Abstract
Background/Objective
Obesity is associated with an inflammatory state that is often characterized by elevated plasma C-reactive protein (CRP) levels. Although coffee is broadly consumed in Western societies, few studies have examined the relationship between obesity, coffee consumption and CRP levels. The objective of the present study was to assess the relationship between obesity, coffee consumption and variation in CRP in postmenopausal, overweight/obese women with or without hormone replacement therapy (HRT) use.
Subjects/Methods
Cross-sectional analyses of 344 healthy sedentary, overweight/obese postmenopausal women (mean age=57.1±6.4 years and mean body mass index [BMI]=36.1±3.9 kg/m2). Plasma CRP levels were measured by a highly sensitive immunoassay that used monoclonal antibodies coated with polystyrene particles. Diet was assessed using the Food Intake and Analysis System semi-quantitative food frequency questionnaire.
Results
Plasma CRP was positively associated with BMI (p<0.001) and negatively associated with coffee consumption (p≤0.05). In women using HRT, plasma CRP was positively associated BMI in women consuming less than one cup of coffee per month (r2=0.15 [p<0.001]), one cup per day (0.14 [p=0.02]) and more than one cup per day (0.12 [p=0.03]). In women who did not use HRT, CRP was associated BMI only in women consuming less than one cup of coffee per day (r2=0.16 [p<0.001]) but not in women consuming one cup per day (0.06 [p=0.10]) or more than one daily cup of coffee (0.03 [p=0.27]).
Conclusion
Among overweight/obese postmenopausal women coffee consumption is negatively associated with CRP. Coffee consumption appears to attenuate the association between BMI and CRP, but only in women not using HRT.
Keywords: Obesity, Coffee, CRP, Postmenopausal women, Hormone replacement Therapy
INTRODUCTION
Both prospective epidemiology studies and intervention studies have shown that plasma C-reactive protein (CRP) levels, a marker of systemic inflammation, are associated with incidence of cardiovascular disease (CVD), independently from other conventional risk factors (Danesh et al 2004). Studies have also reported an association between plasma CRP levels and diabetes incidence (Dehghan et al 2007). Based on these observations, correlates of plasma CRP levels need to be better established in order to potentially target the determinants of plasma CRP levels and hopefully reduce its related risk of CVD/diabetes. Although obesity, particularly abdominal obesity is associated directly with plasma CRP levels (Lemieux et al 2001, Nakamura et al 2008) the variance in CRP accounted for by weight is relatively small. It is increasingly recognized that specific dietary patterns are associated with plasma CRP levels (Nanri et al 2007). However, these dietary patterns remain poorly investigated.
In the Western world, coffee consumption is ubiquitous and represents the only liquid, other than water, that is consumed almost everyday by a majority of adults (Storey et al 2006). Coffee consumption has been associated with a decreased risk of type 2 diabetes, CVD, breast cancer and inflammatory diseases (Andersen et al 2006, Ganmaa et al 2008, Salazar-Martinez et al 2004, van Dam and Feskens 2002). Despite these observations, the exact mechanisms for these associations remain largely unexplained. Additionally, there is limited evidence to suggest that coffee consumption is associated with lower CRP levels (Kotani et al 2008, Lopez-Garcia et al 2006). Conversely, it has been shown that postmenopausal women using hormone replacement therapy (HRT) are characterized by higher plasma CRP levels (Ridker et al 1999). Whether coffee consumption would alter the relationship between BMI and CRP in postmenopausal women using or not using HRT is unknown.
The objective of the present study was to examine the relationship between coffee consumption, obesity and plasma CRP levels in a sample of overweight/obese, postmenopausal women and to investigate whether HRT might alter these associations.
MATERIALS AND METHODS
Study Design
The present analyses were conducted on the baseline examination data of postmenopausal women enrolled in the Dose-Response to Exercise in postmenopausal Women (DREW) trial. A complete description of DREW design and methods has been previously reported (Church et al 2007, Morss et al 2004). The study was a randomized, dose-response exercise trial with a no-exercise control group and 3 exercise groups with incrementally higher doses of energy expenditure. The results of the primary endpoints of this protocol have been previously published (Church et al 2007). We certify that all applicable institutional regulations concerning the ethical use of human volunteers were followed during this research. The research protocol was reviewed and approved annually by the Cooper Institute's institutional review board and subsequently approved by the Pennington Biomedical Research Center IRB for the continued analysis and publication of relevant research findings. Written informed consent was obtained from all participants prior to their inclusion in the study.
Study Participants
A total of 4545 telephone screening interviews between April 2001 and June 2005 were conducted, which ultimately resulted in enrolling 464 postmenopausal, sedentary, overweight/obese [body mass index (BMI) of 25.0–43.0 kg/m2] women aged 45 to 75 years with elevated blood pressure (systolic blood pressure (SBP) ranging from 120.0 to 159.9 mmHg). Exclusion criteria included history of stroke, heart attack, or any serious medical condition that prevented participants from adhering to the protocol or exercising safely. Women were excluded from this study if they had significant cardiovascular disease or disorders (including but not limited to arrhythmias, myocarditis, cardiomyopathy, congestive heart failure, heart disease, stroke or transient ischemic cerebral attacks, peripheral vascular disease with intermittent claudication, acute, chronic or recurrent thrombophlebitis). Women were also excluded if they had a 10-year risk above 10% as indicated by NCEP Panel III report. Diet was assessed once at the beginning of the study using the Food Intake Analysis System semi-quantitative food frequency questionnaire (University of Texas 1996). Women reporting taking HRT were on a stable dose for a minimum of 6 months.
Plasma CRP levels were measured by a highly sensitive immunoassay that used monoclonal antibodies coated with polystyrene particles. The assay was performed with a Behring BN-100 nephelometer (Dade Behring) according to the methods described by the manufacturer (Ledue et al 1998). The coefficient of variation for C-reactive protein in this analysis was 1.0. Individuals with missing coffee data (n=9), who smoked (n=27), taking statins (n=75) or with missing hormone therapy data (n=7) were excluded resulting in 344 individuals with complete data.
Weight was measured on an electronic scale (Siemens Medical Solutions, Malvern, PA) and height was measured using a standard stadiometer. This scale was calibrated weekly. BMI was calculated as weight in kilograms divided by height in meters squared. Cardiorespiratory fitness was measured using a Lode Excalibur Sport cycle ergometer as previously described (Church et al 2007). Blood pressure was measured after a 30 minute rest period using a Colin STBP-780 automated blood pressure unit with participants in the recumbent position.
Statistical analyses
Women were categorized based on coffee consumption into the following groups: no coffee consumption, 1 cup/month to 6 cups/week, 1 cup/day to 13 cups/week or ≥2 cups/ day. We computed the arithmetic mean and standard deviation of each variable by categories of coffee consumption. For dichotomous variables we calculated percentages. Due to the skewed distribution of CRP, we log-transformed CRP values for statistical analyses based on the general linear model, with results expressed as geometric means. We compared geometric mean CRP values across categories of coffee consumption with progressively increased adjustment. Model 1 was adjusted for age. Model 2 was further adjusted for hormone replacement therapy. Model 3 was further adjusted for ethnicity and model 4 was further adjusted for BMI, fitness, alcohol use and arthritis. Because individuals who consumed no coffee had a similar mean CRP as individuals who consumed 1 cup/month to 6 cups/week, we collapsed these two coffee intake categories. We also examined the geometric mean CRP across levels of either BMI or coffee consumption in women using and not using HRT and tested the differences between each group using general linear models. We categorized individuals as overweight (25.0 kg/m2 ≤ BMI <30.0 kg/m2) obese class I (30.0 kg/m2 ≤ BMI <35.0 kg/m2) or obese class II or above (BMI ≥35.0 kg/m2) using established cut-offs. Using linear regression models, we examined the association between BMI and transformed CRP values within levels of coffee consumption in women using and not using HRT. The significance of interactions was assessed by multiple regression analyses. All analyses were performed using commercially available software.
RESULTS
Baseline anthropometric and metabolic characteristics are presented in Table 1 for the whole sample and across levels of coffee consumption. Participants included in the present study were apparently healthy middle-aged obese women. One hundred and sixty (47%) women reported to be currently using HRT. Higher levels of coffee consumption were associated with older age and being Caucasian. None of the other CVD risk factors nor anthropometric measures were associated with coffee consumption. Across coffee consumption categories, women who reported using hormone replacement therapy had similar BMI adiposity indices (data not shown).
Table 1.
Baseline Participant Characteristics*
| Coffee Consumption |
||||||
|---|---|---|---|---|---|---|
| All | None | 1 cup/month to 6 cups/wk | 1 cup/day to 13 cups/wk | ≥2 cups/ day | P -Value ‡ | |
| Characteristics | (n = 344) | (n=104) | (n=86) | (n=89) | (n=65) | |
| Age, y | 57.1 (6.4) | 55.5 (6) | 55.8 (6) | 59.2 (6.2) | 58.7 (6.7) | <0.001 |
| Ethnicity, % | ||||||
| Caucasian | 64.8 | 52.0 | 53.5 | 72 | 90.8 | <0.001 |
| African American | 28.2 | 39 | 39.5 | 19 | 7.7 | |
| Hispanic/other | 7 | 9 | 7 | 9 | 1.5 | |
| Current hormone therapy, % | 46.5 | 43.3 | 47.7 | 49.4 | 46.2 | 0.85 |
| Arthritis, % | 27 | 23.1 | 23.3 | 38.2 | 23.1 | 0.06 |
| Alcohol use, g/day | 1.7 (2.4) | 1.2 (2) | 1.9 (2.4) | 1.9 (2.6) | 1.7 (2.4) | 0.17 |
| Weight, kg | 84.0 (12.1) | 84 (12.5) | 85.2 (11.3) | 83.3 (12.8) | 83.6 (11.6) | 0.75 |
| Waist circumference, cm | 100.6 (12.2) | 101.1 (11.3) | 99.9 (12.3) | 101.6 (13.7) | 99.2 (11.3) | 0.59 |
| Body mass index, kg/m2† | 31.6 (3.9) | 31.6 (3.8) | 31.9 (3.4) | 31.5 (4.3) | 31.5 (3.6) | 0.87 |
| Cardiorespiratory fitness, ml/kg per min | 15.4 (3.2) | 15.1 (3.2) | 15.9 (3.1) | 15.6 (2.9) | 15.0 (3.8) | 0.27 |
| LDL-C, mg/dL | 121.0 (26.5) | 122.9 (24.7) | 122.8 (26.3) | 118.3 (26) | 119.0 (30) | 0.52 |
| HDL-C, mg/dL | 58.5 (14.8) | 57.3 (12.4) | 58.0 (12.9) | 60.1 (16.8) | 58.8 (17.3) | 0.61 |
| Triglycerides, mg/dL | 130.1 (66.9) | 128.9 (65.1) | 130.0 (58.9) | 129.0 (75.6) | 133.5 (68.6) | 0.97 |
| Fasting glucose, mg/dL | 94.4 (9.8) | 92.6 (10.7) | 95.1 (10.2) | 95.1 (9) | 95.5 (8.5) | 0.14 |
| Blood pressure, mm Hg | ||||||
| Systolic | 140.6 (13.1) | 138.8 (12.6) | 140.5 (11.4) | 140.6 (13.3) | 143.8 (15.4) | 0.12 |
| Diastolic | 81.5 (8.4) | 81.3 (8) | 83.8 (7.5) | 80.3 (9.5) | 80.5 (8.2) | 0.02 |
Abbreviations: LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; HR, heart rate; VO2, volume of oxygen consumed
Continuous variables presented as means (SD) and dichotomous variables presented as percentages.
Calculated as weight in kilograms divided by height in meters squared
For between group differences
Table 2 shows median and mean plasma CRP levels across levels of coffee consumption. Greater coffee consumption was significantly associated with lower CRP levels (p for trend = 0.002). Further adjustment for potential confounders including age, BMI, hormone replacement therapy, ethnicity, fitness, alcohol use and arthritis had no meaningful effect on the association (p for trend=0.005). We have studied the relationship of CRP several other dietary component (total energy intake, alcohol intake, saturated fat intake and fiber intake) and found that only coffee intake was associated with CRP (data not shown).
Table 2.
C-reactive protein across categories of coffee consumption.
| Coffee Consumption | |||||
|---|---|---|---|---|---|
| None | 1 cup/month to 6 cups/wk | 1 cup/day to 13 cups/wk | ≥2 cups/ day | P for trend | |
| Median CRP | 5.0 [1.7,8.7] | 5.3 [2.4, 9.3] | 3.3 [1.4, 7.7] | 3.1 [1.7, 5.5] | N/A |
| Covariates | |||||
| None | 4.1 (3.3, 5.0) | 4.3 (3.4, 5.4) | 3.2 (2.5, 3.9) | 2.9 (2.2, 3.6) | 0.002 |
| Model 1 | 4.1 (3.4, 5.0) | 4.2 (3.4, 5.2) | 3.2 (2.6, 3.9) | 2.9 (2.2, 3.7) | 0.002 |
| Model 2 | 4.2 (3.5, 5.1) | 4.2 (3.4, 5.1) | 3.1 (2.5, 3.8) | 2.9 (2.3, 3.6) | 0.001 |
| Model 3 | 4.1 (3.4, 5.0) | 4.1 (3.3, 5.0) | 3.1 (2.6, 3.9) | 3.0 (2.4, 3.8) | 0.005 |
| Model 4 | 4.1 (3.4, 4.9) | 4.1 (3.3, 5.0) | 3.1 (2.6, 3.8) | 3.0 (2.4, 3.8) | 0.005 |
Geometric means with adjustment for CRP levels are shown. Model 1 was adjusted for age and BMI. Model 2 was further adjusted for hormone replacement therapy. Model 3 was further adjusted for ethnicity and model 4 was further adjusted for fitness, alcohol use and arthritis. Data is expressed as mean (95% CI) or median (IQR).
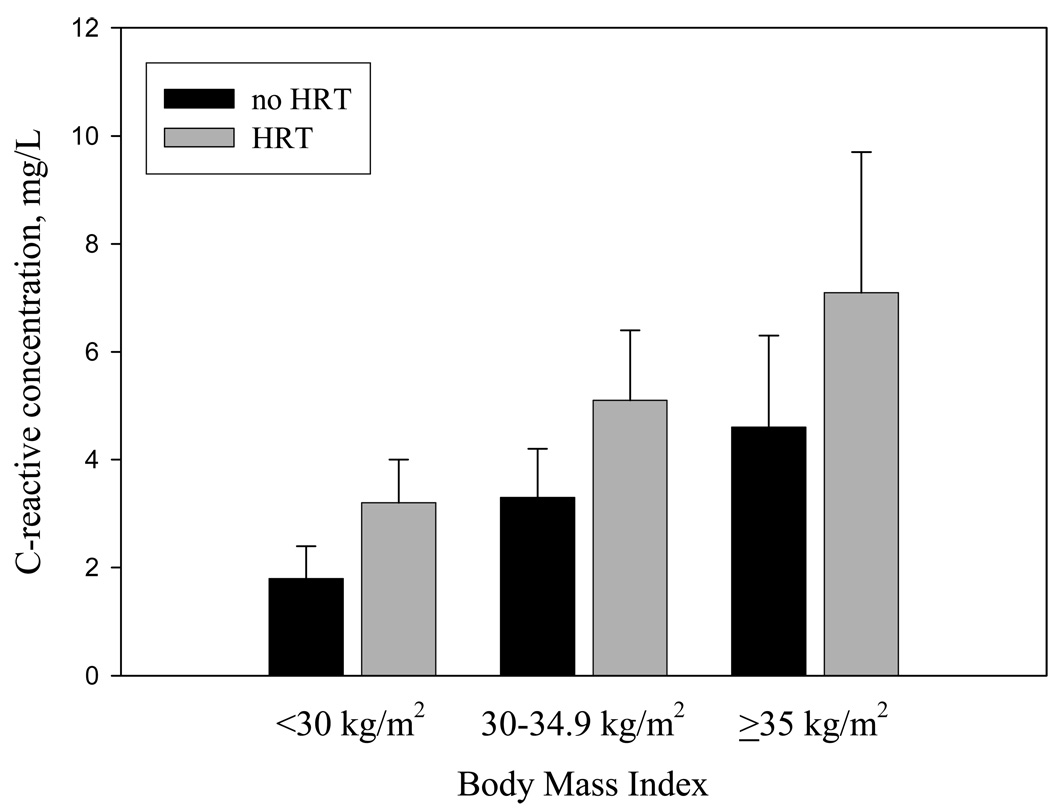
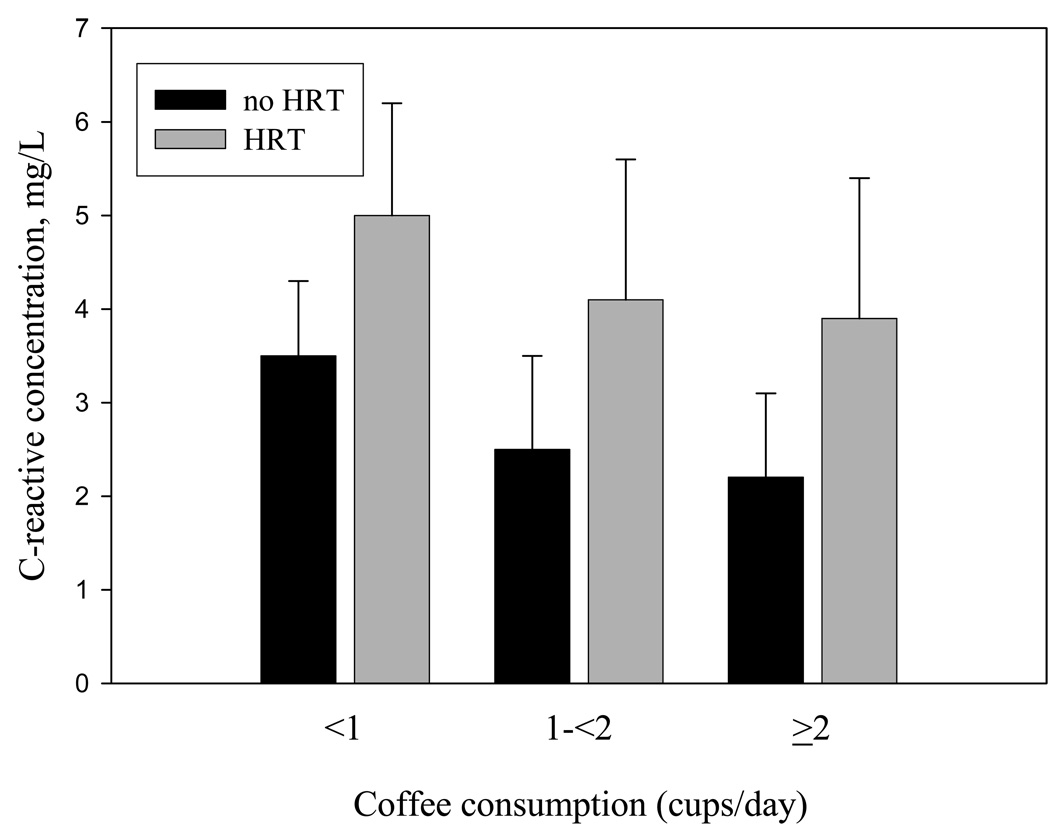
Figure 1 depicts plasma CRP levels in women classified on the basis of BMI and HRT use. In women using or not using HRT, CRP was positively associated with BMI (p for trend < 0.001 for each). Figure 2 shows plasma CRP levels in women classified on the basis of coffee consumption and HRT use. As reported in Table 2, women with an elevated coffee consumption had lower plasma CRP levels. In women using and not using HRT, CRP was inversely associated with coffee consumption (P trend = 0.02 and 0.05, respectively).
Figure 1.
Plasma C-reactive protein levels in women classified on the basis of body mass index (BMI<30 kg/m2, 30–35 kg/m2 and ≥35 kg/m2) and hormone replacement therapy (HRT) use. P<0.001 for trend in CRP levels across BMI categories (in women using HRT and in women not using HRT).
Figure 2.
Plasma C-reactive protein levels in women classified on the basis of coffee consumption (1 cup/month to 6 cups/week, 1 cup/day to 13 cups/week, ≥2 cups/ day) and hormone replacement therapy (HRT) use. P=0.02 for trend in CRP levels across coffee consumption categories in women using HRT and p=0.05 in women not using HRT.
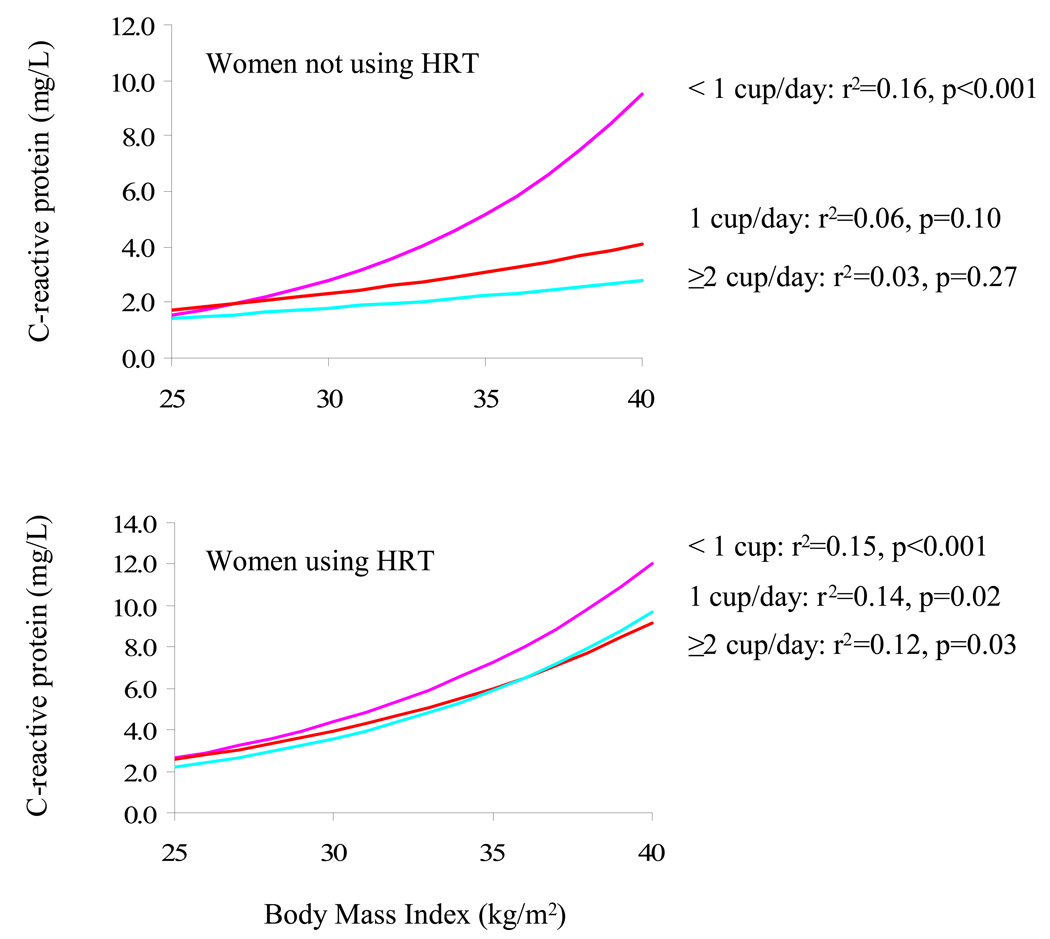
Figure 3 depicts the BMI and CRP relation within levels of coffee consumption in women not using HRT (top) and using HRT (bottom). In women using HRT, there was a positive relationship between BMI and CRP levels for all three coffee consumption categories. In women not using HRT, there was a positive association between BMI and CRP in women that drank <1 cup of coffee per day. Using a formal test for interaction, we found no association between BMI and CRP in women that do not use HRT and drink one or more cups of coffee per day (p=0.62). In women not using HRT, the coffee-by-BMI interaction analysis just missed obtaining statistical significance (p = 0.08).
Figure 3.
Linear regression and associated regression coefficients between body mass index and plasma C-reactive protein levels in women not using hormone replacement therapy (top) and in women using hormone replacement therapy (bottom) further classified on the basis of coffee consumption (1 cup/month to 6 cups/week [violet], 1 cup/day to 13 cups/week [red] or ≥2 cups/ day [turquoise]).
DISCUSSION
Results of the present study suggest that the relationship between obesity and plasma CRP levels is influenced by coffee consumption in overweight/obese, postmenopausal women who do not use HRT. As the positive relationship between obesity and plasma levels of CRP has been well-described in several studies, we found that, in women who reported drinking at least one daily cup of coffee, BMI was not associated with CRP. However, our results also suggest that this observation is limited to postmenopausal women who do not use HRT. As approximately half of postmenopausal U.S. women report using HRT (Brett and Chong 2001), we believe that these findings could have an important impact in terms of chronic diseases prevention. Indeed, it is well recognized that women using HRT are characterized by increased plasma CRP levels (Cooper et al 2007, Ridker et al 1999). In our study, we found that this relationship was also independent from coffee consumption. Although the joint effects of obesity and HRT (or oral contraceptives) on plasma CRP levels have been previously documented (Buchbinder et al 2008), our study suggests that coffee consumption might modify these relationships. To the best of our knowledge, the present study is the first ever conducted on this topic.
A few studies have investigated the association between coffee consumption and low-grade inflammation biomarkers such as CRP. In the ATTICA study, Zampelas and colleagues (Zampelas et al 2004) investigated the relationship between coffee consumption and inflammatory markers in 1528 apparently healthy women (mean age=45±13 years) and reported that coffee consumption was positively associated with CRP levels. In that study, compared to women who did not drink coffee, those who consummated more than 400 mL/day had approximately 38% higher CRP levels. However, it is worth mentioning that although CRP levels were higher, mean plasma levels of CRP women drinking more than 400 mL/day remained in what is considered as a “normal” CRP range. The fact that our study population was limited to overweight/obese postmenopausal women compared to a study sample representative of women living in a Greek province (Attica) might explain, to a certain extent, this discrepancy. Similarly to what we observed in the present study, results of the Nurses’ Health Study suggest that plasma CRP levels were lower in women who reported drinking more than two cups of coffee per day compared with women who reported drinking less than one cup of coffee per day (Lopez-Garcia et al 2006). However, this observation was limited to women with type 2 diabetes as healthy women had similar CRP levels across coffee consumption categories. It is of interest to mention that diabetic women of that cohort had BMIs and blood pressure values which were similar to those assessed in our study sample. Finally, in a sample of 459 community-living Japanese women aged 23–83, Kotani et al. (Kotani et al 2008) reported that CRP was lower in women who reported consuming at least one cup of coffee per day compared to women who consumed less than one cup of coffee per day. Although they mentioned that this difference was higher in women aged >60 years of age, they did not investigate whether this relationship was still observed in women using or not using HRT.
Over the past few years, studies have reported that coffee consumption was associated with a lower risk of type 2 diabetes an inflammatory diseases in women (Andersen et al 2006, van Dam and Hu 2005). However, very few studies have examined how this relationships could be explained from a pathophysiological standpoint. Chung et al. (Chung et al 2004) have shown that treatment of HepG2 cells with caffeic acid, a naturally occurring phenolic compound found in coffee might inhibit the function of the transcription nuclear factor κB, an important mediator of the inflammatory response. Although coffee might have anti-inflammatory or anti-oxidation properties, there is little pathophysiological evidence to support the results observed in the present study. Moreover, whether the relationship between HRT and CRP is the consequence of increased systemic inflammation or rather the consequence of the effects of estrogens on hepatic CRP production remains unclear (Ridker et al 1999, Silvestri et al 2003) and the possible biological pathways though which coffee might alter the relationship between obesity and CRP in postmenopausal women who do not use HRT need to be established.
Because of the cross-sectional design of our study, we cannot infer causality from our results. The prospective relationship between obesity, coffee consumption and plasma CRP levels and associated CVD and type 2 diabetes risk should be further investigated in population and/or intervention studies. Moreover, as our study sample was limited to overweight/obese postmenopausal women, we believe that this relationship should be documented in nonobese women from various ethnicities as well as in men. Similarly, since our study population was limited in size, lack of statistical power may have limited our ability to detect associations, especially for the analyses which required separating the study population into six groups. One other limitation of our study is the fact that coffee consumption was self-reported and that the food frequency questionnaire that was used did not enable us to measure the intake of the principal bioactive component of coffee: caffeine. Women of the study took the questionnaire once at the beginning of the study and therefore, we could not perform repeated assessment of coffee intake. Finally, the difference between consumption of boiled vs. filtered coffee was not assessed. However, these limitations would introduce an increased random measurement error that would likely attenuate the associations reported in the present study.
In conclusion, we found that BMI was positively associated with variations in plasma CRP levels and that coffee consumption was inversely associated with CRP, more specifically in overweight/obese postmenopausal women who did not take HRT. Our study also suggests that coffee consumption alters the relationship between obesity and plasma CRP levels in these women. In women who reported using HRT, BMI was positively associated with plasma CRP levels, with coffee consumption having no impact on this relationship. As the relationship between coffee consumption and CVD risk is not fully established, results of the present study do not support the view that coffee consumption could be deleterious to cardiovascular health. Rather, based on the results of our study, coffee consumption is associated with reduced levels of an inflammatory marker predictive of CVD risk in overweight/obese postmenopausal women who do not use HRT. However, whether coffee consumption should be encouraged to reduce CVD and/or type 2 diabetes risk associated with low-grade inflammation still needs to be further investigated. Additional studies and randomized trials will shed light on the specific mechanisms by which coffee consumption could possibly be linked to low-grade inflammation.
ACKNOWLEDGEMENTS
The authors would like to thank the participants and the staff and the Scientific Advisory Board of the Cooper Clinic Institute. Dr. Després is Scientific Director of the International Chair on Cardiometabolic Risk, which is supported by an unrestricted grant from Sanofi Aventis awarded to Université Laval. This study was supported by a grant HL66262 and HL071900 from the National Institutes of Health
References
- Andersen LF, Jacobs DR, Jr, Carlsen MH, Blomhoff R. Consumption of coffee is associated with reduced risk of death attributed to inflammatory and cardiovascular diseases in the Iowa Women's Health Study. Am J Clin Nutr. 2006;83:1039–1046. doi: 10.1093/ajcn/83.5.1039. [DOI] [PubMed] [Google Scholar]
- Brett KM, Chong Y. Hormone Replacement Therapy: Knowledge and Use in the United States. Hyattsville, Maryland: National Center for Health Statistics; 2001. [Google Scholar]
- Buchbinder S, Kratzsch J, Fiedler GM, Yar V, Brugel M, Leichtle A, et al. Body weight and oral contraceptives are the most important modulators of serum CRP levels. Scand J Clin Lab Invest. 2008;68:140–144. doi: 10.1080/00365510701487727. [DOI] [PubMed] [Google Scholar]
- Chung TW, Moon SK, Chang YC, Ko JH, Lee YC, Cho G, et al. Novel and therapeutic effect of caffeic acid and caffeic acid phenyl ester on hepatocarcinoma cells: complete regression of hepatoma growth and metastasis by dual mechanism. Faseb J. 2004;18:1670–1681. doi: 10.1096/fj.04-2126com. [DOI] [PubMed] [Google Scholar]
- Church TS, Earnest CP, Skinner JS, Blair SN. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: a randomized controlled trial. JAMA. 2007;297:2081–2091. doi: 10.1001/jama.297.19.2081. [DOI] [PubMed] [Google Scholar]
- Cooper BC, Burger NZ, Toth MJ, Cushman M, Sites CK. Insulin resistance with hormone replacement therapy: associations with markers of inflammation and adiposity. Am J Obstet Gynecol. 2007;196:123, e121–e127. doi: 10.1016/j.ajog.2006.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- Dehghan A, van Hoek M, Sijbrands EJ, Stijnen T, Hofman A, Witteman JC. Risk of type 2 diabetes attributable to C-reactive protein and other risk factors. Diabetes Care. 2007;30:2695–2699. doi: 10.2337/dc07-0348. [DOI] [PubMed] [Google Scholar]
- Ganmaa D, Willett WC, Li TY, Feskanich D, van Dam RM, Lopez-Garcia E, et al. Coffee, tea, caffeine and risk of breast cancer: a 22-year follow-up. International journal of cancer. 2008;122:2071–2076. doi: 10.1002/ijc.23336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani K, Tsuzaki K, Sano Y, Maekawa M, Fujiwara S, Hamada T, et al. The relationship between usual coffee consumption and serum C-reactive protein level in a Japanese female population. Clin Chem Lab Med. 2008;46:1434–1437. doi: 10.1515/CCLM.2008.286. [DOI] [PubMed] [Google Scholar]
- Ledue TB, Weiner DL, Sipe JD, Poulin SE, Collins MF, Rifai N. Analytical evaluation of particle-enhanced immunonephelometric assays for C-reactive protein, serum amyloid A and mannose-binding protein in human serum. Ann Clin Biochem. 1998;35(Pt 6):745–753. doi: 10.1177/000456329803500607. [DOI] [PubMed] [Google Scholar]
- Lemieux I, Pascot A, Prud'homme D, Alméras N, Bogaty P, Nadeau A, et al. Elevated C-reactive protein: another component of the atherothrombotic profile of abdominal obesity. Arterioscler Thromb Vasc Biol. 2001;21:961–967. doi: 10.1161/01.atv.21.6.961. [DOI] [PubMed] [Google Scholar]
- Lopez-Garcia E, van Dam RM, Qi L, Hu FB. Coffee consumption and markers of inflammation and endothelial dysfunction in healthy and diabetic women. Am J Clin Nutr. 2006;84:888–893. doi: 10.1093/ajcn/84.4.888. [DOI] [PubMed] [Google Scholar]
- Morss GM, Jordan AN, Skinner JS, Dunn AL, Church TS, Earnest CP, et al. Dose Response to Exercise in Women aged 45–75 yr (DREW): design and rationale. Med Sci Sports Exerc. 2004;36:336–344. doi: 10.1249/01.MSS.0000113738.06267.E5. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Ito H, Egami Y, Kaji Y, Maruyama T, Koike G, et al. Waist circumference is the main determinant of elevated C-reactive protein in metabolic syndrome. Diabetes Res Clin Pract. 2008;79:330–336. doi: 10.1016/j.diabres.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Nanri A, Moore MA, Kono S. Impact of C-reactive protein on disease risk and its relation to dietary factors. Asian Pac J Cancer Prev. 2007;8:167–177. [PubMed] [Google Scholar]
- Ridker PM, Hennekens CH, Rifai N, Buring JE, Manson JE. Hormone replacement therapy and increased plasma concentration of C-reactive protein. Circulation. 1999;100:713–716. doi: 10.1161/01.cir.100.7.713. [DOI] [PubMed] [Google Scholar]
- Salazar-Martinez E, Willett WC, Ascherio A, Manson JE, Leitzmann MF, Stampfer MJ, et al. Coffee consumption and risk for type 2 diabetes mellitus. Ann Intern Med. 2004;140:1–8. doi: 10.7326/0003-4819-140-1-200401060-00005. [DOI] [PubMed] [Google Scholar]
- Silvestri A, Gebara O, Vitale C, Wajngarten M, Leonardo F, Ramires JA, et al. Increased levels of C-reactive protein after oral hormone replacement therapy may not be related to an increased inflammatory response. Circulation. 2003;107:3165–3169. doi: 10.1161/01.CIR.0000074208.02226.5E. [DOI] [PubMed] [Google Scholar]
- Storey ML, Forshee RA, Anderson PA. Beverage consumption in the US population. J Am Diet Assoc. 2006;106:1992–2000. doi: 10.1016/j.jada.2006.09.009. [DOI] [PubMed] [Google Scholar]
- University of Texas H. Food Intake Analysis System (computer program) Version 3.0 Austin. 1996. [Google Scholar]
- van Dam RM, Feskens EJ. Coffee consumption and risk of type 2 diabetes mellitus. Lancet. 2002;360:1477–1478. doi: 10.1016/S0140-6736(02)11436-X. [DOI] [PubMed] [Google Scholar]
- van Dam RM, Hu FB. Coffee consumption and risk of type 2 diabetes: a systematic review. JAMA. 2005;294:97–104. doi: 10.1001/jama.294.1.97. [DOI] [PubMed] [Google Scholar]
- Zampelas A, Panagiotakos DB, Pitsavos C, Chrysohoou C, Stefanadis C. Associations between coffee consumption and inflammatory markers in healthy persons: the ATTICA study. Am J Clin Nutr. 2004;80:862–867. doi: 10.1093/ajcn/80.4.862. [DOI] [PubMed] [Google Scholar]