Abstract
The dynorphin-like peptides have profound effects on the state of the brain reward system and human and animal behavior. The dynorphin-like peptides affect locomotor activity, food intake, sexual behavior, anxiety-like behavior, and drug intake. Stimulation of kappa-opioid receptors, the endogenous receptor for the dynorphin-like peptides, inhibits dopamine release in the striatum (nucleus accumbens and caudate putamen) and induces a negative mood state in humans and animals. The administration of drugs of abuse increases the release of dopamine in the striatum and mediates the concomitant release of dynorphin-like peptides in this brain region. The reviewed studies suggest that chronic drug intake leads to an upregulation of the brain dynorphin system in the striatum and in particular in the dorsal part of the striatum/caudate putamen. This might inhibit drug-induced dopamine release and provide protection against the neurotoxic effects of high dopamine levels. After the discontinuation of chronic drug intake these neuroadaptations remain unopposed which has been suggested to contribute to the negative emotional state associated with drug withdrawal and increased drug intake. Kappa-opioid receptor agonists have also been shown to inhibit calcium channels. Calcium channel inhibitors have antidepressant-like effects and inhibit the release of norepinephrine. This might explain that in some studies kappa-opioid receptor agonists attenuate nicotine and opioid withdrawal symptomatology. A better understanding of the role of dynorphins in the regulation of brain reward function might contribute to the development of novel treatments for mood disorders and other disorders that stem from a dysregulation of the brain reward system.
Keywords: Dynorphins, neuropeptides, brain reward function, negative mood state, drug addiction
1. Introduction
The brain reward system plays a critical role in guiding behavior in humans and animals. Mood states have been shown to affect food intake, sexual activity, drug intake, and cognitive processes (American Psychiatric Association, 2000; Brand et al., 1992; Gomez et al., 2006). Many psychiatric and neurological disorders have been associated with a severe dysregulation of the brain reward system. Prolonged episodes of depressed mood are common in patients with schizophrenia, epilepsy, Parkinson’s disease, Alzheimer’s disease, and eating disorders (Gucciardi et al., 2004; Kanner, 2003; Lyketsos and Olin, 2002; Reijnders et al., 2008). A prolonged state of euphoria, which leads to increased risk taking behavior and hypersexuality, is one of the core symptoms of bipolar disorder (American Psychiatric Association, 2000). Environmental factors and drugs of abuse can have profound acute and protracted effects on the state of the brain reward system. Exposure to severe stressors may lead to a compromised brain reward system and an enriched nurturing environment early in life protects against depression (Levine, 2005; Plotsky et al., 2005). The administration of specific drugs of abuse leads to euphoria which has been suggested to play an important role in the initiation of drug intake (Koob et al., 1998). Prolonged drug intake induces neuroadaptations which can mediate a severe acute and protracted negative emotional state upon the cessation of drug use (Bruijnzeel and Gold, 2005; Koob and Le Moal, 2005). Neuropeptide systems have been shown to play a critical role in the regulation of mood states and drugs of abuse can have a long term effects on brain neuropeptide systems. For example, chronic administration of drugs of abuse leads to a hyperactivity of brain corticotropin-releasing factor (CRF) systems which has been associated with depression, drug withdrawal, and drug craving (Bruijnzeel and Gold, 2005; Koob, 2008; Shaham et al., 2000; Sinha, 2001). Drugs that block CRF receptors have been proposed as novel treatments for major depressive disorder and addiction (Stahl and Wise, 2008).
The brain dopaminergic system has been shown to play a critical role in signaling reward and establishing stimulus reward associations (Fouriezos et al., 1978; Robbins and Everitt, 1982; Spyraki et al., 1982; Spyraki et al., 1983; Wise et al., 1978). Food intake, drug intake, and sexual behavior increase dopamine release in the striatum and dopamine receptor antagonists prevent these behaviors (Church et al., 1987; Hernandez and Hoebel, 1988; Pfaus et al., 1990; Wise, 2004). The striatum includes the the caudate putamen (dorsal striatum), the nucleus accumbens (ventral striatum), and the olfactory tubercle (Heimer and Wilson, 1975). Although the great majority of the studies have focused on the role of the nucleus accumbens in brain reward function, evidence indicates that the caudate putamen also plays a critical role in regulating the state of the brain reward system (Apicella et al., 1991; Koepp et al., 1998). It has been demonstrated that dynorphin-like peptides and their receptors (i.e., the kappa-opioid receptor) play a critical role in regulating the state of the brain reward system by modulating dopamine release in the striatum (Di Chiara and Imperato, 1988b; Maisonneuve et al., 1994). Dynorphin-like peptides inhibit the release of dopamine in the striatum and may therefore play a critical role in the negative feedback regulation of dopamine release (Figure 1). Kappa-opioid receptor antagonists stimulate the release of dopamine in the striatum and have antidepressant-like effects (Beardsley et al., 2005; Pliakas et al., 2001; You et al., 1999). This suggests that endogenous dynorphin-like peptides exert tonic inhibitory control over dopamine release in the striatum. The aim of this review is to provide a detailed overview of the role of dynorphin-like peptides in regulating brain reward function. First, an overview will be provided of the expression patterns of the dynorphin-like peptides and the kappa-opioid receptors in the brain. Then the behavioral and neurochemical effects of kappa-opioid receptor agonists and antagonists will be discussed and an overview of the effects of drug of abuse on the expression of dynorphin-like peptides will be provided. Finally, it will be discussed how drug-induced changes in the expression of dynorphin-like peptides can contribute to the development of prolonged negative mood states and drug addiction.
Figure 1.
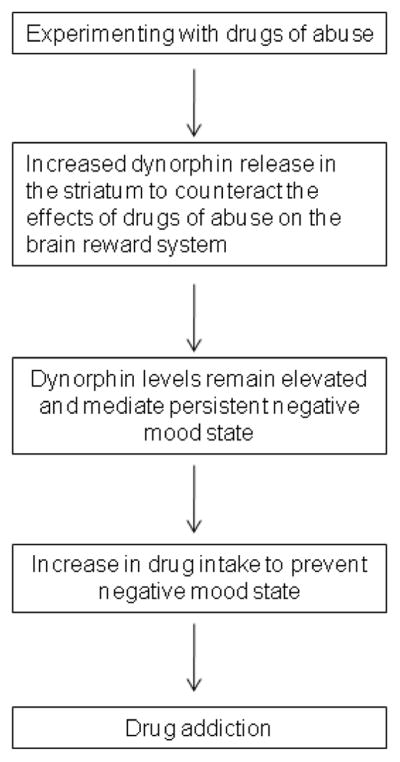
Role of dynorphin-like peptides in the development of drug addiction. This diagram indicates that drug abuse induces counteradaptive processes such as an increased release of dynorphin in the striatum to limit the effects of drugs of abuse on the brain reward system. Persistent elevated dynorphin levels may lead to a further increase in drug intake (i.e., negative reinforcement) to temporarily diminish the negative mood mediated by dynorphin. The perpetual cycle of negative mood states and drug intake to prevent negative mood states may lead to the development of a drug addiction (i.e., compulsive drug use and loss of control over drug intake).
2. Dynorphin-like peptides
2.1 Dynorphin A and related peptides
The opioids in the brain are derived from three distinct prohormones: proopiomelanocortin (POMC), proenkephalin, and prodynorphin. The expression of POMC is restricted to the arcuate nucleus of the hypothalamus and the nucleus of the solitary tract (Nakanishi et al., 1979). The enzymatic cleavage of POMC leads to the formation of melanocortins and endorphins. The melanocortins include adrenocorticotropic hormone (ACTH), α-melanocyte stimulating hormone (MSH), β-MSH, and γ-MSH and play an important role in the control of the hypothalamic-pituitary-adrenal (HPA) axis, food intake, and skin and hair pigmentation (de Wied, 1993; Fan et al., 1997). The endorphins include α-endorphin, β-endorphin, and γ-endorphin. Beta-endorphin has potent analgesic properties, potentiates brain reward function, and is self-administered by animals (Amalric et al., 1987; Tseng et al., 1976; van Ree et al., 1979). The central role of α-endorphin and γ-endorphin is not completely understood yet. There is some evidence to suggest that α-endorphin and γ-endorphin have opposite effects on brain reward function. De Wied and colleagues demonstrated that α-endorphin potentiated self-stimulation of the ventral tegmental area/substantia nigra region and (Des-Tyr)-γ-endorphin inhibited self-stimulation in this region (Dorsa et al., 1979). (Des-Tyr)-γ-endorphin is similar to γ-endorphin with the exception that the tyrosine at position one is removed. This finding suggests that α-endorphin stimulates the brain reward system and γ-endorphin inhibits the brain reward system. The prohormone proenkephalin is widely expressed in the brain (Noda et al., 1982). Proenkephalin positive neurons have been detected in the bed nucleus of the stria terminalis, the central nucleus of the amygdala, the caudate putamen, and various hypothalamic nuclei including the paraventricular hypothalamic nucleus (Sukhov et al., 1995). Proenkephalin is enzymatically cleaved into leu-enkephalin, met-enkepahalin, and two carboxyl (C)-terminally extended met-enkephalins: met-enkephalin-Arg6-Phe7 and met-enkephalin-Arg6-Gly7-Leu8 (Comb et al., 1982; Hughes et al., 1975). The enkephalins have been suggested to potentiate brain reward function and also mediate pro-inflammatory effects during the early stage of an immunoresponse (Ignatov et al., 1981; Phillips and Lepiane, 1982; Salzet and Tasiemski, 2001; Smith et al., 1982).
During the 1980’s and early 1990’s the rat, mouse, porcine, and human prodynorphin genes were cloned (Civelli et al., 1985; Gulya et al., 1993; Horikawa et al., 1983; Kakidani et al., 1982). Northern blotting revealed high levels of prodynorphin mRNA in the hypothalamus, striatum, hippocampus, and relatively low levels in the midbrain, nucleus of the solitary tract, and the brainstem (Civelli et al., 1985). More recent studies demonstrated that prodynorphin mRNA is expressed in both the caudate putamen and the nucleus accumbens (Di Benedetto et al., 2004). Prodynorphin is enzymatically processed into a variety of neuropeptides (Table 1). The partial amino acid sequence (Tyr1-Gly2-Gly3-Phe4-Leu5-Arg6-Arg7-Ile8-Arg9-Pro10-Lys11-Leu12-Lys13) of dynorphin was first described by Goldstein and colleagues (Goldstein et al., 1979). This novel tridecapeptide was extremely potent in inhibiting electrically stimulated muscle contractions in guinea pig ileum preparations compared to previously discovered peptides such as leu-enkephalin and β-endorphin and was therefore named dynorphin after the Greek word dynamis (power)(Goldstein et al., 1979). The complete amino acid sequence of dynorphin (Tyr1-Gly2-Gly3-Phe4-Leu5-Arg6-Arg7-Ile8-Arg9-Pro10-Lys11-Leu12-Lys13-Trp14-Asp15-Asn16-Gln17) was described 2 years later by the same research group (Goldstein et al., 1981). Dynorphin (1-17) is as potent as dynorphin (1-13) in the guinea pig ileum bioassay, thus indicating that the four C-terminus amino acids do not contribute to the potency of dynorphin (1-17) (Goldstein et al., 1981). Shortly after the discovery of dynorphin (1-17), Fischli and colleagues described a 32-amino acid neuropeptide that incorporates dynorphin (1-17) at the amino (N)-terminus and a novel 13-amino acid dynorphin-like peptide at the C-terminus. The 13-amino acid peptide at the C-terminus was named dynorphin B and the previously described dynorphin was now called dynorphin A (Fischli et al., 1982). The 32-amino acid peptide that incorporates dynorphin A and dynorphin B was called dynorphin (1-32) or big dynorphin. Minamino and colleagues isolated an 8-amino acid peptide from the porcine hypothalamus, dynorphin A (1-8), that is comprised of the 8 N-terminal amino acids of dynorphin A (Minamino et al., 1980). Two other peptides that are processed from prodynorphin are α-neoendorphin and β-neoendorphin. Alpha neoendorphin was first isolated from porcine hypothalami and further chemical analyses revealed that this was a 10-amino acid peptide that has the same 6 N-terminal amino acids as dynorphin A and dynorphin B (Kangawa et al., 1981; Kangawa and Matsuo, 1979). Beta-neoendorphin has the same amino acid sequence as α-neoendorphin with the exception that it lacks the amino acid lysine at position 10.
Table 1.
Dynorphin-like peptides derived from the prodynorphin precursor.
| Peptide | Amino acid sequence |
|---|---|
| a Dynorphin A(1–8) | Tyr1-Gly-Gly-Phe-Leu-Arg-Arg-Ile8 |
| b Dynorphin A(1–13) | Tyr1-Gly-Gly-Phe-Leu-Arg-Arg-Ile-Arg-Pro-Lys-Leu-Lys13 |
| c Dynorphin A(1–17) | Tyr1-Gly-Gly-Phe-Leu-Arg-Arg-Ile-Arg-Pro-Lys-Leu-Lys-Trp-Asp-Asn-Gln17 |
| d Dynorphin A(1–32) | Tyr1-Gly-Gly-Phe-Leu-Arg-Arg-Ile-Arg-Pro-Lys-Leu-Lys-Trp-Asp-Asn-Gln-Lys-Arg-Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Gln-Phe-Lys-Val-Val-Thr32 |
| d Dynorphin B(1–13) | Tyr1-Gly-Gly-Phe-Leu-Arg-Arg-Gln-Phe-Lys-Val-Val-Thr13 |
| e Leumorphin | Tyr1-Gly-Gly-Phe-Leu-Arg-Arg-Gln-Phe-Lys-Val-Val-Thr-Arg-Ser-Gln-Glu-Asp-Pro-Asn-Ala-Tyr-Tyr-Glu-Glu-Leu-Phe-Asp-Val29 |
| fα-neoendorphin | Tyr1-Gly-Gly-Phe-Leu-Arg-Lys-Tyr-Pro-Lys10 |
| gβ-neoendorphin | Tyr1-Gly-Gly-Phe-Leu-Arg-Lys-Tyr-Pro9 |
Citations:
Numerous studies have investigated the expression profile of dynorphin A and a few studies have investigated the expression profile of the other dynorphin family members. These studies suggest that there is a strong overlap in the expression patterns of the dynorphin family members and many dynorphin-like peptides are co-expressed in the same neurons (Weber et al., 1981; Weber et al., 1982; Weber and Barchas, 1983). All the studies that investigated the dynorphin A protein expression in the brain point toward a similar expression pattern. High levels of dynorphin A have been detected in the medial forebrain bundle/lateral hypothalamic area and substantia nigra. Somewhat lower levels of dynorphin A have been detected in the hippocampus, nucleus accumbens, caudate putamen, lateral subnucleus of the paraventricular hypothalamic nucleus, the supraoptic nucleus, and the nucleus of the solitary tract (Goldstein and Ghazarossian, 1980; Khachaturian et al., 1982; Weber et al., 1982). High levels of dynorphin B fibers and terminals have been detected in the substantia nigra, nucleus accumbens, ventral pallidum, internal capsule, and the hippocampal formation. Somewhat lower levels have been detected in the caudate putamen, central nucleus of the amygdala, bed nucleus of the stria terminalis, and various hypothalamic nuclei including the paraventricular hypothalamic nucleus and the supraoptic nucleus (Weber and Barchas, 1983). The latter study points towards a somewhat more widespread distribution of dynorphin B compared to the distribution of dynorphin A (Khachaturian et al., 1982; Weber et al., 1982; Weber and Barchas, 1983). It was suggested that the background staining with the dynorphin B antiserum is lower than with dynorphin A antiserum and therefore a more widespread distribution of dynorphin B might have been detected (Weber and Barchas, 1983). The distribution pattern of α-neoendorphin and β-neoendorphin is similar to that of dynorphin A (Weber et al., 1982; Zamir et al., 1984a). High levels of α-neoendorphin and β-neoendorphin have been detected in the nucleus accumbens, caudate putamen, substantia nigra, ventral tegmental area, the amygdala complex, bed nucleus of the stria terminalis, and various hypothalamic nuclei (Zamir et al., 1984a; Zamir et al., 1984b).
2.2 Kappa-opioid receptors
Pharmacological studies conducted during the 1970’s provided evidence for the existence four types of opioid receptors. Martin and colleagues identified three types of opioid receptors: the mu (μ)-opioid receptor, the kappa (κ)-opioid receptor, and the sigma (σ)-opioid receptor (MARTIN et al., 1976). Lord and colleagues provided evidence for the existence of a fourth opioid receptor which was called the delta (δ)-opioid receptor (Lord et al., 1977). Follow-up research suggested that the sigma receptor is not an opioid receptor as opioid receptor agonists bind with an extremely low affinity to this receptor and drugs such as phencyclidine (PCP) bind to this receptor (Chen et al., 1993; Quirion et al., 1981). The most recently discovered opioid receptor is the ORL1 (opioid receptor like) receptor and the endogenous ligand for this receptor is nociceptin/orphanin FQ (Meunier et al., 1995; Mollereau et al., 1994). It has been suggested that the ORL1 receptor should be called the NOP1 receptor after the name of the endogenous ligand for this receptor (nociceptin/orphanin FQ peptide) (Mogil and Pasternak, 2001).
Pharmacological studies have provided some evidence for the existence of multiple kappa-opioid receptor subtypes, however, so far only one kappa-opioid receptor has been cloned and the existence of multiple kappa-opioid receptors has been questioned (Connor and Kitchen, 2006; Traynor, 1989). The kappa-opioid receptor has been cloned from many species including rat, mouse, guinea pig, and human (Meng et al., 1993; Simonin et al., 1995; Xie et al., 1994; Yasuda et al., 1993; Zhu et al., 1995). Attali and colleagues described two types of kappa-opioid receptors in the spinal cord of guinea-pigs. One of the kappa-opioid receptors binds the peptide (D-Ala2, D-Leu5)-enkephalin (DADLE) with a high affinity and the other kappa-opioid receptor binds DADLE with a low affinity (Attali et al., 1982). Furthermore, evidence has been provided for U-69593 sensitive high-affinity binding sites (κ1) and U-69593 insensitive low affinity binding sites (κ2) (Zukin et al., 1988). Jordan and Levi have provided a possible explanation for the fact that pharmacological studies provide evidence for the existence of multiple kappa-opioid receptors. They demonstrated that fully functional kappa-opioid receptors and delta-opioid receptors can form heterodimers (Jordan and Devi, 1999). The original kappa-opioid receptor has a high affinity for U-69593 and the kappa-delta receptor heterodimer has a low affinity for U-69593. Despite that the kappa-delta heterodimer has a low affinity for U-69593 it has a high affinity for the endogenous ligand dynorphin A. Thus, the kappa-opioid receptor can form heterodimers with the delta-opioid receptor which leads to a decrease in affinity for compounds such as U-69593. The existence of a third naloxone benzoylhydrazone (NalBzoH) sensitive kappa-opioid receptor (k3) has been proposed (Clark et al., 1989). More recent studies demonstrated that the putative κ3 receptor agonist NalBzoH is a partial mu, kappa, and delta-opioid receptor agonist and an ORL1/NOP1 receptor antagonist (Olianas et al., 2006). Therefore, at this point in time there is not sufficient evidence for the existence of the κ3 receptor (Connor and Kitchen, 2006).
The anatomical distribution of the kappa-opioid receptors would suggest an important role for the endogenous kappa-opioid receptor agonists in brain reward function. High levels of kappa-opioid receptor mRNA have been detected in the ventral tegmental area, substantia nigra, nucleus accumbens, caudate putamen, claustrum, endoperiform nucleus, various hypothalamic nuclei, and the amygdala of the rat brain (Meng et al., 1993). A similar expression profile has been detected in the human brain (Simonin et al., 1995; Zhu et al., 1995). Overall there is a strong overlap in the expression of mRNA for the kappa-opioid receptor and binding sites for the dynorphin-like peptides. Immunohistochemical and autoradiography studies indicate relatively high levels of kappa-opioid receptors in the nucleus accumbens shell and core, caudate putamen, claustrum, endoperiform nucleus, ventral pallidum, medial habenula, bed nucleus of the stria terminals, amygdala, and relatively low levels in the ventral tegmental area and substantia nigra (Mansour et al., 1987; Mansour et al., 1996). There is somewhat of a discrepancy between kappa-opioid receptor mRNA and kappa-opioid receptor binding sites in the ventral tegmental area and in the substantia nigra. The ventral tegmental area and substantia nigra have relatively high levels of kappa-opioid receptor mRNA and low levels of kappa-opioid receptor binding sites. Therefore, it has been suggested that the kappa-opioid receptors are produced in the ventral tegmental area and substantia nigra and then transported to the nucleus accumbens and caudate putamen where they are expressed on presynaptic terminals and control the release of dopamine (Britt and McGehee, 2008; Mansour et al., 1995).
The kappa-opioid receptor is a G protein-coupled receptor and stimulation of the kappa-opioid receptor leads to the inhibition of adenylyl cyclase (Attali et al., 1989; Konkoy and Childers, 1989; Prather et al., 1995). The kappa-opioid receptor also plays a role in the regulation of calcium currents. The kappa-opioid receptor is coupled to an N-type calcium channel and stimulation of the kappa-opioid receptor inhibits calcium currents (Tallent et al., 1994). N-type calcium channels are expressed on presynaptic terminals and play a critical role in the release of neurotransmitters (Evans and Zamponi, 2006). Therefore, dynorphin-like peptides may inhibit the release of neurotransmitters by inhibiting N-type calcium channels. Furthermore, kappa-opioid receptor agonists have been shown to stimulate inwardly rectifying (i.e., ion conductance depends on membrane potential) potassium channels (Henry et al., 1995; Lewin et al., 2007). The inward rectifying potassium current is a flow of potassium ions from the outside to the inside of the cell (Lu, 2004). The potassium conductance increases when the membrane potential becomes more negative and the potassium conductance decreases when the membrane depolarizes. The inward rectifying potassium channels play an important role in maintaining the resting potential of neurons (Lu, 2004).
3. Kappa-opioid receptor ligands and behavior
3.1 Behavioral effects of kappa-opioid receptor agonists
A variety of animal models have been used to investigate the effects of kappa-opioid receptor agonists on brain reward function. Kappa-opioid receptor agonists have been intensively investigated in place conditioning procedures. Place conditioning sessions consist of a pre-test, a conditioning phase, and a post-test. During the pre-test the animals can freely explore the multicompartment test apparatus and how much time the rats spend in each of the compartments is assessed. During the conditioning sessions one of the compartments is paired with the drug and the other compartment is paired with vehicle. During the post-test it is determined if the preference for the drug-paired compartment has changed compared to the pre-test. Animals that are being treated with a drug that induces a positive mood state often develop a preference for the drug paired compartment (i.e., conditioned place preference). Conversely, animals will avoid a compartment that has been paired with a drug that induces a negative mood state (i.e., conditioned place aversion). Accumulating evidence indicates that kappa-opioid receptor agonists such as U-50488, U-69593, and salvinorin A induce conditioned place aversion in rodents (Iwamoto, 1985; Shippenberg and Herz, 1987; Suzuki et al., 1993; Zhang et al., 2005). The place aversion induced by the kappa-opioid receptor agonists can be blocked by pretreatment with the specific kappa-opioid receptor antagonist nor-binaltorphimine (nor-BNI) (Zhang et al., 2005). Intracranial self-stimulation (ICSS) studies support the observation that kappa-opioid receptor agonists induce a negative mood state. Kappa-opioid receptor agonists elevate brain reward thresholds in rats (Carlezon, Jr. et al., 2006; Todtenkopf et al., 2004; Tomasiewicz et al., 2008). This indicates that the kappa-opioid receptor agonists decrease the sensitivity to rewarding electrical stimuli. An inability to experience pleasure (i.e., anhedonia) is one of the core symptoms of depression (American Psychiatric Association, 2000).
The rat forced swim test is widely used to screen for novel antidepressant drugs. In this test the rats are placed in a cylinder with water on two consecutive days (15 minutes first session and 5 minutes second session) and the duration that the rats spend swimming, climbing, and immobile is assessed on day 2. Antidepressant drugs that block the reuptake of noradrenaline decrease immobility and increase climbing in this test and antidepressants that block the reuptake of serotonin decrease immobility and increase swimming (Cryan et al., 2002). Treatments that induce a negative mood state such as for example amphetamine withdrawal increase immobility and decrease climbing in the forced swim test (Cryan et al., 2003). The kappa-opioid receptor agonists U-69593 and salvinorin A increase immobility and decrease swimming in the forced swim test (Carlezon, Jr. et al., 2006; Mague et al., 2003). This indicates that kappa-opioid receptor agonists induce a behavioral response in the forced swim test that is similar to that induced by amphetamine withdrawal and opposite to that of acute treatment with an antidepressant drug. Furthermore, the kappa-opioid receptor agonist U-50488 has been shown to decrease male sexual behavior in rats as indicated by a decrease in female directed behavior, an increase in copulation and ejaculation latencies, and a decrease in the number of ejaculations (Leyton and Stewart, 1992). Similar decreases in sexual motivation have been reported in rats during amphetamine withdrawal, which is considered a model for depression, and in humans during a major depressive episode (Barr et al., 1999; Kennedy et al., 1999). Bals-Kubic and colleagues investigated the brain sites via which the kappa-opioid receptor agonists might mediate the negative mood state (Bals-Kubik et al., 1993). It was shown that the administration of U-50488 or the dynorphin A analog E-2078 into the ventral tegmental area, nucleus accumbens, prefrontal cortex, and lateral hypothalamus induces conditioned place aversion in rats. The administration of U-50488 or E-2078 into the substantia nigra or the caudate putamen did not induce conditioned place aversion.
The acute effects of kappa-opioid receptor agonists have also been investigated in humans. Pfeiffer and colleagues investigated the subjective effects of the kappa-opioid receptor agonist MR 2033 (Pfeiffer et al., 1986). Subjects treated with a low dose of MR 2033 reported increased anxiety, racing thoughts, and discomfort. Subjects who were treated with higher doses of MR 2033 reported that this drug induced severe disturbances in the perception of time and space, visual hallucinations, and depersonalization (Pfeiffer et al., 1986). Some subjects also displayed loss of self-control. Follow-up studies have reported that other kappa-opioid receptor agonists (e.g., enadoline and U-62066) also induce visual hallucinations, agitation, sedation, and dysphoria (Rimoy et al., 1994; Walsh et al., 2001). Taken together, these studies suggest that kappa-opioid receptor agonists induce a negative mood state in rodents and humans. A recent study suggests that very low doses of kappa-opioid receptor agonists may induce positive mood states and high doses of kappa-opioid receptor agonists induce negative mood states (Braida et al., 2008). Braida and colleagues demonstrated that very low doses of salvinorin A (0.1–40 μg/kg, sc) induced conditioned place preference in rats and a high dose of salvinorin A (160 μg/kg, sc) induced conditioned place aversion. Salvinorin A-induced conditioned place preference could be blocked by the kappa-opioid receptor antagonist nor-BNI. On a similar note, rats self-administer low doses of salvinorin A (0.1 and 0.5 μg per infusion) and do not self-administer a high dose of salvinorin A (1 μg per infusion) (Braida et al., 2008). These findings suggest that additional studies are needed to investigate the effects of low doses of kappa-opioid receptor agonists on the brain reward system. For example, Braida and colleagues demonstrated that doses up to 40 μg/kg (sc) induce a positive mood state and higher doses are ineffective or induce a negative mood state (Braida et al., 2008). The lowest dose tested by Carlezon and colleagues in the forced swim test and ICSS procedure was 125 μg/kg (ip). Therefore it cannot be ruled out that lower doses might have had an antidepressant-like effect in the forced swim test or would have lowered brain reward thresholds in the ICSS procedure (Carlezon, Jr. et al., 2006).
3.2 Behavioral effects of kappa-opioid receptor antagonists
The effects of kappa-opioid receptor antagonists have been investigated in animal models that are widely used to screen for novel antidepressants (e.g., forced swim test and learned helplessness paradigm) and anxiolytics (e.g., elevated plus maze test). Overall, these studies indicate that the behavioral effects of the kappa-opioid receptor antagonists are opposite to those of high doses of the kappa-opioid receptor agonists. Kappa-opioid receptor antagonists decrease immobility in the forced swim test and increase swimming or climbing (Beardsley et al., 2005; Mague et al., 2003). Similar behavioral effects have been observed in rats in the forced swim test after the administration of antidepressants (Cryan et al., 2002). The antidepressant-like effects of the kappa-opioid receptor antagonists have also been explored in the learned helplessness paradigm. In this test the rats are exposed to inescapable footshocks and are then tested for shock avoidance learning at a later time point. The rats exposed to the inescapable footshocks display a deficit in shock avoidance learning and this effect can be prevented by treatment with antidepressants prior to the shock avoidance learning session (Leshner et al., 1979; Seligman and Beagley, 1975; Telner and Singhal, 1981). Similar to the antidepressants, the kappa-opioid receptor antagonist nor-BNI decreases the number of escape failures in the learned helplessness paradigm (Newton et al., 2002).
Conflicting findings have been reported with regard to the brain sites by which nor-BNI mediates its antidepressant-like effects in the learned helplessness paradigm. Shirayama and colleagues reported that the administration of nor-BNI into the dentate gyrus of the hippocampus, the CA3 region of the hippocampus, the nucleus accumbens shell, and the nucleus accumbens core decreases the number of escape failures in the learned helplessness paradigm (Shirayama et al., 2004). In contrast, Newton and colleagues reported that the administration of nor-BNI into the nucleus accumbens, but not into the dentate gyrus of the hippocampus, decreases the number of escape failures in the learned helplessness paradigm (Newton et al., 2002). It is unlikely that this discrepancy was due to the doses of nor-BNI used as in both studies the same dose of nor-BNI was administered into the dentate gyrus (2.5 μg/side). The discrepancy in the effectiveness of nor-BNI administered into the dentate gyrus to attenuate learned helplessness might be due to the location of the injection sites. Shirayama administered the nor-BNI anterior posterior (AP) −3.8 mm, medial lateral (ML) ± 2.0 mm, dorsal ventral (DV) −3.2 mm from dura, which is the location of the dentate gyrus (Paxinos and Watson, 1998). In contrast, Newton administered the nor-BNI at AP −3.8 mm, ML ± 2.0 mm, DV −6.5 mm from skull (Newton et al., 2002). The latter injection site is about 2 mm ventral of the dentate gyrus and a histological reconstruction of the injection sites was not provided (Paxinos and Watson, 1998). Therefore, these findings suggest that blockade of kappa-opioid receptors in the dentate gyrus leads to an antidepressant-like effect in the learned helplessness paradigm (Shirayama et al., 2004).
Evidence indicates that kappa-opioid receptor antagonists also have anxiolytic-like effects. The kappa-opioid receptor antagonists nor-BNI and JDTic increase the percentage of time in the open arms and the number of open arm entries in the elevated plus maze test (Knoll et al., 2007). The same kappa-opioid receptor antagonists also decrease conditioned fear in the fear potentiated startle paradigm without affecting baseline startle or shock reactivity (Knoll et al., 2007). Stressors can induce a negative mood state in humans and chronic exposure to stressors can lead to the development of anxiety and depressive disorders (Bolger et al., 1989). The negative mood state associated with exposure to stressors can be paired with a specific physical context or other environmental cues such as smells. Recent evidence suggests that stress-induced negative mood states are at least partly mediated by the release of dynorphins. Chavkin and colleagues paired the smell of an imitation almond extract with a forced swim experience in mice. When the mice were subsequently tested in a T-maze with a Nestlett with the almond extract on one of the arms the mice avoided the arm with the Nestlett (Land et al., 2008). Neither the mice that were pretreated with nor-BNI prior to the forced swim sessions, nor the prodynorphin knockout mice displayed an aversion to the Nestletts with the almond extract. On a similar note, mice pretreated with nor-BNI and prodynorphin knockout mice did not avoid a compartment in which they had received footshocks (Land et al., 2008). The administration of nor-BNI to mice or genetic deletion of the prodynorphin gene decreases the amount of time spent in a submissive social defeat posture after the mice have been place in the home cage of an aggressive male mouse (McLaughlin et al., 2006). The latter finding confirms that blockade of dynorphin signaling protects against the effects of a severe stressful experience. The neuropeptide CRF has been suggested to play an important role in psychiatric disorders such as depression and drug addictions (Bruijnzeel and Gold, 2005; Koob, 2008; Shaham et al., 2000; Sinha, 2001). The administration of CRF to rodents leads to increased anxiety-like behavior and a negative mood state as reflected by CRF-induced place aversion and elevations in brain reward thresholds in the ICSS procedure (Cador et al., 1992; Macey et al., 2000). Interestingly, CRF does not induce place aversion in mice pretreated with nor-BNI or in prodynorphin knockout mice (Land et al., 2008). This suggests that the CRF-induced negative mood state is at least partly mediated by the endogenous release of dynorphin-like peptides.
3.3 Neurochemical effects of kappa-opioid receptor ligands
Dopamine signaling has been suggested to play an important role in mediating the acute rewarding effects of drugs of abuse and natural reinforcers such as food and sex (Church et al., 1987; Koob, 2009; Malmnas, 1976; Robbins and Everitt, 1982; Wise et al., 1978). The mesolimbic dopaminergic system is compromised of dopaminergic projections from the ventral tegmental area to the nucleus accumbens, amygdala, hippocampus, and the medial prefrontal cortex. Drugs of abuse and natural reinforcers have been shown to increase dopamine levels in the projection areas of the ventral tegmental area (Church et al., 1987; Hernandez and Hoebel, 1988; Pfaus et al., 1990; Wise, 2004). In contrast, drug withdrawal, which leads to a negative mood state, has been associated with decreased dopamine levels in the nucleus accumbens (Weiss et al., 1992; Weiss et al., 1996). Extensive evidence points toward an important role for the dynorphin/kappa opioid receptor system in the regulation of dopamine release. The subcutaneous administration of the kappa-opioid receptor agonists U-50488, bremazocine, and tifluadome decreases extracellular dopamine levels in the nucleus accumbens (Di Chiara and Imperato, 1988b). In contrast, the subcutaneous administration of the kappa-opioid receptor antagonist nor-BNI leads to increased dopamine levels in the nucleus accumbens (Maisonneuve et al., 1994). Distinct groups of ventral tegmental area neurons project to the nucleus accumbens and the prefrontal cortex (Fallon, 1981; Swanson, 1982). It is interesting to note that the ventral tegmental area dynorphin/kappa-opioid receptor system plays a differential role in the regulation of neurons that project to the nucleus accumbens and the prefrontal cortex. Electrophysiological studies indicate that the kappa-opioid receptor agonist U-69593 inhibits dopaminergic neurons in the ventral tegmental area that project to the medial prefrontal cortex and does not inhibit neurons that project to the nucleus accumbens (Margolis et al., 2006). In addition, the administration of U-69593 into the ventral tegmental area decreases dopamine levels in the medial prefrontal cortex and does not affect dopamine levels in the nucleus accumbens (Margolis et al., 2006). This indicates that dopamine release in the medial prefrontal cortex, but not in the nucleus accumbens, is regulated by kappa-opioid receptors in the ventral tegmental area. Extensive evidence indicates that dopamine release in the nucleus accumbens is regulated by kappa-opioid receptors on presynpatic terminals in this brain site (Meshul and McGinty, 2000). The kappa-opioid receptor agonist U-50488 inhibits the electrically evoked release of [3H]dopamine from superfused nucleus accumbens slices (Heijna et al., 1990). In addition, the administration of the kappa-opioid receptor agonists U-69593 and U-50488 into the nucleus accumbens lowers extracellular dopamine levels in this brain site (Donzanti et al., 1992; Spanagel et al., 1992).
The great majority (~ 90%) of the neurons in the nucleus accumbens are GABAergic medium spiny neurons (Gerfen, 2004). In addition to the medium spiny neurons the nucleus accumbens contains populations of GABAergic interneurons, cholinergic interneurons, and nitric oxide synthase (NOS) containing interneurons (Nicola et al., 2000). The GABAergic medium spiny neurons and interneurons in the nucleus accumbens receive extensive glutamatergic input from the amygdala, hippocampus, and prefrontal cortex (Floresco et al., 2001; Groenewegen et al., 1987; Pennartz et al., 1994). Dysregulation of glutamatergic and GABAergic transmission in the nucleus accumbens has been associated with negative mood states. For example, the opioid receptor antagonist naloxone induces a potent, 300%, increase in glutamate levels in the nucleus accumbens of morphine dependent animals and does not affect glutamate levels in the accumbens of control animals (Nicola et al., 2000). Opioid receptor antagonists induce a negative mood state in opioid dependent animals and do not affect mood states in drug-free control animals (Bruijnzeel et al., 2006; Schulteis et al., 1994). Furthermore, the administration of glutamate into the nucleus accumbens decreases swimming in the rat forced swim test and the administration of the N-methyl-D-aspartate (NMDA) antagonists dizocilpine or 2-amino-5-phosphonovalerate in the same brain site increases swimming in the rat forced swim test (Rada et al., 2003). The observation that the administration of NMDA receptor antagonists into the nucleus accumbens increases swimming in the forced swim test suggests that these compounds have an antidepressant-like effects when administered into this brain site. Morphine and cocaine withdrawal has been associated with an increased release of GABA in the nucleus accumbens, which suggests that elevated levels of GABA in the nucleus accumbens might mediate negative mood states (Chieng and Williams, 1998; Xi et al., 2003). Taken together, these studies suggest that increased GABAergic and glutamatergic transmission in the nucleus accumbens can contribute to negative mood states. Therefore, it is somewhat surprising to note that kappa-opioid receptor agonists, which induce negative mood states, decrease GABA and glutamate levels in the nucleus accumbens (Hjelmstad and Fields, 2001; Hjelmstad and Fields, 2003; Rawls and McGinty, 1998). At this point in time, extensive evidence points toward a role for the dynorphin-like peptides, GABA, and glutamate in the nucleus accumbens in the regulation of brain reward function. It is, however, not known how the simultaneous release of these neurotransmitters affects brain reward function. Future studies are needed to delineate the complex interactions between the dynorphin-like peptides, GABA, and glutamate in the nucleus accumbens in regulating brain reward function.
The nigrostriatal dopaminergic system consists of dopaminergic projections from the substantia nigra to the caudate putamen. The nigrostriatal dopaminergic systems plays an important role in movement control and the loss of dopaminergic neurons in the substantia nigra leads to Parkinson’s disease (Jellinger, 2007; Lewitt, 2008). The loss of the dopaminergic neurons in the substantia nigra has also been suggested to lead to depression (Lemke, 2008). The dynorphin/kappa opioid receptor system plays an important role in the regulation of dopamine release in the caudate putamen. Subcutaneous administration of the kappa-opioid receptor agonists decreases extracellular dopamine levels in the caudate putamen (Di Chiara and Imperato, 1988b; Zhang et al., 2005). You and colleagues performed a detailed investigation into the role of kappa-opioid receptors in the caudate putamen and substantia nigra in the regulation of dopamine release in the caudate putamen (You et al., 1999). They demonstrated that the administration of the kappa-opioid receptor agonist U-50488 into the substantia nigra or the caudate putamen decreases extracellular dopamine levels in the caudate putamen. The administration of nor-BNI into the substantia nigra or caudate putamen increases dopamine release in the caudate putamen. Overall, these findings suggest that dopamine release in the caudate putamen is regulated by the endogenous release of dynorphin-like peptides in the caudate putamen and the substantia nigra. Dynorphin-like peptides in the caudate putamen might attenuate the release of dopamine in this brain site via two distinct mechanisms. First, the dynorphin-like peptides might bind to the presynaptic kappa-opioid receptors on dopaminergic terminals and thereby decrease the release of dopamine. Second, the release of dynorphin-like peptides into the caudate putamen might reduce the release of dopamine in this brain site via the activation of a negative feedback pathway. This is supported by the observation that the administration of the kappa-opioid receptor agonist U-50488 into the caudate putamen inhibits the firing of dopaminergic neurons in the substantia nigra (Walker et al., 1987). The effects of the administration of kappa-opioid receptor agonists into the caudate putamen on the firing rate of substantia nigra neurons are opposite to those of the effects of dopamine and mu-opioid receptor agonists. The administration of dopamine, haloperidol, and morphine into the caudate putamen has been shown to increase the spontaneous firing of neurons in the substantia nigra and thereby stimulate dopamine release in the caudate putamen (Chesselet et al., 1981; Finnerty and Chan, 1979; Iwatsubo and Clouet, 1977).
3.4 Kappa-opioid receptor ligands and natural reinforcers
Extensive preclinical evidence indicates that kappa-opioid receptors play an important role in food intake. Kappa-opioid receptor agonists have been shown to increase the intake of regular lab chow and highly palatable foods (sweetened milk, sucrose pellets, sucrose solution) in hungry and sated rats (Badiani et al., 2001; Cooper et al., 1985; Locke et al., 1982; Morley and Levine, 1981; Ruegg et al., 1997). In contrast, the kappa-opioid receptor antagonist nor-BNI decreases food intake and prevents food intake induced by kappa-opioid receptor agonists (Beczkowska et al., 1992; Levine et al., 1990). These findings suggest that stimulation of kappa-opioid receptors increases feeding irrespective of the satiety status and the palatability of the available food source. Relatively little research has been conducted in order to investigate the neuronal substrates via which dynorphin-like peptides increase food intake. At this point in time, two brain sites have been identified by which the dynorphin-like peptides may increase food intake. The administration of dynorphin A into the medial hypothalamus and the ventral tegmental area has been shown to increase food intake in rats (Gosnell, 1988) (Hamilton and Bozarth, 1988). Dynorphin A (1-13) binds with a high affinity to kappa-opioid receptors but also with a low affinity to mu-opioid receptors and therefore the dose-response curves of dynorphin A (1-13) and the mu-opioid receptor agonist morphine were compared. It was shown that dynorphin A (1-13) was about 50,000 times more potent than morphine in eliciting feeding behavior, which suggests that the effects of dynorphin A (1-13) were mediated via the activation of the kappa-opioid receptor. Nencini and Stewart reported that the administration of a kappa-opioid receptor agonist, U-50488, in the ventral tegmental area does not stimulate food intake (Nencini and Stewart, 1990). There was, however, a major difference between these two studies in the way the food intake was measured. Hamilton and Bozarth recorded feeding behavior during the first 15 minutes after the administration of dynorphin A (1-13) (Hamilton and Bozarth, 1988). In contrast, Nencini and Steward recorded the amount of food consumed after 2 hours and 5 hours (Nencini and Stewart, 1990). Therefore, it might be possible that the activation of kappa-opioid receptors in the ventral tegmental area induces a short burst of food intake which cannot be detected when food intake is investigated over a 2 or 5 hour time period.
Some evidence suggests that the effects of dynorphin-like peptides on food intake depend on the emotional state of the animal. Mild tail pinches have been shown to increase food intake in sated rats (Antelman and Caggiula, 1977). Tail pinch-induced food intake is at least partly mediated by the release of dopamine in the dorsal striatum and the endogenous release of opioids. This is supported by the observation that lesioning of the dopaminergic nigrostriatal bundle or the administration of the nonspecific opioid receptor antagonist naloxone inhibits tail pinch-induced food intake in rats (Antelman and Szechtman, 1975; Morley and Levine, 1980). Very little research has been conducted to investigate the role of dynorphin-like peptides in tail pinch-induced food intake. However, tail pinch stress has been shown to decrease dynorphin A (1-13) levels in the cortex but not in the hypothalamus (Morley et al., 1982). Additional studies are needed to investigate if the tail pinch-induced release of dynorphin-like peptides in the cortex affects tail pinch-induced food intake. In contrast to the mild tail pinch stressor, more severe stressors such as restraint stress decrease food intake in rats (Harris et al., 1998). The effects of restraint stress on food intake are at least partly mediated by the endogenous release of CRF. The CRF receptor antagonist α-helical CRF(9-41) prevents restraint stress-induced hypophagia and weight loss (Smagin et al., 1999). The administration of CRF into the lateral ventricles has been shown to decrease food intake in rats (Britton et al., 1982). CRF has also been shown to suppress dynorphin A (1-13) induced feeding in rats (Levine et al., 1983). The observation that CRF inhibits dynorphin A (1-13)-induced food intake is in line with previous observations that indicate that CRF is an extremely potent inhibitor of food intake. CRF has been shown to inhibit feeding induced by the GABA type A agonist muscimol, norepinephrine, insulin, and severe, 30 hours, of food deprivation (Levine et al., 1983; Morley and Levine, 1982). Taken together, these findings suggest that CRF counteracts the effects of dynorphin-like peptides on food intake.
Extensive evidence points toward a role of kappa-opioid receptor signaling in male sexual behavior. Kappa-opioid receptor agonists have been shown to decrease sexual behavior in male rats, rabbits, and newts (Agmo et al., 1994; Deviche and Moore, 1987; Leyton and Stewart, 1992). Very little research has been done to investigate the neurobiological substrates that mediate the decrease in sexual behavior induced by kappa-opioid receptor agonists. Some evidence suggests that endogenous dynorphin-like peptides may decrease sexual behavior by stimulating kappa-opioid receptors in the ventral tegmental area, the nucleus accumbens, and the medial preoptic area (Leyton and Stewart, 1992). The administration of the kappa-opioid receptor antagonist nor-BNI into the ventral tegmental area, nucleus accumbens, or medial preoptic area increases female-directed behavior (Leyton and Stewart, 1992). In addition, the administration of nor-BNI in these brain sites prevented some of the sexual inhibitory effects (e.g, decreased female directed behavior, decreased number of ejaculations, increased copulation and ejaculation latencies) that were observed after the systemic administration of the kappa-opioid receptor agonist U-50488.
4. Kappa-opioid receptor ligands and drug abuse
4.1 Drugs of abuse and adaptations in dynorphin signaling
The noncontingent administration of drugs of abuse and the self-administration of drugs of abuse leads to increased extracellular dopamine levels in the mesolimbic and the nigrostriatal dopaminergic system (Cadoni and Di, 1999; Paulson and Robinson, 1995; Yoshida et al., 1993). The drug-induced dopamine release in the nucleus accumbens has been suggested to play an important role in the initiation and maintenance of drug self-administration and establishing stimulus–reward associations (Fouriezos et al., 1978; Robbins et al., 1989; Robbins and Everitt, 1982; Spyraki et al., 1982; Spyraki et al., 1983; Spyraki et al., 1987; Wise et al., 1978). Although the great majority of the studies have focused on the role of dopamine in the nucleus accumbens in brain reward function, accumulating evidence suggests that the caudate putamen also plays an important role in signaling reward. Schultz and colleagues investigated the effects of the presentation of a liquid-reward in a go-nogo task on the activity of neurons in the nucleus accumbens and the caudate putamen of monkeys (Apicella et al., 1991). They demonstrated that the presentation of the liquid reward increased the activity of neurons in the caudate putamen and the nucleus accumbens. A positron emission tomography (PET) study demonstrated that playing video games for a monetary incentive increased dopamine transmission in the caudate putamen and the nucleus accumbens (Koepp et al., 1998). Furthermore, when test subjects in a functional Magnetic Resonance Imaging (fMRI) study were shown pictures of people with whom they were intensely in love they displayed increased activity in the ventral tegmental area and the caudate nucleus (Aron et al., 2005).
Drugs of abuse induce feelings of euphoria and a dramatic release of dopamine in the mesolimbic and nigrostriatal dopaminergic systems. The drug induced release of dopamine in the brain is much greater than that induced by any natural reinforcer. For example, dopamine levels in the nucleus accumbens are 1000% of those of baseline levels after the administration of amphetamine and dopamine levels in the same brain site are 140% of baseline levels after sex in male rats (Di Chiara and Imperato, 1988a; Fiorino and Phillips, 1999). High levels of dopamine have neurotoxic effects as indicated by a degeneration of dopaminergic terminals and apoptosis in the striatum (Krasnova and Cadet, 2009). Extensive evidence indicates that drugs of abuse induce the release of dynorphin-like peptides (Sivam, 1989; Smiley et al., 1990), which might be a compensatory response to prevent highly elevated dopamine levels and the accompanying neurotoxic effects. This is supported by the observation that kappa-opioid receptor agonists protect against some of the neurotoxic effects of methamphetamine. The administration of methamphetamine to mice (10 mg/kg, 4 time in 1 day) leads to a long-term decreased methamphetamine and potassium-induced dopamine release in the caudate putamen (El Daly et al., 2000), which is likely caused by the neurotoxic effects of high levels of dopamine (Krasnova and Cadet, 2009). The administration of the kappa-opioid receptor agonist U-69593 prior to two of the four methamphetamine injections prevented the methamphetamine-induced effects on dopamine transmission. Recent evidence indicates that kappa-opioid receptor agonists may also prevent some of the molecular effects of drugs of abuse. Drugs of abuse and dopamine increase the phosphorylation of DARPP-32 (dopamine- and cyclic AMP-regulated phosphoprotein, Mr = 32,000) at threonine-34 and decrease the phosphorylation at threonine-75 via a dopamine D1 receptor dependent mechanism (Hemmings, Jr. et al., 1984b; Hemmings, Jr. et al., 1984a; Nishi et al., 2000; Svenningsson et al., 2000). The phosphorylation and dephosphorylation of DARPP-32 has been shown to affect many of the behavioral, neurochemical, and electrophysiological effects of drugs of abuse (Borgkvist and Fisone, 2007; Fienberg and Greengard, 2000). In a recent study it was shown that cocaine increases the phosphorylation of DARPP-32 at threonine-34 in the caudate putamen, prefrontal cortex, and the hippocampus. Pretreatment with the kappa-opioid receptor agonist U-69593 prevents the cocaine-induced phosphorylation of DARPP-32 at threonine-34 in these brain sites (D’Addario et al., 2007).
It is interesting to note that a majority of the studies indicate that chronic cocaine increases dynorphin-like peptide levels in the nigrostriataldopaminergic system but not in the mesolimbic dopaminergic system. Cocaine self-administration in rats and monkeys increases prodynorphin mRNA in the caudate putamen but not in the nucleus accumbens (Daunais et al., 1993; Daunais et al., 1995; Fagergren et al., 2003; Schlussman et al., 2005). In contrast to the aforementioned studies, Schlussman and colleagues demonstrated that cocaine self-administration in rats leads to an increase in dynorphin mRNA levels in the nucleus accumbens and the caudate putamen (Hurd et al., 1992). The difference between these studies might be due to differences in cocaine administration schedules, rat strains, and inconsistencies in the selection of brain sections. In addition to cocaine, the administration of alcohol, amphetamine, methamphetamine, morphine, and MDMA (3,4-methylenedioxymethamphetamine) has been shown to increase dynorphin levels in the nigrostriatal dopaminergic system (Gulya et al., 1993; Hanson et al., 1987; Hanson et al., 1988; Singh et al., 1991; Trujillo and Akil, 1990). Trujillo and Akil compared the effects of chronic morphine and chronic amphetamine on the expression of dynorphin-like peptides in the nucleus accumbens and the caudate putamen (Trujillo and Akil, 1990). Chronic amphetamine increased dynorphin A (1-8), dynorphin A (1-17), dynorphin B (1-13), and α-neoendorphin levels in the caudate putamen. Chronic amphetamine did not induce significant increases in the levels of these peptides in the nucleus accumbens. Chronic morphine significantly increased dynorphin A (1-8) and α-neoendorphin levels in the caudate putamen and also induced a non-significant increase in dynorphin A (1-17), 52%, and dynorphin B (1-13), 45%, in this brain site. Chronic morphine did not affect the dynorphin-like peptide levels in the nucleus accumbens. These findings suggest that psychostimulants and opioids might have similar effects on dynorphin-like peptide levels in the caudate putamen. A recent study demonstrated that a single injection of nicotine increases dynorphin B (1-13) and prodynorphin mRNA levels in mice. It was shown that dynorphin B (1-13) levels in the nucleus accumbens and the caudate putamen were increased 1 hour and 18 hours after the administration of nicotine (Isola et al., 2009). The prodynorphin mRNA levels were increased in the nucleus accumbens and the caudate putamen 3 hours after the administration of nicotine. A detailed analysis of the effects of nicotine on prodynorphin mRNA levels in the caudate putamen indicated that a single injection of nicotine increased prodynorphin mRNA levels in the caudate putamen for about 24 hours.
Drug abuse also leads to increased dynorphin levels in the human brain. Hurd and Herkenham investigated the effects of cocaine abuse on prodynorphin mRNA levels in the nucleus accumbens and caudate putamen (Hurd and Herkenham, 1993). It was shown that cocaine abuse leads to a significant increase in prodynorphin mRNA levels in the putamen, but not in the caudate or the nucleus accumbens. There was a trend toward an increase in prodynorphin mRNA levels in the putamen of subjects with a history of cocaine abuse. In a more recent study the effects of cocaine abuse on dynorphin A (1-17) levels in the nucleus accumbens and caudate putamen of subjects with a history of cocaine abuse was investigated (Frankel et al., 2008). It was demonstrated that chronic cocaine abuse leads to an increase in dynorphin A (1-17) levels in the caudate, but not in the putamen or the nucleus accumbens. There was, however, a trend towards an increase in dynorphin A (1-17) in the putamen of cocaine abusers. These studies with human brain samples suggest that chronic cocaine abuse leads to an increase in dynorphin production in the caudate putamen but not in the nucleus accumbens. Increased prodynorphin mRNA levels have been detected in the caudate nuclei of suicide victims compared to those of normal controls and patients with schizophrenia (Hurd et al., 1997). Therefore, the cocaine-induced hyperactivity of the dorsal striatal dynorphin system could possibly contribute to the high suicide rate among cocaine abusers (Marzuk et al., 1992).
Cocaine abuse also leads to a dramatic increase in dynorphin A levels in the ventral pallidum (Frankel et al., 2008). The dynorphin A (1-17) level in the ventral pallidum of chronic cocaine abusers was about 450% of that of controls while the dynorphin A (1-17) level in the caudate of chronic cocaine abusers was about 200% of that of controls (Frankel et al., 2008). The ventral pallidum is a ventral extension of the globus pallidus and has been suggested to play an important role in regulating brain reward function (Noback et al., 2005; Smith et al., 2009). Dopaminergic neurons from the ventral tegmental area project to the ventral pallidum and the administration of a dopamine D1 receptor agonist into the ventral pallidum increases the firing rate of a majority, 69%, of the neurons in this brain site (Klitenick et al., 1992; Maslowski and Napier, 1991). Drugs of abuse such as alcohol and cocaine increases the release of dopamine in the ventral pallidum (Melendez et al., 2003; Sizemore et al., 2000). In addition, the administration of cocaine or amphetamine into the ventral pallidum induces conditioned place preference (Gong et al., 1996). Furthermore, 6-hydroxydopamine-induced lesioning of dopaminergic terminals in the ventral pallidum prevents the development of cocaine-induced conditioned place preference (Gong et al., 1997). Taken together, these findings indicate that drug-induced dopamine release in the ventral pallidum plays a critical role in signaling the rewarding effects of drugs of abuse. It is suggested here that the increase in dynorphin levels in the ventral pallidum of chronic cocaine abusers might be an adaptive response to attenuate the cocaine-induced dopamine release in this brain site (Frankel et al., 2008). The administration of heroin to rats has also been shown to induce a strong increase in dynorphin B levels in the globus pallidus (Weissman and Zamir, 1987). This suggests that opioids might have similar effects on dynorphin-like peptide levels in the globus pallidus/ventral pallidum complex as psychostimulants. Taken together, the above discussed literature indicates that drugs of abuse increase the release of dopamine in the mesolimbic and nigrostriatal dopaminergic systems. The concomitant release of the dynorphin-like peptides in the caudate putamen and to a lesser degree in the nucleus accumbens might act as negative feedback loop to decrease dopamine release.
4.2 Kappa-opioid receptor ligands and drug reward
Evidence indicates that kappa-opioid receptor agonists attenuate the behavioral effects of psychostimulants. The effects of kappa-opioid receptor agonists have been more thoroughly evaluated on the rewarding and locomotor effects of cocaine than on the effects of nicotine, amphetamine, or other psychostimulants. The kappa-opioid receptor agonists U-69593 and U-50488 have been shown to prevent the acute locomotor effects of cocaine (Heidbreder et al., 1995). In addition, pretreatment with the kappa-opioid receptor agonists prevents the development of cocaine-induced locomotor sensitization (Heidbreder et al., 1993; Heidbreder et al., 1995). Kappa-opioid receptor agonists reduce the self-administration of cocaine in rats, mice, and rhesus monkeys (Glick et al., 1995; Kuzmin et al., 1997; Negus et al., 1997; Schenk et al., 1999). In addition, pretreatment with kappa-opioid receptor agonists prevents the development of cocaine-induced conditioned place preference in mice and rats (Suzuki et al., 1992; Zhang et al., 2004). Finally, cocaine lowers the brain reward thresholds of rats in the ICSS procedure. A lowering of brain reward thresholds in the ICSS procedure is indicative of a potentiation of brain reward function. Pretreatment with kappa-opioid receptor agonists prevents the cocaine-induced lowering in brain reward thresholds (Tomasiewicz et al., 2008). Taken together, these findings indicate that kappa-opioid receptor agonists prevent the locomotor and the rewarding effects of cocaine. Some evidence has been provided to suggest that kappa-opioid receptor agonists attenuate the behavioral effects of amphetamine and nicotine. Gray and colleagues demonstrated that amphetamine induces a strong increase in activity in rats (e.g., locomotion, rearing, grooming) and this effect was partially blocked by pretreatment with the kappa-opioid receptor agonist U-69593 (Gray et al., 1999). Furthermore, pretreatment with the kappa-opioid receptor agonists U-50488, U-69593, and CI-977 prevents the nicotine-induced increase in locomotor activity in rats (Hahn et al., 2000). At this point in time, it is not known if the kappa-opioid receptor agonists attenuate the rewarding effects of nicotine, amphetamine, and other psychostimulants. Stimulation of kappa-opioid receptors has also been reported to attenuate some of the behavioral effects of the mu-opioid receptor agonist morphine. Morphine has been shown to induce hyperactivity in mice and this effect can be prevented by pretreatment with the kappa-opioid receptor agonist TRK-820 (Tsuji et al., 2001). Kappa-opioid receptor agonists have also been shown to decrease the self-administration of morphine in rats and mice at doses that do not affect the self-administration of water (Glick et al., 1995; Kuzmin et al., 1997). In addition, low doses of kappa-opioid receptor agonists that do not induce place aversion alone prevent morphine-induced conditioned place preference in rats and mice (Bolanos et al., 1996; Funada et al., 1993). Thus, the aforementioned studies indicate that low, non-aversive doses of kappa-opioid receptor agonists decrease the rewarding effects of morphine in rats and mice.
4.3 Kappa-opioid receptor ligands and drug withdrawal
Drug withdrawal leads to a severe negative emotional state and has been associated with a decreased release of dopamine in the brain (Parsons et al., 1991; Paulson and Robinson, 1996; Weiss et al., 1992). It is hypothesized here that a continued increased production and release of dynorphin-like peptides during the withdrawal phase contributes to the decreased dopamine release and the negative affective state associated with drug withdrawal. Extensive evidence points toward an increased production and release of dynorphin-like peptides during the acute and protracted drug withdrawal phase. Isola and colleagues investigated the effects of nicotine withdrawal in mice on dynorphin B (1-13) protein levels and prodynorphin mRNA in the striatum (nucleus accumbens and caudate putamen combined). The dynorphin B (1-13) levels were decreased from 4 to 72 hours after the discontinuation of nicotine administration. A decrease in dynorphin B (1-13) levels is indicative of an increased release of dynorphin B (1-13). The prodynorphin mRNA levels were increased from 8 hours until at least 96 hours after the discontinuation of nicotine administration. It is interesting to note that the decrease in dynorphin B (1-13) levels parallels the deficit in brain reward function associated with nicotine withdrawal (Bruijnzeel et al., 2007). In a previous study we reported that the brain reward thresholds of rats are elevated from 3–96 hours after discontinuation of nicotine administration (Bruijnzeel et al., 2007). Prodynorphin mRNA levels in the nucleus accumbens are also increased during acute alcohol withdrawal (Przewlocka et al., 1997). The prodynorphin mRNA levels were increased 24 and 48 hours post alcohol administration in rats and were returned to baseline levels 96 hours post alcohol administration (Przewlocka et al., 1997). Lindholm and colleagues reported that the dynorphin B (1-13) levels are also increased in the nucleus accumbens of rats 21 days after a period of chronic alcohol administration (Lindholm et al., 2000). The increased dynorphin B (1-13) levels in the nucleus accumbens might contribute to the protracted negative affective state associated with the discontinuation of chronic alcohol intake (Rylkova et al., 2008). Discontinuation of chronic cocaine administration also leads to increased prodynorphin mRNA levels in the lateral hypothalamus of rats (Zhou et al., 2008). Therefore, a hyperactivity of dynorphin systems in the hypothalamus might contribute to the negative emotional state associated with cocaine withdrawal.
A hyperactivity of the dynorphin systems might also play a role in alcohol intake in alcohol dependent patients. Rodents self-administer alcohol and the alcohol intake is greatly increased when the rats have been chronically exposed to alcohol vapor (O’Dell et al., 2004). Walker and Koob demonstrated that the kappa-opioid receptor antagonist nor-BNI decreases the self-administration of alcohol in alcohol dependent animals which have been chronically exposed to alcohol vapor and does not affect alcohol self-administration in non-dependent animals (Walker and Koob, 2008a). The same investigators proposed that chronic alcohol intake leads to a hyperactivity of the brain dynorphin systems. After the discontinuation of alcohol intake the increased release of dynorphin may lead to a negative emotional state, craving, and high levels of alcohol intake when alcohol becomes available. Blockade of the kappa-opioid receptors may decrease alcohol intake by attenuating the negative emotional state associated with alcohol withdrawal (Walker and Koob, 2008a; Walker and Koob, 2008b).
Additional support for a role of dynorphin in drug withdrawal is provided by the observation that kappa-opioid receptor knockout mice display fewer naloxone-precipitated somatic withdrawal signs (body tremor, ptosis, jumping sniffing, and diarrhea) than wild-type controls (Simonin et al., 1998). The above discussed studies suggest that a hyperactivity of the brain dynorphin systems at least partly mediates some of the somatic and affective drug withdrawal signs. It should be noted, however, that there is evidence to suggest that kappa-opioid receptor agonists could potentially attenuate opioid and nicotine withdrawal. For example, the kappa-opioid receptor agonists U-50488 and TRK-820 have been reported to prevent mecamylamine-induced conditioned place aversion in nicotine dependent animals (Ise et al., 2002). The latter study seems to be in conflict with other studies that demonstrated that kappa-opioid receptor agonists induce conditioned place aversion (Mucha and Herz, 1985; Zhang et al., 2005). Mucha and Herz reported that 1 mg/kg of U-50488 induces conditioned place aversion and Ise and colleagues reported that the same dose of U-50488 completely prevented nicotine withdrawal-induced conditioned place aversion (Ise et al., 2002; Mucha and Herz, 1985). It might be possible that in the aforementioned studies U-50488 mediated its effects in control animals and animals withdrawing from nicotine via a distinct molecular mechanism. Kappa-opioid receptor antagonists are potent calcium channel inhibitors (Macdonald and Werz, 1986; Tallent et al., 1994; Werz and Macdonald, 1984). Calcium channel inhibitors have been shown to attenuate mecamylamine-precipitated somatic withdrawal signs in rats (Biala and Weglinska, 2005). Furthermore, calcium channel inhibitors have also been shown to possess antidepressant and anxiolytic-like properties and inhibit alcohol, morphine, and benzodiazepine withdrawal (Pucilowski, 1992). Therefore, the kappa-opioid receptor agonist U-50488 might have prevented nicotine withdrawal induced place aversion by inhibiting calcium channels. Kappa-opioid receptor agonists also inhibit dopamine release in the nucleus accumbens and caudate putamen of drug free animals (Di Chiara and Imperato, 1988b). Decreased dopamine levels in the striatum have been associated with negative emotional states and therefore the kappa-opioid receptor agonist U-50488 might have induced place aversion in the drug free animals by inhibiting dopamine release in the nucleus accumbens and caudate putamen. Future studies are needed to investigate if chronic drug administration alters the neurochemical response to kappa-opioid receptor agonists. For example, it is not known if chronic drug administration affects the coupling of kappa-opioid receptors to calcium channels and if kappa-opioid receptor agonists inhibit the release of dopamine in the striatum of drug dependent animals to a similar degree as in drug naive animals.
The stimulation of kappa-opioid receptors has been suggested to decrease opioid withdrawal signs. Tulunay and colleagues reported that dynorphin B (1-13) suppresses spontaneous jumping in morphine withdrawing mice (Tulunay et al., 1981). In addition, the intravenous administration of dynorphin B decreases heroin withdrawal symptoms (yawning, diarrhea, running nose, etc) in humans (Wen et al., 1984; Wen and Ho, 1982). In contrast, the kappa-opioid receptor antagonist nor-BNI potentiates naloxone-induced place aversion in morphine dependent rats and increases the number of naloxone-induced somatic withdrawal signs (Spanagel et al., 1994). Taken together, these findings suggest that stimulation of kappa-opioid receptors ameliorates opioid withdrawal signs. The affective and somatic opioid withdrawal signs are at least partly mediated by an increased release of norepinephrine in the brain (Delfs et al., 2000; Gold et al., 1978). It is hypothesized here that kappa-opioid receptor agonists attenuate opioid withdrawal by decreasing the opioid withdrawal induced release of norepinephrine. The kappa-opioid receptor is coupled to an N-type calcium channel and activation of the kappa-opioid receptors inhibits N-type calcium currents (Tallent et al., 1994). Drugs that block the N-type calcium channels have been shown to block norepinephrine release from sympathetic neurons (Hirning et al., 1988). This is in line with the observation that dynorphin A (1-13) and the kappa-opioid receptor agonist ethylketocyclazocine decrease the potassium evoked release of [3H]noradrenaline from guinea pig cortical slices (Kinouchi et al., 1989). In addition, the kappa-opioid receptor agonist U-50488 has been shown to inhibit norepinephrine release from brain slices (Werling et al., 1988). Future studies are needed to investigate if the kappa-opioid receptor agonists prevent norepinephrine release in the brain associated with opioid withdrawal (Done et al., 1992; Rossetti et al., 1993). The stimulation of the kappa-opioid receptors has also been shown to prevent alcohol withdrawal-induced seizures. Beadles-Bohling and Wiren investigated the effects of the kappa-opioid receptor antagonist nor-BNI and the kappa-opioid receptor agonist U-50488 on handling-induced convulsions in alcohol withdrawing mice. It was shown that U-50488 reduced handling-induced convulsions and nor-BNI increased handling-induced convulsions (Beadles-Bohling and Wiren, 2006). Alcohol withdrawal-induced seizures are at least partly mediated by an increased glutamate release in the brain and stimulation of kappa-opioid receptors has been shown to decrease glutamate release (Hjelmstad and Fields, 2003; Tsai et al., 1995; Tsai and Coyle, 1998). Therefore, kappa-opioid receptor agonists might prevent alcohol withdrawal-induced seizures by decreasing glutamatergic transmission.
5. Concluding remarks
The reviewed studies unequivocally indicate that the dynorphin-like peptides play an important role in regulating the state of the brain reward system. Overall these studies indicate that stimulation of kappa-opioid receptors leads to a negative emotional state by inhibiting the release of dopamine in the striatum. The same mechanism might play a critical role in protecting the brain from the neurotoxic effects of drugs of abuse such as methamphetamine. The behavioral and neurochemical effects of kappa-opioid receptor agonists are opposite to those of kappa-opioid receptor antagonists. Kappa-opioid receptor antagonists have been shown to have potent antidepressant-like effects and prevent stress-induced negative emotional states (Land et al., 2008; Mague et al., 2003; Pliakas et al., 2001). The studies discussed in this review suggest that chronic drug intake induces neuroadaptations in the brain dynorphin system that inhibit drug-induced dopamine release. The increased production of dynorphin-like peptides may initially counteract the effects of drugs of abuse but might induce a negative emotional state when drug intake ceases and these adaptations remain unopposed. It should be noted, however, that there is evidence that suggests that kappa-opioid receptor agonists attenuate drug withdrawal. Kappa-opioid receptor agonists have been shown to regulate ion channels and glutamatergic, GABAergic, and noradrenergic transmission (Hirning et al., 1988; Hjelmstad and Fields, 2003; Kinouchi et al., 1989; Tallent et al., 1994). Therefore, kappa-opioid receptor agonists may attenuate drug withdrawal symptomatology by decreasing glutamatergic, GABAergic, or noradrenergic transmission in the brain. It cannot be ruled out that the effects of kappa-opioid receptor agonists/antagonists are brain site specific. For example, it could be hypothesized that the administration of kappa-opioid receptor antagonists into the striatum prevents drug withdrawal by increasing dopamine levels. In contrast, kappa-opioid receptor agonists could prevent drug withdrawal by inhibiting glutamatergic, GABAergic, or noradrenergic transmission in brain sites that mediate negative mood states such as the central nucleus of the amygdala or the bed nucleus of the stria terminalis.
It is interesting to note that the drug induced adaptations in the dynorphin system are most pronounced in brain areas such as the caudate putamen, globus pallidus, and the ventral pallidum (Daunais et al., 1995; Frankel et al., 2008; Weissman and Zamir, 1987). It was originally thought that the role of these brain areas was limited to controlling motor function. However, accumulating evidence demonstrates that these areas play a pivotal role in regulating mood states. Future studies are needed to investigate the role of the dynorphin-like peptides in the caudate putamen, globus pallidus, and ventral pallidum in regulating the state of the brain reward system. The dynorphin-like peptides have also been shown to play a critical role in regulating dopamine release in the nucleus accumbens. Therefore they may play a critical role in preventing the development of drug addictions by attenuating the rewarding effects of drugs of abuse. Taken together, these studies would advocate investigating the role of dynorphin-like peptides and their receptors in the basal ganglia in regulating the state of the brain reward systems. A better understanding of the role of the dynorphin system might contribute to the development of novel treatments for mood disorders, drug addictions, and other disorders that stem from a dysregulation of the brain reward system.
Acknowledgments
This research was funded by National Institute on Drug Abuse grants DA023575 and DA020502 and a Flight Attendant Medical Research Institute Young Clinical Scientist Award (Grant nr. 52312) to AB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agmo A, Paredes RG, Contreras JL. Opioids and sexual behavior in the male rabbit: the role of central and peripheral opioid receptors. J Neural Transm Gen Sect. 1994;97:211–223. doi: 10.1007/BF02336142. [DOI] [PubMed] [Google Scholar]
- Amalric M, Cline EJ, Martinez JL, Jr, Bloom FE, Koob GF. Rewarding properties of beta-endorphin as measured by conditioned place preference. Psychopharmacology (Berl) 1987;91:14–19. doi: 10.1007/BF00690919. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Press; Washington, DC: 2000. [Google Scholar]
- Antelman SM, Caggiula AR. Norepinephrine-dopamine interactions and behavior. Science. 1977;195:646–653. doi: 10.1126/science.841304. [DOI] [PubMed] [Google Scholar]
- Antelman SM, Szechtman H. Tail pinch induces eating in sated rats which appears to depend on nigrostriatal dopamine. Science. 1975;189:731–733. doi: 10.1126/science.1154024. [DOI] [PubMed] [Google Scholar]
- Apicella P, Ljungberg T, Scarnati E, Schultz W. Responses to reward in monkey dorsal and ventral striatum. Exp Brain Res. 1991;85:491–500. doi: 10.1007/BF00231732. [DOI] [PubMed] [Google Scholar]
- Aron A, Fisher H, Mashek DJ, Strong G, Li H, Brown LL. Reward, motivation, and emotion systems associated with early-stage intense romantic love. J Neurophysiol. 2005;94:327–337. doi: 10.1152/jn.00838.2004. [DOI] [PubMed] [Google Scholar]
- Attali B, Gouarderes C, Mazarguil H, Audigier Y, Cros J. Differential interaction of opiates to multiple “kappa” binding sites in the guinea-pig lumbo-sacral spinal cord. Life Sci. 1982;31:1371–1375. doi: 10.1016/0024-3205(82)90384-8. [DOI] [PubMed] [Google Scholar]
- Attali B, Saya D, Vogel Z. Kappa-opiate agonists inhibit adenylate cyclase and produce heterologous desensitization in rat spinal cord. J Neurochem. 1989;52:360–369. doi: 10.1111/j.1471-4159.1989.tb09130.x. [DOI] [PubMed] [Google Scholar]
- Badiani A, Rajabi H, Nencini P, Stewart J. Modulation of food intake by the kappa opioid U-50,488H: evidence for an effect on satiation. Behav Brain Res. 2001;118:179–186. doi: 10.1016/s0166-4328(00)00325-9. [DOI] [PubMed] [Google Scholar]
- Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–495. [PubMed] [Google Scholar]
- Barr AM, Fiorino DF, Phillips AG. Effects of withdrawal from an escalating dose schedule of d-amphetamine on sexual behavior in the male rat. Pharmacol Biochem Behav. 1999;64:597–604. doi: 10.1016/s0091-3057(99)00156-2. [DOI] [PubMed] [Google Scholar]
- Beadles-Bohling AS, Wiren KM. Anticonvulsive effects of kappa-opioid receptor modulation in an animal model of ethanol withdrawal. Genes Brain Behav. 2006;5:483–496. doi: 10.1111/j.1601-183X.2005.00200.x. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Howard JL, Shelton KL, Carroll FI. Differential effects of the novel kappa opioid receptor antagonist, JDTic, on reinstatement of cocaine-seeking induced by footshock stressors vs cocaine primes and its antidepressant-like effects in rats. Psychopharmacology (Berl) 2005;183:118–126. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- Beczkowska IW, Bowen WD, Bodnar RJ. Central opioid receptor subtype antagonists differentially alter sucrose and deprivation-induced water intake in rats. Brain Res. 1992;589:291–301. doi: 10.1016/0006-8993(92)91289-q. [DOI] [PubMed] [Google Scholar]
- Biala G, Weglinska B. Blockade of the expression of mecamylamine-precipitated nicotine withdrawal by calcium channel antagonists. Pharmacol Res. 2005;51:483–488. doi: 10.1016/j.phrs.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Bolanos CA, Garmsen GM, Clair MA, McDougall SA. Effects of the kappa-opioid receptor agonist U-50,488 on morphine-induced place preference conditioning in the developing rat. Eur J Pharmacol. 1996;317:1–8. doi: 10.1016/s0014-2999(96)00698-x. [DOI] [PubMed] [Google Scholar]
- Bolger N, DeLongis A, Kessler RC, Schilling EA. Effects of daily stress on negative mood. J Pers Soc Psychol. 1989;57:808–818. doi: 10.1037//0022-3514.57.5.808. [DOI] [PubMed] [Google Scholar]
- Borgkvist A, Fisone G. Psychoactive drugs and regulation of the cAMP/PKA/DARPP-32 cascade in striatal medium spiny neurons. Neurosci Biobehav Rev. 2007;31:79–88. doi: 10.1016/j.neubiorev.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Braida D, Limonta V, Capurro V, Fadda P, Rubino T, Mascia P, Zani A, Gori E, Fratta W, Parolaro D, Sala M. Involvement of kappa-opioid and endocannabinoid system on Salvinorin A-induced reward. Biol Psychiatry. 2008;63:286–292. doi: 10.1016/j.biopsych.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Brand AN, Jolles J, Gispen-de WC. Recall and recognition memory deficits in depression. J Affect Disord. 1992;25:77–86. doi: 10.1016/0165-0327(92)90095-n. [DOI] [PubMed] [Google Scholar]
- Britt JP, McGehee DS. Presynaptic opioid and nicotinic receptor modulation of dopamine overflow in the nucleus accumbens. J Neurosci. 2008;28:1672–1681. doi: 10.1523/JNEUROSCI.4275-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton DR, Koob GF, Rivier J, Vale W. Intraventricular corticotropin-releasing factor enhances behavioral effects of novelty. Life Sci. 1982;31:363–367. doi: 10.1016/0024-3205(82)90416-7. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Gold MS. The role of corticotropin-releasing factor-like peptides in cannabis, nicotine, and alcohol dependence. Brain Res Brain Res Rev. 2005;49:505–528. doi: 10.1016/j.brainresrev.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Lewis B, Bajpai LK, Morey TE, Dennis DM, Gold M. Severe deficit in brain reward function associated with fentanyl withdrawal in rats. Biol Psychiatry. 2006;59:477–480. doi: 10.1016/j.biopsych.2005.07.020. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Zislis G, Wilson C, Gold MS. Antagonism of CRF receptors prevents the deficit in brain reward function associated with precipitated nicotine withdrawal in rats. Neuropsychopharmacology. 2007;32:955–963. doi: 10.1038/sj.npp.1301192. [DOI] [PubMed] [Google Scholar]
- Cadoni C, Di CG. Reciprocal changes in dopamine responsiveness in the nucleus accumbens shell and core and in the dorsal caudate-putamen in rats sensitized to morphine. Neuroscience. 1999;90:447–455. doi: 10.1016/s0306-4522(98)00466-7. [DOI] [PubMed] [Google Scholar]
- Cador M, Ahmed SH, Koob GF, Le MM, Stinus L. Corticotropin-releasing factor induces a place aversion independent of its neuroendocrine role. Brain Res. 1992;597:304–309. doi: 10.1016/0006-8993(92)91487-y. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Beguin C, Dinieri JA, Baumann MH, Richards MR, Todtenkopf MS, Rothman RB, Ma Z, Lee DY, Cohen BM. Depressive-Like Effects of the {kappa}-Opioid Receptor Agonist Salvinorin A on Behavior and Neurochemistry in Rats. J Pharmacol Exp Ther. 2006;316:440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- Chen JC, Smith ER, Cahill M, Cohen R, Fishman JB. The opioid receptor binding of dezocine, morphine, fentanyl, butorphanol and nalbuphine. Life Sci. 1993;52:389–396. doi: 10.1016/0024-3205(93)90152-s. [DOI] [PubMed] [Google Scholar]
- Chesselet MF, Cheramy A, Reisine TD, Glowinski J. Morphine and delta-opiate agonists locally stimulate in vivo dopamine release in cat caudate nucleus. Nature. 1981;291:320–322. doi: 10.1038/291320a0. [DOI] [PubMed] [Google Scholar]
- Chieng B, Williams JT. Increased opioid inhibition of GABA release in nucleus accumbens during morphine withdrawal. J Neurosci. 1998;18:7033–7039. doi: 10.1523/JNEUROSCI.18-17-07033.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church WH, Justice JB, Jr, Byrd LD. Extracellular dopamine in rat striatum following uptake inhibition by cocaine, nomifensine and benztropine. Eur J Pharmacol. 1987;139:345–348. doi: 10.1016/0014-2999(87)90592-9. [DOI] [PubMed] [Google Scholar]
- Civelli O, Douglass J, Goldstein A, Herbert E. Sequence and expression of the rat prodynorphin gene. Proc Natl Acad Sci U S A. 1985;82:4291–4295. doi: 10.1073/pnas.82.12.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JA, Liu L, Price M, Hersh B, Edelson M, Pasternak GW. Kappa opiate receptor multiplicity: evidence for two U50,488-sensitive kappa 1 subtypes and a novel kappa 3 subtype. J Pharmacol Exp Ther. 1989;251:461–468. [PubMed] [Google Scholar]
- Comb M, Seeburg PH, Adelman J, Eiden L, Herbert E. Primary structure of the human Met- and Leu-enkephalin precursor and its mRNA. Nature. 1982;295:663–666. doi: 10.1038/295663a0. [DOI] [PubMed] [Google Scholar]
- Connor M, Kitchen I. Has the sun set on kappa3-opioid receptors? Br J Pharmacol. 2006;147:349–350. doi: 10.1038/sj.bjp.0706603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper SJ, Jackson A, Morgan R, Carter R. Evidence for opiate receptor involvement in the consumption of a high palatability diet in nondeprived rats. Neuropeptides. 1985;5:345–348. doi: 10.1016/0143-4179(85)90024-1. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Hoyer D, Markou A. Withdrawal from chronic amphetamine induces depressive-like behavioral effects in rodents. Biol Psychiatry. 2003;54:49–58. doi: 10.1016/s0006-3223(02)01730-4. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- D’Addario C, Di BM, Candeletti S, Romualdi P. The kappa-opioid receptor agonist U-69593 prevents cocaine-induced phosphorylation of DARPP-32 at Thr(34) in the rat brain. Brain Res Bull. 2007;73:34–39. doi: 10.1016/j.brainresbull.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Daunais JB, Roberts DC, McGinty JF. Cocaine self-administration increases preprodynorphin, but not c-fos, mRNA in rat striatum. Neuroreport. 1993;4:543–546. doi: 10.1097/00001756-199305000-00020. [DOI] [PubMed] [Google Scholar]
- Daunais JB, Roberts DC, McGinty JF. Short-term cocaine self administration alters striatal gene expression. Brain Res Bull. 1995;37:523–527. doi: 10.1016/0361-9230(95)00049-k. [DOI] [PubMed] [Google Scholar]
- de Wied D. Melanotropins as neuropeptides. Ann N Y Acad Sci. 1993;680:20–28. doi: 10.1111/j.1749-6632.1993.tb19672.x. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- Deviche P, Moore FL. Opioid kappa-receptor agonists suppress sexual behaviors in male rough-skinned newts (Taricha granulosa) Horm Behav. 1987;21:371–383. doi: 10.1016/0018-506x(87)90021-3. [DOI] [PubMed] [Google Scholar]
- Di Benedetto M, D’Addario C, Collins S, Izenwasser S, Candeletti S, Romualdi P. Role of serotonin on cocaine-mediated effects on prodynorphin gene expression in the rat brain. J Mol Neurosci. 2004;22:213–222. doi: 10.1385/JMN:22:3:213. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988a;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther. 1988b;244:1067–1080. [PubMed] [Google Scholar]
- Done C, Silverstone P, Sharp T. Effect of naloxone-precipitated morphine withdrawal on noradrenaline release in rat hippocampus in vivo. Eur J Pharmacol. 1992;215:333–336. doi: 10.1016/0014-2999(92)90052-6. [DOI] [PubMed] [Google Scholar]
- Donzanti BA, Althaus JS, Payson MM, Von Voigtlander PF. Kappa agonist-induced reduction in dopamine release: site of action and tolerance. Res Commun Chem Pathol Pharmacol. 1992;78:193–210. [PubMed] [Google Scholar]
- Dorsa DM, van Ree JM, de WD. Effects of [Des-Tyr 1]-gamma-endorphin and alpha-endorphin on substantia nigra self-stimulation. Pharmacol Biochem Behav. 1979;10:899–905. doi: 10.1016/0091-3057(79)90065-0. [DOI] [PubMed] [Google Scholar]
- El Daly E, Chefer V, Sandill S, Shippenberg TS. Modulation of the neurotoxic effects of methamphetamine by the selective kappa-opioid receptor agonist U69593. J Neurochem. 2000;74:1553–1562. doi: 10.1046/j.1471-4159.2000.0741553.x. [DOI] [PubMed] [Google Scholar]
- Evans RM, Zamponi GW. Presynaptic Ca2+ channels--integration centers for neuronal signaling pathways. Trends Neurosci. 2006;29:617–624. doi: 10.1016/j.tins.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Fagergren P, Smith HR, Daunais JB, Nader MA, Porrino LJ, Hurd YL. Temporal upregulation of prodynorphin mRNA in the primate striatum after cocaine self-administration. Eur J Neurosci. 2003;17:2212–2218. doi: 10.1046/j.1460-9568.2003.02636.x. [DOI] [PubMed] [Google Scholar]
- Fallon JH. Collateralization of monoamine neurons: mesotelencephalic dopamine projections to caudate, septum, and frontal cortex. J Neurosci. 1981;1:1361–1368. doi: 10.1523/JNEUROSCI.01-12-01361.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature. 1997;385:165–168. doi: 10.1038/385165a0. [DOI] [PubMed] [Google Scholar]
- Fienberg AA, Greengard P. The DARPP-32 knockout mouse. Brain Res Brain Res Rev. 2000;31:313–319. doi: 10.1016/s0165-0173(99)00047-8. [DOI] [PubMed] [Google Scholar]
- Finnerty EP, Chan SH. Morphine suppression of substantia nigra zona reticulata neurons in the rat: implicated role for a novel striatonigral feedback mechanism. Eur J Pharmacol. 1979;59:307–310. doi: 10.1016/0014-2999(79)90296-6. [DOI] [PubMed] [Google Scholar]
- Fiorino DF, Phillips AG. Facilitation of sexual behavior and enhanced dopamine efflux in the nucleus accumbens of male rats after D-amphetamine-induced behavioral sensitization. J Neurosci. 1999;19:456–463. doi: 10.1523/JNEUROSCI.19-01-00456.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischli W, Goldstein A, Hunkapiller MW, Hood LE. Isolation and amino acid sequence analysis of a 4,000-dalton dynorphin from porcine pituitary. Proc Natl Acad Sci U S A. 1982;79:5435–5437. doi: 10.1073/pnas.79.17.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci. 2001;21:4915–4922. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouriezos G, Hansson P, Wise RA. Neuroleptic-induced attenuation of brain stimulation reward in rats. J Comp Physiol Psychol. 1978;92:661–671. doi: 10.1037/h0077500. [DOI] [PubMed] [Google Scholar]
- Frankel PS, Alburges ME, Bush L, Hanson GR, Kish SJ. Striatal and ventral pallidum dynorphin concentrations are markedly increased in human chronic cocaine users. Neuropharmacology. 2008;55:41–46. doi: 10.1016/j.neuropharm.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funada M, Suzuki T, Narita M, Misawa M, Nagase H. Blockade of morphine reward through the activation of kappa-opioid receptors in mice. Neuropharmacology. 1993;32:1315–1323. doi: 10.1016/0028-3908(93)90026-y. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. Basal Ganglia. In: Paxinos G, editor. The Rat Nervous System. Elsevier Academic Press; San Diego, CA: 2004. pp. 455–509. [Google Scholar]
- Glick SD, Maisonneuve IM, Raucci J, Archer S. Kappa opioid inhibition of morphine and cocaine self-administration in rats. Brain Res. 1995;681:147–152. doi: 10.1016/0006-8993(95)00306-b. [DOI] [PubMed] [Google Scholar]
- Gold MS, Redmond DE, Jr, Kleber HD. Clonidine blocks acute opiate- withdrawal symptoms. Lancet. 1978;2:599–602. doi: 10.1016/s0140-6736(78)92823-4. [DOI] [PubMed] [Google Scholar]
- Goldstein A, Fischli W, Lowney LI, Hunkapiller M, Hood L. Porcine pituitary dynorphin: complete amino acid sequence of the biologically active heptadecapeptide. Proc Natl Acad Sci U S A. 1981;78:7219–7223. doi: 10.1073/pnas.78.11.7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A, Ghazarossian VE. Immunoreactive dynorphin in pituitary and brain. Proc Natl Acad Sci U S A. 1980;77:6207–6210. doi: 10.1073/pnas.77.10.6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A, Tachibana S, Lowney LI, Hunkapiller M, Hood L. Dynorphin-(1–13), an extraordinarily potent opioid peptide. Proc Natl Acad Sci U S A. 1979;76:6666–6670. doi: 10.1073/pnas.76.12.6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez RG, Fleming SH, Keller J, Flores B, Kenna H, DeBattista C, Solvason B, Schatzberg AF. The neuropsychological profile of psychotic major depression and its relation to cortisol. Biol Psychiatry. 2006;60:472–478. doi: 10.1016/j.biopsych.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Gong W, Neill D, Justice JB., Jr Conditioned place preference and locomotor activation produced by injection of psychostimulants into ventral pallidum. Brain Res. 1996;707:64–74. doi: 10.1016/0006-8993(95)01222-2. [DOI] [PubMed] [Google Scholar]
- Gong W, Neill D, Justice JB., Jr 6-Hydroxydopamine lesion of ventral pallidum blocks acquisition of place preference conditioning to cocaine. Brain Res. 1997;754:103–112. doi: 10.1016/s0006-8993(97)00059-0. [DOI] [PubMed] [Google Scholar]
- Gosnell BA. Involvement of mu opioid receptors in the amygdala in the control of feeding. Neuropharmacology. 1988;27:319–326. doi: 10.1016/0028-3908(88)90050-0. [DOI] [PubMed] [Google Scholar]
- Gray AM, Rawls SM, Shippenberg TS, McGinty JF. The kappa-opioid agonist, U-69593, decreases acute amphetamine-evoked behaviors and calcium-dependent dialysate levels of dopamine and glutamate in the ventral striatum. J Neurochem. 1999;73:1066–1074. doi: 10.1046/j.1471-4159.1999.0731066.x. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Vermeulen-Van der ZE, te KA, Witter MP. Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience. 1987;23:103–120. doi: 10.1016/0306-4522(87)90275-2. [DOI] [PubMed] [Google Scholar]
- Gucciardi E, Celasun N, Ahmad F, Stewart DE. Eating Disorders. BMC Womens Health. 2004;4(Suppl 1):S21. doi: 10.1186/1472-6874-4-S1-S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulya K, Orpana AK, Sikela JM, Hoffman PL. Prodynorphin and vasopressin mRNA levels are differentially affected by chronic ethanol ingestion in the mouse. Brain Res Mol Brain Res. 1993;20:1–8. doi: 10.1016/0169-328x(93)90105-x. [DOI] [PubMed] [Google Scholar]
- Hahn B, Stolerman IP, Shoaib M. Kappa-opioid receptor modulation of nicotine-induced behaviour. Neuropharmacology. 2000;39:2848–2855. doi: 10.1016/s0028-3908(00)00119-2. [DOI] [PubMed] [Google Scholar]
- Hamilton ME, Bozarth MA. Feeding elicited by dynorphin (1–13) microinjections into the ventral tegmental area in rats. Life Sci. 1988;43:941–946. doi: 10.1016/0024-3205(88)90271-8. [DOI] [PubMed] [Google Scholar]
- Hanson GR, Merchant KM, Letter AA, Bush L, Gibb JW. Methamphetamine-induced changes in the striatal-nigral dynorphin system: role of D-1 and D-2 receptors. Eur J Pharmacol. 1987;144:245–246. doi: 10.1016/0014-2999(87)90527-9. [DOI] [PubMed] [Google Scholar]
- Hanson GR, Merchant KM, Letter AA, Bush L, Gibb JW. Characterization of methamphetamine effects on the striatal-nigral dynorphin system. Eur J Pharmacol. 1988;155:11–18. doi: 10.1016/0014-2999(88)90397-4. [DOI] [PubMed] [Google Scholar]
- Harris RB, Zhou J, Youngblood BD, Rybkin II, Smagin GN, Ryan DH. Effect of repeated stress on body weight and body composition of rats fed low- and high-fat diets. Am J Physiol. 1998;275:R1928–R1938. doi: 10.1152/ajpregu.1998.275.6.R1928. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Babovic-Vuksanovic D, Shoaib M, Shippenberg TS. Development of behavioral sensitization to cocaine: influence of kappa opioid receptor agonists. J Pharmacol Exp Ther. 1995;275:150–163. [PubMed] [Google Scholar]
- Heidbreder CA, Goldberg SR, Shippenberg TS. The kappa-opioid receptor agonist U-69593 attenuates cocaine-induced behavioral sensitization in the rat. Brain Res. 1993;616:335–338. doi: 10.1016/0006-8993(93)90228-f. [DOI] [PubMed] [Google Scholar]
- Heijna MH, Padt M, Hogenboom F, Portoghese PS, Mulder AH, Schoffelmeer AN. Opioid receptor-mediated inhibition of dopamine and acetylcholine release from slices of rat nucleus accumbens, olfactory tubercle and frontal cortex. Eur J Pharmacol. 1990;181:267–278. doi: 10.1016/0014-2999(90)90088-n. [DOI] [PubMed] [Google Scholar]
- Heimer L, Wilson RD. The subcortical projections of the allocortex: similarities in the neural associations of the hippocampus, the piriform cortex, and the neocortex. Golgi centenial symposium proceedings Raven; New York: 1975. pp. 177–193. [Google Scholar]
- Hemmings HC, Jr, Greengard P, Tung HY, Cohen P. DARPP-32, a dopamine-regulated neuronal phosphoprotein, is a potent inhibitor of protein phosphatase-1. Nature. 1984a;310:503–505. doi: 10.1038/310503a0. [DOI] [PubMed] [Google Scholar]
- Hemmings HC, Jr, Williams KR, Konigsberg WH, Greengard P. DARPP-32, a dopamine- and adenosine 3′:5′-monophosphate-regulated neuronal phosphoprotein. I Amino acid sequence around the phosphorylated threonine. J Biol Chem. 1984b;259:14486–14490. [PubMed] [Google Scholar]
- Henry DJ, Grandy DK, Lester HA, Davidson N, Chavkin C. Kappa-opioid receptors couple to inwardly rectifying potassium channels when coexpressed by Xenopus oocytes. Mol Pharmacol. 1995;47:551–557. [PubMed] [Google Scholar]
- Hernandez L, Hoebel BG. Feeding and hypothalamic stimulation increase dopamine turnover in the accumbens. Physiol Behav. 1988;44:599–606. doi: 10.1016/0031-9384(88)90324-1. [DOI] [PubMed] [Google Scholar]
- Hirning LD, Fox AP, McCleskey EW, Olivera BM, Thayer SA, Miller RJ, Tsien RW. Dominant role of N-type Ca2+ channels in evoked release of norepinephrine from sympathetic neurons. Science. 1988;239:57–61. doi: 10.1126/science.2447647. [DOI] [PubMed] [Google Scholar]
- Hjelmstad GO, Fields HL. Kappa opioid receptor inhibition of glutamatergic transmission in the nucleus accumbens shell. J Neurophysiol. 2001;85:1153–1158. doi: 10.1152/jn.2001.85.3.1153. [DOI] [PubMed] [Google Scholar]
- Hjelmstad GO, Fields HL. Kappa opioid receptor activation in the nucleus accumbens inhibits glutamate and GABA release through different mechanisms. J Neurophysiol. 2003;89:2389–2395. doi: 10.1152/jn.01115.2002. [DOI] [PubMed] [Google Scholar]
- Horikawa S, Takai T, Toyosato M, Takahashi H, Noda M, Kakidani H, Kubo T, Hirose T, Inayama S, Hayashida H. Isolation and structural organization of the human preproenkephalin B gene. Nature. 1983;306:611–614. doi: 10.1038/306611a0. [DOI] [PubMed] [Google Scholar]
- Hughes J, Smith TW, Kosterlitz HW, Fothergill LA, Morgan BA, Morris HR. Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature. 1975;258:577–580. doi: 10.1038/258577a0. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Brown EE, Finlay JM, Fibiger HC, Gerfen CR. Cocaine self-administration differentially alters mRNA expression of striatal peptides. Brain Res Mol Brain Res. 1992;13:165–170. doi: 10.1016/0169-328x(92)90058-j. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Herkenham M. Molecular alterations in the neostriatum of human cocaine addicts. Synapse. 1993;13:357–369. doi: 10.1002/syn.890130408. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Herman MM, Hyde TM, Bigelow LB, Weinberger DR, Kleinman JE. Prodynorphin mRNA expression is increased in the patch vs matrix compartment of the caudate nucleus in suicide subjects. Mol Psychiatry. 1997;2:495–500. doi: 10.1038/sj.mp.4000319. [DOI] [PubMed] [Google Scholar]
- Ignatov I, Kovalenko VS, Andreev BV, Titov MI. Effect of leu- and met-enkephalins on the brain’s reinforcement systems. Biull Eksp Biol Med. 1981;92:33–35. [PubMed] [Google Scholar]
- Ise Y, Narita M, Nagase H, Suzuki T. Modulation of kappa-opioidergic systems on mecamylamine-precipitated nicotine-withdrawal aversion in rats. Neurosci Lett. 2002;323:164–166. doi: 10.1016/s0304-3940(02)00074-5. [DOI] [PubMed] [Google Scholar]
- Isola R, Zhang H, Tejwani GA, Neff NH, Hadjiconstantinou M. Acute nicotine changes dynorphin and prodynorphin mRNA in the striatum. Psychopharmacology (Berl) 2009;201:507–516. doi: 10.1007/s00213-008-1315-4. [DOI] [PubMed] [Google Scholar]
- Iwamoto ET. Place-conditioning properties of mu, kappa, and sigma opioid agonists. Alcohol Drug Res. 1985;6:327–339. [PubMed] [Google Scholar]
- Iwatsubo K, Clouet DH. Effects of morphine and haloperidol on the electrical activity of rat nigrostriatal neurons. J Pharmacol Exp Ther. 1977;202:429–436. [PubMed] [Google Scholar]
- Jellinger KA. Morphological substrates of parkinsonism with and without dementia: a retrospective clinico-pathological study. J Neural Transm Suppl. 2007:91–104. doi: 10.1007/978-3-211-73574-9_12. [DOI] [PubMed] [Google Scholar]
- Jordan BA, Devi LA. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399:697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakidani H, Furutani Y, Takahashi H, Noda M, Morimoto Y, Hirose T, Asai M, Inayama S, Nakanishi S, Numa S. Cloning and sequence analysis of cDNA for porcine beta-neo-endorphin/dynorphin precursor. Nature. 1982;298:245–249. doi: 10.1038/298245a0. [DOI] [PubMed] [Google Scholar]
- Kangawa K, Matsuo H. alpha-Neo-endorphin: a “big” Leu-enkephalin with potent opiate activity from porcine hypothalami. Biochem Biophys Res Commun. 1979;86:153–160. doi: 10.1016/0006-291x(79)90394-2. [DOI] [PubMed] [Google Scholar]
- Kangawa K, Minamino N, Chino N, Sakakibara S, Matsuo H. The complete amino acid sequence of alpha-neo-endorphin. Biochem Biophys Res Commun. 1981;99:871–878. doi: 10.1016/0006-291x(81)91244-4. [DOI] [PubMed] [Google Scholar]
- Kanner AM. Depression in epilepsy: prevalence, clinical semiology, pathogenic mechanisms, and treatment. Biol Psychiatry. 2003;54:388–398. doi: 10.1016/s0006-3223(03)00469-4. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Dickens SE, Eisfeld BS, Bagby RM. Sexual dysfunction before antidepressant therapy in major depression. J Affect Disord. 1999;56:201–208. doi: 10.1016/s0165-0327(99)00050-6. [DOI] [PubMed] [Google Scholar]
- Khachaturian H, Watson SJ, Lewis ME, Coy D, Goldstein A, Akil H. Dynorphin immunocytochemistry in the rat central nervous system. Peptides. 1982;3:941–954. doi: 10.1016/0196-9781(82)90063-8. [DOI] [PubMed] [Google Scholar]
- Kinouchi K, Maeda S, Saito K, Inoki R, Fukumitsu K, Yoshiya I. Effects of pentazocine and other opioids on the potassium-evoked release of [3H]noradrenaline from guinea pig cortical slices. Eur J Pharmacol. 1989;164:63–68. doi: 10.1016/0014-2999(89)90231-8. [DOI] [PubMed] [Google Scholar]
- Klitenick MA, Deutch AY, Churchill L, Kalivas PW. Topography and functional role of dopaminergic projections from the ventral mesencephalic tegmentum to the ventral pallidum. Neuroscience. 1992;50:371–386. doi: 10.1016/0306-4522(92)90430-a. [DOI] [PubMed] [Google Scholar]
- Knoll AT, Meloni EG, Thomas JB, Carroll FI, Carlezon WA., Jr Anxiolytic-like effects of kappa-opioid receptor antagonists in models of unlearned and learned fear in rats. J Pharmacol Exp Ther. 2007;323:838–845. doi: 10.1124/jpet.107.127415. [DOI] [PubMed] [Google Scholar]
- Koepp MJ, Gunn RN, Lawrence AD, Cunningham VJ, Dagher A, Jones T, Brooks DJ, Bench CJ, Grasby PM. Evidence for striatal dopamine release during a video game. Nature. 1998;393:266–268. doi: 10.1038/30498. [DOI] [PubMed] [Google Scholar]
- Konkoy CS, Childers SR. Dynorphin-selective inhibition of adenylyl cyclase in guinea pig cerebellum membranes. Mol Pharmacol. 1989;36:627–633. [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology 56 Suppl. 2009;1:18–31. doi: 10.1016/j.neuropharm.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res Rev. 2009;60:379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin AV, Semenova S, Gerrits MA, Zvartau EE, van Ree JM. Kappa-opioid receptor agonist U50,488H modulates cocaine and morphine self-administration in drug-naive rats and mice. Eur J Pharmacol. 1997;321:265–271. doi: 10.1016/s0014-2999(96)00961-2. [DOI] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke MR. Depressive symptoms in Parkinson’s disease. Eur J Neurol . 2008;15(Suppl 1):21–25. doi: 10.1111/j.1468-1331.2008.02058.x. [DOI] [PubMed] [Google Scholar]
- Leshner AI, Remler H, Biegon A, Samuel D. Desmethylimipramine (DMI) counteracts learned helplessness in rats. Psychopharmacology (Berl) 1979;66:207–208. doi: 10.1007/BF00427633. [DOI] [PubMed] [Google Scholar]
- Levine AS, Grace M, Billington CJ, Portoghese PS. Nor-binaltorphimine decreases deprivation and opioid-induced feeding. Brain Res. 1990;534:60–64. doi: 10.1016/0006-8993(90)90112-o. [DOI] [PubMed] [Google Scholar]
- Levine AS, Rogers B, Kneip J, Grace M, Morley JE. Effect of centrally administered corticotropin releasing factor (CRF) on multiple feeding paradigms. Neuropharmacology. 1983;22:337–339. doi: 10.1016/0028-3908(83)90249-6. [DOI] [PubMed] [Google Scholar]
- Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology. 2005;30:939–946. doi: 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Lewin B, Cassimeris L, Lingappa VR, Plopper G. Cells. Jones & Bartlett Publishers; Sudbury, MA: 2007. [Google Scholar]
- Lewitt PA. Levodopa for the treatment of Parkinson’s disease. N Engl J Med. 2008;359:2468–2476. doi: 10.1056/NEJMct0800326. [DOI] [PubMed] [Google Scholar]
- Leyton M, Stewart J. The stimulation of central kappa opioid receptors decreases male sexual behavior and locomotor activity. Brain Res. 1992;594:56–74. doi: 10.1016/0006-8993(92)91029-e. [DOI] [PubMed] [Google Scholar]
- Lindholm S, Ploj K, Franck J, Nylander I. Repeated ethanol administration induces short- and long-term changes in enkephalin and dynorphin tissue concentrations in rat brain. Alcohol. 2000;22:165–171. doi: 10.1016/s0741-8329(00)00118-x. [DOI] [PubMed] [Google Scholar]
- Locke KW, Brown DR, Holtzman SG. Effects of opiate antagonists and putative mu- and kappa-agonists on milk intake in rat and squirrel monkey. Pharmacol Biochem Behav. 1982;17:1275–1279. doi: 10.1016/0091-3057(82)90133-2. [DOI] [PubMed] [Google Scholar]
- Lord JA, Waterfield AA, Hughes J, Kosterlitz HW. Endogenous opioid peptides: multiple agonists and receptors. Nature. 1977;267:495–499. doi: 10.1038/267495a0. [DOI] [PubMed] [Google Scholar]
- Lu Z. Mechanism of rectification in inward-rectifier K+ channels. Annu Rev Physiol. 2004;66:103–129. doi: 10.1146/annurev.physiol.66.032102.150822. [DOI] [PubMed] [Google Scholar]
- Lyketsos CG, Olin J. Depression in Alzheimer’s disease: overview and treatment. Biol Psychiatry. 2002;52:243–252. doi: 10.1016/s0006-3223(02)01348-3. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Werz MA. Dynorphin A decreases voltage-dependent calcium conductance of mouse dorsal root ganglion neurones. J Physiol. 1986;377:237–249. doi: 10.1113/jphysiol.1986.sp016184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey DJ, Koob GF, Markou A. CRF and urocortin decreased brain stimulation reward in the rat: reversal by a CRF receptor antagonist. Brain Res. 2000;866:82–91. doi: 10.1016/s0006-8993(00)02229-0. [DOI] [PubMed] [Google Scholar]
- Mague SD, Pliakas AM, Todtenkopf MS, Tomasiewicz HC, Zhang Y, Stevens WC, Jr, Jones RM, Portoghese PS, Carlezon WA., Jr Antidepressant-like effects of kappa-opioid receptor antagonists in the forced swim test in rats. J Pharmacol Exp Ther. 2003;305:323–330. doi: 10.1124/jpet.102.046433. [DOI] [PubMed] [Google Scholar]
- Maisonneuve IM, Archer S, Glick SD. U50,488, a kappa opioid receptor agonist, attenuates cocaine-induced increases in extracellular dopamine in the nucleus accumbens of rats. Neurosci Lett. 1994;181:57–60. doi: 10.1016/0304-3940(94)90559-2. [DOI] [PubMed] [Google Scholar]
- Malmnas CO. The significance of dopamine, versus other catecholamines, for L-dopa induced facilitation of sexual behavior in the castrated male rat. Pharmacol Biochem Behav. 1976;4:521–526. doi: 10.1016/0091-3057(76)90191-x. [DOI] [PubMed] [Google Scholar]
- Mansour A, Burke S, Pavlic RJ, Akil H, Watson SJ. Immunohistochemical localization of the cloned kappa 1 receptor in the rat CNS and pituitary. Neuroscience. 1996;71:671–690. doi: 10.1016/0306-4522(95)00464-5. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci. 1995;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J Neurosci. 1987;7:2445–2464. [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Chefer VI, Shippenberg TS, Hjelmstad GO, Fields HL. Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc Natl Acad Sci U S A. 2006;103:2938–2942. doi: 10.1073/pnas.0511159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN WR, EADES CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976;197:517–532. [PubMed] [Google Scholar]
- Marzuk PM, Tardiff K, Leon AC, Stajic M, Morgan EB, Mann JJ. Prevalence of cocaine use among residents of New York City who committed suicide during a one-year period. Am J Psychiatry. 1992;149:371–375. doi: 10.1176/ajp.149.3.371. [DOI] [PubMed] [Google Scholar]
- Maslowski RJ, Napier TC. Dopamine D1 and D2 receptor agonists induce opposite changes in the firing rate of ventral pallidal neurons. Eur J Pharmacol. 1991;200:103–112. doi: 10.1016/0014-2999(91)90672-d. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology. 2006;31:1241–1248. doi: 10.1038/sj.npp.1300872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI, Rodd-Henricks ZA, McBride WJ, Murphy JM. Alcohol stimulates the release of dopamine in the ventral pallidum but not in the globus pallidus: a dual-probe microdialysis study. Neuropsychopharmacology. 2003;28:939–946. doi: 10.1038/sj.npp.1300081. [DOI] [PubMed] [Google Scholar]
- Meng F, Xie GX, Thompson RC, Mansour A, Goldstein A, Watson SJ, Akil H. Cloning and pharmacological characterization of a rat kappa opioid receptor. Proc Natl Acad Sci U S A. 1993;90:9954–9958. doi: 10.1073/pnas.90.21.9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshul CK, McGinty JF. Kappa opioid receptor immunoreactivity in the nucleus accumbens and caudate-putamen is primarily associated with synaptic vesicles in axons. Neuroscience. 2000;96:91–99. doi: 10.1016/s0306-4522(99)90481-5. [DOI] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Minamino N, Kangawa K, Chino N, Sakakibara S, Matsuo H. Beta-neo-endorphin, a new hypothalamic “big” Leu-enkephalin of porcine origin: its purification and the complete amino acid sequence. Biochem Biophys Res Commun. 1981;99:864–870. doi: 10.1016/0006-291x(81)91243-2. [DOI] [PubMed] [Google Scholar]
- Minamino N, Kangawa K, Fukuda A, Matsuo H, Iagarashi M. A new opioid octapeptide related to dynorphin from porcine hypothalamus. Biochem Biophys Res Commun. 1980;95:1475–1481. doi: 10.1016/s0006-291x(80)80063-5. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Pasternak GW. The molecular and behavioral pharmacology of the orphanin FQ/nociceptin peptide and receptor family. Pharmacol Rev. 2001;53:381–415. [PubMed] [Google Scholar]
- Mollereau C, Parmentier M, Mailleux P, Butour JL, Moisand C, Chalon P, Caput D, Vassart G, Meunier JC. ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett. 1994;341:33–38. doi: 10.1016/0014-5793(94)80235-1. [DOI] [PubMed] [Google Scholar]
- Morley JE, Elson MK, Levine AS, Shafer RB. The effects of stress on central nervous system concentrations of the opioid pepti, dedynorphin. Peptides. 1982;3:901–906. doi: 10.1016/0196-9781(82)90058-4. [DOI] [PubMed] [Google Scholar]
- Morley JE, Levine AS. Stress-induced eating is mediated through endogenous opiates. Science. 1980;209:1259–1261. doi: 10.1126/science.6250222. [DOI] [PubMed] [Google Scholar]
- Morley JE, Levine AS. Dynorphin-(1–13) induces spontaneous feeding in rats. Life Sci. 1981;29:1901–1903. doi: 10.1016/0024-3205(81)90522-1. [DOI] [PubMed] [Google Scholar]
- Morley JE, Levine AS. Corticotrophin releasing factor, grooming and ingestive behavior. Life Sci. 1982;31:1459–1464. doi: 10.1016/0024-3205(82)90007-8. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Herz A. Motivational properties of kappa and mu opioid receptor agonists studied with place and taste preference conditioning. Psychopharmacology (Berl) 1985;86:274–280. doi: 10.1007/BF00432213. [DOI] [PubMed] [Google Scholar]
- Nakanishi S, Inoue A, Kita T, Nakamura M, Chang AC, Cohen SN, Numa S. Nucleotide sequence of cloned cDNA for bovine corticotropin-beta-lipotropin precursor. Nature. 1979;278:423–427. doi: 10.1038/278423a0. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Portoghese PS, Lin CE. Effects of kappa opioids on cocaine self-administration by rhesus monkeys. J Pharmacol Exp Ther. 1997;282:44–55. [PubMed] [Google Scholar]
- Nencini P, Stewart J. Chronic systemic administration of amphetamine increases food intake to morphine, but not to U50-488H, microinjected into the ventral tegmental area in rats. Brain Res. 1990;527:254–258. doi: 10.1016/0006-8993(90)91144-6. [DOI] [PubMed] [Google Scholar]
- Newton SS, Thome J, Wallace TL, Shirayama Y, Schlesinger L, Sakai N, Chen J, Neve R, Nestler EJ, Duman RS. Inhibition of cAMP response element-binding protein or dynorphin in the nucleus accumbens produces an antidepressant-like effect. J Neurosci. 2002;22:10883–10890. doi: 10.1523/JNEUROSCI.22-24-10883.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM, Surmeier J, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- Nishi A, Bibb JA, Snyder GL, Higashi H, Nairn AC, Greengard P. Amplification of dopaminergic signaling by a positive feedback loop. Proc Natl Acad Sci U S A. 2000;97:12840–12845. doi: 10.1073/pnas.220410397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noback CR, Demarest RJ, Ruggiero DA. The Human Nervous System: Structure and Function. Humana Press; Totowa, NJ: 2005. [Google Scholar]
- Noda M, Furutani Y, Takahashi H, Toyosato M, Hirose T, Inayama S, Nakanishi S, Numa S. Cloning and sequence analysis of cDNA for bovine adrenal preproenkephalin. Nature. 1982;295:202–206. doi: 10.1038/295202a0. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Olianas MC, Concas D, Onali P. Agonist activity of naloxone benzoylhydrazone at recombinant and native opioid receptors. Br J Pharmacol. 2006;147:360–370. doi: 10.1038/sj.bjp.0706601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons LH, Smith AD, Justice JB., Jr Basal extracellular dopamine is decreased in the rat nucleus accumbens during abstinence from chronic cocaine. Synapse. 1991;9:60–65. doi: 10.1002/syn.890090109. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Robinson TE. Amphetamine-induced time-dependent sensitization of dopamine neurotransmission in the dorsal and ventral striatum: a microdialysis study in behaving rats. Synapse. 1995;19:56–65. doi: 10.1002/syn.890190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson PE, Robinson TE. Regional differences in the effects of amphetamine withdrawal on dopamine dynamics in the striatum. Analysis of circadian patterns using automated on-line microdialysis. Neuropsychopharmacology. 1996;14:325–337. doi: 10.1016/0893-133X(95)00141-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 1998. [DOI] [PubMed] [Google Scholar]
- Pennartz CM, Groenewegen HJ, Lopes da Silva FH. The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog, Neurobiol. 1994;42:719–761. doi: 10.1016/0301-0082(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Damsma G, Nomikos GG, Wenkstern DG, Blaha CD, Phillips AG, Fibiger HC. Sexual behavior enhances central dopamine transmission in the male rat. Brain Res. 1990;530:345–348. doi: 10.1016/0006-8993(90)91309-5. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- Phillips AG, Lepiane FG. Reward produced by microinjection of (D-Ala2),Met5-enkephalinamide into the ventral tegmental area. Behav Brain Res. 1982;5:225–229. doi: 10.1016/0166-4328(82)90057-2. [DOI] [PubMed] [Google Scholar]
- Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30:2192–2204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- Prather PL, McGinn TM, Claude PA, Liu-Chen LY, Loh HH, Law PY. Properties of a kappa-opioid receptor expressed in CHO cells: interaction with multiple G-proteins is not specific for any individual G alpha subunit and is similar to that of other opioid receptors. Brain Res Mol Brain Res. 1995;29:336–346. doi: 10.1016/0169-328x(94)00264-f. [DOI] [PubMed] [Google Scholar]
- Przewlocka B, Turchan J, Lason W, Przewlocki R. Ethanol withdrawal enhances the prodynorphin system activity in the rat nucleus accumbens. Neurosci Lett. 1997;238:13–16. doi: 10.1016/s0304-3940(97)00829-x. [DOI] [PubMed] [Google Scholar]
- Pucilowski O. Psychopharmacological properties of calcium channel inhibitors. Psychopharmacology (Berl) 1992;109:12–29. doi: 10.1007/BF02245476. [DOI] [PubMed] [Google Scholar]
- Quirion R, Hammer RP, Jr, Herkenham M, Pert CB. Phencyclidine (angel dust)/sigma “opiate” receptor: visualization by tritium-sensitive film. Proc Natl Acad Sci U S A. 1981;78:5881–5885. doi: 10.1073/pnas.78.9.5881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada P, Moreno SA, Tucci S, Gonzalez LE, Harrison T, Chau DT, Hoebel BG, Hernandez L. Glutamate release in the nucleus accumbens is involved in behavioral depression during the PORSOLT swim test. Neuroscience. 2003;119:557–565. doi: 10.1016/s0306-4522(03)00162-3. [DOI] [PubMed] [Google Scholar]
- Rawls SM, McGinty JF. Kappa receptor activation attenuates L-trans-pyrrolidine-2,4-dicarboxylic acid-evoked glutamate levels in the striatum. J Neurochem. 1998;70:626–634. doi: 10.1046/j.1471-4159.1998.70020626.x. [DOI] [PubMed] [Google Scholar]
- Reijnders JS, Ehrt U, Weber WE, Aarsland D, Leentjens AF. A systematic review of prevalence studies of depression in Parkinson’s disease. Mov Disord. 2008;23:183–189. doi: 10.1002/mds.21803. [DOI] [PubMed] [Google Scholar]
- Rimoy GH, Wright DM, Bhaskar NK, Rubin PC. The cardiovascular and central nervous system effects in the human of U-62066E. A selective opioid receptor agonist. Eur J Clin Pharmacol. 1994;46:203–207. doi: 10.1007/BF00192549. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Cador M, Taylor JR, Everitt BJ. Limbic-striatal interactions in reward-related processes. Neurosci Biobehav Rev. 1989;13:155–162. doi: 10.1016/s0149-7634(89)80025-9. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Functional studies of the central catecholamines. Int Rev Neurobiol. 1982;23:303–365. doi: 10.1016/s0074-7742(08)60628-5. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Longu G, Mercuro G, Gessa GL. Extraneuronal noradrenaline in the prefrontal cortex of morphine-dependent rats: tolerance and withdrawal mechanisms. Brain Res. 1993;609:316–320. doi: 10.1016/0006-8993(93)90889-u. [DOI] [PubMed] [Google Scholar]
- Ruegg H, Yu WZ, Bodnar RJ. Opioid-receptor subtype agonist-induced enhancements of sucrose intake are dependent upon sucrose concentration. Physiol Behav. 1997;62:121–128. doi: 10.1016/s0031-9384(97)00151-0. [DOI] [PubMed] [Google Scholar]
- Rylkova D, Boissoneault J, Isaac S, Prado M, Shah HP, Bruijnzeel AW. Effects of NPY and the specific Y1 receptor agonist [D-His(26)]-NPY on the deficit in brain reward function and somatic signs associated with nicotine withdrawal in rats. Neuropeptides. 2008;42:215–227. doi: 10.1016/j.npep.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzet M, Tasiemski A. Involvement of pro-enkephalin-derived peptides in immunity. Dev Comp Immunol. 2001;25:177–185. doi: 10.1016/s0145-305x(00)00047-1. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B, Shippenberg TS. U69593, a kappa-opioid agonist, decreases cocaine self-administration and decreases cocaine-produced drug-seeking. Psychopharmacology (Berl) 1999;144:339–346. doi: 10.1007/s002130051016. [DOI] [PubMed] [Google Scholar]
- Schlussman SD, Zhou Y, Bailey A, Ho A, Kreek MJ. Steady-dose and escalating-dose “binge” administration of cocaine alter expression of behavioral stereotypy and striatal preprodynorphin mRNA levels in rats. Brain Res Bull. 2005;67:169–175. doi: 10.1016/j.brainresbull.2005.04.018. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Gold LH, Stinus L, Koob GF. Relative sensitivity to naloxone of multiple indices of opiate withdrawal: a quantitative dose-response analysis. J Pharmacol Exp Ther. 1994;271:1391–1398. [PubMed] [Google Scholar]
- Seligman ME, Beagley G. Learned helplessness in the rat. J Comp Physiol Psychol. 1975;88:534–541. doi: 10.1037/h0076430. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Herz A. Place preference conditioning reveals the involvement of D1-dopamine receptors in the motivational properties of mu- and kappa-opioid agonists. Brain Res. 1987;436:169–172. doi: 10.1016/0006-8993(87)91571-x. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Ishida H, Iwata M, Hazama GI, Kawahara R, Duman RS. Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J Neurochem. 2004;90:1258–1268. doi: 10.1111/j.1471-4159.2004.02589.x. [DOI] [PubMed] [Google Scholar]
- Simonin F, Gaveriaux-Ruff C, Befort K, Matthes H, Lannes B, Micheletti G, Mattei MG, Charron G, Bloch B, Kieffer B. kappa-Opioid receptor in humans: cDNA and genomic cloning, chromosomal assignment, functional expression, pharmacology, and expression pattern in the central nervous system. Proc Natl Acad Sci U S A. 1995;92:7006–7010. doi: 10.1073/pnas.92.15.7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonin F, Valverde O, Smadja C, Slowe S, Kitchen I, Dierich A, Le MM, Roques BP, Maldonado R, Kieffer BL. Disruption of the kappa-opioid receptor gene in mice enhances sensitivity to chemical visceral pain, impairs pharmacological actions of the selective kappa-agonist U-50,488H and attenuates morphine withdrawal. EMBO J. 1998;17:886–897. doi: 10.1093/emboj/17.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NA, Midgley LP, Bush LG, Gibb JW, Hanson GR. N-Methyl-D-aspartate receptors mediate dopamine-induced changes in extrapyramidal and limbic dynorphin systems. Brain Res. 1991;555:233–238. doi: 10.1016/0006-8993(91)90346-w. [DOI] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Sivam SP. Cocaine selectively increases striatonigral dynorphin levels by a dopaminergic mechanism. J Pharmacol Exp Ther. 1989;250:818–824. [PubMed] [Google Scholar]
- Sizemore GM, Co C, Smith JE. Ventral pallidal extracellular fluid levels of dopamine, serotonin, gamma amino butyric acid, and glutamate during cocaine self-administration in rats. Psychopharmacology (Berl) 2000;150:391–398. doi: 10.1007/s002130000456. [DOI] [PubMed] [Google Scholar]
- Smagin GN, Howell LA, Redmann S, Jr, Ryan DH, Harris RB. Prevention of stress-induced weight loss by third ventricle CRF receptor antagonist. Am J Physiol. 1999;276:R1461–R1468. doi: 10.1152/ajpregu.1999.276.5.R1461. [DOI] [PubMed] [Google Scholar]
- Smiley PL, Johnson M, Bush L, Gibb JW, Hanson GR. Effects of cocaine on extrapyramidal and limbic dynorphin systems. J Pharmacol Exp Ther. 1990;253:938–943. [PubMed] [Google Scholar]
- Smith BR, Brown ZW, Amit Z. Intraventricular self-administration of leucine- enkephalin by laboratory rats. Life Sci. 1982;31:1527–1530. doi: 10.1016/0024-3205(82)90042-x. [DOI] [PubMed] [Google Scholar]
- Smith KS, Tindell AJ, Aldridge JW, Berridge KC. Ventral pallidum roles in reward and motivation. Behav Brain Res. 2009;196:155–167. doi: 10.1016/j.bbr.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Almeida OF, Bartl C, Shippenberg TS. Endogenous kappa-opioid systems in opiate withdrawal: role in aversion and accompanying changes in mesolimbic dopamine release. Psychopharmacology (Berl) 1994;115:121–127. doi: 10.1007/BF02244761. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci U S A. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyraki C, Fibiger HC, Phillips AG. Attenuation by haloperidol of place preference conditioning using food reinforcement. Psychopharmacology (Berl) 1982;77:379–382. doi: 10.1007/BF00432775. [DOI] [PubMed] [Google Scholar]
- Spyraki C, Fibiger HC, Phillips AG. Attenuation of heroin reward in rats by disruption of the mesolimbic dopamine system. Psychopharmacology (Berl) 1983;79:278–283. doi: 10.1007/BF00427827. [DOI] [PubMed] [Google Scholar]
- Spyraki C, Nomikos GG, Varonos DD. Intravenous cocaine-induced place preference: attenuation by haloperidol. Behav Brain Res. 1987;26:57–62. doi: 10.1016/0166-4328(87)90016-7. [DOI] [PubMed] [Google Scholar]
- Stahl SM, Wise DD. The potential role of a corticotropin-releasing factor receptor-1 antagonist in psychiatric disorders. CNS Spectr. 2008;13:467–483. doi: 10.1017/s1092852900016709. [DOI] [PubMed] [Google Scholar]
- Sukhov RR, Walker LC, Rance NE, Price DL, Young WS., III Opioid precursor gene expression in the human hypothalamus. J Comp Neurol. 1995;353:604–622. doi: 10.1002/cne.903530410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Funada M, Narita M, Misawa M, Nagase H. Morphine-induced place preference in the CXBK mouse: characteristics of mu opioid receptor subtypes. Brain Res. 1993;602:45–52. doi: 10.1016/0006-8993(93)90239-j. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Shiozaki Y, Masukawa Y, Misawa M, Nagase H. The role of mu- and kappa-opioid receptors in cocaine-induced conditioned place preference. Jpn J Pharmacol. 1992;58:435–442. doi: 10.1254/jjp.58.435. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Lindskog M, Ledent C, Parmentier M, Greengard P, Fredholm BB, Fisone G. Regulation of the phosphorylation of the dopamine- and cAMP-regulated phosphoprotein of 32 kDa in vivo by dopamine D1, dopamine D2, and adenosine A2A receptors. Proc Natl Acad Sci U S A. 2000;97:1856–1860. doi: 10.1073/pnas.97.4.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Tallent M, Dichter MA, Bell GI, Reisine T. The cloned kappa opioid receptor couples to an N-type calcium current in undifferentiated PC-12 cells. Neuroscience. 1994;63:1033–1040. doi: 10.1016/0306-4522(94)90570-3. [DOI] [PubMed] [Google Scholar]
- Telner JI, Singhal RL. Effects of nortriptyline treatment on learned helplessness in the rat. Pharmacol Biochem Behav. 1981;14:823–826. doi: 10.1016/0091-3057(81)90367-1. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 2004;172:463–470. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- Tomasiewicz HC, Todtenkopf MS, Chartoff EH, Cohen BM, Carlezon WA., Jr The kappa-opioid agonist U69,593 blocks cocaine-induced enhancement of brain stimulation reward. Biol Psychiatry. 2008;64:982–988. doi: 10.1016/j.biopsych.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynor J. Subtypes of the kappa-opioid receptor: fact or fiction? Trends Pharmacol Sci. 1989;10:52–53. doi: 10.1016/0165-6147(89)90074-6. [DOI] [PubMed] [Google Scholar]
- Trujillo KA, Akil H. Pharmacological regulation of striatal prodynorphin peptides. Prog Clin Biol Res. 1990;328:223–226. [PubMed] [Google Scholar]
- Tsai G, Coyle JT. The role of glutamatergic neurotransmission in the pathophysiology of alcoholism. Annu Rev Med. 1998;49:173–184. doi: 10.1146/annurev.med.49.1.173. [DOI] [PubMed] [Google Scholar]
- Tsai G, Gastfriend DR, Coyle JT. The glutamatergic basis of human alcoholism. Am J Psychiatry. 1995;152:332–340. doi: 10.1176/ajp.152.3.332. [DOI] [PubMed] [Google Scholar]
- Tseng LF, Loh HH, Li CH. Beta-Endorphin as a potent analgesic by intravenous injection. Nature. 1976;263:239–240. doi: 10.1038/263239a0. [DOI] [PubMed] [Google Scholar]
- Tsuji M, Takeda H, Matsumiya T, Nagase H, Narita M, Suzuki T. The novel kappa-opioid receptor agonist TRK-820 suppresses the rewarding and locomotor-enhancing effects of morphine in mice. Life Sci. 2001;68:1717–1725. doi: 10.1016/s0024-3205(01)00957-2. [DOI] [PubMed] [Google Scholar]
- Tulunay FC, Jen MF, Chang JK, Loh HH, Lee NM. Possible regulatory role of dynorphin on morphine- and beta-endorphin-induced analgesia. J Pharmacol Exp Ther. 1981;219:296–298. [PubMed] [Google Scholar]
- van Ree JM, Smyth DG, Colpaert FC. Dependence creating properties of lipotropin C-fragment (beta-endorphin): evidence for its internal control of behavior. Life Sci. 1979;24:495–502. doi: 10.1016/0024-3205(79)90170-x. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology. 2008a;33:643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Regarding “Dynorphin is a downstream effector of striatal BDNF regulation of ethanol intake”. FASEB J. 2008b;22:2113–2114. doi: 10.1096/fj.08-0702LTR. [DOI] [PubMed] [Google Scholar]
- Walker JM, Thompson LA, Frascella J, Friederich MW. Opposite effects of mu and kappa opiates on the firing-rate of dopamine cells in the substantia nigra of the rat. Eur J Pharmacol. 1987;134:53–59. doi: 10.1016/0014-2999(87)90130-0. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Strain EC, Abreu ME, Bigelow GE. Enadoline, a selective kappa opioid agonist: comparison with butorphanol and hydromorphone in humans. Psychopharmacology (Berl) 2001;157:151–162. doi: 10.1007/s002130100788. [DOI] [PubMed] [Google Scholar]
- Weber E, Barchas JD. Immunohistochemical distribution of dynorphin B in rat brain: relation to dynorphin A and alpha-neo-endorphin systems. Proc Natl Acad Sci U S A. 1983;80:1125–1129. doi: 10.1073/pnas.80.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber E, Roth KA, Barchas JD. Colocalization of alpha-neo-endorphin and dynorphin immunoreactivity in hypothalamic neurons. Biochem Biophys Res Commun. 1981;103:951–958. doi: 10.1016/0006-291x(81)90902-5. [DOI] [PubMed] [Google Scholar]
- Weber E, Roth KA, Barchas JD. Immunohistochemical distribution of alpha-neo-endorphin/dynorphin neuronal systems in rat brain: evidence for colocalization. Proc Natl Acad Sci U S A. 1982;79:3062–3066. doi: 10.1073/pnas.79.9.3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Markou A, Lorang MT, Koob GF. Basal extracellular dopamine levels in the nucleus accumbens are decreased during cocaine withdrawal after unlimited-access self-administration. Brain Res. 1992;593:314–318. doi: 10.1016/0006-8993(92)91327-b. [DOI] [PubMed] [Google Scholar]
- Weiss F, Parsons LH, Schulteis G, Hyytia P, Lorang MT, Bloom FE, Koob GF. Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. J Neurosci. 1996;16:3474–3485. doi: 10.1523/JNEUROSCI.16-10-03474.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman BA, Zamir N. Differential effects of heroin on opioid levels in the rat brain. Eur J Pharmacol. 1987;139:121–123. doi: 10.1016/0014-2999(87)90506-1. [DOI] [PubMed] [Google Scholar]
- Wen HL, Ho WK. Suppression of withdrawal symptoms by dynorphin in heroin addicts. Eur J Pharmacol. 1982;82:183–186. doi: 10.1016/0014-2999(82)90509-x. [DOI] [PubMed] [Google Scholar]
- Wen HL, Ho WK, Wen PY. Comparison of the effectiveness of different opioid peptides in suppressing heroin withdrawal. Eur J Pharmacol. 1984;100:155–162. doi: 10.1016/0014-2999(84)90217-6. [DOI] [PubMed] [Google Scholar]
- Werling LL, McMahon PN, Cox BM. Selective tolerance at mu and kappa opioid receptors modulating norepinephrine release in guinea pig cortex. J Pharmacol Exp Ther. 1988;247:1103–1106. [PubMed] [Google Scholar]
- Werz MA, Macdonald RL. Dynorphin reduces calcium-dependent action potential duration by decreasing voltage-dependent calcium conductance. Neurosci Lett. 1984;46:185–190. doi: 10.1016/0304-3940(84)90439-7. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wise RA, Spindler J, deWit H, Gerberg GJ. Neuroleptic-induced “anhedonia” in rats: pimozide blocks reward quality of food. Science. 1978;201:262–264. doi: 10.1126/science.566469. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Ramamoorthy S, Shen H, Lake R, Samuvel DJ, Kalivas PW. GABA transmission in the nucleus accumbens is altered after withdrawal from repeated cocaine. J Neurosci. 2003;23:3498–3505. doi: 10.1523/JNEUROSCI.23-08-03498.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie GX, Meng F, Mansour A, Thompson RC, Hoversten MT, Goldstein A, Watson SJ, Akil H. Primary structure and functional expression of a guinea pig kappa opioid (dynorphin) receptor. Proc Natl Acad Sci U S A. 1994;91:3779–3783. doi: 10.1073/pnas.91.9.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda K, Raynor K, Kong H, Breder CD, Takeda J, Reisine T, Bell GI. Cloning and functional comparison of kappa and delta opioid receptors from mouse brain. Proc Natl Acad Sci U S A. 1993;90:6736–6740. doi: 10.1073/pnas.90.14.6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Yokoo H, Tanaka T, Mizoguchi K, Emoto H, Ishii H, Tanaka M. Facilitatory modulation of mesolimbic dopamine neuronal activity by a mu-opioid agonist and nicotine as examined with in vivo microdialysis. Brain Res. 1993;624:277–280. doi: 10.1016/0006-8993(93)90087-4. [DOI] [PubMed] [Google Scholar]
- You ZB, Herrera-Marschitz M, Terenius L. Modulation of neurotransmitter release in the basal ganglia of the rat brain by dynorphin peptides. J Pharmacol Exp Ther. 1999;290:1307–1315. [PubMed] [Google Scholar]
- Zamir N, Palkovits M, Brownstein MJ. Distribution of immunoreactive beta-neo-endorphin in discrete areas of the rat brain and pituitary gland: comparison with alpha-neo-endorphin. J Neurosci. 1984a;4:1248–1252. doi: 10.1523/JNEUROSCI.04-05-01248.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir N, Palkovits M, Brownstein MJ. The distribution of immunoreactive alpha-neo-endorphin in the central nervous system of the rat. J Neurosci. 1984b;4:1240–1247. doi: 10.1523/JNEUROSCI.04-05-01240.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Effect of the kappa opioid agonist R-84760 on cocaine-induced increases in striatal dopamine levels and cocaine-induced place preference in C57BL/6J mice. Psychopharmacology (Berl) 2004;173:146–152. doi: 10.1007/s00213-003-1716-3. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Effects of the plant-derived hallucinogen salvinorin A on basal dopamine levels in the caudate putamen and in a conditioned place aversion assay in mice: agonist actions at kappa opioid receptors. Psychopharmacology (Berl) 2005;179:551–558. doi: 10.1007/s00213-004-2087-0. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Cui CL, Schlussman SD, Choi JC, Ho A, Han JS, Kreek MJ. Effects of cocaine place conditioning, chronic escalating-dose “binge” pattern cocaine administration and acute withdrawal on orexin/hypocretin and preprodynorphin gene expressions in lateral hypothalamus of Fischer and Sprague-Dawley rats. Neuroscience. 2008;153:1225–1234. doi: 10.1016/j.neuroscience.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Chen C, Xue JC, Kunapuli S, DeRiel JK, Liu-Chen LY. Cloning of a human kappa opioid receptor from the brain. Life Sci. 1995;56:201–207. doi: 10.1016/0024-3205(94)00507-o. [DOI] [PubMed] [Google Scholar]
- Zukin RS, Eghbali M, Olive D, Unterwald EM, Tempel A. Characterization and visualization of rat and guinea pig brain kappa opioid receptors: evidence for kappa 1 and kappa 2 opioid receptors. Proc Natl Acad Sci U S A. 1988;85:4061–4065. doi: 10.1073/pnas.85.11.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]


