Summary
Recent reports describe several molecular entities common to the immune and nervous systems. Salient among them are the chemokines and their receptors, which play remarkably varied and potent roles in immunobiology and neurobioloogy. This review limns several illustrative examples and presents general principles of chemokine action which are manifest in both systems:
chemokines tend equally to arrest cells and to make them move, in the process of positioning target cells with spatiotemporal precision;’
signaling and non-signaling receptors collaborate to adjust the chemokine environment for maximal efficacy;
expression of a single chemokine receptor on circulating blood cells and parenchymal cells is often used to coordinate complex tissue responses.
The task before investigators is to integrate knowledge of the roles of key receptors such as CXCR4, CXCR2, CX3CR1, CCR2 (as well as their ligands and other multifunctional chemokine receptors) into a coherent account of events during pathologic processes, in order to guide therapeutic development.
Classification and organization of the chemokine system
The immune and nervous systems have recently been shown to comprise a number of molecules shared in common. Such findings were not unexpected given the complexity of the immune and nervous systems, but the specific molecules and processes involved have often been fascinatingly surprising. Chemokines and their receptors are prominent examples of joint use by the immune and nervous systems, yet seemed initially to be completely devoted to assisting the function of the immune system.
The chemokine universe is comprised of approximately 50 peptides and 20 receptors in humans, with homologues, orthologs and related peptides in other vertebrate species (Charo and Ransohoff, 2006; Rot and von Andrian, 2004). Chemokines are divided into families and signal to corresponding families of chemokine receptors (for example, CXC chemokine action is mediated by CXC chemokine receptors). Chemokine receptors are G-protein coupled receptors (GPCRs) and act specifically through pertussis toxin-sensitive Gαi components. Chemokine-specific GPCRs are drug targets, and the biotech/pharmaceutical industry has mounted substantial efforts to modulate chemokine receptor activity, heightening the medical importance of understanding how chemokines regulate inflammatory disease. First identified by their ability to mediate leukocyte chemoattraction in vitro, chemokines are now recognized to govern a wide array of leukocyte functions during inflammation and immunity.
The numerical mis-match between chemokines and receptors makes it immediately apparent that ligand-receptor relationships may not be simple, and this suspicion has proven to be almost appallingly accurate (Rot and von Andrian, 2004). Several chemokine/receptor pairs are exclusive; for other chemokine receptors, responses can be elicited by as many as 10 individual ligands. Conversely, some chemokines can productively signal to as many as three receptors. There have been several excellent reviews detailing ligand-receptor relationships, as well as a hyperlinked website, current through April, 2006 (http://cytokine.medic.kumamoto-u.ac.jp/CFC/CK/Chemokine.html).
Chemokines deliver astonishingly diverse and context-specific signals. The remarkable versatility and functional flexibility of the chemokine system is conferred by 1) their large number; 2) their tightly-regulated transcriptional expression; 3) their ability to interact with binding moieties (such as glycosaminoglycans or non-signaling receptors) after secretion; and 4) their proteolytic processing (Ransohoff, 2003). Lending further plasticity for regulated expression, chemokine genes can be subject to copy-number polymorphism. Chemokine expression, particularly during inflammation, is primarily regulated by inducible transcription, followed by translation, secretion and turnover. However, two chemokines (CXCL16; CX3CL1) are expressed as transmembrane components which are regulated by cleavage by members of the ADAM (A Disintegrin And Metalloprotease) family enzymes, at least for action at a distance as soluble chemoattractants. Membrane-bound CX3CL1 and CXCL16 can also serve as adhesion molecules for receptor-bearing cells.
The chemokine ligand super-family is further partitioned into sub-groups of the largest (CC chemokines; 28 members) and second-largest (CXC chemokines; 16 members) families. Genomic organization helps to give order to this bewilderingly large super-family (Colobran et al., 2007). Most human CXC chemokines are encoded at chromosomal location 4q12-21, with the majority of CC chemokine found at 17q11-21 and these loci are often syntenic in other mammalian species. Chemokine sub-group members, encoded in multigene arrays, are functionally related. For example, CXCL9, CXCL10, CXCL11, three CXC-family IFN-gamma-inducible chemokines which signal to a single receptor CXCR3 (which is regulated by the Th1-associated transcription factor T-bet), are clustered together separated by at most a few dozen kilobases. A similar array contains eosinophil-attracting eotaxin peptides CCL24 and CCL26, members of the CC family. ‘Solitary’ chemokines, such as CXCL12 at Chr.10q11 and CXCL16 at 17p13 found outside multi-gene arrays, are noted to be paired exclusively with individual signaling receptors and to have distinct functions. With respect to CXCL9, CXCL10 and CXCL11, even though their genomic, structural and in-vitro-functional similarities give an appearance of conspicuous redundancy, careful analysis of cells expressing engineered receptors demonstrated selective signaling pathways suggesting distinct functions (Colvin et al., 2004). This concept was confirmed with the demonstration that CXCL9 played a crucial and non-redundant role in anti-tumor immunity (Gorbachev et al., 2007). Not surprisingly, the role of CXCR3 in T cell trafficking during Th1 immune responses has proven surprisingly subtle and intricate (Koch et al., 2009; Lord et al., 2005; Liu et al., 2006b; Liu et al., 2005). It is accordingly accurate to speak both of redundancy and specificity in the chemokine system (Mantovani, 1999; Charo and Ransohoff, 2006; Rot and von Andrian, 2004).
Chemokines mediate effects by regulating cell-cell interactions
The eponymous action of chemokines towards responsive cells is gradient dependent chemoattraction in-vitro. It seems intuitively appealing to assign chemokines a role in leukocyte trafficking based purely on their ability to drive gradient dependent migration; this notion however is incomplete. Rather, chemokine action causes cells initially to arrest, rather than move, during the multi-step process of leukocyte extravasation (Butcher, 1991) under flow (Alon et al., 2003; Alon and Feigelson, 2002; Cinamon et al., 2004; Cinamon et al., 2001a; Cinamon et al., 2001b; Laudanna and Alon, 2006; Schreiber et al., 2007; Ransohoff et al., 2007). Perhaps most significantly, chemokines mediate both clustering and conformational changes of integrins, leading to high-affinity and -avidity integrin interactions with Cell Adhesion Molecules (CAMs) on vascular endothelial cells.
Chemokine/chemokine receptor signaling (or action through closely-related chemoattractant receptors) is therefore essential for leukocyte-endothelial recognition, which regulates leukocyte trafficking. Leukocyte extravasation requires multiple chemokine-mediated signals. As noted above, the first signal, delivered by chemokines immobilized on the luminal surface of the endothelial cell, helps convert leukocyte rolling under flow on endothelium into arrest, by inducing Rho GTPase family signaling that causes conformational change and redistribution of leukointegrins (Ward, 2009). These alterations in their shape and distribution are required for firm adhesion of leukocyte integrins (such as LFA-1) to CAMs (such as ICAM-1) on endothelium (Shulman et al., 2009). Additional chemokine signals are implicated in ‘crawling’ of leukocytes across endothelium in search of a suitable locus for extravasation. Finally, chemokines are implicated in extravasation itself through inducing cytoskeletal reorganization and chemotaxis towards abluminal chemokines in inflamed tissues. In one classic example, CCL21 is an arrest receptor for lymphocytes rolling on High Endothelial Venules (HEV) of peripheral lymph nodes (Stein et al., 2000). In the context of leukocyte trafficking, chemokines and their receptors are grouped as homeostatic (chemokines expressed constitutively in organs such as in lymph nodes and spleen, with receptors on leukocytes homing to those organs) or inflammatory (chemokines induced on-demand at sites of inflammation, with cognate receptors on infiltrating leukocytes) (Charo and Ransohoff, 2006).
As predicted, gene targeting studies showed that inflammatory chemokine receptors such as CCR2 and CXCR2 are essential for responses to a wide variety of infectious and inflammatory challenges (Charo and Ransohoff, 2006). Studies of the homeostatic chemokine receptors such as CCR7 and CXCR5 led to a paradigm refinement (if not shift): in early gene-targeting experiments, CXCR5 (Forster et al., 1996), and later CCR7, were implicated in developmental organogenesis for lymphoid tissues, as well as in lymphocyte homing to lymph nodes (Lipp and Muller, 2003). These striking findings showed for the first time that chemokine receptors mediated cell migration during development, as well as during inflammatory and immune processes in the postnatal organism.
Non-signaling chemokine receptor-like molecules help localize chemokines within tissues
Considerable effort seems to have been devoted to maintenance of appropriate fluid and tissue levels of chemokines. For example, there are several chemokine receptor-like molecules (D6; Duffy Antigen Receptor for Chemokines: DARC; CCX-CKR; CCRL2 and possibly CXCR7) whose primary function seems to be adjusting ambient concentrations of their chemokine ligands (Locati et al., 2005; Mantovani et al., 2006). In some cases, these receptor-like molecules do not signal by the canonical GPCR pathways but rather internalize chemokines either to translocate them elsewhere or to dispose of them. From evaluation of chemokine receptor knockout mice, it is now apparent that the ‘signaling’ receptors similarly modulate the chemokine environment (Cardona et al., 2008; Mantovani and Locati, 2008). This function of chemokine receptors will need to be taken into account, as therapeutic chemokine receptor blockade becomes a more prevalent treatment for disease (Charo and Ransohoff, 2006).
The chemokine receptor-like molecules that lack G-protein coupling mediate important functions in chemokine biology (Rot and von Andrian, 2004). By convention, these molecules, which fail to mediate chemoattraction in-vitro and don't increase cytoplasmic calcium levels, are termed “non-signaling” receptors, although they clearly couple the presence of their ligands to cellular responses. The non-signaling receptors are closely related to chemokine receptors, being ‘seven-spanning’ plasma membrane components. The best-characterized are DARC and D6, each of which efficiently binds and internalizes numerous chemokines. Expressed on post-capillary venular endothelium, DARC supports leukocyte recruitment to tissues, through binding inflammatory chemokines abluminally, and internalizing them for transcytosis and immobilization on the lumenal aspect of the capillary, to signal to rolling leukocytes (Pruenster et al., 2009). Parenthetically, DARC has also been widely studied for its role in P. vivax invasion of erythrocytes (Horuk et al., 1993). On red cells DARC has long been proposed to carry out physiological scavenging of the unbound plasma fraction of its approximate dozen of chemokine ligands. This speculation was confirmed, with the recent demonstration that post-transfusion pulmonary inflammation is caused in part by the loss of DARC's scavenging activity on banked erythrocytes (Mangalmurti et al., 2009). DARC therefore plays an exquisitely subtle role in inflammation, removing surplus plasma chemokines which could mediate harmful, indiscriminate inflammation, while orchestrating leukocyte entry into tissues harboring pathogens or sites of damage.
Encoded at human 3p21, near a cluster of chemokine receptor genes, D6 binds at least 12 inflammatory CC chemokines, internalizes them and targets them for degradation, through constitutive recycling between plasma membrane and endocytic vesicles at an extraordinarily rapid rate (Locati et al., 2005). D6 is expressed constitutively on lymphatic endothelium and, inducibly, on leukocytes (Graham and McKimmie, 2006), and the functional importance of D6 on leukocytes versus lymphatic endothelium is an unresolved question (Graham and McKimmie, 2006). Topical phorbol ester or intradermal complete Freund's adjuvant (CFA) caused remarkably enhanced and sustained inflammation of the skin of D6-/- mice, due to persistently elevated local chemokine levels (Martinez de la Torre et al., 2005; Jamieson et al., 2005).
In this context, D6 is a ‘scavenger’ receptor and plays a major anti-inflammatory role. Studies of autoimmune CNS inflammation frequently involve a disease model termed experimental autoimmune encephalomyelitis (EAE). A surrogate for the inflammatory aspect of the human disorder multiple sclerosis (MS), EAE is typically generated by immunization with peptide fragments derived from myelin proteins emulsified in powerful adjuvants. Mice thus immunized develop CNS inflammation including hematogenous leukocyte infiltrates accompanied by motor signs about two weeks following immunization.
We speculated that D6-/- mice might show a worsened course of EAE, if chemokine clearance from the CNS were impaired. Unexpectedly, D6-/- mice did not generate efficient encephalitogenic responses to immunization with MOG35-55 peptide/CFA and were relatively resistant to EAE (Liu et al., 2006a), possibly because dendritic cells trying to access lymphatics were trapped in the ‘hyper-inflamed’ immunization site. The role of D6 in immunity might therefore be to remove inflammatory chemokines and allow DCs exiting tissue to respond to homeostatic chemokine present on the lymphatic endothelial lumen. It should be emphasized that the D6 story is far from complete: the functional significance of its expression on lymphatic endothelium and leukocytes remains enigmatic, as does its physiological significance in adjusting the chemokine environment in the diverse settings (inflammation; cancer; pregnancy) in which it's been accorded physiological significance (Borroni et al., 2009; Borroni et al., 2008; Martinez de la et al., 2007; Nibbs et al., 2007).
A unifying concept from studying DARC and D6, two non-signaling receptors is that they exert uniquely disparate functions by virtue of their expression on parenchymal or vascular elements, as compared with their expression on circulating or extravasated blood cells. A cardinal example is DARC, where expression on post-capillary venules mediates one set of effects while its presence on erythrocytes mediates separate and distinct functions.
Chemokines are selective leukocyte chemoattractants in EAE and are implicated in disease pathogenesis
The Central Nervous System (CNS) is immune-privileged by virtue of lacking resident dendritic cells (DCs) (Galea et al., 2007). Equally important for the preservation of its post-mitotic and fragile cells, the CNS possesses attributes which modify inflammation. These characteristics include the blood-brain barrier (BBB) (Bechmann et al., 2007), and a tendency to recruit hematogenous cells as porcupines make love: very carefully. Cell loss after administration of the excitotoxin kainic acid (KA) involves neuronal necrosis and provides one well characterized example. Different to such injury in peripheral tissues (where necrotic injury elicits a rapid cellular response dominated by neutrophils), CNS leukocyte infiltrates after KA appear following a delay and consist primarily of mononuculear phagocytes (Bell and Perry, 1995).
If we consider the phenomenon of stringent control over leukocyte entry into the CNS (Engelhardt and Ransohoff, 2005), and also take account of the importance of chemokines for specificity in leukocyte migration, it is not surprising that early efforts in the field of ‘neurochemokinology’ involved analysis of the functions of chemokines and their receptors in leukocyte trafficking to the inflamed CNS. Much of this work utilized the EAE model of autoimmune inflammatory mechanisms, which are believed to be implicated in MS.
Trafficking of some but not all blood derived leukocyte cell types to the inflamed CNS of animals with EAE and other model conditions have been attributed to specific chemokines and their receptors. Importantly, disruption of chemokine-mediated leukocyte trafficking also alters disease expression. However, major issues remain unresolved. Although T lymphocytes are required for EAE pathogenesis, no chemokine receptor is known to play a non-redundant, essential role for migration of CD4+ T lymphocytes to the CNS during EAE. Below are examples of current knowledge of chemokine receptors that regulate the migration of leukocyte populations to the inflamed CNS of mice with EAE and other model disorders.
CCL2 and CCR2 mediate monocyte accumulation and tissue injury in the CNS during EAE
Monocytes accumulate in large numbers in the inflamed CNS of mice with EAE and give rise to macrophages which carry out diverse functions including directly damaging myelin and axons; clearance of tissue debris; and production of cytokines. Macrophages are pathogenic in EAE as shown by depletion studies (Brosnan et al., 1981). Monocytes which enter the CNS are Ly6Chi (King et al., 2009), and belong to a monocyte subset that preferentially uses CCR2 for trafficking to inflamed tissues (Geissmann et al., 2003). Even with a potent immunization regimen, CCL2-/- mice with EAE exhibited extremely mild disease, with near-complete lack of infiltrating monocytes, and minimal demyelination (Huang et al., 2001). CCR2 was identified as the receptor responsible for CCL2 action in EAE (Fife et al., 2000; Izikson et al., 2000). Immunization with thrice higher antigen concentrations than conventionally used, drove atypical neutrophilic EAE in CCR2-/- mice, underlining the role of CCR2 for monocytic infiltrates (Gaupp et al., 2003). Blocking CCR2 with neutralizing antibodies also suppressed EAE, with particular efficacy for relapsing disease (Mahad and Ransohoff, 2003). The clinical importance of this work was suggested by reports of altered concentrations of CCL2 in the CSF of MS patients. Specifically, MS patients exhibited reduced amounts of CSF CCL2, particularly during active disease, whereas most neuroinflammatory diseases (such as viral encephalitis or HIV-associated dementia) feature very high CSF CCL2 concentrations. In-vitro studies suggested that CCL2 in the CNS extracellular space (in equilibrium with the CSF), might be consumed by CCR2+ migrating cells (Mahad et al., 2006). Using a variety of additional models of CNS inflammation and injury, it became evident that CCL2 signals to CCR2 to recruit monocytes to the inflamed CNS (Mildner et al., 2007; Ransohoff, 2007).
CX3CR1, fractalkine and natural killer (NK) cells
NK cells mediate regulatory effects in EAE, as suggested more than a decade ago, by the occurrence of greatly worsened EAE in mice treated with depleting anti-NK1.1 antibodies (Zhang et al., 1997). Later experiments demonstrated that CX3CR1 was essential for recruitment of NK cells (but not NK-T cells, T lymphocytes or monocytes) into EAE tissues (Huang et al., 2006; Shi and Van Kaer, 2006). Mice lacking CX3CR1 developed EAE of equivalent severity to mice depleted of NK cells by passive immunization with anti-NK1.1 antibodies. Interestingly, individuals with MS had lower numbers of CX3CR1+ NK cells than did relevant controls, and there was a relationship between frequency of circulating CX3CR1+ NK cells and MS disease activity (Infante-Duarte et al., 2005).
CXCR2 and migration of neutrophils to the CNS
In many circumstances, neutrophils are poorly recruited to the CNS parenchyma and monocytes seem to be preferred. At the early phases of some EAE models substantial numbers of neutrophils are observed, and there is indirect evidence that they exert a pathogenic function (McColl et al., 1998). CXCR2-/- mice were relatively refractory to developing EAE and neutrophils were not detected in the CNS of mutant mice after immunization. Most tellingly, transfer of small numbers of wild-type neutrophils rescued the ability of CXCR2-/- mice to exhibit signs of EAE (Carlson et al., 2008).
Importantly several chemokine system elements studied in the EAE system are homologous in humans and mice. For example, human CXCR2 functions equivalently to the murine ortholog in ‘knock-in’ mice (Mihara et al., 2005). Furthermore, chemokine receptors are drug targets (Charo and Ransohoff, 2006). Therefore, where appropriate, insights from this research might readily be applied to treating human inflammatory CNS diseases such as MS.
On beyond immunity: Chemokines regulate development and physiology of the nervous system
Beginning in 1998 (Ma et al., 1998; Zou et al., 1998; Tachibana et al., 1998), it was discovered that mice lacking CXCR4 or its ligand CXCL12 harbored extensive neurodevelopmental defects (Li and Ransohoff, 2008), with prominent malpositioning of neurons of the cerebellum, dentate gyrus, trigeminal ganglia, dorsal root ganglia and cortical interneurons as well as aberrant initial trajectory of spinal motor axons (Lazarini et al., 2003). The same year (1998), it was shown that chemokine CXCL1, in the presence of PDGF, stimulated proliferation of a key population of glial cells, the oligodendrocyte progenitor cells (OPCs) (Robinson et al., 1998). It was later found that CXCR2 and its ligand CXCL1 helped determine both positioning and numbers of oligodendrocytes in the developing spinal cord, by acting as an ‘arrest receptor’ and proliferative signal for OPCs (Tsai et al., 2002). These reports about CXCR4's actions towards neurons and the role of CXCR2 in gliogenesis sparked reconsideration of contemporary phylogenetic research, culminating in the proposal that CXC chemokines emerged at the dawn of vertebrate evolution, to pattern the nervous system (Huising et al., 2003).
In retrospect, it seems unsurprising that chemokines are broadly involved in organ patterning. Chemokine receptors had been shown to organize secondary lymphoid organs during development (Forster et al., 1996). Further, chemokines and chemokine receptors can be identified in the most primitive vertebrates, the agnathous fish E. burgeri (hagfish), but not in invertebrates (DeVries et al., 2006).
Despite their function during development it seems somewhat perplexing that chemokine receptors are expressed on adult neural cells and mediate physiological or repair functions in the adult CNS (Imitola et al., 2004; Tran and Miller, 2003). However, by analogy with the neurotrophins (Arevalo and Chao, 2005; Chao et al., 2006; Zampieri and Chao, 2006), it seems that chemokines and their receptors play selected roles in nervous system development and are then retained for distinct purposes during postnatal and adult life. A first workshop on “Chemokines and chemokine receptors in the Nervous System” (Trettel et al., 2008) was held in Fall, 2007 and addressed this topic among many others.
A technical note: Lack of suitable antibodies for immunohistochemistry has misdirected research into the functions of chemokine receptors in the CNS
The most powerful means to localize a receptor in tissue is through immunohistochemistry, which simultaneously reports the presence of protein, its cell of origin and its subcellular localization. Sadly, production of specific and sensitive antibodies to chemokine receptors has proven devilishly difficult. As a result, many preliminary reports using immunohistochemistry to analyze chemokine receptor expression by CNS cells could not be confirmed by definitive studies that included evaluation of wild-type and knockout mice. Standards for CNS tissue immunohistochemistry have been proposed and it would be prudent to regard reports using immunohistochemistry to describe chemokine receptors in CNS tissues as preliminary until all criteria enunciated therein have been fulfilled (Saper and Sawchenko, 2003).
“Different strokes for different folks”: Chemokines and their receptors play strikingly different roles in the differing contexts of immunobiology and neurobiology
In several cases, chemokine receptors can be found both on circulating leukocytes and on parenchymal cells of the CNS, including neuroepithelial cells. In such cases, four of which are illustrated below, potentially decisive roles are played by these receptors and their ligands in neuroinflammatory processes. This attribute makes these chemokine/receptor pairs particularly worthy of attention for neuroimmunologists and neuroinflammation researchers.
CXCR4/CXCR7/CXCL12
CXCR4 has been termed the ‘ancestral chemokine receptor’ because its homologs are most readily identified in phylogenetic studies of chemokine receptors. The unique ligand for CXCR4 is CXCL12, and the pair was considered monogamous until recent reports that characterized RDC1 (an orphan GPCR) as CXCR7, the second CXCL12 receptor (Sierro et al., 2007; Burns et al., 2006).
Along with showing the earliest expression of any chemokine system elements during embryogenesis, the CXCR4/CXCL12 signaling pair have a bewilderingly large array of developmental functions. These properties seem most pertinent for development of the cardiovascular/hematopoietic, nervous and urogenital systems, all of which show abnormalities in knockout mice. During adult life, CXCR4 is expressed on circulating lymphocytes, neutrophils and monocytes. CXCR4 does not seem however to regulate leukocyte accumulation in tissues but does play a role in lymphocyte trafficking within lymph nodes (Okada et al., 2002). Interestingly, distribution of cells within inflamed CNS lesions is in part modulated by interactions between CXCL12, which is normally immobilized at the abluminal surface of cerebral microvessels, and CXCR4 on leukocytes, which are thereby retained in perivascular spaces (McCandless et al., 2008b; McCandless et al., 2006). There is preliminary evidence that these observations might be pertinent for human disease (Moll et al., 2009; McCandless et al., 2008a).
Studies of CXCR4 in zebrafish nervous system development provided results that exemplified how decoy receptors might shape chemokine gradients to optimize migratory competence. During zebrafish embryogenesis, approximately 100 migrating cells constitute the primordium which will give rise to posterior lateral line neurons that sense water currents. These cells migrate with anterior-posterior directionality, in a uniform concentration of SDF1a, a CXCL12 homolog. Cells at the leading edge of the migrating cell band express CXCR4b, while those at the trailing border express CXCR7. Antisense-mediated knockdown of either CXCR4b or CXCR7 partially impairs migration. Suppression of the CXCL12a ligand, or of both receptors, produces marked defects in migration, halting the primordium. One interpretation (Dambly-Chaudiere et al., 2007) of these findings is that CXCR4b is the signaling receptor for migration, while CXCR7 internalizes ligand and establishes the chemokine gradient (Tiveron and Cremer, 2008). These findings illustrate a principle of chemokine action: that non-signaling receptors can assist in generating chemokine gradients for migration. Applications of these insights to mammalian neurochemokinology are eagerly awaited.
The most obvious adult function of CXCR4 seems related to its expression on a large proportion of tissue stem cells. For example, mobilization of CD34+ hematopoietic stem cells from bone marrow stores can be mediated directly by pharmacological blockade of CXCR4 using AMD3100, one of two FDA-approved drugs targeting chemokine receptors. In the postnatal CNS, CXCR4 functions include modulating GABAergic inputs to dentate gyrus neural progenitor cells, thereby providing regulatory control over proliferation during adult neurogenesis (Bhattacharyya et al., 2008; Kolodziej et al., 2008). These two functions could not be more dissimilar and incarnate the diverse functions of chemokines and chemokine receptors in the nervous and immune systems.
A final neurobiological function of postnatal CXCR4/CXCL12 concerns two relatively new concepts: first, that of gliotransmitters, by which astrocytes participate actively in synaptic transmission (Chen et al., 2006); and second that cytokine TNFα also functions physiologically at CNS synapses (Stellwagen and Malenka, 2006). In an elegant series of studies (Bezzi et al., 2001; Cali et al., 2008) it has been shown that CXCL12 signaling to CXCR4 modulates astrocyte exocytosis of the excitatory neurotransmitter glutamate and that subsequent signaling elicits rapid release of TNFα. This mechanism cleverly uses available materials to support normal synaptic physiology but carries hidden dangers (Allen and Attwell, 2001). If nearby microglia which also express CXCR4 and TNF receptors, respond to these products with augmented TNF release, neuronal damage can ensue, both because of direct toxic effects of TNF and by virtue of impaired astrocyte uptake of glutamate which, present in excess, can engage a neurotoxic process termed excitotoxicity.
CXCR2 and its ligands
Chemokines received their initial attention because their index member CXCL8/IL8 was the first leukocyte chemoattractant which could signal to neutrophils and not to monocytes, unlike classical chemoattractants such as the complement anaphylatoxin C5a (Ransohoff, 2005). One neutrophil receptor for CXCL8, designated CXCR2, was among the first to be cloned and deleted from the murine genome (Cacalano et al., 1994). Of the CXCR2 ligands, CXCL1/GRO-alpha has the most extensively documented biology. This remarkable peptide has been discovered and rediscovered in contexts as varied as inflammation (Reutershan, 2006), cancer biology (Anisowicz et al., 1987; Luan et al., 1997; Acosta et al., 2008); cell proliferation (Cochran et al., 1983); wound healing (Martins-Green and Hanafusa, 1997); angiogenesis (Strieter et al., 2004); and neuroglial cell biology (Tran and Miller, 2003).
The exceptional versatility of CXCR2 is best shown through its implication in cutaneous wound healing (Devalaraja et al., 2000; Milatovic et al., 2003). When full thickness skin lesions were characterized in CXCR2-/- mice, the data suggested that CXCR2 was responsible for 1) attracting neutrophils into the skin breach; 2) promoting angiogenesis through expression on angioblasts; and 3) fostering epithelialization via its presence on keratinocytes (Zaja-Milatovic and Richmond, 2008). This concerted action towards mesenchymal, hematopoietic and epithelial cells during a single tissue repair process lasting only several days is unprecedented in chemokine biology.
Initial studies of oligodendroglial progenitors, purified from neonatal rodent spinal cord, showed that CXCL1, while devoid of intrinsic mitogenic properties, could synergize with platelet derived growth factor (PDGF) to drive a fourfold increased proliferative response as compared with PDGF alone (Robinson et al., 1998). Further evaluation in immediately postnatal mice revealed that migrating oligodendrocyte progenitor cells (OPCs) encountered a locally elevated concentration of CXCL1 in the terrain destined to give rise to ventral spinal cord white matter. A combination of assays including in vitro migration studies and slice culture preparations showed that CXCL1 enhanced the interactions between migrating OPCs and the tissue substrate, leading to stalled movement. At the site of arrest, OPCs found themselves in high concentrations of both CXCL1 and PDGF, proliferated vigorously, then differentiated and myelinated axons (Tsai et al., 2002; Tran and Miller, 2003). Ventral CXCL1 expression receded during the first postnatal week, to be succeeded by dorsal CXCL1 production, which promoted myelination of this portion of the spinal cord during the second postnatal week. Mice lacking CXCR2 exhibited a somewhat predictable phenotype: migrating OPCs failed to arrest in the presumptive white matter and accumulated at the tissue edge, the pial surface of the developing spinal cord. Because these misplaced OPCs found axons in a dysregulated sequence, early myelin formation was shifted from the white matter towards the pial surface, and the normal G-ratio, which expresses the tight relationship between axon caliber and myelin thickness, was perturbed (Padovani-Claudio et al., 2006).
As noted above, the presence of CXCR2+ neutrophils was sufficient to render CXCR2-/- mice susceptible to EAE (Carlson et al., 2008). Notably however, resultant disease was mild, raising the question whether other effects of CXCL1 and CXCR2 might be involved in disease severity. The components of the CXCL1/CXCR2 signaling system are present in the CNS of mice with EAE and humans with MS (Omari et al., 2006; Omari et al., 2005; Glabinski et al., 1997). Furthermore, an excess of CXCL1 in transgenic mice appears to modulate disease, although the mechanism for this effect remains uncertain (Omari et al., 2009). Further studies of genetic and disease models may help to clarify the specific functions of CXCR2 and its ligands during inflammatory demyelination and other disease states.
“Location! Location! Location!” CX3CR1 and fractalkine
CX3CL1/fractalkine has been fascinating and frustrating researchers since its first description (Schall, 1997). Fractalkine is an unusual chemokine, one of two expressed as a primary single-pass transmembrane molecule which is capable of mediating firm monocyte-endothelial adhesion under flow, or chemoattraction, following proteolytic release. Fractalkine's function towards circulating monocytes has become increasingly clear due to convergent lines of investigation. Knockout mice (CX3CR1-/-) were shown to have lessened atherosclerosis due to reduced monocyte infiltration of atheromata, in relevant models (Charo, 2001; Lesnik et al., 2003). Adding spice to the story, humans (about 30% of the Caucasian population) with a variant form of the receptor that blunted adhesive signaling, also showed delayed atherosclerotic endpoints (McDermott et al., 2003; McDermott et al., 2001; Moatti et al., 2001). Another contribution to understanding fractalkine biology came from delineating two subsets of circulating monocytes, one of which expressed low levels of Ly6C but high levels of CX3CR1 (Geissmann et al., 2003; Geissmann et al., 2008).
The distinct role of fractalkine/CX3CR1 in the CNS arises from the expression pattern of the ligand. In particular, vascular structures (endothelial or smooth muscle cells) expresses fractalkine except within the CNS (Sunnemark et al., 2005), where fractalkine is produced by neurons (Harrison et al., 1998). Neurons constitutively produce substantial amounts of soluble fractalkine through ongoing transcription, membrane insertion and proteolytic release (Cardona et al., 2008). The fractalkine receptor CX3CR1 is present in the CNS only on microglia (Cardona et al., 2006). Evaluation of several pathological processes which did not involve BBB compromise (and therefore did not entail large scale entry of peripheral leukocytes) showed that signaling through the fractalkine receptor reduced neuronal damage (Cardona et al., 2006). One potential mechanism for this protective effect was demonstrated in-vitro: microglia exposed to fractalkine produce adenosine which signals to protect hippocampal neurons from excitotoxic challenge, through the A1 receptor (Lauro et al., 2008). The potential practical relevance of fractalkine's ability to inhibit microglial neurotoxicity was illustrated vividly by increased liability to age-related macular degeneration in individuals expressing the variant form of the receptor (Combadiere et al., 2007; Tuo et al., 2004).
CCR2 and CCL2: Multiple roles in pain states
As noted above, CCR2 is expressed by a subpopulation of monocytes which are typified by the presence of high levels of membrane Ly6C, and CCR2 is required for entry of monocytes into diverse inflammatory sites including the CNS (Charo and Ransohoff, 2006). CCL2 is the principal ligand for trafficking of CCR2+ ‘inflammatory’ monocytes into the nervous system (Mahad et al., 2006). Mice lacking CCR2 and CCL2 were produced in the late 1990s and were both viable and fertile, with their study yielding remarkable insights into their biology (Charo and Peters, 2003; Gu et al., 1999). Subsequent studies showed potentially distinctive roles for CCR2 and the monocytes whose function it governed, in models of Alzheimer's disease and age-related macular degeneration (El et al., 2007; Ambati et al., 2003). CCR2-/- mice were resistant to models of pain (Abbadie et al., 2003) and, remarkably, both inflammatory pain (elicited by injecting irritants) and neuropathic pain (induced by nerve injury) were about equally affected. Initial evaluation of the affected tissues demonstrated upregulation of CCR2 in the dorsal root ganglia (DRG), in the injured nerve and in the inflamed injection-site. It seemed plausible that pain pathways were modulated by the presence of CCR2+ inflammatory monocytes and that loss of CCR2 signaling ameliorated pain by dampening macrophage-mediated inflammation. Concordant results were obtained through study of transgenic mice that over-expressed CCL2 in the CNS under control of the astrocyte-specific GFAP promoter (Menetski et al., 2007).
However, the situation became more complex with the surprising demonstration that DRG neurons upregulated both ligand CCL2 and receptor CCR2 in the context of chronic nerve root damage. Furthermore, CCL2 was packaged in synaptic-like vesicles and its action at CCR2 led to pain-promoting responses, including sensitization of the Transient Receptor Potential Vanilloid Receptor subtype 1 (TRPV1) ion channel (Jung et al., 2008; White et al., 2005; White and Wilson, 2008). Taken together, these results indicate that CCR2 plays a complex, multifarious role in pain states, both through its expected function on inflammatory monocytes and via its startling and novel properties as an inducible neuromodulatory receptor on DRG neurons (Abbadie, 2005).
Conclusions
From the perspective of neuroinflammation studies, chemokine research has followed a long and complex odyssey beginning with subset-specific chemoattractants, which seemed likely to mediate the cautious recruitment of leukocytes into the CNS and PNS. Along the way, chemokine receptors were discovered on neural cells, and other CNS-resident cells were shown capable of elaborating chemokine ligands. With the recognition that chemokines and their receptors on these diverse cell types can interact during pathological processes, bewildering and tantalizing vistas of cross-system signaling between the CNS and the immune system become apparent. Deciphering this signaling will place researchers in a position to deploy their insights in the service of novel strategies to treat neurological disease.
Figure 1.
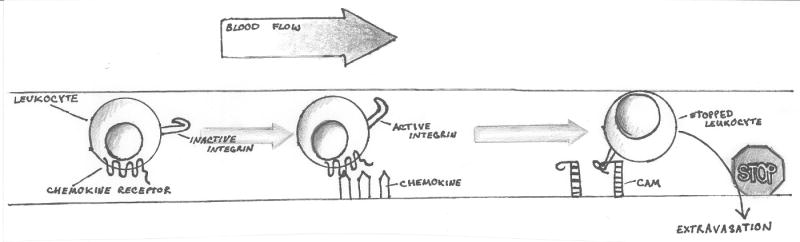
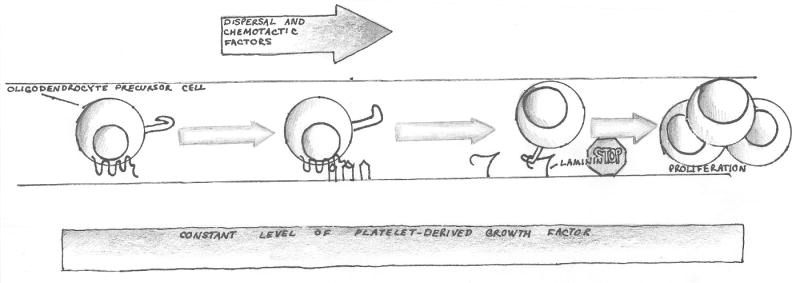
Figure 1a. Chemokine receptor signaling mediates arrest of moving cells in the immune system.
The cartoon illustrates the function of chemokine receptor signaling in the early phases of leukocyte extravasation under flow. Initial leukocyte-endothelial interactions are loose and reversible. Chemokines are immobilized in the vascular lumen by interactions with glycosaminoglycans or by presentation through molecules such as the Duffy Antigen Receptor for Chemokines (DARC). Chemokine receptor signaling converts inactive integrin to active integrin, capable of high-affinity interaction with Cell Adhesion Molecules (CAMs) on the endothelial luminal surface. This interaction arrests the leukocyte under flow and extravasation of the leukocyte often follows.
Figure 1b. Chemokine receptor signaling mediates arrest of moving cells in the developing nervous system.
The lower panel shows an oligodendrocyte precursor cell (OPC) moving through the presumptive white matter of the post-natal rodent spinal cord. Chemokine CXCL1 is expressed focally in these tissues and signals to CXCR2 on the OPC. In an in-vitro model, signaling through CXCR2 leads to increased interaction between the OPC and a laminin substrate. Studies using purified OPCs in vitro, and in vivo analysis of CXCR2-/- mice suggest that arrest of the OPC in a high local concentration of CXCL1 and PDGF (which is present throughout the developing spinal cord) drives a burst of OPC proliferation.
Figure 2. Differential expression of fractalkine/CX3CL1 in the peripheral vascular system and in the central nervous system (CNS) leads to disparate functional roles.
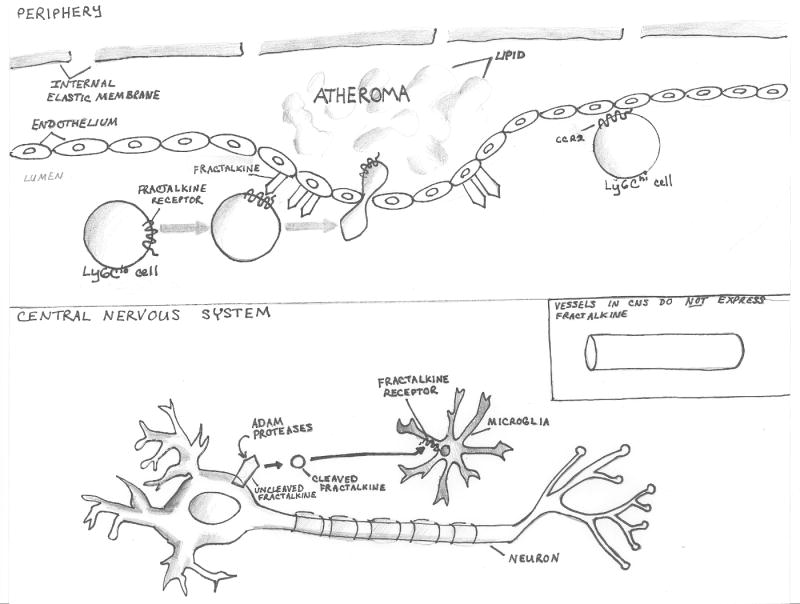
In the periphery (upper panel), fractalkine is expressed on the endothelium as a single-pass transmembrane component, which can mediate firm arrest under flow. Ly6Clo monocytes express high levels of CX3CR1/fractalkine receptor, and can be recruited into a developing atheroma through CX3CL1/CX3CR1 interactions. Both CX3CR1-/- mice and individuals harboring a polymorphic variant of CX3CR1 (CX3CR1I249/M280) which acts as a dominant negative inhibitor of signaling are relatively protected from atherosclerosis. Atheromata are also infiltrated by Ly6Chi monocytes via signaling to CCR2.
Fractalkine is not present on CNS vessels. Instead, fractalkine is expressed by neurons, where it can be cleaved and released by ADAM proteases, so that the healthy adult mouse brain contains 150-300pg/mg protein of soluble fractalkine. Microglia uniformly express CX3CR1 and respond to ligand by modulating effector functions in a manner that limits toxicity to neurons. Individuals bearing the CX3CR1I249/M280 variant are at increased risk for Age-related Macular Degeneration (AMD), an inflammatory neurodegenerative disorder, possibly because of neurotoxicity mediated by retinal microglia.
Figure 3.
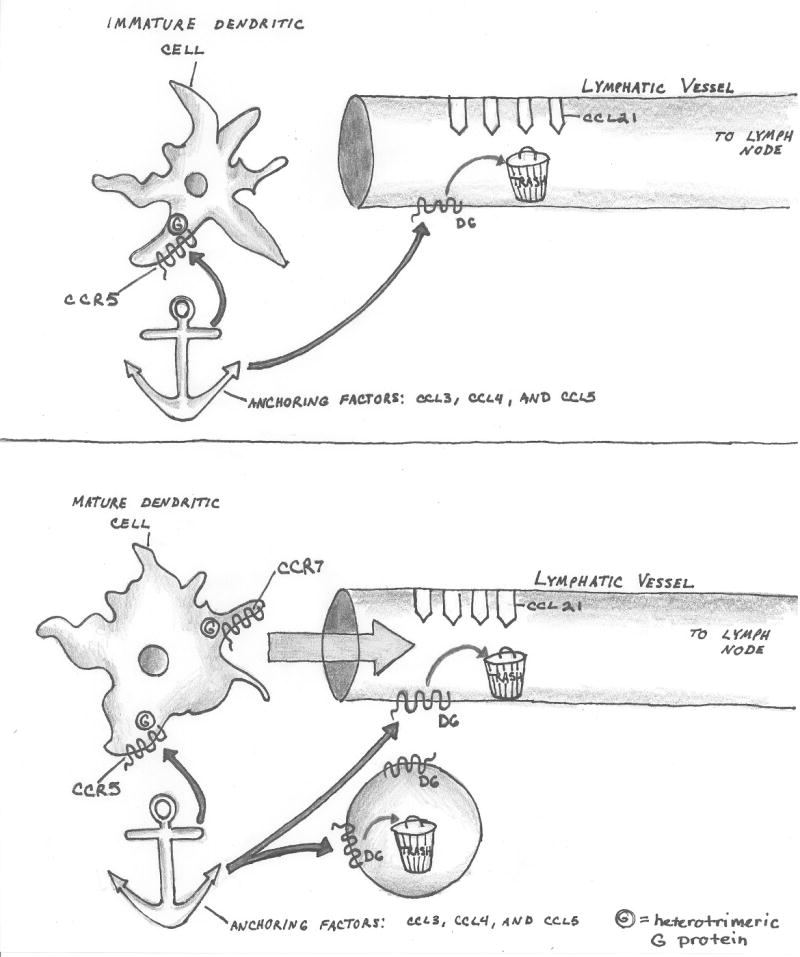
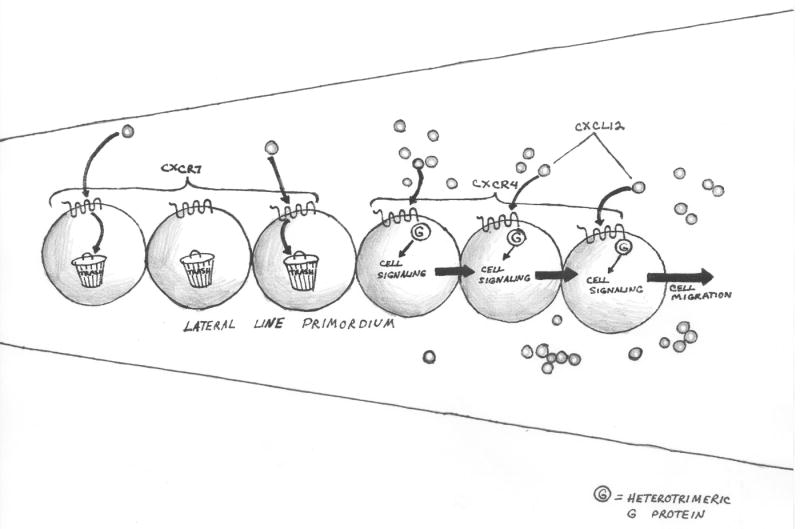
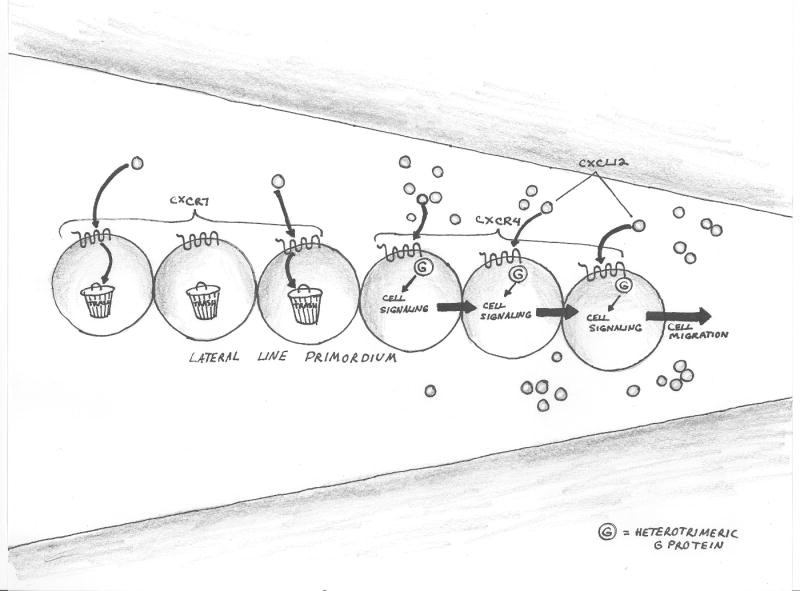
Figure 3A-B. Nonsignaling ‘scavenger’ chemokine receptor-like molecules adjust the chemokine environment to optimize immune function.
In inflamed tissues, an immature dendritic cell (3A) encounters a high concentration of chemokines (CCL3, CCL4, CCL5) which all signal to CCR5 and ‘anchor’ the cell in place, while it's processing antigen and upregulating functions associated with antigen presentation. During this time, D6, a nonsignaling scavenger receptor on lymphatic endothelium, clears excess inflammatory CC chemokines.
After maturation (3B), the dendritic cell downregulates CCR5, limiting signaling from CCL3, CCL4 and CCL5. The mature dendritic cell also upregulates CCR7. At the same time, other leukocytes upregulate D6 and assist in removing these inflammatory chemokines (but not the homeostatic chemokine CCL21) from the environment. The culmination of reduced signaling from inflammatory chemokines and suppressed signaling from inflammatory chemokines favors entry of the dendritic cell into the afferent lymphatic vessel, whose lumen is decorated with CCL21. The dendritic cell will subsequently traffick to the draining lymph node.
Figure 3C. Nonsignaling ‘scavenger’ chemokine receptor-like molecules adjust the chemokine environment to optimize neuron precursor migration during development.
The lateral line primordium comprises a population of cells, organized in a coherent linear aggregate, whose migration is mediated by chemokine CXCL12. Cells at the leading edge express the signaling receptor CXCR4. Cells at the trailing edge express CXCR7 and enhance gradient steepness, by removing CXCL12.
Acknowledgments
I thank past and recent members of the Ransohoff lab for their dedication and hard work; Joyce Ma (University of California, Davis) for critical review of the manuscript and Sara Hardy (Cleveland Clinic Lerner College of Medicine of CWRU) for superb renderings of draft figures.
Research in my laboratory has received support from the US National Institutes of Health; the National Multiple Sclerosis Society; the Charles A. Dana Foundation; the Robert Packard Center for ALS Research at Johns Hopkins; the Nancy Davis Center Without Walls; and the Williams Family Fund for MS Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Abbadie C. Chemokines, chemokine receptors and pain. Trends Immunol. 2005;26:529–534. doi: 10.1016/j.it.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Abbadie C, Lindia JA, Cumiskey AM, Peterson LB, Mudgett JS, Bayne EK, DeMartino JA, Macintyre DE, Forrest MJ. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc Natl Acad Sci U S A. 2003;100:7947–7952. doi: 10.1073/pnas.1331358100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta JC, O'Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Fumagalli M, Da CM, Brown C, Popov N, Takatsu Y, Melamed J, dda di FF, Bernard D, Hernando E, Gil J. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008;133:1006–1018. doi: 10.1016/j.cell.2008.03.038. [DOI] [PubMed] [Google Scholar]
- Allen NJ, Attwell D. A chemokine-glutamate connection. Nat Neurosci. 2001;4:676–678. doi: 10.1038/89443. [DOI] [PubMed] [Google Scholar]
- Alon R, Feigelson S. From rolling to arrest on blood vessels: leukocyte tap dancing on endothelial integrin ligands and chemokines at sub-second contacts. Semin Immunol. 2002;14:93–104. doi: 10.1006/smim.2001.0346. [DOI] [PubMed] [Google Scholar]
- Alon R, Grabovsky V, Feigelson S. Chemokine induction of integrin adhesiveness on rolling and arrested leukocytes local signaling events or global stepwise activation? Microcirculation. 2003;10:297–311. doi: 10.1038/sj.mn.7800195. [DOI] [PubMed] [Google Scholar]
- Ambati J, Anand A, Fernandez S, Sakurai E, Lynn BC, Kuziel WA, Rollins BJ, Ambati BK. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med. 2003;9:1390–1397. doi: 10.1038/nm950. [DOI] [PubMed] [Google Scholar]
- Anisowicz A, Bardwell L, Sager R. Constitutive overexpression of a growth-regulated gene in transformed Chinese hamster and human cells. Proc Natl Acad Sci U S A. 1987;84:7188–7192. doi: 10.1073/pnas.84.20.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arevalo JC, Chao MV. Axonal growth: where neurotrophins meet Wnts. Curr Opin Cell Biol. 2005;17:112–115. doi: 10.1016/j.ceb.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Bechmann I, Galea I, Perry VH. What is the blood-brain barrier (not)? Trends Immunol. 2007;28:5–11. doi: 10.1016/j.it.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Bell MD, Perry VH. Adhesion molecule expression on murine cerebral endothelium following the injection of a proinflammagen or during acute neuronal degeneration. J Neurocytol. 1995;24:695–710. doi: 10.1007/BF01179819. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, de CE, Vescovi A, Bagetta G, Kollias G, Meldolesi J, Volterra A. CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–710. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya BJ, Banisadr G, Jung H, Ren D, Cronshaw DG, Zou Y, Miller RJ. The chemokine stromal cell-derived factor-1 regulates GABAergic inputs to neural progenitors in the postnatal dentate gyrus. J Neurosci. 2008;28:6720–6730. doi: 10.1523/JNEUROSCI.1677-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borroni EM, Bonecchi R, Buracchi C, Savino B, Mantovani A, Locati M. Chemokine decoy receptors: new players in reproductive immunology. Immunol Invest. 2008;37:483–497. doi: 10.1080/08820130802191318. [DOI] [PubMed] [Google Scholar]
- Borroni EM, Buracchi C, Savino B, Pasqualini F, Russo RC, Nebuloni M, Bonecchi R, Mantovani A, Locati M. Role of the chemokine scavenger receptor D6 in balancing inflammation and immune activation. Methods Enzymol. 2009;460:231–243. doi: 10.1016/S0076-6879(09)05211-2. [DOI] [PubMed] [Google Scholar]
- Brosnan CF, Bornstein MB, Bloom BR. The effects of macrophage depletion on the clinical and pathologic expression of experimental allergic encephalomyelitis. J Immunol. 1981;126:614–620. [PubMed] [Google Scholar]
- Burns JB, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold MET, Sunshine MJ, Littman DR, Kuo CJ, Wei K, McMaster BE, Wright K, Howard MC, Schall TJ. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Cacalano G, Lee J, Kikly K, Ryan AM, Pitts-Meek S, Hultgren B, Wood WI, Moore MW. Neutrophil and B cell expansion in mice that lack the murine IL-8 receptor homolog. Science. 1994;265:682–684. doi: 10.1126/science.8036519. [DOI] [PubMed] [Google Scholar]
- Cali C, Marchaland J, Regazzi R, Bezzi P. SDF 1-alpha (CXCL12) triggers glutamate exocytosis from astrocytes on a millisecond time scale: imaging analysis at the single-vesicle level with TIRF microscopy. J Neuroimmunol. 2008;198:82–91. doi: 10.1016/j.jneuroim.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- Cardona AE, Sasse ME, Mizutani M, Cardona SM, Liu L, Savarin C, Hu T, Ransohoff RM. Scavenging roles of chemokine receptors: chemokine receptor deficiency is associated with increased levels of ligand in circulation and tissues. Blood. 2008;112:256–263. doi: 10.1182/blood-2007-10-118497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson T, Kroenke M, Rao P, Lane TE, Segal B. The Th17-ELR+ CXC chemokine pathway is essential for the development of central nervous system autoimmune disease. J Exp Med. 2008;205:811–823. doi: 10.1084/jem.20072404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MV, Rajagopal R, Lee FS. Neurotrophin signalling in health and disease. Clin Sci (Lond) 2006;110:167–173. doi: 10.1042/CS20050163. [DOI] [PubMed] [Google Scholar]
- Charo IF. Fractalkine and atherosclerosis: a new role for a curious chemokine. Blood. 2001;97:1905A–1905. [Google Scholar]
- Charo IF, Peters W. Chemokine receptor 2 (CCR2) in atherosclerosis, infectious diseases, and regulation of T-cell polarization. Microcirculation. 2003;10:259–264. doi: 10.1038/sj.mn.7800191. [DOI] [PubMed] [Google Scholar]
- Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- Chen XK, Xiong YF, Zhou Z. “Kiss-and-run” exocytosis in astrocytes. Neuroscientist. 2006;12:375–378. doi: 10.1177/1073858406291588. [DOI] [PubMed] [Google Scholar]
- Cinamon G, Grabovsky V, Winter E, Franitza S, Feigelson S, Shamri R, Dwir O, Alon R. Novel chemokine functions in lymphocyte migration through vascular endothelium under shear flow. J Leukoc Biol. 2001a;69:860–866. [PubMed] [Google Scholar]
- Cinamon G, Shinder V, Alon R. Shear forces promote lymphocyte migration across vascular endothelium bearing apical chemokines. Nat Immunol. 2001b;2:515–522. doi: 10.1038/88710. [DOI] [PubMed] [Google Scholar]
- Cinamon G, Shinder V, Shamri R, Alon R. Chemoattractant signals and beta 2 integrin occupancy at apical endothelial contacts combine with shear stress signals to promote transendothelial neutrophil migration. J Immunol. 2004;173:7282–7291. doi: 10.4049/jimmunol.173.12.7282. [DOI] [PubMed] [Google Scholar]
- Cochran BH, Reffel AC, Stiles CD. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983;33:939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- Colobran R, Pujol-Borrell R, Armengol MP, Juan M. The chemokine network. I. How the genomic organization of chemokines contains clues for deciphering their functional complexity. Clin Exp Immunol. 2007;148:208–217. doi: 10.1111/j.1365-2249.2007.03344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin RA, Campanella GS, Sun J, Luster AD. Intracellular domains of CXCR3 that mediate CXCL9, CXCL10, and CXCL11 function. J Biol Chem. 2004;279:30219–30227. doi: 10.1074/jbc.M403595200. [DOI] [PubMed] [Google Scholar]
- Combadiere C, Feumi C, Raoul W, Keller N, Rodero M, Pezard A, Lavalette S, Houssier M, Jonet L, Picard E, Debre P, Sirinyan M, Deterre P, Ferroukhi T, Cohen SY, Chauvaud D, Jeanny JC, Chemtob S, Behar-Cohen F, Sennlaub F. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J Clin Invest. 2007;117:2920–2928. doi: 10.1172/JCI31692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dambly-Chaudiere C, Cubedo N, Ghysen A. Control of cell migration in the development of the posterior lateral line: antagonistic interactions between the chemokine receptors CXCR4 and CXCR7/RDC1. BMC Dev Biol. 2007;7:23. doi: 10.1186/1471-213X-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devalaraja RM, Nanney LB, Du J, Qian Q, Yu Y, Devalaraja MN, Richmond A. Delayed wound healing in CXCR2 knockout mice. J Invest Dermatol. 2000;115:234–244. doi: 10.1046/j.1523-1747.2000.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries ME, Kelvin AA, Xu L, Ran L, Robinson J, Kelvin DJ. Defining the origins and evolution of the chemokine/chemokine receptor system. J Immunol. 2006;176:401–415. doi: 10.4049/jimmunol.176.1.401. [DOI] [PubMed] [Google Scholar]
- El KJ, Toft M, Hickman SE, Means TK, Terada K, Geula C, Luster AD. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007;13:432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- Engelhardt B, Ransohoff RM. The ins and outs of T-lymphocyte trafficking to the CNS: anatomical sites and molecular mechanisms. Trends Immunol. 2005;26:485–495. doi: 10.1016/j.it.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Fife BT, Huffnagle GB, Kuziel WA, Karpus WJ. CC chemokine receptor 2 is critical for induction of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192:899–906. doi: 10.1084/jem.192.6.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster R, Mattis AE, Kremmer E, Wolf E, Brem G, Lipp M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1037–1047. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- Galea I, Bechmann I, Perry VH. What is immune privilege (not)? Trends Immunol. 2007;28:12–18. doi: 10.1016/j.it.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Gaupp S, Pitt D, Kuziel WA, Cannella B, Raine CS. Experimental autoimmune encephalomyelitis (EAE) in CCR2(-/-) mice: susceptibility in multiple strains. Am J Pathol. 2003;162:139–150. doi: 10.1016/S0002-9440(10)63805-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann F, Auffray C, Palframan R, Wirrig C, Ciocca A, Campisi L, Narni-Mancinelli E, Lauvau G. Blood monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T-cell responses. Immunol Cell Biol. 2008;86:398–408. doi: 10.1038/icb.2008.19. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- Glabinski AR, Tani M, Strieter RM, Tuohy VK, Ransohoff RM. Synchronous synthesis of alpha- and beta-chemokines by cells of diverse lineage in the central nervous system of mice with relapses of chronic experimental autoimmune encephalomyelitis. Am J Pathol. 1997;150:617–630. [PMC free article] [PubMed] [Google Scholar]
- Gorbachev AV, Kobayashi H, Kudo D, Tannenbaum CS, Finke JH, Shu S, Farber JM, Fairchild RL. CXC chemokine ligand 9/monokine induced by IFN-gamma production by tumor cells is critical for T cell-mediated suppression of cutaneous tumors. J Immunol. 2007;178:2278–2286. doi: 10.4049/jimmunol.178.4.2278. [DOI] [PubMed] [Google Scholar]
- Graham GJ, McKimmie CS. Chemokine scavenging by D6: a movable feast? Trends Immunol. 2006;27:381–386. doi: 10.1016/j.it.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Gu L, Tseng SC, Rollins BJ. Monocyte chemoattractant protein-1. Chem Immunol. 1999;72:7–29. doi: 10.1159/000058723. [DOI] [PubMed] [Google Scholar]
- Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, Streit WJ, Salafranca MN, Adhikari S, Thompson DA, Botti P, Bacon KB, Feng L. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci U S A. 1998;95:10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horuk R, Chitnis CE, Darbonne WC, Colby TJ, Rybicki A, Hadley TJ, Miller LH. A receptor for the malarial parasite Plasmodium vivax: the erythrocyte chemokine receptor. Science. 1993;261:1182–1184. doi: 10.1126/science.7689250. [DOI] [PubMed] [Google Scholar]
- Huang D, Shi FD, Jung S, Pien GC, Wang J, Salazar-Mather TP, He TT, Weaver JT, Ljunggren HG, Biron CA, Littman DR, Ransohoff RM. The neuronal chemokine CX3CL1/fractalkine selectively recruits NK cells that modify experimental autoimmune encephalomyelitis within the central nervous system. FASEB J. 2006;20:896–905. doi: 10.1096/fj.05-5465com. [DOI] [PubMed] [Google Scholar]
- Huang DR, Wang J, Kivisakk P, Rollins BJ, Ransohoff RM. Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J Exp Med. 2001;193:713–726. doi: 10.1084/jem.193.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huising MO, Stet RJ, Kruiswijk CP, Savelkoul HF, Lidy Verburg-van Kemenade BM. Molecular evolution of CXC chemokines: extant CXC chemokines originate from the CNS. Trends Immunol. 2003;24:307–313. doi: 10.1016/s1471-4906(03)00120-0. [DOI] [PubMed] [Google Scholar]
- Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, Snyder EY, Khoury SJ. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante-Duarte C, Weber A, Kratzschmar J, Prozorovski T, Pikol S, Hamann I, Bellmann-Strobl J, Aktas O, Dorr J, Wuerfel J, Sturzebecher CS, Zipp F. Frequency of blood CX3CR1-positive natural killer cells correlates with disease activity in multiple sclerosis patients. FASEB J. 2005;19:1902–1904. doi: 10.1096/fj.05-3832fje. [DOI] [PubMed] [Google Scholar]
- Izikson L, Klein RS, Charo IF, Weiner HL, Luster AD. Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2. J Exp Med. 2000;192:1075–1080. doi: 10.1084/jem.192.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson T, Cook DN, Nibbs RJ, Rot A, Nixon C, McLean P, Alcami A, Lira SA, Wiekowski M, Graham GJ. The chemokine receptor D6 limits the inflammatory response in vivo. Nat Immunol. 2005;6:403–411. doi: 10.1038/ni1182. [DOI] [PubMed] [Google Scholar]
- Jung H, Toth PT, White FA, Miller RJ. Monocyte chemoattractant protein-1 functions as a neuromodulator in dorsal root ganglia neurons. J Neurochem. 2008;104:254–263. doi: 10.1111/j.1471-4159.2007.04969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King IL, Dickendesher TL, Segal BM. Circulating Ly-6C+ myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease. Blood. 2009;113:3190–3197. doi: 10.1182/blood-2008-07-168575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziej A, Schulz S, Guyon A, Wu DF, Pfeiffer M, Odemis V, Hollt V, Stumm R. Tonic activation of CXC chemokine receptor 4 in immature granule cells supports neurogenesis in the adult dentate gyrus. J Neurosci. 2008;28:4488–4500. doi: 10.1523/JNEUROSCI.4721-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudanna C, Alon R. Right on the spot. Chemokine triggering of integrin-mediated arrest of rolling leukocytes. Thromb Haemost. 2006;95:5–11. [PubMed] [Google Scholar]
- Lauro C, Di AS, Cipriani R, Sobrero F, Antonilli L, Brusadin V, Ragozzino D, Limatola C. Activity of adenosine receptors type 1 Is required for CX3CL1-mediated neuroprotection and neuromodulation in hippocampal neurons. J Immunol. 2008;180:7590–7596. doi: 10.4049/jimmunol.180.11.7590. [DOI] [PubMed] [Google Scholar]
- Lazarini F, Tham TN, Casanova P, Arenzana-Seisdedos F, Dubois-Dalcq M. Role of the alpha-chemokine stromal cell-derived factor (SDF-1) in the developing and mature central nervous system. Glia. 2003;42:139–148. doi: 10.1002/glia.10139. [DOI] [PubMed] [Google Scholar]
- Lesnik P, Haskell CA, Charo IF. Decreased atherosclerosis in CX3CR1-/- mice reveals a role for fractalkine in atherogenesis. J Clin Invest. 2003;111:333–340. doi: 10.1172/JCI15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Ransohoff RM. Multiple roles of chemokine CXCL12 in the central nervous system: A migration from immunology to neurobiology. Prog Neurobiol. 2008;84:116–131. doi: 10.1016/j.pneurobio.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp M, Muller G. Shaping up adaptive immunity: the impact of CCR7 and CXCR5 on lymphocyte trafficking. Verh Dtsch Ges Pathol. 2003;87:90–101. [PubMed] [Google Scholar]
- Liu L, Callahan MK, Huang D, Ransohoff RM. Chemokine receptor CXCR3: an unexpected enigma. Curr Top Dev Biol. 2005;68:149–181. doi: 10.1016/S0070-2153(05)68006-4. [DOI] [PubMed] [Google Scholar]
- Liu L, Graham GJ, Damodaran A, Hu T, Lira SA, Sasse M, Canasto-Chibuque C, Cook DN, Ransohoff RM. Cutting edge: the silent chemokine receptor d6 is required for generating T cell responses that mediate experimental autoimmune encephalomyelitis. J Immunol. 2006a;177:17–21. doi: 10.4049/jimmunol.177.1.17. [DOI] [PubMed] [Google Scholar]
- Liu L, Huang D, Matsui M, He TT, Hu T, DeMartino J, Lu B, Gerard C, Ransohoff RM. Severe disease, unaltered leukocyte migration, and reduced IFN-{gamma} production in CXCR3-/- mice with experimental autoimmune encephalomyelitis. J Immunol. 2006b;176:4399–4409. doi: 10.4049/jimmunol.176.7.4399. [DOI] [PubMed] [Google Scholar]
- Locati M, Torre YM, Galliera E, Bonecchi R, Bodduluri H, Vago G, Vecchi A, Mantovani A. Silent chemoattractant receptors: D6 as a decoy and scavenger receptor for inflammatory CC chemokines. Cytokine Growth Factor Rev. 2005;16:679–686. doi: 10.1016/j.cytogfr.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Lord G, Rao RM, Choe H, Sullivan BM, Lichtman AH, Luscinskas FW, Glimcher LH. T-bet is required for optimal pro-inflammatory CD4+ T cell trafficking. Blood. 2005 doi: 10.1182/blood-2005-04-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan J, Shattuck-Brandt R, Haghnegahdar H, Owen JD, Strieter R, Burdick M, Nirodi C, Beauchamp D, Johnson KN, Richmond A. Mechanism and biological significance of constitutive expression of MGSA/GRO chemokines in malignant melanoma tumor progression. J Leukoc Biol. 1997;62:588–597. doi: 10.1002/jlb.62.5.588. [DOI] [PubMed] [Google Scholar]
- Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4 or SDF-1 deficient mice. Proc Natl Acad Sci U S A. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahad D, Callahan MK, Williams KA, Ubogu EE, Kivisakk P, Tucky B, Kidd G, Kingsbury GA, Chang A, Fox RJ, Mack M, Sniderman MB, Ravid R, Staugaitis SM, Stins MF, Ransohoff RM. Modulating CCR2 and CCL2 at the blood-brain barrier: relevance for multiple sclerosis pathogenesis. Brain. 2006;129:212–223. doi: 10.1093/brain/awh655. [DOI] [PubMed] [Google Scholar]
- Mahad DJ, Ransohoff RM. The role of MCP-1 (CCL2) and CCR2 in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE) Semin Immunol. 2003;15:23–32. doi: 10.1016/s1044-5323(02)00125-2. [DOI] [PubMed] [Google Scholar]
- Mangalmurti NS, Xiong Z, Hulver M, Ranganathan M, Liu XH, Oriss T, Fitzpatrick M, Rubin M, Triulzi D, Choi A, Lee JS. Loss of red cell chemokine scavenging promotes transfusion-related lung inflammation. Blood. 2009;113:1158–1166. doi: 10.1182/blood-2008-07-166264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A. The chemokine system: redundancy for robust outputs. Immunol Today. 1999;20:254–257. doi: 10.1016/s0167-5699(99)01469-3. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Bonecchi R, Locati M. Tuning inflammation and immunity by chemoattractant decoy receptors: the sound of silence. Nat Rev Immunol. 2006;6:907–918. doi: 10.1038/nri1964. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Locati M. Housekeeping by chemokine scavenging. Blood. 2008;112:215–216. doi: 10.1182/blood-2008-04-149237. [DOI] [PubMed] [Google Scholar]
- Martinez de la Torre Y, Locati M, Buracchi C, Dupor J, Cook DN, Bonecchi R, Nebuloni M, Rukavina D, Vago L, Vecchi A, Lira SA, Mantovani A. Increased inflammation in mice deficient for the chemokine decoy receptor D6. Eur J Immunol. 2005;35:1342–1346. doi: 10.1002/eji.200526114. [DOI] [PubMed] [Google Scholar]
- Martinez de la TY, Buracchi C, Borroni EM, Dupor J, Bonecchi R, Nebuloni M, Pasqualini F, Doni A, Lauri E, Agostinis C, Bulla R, Cook DN, Haribabu B, Meroni P, Rukavina D, Vago L, Tedesco F, Vecchi A, Lira SA, Locati M, Mantovani A. Protection against inflammation- and autoantibody-caused fetal loss by the chemokine decoy receptor D6. Proc Natl Acad Sci U S A. 2007;104:2319–2324. doi: 10.1073/pnas.0607514104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins-Green M, Hanafusa H. The 9E3/CEF4 gene and its product the chicken chemotactic and angiogenic factor (cCAF): potential roles in wound healing and tumor development. Cytokine Growth Factor Rev. 1997;8:221–232. doi: 10.1016/s1359-6101(97)00016-6. [DOI] [PubMed] [Google Scholar]
- McCandless EE, Piccio L, Woerner BM, Schmidt RE, Rubin JB, Cross AH, Klein RS. Pathological expression of CXCL12 at the blood-brain barrier correlates with severity of multiple sclerosis. Am J Pathol. 2008a;172:799–808. doi: 10.2353/ajpath.2008.070918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCandless EE, Wang Q, Woerner BM, Harper JM, Klein RS. CXCL12 limits inflammation by localizing mononuclear infiltrates to the perivascular space during experimental autoimmune encephalomyelitis. J Immunol. 2006;177:8053–8064. doi: 10.4049/jimmunol.177.11.8053. [DOI] [PubMed] [Google Scholar]
- McCandless EE, Zhang B, Diamond MS, Klein RS. CXCR4 antagonism increases T cell trafficking in the central nervous system and improves survival from West Nile virus encephalitis. Proc Natl Acad Sci U S A. 2008b;105:11270–11275. doi: 10.1073/pnas.0800898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McColl SR, Staykova MA, Wozniak A, Fordham S, Bruce J, Willenborg DO. Treatment with anti-granulocyte antibodies inhibits the effector phase of experimental autoimmune encephalomyelitis. J Immunol. 1998;161:6421–6426. [PubMed] [Google Scholar]
- McDermott DH, Fong AM, Yang Q, Sechler JM, Cupples LA, Merrell MN, Wilson PW, D'Agostino RB, O'Donnell CJ, Patel DD, Murphy PM. Chemokine receptor mutant CX3CR1-M280 has impaired adhesive function and correlates with protection from cardiovascular disease in humans. J Clin Invest. 2003;111:1241–1250. doi: 10.1172/JCI16790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott DH, Halcox JP, Schenke WH, Waclawiw MA, Merrell MN, Epstein N, Quyyumi AA, Murphy PM. Association between polymorphism in the chemokine receptor CX3CR1 and coronary vascular endothelial dysfunction and atherosclerosis. Circ Res. 2001;89:401–407. doi: 10.1161/hh1701.095642. [DOI] [PubMed] [Google Scholar]
- Menetski J, Mistry S, Lu M, Mudgett JS, Ransohoff RM, DeMartino JA, Macintyre DE, Abbadie C. Mice overexpressing chemokine ligand 2 (CCL2) in astrocytes display enhanced nociceptive responses. Neuroscience. 2007;149:706–714. doi: 10.1016/j.neuroscience.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Mihara K, Smit MJ, Krajnc-Franken M, Gossen J, Rooseboom M, Dokter W. Human CXCR2 (hCXCR2) takes over functionalities of its murine homolog in hCXCR2 knockin mice. Eur J Immunol. 2005;35:2573–2582. doi: 10.1002/eji.200526021. [DOI] [PubMed] [Google Scholar]
- Milatovic S, Nanney LB, Yu Y, White JR, Richmond A. Impaired healing of nitrogen mustard wounds in CXCR2 null mice. Wound Repair Regen. 2003;11:213–219. doi: 10.1046/j.1524-475x.2003.11310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M, Heikenwalder M, Bruck W, Priller J, Prinz M. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci. 2007;10:1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- Moatti D, Faure S, Fumeron F, Amara M, Seknadji P, McDermott DH, Debre P, Aumont MC, Murphy PM, de Prost D, Combadiere C. Polymorphism in the fractalkine receptor CX3CR1 as a genetic risk factor for coronary artery disease. Blood. 2001;97:1925–1928. doi: 10.1182/blood.v97.7.1925. [DOI] [PubMed] [Google Scholar]
- Moll NM, Cossoy MB, Fisher E, Staugaitis SM, Tucky BH, Rietsch AM, Chang A, Fox RJ, Trapp BD, Ransohoff RM. Imaging correlates of leukocyte accumulation and CXCR4/CXCL12 in multiple sclerosis. Arch Neurol. 2009;66:44–53. doi: 10.1001/archneurol.2008.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibbs RJ, Gilchrist DS, King V, Ferra A, Forrow S, Hunter KD, Graham GJ. The atypical chemokine receptor D6 suppresses the development of chemically induced skin tumors. J Clin Invest. 2007;117:1884–1892. doi: 10.1172/JCI30068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Ngo VN, Ekland EH, Forster R, Lipp M, Littman DR, Cyster JG. Chemokine requirements for B cell entry to lymph nodes and Peyer's patches. J Exp Med. 2002;196:65–75. doi: 10.1084/jem.20020201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omari KM, John G, Lango R, Raine CS. Role for CXCR2 and CXCL1 on glia in multiple sclerosis. Glia. 2006;53:24–31. doi: 10.1002/glia.20246. [DOI] [PubMed] [Google Scholar]
- Omari KM, John GR, Sealfon SC, Raine CS. CXC chemokine receptors on human oligodendrocytes: implications for multiple sclerosis. Brain. 2005;128:1003–1015. doi: 10.1093/brain/awh479. [DOI] [PubMed] [Google Scholar]
- Omari KM, Lutz SE, Santambrogio L, Lira SA, Raine CS. Neuroprotection and remyelination after autoimmune demyelination in mice that inducibly overexpress CXCL1. Am J Pathol. 2009;174:164–176. doi: 10.2353/ajpath.2009.080350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padovani-Claudio DA, Liu L, Ransohoff RM, Miller RH. Alterations in the oligodendrocyte lineage, myelin, and white matter in adult mice lacking the chemokine receptor CXCR2. Glia. 2006;54:471–483. doi: 10.1002/glia.20383. [DOI] [PubMed] [Google Scholar]
- Pruenster M, Mudde L, Bombosi P, Dimitrova S, Zsak M, Middleton J, Richmond A, Graham GJ, Segerer S, Nibbs RJ, Rot A. The Duffy antigen receptor for chemokines transports chemokines and supports their promigratory activity. Nat Immunol. 2009;10:101–108. doi: 10.1038/ni.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM. Snip-snip, kill-kill: truncated SDF-1 and HIV-associated neurodegeneration. Nat Neurosci. 2003;6:1009–1011. doi: 10.1038/nn1003-1009. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM. Selective leukocyte chemoattractants emerge from the primeval sup(ernatants) J Immunol. 2005;175:5567–5568. doi: 10.4049/jimmunol.175.9.5567. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM. Microgliosis: the questions shape the answers. Nat Neurosci. 2007;10:1507–1509. doi: 10.1038/nn1207-1507. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Man S, Ubogu EE. “Doing the locomotion” with the multistep paradigm. Blood. 2007;109:1342–1343. [Google Scholar]
- Reutershan J. CXCR2--the receptor to hit? Drug News Perspect. 2006;19:615–623. doi: 10.1358/dnp.2006.19.10.1068009. [DOI] [PubMed] [Google Scholar]
- Robinson S, Tani M, Strieter RM, Ransohoff RM, Miller RH. The chemokine growth-regulated oncogene-alpha promotes spinal cord oligodendrocyte precursor proliferation. J Neurosci. 1998;18:10457–10463. doi: 10.1523/JNEUROSCI.18-24-10457.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- Saper CB, Sawchenko PE. Magic peptides, magic antibodies: guidelines for appropriate controls for immunohistochemistry. J Comp Neurol. 2003;465:161–163. doi: 10.1002/cne.10858. [DOI] [PubMed] [Google Scholar]
- Schall T. Fractalkine--a strange attractor in the chemokine landscape. Immunol Today. 1997;18:147. doi: 10.1016/s0167-5699(97)84655-5. [DOI] [PubMed] [Google Scholar]
- Schreiber T, Shinder V, Cain D, Alon R, Sackstein R. Shear flow-dependent integration of apical and subendothelial chemokines in T cell transmigration: implications for locomotion and the “multi-step paradigm”. Blood. 2007;109:1381–1386. doi: 10.1182/blood-2006-07-032995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi FD, Van Kaer L. Reciprocal regulation between natural killer cells and autoreactive T cells. Nat Rev Immunol. 2006;6:751–760. doi: 10.1038/nri1935. [DOI] [PubMed] [Google Scholar]
- Shulman Z, Shinder V, Klein E, Grabovsky V, Yeger O, Geron E, Montresor A, Bolomini-Vittori M, Feigelson SW, Kirchhausen T, Laudanna C, Shakhar G, Alon R. Lymphocyte crawling and transendothelial migration require chemokine triggering of high-affinity LFA-1 integrin. Immunity. 2009;30:384–396. doi: 10.1016/j.immuni.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierro F, Biben C, Martinez-Munoz L, Mellado M, Ransohoff RM, Li M, Woehl B, Leung H, Groom J, Batten M, Harvey RP, Martinez A, Mackay CR, Mackay F. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc Natl Acad Sci U S A. 2007;104:14759–14764. doi: 10.1073/pnas.0702229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JV, Rot A, Luo Y, Narasimhaswamy M, Nakano H, Gunn MD, Matsuzawa A, Quackenbush EJ, Dorf ME, von Andrian UH. The CC chemokine thymus-derived chemotactic agent 4 (TCA-4, secondary lymphoid tissue chemokine, 6Ckine, exodus-2) triggers lymphocyte function-associated antigen 1-mediated arrest of rolling T lymphocytes in peripheral lymph node high endothelial venules. J Exp Med. 2000;191:61–76. doi: 10.1084/jem.191.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Strieter RM, Belperio JA, Phillips RJ, Keane MP. CXC chemokines in angiogenesis of cancer. Semin Cancer Biol. 2004;14:195–200. doi: 10.1016/j.semcancer.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Sunnemark D, Eltayeb S, Nilsson M, Wallstrom E, Lassmann H, Olsson T, Berg AL, Ericsson-Dahlstrand A. CX3CL1 (fractalkine) and CX3CR1 expression in myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis: kinetics and cellular origin. J Neuroinflammation. 2005;2:17. doi: 10.1186/1742-2094-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S, Kishimoto T, Nagasawa T. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- Tiveron MC, Cremer H. CXCL12/CXCR4 signalling in neuronal cell migration. Curr Opin Neurobiol. 2008;18:237–244. doi: 10.1016/j.conb.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Tran PB, Miller RJ. Chemokine receptors: signposts to brain development and disease. Nat Rev Neurosci. 2003;4:444–455. doi: 10.1038/nrn1116. [DOI] [PubMed] [Google Scholar]
- Trettel F, Di AS, Limatola C, Ransohoff RM. Chemokines and chemokine receptors in the nervous system Rome, 27/28 October, 2007. J Neuroimmunol. 2008;198:1–8. doi: 10.1016/j.jneuroim.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Tsai HH, Frost E, To V, Robinson S, ffrench-Constant C, Geertman R, Ransohoff RM, Miller RH. The chemokine receptor CXCR2 controls positioning of oligodendrocyte precursors in developing spinal cord by arresting their migration. Cell. 2002;110:373–383. doi: 10.1016/s0092-8674(02)00838-3. [DOI] [PubMed] [Google Scholar]
- Tuo J, Smith BC, Bojanowski CM, Meleth AD, Gery I, Csaky KG, Chew EY, Chan CC. The involvement of sequence variation and expression of CX3CR1 in the pathogenesis of age-related macular degeneration. FASEB J. 2004;18:1297–1299. doi: 10.1096/fj.04-1862fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SG. Millipede-like lymphocyte crawling: feeling the way with filopodia. Immunity. 2009;30:315–317. doi: 10.1016/j.immuni.2009.03.002. [DOI] [PubMed] [Google Scholar]
- White FA, Sun J, Waters SM, Ma C, Ren D, Ripsch M, Steflik J, Cortright DN, LaMotte RH, Miller RJ. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc Natl Acad Sci U S A. 2005;102:14092–14097. doi: 10.1073/pnas.0503496102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FA, Wilson NM. Chemokines as pain mediators and modulators. Curr Opin Anaesthesiol. 2008;21:580–585. doi: 10.1097/ACO.0b013e32830eb69d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaja-Milatovic S, Richmond A. CXC chemokines and their receptors: a case for a significant biological role in cutaneous wound healing. Histol Histopathol. 2008;23:1399–1407. doi: 10.14670/hh-23.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampieri N, Chao MV. Mechanisms of neurotrophin receptor signalling. Biochem Soc Trans. 2006;34:607–611. doi: 10.1042/BST0340607. [DOI] [PubMed] [Google Scholar]
- Zhang B, Yamamura T, Kondo T, Fujiwara M, Tabira T. Regulation of experimental autoimmune encephalomyelitis by natural killer (NK) cells. J Exp Med. 1997;186:1677–1687. doi: 10.1084/jem.186.10.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]


