Abstract
Normal tissue development and function are regulated by the interplay between cells and their surrounding extracellular matrix (ECM). The ECM provides biochemical and mechanical contextual information that is conveyed from the cell membrane through the cytoskeleton to the nucleus to direct cell phenotype. Cells, in turn, remodel the ECM and thereby sculpt their local microenvironment. Here we review the mechanisms by which cells interact with, respond to, and influence the ECM, with particular emphasis placed on the role of this bidirectional communication during tissue morphogenesis. We also discuss the implications for successful engineering of functional tissues ex vivo.
Keywords: mechanotransduction, dynamic reciprocity, pattern formation
1. Introduction
The extracellular matrix (ECM) arguably influences almost every decision made by the cells it contacts. ECM provides mechanical support and biochemical signals, both of which can affect cytoskeletal structure, chromatin organization, and gene transcription. Cells, in turn, reorganize and remodel the ECM and thus play an active role in sculpting their surrounding environment and in directing their own phenotypes. This concept of bidirectional communication from the ECM to the cytoskeleton to the nucleus and back again was put forth almost at the same time as the community recognized the likely existence of ECM receptors [1, 2] and appears to play a fundamental role in tissue development, differentiation, homeostasis, and disease progression.
ECM and its receptors are highly conserved. ECM proteins, especially components of the basement membrane, are found in the tissues of nearly all multicellular animals. Basement membrane forms at the blastula stage very early during mammalian embryonic development. Embryos with homozygous null mutations in laminin genes that prevent assembly of the basement membrane die early after implantation (reviewed in [3]). Dynamic reciprocal communication between cells and ECM is now considered to be essential during tissue morphogenesis and wound healing. Here, we review the mechanisms by which cells respond to and remodel the ECM, with a specific focus on implications for the development and morphogenesis of native and engineered tissues.
2. ECM signaling to the nucleus – biochemical
Cells interact with the ECM via transmembrane receptors, including members of the integrin and dystroglycan families. Integrins comprise a large conserved family of α and β subunits which combine to form heterodimers that bind selectively to different ligands. Each subunit has a large extracellular domain, a single transmembrane domain, and a short cytoplasmic tail which contains well-defined motifs that act as binding sites for various structural and signaling proteins. ECM adhesion sites are thus multiprotein complexes that connect the ECM to the cytoskeleton and serve as platforms for key signaling proteins. Over 150 distinct proteins have been found at the cytoplasmic face of integrin-mediated adhesions [4]. Integrins and their downstream signaling are critical for development, as knockout mice for most integrin subunits are embryonic (or perinatal) lethal [5].
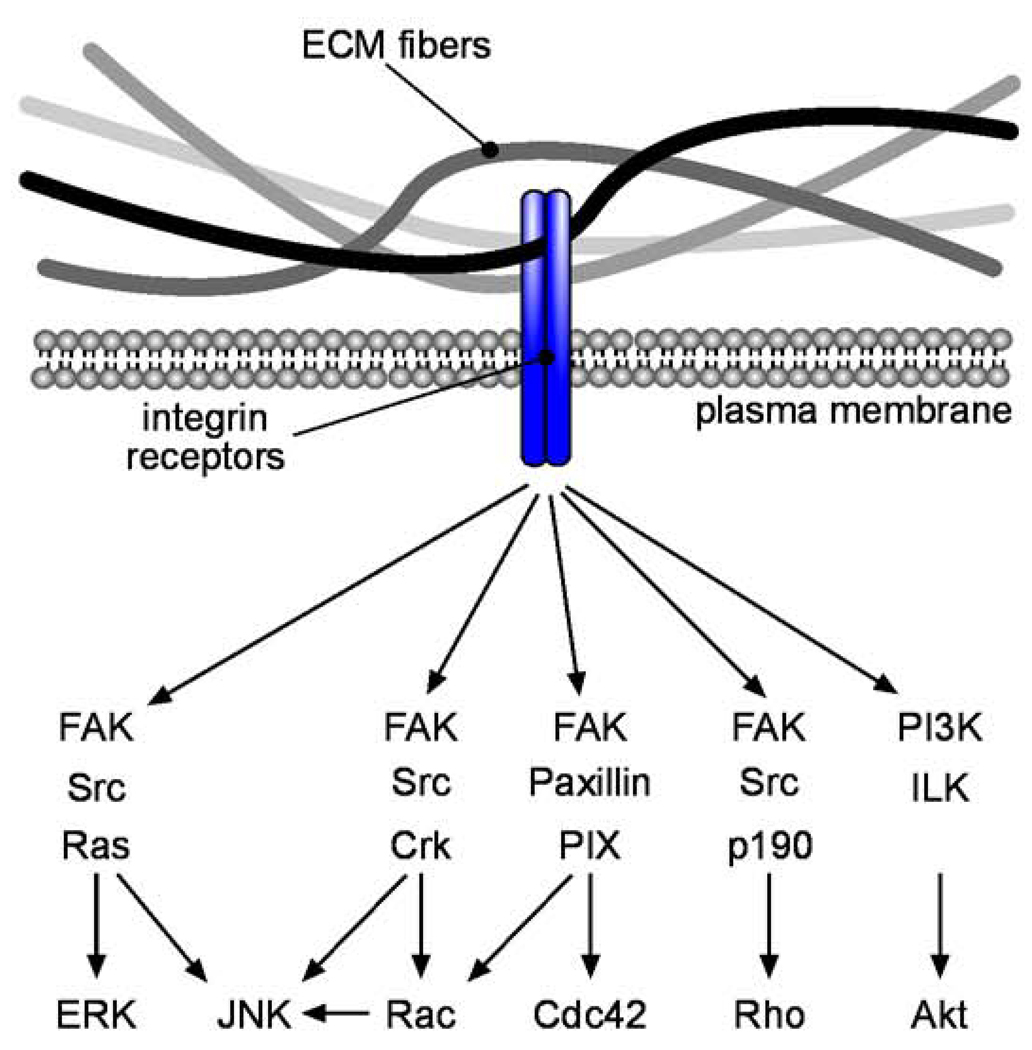
Integrin engagement leads to several downstream signaling events that can ultimately alter cell fate (Figure 1). Focal adhesion kinase (FAK) is a non-receptor tyrosine kinase that is activated by integrin signaling. Autophosphorylation of FAK creates high-affinity binding sites for Src-homology 2 (SH2) domains, leading to an interaction with Src-family kinases. Src in turn activates the Ras-extracellular signal-regulated kinase (ERK) signaling cascade, which modulates the contractile properties of the cell by regulating myosin dynamics through myosin light chain kinase. ERK also relays extracellular information by translocating to the nucleus, where it stimulates gene expression by phosphorylating transcription factors, including ternary complex factors (TCFs) such as Elk-1, which regulate genes by binding to serum response elements (SREs). In addition to signaling through the Ras-ERK pathway, integrin engagement leads to signaling through several other kinase pathways, including phosphatidylinositol 3' kinase (PI3K)/Akt, integrin-linked kinase (ILK), and Jun-N-terminal kinase (JNK). FAK, ILK, and JNK are all required for normal development: FAK, ILK, and JNK1/JNK2 knockout mice are embryonic lethal [6–8]. The final outcomes of these kinase signals generated by adhesion complexes often depend in part on the mechanical properties of the ECM (see below).
Figure 1. Biochemical signaling from ECM to the nucleus.
Integrin signaling activates a large number of pathways, many of which induce cytoskeletal alterations. Kinase signaling cascades lead to gene transcription changes in the nucleus.
2.1 Signaling to cell survival pathways
In most types of adherent cells, detachment from the ECM induces a specific apoptotic pathway called anoikis [9]. Avoidance of anoikis leading to adhesion-independent cell growth is thought to be a key step in neoplastic transformation. In untransformed cells, detachment causes pro-apoptotic proteins to translocate to the mitochondrial membrane, leading to mitochondrial depolarization, release of cytochrome C into the cytoplasm, and activation of caspases (reviewed in [10]). Activated caspase-3 initiates a proteolytic cascade, causing cleavage of signaling molecules such as FAK and structural proteins such as nuclear lamins. The caspase cascade also induces specific enzymes responsible for DNA fragmentation, including caspase-activated DNase. The anoikis response is highly regulated throughout development and homeostasis, and can be ECM-specific in certain cell types. For example, basement membrane proteins, but not interstitital collagens, prevent apoptosis of mammary epithelial cells [11]. Integrin-mediated signaling through FAK, ILK, Src, PI3K, and ERK have all been shown to protect against anoikis (reviewed in [12]).
Detachment from ECM is not a universal death sentence, however, as recent studies have revealed specific survival pathways that are induced upon cell detachment. Autophagy is a highly regulated lysosomal self-digestion process that is stimulated upon cell detachment and promotes cell survival, in part by delaying induction of anoikis. For example, lack of integrin engagement has been shown to induce autophagy during cavitation of the lumen of mammary epithelial acini [13, 14]. A similar process has been observed for lumen cavitation during embryoid body formation [15], suggesting a conserved role during tissue development. The signaling pathways between the ECM and induction of autophagy remain to be elucidated, but involve increased expression of autophagy-related genes (reviewed in [16]).
2.2 Signaling to cell proliferation
Cell adhesion to the ECM elicits the same types of signals as do mitogens, and growth factor receptors are often found within adhesion complexes [17]. It is not surprising, then, that the proliferation of most adherent cell types involves cooperation between ECM receptors and growth factor or hormone receptor signaling. In non-transformed cells, progression through the cell cycle depends on regulated expression of cyclins, which form complexes with cyclin-dependent kinases (CDKs). Cyclin D1 is critical for progression through G1 phase, and its expression requires signaling from both the ECM and growth factors (reviewed in [18]). For example, coordinated signaling through the ERK cascade stimulates the expression of AP-1 transcription factors; an AP-1 binding site is found in the promoter of the cyclin D1 gene. Crosstalk is also important for hormone-mediated regulation of cell proliferation. Proliferation of mammary epithelial cells during pregnancy is regulated by estrogen and progesterone, but progression of these cells through the cell cycle requires simultaneous adhesion to basement membrane proteins [19, 20]. This may be due in part to upregulation of estrogen receptor expression by the basement membrane [21]. Promoter regions of several ECM-regulated genes have been found to contain an ECM-response element, but the signaling between ECM to these regulatory sites is still an active area of investigation (reviewed in [22]).
2.3 Signaling to differentiation
ECM provides contextual signaling in the functional differentiation of cells from a number of organs, including those from the mammary gland, lung, and liver. Although lactogenic hormones such as prolactin induce mammary differentiation and milk protein secretion, sustained signaling through the prolactin receptor requires anchorage of β1-integrins [23]. This is due in part to specific integrin-mediated signals as well as to integrin-mediated establishment of basolateral polarity, which exposes the prolactin receptor to its ligand [24]. Type II alveolar epithelial cells, the cuboidal cells of the lung responsible for secreting surfactant, are also highly regulated by ECM composition; in the absence of appropriate cues, these cells differentiate into type I cells. This switch between phenotypes appears to be regulated in part by the degree of heparin sulfation within the surrounding ECM [25]. The differentiated function of hepatocytes has been explored in depth using ECM microarrays, which revealed that adhesion to collagen IV positively regulates albumin production [26].
A similar role for ECM is being uncovered in regulation of stem cell fate. Stem cells reside within niches, specialized microenvironments that promote both self-renewal and asymmetric division. Exit from the niche induces stem cell differentiation. Circumstantial evidence suggests that ECM adhesion may be a defining feature of stem cells: high levels of β1-integrin are a marker of stem cells of the mammary epithelium [27], the hair follicle and epidermis [28], and the central nervous system [29]. The niche, therefore, would be expected to include specific ECM proteins depending on the tissue or organ. This appears to be true, as neural stem cells within the subventricular zone of the adult brain reside in regions enriched for laminin-containing ECM [30] and epidermal stem cells lie adjacent to the basement membrane [31]. Similarly, LaBarge et al used combinatorial microenvironments to identify laminin-111 as a major putative component of the niche surrounding progenitor cells of the human mammary gland [32]. The signaling pathways downstream of ECM adhesion that regulate stem cell fate are an active area of investigation.
3. ECM signaling to the nucleus – mechanical
3.1 Mechanotransduction
The multitude of cells and tissues within multicellular organisms function as a single coordinated living unit by communicating signals, which in addition to biochemical, can be mechanical in origin. The mechanical state of a tissue is maintained and modulated by the cytoskeletal contractile activity of the cells along with the surrounding ECM. A growing body of evidence supports the notion that the mechanical properties of the ECM, in addition to providing structural support to cells, furnish instructive signals, which cells sense, integrate, and interpret to effect response, a process termed mechanotransduction [33].
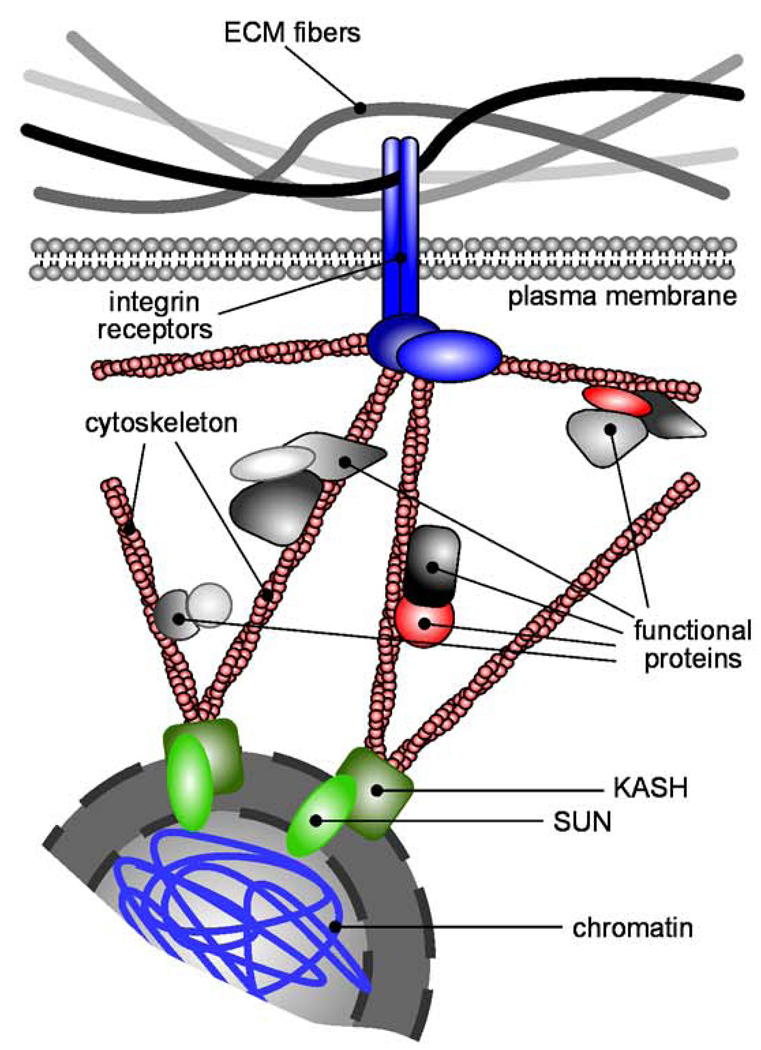
Two kin theories aimed at explaining the phenomenon whereby the mechanical environment and perturbations therein can elicit cellular response are Ingber’s tensegrity [34–36] and Bissell’s dynamic reciprocity [1, 22] models, which appeared around the same time. Both of these models postulate the existence of physical continuity within tissues, extending from the ECM to the nucleus itself (Figure 2). It is this architectural network that allows external mechanical information to be transmitted to the interior of the cell and the nucleus, where it could affect structural rearrangements ultimately resulting in changes in gene expression. Experimental studies have confirmed many aspects of these theories. It is now widely recognized that the actin cytoskeleton is mechanically coupled to ECM proteins at focal adhesions comprised of integrins and other structural and signaling proteins [37]. The physical continuity at the opposite end – between the cytoskeleton and the nucleus – is still largely speculative. Nevertheless, a number of molecular candidates that bridge chromatin and the cytoskeleton have been identified (reviewed in [38]). Actin fibers bind the KASH domain of nesprins, large proteins that span the outer nuclear membrane and project into the cytoplasm [39], which in turn interact with the SUN domain of proteins integral to the inner nuclear membrane, providing a mechanical bridge between the cytoskeleton and the nuclear membrane [40]. Furthermore, the nuclear lamina, a thin mesh-like structure associated with the inner nuclear membrane, forms scaffolds which bind the SUN/KASH complex [41]. Finally, the nuclear lamina is coupled to chromatin either directly or indirectly through associated proteins [38]. The molecular sequence described here forms a physical link that spans the distance between ECM and chromatin, theoretically allowing for outside-in force transmission. The concept of ECM-originating force being channeled to the nucleus is confirmed by “intracellular” traction force microscopy studies, where force applied locally to apical integrins is transmitted to basal focal adhesions and the nucleus [42, 43]. Other studies demonstrate that exerting force on integrins by manipulating bound magnetic microbeads leads to cytoskeletal rearrangements, organelle movement, nuclear shape changes, and nucleolar rearrangements [44, 45].
Figure 2. Mechanical signaling from ECM to the nucleus.
The cytoskeleton physically links the ECM to chromatin and functional proteins in the cytoplasm. Mechanical perturbations in the ECM can propagate to the interior of the cell, structurally rearranging proteins and DNA tethered to the cytoskeleton and thus altering biochemical signaling.
3.2 Tension-mediated signaling
How does the physical continuity between the ECM and nucleus enable mechanical signaling? One hypothesis is that the cytoplasm is a site of solid-state biochemistry: Many of the molecules that mediate DNA synthesis, transcription, protein synthesis, and signal transduction do not diffuse freely in the cytoplasm, but rather are immobilized on cytoskeletal fibers [36, 46]. Thus, any mechanical perturbations taking place in the ECM can propagate to the cell’s interior and cause structural rearrangement of the cytoskeleton and the immobilized proteins, potentially altering their activity. Studies of molecular mechanics reveal a number of structural motifs that respond to applied force (reviewed by [33]). Mechanical forces can expose cryptic protein sequences that are otherwise unavailable in the unstressed state for interaction with cell surface receptors. For instance, contractile force generated by cells is sufficient to partially unfold fibronectin [47]. In addition, many enzymes exhibit altered activity in response to mechanical stress (reviewed in [48, 49]), enabling another mechanism for transduction of mechanical into biochemical signals.
Mechanotransduction may proceed through direct mechanical impingement upon chromatin, initiated by cues transmitted from the cell exterior. The work of Dalby et al demonstrates that changes in substratum adhesion of fibroblasts leads to changes in the position of chromosomes and altered gene expression [50–52]. One could speculate that, in analogy to force-mediated protein unfolding, mechanical stresses experienced by chromatin may induce unraveling of certain DNA motifs, making them more accessible to transcription factors and thus influencing gene expression. Physical forces open mechanosensitive ion channels that are tethered to ECM or cytoskeletal filaments, regulating ionic transport into the cell [53, 54]. Applying sustained nanonewton-level force to integrins results in calcium influx [55], and applying shear to integrins triggers the cAMP signaling cascade, which activates transcription of genes containing multiple cAMP-response elements [56]. In these studies, force applied to other transmembrane receptors does not influence signaling, indicating that the response is specific to integrins. Perturbing the cell mechanical environment by increasing ECM stiffness activates the small GTPase Rho [57]. Active Rho promotes actomyosin stress fiber assembly [58], rendering the cell mechanically more tensed [59]. The balance between two effectors of Rho, mDia and Rho kinase, regulates the G1/S-phase transition via the degradation of p27, a CDK inhibitor [60]. Stiffer matrices have also been shown to disrupt cadherin-based intercellular junctions, leading to loss of tissue architecture and altered junctional localization of β-catenin [57].
3.3 Stem cells and morphogenesis
Increasing evidence implicates the tensional state of a tissue, to a great extent dictated by the ECM, as a key regulator of differentiation, morphogenesis, homeostasis, and disease progression. Cellular geometry and spreading control the transition of cells between proliferation and apoptosis [61], as well as the ability of a cell to undergo epithelial-mesenchymal transition (EMT) [62]. The mechanical properties of the microenvironment control the differentiation of stem cells: Human mesenchymal stem cells differentiate down lineages depending upon matrix stiffness [63]. By micropatterning endothelial and epithelial cells on adhesive islands of controlled geometry, Nelson et al found that patterns of cell proliferation correlate with patterns of mechanical tension within tissues [64]. These mechanically-regulated basic cellular processes (proliferation, differentiation, apoptosis) are finely orchestrated within the developing embryo to give rise to a complex multicellular organism. Thus, one could imagine a role for physical force in the control of development on a larger scale. Indeed, a number of recent studies implicate mechanics as a regulator of embryogenesis (reviewed in [65]). Mesoderm and notochord stiff enough to resist buckling are needed during Xenopus laevis gastrulation [66, 67], and actomyosin contractility is required for dorsal closure in the Drosophila melanogaster embryo [68, 69]. Twist – a master regulator of cell shape changes during gastrulation – has been implicated as a major player in the mechanical control of embryogenesis. Applying both tensile and compressive force on Drosophila embryos results in increased Twist expression [70, 71], whereas laser ablation of dorsal epithelium leads to reduced levels of Twist [70].
Tissue contractility has also been implicated in organogenesis during embryonic development. Down-modulating Rho-mediated tension inhibits branching morphogenesis of the embryonic lung [72] and kidney [73], whereas increasing tension promotes branching [74]. The mechanotransduction pathways that regulate branching are unclear. Moore et al observed that branch initiation is preceded by local thinning of the basement membrane and speculated that the budding process is foremost mechanical – ECM degradation physically enables the adjacent pre-stressed epithelium to invade, analogous to a “run in a stocking” [72]. The events that prompt the initial ECM degradation, however, are unknown. One could also speculate that the mechanical differentials originate in the epithelium rather than the stroma. Based on results showing that cells exposed to elevated mechanical stress exhibit high proliferation rates [64] and higher responsiveness to growth factors [75], one could imagine that epithelial regions experiencing higher stress could acquire a more invasive motile phenotype and thereby undergo branching.
4. ECM remodeling – biochemical
The cell responds to its microenvironment, but also plays an active role in sculpting the surrounding ECM. During tissue development, differentiation, and homeostasis, the ECM is continuously remodeled by its resident cells. The ECM is comprised of a large variety of proteins, proteoglycans, and hyaluronan, and the synthesis of each molecule depends on organ, disease status, and age. In addition, many of the protein constituents of the ECM are regulated by alternative splicing. At least 20 different isoforms of fibronectin are expressed in humans, with higher expression of specific isoforms during embryonic development, wound healing, angiogenesis, and fibrosis (reviewed in [76]). Hyaluronan also shows age-dependent expression patterns, with high expression during embryogenesis and decreasing expression as the organism ages [77]. Hyaluronan synthase-2 is required for embryonic development; null embryos die of severe heart defects midway through gestation, including a lack of heart valves due to endocardial cushion cells failing to undergo EMT [78]. Our basic understanding of tissue development and morphogenesis would be significantly enhanced by revelation of the signals and mechanisms that differentially and temporally regulate ECM expression.
The structure and biochemical properties of existing ECM polymers are altered via proteolytic cleavage. Several families of proteases, including matrix metalloproteinases (MMPs), serine proteases, and cysteine proteases are actively secreted by cells. The MMP family consists of more than 20 structurally related zinc-dependent proteases that can degrade ECM proteins, cell surface receptors, and other molecules. Most family members are secreted from the cell, including collagenase-1/MMP1 (which was identified in studies of tadpole tail metamorphosis [79]), but others are associated with the cell membrane and provide localized proteolysis. Cells use MMPs to remove steric barriers during migration, to produce cleavage fragments with unique biological activities, and to remodel intercellular junctions during morphogenesis (reviewed in [80]). Despite their broad usage and highly regulated expression and activation, MMP knockout mice survive to birth, suggesting either that other proteases dominate embryonic remodeling or that MMPs are highly functionally redundant. There is also ample crosstalk between members of the different protease families during ECM remodeling. Intracellular serine proteases, such as furins, cleave and activate the transmembrane MMP, MMP14. Urokinase plasminogen activator (uPA), also a serine protease, activates plasmin, which can cleave ECM proteins as well as activate MMPs. Misregulation of protease function is a hallmark of diseases including chronic inflammation and cancer.
5. ECM remodeling – mechanical
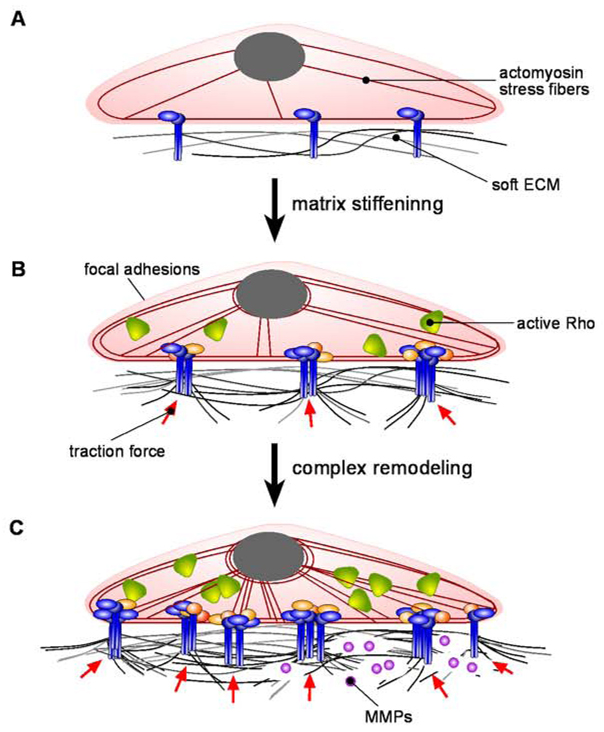
In addition to biochemical signals, the ECM is remodeled by mechanical force (Figure 3). Early experiments in which cells were plated on silicone rubber substrata [81], as well as later studies using techniques such as traction force microscopy [82, 83] and subcellular-scale microarrays of posts [84] all demonstrated that cells pull on their substrata with nanonewton-scale forces. It is these forces, generated at integrin-ligand interaction points, which actively remodel the fibrous three-dimensional (3D) matrices in which cells reside in vivo. Confocal reflection microscopy studies revealed that migrating cells such as fibroblasts and tumor cells radially align surrounding matrix fibers towards the cell body in structures ranging from 50 to 200 µm in length [85]. Fibroblasts embedded in 3D collagen gels can deform and compact their matrix by approximately two orders of magnitude [86]. Mechanical remodeling of the ECM is dependent on the contractile activity of the cells. Activation of the Rho pathway and the subsequent assembly of stress fibers results in integrin clustering, focal adhesion maturation, and elevated traction forces at the cell-matrix interface [57, 63]. This contractility leads to assembly of fibronectin matrix: Inhibition of actomyosin stress fiber formation results in reduced fibronectin fibril assembly, whereas stimulation of Rho- or myosin light chain kinase-mediated contractility promotes assembly [87]. Inhibiting cellular contractility also abolishes collagen gel contraction [88].
Figure 3. Mechanical remodeling of the ECM.
(A) Cells residing within soft matrices are minimally mechanically active. (B) ECM stiffening causes Rho activation, stress fiber formation, integrin clustering and force generation. (C) These events can elicit a complex response: positive mechanical feedback resulting in further matrix compaction and stiffening, or proteolytic degradation of the ECM through increased MMP production.
Mechanical remodeling of the ECM is often self-regulated and the result of cell-matrix crosstalk. Mechanical stiffening of the ECM itself can lead to Rho activation [57], which, as discussed above, results in mechanical remodeling of the matrix through focal adhesion maturation and increased force generation by the cell. These processes ultimately lead to increased matrix traction and compaction, further elevating local ECM stiffness. This positive feedback is balanced by negative regulation, in the form of mechanically-induced biochemical matrix remodeling. The mechanical state of the ECM can elicit a complex proteolytic response in the cell through altered production levels of MMP2 and MMP9, responsible for matrix turnover. In particular, it has been demonstrated that the levels of both proteases dramatically increase upon mechanical loading of soft matrices, whereas mechanical loading of stiff matrices leads to an increase in MMP9 production only [89]. Thus, two mechanical parameters that characterize the ECM – stiffness and load – both control the extent of matrix remodeling through biochemical degradation, which ultimately results in altered mechanical properties. This tightly regulated cell-matrix crosstalk could be an essential mechanism by which tissues finely tune their mechanical state, thus adapting to changes during development, disease progression, or aging.
6. ECM in engineered tissues
Functional engineered tissues, whether for use in regenerative medicine or in studies of basic biology, must include both the cells and their surrounding ECM. As discussed above, cells within native tissues are constantly communicating with and influencing their microenvironments; the same is true for those in man-made tissues. One challenge in the field is to build tissues containing ECMs that recapitulate both the biochemical and mechanical properties of native ECMs in vivo. For tissues to undergo proper development, differentiation, and homeostasis, the ECM necessarily contains signaling moieties and cleavage sites to enable appropriate remodeling. The ECM is also not a homogeneous mixture of proteins – it follows the heterogeneity of the tissue and itself is heterogeneous on the micrometer length scale.
Strategies to mimic native ECM have been reviewed extensively elsewhere [90–92]. Efforts are currently biased towards synthetic ECMs in which a biologically inert polymer, such as polyethylene glycol, is modified to contain sites that support cell adhesion and/or proteolytically-mediated degradation [93]. The advantages of this strategy are the high degree of control and batch-to-batch consistency; one major disadvantage is the inherent presumption that we currently understand all of the signaling properties endowed by any one particular ECM molecule within a given tissue. An orthogonal strategy is to use chemically modified natural ECM polymers, such as derivatives of hyaluronan [94]. Finally, unmodified natural ECM molecules, including type I collagen and fibrin, have been used extensively in studies aimed at recapitulating native tissue structure. Aside from maintaining known and undiscovered adhesion moities, natural ECMs can be manipulated to achieve microscale heterogeneities, including channels and networks [95–97], which permit engineering of functional microscale tissues.
7. Perspective
Cells and the ECM communicate in a dialogue that is dynamic and constantly evolving over the lifetime of the tissue. Cell and developmental biologists are actively trying to understand the signals conveyed by the ECM and how those signals are translated into functional cellular responses. Engineers, on the other hand, are trying to mimic the ECM half of the conversation. Advances in building engineered tissues will require designer ECMs, either synthetic or natural, that not only “speak” to cells, but also “listen” to them.
Acknowledgements
The authors are supported by grants from the NIH (GM083997 and CA128660), Susan G. Komen for the Cure, and the David & Lucile Packard Foundation. C.M.N. holds a Career Award at the Scientific Interface from the Burroughs Wellcome Fund.
Abbreviations
- CDK
cyclin-dependent kinase
- ECM
extracellular matrix
- EMT
epithelial-mesenchymal transition
- ERK
extracellular signal-regulated kinase
- FAK
focal adhesion kinase
- ILK
integrin-linked kinase
- PI3K
phosphatidylinositol 3' kinase
- JNK
Jun-N-terminal kinase
- MMP
matrix metalloproteinase
- SH2
Src-homology 2
- 3D
three-dimension(al)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bissell MJ, Hall HG, Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- 2.Ingber DE, Jamieson JD. Cells as tensegrity structures: architectural regulation of histodifferentiation by physical forces transduced over basement membrane. In: Andersson LC, Gahmberg CG, Ekblom P, editors. Gene expression during normal and malignant differentiation. Orlando, FL: Academic Press; 1985. pp. 13–32. [Google Scholar]
- 3.Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–284. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- 4.Zaidel-Bar R, Itzkovitz S, Ma'ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9:858–867. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fassler R, Georges-Labouesse E, Hirsch E. Genetic analyses of integrin function in mice. Curr Opin Cell Biol. 1996;8:641–646. doi: 10.1016/s0955-0674(96)80105-0. [DOI] [PubMed] [Google Scholar]
- 6.Furuta Y, Ilic D, Kanazawa S, Takeda N, Yamamoto T, Aizawa S. Mesodermal defect in late phase of gastrulation by a targeted mutation of focal adhesion kinase, FAK. Oncogene. 1995;11:1989–1995. [PubMed] [Google Scholar]
- 7.Sakai T, Li S, Docheva D, et al. Integrin-linked kinase (ILK) is required for polarizing the epiblast, cell adhesion, and controlling actin accumulation. Genes Dev. 2003;17:926–940. doi: 10.1101/gad.255603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuan CY, Yang DD, Samanta Roy DR, Davis RJ, Rakic P, Flavell RA. The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron. 1999;22:667–676. doi: 10.1016/s0896-6273(00)80727-8. [DOI] [PubMed] [Google Scholar]
- 9.Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddig PJ, Juliano RL. Clinging to life: cell to matrix adhesion and cell survival. Cancer Metastasis Rev. 2005;24:425–439. doi: 10.1007/s10555-005-5134-3. [DOI] [PubMed] [Google Scholar]
- 11.Pullan S, Wilson J, Metcalfe A, et al. Requirement of basement membrane for the suppression of programmed cell death in mammary epithelium. J Cell Sci. 1996;109(Pt 3):631–642. doi: 10.1242/jcs.109.3.631. [DOI] [PubMed] [Google Scholar]
- 12.Chiarugi P, Giannoni E. Anoikis: a necessary death program for anchorage-dependent cells. Biochem Pharmacol. 2008;76:1352–1364. doi: 10.1016/j.bcp.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 13.Debnath J, Mills KR, Collins NL, Reginato MJ, Muthuswamy SK, Brugge JS. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell. 2002;111:29–40. doi: 10.1016/s0092-8674(02)01001-2. [DOI] [PubMed] [Google Scholar]
- 14.Fung C, Lock R, Gao S, Salas E, Debnath J. Induction of autophagy during extracellular matrix detachment promotes cell survival. Mol Biol Cell. 2008;19:797–806. doi: 10.1091/mbc.E07-10-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qu X, Zou Z, Sun Q, et al. Autophagy gene-dependent clearance of apoptotic cells during embryonic development. Cell. 2007;128:931–946. doi: 10.1016/j.cell.2006.12.044. [DOI] [PubMed] [Google Scholar]
- 16.Lock R, Debnath J. Extracellular matrix regulation of autophagy. Curr Opin Cell Biol. 2008;20:583–588. doi: 10.1016/j.ceb.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danen EH, Yamada KM. Fibronectin, integrins, and growth control. J Cell Physiol. 2001;189:1–13. doi: 10.1002/jcp.1137. [DOI] [PubMed] [Google Scholar]
- 18.Klein EA, Assoian RK. Transcriptional regulation of the cyclin D1 gene at a glance. J Cell Sci. 2008;121:3853–3857. doi: 10.1242/jcs.039131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie J, Haslam SZ. Extracellular matrix regulates ovarian hormone-dependent proliferation of mouse mammary epithelial cells. Endocrinology. 1997;138:2466–2473. doi: 10.1210/endo.138.6.5211. [DOI] [PubMed] [Google Scholar]
- 20.Haslam SZ, Woodward TL. Host microenvironment in breast cancer development: epithelial-cell-stromal-cell interactions and steroid hormone action in normal and cancerous mammary gland. Breast Cancer Res. 2003;5:208–215. doi: 10.1186/bcr615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novaro V, Roskelley CD, Bissell MJ. Collagen-IV and laminin-1 regulate estrogen receptor alpha expression and function in mouse mammary epithelial cells. J Cell Sci. 2003;116:2975–2986. doi: 10.1242/jcs.00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2006;22:287–309. doi: 10.1146/annurev.cellbio.22.010305.104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naylor MJ, Li N, Cheung J, et al. Ablation of beta1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. J Cell Biol. 2005;171:717–728. doi: 10.1083/jcb.200503144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu R, Nelson CM, Muschler JL, Veiseh M, Vonderhaar BK, Bissell MJ. Sustained activation of STAT5 is essential for chromatin remodeling and maintenance of mammary-specific function. J Cell Biol. 2009;184:57–66. doi: 10.1083/jcb.200807021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leiner KA, Newman D, Li CM, Walsh E, Khosla J, Sannes PL. Heparin and fibroblast growth factors affect surfactant protein gene expression in type II cells. Am J Respir Cell Mol Biol. 2006;35:611–618. doi: 10.1165/rcmb.2006-0159OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flaim CJ, Chien S, Bhatia SN. An extracellular matrix microarray for probing cellular differentiation. Nat Methods. 2005;2:119–125. doi: 10.1038/nmeth736. [DOI] [PubMed] [Google Scholar]
- 27.Pontier SM, Muller WJ. Integrins in mammary-stem-cell biology and breast-cancer progression--a role in cancer stem cells? J Cell Sci. 2009;122:207–214. doi: 10.1242/jcs.040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watt FM. Role of integrins in regulating epidermal adhesion, growth and differentiation. Embo J. 2002;21:3919–3926. doi: 10.1093/emboj/cdf399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campos LS. Beta1 integrins and neural stem cells: making sense of the extracellular environment. Bioessays. 2005;27:698–707. doi: 10.1002/bies.20256. [DOI] [PubMed] [Google Scholar]
- 30.Shen Q, Wang Y, Kokovay E, et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008;3:289–300. doi: 10.1016/j.stem.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuchs E. Finding One's Niche in the Skin. Cell Stem Cell. 2009;4:499–502. doi: 10.1016/j.stem.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Labarge MA, Nelson CM, Villadsen R, et al. Human mammary progenitor cell fate decisions are products of interactions with combinatorial microenvironments. Integr Biol. 2009;1:70–79. doi: 10.1039/b816472j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nat Rev Mol Cell Biol. 2006;7:265–275. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- 34.Ingber DE, Tensegrity I. Cell structure and hierarchical systems biology. J Cell Sci. 2003;116:1157–1173. doi: 10.1242/jcs.00359. [DOI] [PubMed] [Google Scholar]
- 35.Ingber DE. Tensegrity II. How structural networks influence cellular information processing networks. J Cell Sci. 2003;116:1397–1408. doi: 10.1242/jcs.00360. [DOI] [PubMed] [Google Scholar]
- 36.Ingber DE. Tensegrity-based mechanosensing from macro to micro. Prog Biophys Mol Biol. 2008;97:163–179. doi: 10.1016/j.pbiomolbio.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burridge K, Fath K, Kelly T, Nuckolls G, Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- 38.Gieni RS, Hendzel MJ. Mechanotransduction from the ECM to the genome: are the pieces now in place? J Cell Biochem. 2008;104:1964–1987. doi: 10.1002/jcb.21364. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Q, Skepper JN, Yang F, et al. Nesprins: a novel family of spectrin-repeat-containing proteins that localize to the nuclear membrane in multiple tissues. J Cell Sci. 2001;114:4485–4498. doi: 10.1242/jcs.114.24.4485. [DOI] [PubMed] [Google Scholar]
- 40.Starr DA, Han M. ANChors away: an actin based mechanism of nuclear positioning. J Cell Sci. 2003;116:211–216. doi: 10.1242/jcs.00248. [DOI] [PubMed] [Google Scholar]
- 41.Zastrow MS, Vlcek S, Wilson KL. Proteins that bind A-type lamins: integrating isolated clues. J Cell Sci. 2004;117:979–987. doi: 10.1242/jcs.01102. [DOI] [PubMed] [Google Scholar]
- 42.Hu S, Chen J, Butler JP, Wang N. Prestress mediates force propagation into the nucleus. Biochem Biophys Res Commun. 2005;329:423–428. doi: 10.1016/j.bbrc.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 43.Hu S, Chen J, Fabry B, et al. Intracellular stress tomography reveals stress focusing and structural anisotropy in cytoskeleton of living cells. Am J Physiol Cell Physiol. 2003;285:C1082–C1090. doi: 10.1152/ajpcell.00159.2003. [DOI] [PubMed] [Google Scholar]
- 44.Maniotis AJ, Bojanowski K, Ingber DE. Mechanical continuity and reversible chromosome disassembly within intact genomes removed from living cells. J Cell Biochem. 1997;65:114–130. [PubMed] [Google Scholar]
- 45.Wang N, Naruse K, Stamenovic D, et al. Mechanical behavior in living cells consistent with the tensegrity model. Proc Natl Acad Sci U S A. 2001;98:7765–7770. doi: 10.1073/pnas.141199598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pollack GH. Cells, gels and the engines of life. Ebner and Sons Publishers; 2001. [Google Scholar]
- 47.Baneyx G, Baugh L, Vogel V. Fibronectin extension and unfolding within cell matrix fibrils controlled by cytoskeletal tension. Proc Natl Acad Sci U S A. 2002;99:5139–5143. doi: 10.1073/pnas.072650799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bustamante C. Of torques, forces, and protein machines. Protein Sci. 2004;13:3061–3065. doi: 10.1110/ps.041064504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khan S, Sheetz MP. Force effects on biochemical kinetics. Annu Rev Biochem. 1997;66:785–805. doi: 10.1146/annurev.biochem.66.1.785. [DOI] [PubMed] [Google Scholar]
- 50.Dalby MJ, Biggs MJ, Gadegaard N, Kalna G, Wilkinson CD, Curtis AS. Nanotopographical stimulation of mechanotransduction and changes in interphase centromere positioning. J Cell Biochem. 2007;100:326–338. doi: 10.1002/jcb.21058. [DOI] [PubMed] [Google Scholar]
- 51.Dalby MJ, Gadegaard N, Herzyk P, Agheli H, Sutherland DS, Wilkinson CD. Group analysis of regulation of fibroblast genome on low-adhesion nanostructures. Biomaterials. 2007;28:1761–1769. doi: 10.1016/j.biomaterials.2006.11.049. [DOI] [PubMed] [Google Scholar]
- 52.Dalby MJ, Gadegaard N, Herzyk P, et al. Nanomechanotransduction and interphase nuclear organization influence on genomic control. J Cell Biochem. 2007;102:1234–1244. doi: 10.1002/jcb.21354. [DOI] [PubMed] [Google Scholar]
- 53.Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004;572:65–68. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 54.Sukharev S, Corey DP. Mechanosensitive channels: multiplicity of families and gating paradigms. Sci STKE 2004. 2004:re4. doi: 10.1126/stke.2192004re4. [DOI] [PubMed] [Google Scholar]
- 55.Matthews BD, Overby DR, Mannix R, Ingber DE. Cellular adaptation to mechanical stress: role of integrins, Rho, cytoskeletal tension and mechanosensitive ion channels. J Cell Sci. 2006;119:508–518. doi: 10.1242/jcs.02760. [DOI] [PubMed] [Google Scholar]
- 56.Meyer CJ, Alenghat FJ, Rim P, Fong JH, Fabry B, Ingber DE. Mechanical control of cyclic AMP signalling and gene transcription through integrins. Nat Cell Biol. 2000;2:666–668. doi: 10.1038/35023621. [DOI] [PubMed] [Google Scholar]
- 57.Paszek MJ, Zahir N, Johnson KR, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 58.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 60.Mammoto A, Huang S, Moore K, Oh P, Ingber DE. Role of RhoA, mDia, and ROCK in cell shape-dependent control of the Skp2-p27kip1 pathway and the G1/S transition. J Biol Chem. 2004;279:26323–26330. doi: 10.1074/jbc.M402725200. [DOI] [PubMed] [Google Scholar]
- 61.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 62.Nelson CM, Khauv D, Bissell MJ, Radisky DC. Change in cell shape is required for matrix metalloproteinase-induced epithelial-mesenchymal transition of mammary epithelial cells. J Cell Biochem. 2008;105:25–33. doi: 10.1002/jcb.21821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 64.Nelson CM, Jean RP, Tan JL, et al. Emergent patterns of growth controlled by multicellular form and mechanics. Proc Natl Acad Sci U S A. 2005;102:11594–11599. doi: 10.1073/pnas.0502575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wozniak MA, Chen CS. Mechanotransduction in development: a growing role for contractility. Nat Rev Mol Cell Biol. 2008;10:34–43. doi: 10.1038/nrm2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Adams DS, Keller R, Koehl MA. The mechanics of notochord elongation, straightening and stiffening in the embryo of Xenopus laevis. Development. 1990;110:115–130. doi: 10.1242/dev.110.1.115. [DOI] [PubMed] [Google Scholar]
- 67.Keller R, Jansa S. Xenopus Gastrulation without a blastocoel roof. Dev Dyn. 1992;195:162–176. doi: 10.1002/aja.1001950303. [DOI] [PubMed] [Google Scholar]
- 68.Hutson MS, Tokutake Y, Chang MS, et al. Forces for morphogenesis investigated with laser microsurgery and quantitative modeling. Science. 2003;300:145–149. doi: 10.1126/science.1079552. [DOI] [PubMed] [Google Scholar]
- 69.Kiehart DP, Galbraith CG, Edwards KA, Rickoll WL, Montague RA. Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila. J Cell Biol. 2000;149:471–490. doi: 10.1083/jcb.149.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Desprat N, Supatto W, Pouille P-A, Beaurepaire E, Farge E. Tissue deformation modulates Twist expression to determine anterior midgut differentiation in Drosophila embyros. Dev Cell. 2008;15:470–477. doi: 10.1016/j.devcel.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 71.Farge E. Mechanical induction of Twist in the Drosophila foregut/stomodeal primordium. Curr Biol. 2003;13:1365–1377. doi: 10.1016/s0960-9822(03)00576-1. [DOI] [PubMed] [Google Scholar]
- 72.Moore KA, Polte T, Huang S, et al. Control of basement membrane remodeling and epithelial branching morphogenesis in embryonic lung by Rho and cytoskeletal tension. Dev Dyn. 2005;232:268–281. doi: 10.1002/dvdy.20237. [DOI] [PubMed] [Google Scholar]
- 73.Michael L, Sweeney DE, Davies JA. A role for microfilament-based contraction in branching morphogenesis of the ureteric bud. Kidney Int. 2005;68:2010–2018. doi: 10.1111/j.1523-1755.2005.00655.x. [DOI] [PubMed] [Google Scholar]
- 74.Moore KA, Huang S, Kong Y, Sunday ME, Ingber DE. Control of embryonic lung branching morphogenesis by the Rho activator, cytotoxic necrotizing factor 1. J Surg Res. 2002;104:95–100. doi: 10.1006/jsre.2002.6418. [DOI] [PubMed] [Google Scholar]
- 75.Folkman J, Moscona A. Role of cell shape in growth control. Nature. 1978;273:345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- 76.White ES, Baralle FE, Muro AF. New insights into form and function of fibronectin splice variants. J Pathol. 2008;216:1–14. doi: 10.1002/path.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Itano N. Simple primary structure, complex turnover regulation and multiple roles of hyaluronan. J Biochem. 2008;144:131–137. doi: 10.1093/jb/mvn046. [DOI] [PubMed] [Google Scholar]
- 78.Camenisch TD, Schroeder JA, Bradley J, Klewer SE, McDonald JA. Heart-valve mesenchyme formation is dependent on hyaluronan-augmented activation of ErbB2-ErbB3 receptors. Nat Med. 2002;8:850–855. doi: 10.1038/nm742. [DOI] [PubMed] [Google Scholar]
- 79.Gross J, Lapiere CM. Collagenolytic activity in amphibian tissues: a tissue culture assay. Proc Natl Acad Sci U S A. 1962;48:1014–1022. doi: 10.1073/pnas.48.6.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Harris AK, Wild P, Stopak D. Silicone rubber substrata: a new wrinkle in the study of cell locomotion. Science. 1980;208:177–179. doi: 10.1126/science.6987736. [DOI] [PubMed] [Google Scholar]
- 82.Dembo M, Wang YL. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys J. 1999;76:2307–2316. doi: 10.1016/S0006-3495(99)77386-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pelham RJ, Jr, Wang Y. High resolution detection of mechanical forces exerted by locomoting fibroblasts on the substrate. Mol Biol Cell. 1999;10:935–945. doi: 10.1091/mbc.10.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tan JL, Tien J, Pirone DM, Gray DS, Bhadriraju K, Chen CS. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proc Natl Acad Sci U S A. 2003;100:1484–1489. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Friedl P, Brocker EB. The biology of cell locomotion within three-dimensional extracellular matrix. Cell Mol Life Sci. 2000;57:41–64. doi: 10.1007/s000180050498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bell E, Ivarsson B, Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci U S A. 1979;76:1274–1278. doi: 10.1073/pnas.76.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhong C, Chrzanowska-Wodnicka M, Brown J, Shaub A, Belkin AM, Burridge K. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J Cell Biol. 1998;141:539–551. doi: 10.1083/jcb.141.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Petroll WM, Ma L, Jester JV. Direct correlation of collagen matrix deformation with focal adhesion dynamics in living corneal fibroblasts. J Cell Sci. 2003;116:1481–1491. doi: 10.1242/jcs.00357. [DOI] [PubMed] [Google Scholar]
- 89.Prajapati RT, Chavally-Mis B, Herbage D, Eastwood M, Brown RA. Mechanical loading regulates protease production by fibroblasts in three-dimensional collagen substrates. Wound Repair Regen. 2000;8:226–237. doi: 10.1046/j.1524-475x.2000.00226.x. [DOI] [PubMed] [Google Scholar]
- 90.Prestwich GD. Simplifying the extracellular matrix for 3-D cell culture and tissue engineering: a pragmatic approach. J Cell Biochem. 2007;101:1370–1383. doi: 10.1002/jcb.21386. [DOI] [PubMed] [Google Scholar]
- 91.Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7:211–224. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 92.Nelson CM, Tien J. Microstructured extracellular matrices in tissue engineering and development. Curr Opin Biotechnol. 2006;17:518–523. doi: 10.1016/j.copbio.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 93.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 94.Serban MA, Scott A, Prestwich GD. Use of hyaluronan-derived hydrogels for three-dimensional cell culture and tumor xenografts, Chapter 10. Curr Protoc Cell Biol. 2008 doi: 10.1002/0471143030.cb1014s40. Unit 10 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Golden AP, Tien J. Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. Lab Chip. 2007;7:720–725. doi: 10.1039/b618409j. [DOI] [PubMed] [Google Scholar]
- 96.Chrobak KM, Potter DR, Tien J. Formation of perfused, functional microvascular tubes in vitro. Microvasc Res. 2006;71:185–196. doi: 10.1016/j.mvr.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 97.Tang MD, Golden AP, Tien J. Fabrication of collagen gels that contain patterned, micrometer-scale cavities. Adv Mater. 2004;16:393–397. [Google Scholar]