Abstract
Malignant astrocytomas are infiltrative and aggressive brain tumors. Conventional forms of therapy have not been effective in controlling this incurable disease. Recent advances in understanding the molecular biology of these tumors have revealed potential mechanisms by which astrocytoma cells undergo tumor initiation, progression and maintenance, as well as possible avenues for targeted therapeutics. Studies on the role of neural stem cells as cells of origin and tumor-propagating cells have also greatly increased our understanding of the biology and clinical behavior of these tumors. An integrated view of the genetics, signal transduction and cell biology of astrocytomas, as well as clinical data from patients, will provide a more useful approach in designing novel therapies for this devastating disease.
BACKGROUND
Gliomas are the most common primary malignancies in the central nervous system (CNS). These tumors exhibit histologic resemblance to glial cells, and are classified based on the predominant tumor cell type(s). Astrocytomas, which are composed of cells that by many morphological and protein expression criteria resemble astrocytes, comprise the majority of these tumors. Astrocytomas are further classified into varying degrees of malignancy---World Health Organization (WHO) grades I to IV (1). Grade I tumors are benign whereas grade II tumors are low-grade malignancies that undergo early diffuse infiltration. Both Grade III (anaplastic astrocytoma) and Grade IV (glioblastoma multiforme) tumors are highly malignant and invasive tumors that are lethal within months to years (2, 3). Malignant astrocytic tumors exhibit cellular heterogeneity, diffuse infiltration and widespread invasion throughout the brain, precluding complete surgical resection. Glioblastoma multiforme (GBM) is resistant to current radiation and chemotherapy protocols, leading to dismal survival outcomes that in contrast to other forms of cancer, have not improved dramatically over the past several decades (4, 5).
It has been almost five years since the landmark study that established the use of temozolomide (TMZ) for concomitant and adjuvant chemotherapy alongside postoperative radiotherapy for GBM patients (6). A more recent study confirmed the modest benefits of this regimen in a 5-year follow-up, and TMZ is now considered a standard treatment for GBM (7). Despite intensive therapeutic strategies involving surgery, radiation and chemotherapy, most patients undergo tumor progression or recurrence. Median survival remains at only 15 months and most, if not all, face certain death (6, 7).
This state of affairs places a premium on novel, more directed therapeutic approaches for this complex, aggressive disease. Recent advances have paved the way towards a better understanding of the biology and clinical behavior of these tumors. In particular, it has been suggested that self-renewing stem cells and cancer cells that possess stem cell properties may be directly involved in brain tumor development (8–11). Genetic lesions in GBMs have also been extensively characterized, and provided a better picture of the genomic changes in human tumors (12, 13). Assessment of all these findings will have direct implications in the development of novel therapies against GBMs.
Neural stem cells in the CNS
Neural stem cells are lifelong self-renewing cells in the CNS that exhibit multipotent differentiation into all neural cell types in the brain, including neurons, astrocytes and oligodendrocytes (14). In the adult mammalian brain, including humans, neural stem cells have been identified in the dentate gyrus and the subventricular zone (SVZ), an extensive germinal layer adjacent to the ependyma that concentrates stem cells on the walls of the lateral ventricles (15). These relatively quiescent neural stem cells periodically give rise to lineage-restricted progenitor cells that undergo limited mitoses before differentiating into mature cells (16). While these cells are purported to be important in olfaction and learning and memory processes in rodents, their role in humans is not as well understood (17).
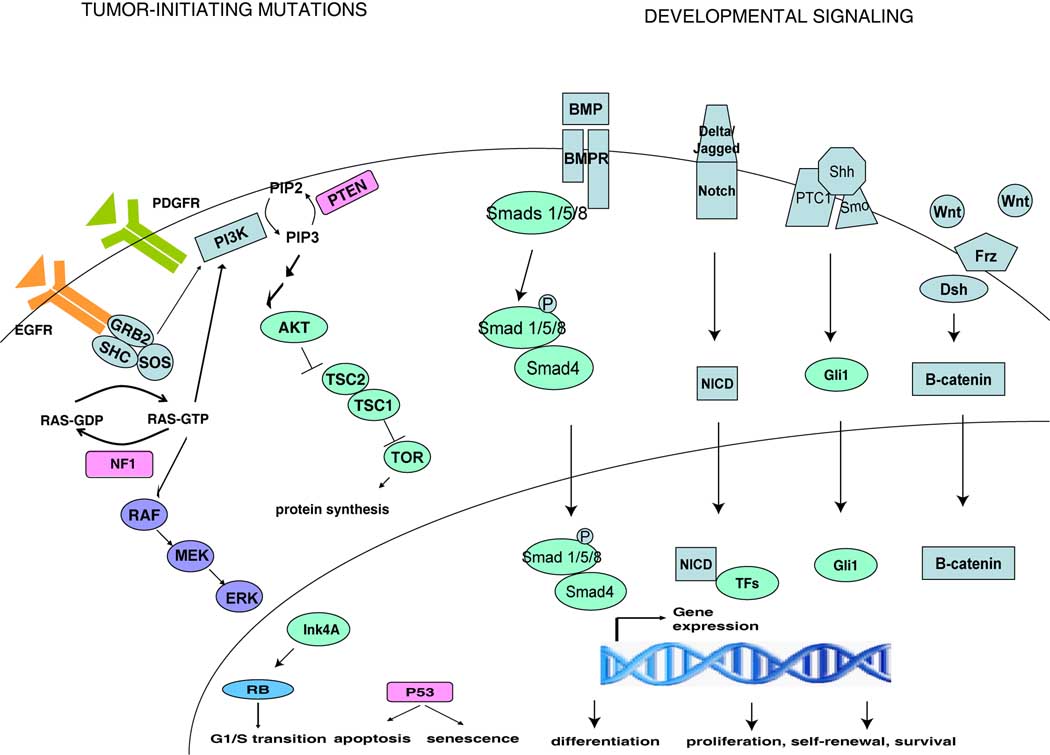
Neural stem cells and their progeny are tightly regulated by a variety of intrinsic and extrinsic factors (see Figure 1). In particular, growth factors signaling via receptor tyrosine kinases (RTK) are important regulators of neural stem cell activity. Ligand activation of the platelet-derived growth factor receptor (PDGFR) promotes self-renewal and differentiation of quiescent neural stem cells, whereas epidermal growth factor receptor (EGFR) activation increases the proliferation of transit amplifying cells (18, 19). Stimulation of these growth factor receptors activates downstream effectors, such as Ras and phosphatidylinositide-3-kinase (PI3K) pathways, which are negatively regulated by the tumor suppressors Nf1 and Pten, respectively. The neurofibromatosis tumor suppressor gene, NF1, encodes the protein neurofibromin, which is a functional RasGAP and responsible for restraining Ras activity in a variety of cellular contexts (20). Pten (phosphatase and tensin homologue on chromosome 10) reverses the PI3K-mediated activation of Akt and its downstream pro-growth and survival signals. Nf1 and Pten have both been shown to function as negative regulators of neural stem cell function. Nf1 deficiency was shown to promote neural stem cell proliferation and survival (21, 22) whereas Pten loss increases neural stem cell proliferation and self-renewal (23, 24). Other tumor suppressors that inhibit unrestrained expansion include the transcription factor p53 and cyclin-dependent kinase inhibitor Ink4A. Loss of p53 increases proliferation in the SVZ neural stem/progenitor cells and provides a growth advantage compared to wild type cells (25, 26), while Ink4A expression correlates with a decline in progenitor function in vivo (27).
Figure 1. Stem Cell-Related Signaling Pathways in Malignant Astrocytomas.
Simplified schematic of genetic alterations and perturbed developmental pathways found in astrocytomas. Mutations in astrocytomas frequently involve cell cycle and apoptosis regulation and growth factor receptor signaling. Receptor tyrosine kinases such as epidermal growth factor receptor (EGFR) and platelet-derived growth factor receptor (PDGFR) are frequently overactivated in malignant astrocytomas, stimulating its downstream effectors, such as Ras and phosphatidylinositide-3-kinase (PI3K). Nf1 and Pten are tumor suppressors that negatively regulate Ras and PI3K signaling, respectively. Other tumor suppressors such as p53, retinoblastoma (Rb) and Ink4A have also been implicated. These pathways have been shown to regulate neural stem cell function. Bone morphogenetic proteins (BMP), Notch, Sonic hedgehog (Shh) and Wnt are developmentally-regulated signaling pathways that are also active in normal neural stem cells but are dysregulated in malignant astrocytomas.
Signaling pathways active during embryonic neural development have also been found to regulate neural stem cells. Classical developmental pathways such as Notch, bone morphogenetic protein (BMP), Sonic hedgehog, and Wnt signaling have been shown to play important roles in maintaining the neurogenic niche (16). Notch signaling via its single-pass transmembrane receptors, typically involved in mediating cell-cell communication, was shown to promote neural stem cell expansion while inhibiting differentiation (28). Bmi-1, a member of the Polycomb family of transcriptional repressors, is required for the self-renewal, but not survival or differentiation, of neural stem cells (29). In contrast BMPs, which signal through the Smad proteins, stimulate differentiation of neural stem cells into the astroglial lineage while inhibiting neuronal differentiation (30). Sonic hedgehog signaling has also been shown to be required for the formation of neural stem cells (31, 32). Exogenous administration of Wnt ligands likewise positively regulates the proliferation and survival of neural stem cells (33, 34).
Cell of Origin of Malignant Astrocytomas
The adult brain was once thought of as a post-mitotic organ. On this basis, tumor development, including that of astrocytomas, was explained to arise through a process of dedifferentiation of mature glia. However, recent advances in stem cell biology and genetically-engineered animal models suggest that dividing cells may give rise to these tumors (35).
Gene mutations found in human tumor genomes have provided important clues to the underlying mechanisms involved in astrocytomas. Genetic alterations in astrocytoma frequently target pathways involved in cell cycle and apoptosis regulation and growth factor receptor signaling. The frequency of certain of these mutations has been further underscored by a recent large-scale sequencing effort which revealed that in human GBMs, somatic mutations were most commonly found in the cancer-associated genes: P53, PTEN, NF1, EGFR, ERBB2 and RB1 (12).
Combinations of oncogene and tumor suppressor mutations sometimes observed in human astrocytomas, as well as viral oncogenes that cause cancer in animals, have been used in the mouse to model astrocytoma development. Overexpression of active forms of Ras, Akt, EGFR, PDGF, v-src or polyoma T-antigen, some in combination with Ink4A/Arf or Pten deletions, lead to astrocytoma formation (36–38). Mutations were induced in embryonic and early postnatal cells and these mice develop tumors with varying levels of penetrance. On the other hand, conditional knockout mice bearing inactivating mutations in human astrocytoma-relevant tumor suppressors Nf1, p53 and/or Pten develop astrocytomas with 100% penetrance (39, 40). Retrospective analysis of these mice demonstrated the earliest lesions to be in the SVZ, suggesting that these tumors may arise from neural stem cells. This hypothesis was directly tested by targeting tumor suppressor inactivating mutations in the adult neurogenic compartment (9). Studies using an inducible nestin-Cre recombinase transgene to direct cre-mediated recombination in neural stem cells and their progeny, or alternatively using stereotactic viral delivery of the cre-expressing adenovirus into the SVZ, showed that inactivation of tumor suppressors in neural precursor cells but not differentiated cell types leads to astrocytoma formation. This provided evidence that neural stem/progenitor cells can give rise to malignant astrocytomas initiated by mutations found in the majority of human GBM patients and that more differentiated cell types were less susceptible to malignant transformation (see Figure 2). Moreover, migration of tumor cells in these mouse models recapitulates the infiltrative phenotype and widespread invasion of tumor cells seen in patients. These studies further showed that cancer-initiating cells have the capacity for multi-lineage differentiation in situ, suggesting that differentiation therapy may be a viable option for blocking the growth of GBM cells (9).
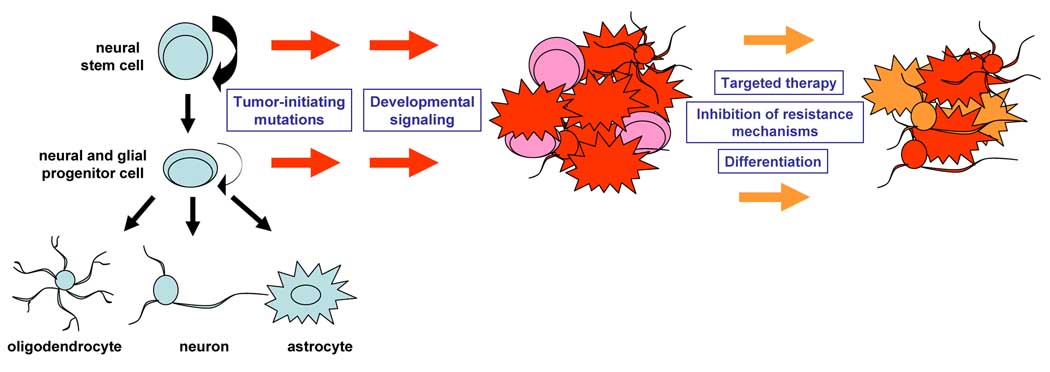
Figure 2. Neural Stem/Progenitor Origin and Stem-Like Cancer Cells in Malignant Astrocytomas and Potential Therapeutic Approaches.
Model of neural stem/progenitor origin posits that targeting of known tumor-initiating mutations in these self-renewing cells give rise to malignant astrocytomas. Activation of developmental signaling pathways may be secondary events during tumorigenesis. Stem-like cancer cells in astrocytomas may represent the more malignant subpopulation of cancer cells and provide a mechanism for therapeutic resistance. Potential avenues for therapeutic intervention may require combinations of targeted therapies against both stem-like and less tumorigenic cancer cells as well as inhibition of resistance mechanisms by targeting cancer stem cells. The stem/progenitor origin and the presence of stem-like cancer cells also paves the way for therapeutic avenues such as differentiation therapy in retarding the growth of malignant astrocytomas.
Stem-like Cancer Cells in Malignant Astrocytomas
Human malignant astrocytomas appear highly heterogeneous. This has suggested a hierarchy in the malignant potential of each of its varying cancer cell types. Indeed, various reports have suggested that there exists a rare population of brain tumor cells that are capable of initiating brain tumor formation in orthotopic transplantation models (10, 41). “Cancer stem cells” or “tumor-propagating cells” have been shown to exhibit resistance to radiation and chemotherapeutic drugs (11, 23). Mouse models have now supported these findings and further established that a precursor-product relationship between cancer stem cells and more differentiated cell types constitutes this heterogeneity (42, 43).
Pathways regulating normal stem cell self-renewal, proliferation, and survival may also be operative in stem-like cancer cells (8, 44), and reactivation of developmental mechanisms may be an important contributor to the aggressive phenotype of cancers such as GBM (45) (see Figure 1). Pten loss and Akt activation were shown to correlate with the aggressive phenotype of stem-like cancer cells (42). BMP receptors are expressed in a subset of human GBM cells and exogenous administration of one of its ligands, BMP4, has been shown to inhibit proliferation and promote differentiation of cancer stem cells (8). Inhibition of hedgehog signaling via cyclopamine or RNA interference was shown to be required for tumor growth and survival (46). Notch and Wnt pathway molecules are also expressed in GBM, and their activation may also contribute to tumorigenesis (47, 48).
These studies do not preclude the possibility, however, that other tumor cell populations may also be tumorigenic but not as aggressive as the “cancer stem cells” (49, 50). However, regardless of whether “cancer stem cells” are rare or common, it appears that tumorigenic cells exhibit properties that mirror those of normal stem cells, albeit in a dysregulated fashion. This raises the possibility that understanding the mechanisms by which these stem-like cancer cells behave may provide clues into slowing the growth of these intractable tumors.
CLINICAL-TRANSLATIONAL ADVANCES
The evolving study of the role that stem cells may play in cancer has impacted astrocytoma research. A neural stem cell origin of astrocytoma whereby self-renewing cells accumulate mutations throughout the lifetime of an individual is consistent with the prevalence of GBM in older patients. It also fits well with the clinically relevant heterogeneous and invasive phenotype seen in patients. If the majority of astrocytomas in humans arise from neural precursor cells, this suggests that there may be a persistent source of pre-tumorigenic cells from self-renewing mutant stem cells. Hence, present therapies may be inadequate in eradicating both tumor cells and primed transformed cells.
Stem-like cancer cells sitting at the top of the hierarchy of tumor cells presents new challenges as well. Do these cells have specific vulnerabilities in terms of essential signaling pathways? Since stem-like cancer cells are also known to usurp normal stem cell self-renewal pathways, are there mechanisms that may operate differently between cancer and normal stem cells? If such cells are eradicated, are less tumorigenic cells able to give rise to these more malignant cells? Are there markers, cell surface or otherwise, that can truly differentiate cancer stem cells from normal cells?
The use of combinations of targeted therapies against key non-overlapping pathways, along with inhibition of mechanisms of therapeutic resistance may provide some benefit (see Figure 2). This may potentially diminish pro-growth and survival signals important for tumor progression and maintenance and may target both stem-like and less tumorigenic cancer cells. Because RTKs are frequently over-activated in GBM, EGFR and PDGFR have been targeted using a variety of tyrosine kinase inhibitors, some of which are in various stages of clinical trials. Inhibitors of RTK downstream effectors, such as Ras, Raf, Akt, and TOR have also been developed (51–53). Whether these inhibitors target stem-like cancer cells needs to established, with some agents showing activity against GBM cancer stem cells (54).
These newer drugs are being tested for use as monotherapy or in combination with other existing therapies. Modest benefit has been reported for some drugs in a subset of patients but effects on survival of these targeted therapies are not clear (52, 55). This accentuates the highly redundant and inter-connected signal transduction mechanisms and genomic instability that are operative in astrocytoma cells. It also suggests that multiple essential non-overlapping pathways, including mechanisms for resistance, need to be addressed, especially since GBMs are highly resistant to all therapies once they recur (51). The reported resistance of stem-like cancer cells to chemotherapy and radiation may be secondary to activation of survival pathways and DNA damage repair, or expression of ATP-binding cassette transporters that can actively efflux xenobiotics from the cell (11, 42). This may partly account for the modest benefits found in the use of these newer drugs so far, and hence, may need to be addressed to achieve greater therapeutic effects.
Inhibition of resistance mechanisms may thus require specific targeting of stem-like cancer cells. Monoclonal antibodies targeting cancer stem cells in leukemias show reduced engraftment and tumor burden (56, 57), and can likewise be applied against astrocytoma cells. Strategies directed against cancer stem cells may also include inhibition of developmental signaling pathways, including notch and sonic hedgehog (46, 47). Another alternative and emerging concept is one of differentiation therapy. The stem/progenitor origin hypothesis, the presence of stem-like cancer cells in malignant astrocytomas, and the fact that some astrocytoma cells spontaneously undergo some form of differentiation, present the possibility that forced differentiation into more “mature”, thus less tumorigenic, cancer cell types may hold promise (Figure 2). Proof of principle for this concept has been provided in other cancers and brain tumors as well (58, 59). In astrocytomas, this was demonstrated by BMP-mediated differentiation of GBM cells, which retarded tumor growth both in vitro and in vivo (8, 60). This suggests that selective activation of pro-differentiation pathways may reduce the tumorigenicity of GBM cells. Meanwhile, high-throughput screens of known drugs, small molecules and novel compounds tested against stem-like and heterogeneous astrocytoma cells have also been undertaken (61). Finally, inter-patient variability in genetic make-up of astrocytomas suggests that treatment regimens tailored to patient molecular profiles may lead to greater therapeutic efficiency and decreased systemic toxicity.
Perspective
The integration of basic and clinical research has greatly increased our understanding of astrocytoma biology. The dismal survival of patients, however, is still the reality and more sophisticated tools are needed to shed light on the intricacies of astrocytoma formation. The interface between human cancer and genetic mouse models will need to be more fully exploited in order to rapidly translate basic science knowledge into practical clinical applications. Moreover, physiologically relevant mouse models can serve as preclinical platforms to rapidly screen novel therapies whereas clinical data on patient response to therapy and its determinants can aid in understanding the behavior of these tumors. A holistic view of the many pathologic processes underlying astrocytomas will hopefully provide unique insights that can translate into effective therapeutic approaches for this incurable disease.
Acknowledgements
We thank Renee McKay for critical reading of this manuscript. This work was supported in part by a Children’s Tumor Foundation Young Investigator Award to S.R.A.L. and NIH (5P50NS052606), US Department of Defense (W81XWH-05-1-0265), and American Cancer Society (RP0408401) grants to L.F.P. L.F.P. is an American Cancer Society Research Professor.
REFERENCES
- 1.Kleihues P, Louis DN, Scheithauer BW, et al. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61:215–225. doi: 10.1093/jnen/61.3.215. discussion 26-9. [DOI] [PubMed] [Google Scholar]
- 2.Furnari FB, Fenton T, Bachoo RM, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 3.Louis DN. Molecular pathology of malignant gliomas. Annu Rev Pathol. 2006;1:97–117. doi: 10.1146/annurev.pathol.1.110304.100043. [DOI] [PubMed] [Google Scholar]
- 4.Zhu Y, Parada LF. The molecular and genetic basis of neurological tumours. Nat Rev Cancer. 2002;2:616–626. doi: 10.1038/nrc866. [DOI] [PubMed] [Google Scholar]
- 5.Maher EA, Furnari FB, Bachoo RM, et al. Malignant glioma: genetics and biology of a grave matter. Genes Dev. 2001;15:1311–1333. doi: 10.1101/gad.891601. [DOI] [PubMed] [Google Scholar]
- 6.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 7.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 8.Piccirillo SG, Reynolds BA, Zanetti N, et al. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444:761–765. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- 9.Alcantara Llaguno S, Chen J, Kwon CH, et al. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell. 2009;15:45–56. doi: 10.1016/j.ccr.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 11.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 12.TCGA. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 15.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- 17.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 18.Jackson EL, Garcia-Verdugo JM, Gil-Perotin S, et al. PDGFR alpha-positive B cells are neural stem cells in the adult SVZ that form glioma-like growths in response to increased PDGF signaling. Neuron. 2006;51:187–199. doi: 10.1016/j.neuron.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36:1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- 20.Le LQ, Parada LF. Tumor microenvironment and neurofibromatosis type I: connecting the GAPs. Oncogene. 2007;26:4609–4616. doi: 10.1038/sj.onc.1210261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hegedus B, Dasgupta B, Shin JE, et al. Neurofibromatosis-1 regulates neuronal and glial cell differentiation from neuroglial progenitors in vivo by both cAMP- and Ras-dependent mechanisms. Cell Stem Cell. 2007;1:443–457. doi: 10.1016/j.stem.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Dasgupta B, Gutmann DH. Neurofibromin regulates neural stem cell proliferation, survival, and astroglial differentiation in vitro and in vivo. J Neurosci. 2005;25:5584–5594. doi: 10.1523/JNEUROSCI.4693-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groszer M, Erickson R, Scripture-Adams DD, et al. PTEN negatively regulates neural stem cell self-renewal by modulating G0-G1 cell cycle entry. Proc Natl Acad Sci U S A. 2006;103:111–116. doi: 10.1073/pnas.0509939103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groszer M, Erickson R, Scripture-Adams DD, et al. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science. 2001;294:2186–2189. doi: 10.1126/science.1065518. [DOI] [PubMed] [Google Scholar]
- 25.Gil-Perotin S, Marin-Husstege M, Li J, et al. Loss of p53 induces changes in the behavior of subventricular zone cells: implication for the genesis of glial tumors. J Neurosci. 2006;26:1107–1116. doi: 10.1523/JNEUROSCI.3970-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meletis K, Wirta V, Hede SM, Nister M, Lundeberg J, Frisen J. p53 suppresses the self-renewal of adult neural stem cells. Development. 2006;133:363–369. doi: 10.1242/dev.02208. [DOI] [PubMed] [Google Scholar]
- 27.Molofsky AV, Slutsky SG, Joseph NM, et al. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Androutsellis-Theotokis A, Leker RR, Soldner F, et al. Notch signalling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442:823–826. doi: 10.1038/nature04940. [DOI] [PubMed] [Google Scholar]
- 29.Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim DA, Tramontin AD, Trevejo JM, Herrera DG, Garcia-Verdugo JM, Alvarez-Buylla A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- 31.Han YG, Spassky N, Romaguera-Ros M, et al. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci. 2008;11:277–284. doi: 10.1038/nn2059. [DOI] [PubMed] [Google Scholar]
- 32.Lai K, Kaspar BK, Gage FH, Schaffer DV. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci. 2003;6:21–27. doi: 10.1038/nn983. [DOI] [PubMed] [Google Scholar]
- 33.Lie DC, Colamarino SA, Song HJ, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 34.Kalani MY, Cheshier SH, Cord BJ, et al. Wnt-mediated self-renewal of neural stem/progenitor cells. Proc Natl Acad Sci U S A. 2008;105:16970–16975. doi: 10.1073/pnas.0808616105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med. 2005;353:811–822. doi: 10.1056/NEJMra043666. [DOI] [PubMed] [Google Scholar]
- 36.Bachoo RM, Maher MA, Ligon KL, et al. Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell. 2002;1:269–277. doi: 10.1016/s1535-6108(02)00046-6. [DOI] [PubMed] [Google Scholar]
- 37.Holland EC, Celestino J, Dai C, Schaefer L, Sawaya RE, Fuller GN. Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nat Genet. 2000;25:55–57. doi: 10.1038/75596. [DOI] [PubMed] [Google Scholar]
- 38.Huse JT, Holland EC. Genetically engineered mouse models of brain cancer and the promise of preclinical testing. Brain Pathol. 2009;19:132–143. doi: 10.1111/j.1750-3639.2008.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu Y, Guignard F, Zhao D, et al. Early inactivation of p53 tumor suppressor gene cooperating with NF1 loss induces malignant astrocytoma. Cancer Cell. 2005;8:119–130. doi: 10.1016/j.ccr.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kwon CH, Zhao D, Chen J, et al. Pten haploinsufficiency accelerates formation of high-grade astrocytomas. Cancer Res. 2008;68:3286–3294. doi: 10.1158/0008-5472.CAN-07-6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Son MJ, Woolard K, Nam DH, Lee J, Fine HA. SSEA-1 is an enrichment marker for tumor-initiating cells in human glioblastoma. Cell Stem Cell. 2009;4:440–452. doi: 10.1016/j.stem.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bleau AM, Hambardzumyan D, Ozawa T, et al. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell. 2009;4:226–235. doi: 10.1016/j.stem.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen J, Alcantara Llaguno SR, Kwon CH, Parada LF. unpublished work. [Google Scholar]
- 44.Ligon KL, Huillard E, Mehta S, et al. Olig2-regulated lineage-restricted pathway controls replication competence in neural stem cells and malignant glioma. Neuron. 2007;53:503–517. doi: 10.1016/j.neuron.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ben-Porath I, Thomson MW, Carey VJ, et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007;17:165–172. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Purow BW, Haque RM, Noel MW, et al. Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1, is critical for glioma cell survival and proliferation. Cancer Res. 2005;65:2353–2363. doi: 10.1158/0008-5472.CAN-04-1890. [DOI] [PubMed] [Google Scholar]
- 48.Alcantara Llaguno SR, Parada LF. unpublished work. [Google Scholar]
- 49.Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kelly PN, Dakic A, Adams JM, Nutt SL, Strasser A. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317:337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- 51.Rich JN, Bigner DD. Development of novel targeted therapies in the treatment of malignant glioma. Nat Rev Drug Discov. 2004;3:430–446. doi: 10.1038/nrd1380. [DOI] [PubMed] [Google Scholar]
- 52.Sathornsumetee S, Rich JN. Designer therapies for glioblastoma multiforme. Ann N Y Acad Sci. 2008;1142:108–132. doi: 10.1196/annals.1444.009. [DOI] [PubMed] [Google Scholar]
- 53.Cavaliere R, Wen PY, Schiff D. Novel therapies for malignant gliomas. Neurol Clin. 2007;25:1141–1171. doi: 10.1016/j.ncl.2007.07.012. x. [DOI] [PubMed] [Google Scholar]
- 54.Griffero F, Daga A, Marubbi D, et al. Different response of human glioma tumor-initiating cells to epidermal growth factor receptor kinase inhibitors. J Biol Chem. 2009;284:7138–7148. doi: 10.1074/jbc.M807111200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Argyriou AA, Kalofonos HP. Molecularly targeted therapies for malignant gliomas. Mol Med. 2009;15:115–122. doi: 10.2119/molmed.2008.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jin L, Lee EM, Ramshaw HS, et al. Monoclonal antibody-mediated targeting of CD123, IL-3 receptor alpha chain, eliminates human acute myeloid leukemic stem cells. Cell Stem Cell. 2009;5:31–42. doi: 10.1016/j.stem.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 57.Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12:1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- 58.Das S, Srikanth M, Kessler JA. Cancer stem cells and glioma. Nat Clin Pract Neurol. 2008;4:427–435. doi: 10.1038/ncpneuro0862. [DOI] [PubMed] [Google Scholar]
- 59.Wang ZG, Delva L, Gaboli M, et al. Role of PML in cell growth and the retinoic acid pathway. Science. 1998;279:1547–1551. doi: 10.1126/science.279.5356.1547. [DOI] [PubMed] [Google Scholar]
- 60.Lee J, Son MJ, Woolard K, et al. Epigenetic-mediated dysfunction of the bone morphogenetic protein pathway inhibits differentiation of glioblastoma-initiating cells. Cancer Cell. 2008;13:69–80. doi: 10.1016/j.ccr.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pollard SM, Yoshikawa K, Clarke ID, et al. Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens. Cell Stem Cell. 2009;4:568–580. doi: 10.1016/j.stem.2009.03.014. [DOI] [PubMed] [Google Scholar]