and found results similar to those we obtained with the Drosophila kinase: Mg2+ATP had a lower Km and a lower maximum velocity than Mg2+GTP (See Figure 1C). With the substitution of Mn2+ for Mg2+, ATP velocity was greatly diminished, and the GTP Km was decreased. (See Figure 1D) Thus, it is likely that the ability of CaMKII to use GTP as a phosphate donor is common to CaMKII from many species, and is not a unique feature of the insect kinase.
ADP is a more effective competitive inhibitor than GDP
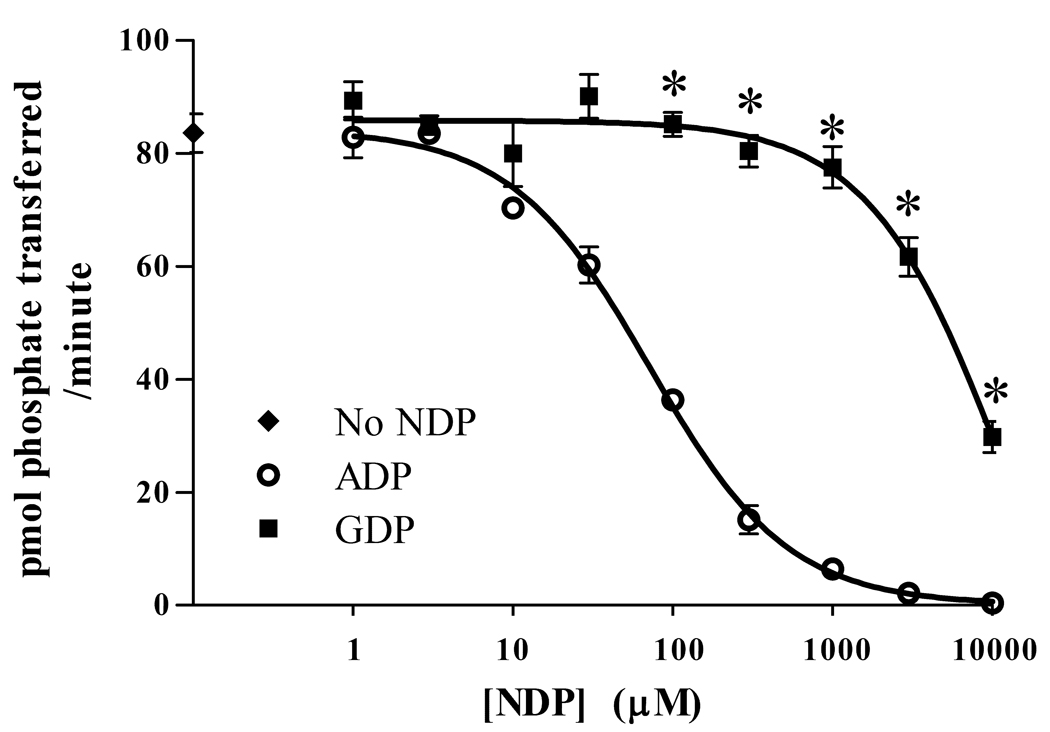
Another possible reason for the difference in Vmax with ATP and GTP is differentially effective competitive inhibition by the ADP and GDP formed after phosphorylation of substrate. If GDP was a lower affinity competitor, it could explain why phosphorylation in GTP, even at high concentrations, does not plateau as reactions done in ATP do. To test this hypothesis, we repeated the activity assay described above, this time adding increasing amounts of ADP or GDP. As shown in Figure 2, ADP does reduce the activity of the kinase to a much greater extent than does GDP. The IC50 for ADP is approximately 72 µM, while the GDP IC50 close to 10 mM, well out of the physiological range. This clearly explains the difference in the Vmax values found in the earlier experiments.
Figure 2.
Phosphorylation by Drosophila CaMKII is more inhibited by ADP than by GDP. AC3 was phosphorylated as before, with 50 µM ATP and increasing amounts of ADP or GDP. Data are expressed as mean ± SEM. N=3. * indicates significant difference between ADP and GDP at a given concentration (Two-tailed T-test, P < 0.01).
Autophosphorylation by GTP
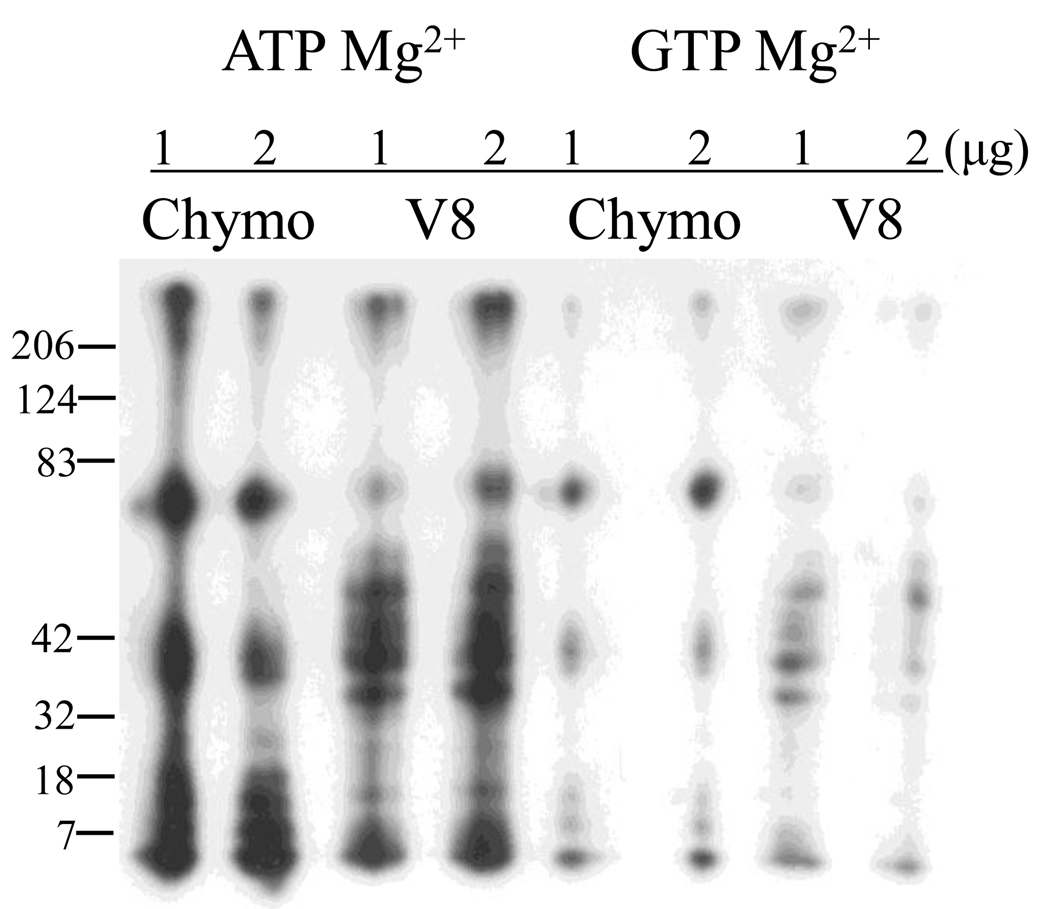
One significant feature of the data presented in Figure 1 is that the maximum phosphorylation velocity was always greater in GTP than in ATP. Since CaMKII is regulated at many levels by autophosphorylation, we wondered if this difference in activity might reflect a difference in autophosphorylation of the kinase in ATP and GTP. Initial experiments indicated that the maximal level of autophosphorylation was equivalent for both nucleotides (data not shown). To ask if nucleotide differentially regulated the sites of autophosphorylation, CaMKII was phosphorylated in the presence of calcium/CaM with either γ-32P ATP or GTP in the presence of Mg2+ or Mn2+ and subjected to partial proteolysis with S. aureus V8 protease and chymotrypsin. This technique generates a “fingerprint” of phosphopeptides that can be used to map differences in site specificity [18]. Figure 3 shows that Mg2+ATP and Mg2+GTP generated similar patterns. This was true irrespective of divalent metal ion (data for Mn2+ not shown), consistent with similar site specificity for the two nucleotides.
Figure 3.
Partial proteolysis of Drosophila CaMKII phosphorylated with ATP and GTP produces identical autophosphorylated fragments. CaMKII was autophosphorylated with γ-32P ATP or GTP and excised from a 9% gel after identification by autoradiography. Protein was digested with either chymotrypsin or S. aureus V8 protease (either 1 or 2 µg total protease) before running on a 15% gel.
Since several regions of CaMKII within the same protease fragment might contain multiple potential autophosphorylation sites, we examined phosphorylation sites directly by mass spectrometry. Only samples prepared in Mg2+ were used since divalent metal ion did not seem to alter phosphopeptide pattern. Samples of CaMKII autophosphorylated in the presence of calcium/CaM with non-radioactive nucleotides were analyzed by mass spectrometry and compared to a sample that had not been autophosphorylated. All of the peptides found with GTP as a phosphate donor were also seen with ATP and/or basal phosphorylation (see Table 2). Interestingly, kinase that had not been subjected to a calcium/CaM stimulation was also found to be phosphorylated, primarily on C-terminal sites that have not been previously identified as phosphorylation sites on this kinase.
Table2.
Mass spectrometry of autophosphorylated Drosophila CaMKII does not identify GTP-specific sites. Untreated (BASAL) Drosophila CaMKII and kinase autophosphorylated with non-radioactive ATP or GTP were analysed by mass spectrometry. Phosphopeptide sequences are shown at left with the phosphorylated residue in bold. + indicates that a particular peptide was found under the indicated condition.
| aa Sequence | aa | BASAL | ATP | GTP | Function |
|---|---|---|---|---|---|
| VASVVHRQETVDC | S280 | + | + | ||
| VASVVHRQETVDC | T287 | + | CaMKII active/Ca2+ independent | ||
| KGEGSQVKESTDSSSTTKEDDDIK | S327 | + | + | + | |
| KGEGSQVKESTDSSSTTKEDDDIK | S332 | + | |||
| KGEGSQVKESTDSSSTTKEDDDIK | T333 | + | + | + | |
| KGEGSQVKESTDSSSTTKEDDDIK | S335 | + | |||
| KGEGSQVKESTDSSSTTKEDDDIK | S336 | + | + | ||
| KGEGSQVKESTDSSSTTKEDDDIK | S337 | + | |||
| KGEGSQVKESTDSSSTTKEDDDIK | T338 | + | + | ||
| KGEGSQVKESTDSSSTTKEDDDIK | T339 | + | + | + | |
| ISGATTFDFIPQK | S505 | + | |||
| ISGATTFDFIPQK | T508 | + | + | ||
| ISGATTFDFIPQK | T509 | + | + |
GTP is used as a phosphate donor in phosphorylation of protein substrates
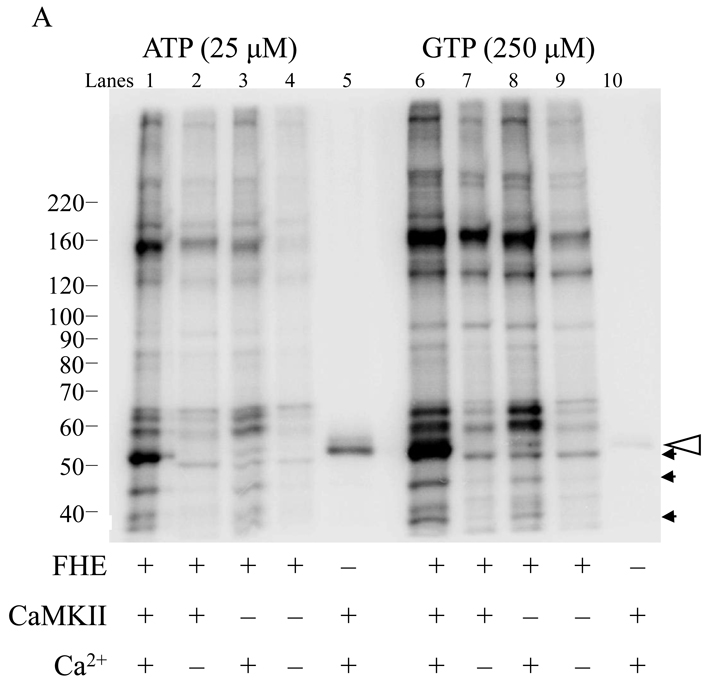
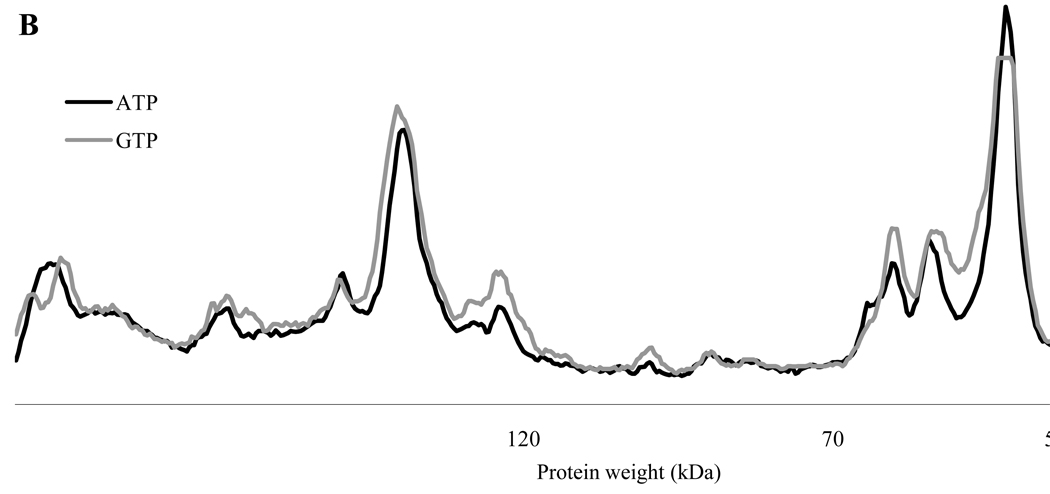
All of the above experiments were done with a peptide specifically designed to be a CaMKII substrate. To determine if GTP could also be used as a phosphate donor with native protein substrates, Drosophila CaMKII was incubated with fly head extract (FHE) and γ-32P Mg2+ATP or Mg2+GTP. A 10-fold greater concentration of Drosophila CaMKII was used for these experiments, in order to differentiate exogenous CaMKII activity from the endogenous activity of the kinases (including CaMKII) in the fly head extract. Figure 4A clearly shows that GTP can be used for substrate phosphorylation of a complex mixture of substrates. Figure 4B shows that the same bands are phosphorylated with ATP and GTP i.e. there do not appear to be substrates that are specific for either ATP or GTP. Interestingly, however, there may be quantitative differences in the ability of certain substrates to be phosphorylated using particular nucleotides. In Figure 4A it is clear that one band is noticeably more phosphorylated with GTP than with ATP. While the band in question is close to 50 kDa in molecular weight, it is unlikely to be CaMKII autophosphorylation, as there is more autophosphorylation in ATP than GTP (see lanes 5 and 10).
Figure 4.
Fly head proteins are similarly phosphorylated by Drosophila CaMKII in the presence of ATP and GTP. Fly head extract (FHE) was prepared from Canton S heads by extraction in RIPA buffer. Reactions were prepared as indicated and proceeded for 2 minutes. (A) Autoradiograph of samples separated on an 8% gel. Reaction components are indicated below each lane. Open arrow indicates position of autophosphorylated CaMKII. Bands that are significantly enhanced by addition of exogenous kinase are indicated by small arrowhead. (B) Densitometric comparison of lanes 1 and 6 (ATP and GTP, respectively) using ImageJ line profile. Results were scaled using specific radionuclide activity.
Discussion
With these experiments, we have shown that CaMKII can indeed use GTP as a phosphate donor for substrate and for autophosphorylation. While several of the key findings from CK2 were replicated, including the improvement in Km for GTP when Mn2+ is substituted for Mg2+, there are some departures from this model as well. In CK2, for instance, the Km for GTP in Mg2+ is only slightly higher than the ATP Km, while in CaMKII, the Km for GTP is 5-fold greater, indicating some selectivity.
Although it is tempting to think that CK2 and CaMKII have similarities in catalytic sites that would account for both being able to use GTP, the evidence does not bear this out. Previous bioinformatics work has compared 11 protein kinases, looking for similarities and differences in their catalytic sites [19] and found that CK2 had two exceptional differences in the canonical recognition sequences Ala-Xaa-Lys-Xaa-Leu in domain II and Leu-Xaa-Asp-Phe-Gly in domain IV; specifically, that the initial Ala was in CK2 a valine, and the phenylalanine in the second catalytic domain was a tryptophan. When these amino acids were mutated back to the canonical form, CK2 showed a decrease in ATP Km to approximately 6 µM and an increase to 70 µM for GTP [20]. In CaMKII, however, these sites are already in the canonical form, and so would not be responsible for the ability to use GTP, although they may help explain the difference in Km values between ATP and GTP. The fact that the ability is conserved from Drosophila to mammals is suggestive of a current function, but could also be a simple preservation of a working catalytic site.
The ability of CaMKII to use GTP as a phosphate donor could be advantageous at times when ATP is depleted in the cytoplasm, especially near the synaptic membrane. Even though the Km for GTP is large, the average GTP levels in the mammalian cytoplasm are several fold higher [11]. This may be even more exaggerated in microdomains like lipid rafts and caveolae, where certain proteins are organized in order to facilitate signal transduction [21; 22] and G-protein coupled receptors are a common element. In these areas, CaMKII could function to produce a steady phosphorylation of substrates, without inhibition by ADP. Whether the active site of CaMKII is specifically sensitive to ADP for purposes of feedback inhibition is another question that must be answered by site-directed mutagenesis.
It is interesting to speculate about the possible in vivo uses for CaMKII, but there are much more practical applications to be found in the laboratory. It is tempting to think that simply changing the cation in a solution (Mg2+ to Mn2+) and using GTP instead of ATP could narrowly activate CaMKII and CK2, instead of a host of protein kinases. This would be especially useful in cases where the substrate is known to be phosphorylated by CaMKII, and inhibition by multiple pharmacological agents would be a concern. Future studies will hopefully elucidate both in vivo and in vitro uses for this rather unique property of CaMKII.
Acknowledgements
We would like to thank XiuXia Sun for purification of Drosophila R3 CaMKII and Neal Waxham (University of Texas Medical School, Houston) for providing purified rat αCaMKII.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Erondu NE, Kennedy MB. Regional distribution of type II Ca2+/calmodulin-dependent protein kinase in rat brain. J Neurosci. 1985;5:3270–3277. doi: 10.1523/JNEUROSCI.05-12-03270.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller SG, Kennedy MB. Regulation of brain type II Ca2+/calmodulin-dependent protein kinase by autophosphorylation: a Ca2+-triggered molecular switch. Cell. 1986;44:861–870. doi: 10.1016/0092-8674(86)90008-5. [DOI] [PubMed] [Google Scholar]
- 3.Wang Z, Palmer G, Griffith LC. Regulation of Drosophila Ca2+/calmodulin-dependent protein kinase II by autophosphorylation analyzed by site-directed mutagenesis. J Neurochem. 1998;71:378–387. doi: 10.1046/j.1471-4159.1998.71010378.x. [DOI] [PubMed] [Google Scholar]
- 4.Colbran RJ, Soderling TR. Calcium/calmodulin-independent autophosphorylation sites of calcium/calmodulin-dependent protein kinase II. Studies on the effect of phosphorylation of threonine 305/306 and serine 314 on calmodulin binding using synthetic peptides. J Biol Chem. 1990;265:11213–11219. [PubMed] [Google Scholar]
- 5.Patton BL, Miller SG, Kennedy MB. Activation of type II calcium/calmodulin-dependent protein kinase by Ca2+/calmodulin is inhibited by autophosphorylation of threonine within the calmodulin-binding domain. J Biol Chem. 1990;265:11204–11212. [PubMed] [Google Scholar]
- 6.Lu CS, Hodge JJ, Mehren J, Sun XX, Griffith LC. Regulation of the Ca2+/CaM-responsive pool of CaMKII by scaffold-dependent autophosphorylation. Neuron. 2003;40:1185–1197. doi: 10.1016/s0896-6273(03)00786-4. [DOI] [PubMed] [Google Scholar]
- 7.Lisman JE. A mechanism for memory storage insensitive to molecular turnover: a bistable autophosphorylating kinase. Proc Natl Acad Sci U S A. 1985;82:3055–3057. doi: 10.1073/pnas.82.9.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leonard AS, Bayer KU, Merrill MA, Lim IA, Shea MA, Schulman H, Hell JW. Regulation of calcium/calmodulin-dependent protein kinase II docking to N-methyl-D-aspartate receptors by calcium/calmodulin and alpha-actinin. J Biol Chem. 2002;277:48441–48448. doi: 10.1074/jbc.M205164200. [DOI] [PubMed] [Google Scholar]
- 9.Sun XX, Hodge JJ, Zhou Y, Nguyen M, Griffith LC. The eag potassium channel binds and locally activates calcium/calmodulin-dependent protein kinase II. J Biol Chem. 2004;279:10206–10214. doi: 10.1074/jbc.M310728200. [DOI] [PubMed] [Google Scholar]
- 10.Griffith LC. Regulation of calcium/calmodulin-dependent protein kinase II activation by intramolecular and intermolecular interactions. J Neurosci. 2004;24:8394–8398. doi: 10.1523/JNEUROSCI.3604-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kornberg A, Baker TA. DNA Replication. New York: W.H. Freeman & Company; 1992. [Google Scholar]
- 12.Rodnight R, Lavin BE. Phosvitin kinase from brain: activation by ions and subcellular distribution. Biochem J. 1964;93:84–91. doi: 10.1042/bj0930084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gatica M, Hinrichs MV, Jedlicki A, Allende CC, Allende JE. Effect of metal ions on the activity of casein kinase II from Xenopus laevis. FEBS Lett. 1993;315:173–177. doi: 10.1016/0014-5793(93)81157-u. [DOI] [PubMed] [Google Scholar]
- 14.Niefind K, Putter M, Guerra B, Issinger OG, Schomburg D. GTP plus water mimic ATP in the active site of protein kinase CK2. Nat Struct Biol. 1999;6:1100–1103. doi: 10.1038/70033. [DOI] [PubMed] [Google Scholar]
- 15.Yde CW, Ermakova I, Issinger OG, Niefind K. Inclining the purine base binding plane in protein kinase CK2 by exchanging the flanking side-chains generates a preference for ATP as a cosubstrate. J Mol Biol. 2005;347:399–414. doi: 10.1016/j.jmb.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Gschwendt M, Kittstein W, Kielbassa K, Marks F. Protein kinase C delta accepts GTP for autophosphorylation. Biochem Biophys Res Commun. 1995;206:614–620. doi: 10.1006/bbrc.1995.1087. [DOI] [PubMed] [Google Scholar]
- 17.Griffith LC, Greenspan RJ. The diversity of calcium/calmodulin-dependent protein kinase II isoforms in Drosophila is generated by alternative splicing of a single gene. J Neurochem. 1993;61:1534–1537. doi: 10.1111/j.1471-4159.1993.tb13650.x. [DOI] [PubMed] [Google Scholar]
- 18.Cleveland DW, Fischer SG, Kirschner MW, Laemmli UK. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977;252:1102–1106. [PubMed] [Google Scholar]
- 19.Hanks SK, Quinn AM, Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988;241:42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- 20.Jakobi R, Traugh JA. Characterization of the phosphotransferase domain of casein kinase II by site-directed mutagenesis and expression in Escherichia coli. J Biol Chem. 1992;267:23894–23902. [PubMed] [Google Scholar]
- 21.Insel PA, Head BP, Patel HH, Roth DM, Bundey RA, Swaney JS. Compartmentation of G-protein-coupled receptors and their signalling components in lipid rafts and caveolae. Biochem Soc Trans. 2005;33:1131–1134. doi: 10.1042/BST20051131. [DOI] [PubMed] [Google Scholar]
- 22.Lajoie P, Goetz JG, Dennis JW, Nabi IR. Lattices, rafts, and scaffolds: domain regulation of receptor signaling at the plasma membrane. J Cell Biol. 2009;185:381–385. doi: 10.1083/jcb.200811059. [DOI] [PMC free article] [PubMed] [Google Scholar]