Abstract
Growth differentiation factor-9 (GDF-9), an oocyte-secreted member of the transforming growth factor β superfamily, progesterone receptor, cyclooxygenase 2 (Cox2; Ptgs2), and the EP2 prostaglandin E2 (PGE2) receptor (EP2; Ptgerep2) are required for fertility in female but not male mice. To define the interrelationship of these factors, we used a preovulatory granulosa cell culture system in which we added recombinant GDF-9, prostaglandins, prostaglandin receptor agonists, or cyclooxygenase inhibitors. GDF-9 stimulated Cox2 mRNA within 2 h, and PGE2 within 6 h; however, progesterone was not increased until 12 h after addition of GDF-9. This suggested that Cox2 is a direct downstream target of GDF-9 but that progesterone synthesis required an intermediate. To determine whether prostaglandin synthesis was required for progesterone production, we analyzed the effects of PGE2 and cyclooxygenase inhibitors on this process. PGE2 can stimulate progesterone synthesis by itself, although less effectively than GDF-9 (3-fold vs. 6-fold increase over 24 h, respectively). Furthermore, indomethacin or NS-398, inhibitors of Cox2, block basal and GDF-9-stimulated progesterone synthesis. However, addition of PGE2 to cultures containing both GDF-9 and NS-398 overrides the NS-398 block in progesterone synthesis. To further define the PGE2-dependent pathway, we show that butaprost, a specific EP2 agonist, stimulates progesterone synthesis and overrides the NS-398 block. In addition, GDF-9 stimulates EP2 mRNA synthesis by a prostaglandin- and progesterone-independent pathway. Thus, GDF-9 induces an EP2 signal transduction pathway which appears to be required for progesterone synthesis in cumulus granulosa cells. These studies further demonstrate the importance of oocyte–somatic cell interactions in female reproduction.
Progesterone and prostaglandins have been shown to play key roles in ovarian physiology, the periovulatory period, and female reproduction. Inhibitors of progesterone synthesis such as epostane block ovulation in a dose-dependent manner (1), and absence of the progesterone receptor in mice prevents release of the oocyte, leading to complete infertility (2, 3). In addition, it has been shown that cumulus cell–oocyte complexes (COCs) synthesize progesterone (4) and that aminoglutethimide, which blocks the formation of pregnenolone, decreases the number of normal oocytes after in vitro maturation (5). Likewise, high doses of indomethacin [an inhibitor of both cyclooxygenase 1 and 2 (Cox1 and Cox2)] or NS-398 (a “specific” Cox2 inhibitor) can block ovulation in rats (1, 6), and absence of Cox2 in mice leads to defects in ovulation, fertilization, and implantation (7). However, studies in rats suggest that the effect on ovulation by indomethacin may be independent of prostaglandin E2 (PGE2); low doses of indomethacin can abolish ovarian PGE2 synthesis without effecting ovulation, and high doses, which inhibit ovulation, also inhibit the synthesis of 15-hydroxyeicosatetraenoic acid methyl ester (15-HETE). In addition, the drug BW755C specifically inhibits 15-HETE production and ovulation in rats (1). These data suggest that 15-HETE and/or other downstream derivatives may be more critical for ovulation than the prostaglandins.
Within the preovulatory follicle, there are two distinct populations of cells: the mural granulosa cells (which line the follicle wall and are closest to the thecal layer) and the cumulus granulosa cells (which are closest to the oocyte). After the luteinizing hormone (LH) surge and before ovulation, the cumulus cells undergo a process called expansion which is due to the extracellular deposition of a hyaluronic acid-rich, gelatinous matrix and “stabilization” of this matrix (8–10). The expansion process is believed to be important for efficient exit of the COC from the ovary, capture by the fimbria of the oviduct, and fertilization (11).
Several studies have also suggested a role for prostaglandins, and in particular PGE2, in maintenance and function of the COC. PGE2 was one of the earliest substances shown to induce cumulus expansion in vitro (12). Additionally, COCs obtained from rats and mice after superovulation synthesize PGE1, PGE2, and PGF2α (4, 13). Treatment of these COCs with indomethacin greatly reduces the in vitro fertilization rate of oocytes, and this effect is reversed if PGE1 and PGE2 are added to the media in the presence of the indomethacin (13).
Four cell surface PGE2 receptor subtypes, EP1–EP4, have been cloned and are expressed in many tissues (reviewed in ref. 14). Recent gene knockout studies have illustrated the essential signaling pathways for PGE2 in the ovary. EP3 receptor knockout mice have mild defects in urinary concentrating ability and impaired febrile responses, but are reproductively normal (15, 16). In addition, although 95% of the EP4 receptor knockout mice died perinatally because of patent ductus arteriosus, the mice that survived this period were fertile similar to the EP3 knockout mice (17, 18). Consistent with the above-mentioned PGE2 data and in contrast to the EP3 and EP4 knockout mice, EP2 receptor knockout mice demonstrate reduced female fertility because of a decreased rate of in vivo fertilization (19–21). This decreased rate of fertilization is due in part to defects in cumulus expansion (21). These data suggest that progesterone and prostaglandins may be critical for maintaining an optimal microenvironment for oocyte survival and fertilization. The interrelationships of progesterone and prostaglandins in these processes are unknown.
Growth differentiation factor 9 (GDF-9) is a glycosylated member of the transforming growth factor β superfamily (22). In mice, GDF-9 is synthesized in oocytes from the primary follicle stage through ovulation (10, 23, 24). Female mice lacking GDF-9 are infertile because of a block at the primary follicle stage, resulting in defects in granulosa cell growth and differentiation, thecal layer formation, and oocyte meiotic competence (25–27). These studies confirmed that the oocyte is not an inert cargo during folliculogenesis but is active in regulating somatic cell function. Because GDF-9 mRNA and protein are synthesized by the oocytes of preovulatory follicles and COCs, we speculated that GDF-9 also played important roles at these stages. Previous studies have also demonstrated that the oocyte is required for cumulus expansion (10, 28–30). We demonstrated that recombinant GDF-9 can induce expansion of complexes in which the oocyte was microsurgically removed (24). Furthermore, recombinant GDF-9 regulates cumulus granulosa cell gene expression and suppresses the expression of genes normally expressed in mural granulosa cells (24). GDF-9 stimulates the synthesis of hyaluronan synthase 2 (Has2) and Cox2 and inhibits the synthesis of urokinase plasminogen activator (uPA) and LH receptor mRNA. In addition, GDF-9 stimulates the synthesis of progesterone in the absence of follicle-stimulating hormone (FSH) over a 24-h period. Thus, GDF-9 is a multifunctional oocyte-secreted protein involved in the regulation of several key ovarian somatic cell functions.
Because of the above-mentioned important roles of GDF-9, progesterone, and prostaglandins in the function and maintenance of the COC, we used an in vitro granulosa culture system to study the interrelationships of these three factors. We demonstrate herein that basal and GDF-9-stimulated progesterone synthesis in preovulatory granulosa cells appears to require a Cox2/PGE2/EP2 dependent pathway and that GDF-9 stimulates both the synthesis of prostaglandins and the expression of the EP2 receptor.
Materials and Methods
Chemicals, Assays, and Generation of Probes.
PGE2 in the culture media was assayed in duplicate at two different dilutions by using an ELISA from Cayman Chemicals (Ann Arbor, MI). Progesterone radioimmunoassays were performed as previously described (24). Indomethacin, PGE2, and NS-398 were obtained from Sigma. Butaprost was a generous gift of Harold Kluender (Bayer Corporation, West Haven, CT). NS-398 (50 mM stock) and butaprost (10 mM stock) were resuspended in DMSO. Indomethacin (1 mg/ml stock) and PGE2 (1 mg/ml stock) were resuspended in 100% ethanol. The mouse EP2 oligonucleotide primers [5′-CAACTGTCCACAAAGGTCAGTCTG-3′ (sense) and 5′-AGGACTGCATACCTTCAGCTGTAC-5′ (antisense)], which span the EP2 intron, were designed based on the published EP2 cDNA and gene sequences (31, 32) and produce a 506-bp cDNA product. Other primer sets are described elsewhere (24).
Production of Recombinant Mouse GDF-9.
The recombinant GDF-9 protein was produced as previously described (24). Briefly, CHO cells carrying the full-length mouse GDF-9 cDNA in the pHTop expression vector (a gift from Genetics Institute, Cambridge, MA) were incubated for 48 h in Opti-MEM reduced serum collection media containing 1 mg/ml heparin (Sigma). The media were harvested, and GDF-9 protein levels were quantitated by SDS/PAGE with subsequent immunoblotting using purified bacterially-derived recombinant GDF-9 as a standard.
Isolation and Culture of Granulosa Cells.
Female CD-1 (ICR) mice (produced at Baylor College of Medicine) 19–21 days old were injected with 7.5 units of Gestyl (Diosynth, Oss, Holland), and mural granulosa cells were harvested from multiple ovaries 44–48 h later as described (24). All granulosa cell assays were performed as described (24) except that FSH was absent in all experiments. After varying periods of culture, nonadherent cells were pelleted from the media, and the media were stored at −20°C. Total RNA was isolated from the granulosa cells by using RNA Stat-60 (Leedo Medical Laboratories, Houston) following the manufacturer's protocol.
Semiquantitative Reverse Transcription (RT)-PCR Analysis.
Oligo (dT)- primed cDNA was synthesized using Superscript reverse transcriptase (GIBCO/BRL) following the manufacturer's protocol. One microliter of each RT reaction (1/20 of total) was used in each 25-μl PCR primed with gene-specific oligonucleotides along with [α-32P]dCTP, and the products were separated by electrophoresis on a 4% polyacrylamide gel. The gels were dried and exposed to autoradiography, or radioactive bands were quantitated by exposure to a Storage Phosphor Screen, analyzed on a Storm 860 scanner with ImageQuant Version 4.2a software (Molecular Dynamics). Statistical significance was determined by Student's t test using Microsoft Excel 98.
Northern Blot Analysis.
Total RNA was isolated from granulosa cells and quantitated by fluorometry using Ribogreen RNA quantitation reagents (Molecular Probes) on a VersaFluor fluorometer (Bio-Rad) using a 485 to 495-nm excitation filter and 515 to 525-nm emission filter. Fifteen micrograms of total RNA of each sample was electrophoresed on a 1.2% agarose/7.6% formaldehyde gel and transferred to Hybond N nylon membrane (Amersham). Probes were labeled with [α-32P]dATP by using the Strip-EZ probe synthesis kit (Ambion, Austin, TX). The membrane was hybridized, washed, and subjected to autoradiography as described (33). Probes were removed from the membrane by using the Strip-EZ removal reagents (Ambion) following the manufacturer's protocol. Signals for each probe were quantitated on a Molecular Dynamics phosphorimager.
Results
GDF-9 Induces Progesterone Synthesis.
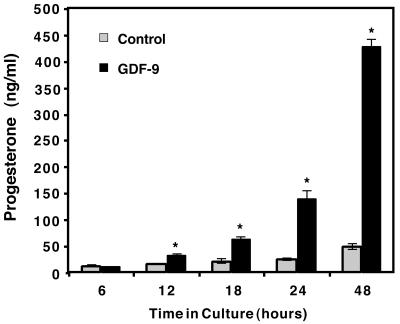
Mural granulosa cells of preovulatory follicles from pregnant mare serum gonadotropin (PMSG)-stimulated mice in a variety of assays behave similarly to cumulus granulosa cells when exposed to oocytes (8, 9, 30) or to GDF-9 (24). Because mural granulosa cells are more abundant and easily separated from oocytes to make an oocyte-free assay system, mural granulosa cells were used in this and all of the following experiments. We have previously demonstrated that granulosa cells produce progesterone when incubated with GDF-9 for 24 h (24). To study the regulation of progesterone synthesis further, we incubated mural granulosa cells in the presence or absence of GDF-9 for 6–48 h (Fig. 1). At and before 6 h, no significant differences between control and GDF-9-treated samples were noted (Fig. 1 and data not shown). By 12 h, a significant two-fold increase in progesterone synthesis became apparent. After 12 h, very modest increases in total progesterone accumulation in the media of control treated granulosa cells are seen, whereas significant increase in GDF-9-stimulated progesterone accumulation continues up through 48 h. By 24 h, there is a 6-fold increase and by 48 h there is an 8.8-fold increase in progesterone production stimulated by GDF-9. Based on these studies, progesterone induction is a late event of GDF-9 treatment compared to the rapid effect seen for Has2, which is induced by 1 h and demonstrates a maximum induction by 5–9 h (24). This may reflect an important role of postovulatory progesterone synthesis while the COC is negotiating the oviduct.
Figure 1.
Time course of progesterone synthesis. Granulosa cells were incubated in culture media lacking FSH in the presence or absence of 100 ng/ml of recombinant mouse GDF-9. Medium was collected at each timepoint and assayed by RIA for progesterone released into the medium. Each timepoint represents the mean ± SEM of three independent samples isolated on the same day. Similar results were obtained in several independent experiments. The asterisk denotes significance (P < 0.05) of control versus GDF-9-treated samples at that timepoint.
GDF-9 Stimulates Cox2 Gene Expression and Prostaglandin Synthesis.
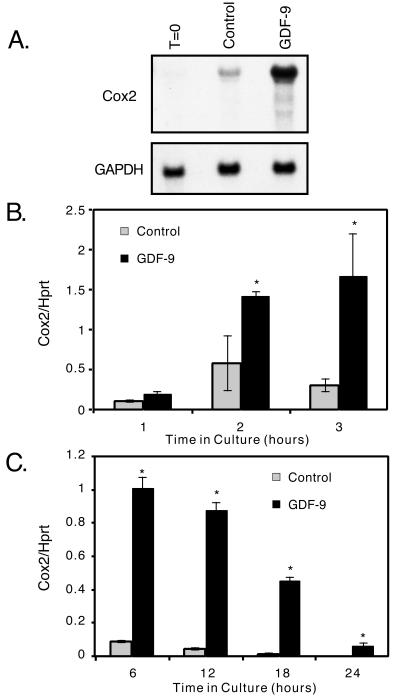
We previously showed that GDF-9 induced Cox2 gene expression after 24 h (24). We were interested in determining whether this effect was secondary to GDF-9-stimulated differentiation of the granulosa cells (and thus a late effect) or whether Cox2 gene expression was regulated more directly by GDF-9. Time course analysis of Cox2 gene expression in granulosa cells cultured in the presence or absence of GDF-9 demonstrated that GDF-9 induced a significant 2.5-fold increase in Cox2 mRNA expression after 2 h, a 5.5-fold increase by 3 h (Fig. 2B), a peak expression between 4 and 8 h (Fig. 2 B and C, and data not shown), and a subsequent decline thereafter (Fig. 2C). However, in the GDF-9-stimulated cells, Cox2 mRNA remains easily detectable at 24 h and actually represents a 49-fold increase over control culture expression levels (Fig. 2C and ref. 24). Northern blot analysis of Cox2 mRNA after a 5-h incubation of the granulosa cells with GDF-9 confirmed the semiquantitative PCR data demonstrating a 13.6-fold increase in Cox2 mRNA (Fig. 2A).
Figure 2.
Regulation of Cox2 mRNA in granulosa cells. (A) Granulosa cells were isolated and incubated for 0 h or 5 h in the presence or absence of 100 ng/ml GDF-9, and the RNA was isolated and analyzed by Northern blotting using a Cox2 cDNA probe or a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe as a quantitative control. (B and C) Time course of Cox2 mRNA synthesis in granulosa cells as measured by using semiquantitative RT-PCR. Granulosa cells were cultured in the presence or absence of recombinant mouse GDF-9 (100 ng/ml), and RT-PCR analyses were performed using Cox2 and hypoxanthine phosphoribosyltransferase (Hprt) (control) primer sets. Each point on the graph represents the mean ± SEM from triplicate samples. The asterisk denotes significance (P < 0.05) of control versus GDF-9-treated samples at that timepoint.
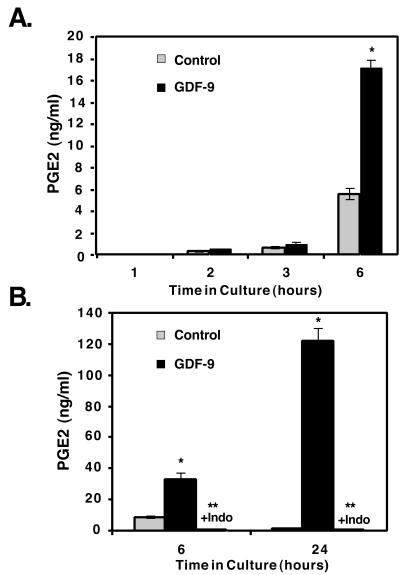
Cox2 catalyzes the rate-limiting first step in prostaglandin production, after which specific prostaglandins, such as PGE2, are subsequently produced. In particular, PGE2 has been shown to be important in ovulation, cumulus expansion, and implantation (7, 12, 34). Therefore, we investigated whether the increase in Cox2 mRNA was reflected in an increase in PGE2 production. PGE2 levels in media from granulosa cells cultured for 1–6 h in the presence or absence of GDF-9 were assayed by a PGE2-specific ELISA (Fig. 3). PGE2 levels are comparable in GDF-9-treated and control samples at 1, 2, and 3 h (Fig. 3A). However, compared with controls, GDF-9-treated cells have produced 3 to 4-fold more PGE2 by 6 h (Fig. 3 A and B), and by 24 h, PGE2 is increased 103-fold (Fig. 3B). This dramatic difference in the relative production by 24 h is secondary to a 3.7-fold increase in the GDF-9 treated cultures between 6 and 24 h and to a drop in PGE2 levels in control-treated cultures presumably from decreased production and increased degradation (Fig. 3B). As expected, treatment of the granulosa cells concurrently with indomethacin, an inhibitor of Cox 1 and Cox 2, completely blocks basal and GDF-9-stimulated PGE2 production, confirming that GDF-9-induced PGE2 production requires de novo synthesis starting from arachadonic acid.
Figure 3.
GDF-9 stimulates PGE2 synthesis in granulosa cells. (A and B) Time course of granulosa cell PGE2 production into the medium in the absence or presence of GDF-9 (100 ng/ml) (A) or in the presence of GDF-9 (100 ng/ml) or presence of GDF-9 (100 ng/ml) and indomethacin (“+Indo”; 1 μg/ml) (B). Single asterisk denotes significance (P < 0.05) of control versus GDF-9-treated samples at that timepoint; double asterisk denotes significance (P < 0.05) of GDF-9- and indomethacin-treated samples versus GDF-9-treated samples at that timepoint.
GDF-9-Stimulated Progesterone Synthesis Requires a Cox2/PGE2/EP2-Dependent Pathway.
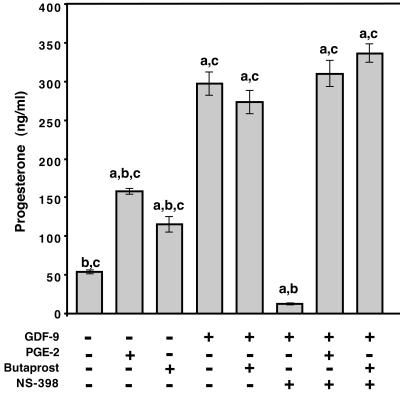
Upon observing the timecourse of GDF-9 induction of prostaglandins and progesterone, we hypothesized that prostaglandins may induce progesterone synthesis. To investigate this hypothesis, progesterone synthesis from granulosa cells cultured in the presence of cyclooxygenase inhibitors and GDF-9 was assayed (Fig. 4). In the presence of either the nonspecific cyclooxygenase inhibitor indomethacin (data not shown) or the Cox2-specific inhibitor, NS-398, progesterone levels dropped to almost undetectable levels (≤13 ng/ml) even in the presence of GDF-9. This reduction in the presence of these cyclooxygenase inhibitors is a 22-fold decrease compared with GDF-9-treated cultures (297 ng/ml) and a 4.3-fold decrease compared with control cultures (54 ng/ml). Cultures containing both NS-398 and PGE2 [100 ng/ml (data not shown) or 500 ng/ml (Fig. 4)] synthesized progesterone at levels comparable to cultures without the cyclooxygenase inhibitor. This demonstrated that the block in progesterone synthesis by the cyclooxygenase inhibitors was due to the lack of prostaglandin production and not due to cell toxicity or other potential effects of the drugs. Interestingly, granulosa cells cultured with NS-398 and synthetic PGE2 [100 ng/ml (data not shown) or 500 ng/ml (Fig. 4)] in the presence of recombinant GDF-9, had 2.7-fold greater progesterone production than cells cultured with PGE2 alone [100 ng/ml (data not shown) or 500 ng/ml (Fig. 4)] even though they contained similar or roughly equivalent concentrations of PGE2.
Figure 4.
GDF-9 stimulated progesterone production depends on prostaglandin synthesis and requires an EP2 receptor pathway. Progesterone production by granulosa cells treated with GDF-9 (100 ng/ml), PGE2 (500 ng/ml), butaprost (10 μM), and/or NS-298 (1 μM) after 24 h of culture. PGE2 and butaprost stimulate progesterone production to similar levels, and GDF-9 is approximately twice as effective at the concentrations used. Butaprost plus GDF-9 is equally effective as GDF-9 alone, suggesting that these two ligands are in the same pathway. NS-398 reduces the progesterone levels to less than baseline levels, and PGE2 or butaprost alone overrides the NS-398 block (data not shown). The production of progesterone by GDF-9 is blocked equally by NS-398 or indomethacin (data not shown) and restored by PGE2 or butaprost. Values are mean ± SEM for triplicate samples. The experiment was repeated on several occasions with different ligand concentrations resulting in similar outcomes. a, P < 0.05 compared with control (no ligand); b, P < 0.05 compared with GDF-9 treatment; c, P < 0.05 compared with GDF-9 plus NS-398 treatment.
Although four PGE2 receptors have been identified, only the EP2 receptor plays a role in the periovulatory period. We therefore hypothesized that the effects of PGE2 on progesterone synthesis were through EP2. To test this hypothesis, we used butaprost, a PGE2 receptor-specific agonist. Similar to PGE2, butaprost can also stimulate a 2-fold increase in progesterone synthesis compared to control (Fig. 4) and can overcome the NS-398 block in the presence (Fig. 4) or absence (data not shown) of GDF-9. Furthermore, butaprost in the presence of GDF-9 and NS-398 (Fig. 4) stimulates a 2.9-fold increase in progesterone synthesis compared with butaprost and NS-398 (data not shown).
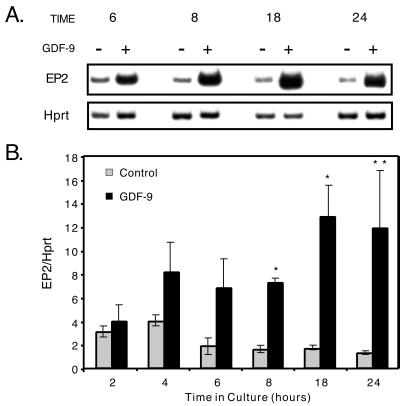
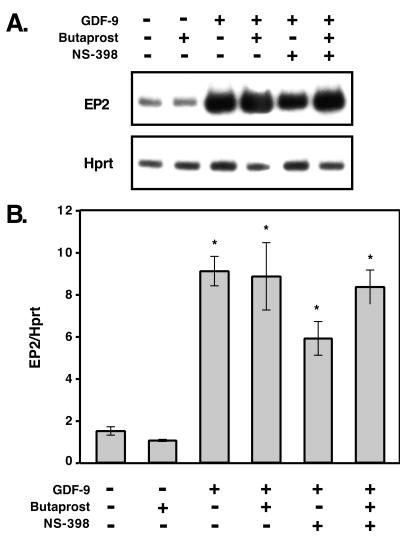
Because PGE2 and the EP2 receptor agonist, butaprost, stimulate progesterone synthesis to similar levels when added alone and are equivalent in the presence of GDF-9 and NS-398 (Fig. 4), PGE2 must be the only prostaglandin required for this process, and EP2 the sole PGE2 receptor necessary for PGE2 stimulation of progesterone synthesis. To address the enhanced effects of GDF-9 on progesterone synthesis independent of PGE2 production, we performed semiquantitative RT-PCR to study the regulation of EP2 mRNA by GDF-9 (Fig. 5). By 4 h of incubation, GDF-9 stimulated a 2-fold increase in EP2 mRNA, and between 6 and 24 h of culture, GDF-9 stimulated a 3.5 to 6.5-fold enhancement in the EP2 mRNA levels. This suggests that GDF-9 also enhances progesterone synthesis by increasing the levels of the EP2 receptor through which the PGE2 can act. To determine whether prostaglandins or progesterone were involved in the induction of the EP2 mRNA, we compared the effects of GDF-9, butaprost, and/or NS-398 on the regulation of EP2 mRNA in granulosa cells after 24 h of culture (Fig. 6). Butaprost did not affect the levels of EP2 mRNA and also did not appear to enhance the effects of GDF-9. The levels of EP2 mRNA continued to be statistically elevated when the granulosa cells were incubated with both GDF-9 and NS-398, which blocked prostaglandin synthesis through Cox2 and also inhibited progesterone production. The data from Fig. 6 demonstrate that stimulation of EP2 is the second pathway (induced by GDF-9 and independent of prostaglandin and progesterone synthesis) that may be important for high level progesterone synthesis in the periovulatory period.
Figure 5.
GDF-9 stimulates EP2 receptor mRNA synthesis. Semiquantitative RT-PCR analysis was used to analyze the expression of EP2 and Hprt (control) in granulosa cells treated with (+) or without (−) GDF-9. (A) Representative samples from 6-, 8-, 18-, and 24-h timepoints are shown. (B) The data are summarized as mean ± SEM for triplicate samples except at the 6-h timepoint, which is mean ± range for duplicate samples for the GDF-9-treated group. Similar results were seen when the experiment was repeated on several independent occasions. Single asterisk denotes significance (P < 0.05) of control versus GDF-9-treated samples at that timepoint; double asterisk denotes significance of P < 0.10.
Figure 6.
GDF-9 induction of EP2 is independent of prostaglandins and progesterone. Semiquantitative RT-PCR analysis was used to analyze the expression of EP2 and Hprt in granulosa cells treated with GDF-9, butaprost, or NS-398 for 24 h. (A) Representative samples incubated under each condition are shown. (B) Mean ± SEM for triplicate samples. Single asterisk denotes significance (P < 0.05).
Discussion
Progesterone signaling is necessary for ovulation (2, 3) and has been implicated as a local regulator in the oviduct during the periovulatory period (4, 5) similar to cumulus cell-produced PGE2. Soon after ovulation, the corpus luteum becomes the major progesterone synthetic factory required for implantation and maintenance of early pregnancy. Studies of preantral follicles have shown that the presence of the oocyte inhibits progesterone synthesis and premature luteinization of granulosa cells (35). However, during the periovulatory period before implantation, oocyte-stimulated local synthesis of progesterone by the cumulus cells may be essential when the COC is free floating within the oviduct lumen and not connected to the systemic blood supply (4, 5). Likewise, as suggested by Koller and colleagues (20), “PGE2 may act through the EP2 receptor to define a microenvironment in the oviduct conducive to fertilization.” In this way, PGE2 could act by (i) binding to EP2 receptors on the cumulus granulosa cells, which in turn support the oocyte, the oviduct, and/or the spermatozoa or (ii) signaling through EP2 receptors in the oviduct, which supports the COC and/or the spermatozoa. Recently, in situ hybridization analysis has shown that the EP2 receptor is most highly expressed in the cumulus cells of preovulatory follicles and that there are defects in the expansion of the cumulus cells in EP2 receptor knockout mice (21). These findings, along with our data, suggest that PGE2 acts in vivo via the first mechanism. This does not rule out additional direct functions of PGE2 in the preovulatory follicle or in the oviduct, such as a direct mediator of matrix reorganization.
Studies by Eppig (12) had initially shown that PGE2 could stimulate, albeit indirectly, the process of cumulus expansion. Other studies over the last two decades had demonstrated that the oocyte was essential to the cumulus-expansion process and that an oocyte-secreted factor was the direct regulator of cumulus expansion (8–10). These studies indicated that an oocyte-secreted factor stimulated hyaluronic acid synthesis and cumulus expansion and inhibited urokinase plasminogen activator (uPA) and LH receptor synthesis (10). The first genetic evidence that the oocyte played an essential role in the regulation of somatic cell function came with the generation of knockout mice lacking the oocyte-secreted factor GDF-9 (25). In the absence of GDF-9, follicles are blocked at the primary follicle stage, lack a thecal layer, and show abnormally increased oocyte growth and defects in oocyte meiotic competence (25–27). Furthermore, recombinant GDF-9 also was shown to have a direct effect on granulosa cells of the preovulatory follicle (24); GDF-9 could replace the oocyte by stimulating cumulus expansion and synthesis of hyaluronan synthase 2, and inhibiting expression of uPA and LHR.
Taken together, the Cox2, EP2, and progesterone receptor knockout studies (2, 3, 7, 19–21), the data from Eppig, Salustri, and colleagues (8–10, 28–30), and the present and published studies from our group (27) indicate an important interrelationship of GDF-9, Cox2, PGE2, and EP2 in cumulus expansion and production of progesterone synthesis. Clearly, GDF-9 produced by the oocyte is a key molecular trigger in this process.
At the time of the LH surge, GDF-9 may induce a series of molecular and physiological changes in the cumulus cells and oocytes; these orchestrated effects include: (i) a rapid induction of Has2 and Cox2 mRNA, (ii) increased synthesis of hyaluronic acid, PGE2 (intermediate events), and one of the PGE2 receptors (EP2), and (iii) increased StAR mRNA. These combined effects appear to be necessary for efficient cumulus expansion and an increase in progesterone synthesis (late effects seen after the rise in PGE2) (see model, Fig. 7). In addition to the inhibitor studies, the relative timecourse for the appearance of PGE2, which precedes the elevation in progesterone and is statistically elevated by 6 h, is consistent with a role of PGE2 in progesterone synthesis in these cells; this does not rule out independent mechanisms of progesterone synthesis regulation in preantral follicles or corpora lutea. In fact, whereas previous studies have demonstrated that gonadotropins can stimulate both progesterone (36) and Cox2 expression (37) and it is possible that these pathways interact indirectly, in the studies presented in this manuscript, no FSH or LH was added to the culture media, demonstrating that GDF-9 alone is sufficient to exert these effects. Thus, we believe that in the surrounding somatic (cumulus) cells, oocyte-derived GDF-9 induces both the enzymatic machinery for the production of prostaglandins and a receptor for autocrine recognition of a specific prostaglandin. Basal cumulus cell progesterone synthesis in our model also requires production of Cox2, which normally is highly expressed in cumulus cells only after the LH surge and is also induced directly by GDF-9 (24). This also suggests that granulosa cell progesterone synthesis in the preovulatory mouse ovary requires products downstream of Cox2. PGE2 binding to EP2 activates an adenylate cyclase pathway, and it is also known that cAMP analogues and phosphodiesterase inhibitors can stimulate cumulus expansion in vitro (38). Based on our data and that of others, we hypothesize that these cAMP analogues act downstream of the EP2 receptor. Thus, in addition to a direct effect of GDF-9 on the synthesis of hyaluronan synthase 2, GDF-9 appears to stimulate a Cox2/PGE2/EP2 pathway which in vivo is required for optimal cumulus expansion. Furthermore, induction of this same Cox2/PGE2/EP2 pathway by GDF-9 is also apparently important for progesterone synthesis by the cumulus granulosa cells. Thus, at least two major functions of PGE2 signaling through EP2 in the periovulatory follicle appear to be enhancement of cumulus expansion and production of progesterone, both of which are necessary for efficient delivery of oocytes into the oviduct, maintenance of oocyte function, and fertilization. Further studies are needed to determine which genes in the progesterone synthetic pathway are directly induced by PGE2, whether StAR mRNA is stimulated indirectly by GDF-9 via induction of the EP2 pathway, and which genes or gene products in addition to Cox2 and EP2 are regulated by GDF-9 in this process.
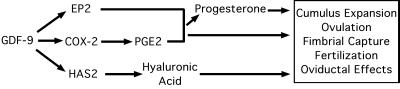
Figure 7.
Model for GDF-9 regulation of prostaglandin and progesterone synthesis in cumulus granulosa cells. Based on the findings of our group (this manuscript and ref. 24), and those of others (2–5, 7, 11, 12, 19–21, 29, 39), we have formulated a simple model for the effects of GDF-9 in the periovulatory period. The combined effects of this extended signal transduction are essential for cumulus expansion, ovulation, fimbrial capture, fertilization, and unknown oviductal effects.
Acknowledgments
We thank Dr. John Eppig for interesting discussions, Ms. Shirley Baker for aid in manuscript preparation, Dr. Amander Clark for help in the production of the recombinant GDF-9, Dr. Harold Kluender for the generous gift of the butaprost, and Drs. Amander Clark and T. Rajendra Kumar for critical review of the manuscript. These studies were supported in part by a sponsored research grant from Genetics Institute and National Institutes of Health grant HD33438 (to M.M.M.). J.A.E., Ph.D. is a student in the Medical Scientist Training Program supported in part by National Institutes of Health Grants GM-07330 and GM-08307 and the Baylor Research Advocates for Student Scientists (BRASS) organization.
Abbreviations
- GDF-9
growth differentiation factor-9
- Cox1 and Cox2
cyclooxygenase 1 and 2
- PGE2
prostaglandin E2
- COC
cumulus cell–oocyte complex
- LH
luteinizing hormone
- FSH
follicle-stimulating hormone
- Hprt
hypoxanthine phosphoribosyltransferase
- RT-PCR
reverse transcription–PCR
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.180295197.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.180295197
References
- 1.Tanaka N, Espey L L, Kawano T, Okamura H. Endocrinol Metab. 1991;23:E170–E174. doi: 10.1152/ajpendo.1991.260.2.E170. [DOI] [PubMed] [Google Scholar]
- 2.Lydon J P, DeMayo F J, Funk C R, Mani S K, Hughes A R, Montgomery C A, Shyamala G, Conneely O M, O'Malley B W. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- 3.Lydon J P, DeMayo F J, Conneely O M, O'Malley B W. J Steroid Biochem Mol Biol. 1996;56:67–77. doi: 10.1016/0960-0760(95)00254-5. [DOI] [PubMed] [Google Scholar]
- 4.Schuetz A W, Dubin N H. Endocrinology. 1981;108:457–463. doi: 10.1210/endo-108-2-457. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X, Armstrong D T. Gamete Res. 1989;23:267–277. doi: 10.1002/mrd.1120230304. [DOI] [PubMed] [Google Scholar]
- 6.Mikuni M, Pall M, Peterson C M, Peterson C A, Hellberg P, Brannstrom M, Richards J S, Hedin L. Biol Reprod. 1998;59:1077–1083. doi: 10.1095/biolreprod59.5.1077. [DOI] [PubMed] [Google Scholar]
- 7.Lim H, Paria B C, Das S K, Dinchuk J E, Langenbach R, Trzaskos J M, Dey S K. Cell. 1997;91:197–208. doi: 10.1016/s0092-8674(00)80402-x. [DOI] [PubMed] [Google Scholar]
- 8.Eppig J J. Semin Dev Biol. 1994;5:51–59. [Google Scholar]
- 9.Salustri A, Camaioni A, D'Alessandris C. Zygote. 1996;4:313–315. doi: 10.1017/s0967199400003312. [DOI] [PubMed] [Google Scholar]
- 10.Elvin J A, Yan C, Matzuk M M. Mol Cell Endocrinol. 2000;159:1–5. doi: 10.1016/s0303-7207(99)00185-9. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Russell P T, Larsen W J. Mol Reprod Dev. 1993;34:87–93. doi: 10.1002/mrd.1080340114. [DOI] [PubMed] [Google Scholar]
- 12.Eppig J J. Biol Reprod. 1981;25:191–195. doi: 10.1095/biolreprod25.1.191. [DOI] [PubMed] [Google Scholar]
- 13.Viggiano J M, Herrero M B, Cebral E, Boquet M G, de Gimeno M F. Prostaglandins Leukotrienes Essent Fatty Acids. 1995;53:261–265. doi: 10.1016/0952-3278(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 14.Coleman R A, Smith W L, Narumiya S. Pharmacol Rev. 1994;46:205–229. [PubMed] [Google Scholar]
- 15.Fleming E F, Athirakul K, Oliverio M I, Key M, Goulet J, Koller B H, Coffman T M. Renal Physiol. 1998;44:F955–F961. doi: 10.1152/ajprenal.1998.275.6.F955. [DOI] [PubMed] [Google Scholar]
- 16.Ushikubi F, Segi E, Sugimoto Y, Murata T, Matsuoka T, Kobayashi T, Hizaki H, Tuboi K, Katsuyama M, Ichikawa A, et al. Nature (London) 1998;395:281–284. doi: 10.1038/26233. [DOI] [PubMed] [Google Scholar]
- 17.Segi E, Sugimoto Y, Yamasaki A, Aze Y, Oida H, Nishimura T, Murata T, Matsuoka T, Ushikubi F, Hirose M, et al. Biochem Biophys Res Commun. 1998;246:7–12. doi: 10.1006/bbrc.1998.8461. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen M, Camenisch T, Snouwaert J N, Hicks E, Coffman T M, Anderson P A W, Malouf N N, Koller B H. Nature (London) 1997;390:78–81. doi: 10.1038/36342. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy C R J, Zhang Y, Brandon S, Guan Y, Coffee K, Funk C D, Magnuson M A, Oates J A, Breyer M D, Breyer R M. Nat Med. 1999;5:217–220. doi: 10.1038/5583. [DOI] [PubMed] [Google Scholar]
- 20.Tilley S L, Audoly L P, Hicks E H, Kim H-S, Flannery P J, Coffman T M, Koller B H. J Clin Invest. 1999;103:1539–1545. doi: 10.1172/JCI6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hizaki H, Segi E, Sugimoto Y, Hirose M, Saji T, Ushikubi F, Matsuoka T, Noda Y, Tanaka T, Yoshida N, et al. Proc Natl Acad Sci USA. 1999;96:10501–10506. doi: 10.1073/pnas.96.18.10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McPherron A C, Lee S-J. J Biol Chem. 1993;268:3444–3449. [PubMed] [Google Scholar]
- 23.McGrath S A, Esquela A F, Lee S-J. Mol Endocrinol. 1995;9:131–136. doi: 10.1210/mend.9.1.7760846. [DOI] [PubMed] [Google Scholar]
- 24.Elvin J A, Clark A T, Wang P, Wolfman N M, Matzuk M M. Mol Endocrinol. 1999;13:1035–1048. doi: 10.1210/mend.13.6.0310. [DOI] [PubMed] [Google Scholar]
- 25.Dong J, Albertini D F, Nishimori K, Kumar T R, Lu N, Matzuk M M. Nature (London) 1996;383:531–535. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- 26.Carabatsos M J, Elvin J A, Matzuk M M, Albertini D F. Dev Biol. 1998;203:373–384. doi: 10.1006/dbio.1998.9087. [DOI] [PubMed] [Google Scholar]
- 27.Elvin J A, Yan C, Wang P, Nishimori K, Matzuk M M. Mol Endocrinol. 1999;13:1018–1034. doi: 10.1210/mend.13.6.0309. [DOI] [PubMed] [Google Scholar]
- 28.Buccione R, Vanderhyden B C, Caron P J, Eppig J J. Dev Biol. 1990;138:16–25. doi: 10.1016/0012-1606(90)90172-f. [DOI] [PubMed] [Google Scholar]
- 29.Salustri A, Yanagishita M, Hascall V C. Dev Biol. 1990;138:26–32. doi: 10.1016/0012-1606(90)90173-g. [DOI] [PubMed] [Google Scholar]
- 30.Salustri A, Ulisse S, Yanagishita M, Hascall V C. J Biol Chem. 1990;265:19517–19523. [PubMed] [Google Scholar]
- 31.Katsuyama M, Nishigaki N, Sugimoto Y, Morimoto K, Negishi M, Narumiya S, Ichikawa A. FEBS Lett. 1995;372:151–156. doi: 10.1016/0014-5793(95)00966-d. [DOI] [PubMed] [Google Scholar]
- 32.Katsuyama M, Sugimoto Y, Okano K, Segi E, Ikegami R, Negishi M, Ichikawa A. Biochem J. 1998;330:1115–1121. doi: 10.1042/bj3301115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahmoudi M, Lin V K. BioTechniques. 1989;7:331–332. [PubMed] [Google Scholar]
- 34.Davis B J, Lennard D E, Lee C A, Tiano H F, Morham S G, Wetsel W C, Langenbach R. Endocrinology. 1999;140:2685–2695. doi: 10.1210/endo.140.6.6715. [DOI] [PubMed] [Google Scholar]
- 35.Vanderhyden B C, Cohen J N, Morley P. Endocrinology. 1993;133:423–426. doi: 10.1210/endo.133.1.8319589. [DOI] [PubMed] [Google Scholar]
- 36.Richards J S. Endocr Rev. 1994;15:725–751. doi: 10.1210/edrv-15-6-725. [DOI] [PubMed] [Google Scholar]
- 37.Sirois J, Levy L O, Simmons L, Richards J S. J Biol Chem. 1993;268:12199–12206. [PubMed] [Google Scholar]
- 38.Eppig J. J Exp Zool. 1979;208:111–120. doi: 10.1002/jez.1402080112. [DOI] [PubMed] [Google Scholar]
- 39.Eppig J J, Peters A H, Telfer E E, Wigglesworth K. Mol Reprod Dev. 1993;34:450–456. doi: 10.1002/mrd.1080340415. [DOI] [PubMed] [Google Scholar]