SUMMARY
Mechanically damaged plasma membrane undergoes rapid calcium-dependent resealing that appears to depend, at least in part, on calpain-mediated cortical cytoskeletal remodeling. Cells null for Capns1, the non-catalytic small subunit present in both m- and μ-calpains, do not undergo calcium-mediated resealing. However, it is not known which of these calpains is needed for repair, or whether other major cytosolic proteinases may participate. Utilizing isozyme-selective siRNAs to decrease expression of Capn1 or Capn2, catalytic subunits of μ- and m-calpains, respectively, in a mouse embryonic fibroblast cell line, we now show that substantial loss of both activities is required to compromise calcium-mediated survival after cell scrape-damage. Using skeletal myotubes derived from Capn3–null mice, we were unable to demonstrate loss of sarcolemma resealing after needle scratch or laser damage. Isolated muscle fibers from Capn3 knockout mice also efficiently repaired laser damage. Employing either a cell line expressing a temperature sensitive E1 ubiquitin ligase, or lactacystin, a specific proteasome inhibitor, it was not possible to demonstrate an effect of the proteasome on calcium-mediated survival after injury. Moreover, several cell-permeant caspase inhibitors were incapable of significantly decreasing survival or inhibiting membrane repair. Taken together with previous studies, the results show that m- or μ-calpain can facilitate repair of damaged plasma membrane. While there was no evidence for the involvement of calpain-3, the proteasome or caspases in early events of plasma membrane repair, our studies do not rule out their participation in downstream events that may link plasma membrane repair to adaptive remodeling after injury.
Keywords: calpain, proteasome, caspase, membrane repair
INTRODUCTION
Cells are constantly exposed to mechanical stresses that produce breaks in their plasma membranes [1–3]. This is readily observed in tissues predicted to be at greatest risk for membrane damage. For example, skeletal muscle myocytes [4] and cardiomyocytes [5], which must routinely contract against mechanical or pressure loads, were shown to allow entry of serum albumin or cell impermeant dyes in their cytosolic compartments, consistent with ongoing contraction-dependent sarcolemmal injury. Recent studies from several laboratories indicate that a discrete mechanism has evolved to repair injured plasma membrane. It is thought that calcium-dependent fusion of intracellular vesicles underlying the ruptured plasma membrane creates a large vesicle patch, which rapidly translocates to the site of damage. There it fuses with the ruptured plasma membrane to quickly restore the permeability barrier that all living cells require.
While the molecular events underlying membrane repair are as yet poorly defined, several candidate repair proteins have been identified; including vSNARE and tSNARE components that regulate vesicle fusion with plasma membrane in exocytosis [6]; the multiple C2 domain protein dysferlin, that appears to be required for efficient repair of damaged skeletal [7] and cardiac [8] muscle sarcolemma; and annexin-A1, a calcium and phospholipid binding protein [9]. Recently, the intracellular calcium-dependent proteinases, calpains, have been shown to be required for calcium-mediated repair of damaged plasma membrane in cultured fibroblasts and some other cell types [10]. It is thought that they clear cortical cytoskeletal remnants at the injury site, facilitating vesicle patch fusion with the torn plasma membrane [3, 11]. Initial studies strongly argued that a ubiquitous heterodimeric calpain, m- or μ-calpain, was required for repair. However, it is still not known if both isozymes have this function. It is conceivable that the participation of other intracellular proteinases could also be indispensable for rapid, complete removal of damaged cortical cytoskeleton, or to catalyze other events required for prompt wound resealing. Inactivating mutations of the CAPN3 gene, encoding the muscle-specific calpain-3, result in a limb-girdle muscular dystrophy: LGMD-2A [12]. Moreover, calpain-3 associates with putative membrane repair proteins at the plasma membrane, including dysferlin [13]. Thus, it would be interesting to know if calpain-3 is also required for repair of wounded skeletal muscle sarcolemma. In addition, other major intracellular proteinases, the proteasome and caspases, participate in a number of physiologic and pathophysiologic processes, and might also contribute to plasma membrane repair. We now provide evidence that m- or μ-calpain can affect repair. However, calpain-3, the proteasome and caspases do not appear to be required.
MATERIALS AND METHODS
Materials
The following siRNA constructs were obtained from Qiagen; Capn1: AA-UAUCCCUCUGCAAACCAAA-dTdT. Capn2: AA-GGUCAGAGGAAGCGUUACA-dTdT. Control siRNA: AA-UUCUCCGAACGUGUCACGU-dTdT. FM1-43 and its paraformaldehyde fixable analog, FM1-43FX, were purchased from Molecular Probes. Calpeptin, lactacystin, kFGF-DEVD aldehyde kFGF-YVAD aldehyde, ZVAD-CH2F, and N-acetyl-YVAD aldehyde were obtained from Calbiochem. ECL extracellular matrix preparation, and 100× insulin-transferrin-selenium stock solution (Gibco product number 51500-056) were purchased from Invitrogen.
Cell culture
Capns1−/−, Capns1+/+, Capns1−/−R, ts20B, and H38-5 mouse fibroblast cell lines were routinely cultured in humidified 5% CO2, in DMEM medium supplemented with 10% FBS. The Capns1 cell lines were cultured at 37 °C. The ts20B and H38-5 cell lines were routinely cultured at 33 °C (permissive temperature for the ts20B cells). To inactivate the mutated E1 ubiquitin ligase in the ts20B cells, they were shifted to 39 °C 16 hours before being scrape-damaged. The H38-5 control cells were heat-treated under identical conditions. The C2C12 mouse myoblast cell line, Capn3−/− myoblasts, and Capn3+/+ myoblasts derived from wild type littermates, were cultured in 37 °C, humidified 5% CO2 in Hams F10 medium containing 20% FBS and 5 ng/ml bFGF. Myotubes were prepared by allowing myoblasts to grow to confluence on culture dishes coated with ECL. Medium was then changed to DMEM supplemented with insulin, transferrin and selenium, and was exchanged for fresh medium every day thereafter. After 4 days, the differentiated myotubes were subjected to needle-scratch injury.
Animals
All procedures on animals were performed according to the European guidelines for the humane care and use of experimental animals. The experiments were approved by the French Ministry of Research and Education (Agreement decision B91 228-101). C57BL/6 mice were purchased from Charles River Laboratories (Les Oncins, France). The Capn3-null mice used in these studies were described in [14].
Calpain expression knockdown studies
The catalytic subunits of m-calpain and μ-calpain in Capns1+/+ SV40-Large T-antigen transformed fibroblasts were downregulated by transfecting with siRNA constructs, employing a standard protocol. Cells were transfected with a Lipofectamine 2000:siRNA complex when approximately 30% confluent in 6-well plates. The conditions recommended by the Lipofectamine 2000 manufacturer (Invitrogen) were followed. The final transfection medium was 0.5 ml Optimem® medium containing 100 nMol of total siRNA complexed with 10 µl of Lipofectamine 2000. Four different conditions were employed: 1) control, 100 nMol of control siRNA; 2) μ-calpain knockdown, 50 nMol of control siRNA plus 50 nMol Capn1 siRNA; 3) m-calpain knockdown, 50 nMol control siRNA plus 50 nMol Capn2 siRNA; 4) double knockdown, 50 nMol each of Capn1 and Capn2 siRNAs. This protocol ensured that the total concentration of siRNA and ratio of siRNA to Lipofectamine was the same in all experimental conditions. Cells were allowed to recover for 48 hours, and then trypsinized and transferred to 12-well plates. The next day, 72 hours after siRNA treatment, the cells were scrape-damaged and analyzed for survival, as described below.
Other samples of siRNA-treated cells were lysed in 25 mM MOPS buffer, pH 7.2, containing 0.5 mM EGTA, 0.5 mM dithiothreitol, 150 mM NaCl, 0.2% Triton X-100, and analyzed for μ- and m-calpain content by casein zymography, as previously described [15]. Briefly, protein in cell lysates was determined by the BCA method [16], and 15 µg of each sample was applied to a 12% non-denaturing polyacrylamide gel co-polymerized with 2 mg casein/ml. After electrophoresis, the gel was incubated in 50 mM Tris-HCl, 5 mM CaCl2 , 5 mM dithiothreitol, pH 7.3. Sixteen hours later, the gel was stained with coomassie blue dye. Calpains were detected by lack of coomassie staining (absence of casein) at their positions in the gel. Bovine m-calpain and human μ-calpain, purified as previously described [17], were applied to the same gel to mark the location of the endogenous calpains. RGB tif images of scanned gels were analyzed for band brightness using the NIH ImageJ program and the default RGB brightness conversion factor. Regions of the zymograms without sample loads provided background brightness levels. Because of low levels of calpain activity in the calpain siRNA-treated samples, no attempt was made to quantitate calpain in the zymograms, and the results should be interpreted as a qualitative assessment of calpain downregulation.
Cell survival assay
Cells were cultured in 12-well plates until 30–50% confluent. They were then scraped with a small plastic cell scraper (Corning product number 3010) in PBS containing 1.5 mM CaCl2 or 2 mM EGTA as previously described [10]. The injured cells were allowed to recover, and assayed for mitochondrial MTT reductase activity [18]. It was previously shown that this assay is consistent with other measures of cell survival, including direct cell counting and clonal survival assays [10]. In some experiments, recovery of plasma membrane integrity one minute after scraping was assessed by trypan blue uptake, as previously described [10]. To investigate the effect of protease inhibitors on survival, cells were cultured with the inhibitors for 2 hours prior to scraping. They were then scraped in buffer containing inhibitor, at the same concentration.
Cell damage protocols
Cells were subjected to needle-scratch damage [10], or laser injury [19] as previously described. Needle-scratch injury was carried out in the presence of 5 µM FM1-43FX in PBS containing 1.5 mM CaCl2, 2 mM EGTA, or 1.5 mM CaCl2 and 20 µM calpeptin. Myotubes derived from Capn3-null mouse myoblasts [20] were used in these experiments. Myotubes scratched in the presence of calpeptin were first pre-incubated for 2 hours in culture medium containing 20 µM calpeptin. One minute after scratching, the myotubes were fixed on ice for 10 min with 4% paraformaldehyde in PBS. The myotubes were washed 4 times with PBS, and subjected to epifluorescence microscopy using a filter set for fluorescein detection. Injured myotubes in a 400× field adjacent to the scratch zone were subjected to image analysis using the NIH ImageJ program. Fluorescence intensity normalized for cell area was compared for myotubes scratched in the presence of calcium, EGTA, or calcium plus calpeptin.
Skeletal muscle myoblasts or derived myotubes from Capn3-null mice [14] were wounded in the presence or absence of 1 mM calcium, using the 820 nm infrared laser of a two photon microscope (LSM 510META, Zeiss and MIRA 900 System, Coherent). Resealing analysis was based on the kinetics and extent of FM1-43 dye entry through open disruptions [19]. The cells were maintained at 37°C during imaging.
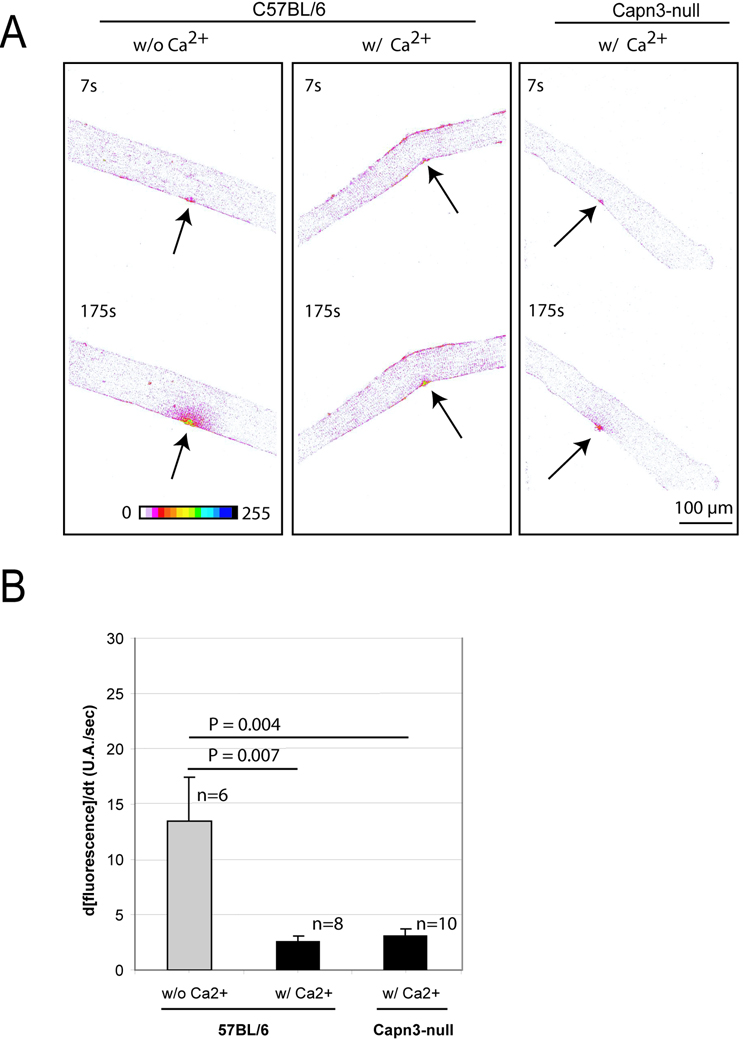
For laser injury of isolated muscle fibers, the following procedure was used. Isolated Flexor Digitorum Brevis (FDB) muscles of 7–8-week old Capn3-null [14] or C57BL/6 mice were digested in DMEM containing 1% collagenase A under slow rotation at 37 °C for 2–3 hours. The digest was washed 4 to 5-times with PBS without calcium or magnesium ion, and resuspended to a final volume of 2 ml in the same buffer. Approximately one-half of the fibers isolated from both Capn3-null and control cells were damaged during the preparation of muscle fibers, as observed by hypercontraction and spontaneous FM1-43 dye uptake. Only undamaged fibers were assessed for repair of laser injury. Before performing the membrane repair assay, the preparation was adjusted to 100 µM calcium and 4 µM FM1-43. To induce damage, an area of the sarcolemma on the membrane of the muscle fiber was irradiated at full power for 1 second with a two-photon confocal laser-scanning microscope. The Multi-Photon Microscope consisted of a Radiance 2100 MP scan head (Bio-Rad) equipped with a mode-locked Titanium-Sapphire laser system (Coherent Verdi-Mira) pumped at 10W and tuned to 800 nm excitation with 100 fs pulses at 76 MHz. The microscope was an inverted Nikon TE300 (Nikon Instech Co.) with Nikon objectives (dry CFI Plan APO, 20× NA0.75). Images were captured for 3 min after the irradiation at 7-s intervals. Images were represented in an inverted 16-color look up table of ImageJ. For every image taken, the fluorescence intensity at the site of the damage was measured by ImageJ on an area of about 0.01 mm2. The rate of fluorescence influx for all fibers in the same group was plotted on a histogram.
Statistical analysis
Except where otherwise indicated, results are presented as experimental mean plus or minus standard deviation. Statistical significance was assessed using Students t-test, and P < 0.05 was considered significant.
RESULTS
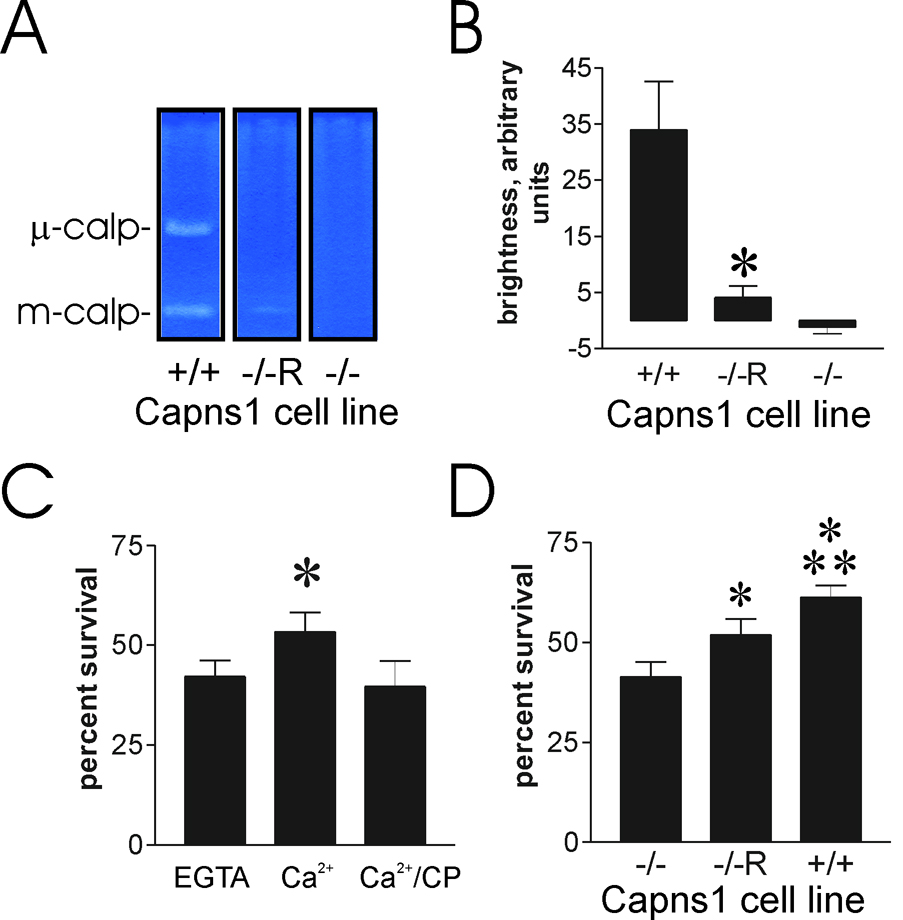
Previous studies demonstrated that SV-40 large T-antigen transformed fibroblasts derived from Capns1-null embryos failed to reseal upon laser injury in the presence of millimolar Ca2+. Moreover, their percent survival after scrape-damage was not increased by Ca2+ [10] To be certain that loss of the calpain small subunit, encoded by Capns1, was responsible for this loss of calcium-dependent membrane repair, it was essential to show gain of function by Capns1 re-expression in the knockout background. Capns1−/−R, a rescued strain that expresses a limited amount of small subunit, was shown to have partially restored calpain activity, assessed by casein zymography, as well as recapitulation of the wild-type cell motility phenotype [21]. We confirmed by casein zymography that m-calpain activity was detectable in the Capns1−/−R cells employed in our studies (fig. 1A). No μ-calpain activity was detected in the Capns1−/−R cells, and, as expected, the Capns1−/− cells displayed neither m-calpain nor μ-calpain activities in the zymogram (fig. 1A). Qualitative assessment of band brightness in the zymograms suggested that the Capns1−/−R cells displayed modest m-calpain activity compared with wild-type (fig. 1B). Several experiments with Capns1−/−R showed that calcium-dependent percent survival after scrape damage was restored (fig. 1C), albeit to a lower extent than observed in wild-type mouse fibroblasts (fig. 1D). This is consistent with the requirement of calpain small subunit for survival. However, because Capns1 encodes the small subunit present in both m- and μ-calpains, these studies could not show which of the ubiquitous, typical calpains was required for cell survival.
Figure 1.
The Capns1−/−R rescued fibroblast cell line expresses limited calpain activity, but shows partial recovery of calcium-mediated cell survival after scraping. A. Casein zymogram of Capns1 cell lines. Cell lysates were subjected to zymography as described in the Materials and Methods section. The μ- and m-calpain bands are shown, as assessed by the migration of purified human μ- and bovine m-calpains, respectively. Lanes are from the same zymogram, but were separated to prevent sample “bleedover”. B. Relative intensity of m-calpain bands from three independent zymography experiments, using the NIH imageJ software program to estimate brightness of the cleared casein zones. C. Capns1−/−R cells were scraped with the indicated additions, as described in the Materials and Methods section, and allowed to recover for 12 hours before assessing cell survival. CP=20 µM calpeptin. Asterisk: P = 0.002 vs EGTA sample (N=6). D. Capns1−/−, Capns1−/−R, and Capns1+/+ fibroblasts were cultured to 30–50% confluence, and scraped in the presence of calcium. Asterisk: P<0.001 vs Capns1−/−. Double asterisk: P<0.001 vs Capns1−/−R.
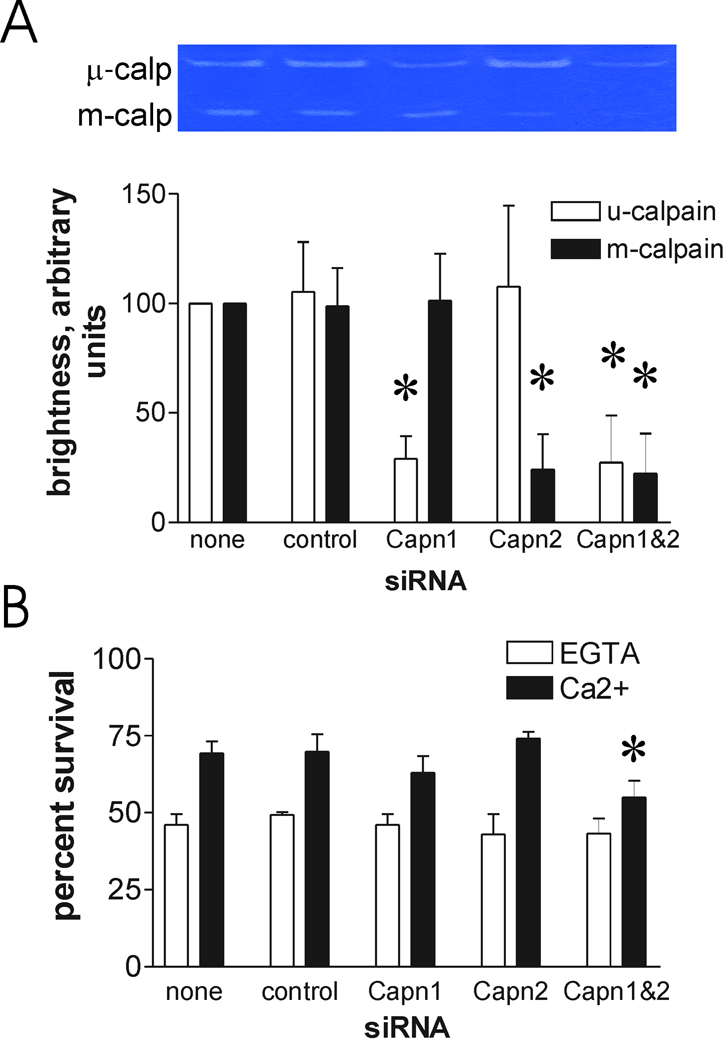
Differential depletion of m- and μ-calpain large subunits has been achieved by siRNA-mediated knockdown [22]. Importantly, depletion of one subunit did not appear to noticeably affect expression of the other. We therefore employed the well-characterized siRNA sequences to deplete expression of m-calpain, μ-calpain, or both large subunits, and assessed the impact on calcium-mediated survival after scrape damage. In three independent experiments, we noted substantial depletion of either m- or μ-calpain activity, as indicated by decreased relative band brightness on casein zymograms, using the protocol described in the Materials and Methods section (fig. 2A). The control siRNA had no apparent influence on expression of either calpain, as assessed by casein zymography, and exposure of cells to both calpain siRNAs together appeared to produce the same effect on their cognate targets as when they were applied separately (fig. 2A). Depletion of either calpain alone had at best a modest effect on survival after scrape damage. However, in three independent studies, this reduction in survival was not statistically significant. In contrast, combining the two siRNAs resulted in a reproducible, but incomplete, inhibition of calcium-mediated survival (fig. 2B). Importantly, there was no significant effect on survival after scraping in the presence of EGTA in this experiment, or in either of the other replicate experiments, arguing against a simple increase in cell toxicity by combining the siRNAs. Taken together with the results presented in figure 1, and with our previous studies that strongly implicated calpains in plasma membrane repair [10, 11], these experiments indicate that either m- or μ-calpain can bring about repair, and that even modest calpain levels are sufficient for at least partial protection.
Figure 2.
Calcium-dependent survival after scraping requires the typical, ubiquitous calpains. A. Capns1+/+ fibroblasts were transfected with the indicated siRNAs. After 72 hours, the cells were lysed and subjected to casein zymography to assess loss of μ- and m-calpain activity. The inset shows results of one experiment. The graph presents data from three experiments. Asterisks: P<0.02 vs corresponding control siRNA. B. Other samples were scraped and viability was determined 16 hours later. Details are provided in the Materials and Methods section. Asterisk: P = 0.005 vs no siRNA addition (N=4).
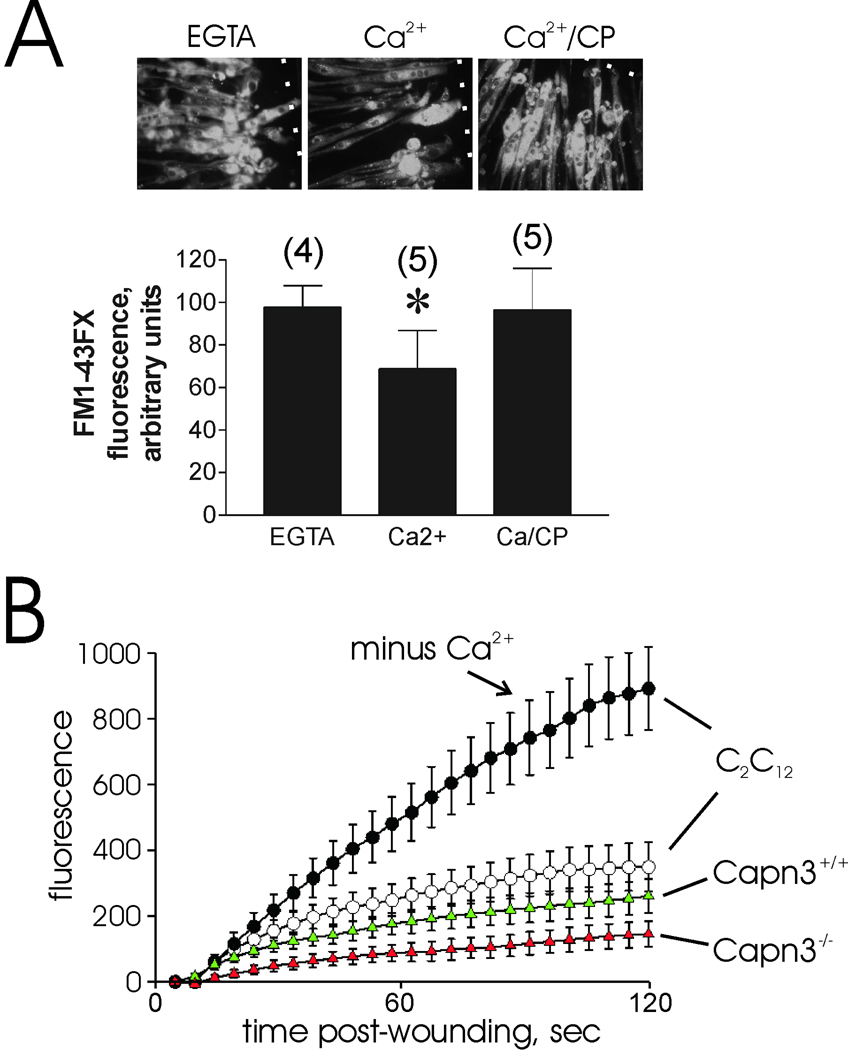
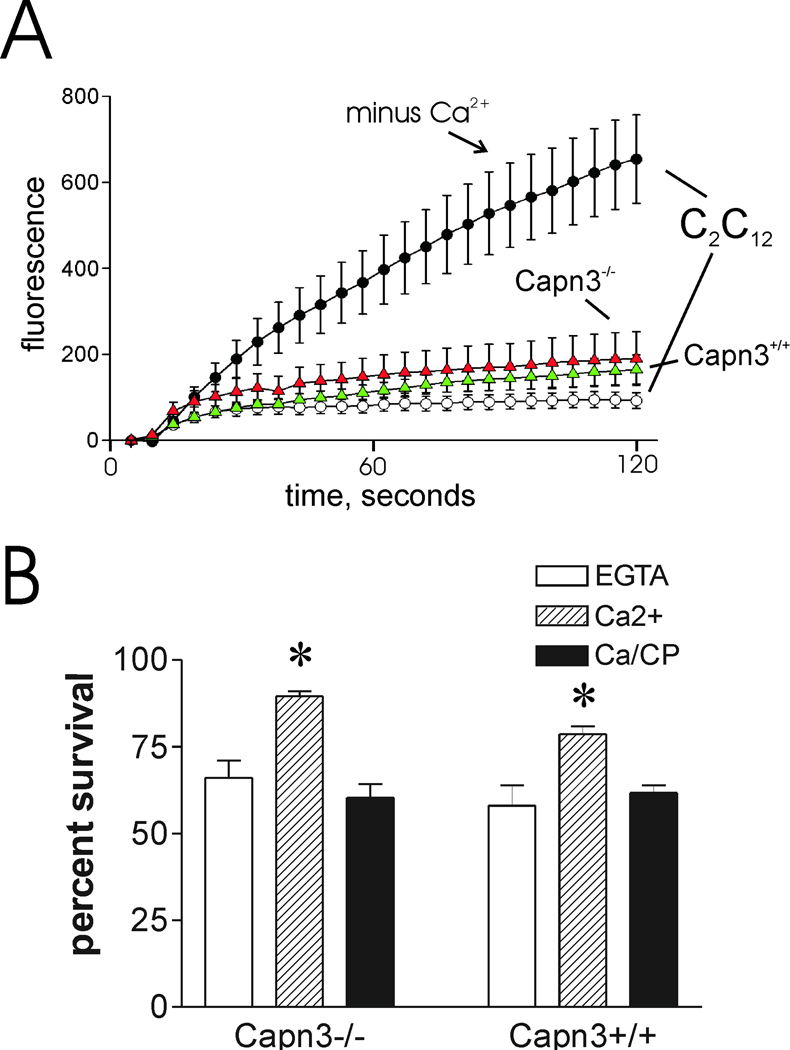
Calpain-3 is localized at the skeletal muscle M-line, Z-band, and the N2A region of the large myofibrillar protein, titin (also called connectin) [23, 24]. While it has been speculated that calpain-3 may participate in sarcolemma repair [25], there is no direct evidence to support this function. In fact, recent studies indicate that nearly all calpain-3 in mature rat muscle fibers is associated with the contractile filament matrix [26]. Nevertheless, it is possible that a minor fraction of calpain-3 is released from contractile filament binding sites following injury, and participates in membrane repair. In keeping with this possibility, calpain-3 appears to be capable of cleaving several sarcolemma-associated proteins, including talin, α-actinin, vinexin, ezrin [27], γ-filamin [28], and β-catenin [29]. To investigate the possible function of calpain-3 in sarcolemma repair, myotubes derived from two calpain-3 knockout mouse strains [14,20] were subjected to membrane damage protocols. FM1-43FX uptake through needle-scratch damage, as described in the Materials and Methods section, was significantly less when calcium was present in the medium (P=0.014 vs EGTA sample, fig. 3A), an indication that Capn3-null myotubes still have the ability to undergo calcium-dependent resealing. Calpeptin prevented the effect of calcium addition, suggesting that μ- and m- calpain- mediated repair is normal in these cells. Capn3-null myotubes recovered from laser injury at least as well as wild type (fig. 3B). Although the Capn3-null myotubes appeared to reseal somewhat better than wild-type in this experiment, this degree of variability is not uncommon within the experimental system employed. Importantly, neither cell type displayed the dramatic, continuous uptake of dye observed when membrane repair is blocked by elimination of calcium (compare C2C12 myotubes ± calcium, fig. 3B), dysferlin deficiency [7], or calpain small subunit ablation [10]. Capn3-null myoblasts also resealed quickly after laser injury (fig. 4A). The cell scraping protocol routinely employed to assess fibroblast survival is not applicable to myotubes, which survive mechanical removal from their growth substrate poorly, if at all. However, it was possible to subject myoblasts to scrape damage. The latter did not appear to bind as avidly to plastic cultureware; thus, they were not injured as much as the fibroblasts we routinely employ, as shown by their increased percent survival under all conditions (fig. 4B). However, it was apparent that loss of calpain-3 did not alter their calcium-dependent survival. As with other cell types, calpeptin completely prevented calcium-dependent survival of the Capn3−/− myoblasts, indicating the involvement of calpains, most likely m- and μ-calpain, in calcium-dependent repair. As a final test of calpain-3 function in repair of intact native muscle fibers, isolated muscle fibers from Capn3−/− mice were subjected to laser injury in the presence of FM 1-43 dye as described in the Materials and Methods section. Consistent with our other studies, the Capn3−/− muscle fibers recovered as well as muscle fibers derived from wild-type mice (fig. 5). Overall, the results of our studies suggest that calpain-3 does not participate significantly in rapid sarcolemma repair after various types of mechanical injury.
Figure 3.
Calcium-dependent repair of damaged sarcolemma is not compromised in myotubes derived from Capn3-null mice. A. Myotubes prepared from Capn3−/− myoblasts were subjected to needle scratch damage in the presence of FM1-43FX dye. The dotted white lines indicate the sites of scratch injury. Dye uptake for the first minute after damage was semi-quantitatively assessed as described in the Materials and Methods section (asterisk: P = 0.014 vs EGTA samples). Numbers in parentheses refer to the total number of scratch sites analyzed in two different culture dish wells. Upper panel depicts typical results for each injury condition. B. Laser injury of Capn3−/− and Capn3+/+ myotubes, as described in the Materials and Methods section. Except where noted, cells were injured in the presence of 1 mM CaCl2. As noted in many previous studies, calcium-mediated resealing of wounded membrane rapidly prevents accumulation of dye, as shown by injury of C2C12 myotubes in the presence of calcium compared with EGTA. Note that both Capn3−/− and Capn3+/+ myotubes (red and green triangles, respectively) resealed efficiently.
Figure 4.
Membrane repair and calcium-dependent cell survival are not significantly compromised in injured Capn3−/− myoblasts. Myoblasts were subjected to laser injury (A) or scrape injury (B) using the standard protocols for each (see Materials and Methods section). Symbols are the same as in figure 3. Asterisks: P < 0.005 vs EGTA samples.
Figure 5.
Comparison of membrane repair capacity between Capn3-null and C57BL/6 mice A. A membrane repair assay was performed on single muscle fibers from C57BL/6 and Capn3-null mice. Images are represented in a “16 colors inverted” look up table (table depicted at the bottom of the left panel) from ImageJ software. Arrows indicate the site of injury. The time at which each picture was taken is also indicated. The two left panels show a representative assay performed on C57BL/6 fibers without (w/o) or with (w/) Ca2+. The right panel shows a representative repair assay performed on Capn3-null fibers. Scale bar = 100 µm. B. Histogram representing the rates of fluorescence influx (Δ[fluorescence]/Δt) in fibers of C57BL/6 w/o Ca2+, w/ Ca2+ and Capn3-null w/ Ca2+. The fibers were sampled from 3 different mice for each condition. Data are means ± s.e.m. Statistical analysis was performed with Student's t-test.
Other major cytosolic proteolytic activities include the ubiquitin-proteasome system, and the caspases. Ubiquitin-signaled proteasomal proteolysis of regulatory proteins mediates many intracellular pathways, including those involved in cell survival [30–32], a function that would logically fit with their possible effect on plasma membrane repair. Caspases are predominantly associated with regulated cell death. However, they appear to have a more diverse role in cell physiology than previously recognized, including the potential of caspase-1 to promote cell survival [33,34]. We therefore determined whether the proteasome or caspases might be required for plasma membrane repair.
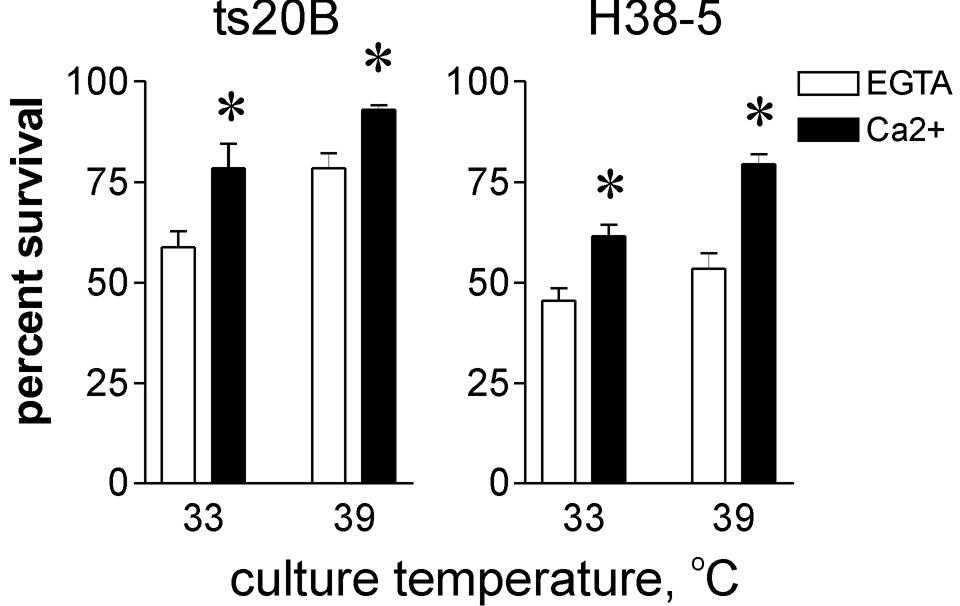
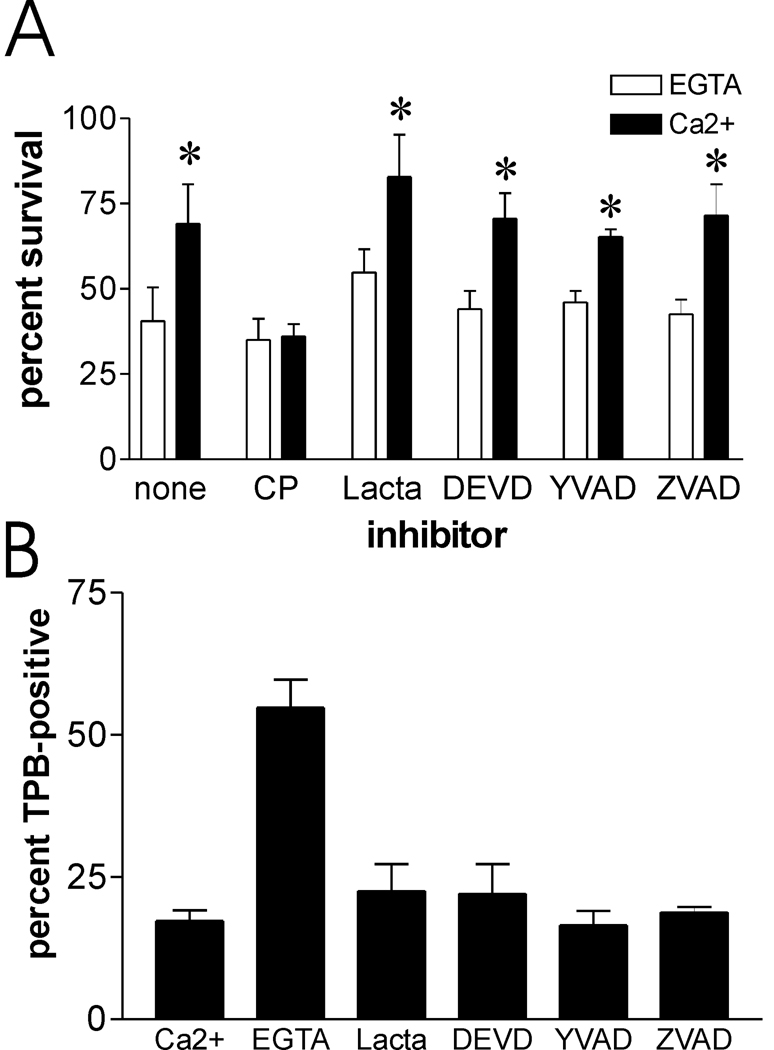
We utilized the ts20b E1-ubiquitin ligase temperature sensitive mutant cell line [35] to investigate the role of the ubiquitin-proteasome system in survival after plasma membrane damage. These cells can produce polyubiquitinated proteasome substrates at 33 °C, but not at 39 °C, thus limiting proteasomal degradation at the higher temperature. The ts20b and the control H38-5 cell lines both displayed calcium-dependent increase in survival after scrape damage, irrespective of their growth at 33 °C or 39 °C, the permissive and restrictive temperatures for ts20b E1-ligase, respectively (fig. 6). Some proteins do not require ubiquitination prior to processing by the proteasome. Therefore, to directly assess its involvement in recovery from plasma membrane damage, proteasome activity in mouse fibroblasts was inhibited by preincubation with the proteasome-specific active site alkylating agent, lactacystin. In the same experiment, other cells were pre-incubated with acetyl-YVAD-CHO or kFGF-DEVD-CHO, cell permeant inhibitors of caspase-1 and caspase-3, respectively. ZVAD-CH2F, a cell permeant pan-caspase inhibitor, was also included in these studies. The concentrations of kFGF-DEVD-CHO and ZVAD-CH2F employed were near the maximum effective doses for inhibition of Capns1+/+ apoptotic cell death induced by exposure to hydroxyurea (data not shown). Survival of scrape-damaged cells was increased by calcium, independent of proteasome or caspase inhibition (fig. 7A). In a separate experiment another caspase-1 inhibitor, kFGF-YVAD-CHO, also had no apparent influence on calcium-mediated survival (data not shown).
Figure 6.
Recovery after scrape-damage is not compromised in a ubiquitin ligase mutant cell line. A ubiquitin E1 ligase temperature sensitive mutant fibroblast cell line (ts20B) and H38-5 control cell line were cultured at permissive (33 °C) or restrictive (39 °C) temperatures prior to scrape-damage and survival analysis as described in the Materials and Methods section. Asterisks: P ≤ 0.02 vs paired EGTA samples (N=4).
Figure 7.
Proteasome and caspases do not appear to contribute to survival after scrape damage, or to acute repair of damaged plasma membrane. A. Capns1+/+ cells were preincubated with protease inhibitors as described in the Materials and Methods section, and then subjected to scrape damage in the presence of calcium and inhibitors. Concentrations of inhibitor used were: calpeptin (CP), 20 µM; lactacystin (lacta), 5 µM; DEVD, 2 µM; YVAD, 2 µM; ZVAD, 10µM. Asterisks: P < 0.01 vs paired EGTA samples (N=4). B. Capns1+/+ cells were treated with inhibitors, scraped in the presence of calcium, and analyzed for trypan blue (TPB) uptake one minute after scraping.
The protease inhibitors, especially the caspase inhibitors, could produce opposing effects on cell survival after membrane damage. They might inhibit membrane resealing, thus promoting cell death, but counteract caspase-dependent apoptosis occurring after membrane damage, thereby promoting survival. To directly assess the influence of the inhibitors on membrane resealing after scrape damage, we employed the trypan blue uptake assay previously utilized to show rapid resealing of Capns1+/+ but not Capns1−/− cells [10]. Consistent with the survival studies, the inhibitors did not significantly delay membrane resealing (fig. 7B). Thus, the cell survival observed upon treatment with these inhibitors appears to be the result of normal plasma membrane repair.
DISCUSSION
Previous studies have highlighted a role for the ubiquitous, typical calpains in plasma membrane repair [10]. However, these studies employed Capns1-null cells or treatment with inhibitors that cannot sufficiently discriminate between the ubiquitous, typical calpains. It was therefore not possible to determine whether endogenously expressed μ- or m-calpain was needed for repair. Capn2-ablated mouse embryos die very early in development, before embryonic fibroblasts can be obtained [36]; therefore, it has not been possible to use this knockout model to differentiate roles of m-calpain and μ-calpain in plasma membrane repair. Our gene knockdown studies (fig. 2) provide the first molecular genetic evidence that either m- or μ-calpain can support calcium-dependent plasma membrane repair. The results are consistent with previous studies showing that pre-loading Capns1-null fibroblasts with either purified m- or μ-calpain restored calcium-mediated recovery following scrape-damage [10]. This broadens the potential scope of calpain-mediated repair, because while many mammalian cells express both m- and μ-calpain, some cells express predominantly one of these two isozymes [37]. Because μ-calpain requires less calcium for activity than m-calpain, it is possible that cells expressing predominantly the former isozyme may have enhanced ability to repair membrane breaks in the presence of less damage, and hence lower localized calcium concentrations. On the other hand, there may be cell or tissue-specific requirements for one or the other ubiquitous calpain. It is worth noting that m-calpain was selectively upregulated in mechanically loaded rat skeletal muscle, while μ-calpain expression was unchanged [38]. Therefore, there appears to be a redundant role for the ubiquitous calpains in membrane repair, and probably other calpain functions as well. However, a degree of selectivity may occur at the tissue level due to upregulation of one isoform over the other.
We were very interested in the possibility that calpain-3 was involved in sarcolemma repair. The dysferlinopathies, LGMD-2B and Miyoshi myopathy, are associated with compromised skeletal muscle sarcolemma repair, owing to mutation of the dysferlin gene. Interestingly, dysferlinopathy is sometimes associated with decreased calpain-3 expression [39], and there is evidence that calpain-3 associates with dysferlin in muscle [13]. While myoblasts do not express the calpain-3 splice variants observed in mature muscle, they do express an alternative splice variant which could potentially participate in membrane repair [40]. Calpain-3 did not appear to affect membrane repair in the myoblast, myotube, and muscle fiber model systems we have employed (fig. 3–fig. 5). Nevertheless, it is an attractive candidate for initiating remodeling of the sarcomere secondary to membrane damage, and it becomes activated in human skeletal muscle in the first 24 hours after eccentric exercise [41]. Calpain-3 is capable of processing several proteins that are required for normal sarcomere structure, or sarcomere-membrane association [27, 28], and might be involved in reorganization of this structure in the first 24 hours after sarcolemma damage. This hypothesis predicts that calpain-3 substrate processing will occur after rapid membrane repair, an idea that can be readily tested in model systems defined in the present work and in previous studies. Recent evidence suggests that AHNAK, a dysferlin- and actin-binding protein, is a substrate for calpain-3 [42], lending further support for its involvement in post-injury sarcolemmal and cortical cytoskeletal remodeling. Assessment of the role of calpain-3 in recovery after membrane repair will require the use of mouse models in long-term studies.
Neither the ubiquitin-proteasome system nor caspases appeared to be required for calcium-mediated membrane repair or survival. However, long-term remodeling of cell or tissue structure after membrane damage may rely on proteasome or caspases. Past studies of human skeletal muscle recovery after moderately damaging eccentric exercise [43] are consistent with this notion. It was found that mcalpain expression was rapidly increased in the first day after exercise. The present study suggests that this may be in response to the initial damage, and in preparation for continued sarcolemma repair in case of immediate further damage. The proteasome system, and lysosomal cathepsins B and L were not upregulated until one to two weeks after damage [43], at a time when they might be involved in long-term muscle remodeling.
ACKNOWLEDGEMENTS
The technical assistance of Elaine Chalfin is gratefully acknowledged.
FUNDING
This work was supported by National Institutes of Health [Award Numbers R21AR054427 and R01AR048177] from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The content is solely the responsibility of the authors, and does not necessarily represent the official views of the National Institute of Arthritis and Musculoskeletal and Skin Diseases or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.McNeil PL, Steinhardt RA. Plasma membrane disruption: repair, prevention, adaptation. Annu Rev Cell Dev Biol. 2003;19:697–731. doi: 10.1146/annurev.cellbio.19.111301.140101. [DOI] [PubMed] [Google Scholar]
- 2.McNeil PL, Kirchhausen T. An emergency response team for membrane repair. Nat Rev Mol Cell Biol. 2005;6:499–505. doi: 10.1038/nrm1665. [DOI] [PubMed] [Google Scholar]
- 3.Han R, Bansal D, Miyake K, Muniz VP, Weiss RM, McNeil PL, Campbell KP. Dysferlin-mediated membrane repair protects the heart from stress-induced left ventricular injury. J Clin Invest. 2007;117:1805–1813. doi: 10.1172/JCI30848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McNeil PL, Khakee R. Disruptions of muscle fiber plasma membranes. Role in exercise-induced damage. Am J Pathol. 1992;140:1097–1109. [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer TA, McNeil PL, Khakee R, Finn P, Kelly RA, Pfeffer MA, Pfeffer JM. Cardiac myocyte membrane wounding in the abruptly pressure-overloaded rat heart under high wall stress. Hypertension. 1997;30:1041–1046. doi: 10.1161/01.hyp.30.5.1041. [DOI] [PubMed] [Google Scholar]
- 6.Bi GQ, Alderton JM, Steinhardt RA. Calcium-regulated exocytosis is required for cell membrane resealing. J Cell Biol. 1995;131:1747–1758. doi: 10.1083/jcb.131.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bansal D, Miyake K, Vogel SS, Groh S, Chen CC, Williamson R, McNeil PL, Campbell KP. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 2003;423:168–172. doi: 10.1038/nature01573. [DOI] [PubMed] [Google Scholar]
- 8.Wenzel K, Geier C, Qadri F, Hubner N, Schulz H, Erdmann B, Gross V, Bauer D, Dechend R, Dietz R, et al. Dysfunction of dysferlin-deficient hearts. J Mol Med. 2007;85:1203–1214. doi: 10.1007/s00109-007-0253-7. [DOI] [PubMed] [Google Scholar]
- 9.McNeil AK, Rescher U, Gerke V, McNeil PL. Requirement for annexin A1 in plasma membrane repair. J Biol Chem. 2006;281:35202–35207. doi: 10.1074/jbc.M606406200. [DOI] [PubMed] [Google Scholar]
- 10.Mellgren RL, Zhang W, Miyake K, McNeil PL. Calpain is required for the rapid, calcium-dependent repair of wounded plasma membrane. J Biol Chem. 2007;282:2567–2575. doi: 10.1074/jbc.M604560200. [DOI] [PubMed] [Google Scholar]
- 11.Mellgren RL, Huang X. Fetuin A stabilizes m-calpain and facilitates plasma membrane repair. J Biol Chem. 2007;282:35868–35877. doi: 10.1074/jbc.M706929200. [DOI] [PubMed] [Google Scholar]
- 12.Richard I, Broux O, Allamand V, Fougerousse F, Chiannilkulchai N, Bourg N, Brenguier L, Devaud C, Pasturaud P, Roudaut C, et al. Mutations in the proteolytic enzyme calpain 3 cause limb-girdle muscular dystrophy type 2A. Cell. 1995;81:27–40. doi: 10.1016/0092-8674(95)90368-2. [DOI] [PubMed] [Google Scholar]
- 13.Huang Y, Verheesen P, Roussis A, Frankhuizen W, Ginjaar I, Haldane F, Laval S, Anderson LV, Verrips T, Frants RR, et al. Protein studies in dysferlinopathy patients using llama-derived antibody fragments selected by phage display. Eur J Hum Genet. 2005;13:721–730. doi: 10.1038/sj.ejhg.5201414. [DOI] [PubMed] [Google Scholar]
- 14.Laure L, Suel L, Roudaut C, Bourg N, Ouali A, Bartoli M, Richard I, Danièle N. Cardiac ankyrin repeat protein is a marker of skeletal muscle pathological remodelling. FEBS J. 2009;276:669–684. doi: 10.1111/j.1742-4658.2008.06814.x. [DOI] [PubMed] [Google Scholar]
- 15.Raser KJ, Posner A, Wang KK. Casein zymography: a method to study mu-calpain, m-calpain, and their inhibitory agents. Arch Biochem Biophys. 1995;319:211–216. doi: 10.1006/abbi.1995.1284. [DOI] [PubMed] [Google Scholar]
- 16.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 17.Mellgren RL. Proteolysis of nuclear proteins by mu-calpain and m-calpain. J Biol Chem. 1991;266:13920–13924. [PubMed] [Google Scholar]
- 18.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Meth. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 19.Miyake K, McNeil PL, Suzuki K, Tsunoda R, Sugai N. An actin barrier to resealing. J Cell Sci. 2001;114:3487–3494. doi: 10.1242/jcs.114.19.3487. [DOI] [PubMed] [Google Scholar]
- 20.Kramerova I, Kudryashova E, Tidball JG, Spencer MJ. Null mutation of calpain 3 (p94) in mice causes abnormal sarcomere formation in vivo and in vitro. Hum Mol Genet. 2004;13:1373–1388. doi: 10.1093/hmg/ddh153. [DOI] [PubMed] [Google Scholar]
- 21.Dourdin N, Bhatt AK, Dutt P, Greer PA, Arthur JS, Elce JS, Huttenlocher A. Reduced cell migration and disruption of the actin cytoskeleton in calpain-deficient embryonic fibroblasts. J Biol Chem. 2001;276:48382–48388. doi: 10.1074/jbc.M108893200. [DOI] [PubMed] [Google Scholar]
- 22.Franco S, Perrin B, Huttenlocher A. Isoform specific function of calpain 2 in regulating membrane protrusion. Exp Cell Res. 2004;299:179–187. doi: 10.1016/j.yexcr.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 23.Sorimachi H, Kinbara K, Kimura S, Takahashi M, Ishiura S, Sasagawa N, Sorimachi N, Shimada H, Tagawa K, Maruyama K, et al. Muscle-specific calpain, p94, responsible for limb girdle muscular dystrophy type 2A, associates with connectin through IS2, a p94- specific sequence. J Biol Chem. 1995;270:31158–31162. doi: 10.1074/jbc.270.52.31158. [DOI] [PubMed] [Google Scholar]
- 24.Huebsch KA, Kudryashova E, Wooley CM, Sher RB, Seburn KL, Spencer MJ, Cox GA. Mdm muscular dystrophy: interactions with calpain 3 and a novel functional role for titin's N2A domain. Hum Mol Genet. 2005;14:2801–2811. doi: 10.1093/hmg/ddi313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glover L, Brown RH., Jr Dysferlin in membrane trafficking and patch repair. Traffic. 2007;8:785–794. doi: 10.1111/j.1600-0854.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 26.Murphy RM, Lamb GD. Endogenous calpain-3 activation is primarily governed by small increases in resting cytoplasmic [Ca2+] and is not dependent of stretch. J Biol Chem. 2009;284:7811–7819. doi: 10.1074/jbc.M808655200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taveau M, Bourg N, Sillon G, Roudaut C, Bartoli M, Richard I. Calpain 3 is activated through autolysis within the active site and lyses sarcomeric and sarcolemmal components. Mol Cell Biol. 2003;23:9127–9135. doi: 10.1128/MCB.23.24.9127-9135.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guyon JR, Kudryashova E, Potts A, Dalkilic I, Brosius MA, Thompson TG, Beckmann JS, Kunkel LM, Spencer MJ. Calpain 3 cleaves filamin C and regulates its ability to interact with gamma- and delta-sarcoglycans. Muscle Nerve. 2003;28:472–483. doi: 10.1002/mus.10465. [DOI] [PubMed] [Google Scholar]
- 29.Kramerova I, Kudryashova E, Wu B, Spencer MJ. Regulation of the M-cadherin-beta-catenin complex by calpain 3 during terminal stages of myogenic differentiation. Mol Cell Biol. 2006;26:8437–8447. doi: 10.1128/MCB.01296-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiarle R, Budel LM, Skolnik J, Frizzera G, Chilosi M, Corato A, Pizzolo G, Magidson J, Montagnoli A, Pagano M, et al. Increased proteasome degradation of cyclin-dependent kinase inhibitor p27 is associated with a decreased overall survival in mantle cell lymphoma. Blood. 2000;95:619–626. [PubMed] [Google Scholar]
- 31.McCracken AA, Brodsky JL. Recognition and delivery of ERAD substrates to the proteasome and alternative paths for cell survival. Curr Top Microbiol Immunol. 2005;300:17–40. doi: 10.1007/3-540-28007-3_2. [DOI] [PubMed] [Google Scholar]
- 32.Seo H, Sonntag KC, Kim W, Cattaneo E, Isacson O. Proteasome activator enhances survival of huntington's disease neuronal model cells. PLoS ONE. 2007;2:e238. doi: 10.1371/journal.pone.0000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keller M, Ruegg A, Werner S, Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell. 2008;132:818–831. doi: 10.1016/j.cell.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 34.Gurcel L, Abrami L, Girardin S, Tschopp J, van der Goot FG. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell. 2006;126:1135–1145. doi: 10.1016/j.cell.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 35.Chowdary DR, Dermody JJ, Jha KK, Ozer HL. Accumulation of p53 in a mutant cell line defective in the ubiquitin pathway. Mol Cell Biol. 1994;14:1997–2003. doi: 10.1128/mcb.14.3.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dutt P, Croall DE, Arthur JS, Veyra TD, Williams K, Elce JS, Greer PA. m-Calpain is required for preimplantation embryonic development in mice. BMC Dev Biol. 2006;6:3. doi: 10.1186/1471-213X-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 38.Spencer MJ, Lu B, Tidball JG. Calpain II expression is increased by changes in mechanical loading of muscle in vivo. J Cell Biochem. 1997;64:55–66. doi: 10.1002/(sici)1097-4644(199701)64:1<55::aid-jcb9>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 39.Anderson LV, Harrison RM, Pogue R, Vafiadaki E, Pollitt C, Davison K, Moss JA, Keers S, Pyle A, Shaw PJ, et al. Secondary reduction in calpain 3 expression in patients with limb girdle muscular dystrophy type 2B and Miyoshi myopathy (primary dysferlinopathies) Neuromuscul Disord. 2000;10:553–559. doi: 10.1016/s0960-8966(00)00143-7. [DOI] [PubMed] [Google Scholar]
- 40.Ojima K, Ono Y, Doi N, Yoshioka K, Kawabata Y, Labeit S, Sorimachi H. Myogenic stage, sarcomere length, and protease activity modulate localization of muscle-specific calpain. J Biol Chem. 2007;282:14493–14504. doi: 10.1074/jbc.M610806200. [DOI] [PubMed] [Google Scholar]
- 41.Murphy RM, Goodman CA, McKenna MJ, Bennie J, Leikis M, Lamb GD. Calpain-3 is autolyzed and hence activated in human skeletal muscle 24 h following a single bout of eccentric exercise. J Appl Physiol. 2007;103:926–931. doi: 10.1152/japplphysiol.01422.2006. [DOI] [PubMed] [Google Scholar]
- 42.Huang Y, de Morree A, van Remoortere A, Bushby K, Frants RR, Dunnen JT, van der Maarel SM. Calpain 3 is a modulator of the dysferlin protein complex in skeletal muscle. Hum Mol Genet. 2008;17:1855–1866. doi: 10.1093/hmg/ddn081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feasson L, Stockholm D, Freyssenet D, Richard I, Duguez S, Beckmann JS, Denis C. Molecular adaptations of neuromuscular disease-associated proteins in response to eccentric exercise in human skeletal muscle. J Physiol. 2002;543:297–306. doi: 10.1113/jphysiol.2002.018689. [DOI] [PMC free article] [PubMed] [Google Scholar]