Abstract
The human follitropin receptor (hFSHR) is a G protein-coupled receptor (GPCR) central to reproductive physiology that is composed of an extracellular domain (ECD) fused to a serpentine region. Using bioluminescence resonance energy transfer (BRET) in living cells, we show that hFSHR dimers form constitutively during their biosynthesis. Mutations in TM1 and TM4 had no effect on hFSHR dimerization, alone or when combined with mutation of Tyr110 in the ECD, a residue predicted to mediate dimerization of the soluble hormone-binding portion of the ECD complexed with FSH (Q. Fan and W. Hendrickson, Nature 433:269–277, 2005). Expressed individually, the serpentine region and a membrane-anchored form of the hFSHR ECD each exhibited homodimerization, suggesting that both domains contribute to dimerization of the full-length receptor. However, even in the context of only the membrane-anchored ECD, mutation of Tyr110 to alanine did not inhibit dimerization. The full-length hFSHR and the membrane-anchored ECD were then each engineered to introduce a consensus site for N-linked glycosylation at residue 110. Despite experimental validation of the presence of carbohydrate on residue 110, we failed to observe disruption of dimerization of either the full-length hFSHR or membrane-anchored ECD containing the inserted glycan wedge. Taken altogether, our data suggest that both the serpentine region and the ECD contribute to hFSHR dimerization and that the dimerization interface of the unoccupied hFSHR does not involve Tyr110 of the ECD.
Keywords: follitropin receptor, FSH receptor, glycoprotein hormone receptor, gonadotropin receptor, G protein-coupled receptor, receptor dimerization
1. Introduction
The human follitropin receptor (hFSHR) is a Family A GPCR integral to both male and female reproductive endocrinology that responds to pituitary FSH. In females, the hFSHR is found on ovarian granulosa cells where it mediates follicle maturation, and in males it is expressed on testicular Sertoli cells where it supports spermatogenesis. As with all GPCRs, the hFSHR contains a serpentine domain composed of seven transmembrane helices (TMs) connected by three extracellular and three intracellular loops that mediates the binding and activation of G proteins, the primary G protein stimulated by the hFSHR being Gs. However, the binding of FSH occurs distally through a series of leucine-rich repeats within the relatively large N-terminal extracellular domain (ECD) of the hFSHR and the N-terminal cysteine-rich region of the mature hFSHR, [1, 2]. There is another cysteine-rich region, termed the hinge or linker region, which anchors the hFSHRHB to the serpentine domain. The hFSHR is most closely related to the human lutropin receptor (hLHR) and human thyrotropin receptor (hTSHR), the other members of the glycoprotein hormone receptor family. Ultimately, how hormone binding to the ECD of a glycoprotein hormone receptor induces the serpentine region to adopt an active conformation remains enigmatic. However, studies suggest that the hinge region may play an important role in transmitting this information between the two domains [3–5].
Our understanding of the structural organization of the hFSHR and how it binds FSH was greatly advanced by the structure of a high-resolution crystal of the hormone-binding domain of the hFSHR (hFSHRHB) complexed with FSH [2, 6]. These studies elegantly demonstrated how the leucine-rich repeats form a horseshoe-like structure with FSH bound to the concave inner surface of the receptor as if in a handclasp. Interestingly, it was found that the hFSHRHB-FSH complex formed low affinity dimers both in solution as well as in the crystal structures and it appeared as if the dimer interface occurred through hydrophobic interactions between the hFSHRHB protomers, with Tyr110 significantly contributing to these interactions [2]. From a variety of experimental approaches, it has been shown that GPCRs form dimers and in some reports higher ordered oligomers as well (see refs. [7–12] for recent reviews). Because most methods examining GPCR self-association cannot distinguish between GPCR dimerization and oligomerization, we utilize the term dimerization herein to imply dimerization and/or oligomerization. Specifically, it has been shown by Thomas et al. that hFSHR self-association can be detected by fluorescence resonance energy transfer (FRET) in fixed cells and by co-immmunoprecipitation of differentially tagged receptors following detergent lysis of co-transfected cells [13]. The present study was undertaken to (i) determine if hFSHR homodimerization could be detected in living cells and, if so, (ii) to determine the relative contributions of the serpentine region, the ECD, and Tyr110 to receptor dimerization.
2. Materials and Methods
2.1 Plasmids and hormones
The hFSHR cDNA was kindly given to us by Ares Advanced Technology (Ares-Serono Group, Randolph, MA). Mutants of the hFSHR were made using standard techniques. The wild-type (wt) and mutant forms of the hFSHR were all modified to contain a myc epitope tag at the N-termimus. For the BRET2 studies, the cDNAs were subcloned into pRluc or pGFP2 vectors (PerkinElmer, Waltham, MA) that insert Renilla luciferase (Rluc) or GFP2, respectively, in-frame at the C-terminus of the protein. The cDNA encoding KvLQT1-RLuc was used as previously described [14] and HERG-GFP2 was a generous gift from Dr. Alvin Shrier (Department of Physiology, McGill University, Montreal, Canada). Plasmids encoding myc-LRP6delN and CD8-GFP2 were kind gifts from Drs. Guizhong Liu (Mount Sinai School of Medicine, New York, NY) and Michel Bouvier (University of Montreal, Quebec, Canada), respectively. To engineer myc-hFSHR(ECD)-LRP6(TM)-Rluc, the myc-LRP6delN construct (in which the extracellular N-terminal domain is deleted) was first subcloned into pRluc. Then the ECD of myc-hFSHR (residues 1–360) was cloned onto the N-terminus. As the CD8-GFP2 construct encoded residues 1–209 and lacked the C-terminal intracellular tail of CD8, we first inserted the CD8 tail (residues 210–236). The hFSHR ECD (residues 1–358) was then spliced onto the N-terminus to yield myc-hFSHR(ECD)-CD8(TM)-GFP2. The full coding sequences of all constructs were determined by the DNA Core of the University of Iowa. Pregnant mare serum gonadotropin (used for the determination of non-specific binding) and highly purified preparations of recombinant human FSH were purchased from Dr. A. Parlow and the National Hormone and Pituitary Program of NIDDK/NIH. FSH was iodinated as previously described for hCG [15].
2.2 Cells and transfections
Human embryonic kidney (HEK) 293 and 293T cells were obtained from the American Type Tissue Collection (Manassas, VA). Cells were maintained and transiently transfected as described previously [16].
2.3 BRET2 assays
HEK293T cells were transiently co-transfected with vectors encoding Rluc-fusion or GFP2-fusion proteins. In a given experiment, the total amount of plasmid transfected was made constant by the addition of empty vector. On the day of the experiment, cells were washed two times with calcium and magnesium free D-PBS, and then detached from the well in 1ml D-PBS. Protein concentrations were measured and equal protein aliquots were distributed into microcentrifuge tubes and collected by gentle centrifugation. The cell pellets were resuspended in a small volume of D-PBS and transferred to a white-bottomed 96 well microplate (white Optiplate; PerkinElmer Life and Analytical Sciences, Waltham, MA) such that all samples were of equal volume and protein concentration. Total fluorescence of the cell suspensions was measured using a POLARstar Optima plate reader (BMG LABTECH, Offenburg, Germany) with an excitation filter at 485 nm and an emission filter at 520 nm and was corrected for the fluorescence measured in cells transfected with empty vector only. The substrate Coelenterazine 400a (Biosynth; Zurich, Switzerland) was then added at a final concentration of 5 μM and readings at 410/80 nm (reflecting the bioluminescence given off by Rluc) and 515/30 nm (reflecting the resonance energy transfer from Rluc to GFP2) were measured simultaneously. Bioluminescence readings were corrected for those obtained from cells transfected with empty vector only. The BRET2 ratio was calculated as the ratio of the light emitted by the receptor-GFP2 (515/30 nm) over the light emitted by the receptor-Rluc (410/80 nm). The BRET2 ratios reported were corrected by subtracting the ratios obtained when receptor-Rluc was expressed alone.
2.4 BRET2 titration curves
For BRET2 saturation curves, HEK293T cells were co-transfected with a fixed concentration of Rluc fusion proteins and increasing concentrations of GFP2 fusion proteins. When more than one curve was generated in a given experiment, the concentrations of plasmids encoding the Rluc fusion proteins were adjusted so that, after substrate addition, bioluminescence values of the Rluc fusion proteins expressed alone were similar. Data were expressed as the net BRET2 ratio, calculated as described above, relative to the ratio of acceptor to donor. The data were plotted using GraphPad Prism (San Diego, CA).
2.5 Subcellular fractionation of membranes
Membranes from transfected HEK293T cells were isolated and separated by sucrose gradient fractionation as previously described [17]. Equal volume samples were taken from each fraction for BRET2 determinations and for Western blot analyses (see below) to detect calnexin (a marker for the endoplasmic reticulum), Na+/K+ ATPase (a marker for the plasma membrane) or the myc-hFSHR fusion proteins. Western blots for myc-hFSHR were also analyzed when the gels were run after applying equal amounts of protein to each well. The conditions for running the SDS gels and probing the Western blots were exactly as described previously [17].
2.6 Hormone desorption experiments
Hormone desorption assays were performed on HEK293 cells transiently transfected with hFSHR as described previously [17] with the following modifications. The preincubation was done using 125I-hFSH (final concentration 200 ng/ml) with or without an excess of pregnant mare serum gonadotropin (final concentration 200 IU/ml). After washing to remove unbound labeled hormone, one group of cells was used to determine t=0 binding and another group was incubated with or without unlabeled hFSH (final concentration 300 ng/ml). At time points thereafter, 125I-hFSH released into the medium was determined by collecting the medium and precipitating intact hormone with tricholoracetic acid. To determine the amount of 125I-hFSH remaining bound to cells, the cells were washed, solubilized and the bound radioactivity counted.
2.7 Carbohydrate analyses
HEK293T cells were transiently transfected and on the day of the experiment they were solubilized as previously described [1] with the following modifications. The protease inhibitors used in all solutions were 3 μM N-ethylmaleimide, 1 μM phenylmethyl sulfonylfluoride, 1 μM pepstatin A, 1 mM leupeptin, 0.1 mg/ml aprotinin, 2 mM EDTA, and 3 mM EGTA. After solubilization, Nonidet P-40 was diluted to 0.1% and the glycerol to 4%. Aliquots containing equal amounts of protein were incubated with shaking overnight at 37C with or without PNGaseF (New England Biolabs, Ipswich, MA) at a final concentration of 5,000 units/ml. A parallel aliquot was incubated overnight at 4C without PNGaseF and compared to the 37C sample with PNGaseF to ensure that receptor degradation did not occur during the 37C incubation. For the full-length hFSHR, an aliquot was incubated an additional 2h at room temperature with 50 mM N-chlorosuccinimide in 4M urea. The samples were resolved by SDS-PAGE and Western blots probed for myc-hFSHR as described above.
2.8 Quantification of cell surface hFSHR and FSH-stimulated cAMP
HEK293 cells were transiently transfected with plasmids encoding HA-tagged wt or mutant hFSHR or, as a control, empty pcDNA3.1 vector. In the same experiment, a group of non-permeabilized cells was used to quantify the cell surface expression of the HA tag by flow cytometry as described [18]. Another group of cells was assayed for specific cell surface binding of a saturating concentration of 125I-FSH as described previously [19]. A third group of cells was used for the determination of FSH-stimulated cAMP as described [19].
3. Results
3.1 hFSHRs form constitutive dimers and/or oligomers
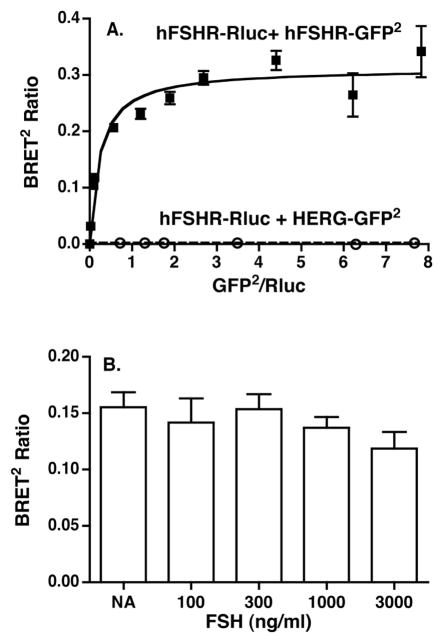
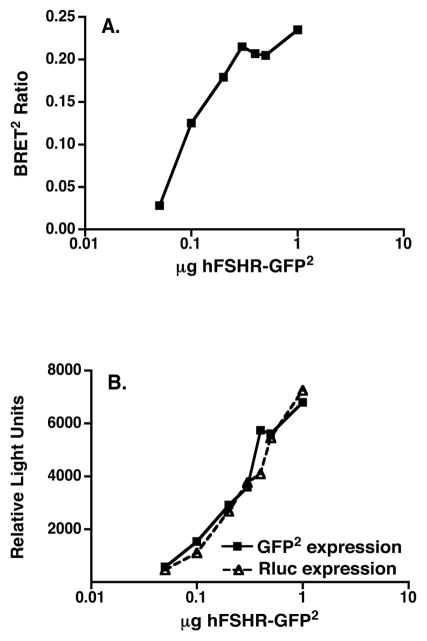
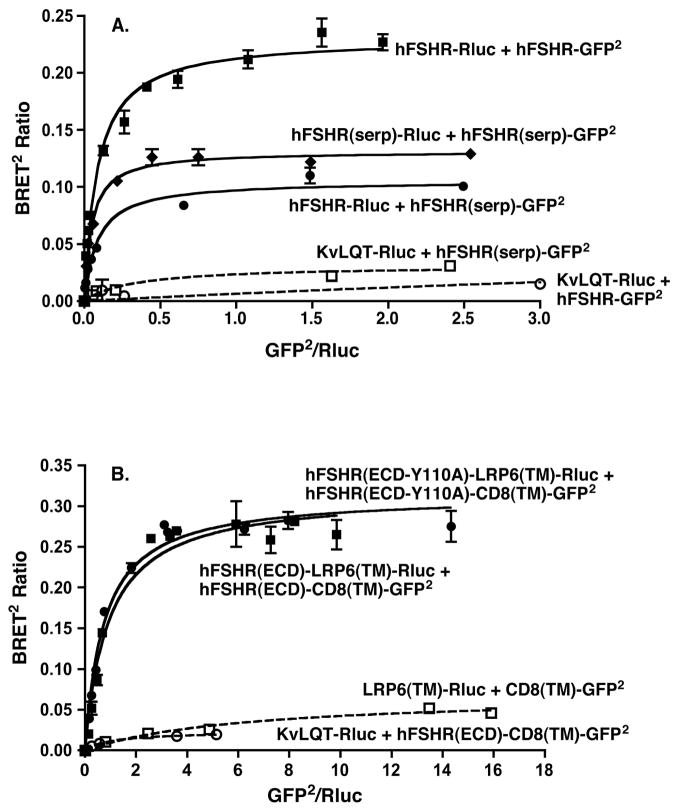
BRET was used to study dimerization of the hFSHR in living cells. Towards this end, titration curves were generated from 293T cells co-transfected with a low and fixed concentration of the energy donor hFSHR-Rluc and increasing concentrations of the energy acceptor hFSHR-GFP2. Upon addition of substrate to cells, increased energy transfer, as determined by the BRET2 ratio, was observed with increased expression ratios of the GFP2 to Rluc fusion proteins (Figure 1A). The plateau observed at the higher ratios of energy acceptor to donor indicates a saturable process and is consistent with that predicted for specific interactions between the two molecules [20]. No energy transfer was observed when cells were co-transfected with hFSHR-Rluc and HERG-GFP2, a voltage-gated potassium channel, further supporting the specificity of hFSHR homodimerization. Preincubation of cells with FSH had no effect on the BRET ratios observed under these conditions (Figure 1B). It should be noted that the BRET methodology employed cannot distinguish between dimerization and higher order oligomerization of the hFSHR. Therefore, as noted above, while we utilize the term dimerization, it should be kept in mind that the hFSHR dimers may in fact be further associated into larger complexes. Additional data to support that hFSHR self-association observed by BRET was not due to random collisions is provided by the data presented in Figure 2A, where cells co-transfected with a fixed ratio of hFSHR-Rluc/hFSHR-GFP2 and decreasing total amounts of receptors displayed resonance energy transfer at very low amounts of receptor (expression levels of which are shown in Figure 2B). Thus, using BRET, the hFSHR can be shown to specifically homodimerize in a constitutive manner in living cells.
Figure 1. BRET2 demonstrates hFSHR homodimerization in living cells.
Panel A: HEK293T cells were transiently transfected with a fixed concentration of hFSHR-Rluc and increasing concentrations of either hFSHR-GFP2 or HERG-GFP2. Data shown are the mean ± SEM of duplicate net BRET2 ratios as a function of GFP2/Rluc expression taken from one experiment that is representative of at least 20 independent experiments. Panel B: HEK293T cells were transiently transfected with hFSHR-Rluc and hFSHR-GFP2 using plasmid concentrations that would yield a submaximal BRET2 ratio. The cells were incubated 40 minutes at RT with the indicated concentrations of FSH immediately prior to the BRET determinations. Data shown are the mean ± SEM of quadruplicate determinations from one experiment. It is representative of two experiments. Similar results were also observed examining the effects of FSH incubations of 20 and 60 minutes at RT and 60 minutes at 37C.
Figure 2. Non-spurious self-association of the hFSHR.
HEK293T cells were transiently transfected with varying total amounts of a fixed ratio (1:5) of hFSHR-Rluc and hFSHR-GFP2. The data shown in Panel A are the net BRET2 ratios as a function of the total amount of plasmid transfected. From the same experiment, the expression of hFSHR-GFP2 (as measured by fluorescence prior to substrate addition) and hFSHR-Rluc (as measured by luminescence) are shown in Panel B. The data shown are representative of at least 3 independent experiments.
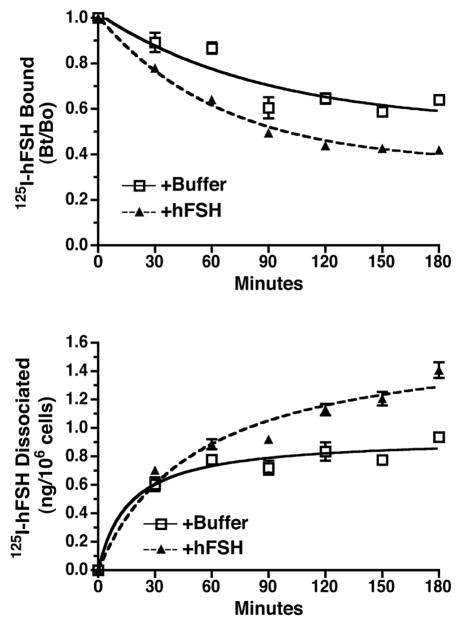
To further confirm dimerization of the hFSHR using a different methodology and to determine if hFSHR dimers were functional, hormone desorption assays were performed. Cells were transfected with hFSHR and then incubated with 125I-FSH to occupy cell surface receptors. After washing to remove the unbound labeled hormone, the cells were incubated for increasing lengths of time in the absence or the presence of unlabeled FSH. It is important to note that upon removal of the unbound 125I-FSH and the replacement of the cells in media lacking 125I-FSH, one has achieved an “infinite dilution” that would prevent the rebinding of 125I-FSH [21]. Consequently, any effects of the subsequent addition of unlabeled hormone on the dissociation of tracer cannot be interpreted as a result of competitive binding to the same binding site. Rather, an effect of the unlabeled hormone on the dissociation kinetics of the tracer would constitute as an allosteric effect, interpreted as most likely reflecting the binding of the unlabeled hormone to one protomer of a GPCR dimer allosterically affecting the conformation of other tracer-bound protomer [22]. As shown in Figure 3 (top panel), pre-bound cell-associated 125I-FSH decreased at a faster rate when unlabeled FSH was present during the second incubation. Concomitantly under these conditions there was an increased release of 125I-FSH into the medium (Figure 3, bottom panel). These data indicate the presence of functional hFSHR dimers on the cell surface.
Figure 3. Functional cell surface hFSHR homodimers as determined by allosteric modulation of hormone binding.
HEK293 cells transiently transfected with a relatively low level of the hFSHR were allowed to bind a saturating concentration of 125I-FSH (200 ng/ml final concentration). After washing to remove unbound 125I-FSH, cells were incubated for the indicated times at RT with buffer only or with a saturating concentration of unlabeled FSH (300 ng/ml final concentration). At the end of the incubation period, the amount of 125I-FSH remaining specifically bound to the cells was determined (Top Panel) and the amount of acid-precipitable cpm in the medium were determined (Bottom Panel). Data shown are the mean ± SEM of triplicate determinations and is representative of three independent experiments.
3.2 Subcellular localization of hFSHR dimers
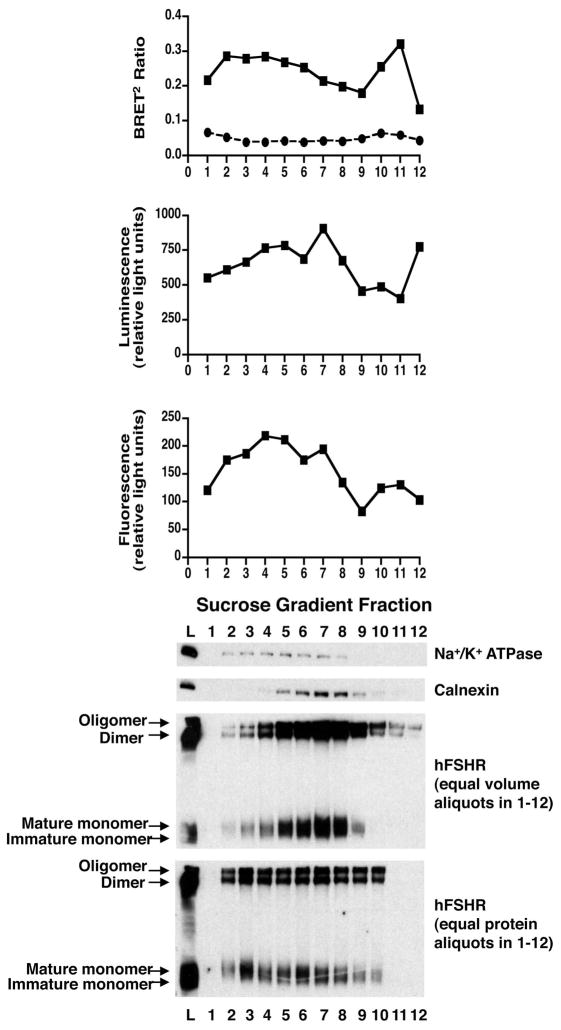
As BRET signals can be generated wherever hFSHR dimers are expressed in the cell, we performed the following experiments to determine the precise subcellular localization of hFSHR dimers. Cells were co-transfected with hFSHR-Rluc and hFSHR-GFP2, membranes were isolated, and these membranes were fractionated by sucrose gradient centrifugation (Figure 4). The expression of hFSHR-Rluc and hFSHR-GFP2 in each fraction was determined by luminescence and fluorescence, respectively (second and third panels). As would be expected, both fusion proteins were expressed in fractions enriched in plasma membranes (i.e., those fractions expressing the plasma membrane marker Na+/K+ATPase) and ER (i.e., those fractions expressing the ER marker calnexin). Importantly, BRET was detected in the plasma membrane and ER fractions as well (top panel), indicating the presence of hFSHR dimers in both cellular compartments. We also examined the expression of the hFSHR in each fraction as determined by Western blotting for myc, which was contained on both hLHR fusion proteins (lower panels). The bands observed on the Western blots are consistent with previously determined molecular weights of monomers of the immature and mature forms of the receptor [23] as well as higher molecular weight forms of the hFSHR that would be consistent with dimeric and oligomeric forms of the receptor. Consistent with the BRET results, the Western blots suggest the presence of hFSHR dimers and oligomers in the plasma membrane and ER. The BRET and Western blot data are consistent and indicate that hFSHR dimers/oligomers are constitutively formed in the ER early in the biosynthetic pathway, suggesting that they are transported to the plasma membrane as self-associated complexes.
Figure 4. hFSHR dimerization in the plasma membrane and ER as determined from subcellular fractionation.
HEK293T cells were transiently transfected with myc-hFSHR-Rluc and myc-hFSHR-GFP2 (closed squares and solid line) or KvLQT-Rluc and myc-hFSHR-GFP2 (open circles and dashed line). Cell membrane lysates were applied to the bottom of a sucrose gradient as described in Materials and Methods and resolved by floatation during centrifugation. Equal volume fractions were analyzed (top to bottom) for hFSHR dimerization as determined by BRET2, hFSHR-Rluc expression as determined by luminescence, hFSHR-GFP2 expression as determined by fluorescence, Na+/K+ ATPase expression as determined by Western blotting, and calnexin expression as determined by Western blotting. The bottom two panels depict hFSHR expression as determined by Western blotting, in the membrane lysate prior to fractionation (L) and each of the fractions. Equal volume aliquots (next to bottom panel) or equal protein aliquots (bottom panel) of the fractions were evaluated. Data shown are representative of at least three independent experiments.
3.3 Both the serpentine and ECD regions of the hFSHR contribute to dimerization/oligomerization
Having established by different approaches that the hFSHR constitutively forms dimers and oligomers, the following experiments were performed to ascertain what region(s) of the receptor contribute to its self-association. As difficult a question as this is for any GPCR, it is that much more complex with the hFSHR because, as with the other glycoprotein hormone receptors, the hFSHR contains a large extracellular domain (ECD) in addition to the prototypical serpentine region. We first addressed the question as to whether the serpentine domain or the ECD alone were each capable of forming homodimers. The serpentine region of the hFSHR, when expressed alone or tagged at the C-terminus with Rluc or GFP2 is not expressed on the cell surface. However, because we have shown that dimerization of the hFSHR initiates in the ER, the intracellular localization of hFSHR(serp) would not impair our ability to measure its dimerization by BRET. As shown in Figure 5A, there is a specific and saturable BRET2 ratio observed upon co-transfection of cells with a fixed concentration of hFSHR(serp)-Rluc and increasing concentrations of hFSHR(serp)-GFP2, indicating that the serpentine core of the hFSHR can form homodimers. The hFSHR serpentine region is also capable of specifically dimerizing with the full-length hFSHR, as shown in the same experiment. The differences in BRETmax values between the different saturation curves cannot readily be interpreted because these values reflect not only potential differences in the distances between energy donor and acceptor and the number of dimeric complexes, but also the relative conformations of energy donors and acceptors [10, 24–26]. Neither the full-length hFSHR nor hFSHR(serp) dimerized to any appreciable extent with KvLQT1-RLuc, another voltage-gated potassium channel used as a negative control. Thus, these data suggest specific homodimerization of the serpentine domain of the hFSHR.
Figure 5. The serpentine region of the hFSHR and membrane-anchored form of the hFSHR ECD are each capable of homodimerizing.
Panels A and B represent different experiments, with Panel A examining dimerization of the serpentine region of the hFSHR and Panel B dimerization of membrane-anchored forms of the hFSHR ECD. In both experiments, HEK293T cells were transfected with the indicated pairs of fusion proteins. The Rluc fusion proteins were held fixed and the concentrations of GFP2 fusion proteins were increased. Data shown are the corrected BRET2 signals as a function of GFP2/Rluc expression. Within a given experiment, the plasmid concentrations were adjusted so that all Rluc fusion proteins in the absence of GFP2 fusion proteins were expressed similarly. The data shown in Panels A and B are each representative of three independent experiments.
It was previously shown that a soluble version of hFSHRHB complexed with FSH formed dimers [2]. To determine if a membrane-anchored form of the complete hFSHR ECD (i.e., containing the hinge region) could form homodimers, we took advantage of LRP6 [27] and CD8 [28] as sources for the membrane-spanning domain. Earlier studies had shown that a chimera containing the hFSHR ECD and the membrane-spanning and cytoplasmic domains of CD8 retained high affinity hormone binding activity [28]. Our preliminary BRET experiments indicated that the TM domain of LRP6 forms homodimers and that TM domain of CD8 similarly homodimerizes. However, when co-expressed together, the TM domains of LRP6 and CD8 do not heterodimerize with each other (shown as one of the negative controls in Figure 4B). Therefore, we created an energy donor fusion protein of the ECD of the hFSHR fused to the TM domain of LRP6, which in turn contained Rluc at the cytoplasmic C-terminus. An energy acceptor fusion protein was created with the hFSHR ECD fused to the TM domain of CD8, which contained GFP2 at the cytoplasmic C-terminus. As shown in Figure 5B, hFSHR(ECD)-LRP6(TM)-Rluc showed saturable dimerization with hFSHR(ECD)-CD8(TM)-GFP2. In light of the negative controls included, it can be concluded that the dimerization between these two fusion proteins was mediated by the ECD of the hFSHR. These data demonstrate that a membrane-anchored form of the ECD of the hFSHR is capable of homodimerization. Thus, both the serpentine domain and ECD domains of the hFSHR contribute to homodimerization.
The following sets of mutants were then analyzed to determine if any would have a significant negative impact on homodimerization of the hFSHR(wt) as determined by BRET saturation curves. Based on underlying theories of resonance energy transfer, it has been argued that BRET50’s (the GFP2/Rluc ratio yielding the half-maximal BRET2) reflects the relative affinities for dimerization of different interacting proteins [24, 26]. Therefore, within each experiment, the BRET50 for the mutant and wt hFSHR were determined and we have presented the data as the ratio of the BRET50 of the mutant relative to the BRET50 for the wt hFSHR (Table 1). hFSHR(W436,W494) and hFSHR(M490,W494,I495), which contained mutations within TM4, were based upon molecular dynamics studies of the serpentine region of the hLHR that predicted TM residues contributing to its dimerization [29]. As determined by confocal microscopy of permeabilized and non-permeabilized cells (data not shown), these two mutants were expressed, but retained in the ER, presumably due to mutation of the highly conserved Trp494. Nonetheless, they still exhibited specific homodimerization, with BRET50’s comparable to the wt hFSHR (Table 1). All other hFSHR mutants we examined (summarized in Table 1) exhibited expression on the cell surface (data not shown). Based on studies on the CCR5 receptor that suggested an inhibition of this receptor by mutation of I52V in TM1 and V150A in TM4 [30], we created the analogous hFSHR(I381A,V49A). In addition, hFSHR(I382A,V383A,M490A,V491A) altered residues in both TM1 and TM4 that had been suggested to be involved in the homodimerization of the α1b-adrenergic receptor [31]. Finally, we also created hFSHR(H485A,S488A,M492A,I495A,F504A), which contained mutations in TM4 predicted to be involved in the dimerization of the dopamine D2 receptor [32]. The results shown in Table 1 indicate that for all mutants examined there was at most a 1.7-fold reduction in the relative propensity for homodimerization. That, coupled with the standard errors of the mean associated with the changes in BRET50 values observed, lead us to conclude that these mutations did not cause meaningful disruptions in the propensity for receptor dimerization.
Table 1.
Effects of Mutations on Dimerization of the hFSHRa
| hFSHR Mutant | Basis for Mutation | Cell Surface Expression | BRET50 mut/BRET50 wt |
|---|---|---|---|
| + | |||
| W436A,W494A | ref. [29] | − | 1.29 ± 0.26 (n=4) |
| M490A,W494A,I495A | ref. [29] | − | 1.50 ± 0.12 (n=3) |
| I381A,V491A | ref. [30] | + | 1.69 ± 0.24 (n=3) |
| I382A,V383A,M490A,V491A | ref. [31] | + | 0.934 ± 0.135 (n=5) |
| H485A,S488A,M492A,I495A,F504A | ref. [32] | + | 1.41 ± 0.26 (n=5) |
| Y110A | ref. [2] | + | 1.74 ± 0.32 (n=7) |
| Y110A,H485A,S488A,M492A,I495A,F504A | refs. [32], [2] | + | 1.29 ± 0.28 (n=4) |
BRET saturation curves were performed in HEK293T cells for a given hFSHR mutant and compared to the wt FSHR within the same experiment. A comparison of the BRET50 values obtained in a given experiment is described as the ratio of the BRET50 for the mutant/BRET50 for the wt hFSHR. Results are presented as the mean ± SEM of the derived ratios for the indicated number of experiments.
3.4 Does Tyr110 in hFSHR contribute to dimerization/oligomerization?
It is likely that these TM mutations may not be particularly disruptive to dimerization of the full-length receptor if the ECD were either the primary mediator of hFSHR dimerization or a contributor whose interactions compensated for loss of interactions within the serpentine domain. Previous crystallographic studies of the soluble hFSHRHB-FSHR complex suggested that Tyr110 contributed to a large number of hydrophobic interactions at the dimerization interface of the ECD [2]. In the context of the full-length hFSHR, we examined mutation of Tyr110 alone and combined with mutations in TM4. Both hFSHR(Y110A) and hFSHR(Y110A,H485A,S488A,M492A,I495A,F504A) formed homodimers with a similar propensity as the wt hFSHR (Table 1). While it could be argued that mutation of only Tyr110 in the context of the full-length hFSHR might be without effect if the serpentine region of the receptor maintained dimerization interactions, the ability of the Y110A,H485A,S488A,M492A,I495A,F504A mutant to dimerize normally was surprising to us.
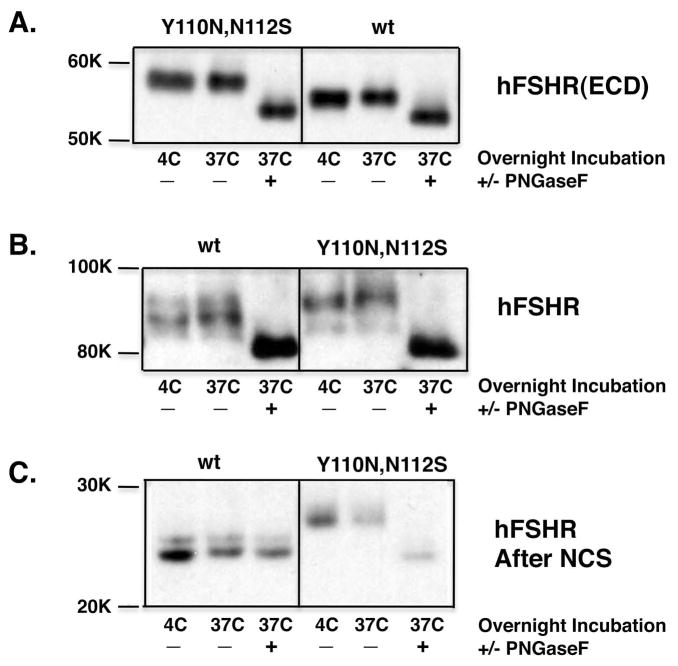
Therefore, we examined whether mutation of Tyr110 was in fact disrupting dimerization mediated by the hFSHR ECD. To address this, we engineered the Y110A mutation in the context of the membrane-anchored hFSHR ECD fusion proteins. As shown in Figure 5B, the BRET saturation curve for the ECD fusion proteins containing the Y110A mutation was indistinguishable from that generated by the wt ECD fusion proteins. To examine this issue further, we also engineered an hFSHR mutant that introduced a consensus site for N-linked glycosylation at codon 110, reasoning that the addition of a large, bulky carbohydrate moiety at this position would undoubtedly disrupt dimerization if it were occurring at that interface. The Y110N,N112S mutation was made in the context of both the full-length hFSHR and the membrane-anchored hFSHR ECD. We first documented that N-linked carbohydrate was in fact attached to the introduced Y110N site. As shown in Figure 6A, the molecular mass of the hFSHR ECD containing the Y110N,N112S mutation is greater than that of the wt ECD. Both the wt and mutant ECDs were converted to a protein with the same decreased molecular mass after treatment with PNGaseF, a glycosidase that cleaves all N-linked carbohydrates. Figure 6B shows results of similar analyses with the full-length hFSHR. Because of the larger mass of the full-length receptor, the increase in molecular mass with the Y110N,N112S mutant, though detectable, is much less apparent. To improve our ability to detect a difference in molecular mass upon introduction of the Y110N,N112S mutation in the full-length hFSHR, we treated both wt and mutant full-length hFSHRs with N-chlorosuccinimide, a chemical reagent that cleaves proteins after tryptophan residues, prior to resolving the samples on the SDS gels [33]. From the sequence of the hFSHR, we had determined that this would generate an N-terminal fragment of the receptor (detectable on the Western blots due to the myc tag at the N-terminus) terminating after Trp186 and thus contain the site for potential N-linked glycosylation we introduced at residue 110, but not contain any other endogenous consensus sites for N-linked glycosylation. As shown in Figure 6C, the N-terminal fragment released from the N-chlorosuccinimide-treated full-length hFSHR more clearly showed a greater molecular mass compared to the N-terminal fragment generated from the Y110N,N112S mutant. The mass of the Y110N,N112S fragment was reduced after treatment with PNGaseF, confirming that the difference in mass is attributable to N-linked carbohydrate. These data confirm that the Y110N,N112S mutant of the full-length hFSHR is indeed glycosylated at residue 110.
Figure 6. hFSHR(Y110N,N112S) causes the addition of N-linked carbohydrate to residue 110.
Panel A: HEK293T cells were transfected with pGFP2 plasmids encoding either wt myc-hFSHR(ECD)-CD8(TM) or myc-hFSHR(ECD-Y110,N112)-CD8(TM) in which a stop codon was inserted directly after the CD8 sequence. Cells were detergent solubilized and incubated overnight at the indicted temperature with or without PNGaseF. Samples were resolved on an 8% polyacrylamide gel and Western blots for the hFSHR were performed using an anti-Myc antibody. Data shown are representative of two independent experiments. Panel B: HEK293T cells were transfected with pcDNA3.1 plasmids encoding either myc-hFSHR(wt) or myc-hFSHR(Y110N,N112S). Cells were detergent solubilized and incubated overnight at the indicted temperature with or without PNGaseF. Samples were resolved on a 5% polyacrylamide gel and Western blots for the hFSHR were performed using an anti-Myc antibody. Data shown are representative of two independent experiments. Panel C: Samples were prepared as in Panel C except that after the overnight incubation with or without PNGaseF, the samples were incubated a further 2h in N-chlorosuccinimide (NCS). Samples were resolved on a 12% polyacrylamide gel and Western blots for the hFSHR were performed using an anti-myc antibody. Data shown are representative of two independent experiments.
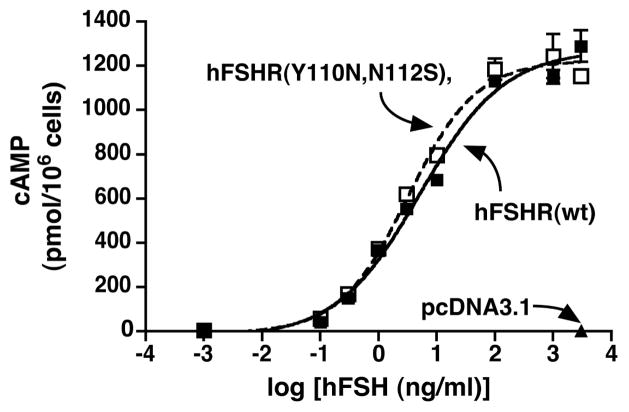
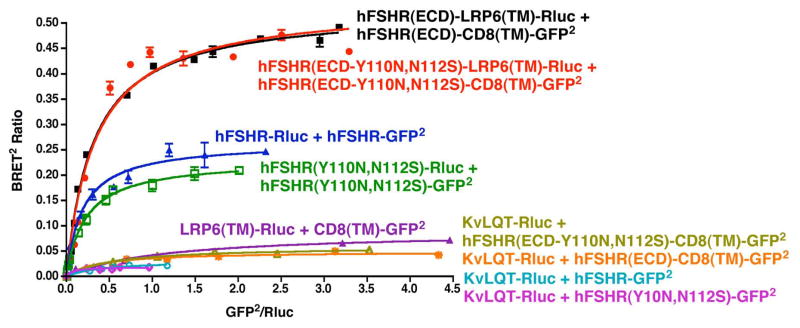
hFSHR(Y110,N112S) was expressed at the cell surface and bound FSH similarly to the wt hFSHR (data not shown). Furthermore, cells expressing the same cell surface densities of wt receptor or Y110N,N112S mutant exhibited similar dose response curves for FSH-stimulated cAMP production (Figure 7). BRET saturation curves showed that the Y110N,N112S mutation in the context of the membrane-anchored form of the hFSHR ECD had no detectable inhibitory effect on dimerization (Figure 8, compare red and black curves). In the context of the full-length hFSHR, a small reduction in the BRETmax was observed as a result of the Y110N,N112S mutation (Figure 8, compare blue and green curves). However, over three similar experiments, there was no difference in the calculated BRET50 values between the wt and Y110,N112S versions of the full-length hFSHR. Taken altogether, the data presented in Figure 6–8 demonstrate that the introduction of N-linked carbohydrate at residue 110 of the hFSHR does not adversely affect the cell surface expression, binding activity, or signaling properties of the full-length FSHR. Importantly, the glycan on residue 110 does not inhibit dimerization of the hFSHR in the context of either the full-length receptor or a membrane-anchored form of the ECD.
Figure 7. Insertion of a glycan wedge at residue 110 of the full-length hFSHR does not impair its binding or signaling properties.
HEK293 cells were transfected with HA-hFSHR(wt), HA-hFSHR(Y110N,N112S), or empty vector pcDNA3.1 as indicated. In the experiment shown, the cell surface densities of wt and mutant hFSHR were similar as indicated by anti-HA flow cytometry results of 2299 and 2220 RLU, respectively and maximal 125I-FSH binding of 6.0 and 5.8 ng/106 cells, respectively. Intracellular cAMP levels were measured in response to increasing concentrations of FSH as shown. Data shown are the mean ± SEM of triplicate determinations within one experiment representative of three independent experiments.
Figure 8. Insertion of a glycan wedge at residue 110 does not impair dimerization of the full-length hFSHR or the hFSHR ECD.
HEK293T cells were transfected with the indicated color matched pairs of fusion proteins. The Rluc fusion proteins were held fixed (and were adjusted so that their expression in the absence of the GFP2 fusion proteins was the same for all the constructs) while the concentrations of GFP2 fusion proteins were increased. Data shown are the corrected BRET signals as a function of GFP2/Rluc expression. The data shown are representative of three independent experiments.
4. Discussion
Using BRET we show that constitutively formed hFSHR dimers can be detected in living cells. BRET studies have similarly demonstrated constitutive dimerization of the hLHR [17, 34] and hTSHR in live cells [34]. We did not observe an effect of FSH on hFSHR dimerization as measured by BRET in intact cells, again consistent with results seen with the hLHR and hTSHR [17, 34]. It could be argued, however, that a large BRET signal arising from intracellular dimerized receptor could obscure a change in BRET signal arising from hormone-occupied cell surface receptor. However, recent studies with the hLHR utilized purified plasma membranes to demonstrate a lack of effect of agonist on BRET2 ratios [17]. Using constitutively active and signaling inactive mutants, it was further shown that there was a lack of correlation between the propensity for homodimerization and the state of activation of the hLHR [17]. Taken altogether, we conclude that the glycoprotein hormone receptors form dimers under basal conditions and that dimer formation or stability does not appear to be influenced by receptor activation.
Many GPCRs have now been shown to form dimers constitutively (see refs. [10, 35] for reviews). Although it has been assumed that the dimers form early in the biosynthetic pathway and are transported to the plasma membrane already self-associated, this has only been experimentally verified for a few GPCRs [17, 36–40]. Therefore, this was directly examined in the present study. Subfractionation studies presented herein demonstrate that hFSHR dimerization is in fact initiated in the ER because BRET was detected in fractions enriched in ER as well as in plasma membranes. In addition, dimeric and oligomeric forms of the hFSHR were evident on Western blots run on the same ER and plasma membrane fractions. The detection of hFSHR dimers early in the biosynthetic pathway suggests that receptor self-association is most likely an obligate step prior to the transport of the hFSHR to the plasma membrane [11, 41, 42]. Interestingly, monomeric forms of the hFSHR were also visible on Western blots of ER and plasma membrane fractions. These observations may reflect an equilibrium between monomeric and self-associated forms of the hFSHR and/or SDS-mediated dissociation of hFSHR self-associated complexes.
Although studies have shown that the β2-adrenergic receptor and rhodopsin each are capable of stimulating G protein when expressed as monomers [43, 44], these observations do not preclude the prevailing view that GPCRs most likely function as dimers [7–10, 45]. While the stoichiometry of G proteins and GPCRs is not firmly established, there are some studies that suggest that a given GPCR dimer engages only a single heterotrimeric G protein [46, 47]. Whether this can be extrapolated to other GPCRs is not yet certain. One of the functional consequences of a GPCR self-associating into a dimeric unit is that this is often associated with negative cooperativity with respect to agonist binding [22]. This was observed for the hFSHR, where we have shown that the addition of unlabeled FSH increased the rate of dissociation of prebound labeled FSH. These observations suggest that the binding of the hormone to one receptor protomer allosterically promotes the dissociation of hormone bound to the other protomer within the hFSHR dimer.
The glycoprotein hormone receptors are unique among Family A GPCRs in that, in addition to the prototypical serpentine region, they contain relatively large hormone-binding extracellular domains. In this respect, they resemble the structural architecture of Family C GPCRs, which also possess large extracellular domains. As discussed above, crystal structures of the hFSHRHB-FSH complex indicated that it formed dimers through the receptor portion of the complex [2, 6]. Because the structure of the hinge region of the hFSHR (or other glycoprotein hormone receptors) has not been determined, it is difficult to accurately predict the orientation of the hFSHRHB-FSH complex relative to the serpentine region of the receptor. As originally proposed by Fan and Hendrickson [2], the dimerized ECD regions of the hFSHR place the serpentine portion of each protomer relatively far apart. However, in light of the large amount of data suggesting dimerization of GPCRs through the serpentine region (as recently discussed in ref. [11]), they subsequently proposed alternative models of the hFSHR that would accommodate their data indicating dimerization through the ECD as well as the prediction that the serpentine region of the receptor would also most likely self-associate [6]. Indeed, our studies show that the hFSHR serpentine region, expressed without the ECD, specifically dimerizes. Even among those GPCRs without sizable extracellular domains, there is little consensus regarding which TMs within the serpentine domain mediate dimerization. This difficulty may reflect the fact that many GPCR dimers further self-associate into larger scale oligomeric structures with multiple dynamic contact points [11, 31, 48, 49]. However, most methods used to detect GPCR dimerization, including a two component resonance energy transfer technique as used here, do not permit the distinction between a receptor dimer and higher ordered oligomer. Therefore, if, as has been suggested [11, 48, 49], the TM regions mediating dimerization differ from those mediating oligomerization, one can envision how disparate results between studies might be obtained.
Based on reports on a few GPCRs where specific TM residues have been suggested to mediate dimerization [30–32], we introduced comparable substitutions within the context of the full-length hFSHR. These mutations did not adversely affect hFSHR dimerization. Nor was an effect observed with substitution of hFSHR TM residues that correspond to amino acids predicted to be involved in dimerization of the hLHR serpentine region as predicted from computational studies [29]. It is possible that even if the serpentine region alone mediated hFSHR dimerization, the residues we mutated in a given construct may not have been sufficient to disrupt self-association. It is also possible that additional interactions between the ECDs maintained dimerization of the full-length hFSHR mutants even if interactions between the serpentine domains were disrupted. Indeed, we show that the ECD of the hFSHR, when expressed in a membrane-anchored form, specifically self-associates. Our data therefore suggest that both the serpentine domain as well as the ECD of the hFSHR participate in receptor self-association. Because BRET detects interactions of proteins that are within 100 Å of each other, the participation of both the ECD and serpentine regions in hFSHR dimerization does not imply that the distances between these two domains are necessarily the same.
The crystal structures of dimeric hFSHRHB-FSH complexes suggest that Tyr110 of the hFSHR participates in a number of hydrophobic interactions at the predicted dimer interface. In our studies, mutation of Tyr110 in the full-length hFSHR was without effect on receptor dimerization when introduced on its own or in conjunction with TM mutations within the serpentine domain. Furthermore, mutation of Tyr110 within the context of the membrane-anchored form of the ECD was without effect. To more rigorously determine if the ECD dimer interface involved Tyr110, we introduced a consensus sequence for N-linked glycosylation with the goal of introducing a large, bulky carbohydrate moiety onto residue 110, following a strategy employed to disrupt dimerization of the GABAB receptor ECD [50]. Although this manipulation did result in glycosylation of residue 110 (now an Asn), no disruption of dimerization was observed for either the full-length hFSH or the membrane-anchored hFSHR ECD. The full-length hFSHR containing the glycan wedge on residue 110 was also shown to be expressed on the cell surface and to have normal hormone binding and signaling properties. While our data appear to conflict with those of Fan and Hendrickson regarding the ECD interface involved in dimerization, our findings do not question the structure of the monomeric form of the hFSHRHB-FSH complex determined [2, 6].
How does one reconcile our data with the structural studies performed on the soluble hFSHRHB-FSH complex? There are several possibilities to consider. Notably, the structural study utilized a soluble form of the hFSHRHB that was co-expressed in insect cells with FSH to yield a secreted FSH-FSHRHB complex. In our studies, the hFSHR was synthesized in the absence of hormone, either in the context of the full-length hFSHR or an artificially membrane-anchored form. Firstly, it is conceivable that the soluble form of the FSH- hFSHRHB complex may have been free to assume an orientation in a dimeric complex that would not be observed when the ECD is constrained by being tethered to the serpentine domain of the hFSHR or an artificial membrane anchor. Secondly, the soluble hormone-occupied hFSHRHB lacked the hinge region of the ECD. While the crystal structure of hFSHRHB is known [2] and the structure of the serpentine domain of the hFSHR can be modeled on the crystal structures of rhodopsin, opsin, or the β2-adrenergic receptor [51–56], there are no structures available yet for the hinge region of a glycoprotein hormone receptor. The presence of the hinge region in the full-length hFSHR and hFSHR ECD may orient the hFSHRHB differently than the soluble hFSHRHB lacking this region. Thirdly, it is possible that while both the hormone-unoccupied form and the hormone-bound form of the ECD may self-associate, the dimer interface may differ between the free and hormone-occupied forms of the receptor. Therefore, while we can conclude that Tyr110 does not appear to be involved in the dimerization of the hFSHR in the basal state, we can not rule out the possibility that hormone binding stabilizes a conformation of the receptor that places Tyr110 at the ECD dimer interface. Along these lines, activation of metabotropic glutamate and GABAB receptors, Family C receptors with large extracellular domains, are thought to be accompanied by intersubunit rearrangements occurring within the context of the GPCR dimer [50, 57]. Interestingly, the addition of carbohydrate to residue 110 did not prevent the FSH-stimulated activation of the hFSHR and subsequent signaling. However, since it has been shown for other GPCRs that monomeric forms of the receptor are capable of stimulating Gs in response to agonist binding [43, 44], it remains possible that the inserted carbohydrate may affect dimerization of the hormone-occupied hFSHR without affecting its signaling capacity. Therefore, other strategies are being considered to further address the role, if any, of Tyr110 in the dimerization interface of the hormone-occupied hFSHR.
In summary, our studies demonstrate obligate and constitutive dimerization of the hFSHR in living cells that involves associations between both the serpentine domains and ECDs. Furthermore, dimerization of the unoccupied hFSHR does not involve Tyr110, as had been suggested for dimerization of the soluble hFSHRHB-FSH complex [2]. Clearly, further studies are warranted to more closely examine the hFSHR dimer interfaces and how these may be altered during receptor activation.
Acknowledgments
We thank Dr. Qing Fan (Columbia University) for helpful discussions. We also thank Ares Advanced Technology (Ares-Serono Group), Dr. Guizhong Liu (Mount Sinai School of Medicine), Dr. Michel Bouvier (University of Montreal) and Dr. Alvin Shrier (McGill University) for their generous gifts of constructs used in the present studies.
Role of the funding source
These studies were supported by NIH grants DK068614 and HD22196 (D.L.S.) and from CIHR (T.E.H.). T.E.H. is a Chercheur National of the Fonds de la Recherche en Santé du Québec. The funding sources did not participate in the study design; in the collection, analysis, and interpretation of the data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Disclosure Statement
The authors have no conflicts of interest to report.
Author Contributions
T.E.H. and D.L.S. designed research; R.G., X.W., X.F., and M.Z performed research; R.G., X.W., X.F., M.Z., T.E.F., and D.L.S. analyzed data; and T.E.H. and D.L.S. wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Davis D, Liu X, Segaloff DL. Mol Endocrinol. 1995;9:159–170. doi: 10.1210/mend.9.2.7776966. [DOI] [PubMed] [Google Scholar]
- 2.Fan QR, Hendrickson WA. Nature. 2005;433:269–277. doi: 10.1038/nature03206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruysters M, Verhoef-Post M, Themmen AP. J Biol Chem. 2008;283:25821–25828. doi: 10.1074/jbc.M804395200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agrawal G, Dighe RR. J Biol Chem. 2009;284:2636–2647. doi: 10.1074/jbc.M808199200. [DOI] [PubMed] [Google Scholar]
- 5.Mizutori Y, Chen CR, McLachlan SM, Rapoport B. Mol Endocrinol. 2008;22:1171–1182. doi: 10.1210/me.2007-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan QR, Hendrickson WA. Mol Cell Endocrinol. 2007;260–262:73–82. doi: 10.1016/j.mce.2005.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bulenger S, Marullo S, Bouvier M. Trends Pharmacol Sci. 2005;26:131–137. doi: 10.1016/j.tips.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Milligan G. Biochim Biophys Acta. 2007;1768:825–835. doi: 10.1016/j.bbamem.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 9.Dalrymple MB, Pfleger KD, Eidne KA. Pharmacol Ther. 2008;118:359–371. doi: 10.1016/j.pharmthera.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Hebert TE, Gales C, Rebois RV. Cell Biochem Biophys. 2006;45:85–109. doi: 10.1385/CBB:45:1:85. [DOI] [PubMed] [Google Scholar]
- 11.Milligan G. Br J Pharmacol. 2008;153(Suppl 1):S216–S229. doi: 10.1038/sj.bjp.0707490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szidonya L, Cserzo M, Hunyady L. J Endocrinol. 2008;196:435–453. doi: 10.1677/JOE-07-0573. [DOI] [PubMed] [Google Scholar]
- 13.Thomas RM, Nechamen CA, Mazurkiewicz JE, Muda M, Palmer S, Dias JA. Endocrinology. 2007;148:1987–1995. doi: 10.1210/en.2006-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rebois RV, Robitaille M, Gales C, Dupre DJ, Baragli A, Trieu P, Ethier N, Bouvier M, Hebert TE. J Cell Sci. 2006;119:2807–2818. doi: 10.1242/jcs.03021. [DOI] [PubMed] [Google Scholar]
- 15.Ascoli M, Puett D. Proc Natl Acad Sci (USA) 1978;75:99–102. doi: 10.1073/pnas.75.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang M, Mizrachi D, Fanelli F, Segaloff DL. J Biol Chem. 2005;280:26169–26176. doi: 10.1074/jbc.M502102200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan R, Feng X, Wu X, Zhang M, Zhang X, Hebert TE, Segaloff DL. J Biol Chem. 2009;284:7483–7494. doi: 10.1074/jbc.M809150200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang M, Feng X, Guan R, Hébert TE, Segaloff DL. Cell Signal. 2009 doi: 10.1016/j.cellsig.2009.07.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang M, Tao YX, Ryan GL, Feng X, Fanelli F, Segaloff DL. J Biol Chem. 2007;282:25527–25539. doi: 10.1074/jbc.M703500200. [DOI] [PubMed] [Google Scholar]
- 20.Kenworthy AK, Edidin M. J Cell Biol. 1998;142:69–84. doi: 10.1083/jcb.142.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Meyts P. J Supramol Struct. 1976;4:241–258. doi: 10.1002/jss.400040211. [DOI] [PubMed] [Google Scholar]
- 22.Springael JY, Urizar E, Costagliola S, Vassart G, Parmentier M. Pharmacol Ther. 2007;115:410–418. doi: 10.1016/j.pharmthera.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Hipkin RW, Sanchez-Yague J, Ascoli M. Mol Endocrinol. 1992;6:2210–2218. doi: 10.1210/mend.6.12.1491699. [DOI] [PubMed] [Google Scholar]
- 24.Mercier JF, Salahpour A, Angers S, Breit A, Bouvier M. J Biol Chem. 2002;277:44925–44931. doi: 10.1074/jbc.M205767200. [DOI] [PubMed] [Google Scholar]
- 25.Milligan G, Bouvier M. FEBS J. 2005;272:2914–2925. doi: 10.1111/j.1742-4658.2005.04731.x. [DOI] [PubMed] [Google Scholar]
- 26.Pfleger KD, Eidne KA. Nat Methods. 2006;3:165–174. doi: 10.1038/nmeth841. [DOI] [PubMed] [Google Scholar]
- 27.Liu G, Bafico A, Harris VK, Aaronson SA. Mol Cell Biol. 2003;23:5825–5835. doi: 10.1128/MCB.23.16.5825-5835.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osuga Y, Kudo M, Kaipia A, Kobilka B, Hsueh AJ. Mol Endocrinol. 1997;11:1659–1668. doi: 10.1210/mend.11.11.0005. [DOI] [PubMed] [Google Scholar]
- 29.Fanelli F. Mol Cell Endocrinol. 2007;260–262:59–64. doi: 10.1016/j.mce.2005.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hernanz-Falcon P, Rodriguez-Frade JM, Serrano A, Juan D, del Sol A, Soriano SF, Roncal F, Gomez L, Valencia A, Martinez AC, Mellado M. Nat Immunol. 2004;5:216–223. doi: 10.1038/ni1027. [DOI] [PubMed] [Google Scholar]
- 31.Lopez-Gimenez JF, Canals M, Pediani JD, Milligan G. Mol Pharmacol. 2007;71:1015–1029. doi: 10.1124/mol.106.033035. [DOI] [PubMed] [Google Scholar]
- 32.Guo W, Shi L, Filizola M, Weinstein H, Javitch JA. Proc Natl Acad Sci (U S A) 2005;102:17495–17500. doi: 10.1073/pnas.0508950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis DP, Rozell TG, Liu X, Segaloff DL. Mol Endocrinol. 1997;11:550–562. doi: 10.1210/mend.11.5.9927. [DOI] [PubMed] [Google Scholar]
- 34.Urizar E, Montanelli L, Loy T, Bonomi M, Swillens S, Gales C, Bouvier M, Smits G, Vassart G, Costagliola S. EMBO J. 2005;24:1954–1964. doi: 10.1038/sj.emboj.7600686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bai M. Cell Signal. 2004;16:175–186. doi: 10.1016/s0898-6568(03)00128-1. [DOI] [PubMed] [Google Scholar]
- 36.Terrillon S, Durroux T, Mouillac B, Breit A, Ayoub MA, Taulan M, Jockers R, Barberis C, Bouvier M. Mol Endocrinol. 2003;17:677–691. doi: 10.1210/me.2002-0222. [DOI] [PubMed] [Google Scholar]
- 37.Salahpour A, Angers S, Mercier JF, Lagace M, Marullo S, Bouvier M. J Biol Chem. 2004;279:33390–33397. doi: 10.1074/jbc.M403363200. [DOI] [PubMed] [Google Scholar]
- 38.Herrick-Davis K, Weaver BA, Grinde E, Mazurkiewicz JE. J Biol Chem. 2006;281:27109–27116. doi: 10.1074/jbc.M604390200. [DOI] [PubMed] [Google Scholar]
- 39.Issafras H, Angers S, Bulenger S, Blanpain C, Parmentier M, Labbe-Jullie C, Bouvier M, Marullo S. J Biol Chem. 2002;277:34666–34673. doi: 10.1074/jbc.M202386200. [DOI] [PubMed] [Google Scholar]
- 40.Overton MC, Blumer KJ. J Biol Chem. 2002;277:41463–41472. doi: 10.1074/jbc.M205368200. [DOI] [PubMed] [Google Scholar]
- 41.Terrillon S, Bouvier M. EMBO Reports. 2004;5:30–34. doi: 10.1038/sj.embor.7400052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dupre DJ, Hebert TE. Cell Signal. 2006;18:1549–1559. doi: 10.1016/j.cellsig.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 43.Whorton MR, Bokoch MP, Rasmussen SG, Huang B, Zare RN, Kobilka B, Sunahara RK. Proc Natl Acad Sci (U S A) 2007;104:7682–7687. doi: 10.1073/pnas.0611448104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bayburt TH, Leitz AJ, Xie G, Oprian DD, Sligar SG. J Biol Chem. 2007;282:14875–14881. doi: 10.1074/jbc.M701433200. [DOI] [PubMed] [Google Scholar]
- 45.Ferre S, Baler R, Bouvier M, Caron MG, Devi LA, Durroux T, Fuxe K, George SR, Javitch JA, Lohse MJ, Mackie K, Milligan G, Pfleger KD, Pin JP, Volkow ND, Waldhoer M, Woods AS, Franco R. Nat Chem Biol. 2009;5:131–134. doi: 10.1038/nchembio0309-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baneres JL, Parello J. J Mol Biol. 2003;329:815–829. doi: 10.1016/s0022-2836(03)00439-x. [DOI] [PubMed] [Google Scholar]
- 47.Damian M, Martin A, Mesnier D, Pin JP, Baneres JL. EMBO J. 2006;25:5693–5702. doi: 10.1038/sj.emboj.7601449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fotiadis D, Liang Y, Filipek S, Saperstein DA, Engel A, Palczewski K. Nature. 2003;421:127–128. doi: 10.1038/421127a. [DOI] [PubMed] [Google Scholar]
- 49.Guo W, Urizar E, Kralikova M, Mobarec JC, Shi L, Filizola M, Javitch JA. EMBO J. 2008;27:2293–2304. doi: 10.1038/emboj.2008.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rondard P, Huang S, Monnier C, Tu H, Blanchard B, Oueslati N, Malhaire F, Li Y, Trinquet E, Labesse G, Pin JP, Liu J. EMBO J. 2008;27:1321–1332. doi: 10.1038/emboj.2008.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 52.Park JH, Scheerer P, Hofmann KP, Choe HW, Ernst OP. Nature. 2008;454:183–187. doi: 10.1038/nature07063. [DOI] [PubMed] [Google Scholar]
- 53.Scheerer P, Park JH, Hildebrand PW, Kim YJ, Krauss N, Choe HW, Hofmann KP, Ernst OP. Nature. 2008;455:497–502. doi: 10.1038/nature07330. [DOI] [PubMed] [Google Scholar]
- 54.Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, Stevens RC. Science. 2007;318:1258–1265. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosenbaum DM, Cherezov V, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, Choi HJ, Yao XJ, Weis WI, Stevens RC, Kobilka BK. Science. 2007;318:1266–1273. doi: 10.1126/science.1150609. [DOI] [PubMed] [Google Scholar]
- 56.Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, Burghammer M, Ratnala VR, Sanishvili R, Fischetti RF, Schertler GF, Weis WI, Kobilka BK. Nature. 2007;450:383–387. doi: 10.1038/nature06325. [DOI] [PubMed] [Google Scholar]
- 57.Brock C, Oueslati N, Soler S, Boudier L, Rondard P, Pin JP. J Biol Chem. 2007;282:33000–33008. doi: 10.1074/jbc.M702542200. [DOI] [PubMed] [Google Scholar]