Abstract
Fragile X-associated tremor/ataxia syndrome (FXTAS) is a neurodegenerative disorder caused by a CGG repeat expansion in the premutation range (55-200) in the fragile X mental retardation 1 gene. Onset is typically in the early seventh decade and men are principally affected. The major signs are cerebellar gait ataxia, intention tremor, frontal executive dysfunction, and global brain atrophy. Other frequent findings are parkinsonism (mild), peripheral neuropathy, psychiatric symptoms (depression, anxiety, agitation), and autonomic dysfunction. The clinical presentation is heterogeneous, with individuals presenting with varied dominating signs, such as tremor, dementia or neuropathy. MR imaging shows atrophy and patchy white matter lesions in the cerebral hemispheres and middle cerebellar peduncles. The latter has been designated the ‘MCP sign’, occurs in about 60% of affected men, and is relatively specific for FXTAS. Affected females generally have less severe disease, less cognitive decline, and some symptoms different from that of men, e.g., muscle pain. Management of FXTAS is complex and includes assessment of the patient's neurological and medical deficits, treatment of symptoms, and provision of relevant referrals, especially genetic counseling. Treatment is empiric, based on anecdotal experience and on knowledge of what works for symptoms of other disorders that also exist in FXTAS. Presently the disorder is under-recognized, since the first published report was in 2001, and since the presentation is variable and mainly consists of a combination of signs common in the elderly. However, accurate diagnosis is critical, for the patient and for the family, as they need education regarding their genetic and health risks.
Keywords: fragile X tremor/ataxia syndrome, FXTAS, fragile X mental retardation 1 gene, treatment
Introduction
Fragile X-associated tremor/ataxia syndrome (FXTAS)(1) is an inherited degenerative disorder that affects aging persons, primarily men, and is associated with an array of neurological symptoms and medical conditions. The disorder is caused by a CGG repeat expansion in the premutation range (55-200) in the 5′ non-coding region of the fragile X mental retardation 1 (FMR1) gene. Expansion of the CGG repeat to >200 results in reduction or absence of fragile X mental retardation protein (FMRP) and, consequentially, fragile X syndrome (FXS), the most common known heritable form of mental retardation and autism. While mutations in FMR1 cause both FXS and FXTAS, the two disorders are distinct, as illustrated in Figure 1. In FXTAS, FMRP is produced in normal or near normal amounts while FMR1 mRNA with the expanded repeat is produced in markedly elevated amounts in leukocytes and brain.(2, 3) The high level of FMR1 mRNA is likely toxic and the cause of the cellular injury responsible for the symptoms. FXTAS is a significant cause of morbidity, especially ataxia in aging men,(4, 5) and is the most common known single-gene form of tremor and ataxia, and perhaps also of dementia, in the older adult population.
Figure 1.
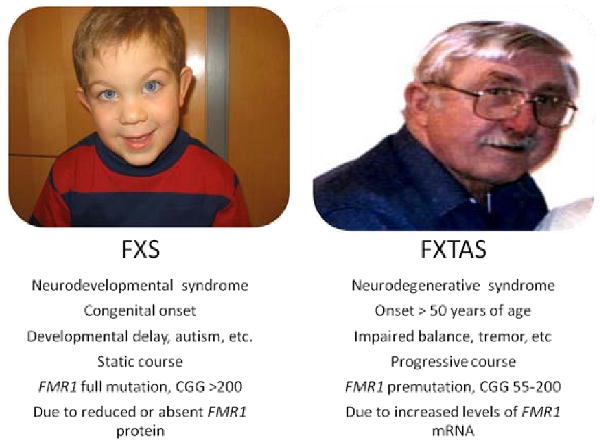
Fragile X Syndrome (FXS) vs. Fragile X-associated Tremor/Ataxia Syndrome (FXTAS). This figure demonstrates that FXS and FXTAS are very different disorders, and that each results from a different length of the repeat expansion that occurs in the same gene, fragile X mental retardation 1 (FMR1). Two males are shown, because males are more affected than females since the gene mutation is on the X chromosome.
Clinical Phenotype
The age of onset of tremor and/or ataxia in males is 61.6 ± 7.9 years (mean ± SD).(6) The classic presentation of FXTAS is a man in his early sixties with progressive cerebellar ataxia, action tremor, parkinsonism and cognitive decline, especially executive dysfunction. Other features, which are present in variable degrees among individually affected persons, are psychiatric disturbances, and autonomic and peripheral neuropathy.(7, 8) The frequency of these various signs are presented in Table 1. However, as shown in Table 2, a number of different clinical features and presentations with varied dominating signs have been reported in persons with FXTAS. Even within families affected persons have disparate clinical presentations, e.g., a presentation that looks like essential tremor in one brother and a classic FXTAS presentation in another.(9)
Table 1. Clinical signs in FXTAS*.
| Clinical Feature | Affected Subjects (%) (n=20) |
|---|---|
| Cerebellar ataxia | |
| Dysarthria | 78 |
| Dysmetria | 93 |
| Postural & gait abnormalities | 86 |
| Tremor | |
| Intention | 70 |
| Resting alone | 10 |
| Parkinsonism | |
| Mild bradykinesia | 57 |
| Mild rigidity | |
| Upper extremity | 71 |
| Lower extremity | 35 |
| Cogwheeling | 5 |
| Peripheral neuropathy | 60 |
| Other medical conditions | |
| Urinary incontinence | 55 |
| Fecal incontinence | 30 |
| Impotence | 80 |
| History of congestive heart failure | 55 |
| Hypertension | 50 |
Adapted from a review of 20 men with FXTAS identified because of a family history of fragile X syndrome, mean age (± SD) 71 ± 6, mean CGG repeat 84 (range 69-99), from Jacquemont et al. (8)
Table 2. FXTAS has a variable clinical phenotype.
| Variably associated features |
| Autonomic dysfunction(30-33) |
| Large-fiber sensory neuropathy(26-28) |
| Psychiatric features: depression, anxiety, irritability(17, 25) |
| Cognitive decline and dementia(21) |
| Oculomotor dysfunction(80) |
| Focal dystonia (limb, cervical, laryngeal)(81, 82) |
| Spastic paraparesis(44) |
| Uncommon presentations |
| Essential tremor-like syndrome(9, 71) |
| MSA-like syndrome(32) |
| Rapidly progressive dementia(83) |
| CMT-like neuropathy(26) |
| Spastic paraparesis(44) |
| Clinical heterogeneity within the same family(9, 84) |
About one in 260 females and one in 813 males in the general population are premutation carriers.(10, 11) Penetrance of FXTAS is age-dependent, with approximately 40% of males over age 50,(12) and 8% of female carriers over age 40(13) developing the disorder. Although large scale epidemiologic studies of FXTAS have not conducted, the predicted lifetime cumulated risk among men in the general population has been estimated to be approximately one in 8,000.(14) Thus, FXTAS is much less common than essential tremor and Parkinson's disease in older adults, but similar in prevalence to that of other neurodegenerative disorders, e.g., other inherited ataxias, amylotrophic lateral sclerosis, and progressive supranuclear palsy.(15)
While the symptoms of FXTAS vary among individuals, almost all affected persons develop problematic cerebellar gait ataxia as the disorder progresses. Unexplained falls are frequent, and the tandem gait is significantly abnormal in about 50% of male carriers over age 50.(Leehey et al., unpublished data) While cerebellar dysfunction is a nearly constant feature that affects the gait, other impairments such as parkinsonism, sensory neuropathy, and weakness also contribute to poor balance. Hypermetria and dysdiadochokinesis are frequently present in the upper extremities.
Action tremor is a common finding, but the severity is quite variable among individuals. Many affected persons, probably due to poor insight related to executive dysfunction, deny having any tremor despite their spouse noting that a mild, intermittent tremor has been present for months or years. Others have an obvious, severe tremor that impairs daily function. Fifty percent of male carriers have at least mild, and 17% at least moderate intention tremor, compared to 25% and 4%, respectively, of age-matched male controls (Leehey et al., unpublished data). The tremor of FXTAS has not been quantitatively studied, but looks like essential tremor. Further, affected persons usually have definite tremor reduction with use of medications commonly prescribed for essential tremor.
Another frequent motor sign is parkinsonism, which is generally mild and mainly manifested by masked facies, generalized rigidity, overall bradykinesia and slow gait. Occasionally affected persons have a parkinsonian posture. Resting tremor is uncommon, and when present, may be due to reoccurrence of the postural tremor in the rest position. Carriers with parkinsonism generally have minor and only transient improvement with dopaminergic medication, suggesting that the underlying mechanism causing parkinsonism is not the same as in primary Parkinson disease. Gait ataxia, intention tremor, and parkinsonism worsen with increasing age, and the severity of tremor and ataxia correlate strongly with increasing CGG repeat size through the premutation range.(16)
Cognitive dysfunction is a very disabling feature in many aging male carriers.(17) Male carriers over age 50 selected without regard to motor signs of FXTAS show normal to mild full scale IQ deficits and significant memory and frontal executive dysfunction.(18-21) Men with motor signs have prominent executive impairment, and as the disease progresses develop global deficits (dementia), affecting intelligence, working memory, declarative learning and memory, remote recall, information processing speed, and temporal sequencing, and perhaps visuospatial functioning. These deficits are suggested to be largely due to the prominent executive dysfunction that occurs in FXTAS.(22) Of note, verbal comprehension and language are relatively preserved.(21) The dementia is of a mixed subcortical - cortical pattern, due to involvement of cerebral and cerebellar white matter, frontal cortex, and hippocampus.(23) Dementia occurs in approximately 40% of men,(23, 24) less often in females(23) and the frequency may be higher in late stage FXTAS.
Psychiatric and behavioral disorders, the latter mainly due to impaired executive function, are also common and disabling features of FXTAS. Males over age 50, selected without regard to motor signs, demonstrate increased rates of anxiety, irritability, agitation, hostility, obsessive-compulsiveness, apathy, and depression.(17, 25) Affected men are unaware of their change in personality and thus will not report these symptoms, but untreated they can lead to significant marital discord. Female carriers have higher rates of depression and obsessive thinking than matched controls.(25)
The peripheral nervous system is also affected in carriers.(1, 8, 26, 27) Males have greater loss of distal lower extremity tendon reflexes and vibration sense than age-matched male controls.(28) Distal leg strength is generally preserved. The neuropathy may be the presenting feature of FXTAS, the only significant neurological finding in a carrier,(26) or asymptomatic. Electrical studies in males with FXTAS document a predominately axonal sensori-motor polyneuropathy.(27) The degree of reflex impairment correlates with severity of ataxia, thus, the neuropathy may contribute to gait difficulties in some affected persons.
Autonomic dysfunction has been described in FXTAS, but controlled studies are needed. In a study of 20 men with FXTAS, 55% reported urinary incontinence and 30% bowel incontinence.(8) Urinary and fecal incontinence mainly occurs in late stage FXTAS.(29, 30) Some individuals have symptomatic orthostasis and syncope.(30-33) Pugliese and colleagues(31) reported a 73 year old man that presented with postprandial hypotension, and was then found to have had hand tremor and 73 CGG repeats in FMR1. There was no family history suggestive of FMR1 related disorders and the patient did not have ataxia. This case report suggests that autonomic impairment can be a presenting and dominant feature of FXTAS. Of note, autonomic nervous system involvement has been documented in neuropathologic studies of paraspinal sympathetic ganglia,(34) myenteric ganglia of the stomach, and subepicardial autonomic ganglia.(30)
Some medical conditions have been reported to be associated with the FMR1 premutation. Hypertension was more common in male (p=.06)(12) and female (p=.002)(13) carriers than controls. Anecdotal evidence suggests that cardiac dysfunction, e.g., arrhythmia, ischemia, and congestive heart failure, may occur more frequently in men with FXTAS. Other disorders also appear to be more common in persons with FXTAS than expected. For example, many men with advancing FXTAS have diet controlled hyperglycemia that leads to diabetes requiring oral therapy. Also, many affected men have episodes of dizziness. In some cases these episodes appear to be due to lightheadedness related to orthostatic hypotension, and in other instances appear to be due to vestibular dysfunction. Studies of hyperglycemia and vestibular dysfunction in FXTAS are needed to determine if and how these are related to the FMR1 premutation.
FXTAS is a progressive disease, but prospective studies on its natural history are lacking. A retrospective study(29) on the progression of tremor and ataxia in 55 men with FXTAS found that after the initial motor sign, usually tremor, median delay of onset of ataxia was two years, onset of falls six years, dependence on a walking aid 15 years, inability to do most daily activities 16 years, and death 21 years. Life expectancy ranged from 5 to 25 years, and other reports cite death occurred in five to seven years after presentation for evaluation.(30, 32-34) Death was due to congestive heart failure, pneumonia, cardiac arrest, or progression of neurologic disease. At end stage affected persons are bedridden, dysphagic, dysarthric, parkinsonian and without bladder or bowel control.(29, 33)
Female Premutation Carriers
Even before the finding that a neurodegenerative disorder, FXTAS, occurs in carriers, females carriers were known to be at increased risk for primary ovarian insufficiency (POI).(35) About 20% develop POI,(36, 37) and it does not appear to be related to the risk of FXTAS.(13) Also, female carriers have been found to have higher rates of depression and anxiety than controls.(25, 38-40) While this could be related to the stress of raising a difficult child with fragile X syndrome, recent studies suggest that depression(41) and anxiety(42) are secondary, at least in part, to the premutation.
FXTAS affects male carriers more(12) partially because of a protective effect of the second X chromosome in females.(43, 44) Most research on FXTAS to date has been in males. The data available on females suggest that the premutation may confer different neurological and medical risks with aging(13, 45) than those that occur in males, due perhaps to hormonal and other, as yet unknown, factors.
Generally female carriers do not develop as much tremor and ataxia as males.(16, 46) A quantitative study(16) showed that females' over age 50 risk for cerebellar ataxia correlated with increasing CGG repeat size only when the activation ratio, the percent of normal FMR1 alleles that are on the active X chromosome, was factored in. Female carriers without FXTAS report more sensory loss (45%), chronic muscle pain (26%), and tremor (12%) than controls.(13) Interestingly, preliminary data suggests that female carriers, regardless of the presence of motor signs, have higher rates of psychogenic signs, including movement disorders and seizures, than expected. (Leehey M & Seritan A, unpublished data).
Some female carriers do develop classic features of FXTAS,(43, 45, 47, 48) but they also have some distinct differences from affected males. Females with FXTAS probably have less cognitive dysfunction than males,(23, 45) although individual case reports document that dementia does occur.(49, 50) Also, chronic muscle pain and a diagnosis of fibromyalgia are frequently reported by affected females. Further, approximately 50% of affected females have been diagnosed with thyroid dysfunction, usually hypothyroidism.(13)
Neuroimaging
Imaging in FXTAS is useful because it typically shows atrophy, white matter changes, and a distinctive abnormality of the middle cerebellar peduncles. These finding are typically more severe in affected males than females.(51) MR imaging reveals atrophy of the cerebrum, cerebellar cortex, corpus callosum and pons. The cerebral and cerebellar white matter has increased T2 and decreased T1 signal intensity. These white matter image changes resemble that typically seen in microvascular ischemia, except that it is often subcortical and patchy.(52) The middle cerebellar peduncles have increased T2 signal in about 60% of affected men and 13% of affected women.(51, 52) This is called the ‘MCP sign’ and is relatively specific for FXTAS. It has also been reported in multiple system atrophy,(53, 54) acquired hepatocerebral degeneration,(55) and recessive ataxia.(54) To date, however, there have been only two patients, one with recessive ataxia and one with multiple system atrophy,(54) reported with the MCP sign that were tested and not found to have the FMR1 premutation.
Some asymptomatic carriers have atrophy also. Brain stem volume was significantly smaller in unaffected male carriers compared to controls.(56) Aging male carriers selected without regard to motor signs had reduced total brain, cerebrum, cerebellum, cerebral cortex, amygdalo-hippocampal complex, and thalamus size.(57, 58)
Diagnosis of FXTAS
The diagnosis of FXTAS is often missed, for two reasons. First, since it has only recently been described,(1) many physicians are not yet familiar with it. Second, the presentation can be quite variable, resulting in misdiagnosis. Correct diagnosis is not only vital for the patient but also for their family; ensuing generations are at high risk for fragile X syndrome. Guidelines to aid the physician in deciding who to test for FXTAS, are shown in Table 3; and generally accepted diagnostic criteria are presented in Table 4.(8) Diagnosis of a premutation carrier requires provision of genetic counseling for the patient and their family.
Table 3. Guidelines for who test for FXTAS.
| Cerebellar ataxia | ≥ age 50 & Unknown cause |
| Action tremor | Presence of cerebellar ataxia, parkinsonism or dementia & ≥ age 50 & Unknown cause |
| Dementia | Presence of cerebellar ataxia, parkinsonism or action tremor & ≥ age 50 & Unknown cause |
| Some FXTAS signs | Middle cerebellar peduncle sign or Patient or family history of premature ovarian insufficiency or Family history of FMR1 related disease |
| Multiple system atrophy, cerebellar subtype* | |
Especially if has action tremor or longer than expected disease duration
Table 4. FXTAS diagnostic criteria in FMR1 premutation carriers(8).
| Diagnostic Categories | |
|---|---|
| Definite: | Presence of one major radiological sign plus one major clinical symptom |
| Probable: | Presence of either one major radiological sign plus one minor clinical symptom or has the two major clinical symptoms |
| Possible: | Presence of one minor radiological sign plus one major clinical symptom |
| Symptom Types | |
| Radiological | |
| Major | MRI white matter lesions in MCPs and or brain stem |
| Minor | MRI white matter lesions in cerebral white matter |
| Minor | Moderate to severe generalized atrophy |
| Clinical | |
| Major | Intention tremor |
| Major | Gait ataxia |
| Minor | Parkinsonism |
| Minor | Moderate to severe short-term memory deficiency |
| Minor | Executive function deficit |
MCP = middle cerebellar peduncle
Management and Therapy
While the patient diagnosed with FXTAS may have presented with a specific complaint, often a motor symptom, the management of FXTAS is complex and involves other considerations. Appropriate management consists of assessment of the patient's neurological and medical deficits, prescription of appropriate medications and rehabilitative services, genetic counseling for the patient and family and provision of relevant referrals. Evaluation for other causes of dementia, particularly reversible contributing causes, such as hypothyroidism, B12 deficiency, folate deficiency and depression is essential. Further, patients need counseling regarding conditions and medications which may worsen FXTAS, e.g. some chemotherapeutic agents, e.g., carboplantin,(59) and major surgery. Referrals are often indicated to urology, rehabilitative medicine, genetic counseling, and social work. The most disabling symptoms are falls, intention tremor, psychiatric problems such as depression, anxiety, agitation, and disinhibition, and cognitive dysfunction, ranging from memory loss to dementia. In addition, many women complain bitterly of fibromyalgia symptoms and some affected persons have severe neuropathic leg pain.(60)
To date there have not been any controlled trials reported that evaluate disease modifying or symptomatic therapies. Thus, intervention is limited to symptomatic therapy. Current recommendations are based on anecdotal reports and on knowledge of what works for symptoms of other disorders that also exist in FXTAS. For example, the action tremor and the parkinsonism in FXTAS may respond to medications used effectively for essential tremor and Parkinson's disease, respectively.
Falls are mainly due to cerebellar dysfunction, which generally does not respond to medical therapy.(61) A few persons with FXTAS and other disorders with cerebellar ataxia have shown at least transient improvement with amantadine at standard doses(62, 63) and with varenicline at 0.5 to 1 mg per day.(64, 65) However anecdotal reports suggest that varenicline may worsen tremor and exacerbate psychiatric symptoms in FXTAS. Gait difficulties may also be caused by parkinsonism, peripheral neuropathy, extensive CNS white matter lesions, and weakness, especially of the proximal lower extremities. Dopaminergic medications improved ataxia in some persons with parkinsonism.(66) Physical therapy aimed at improving strength and flexibility may reduce falls and improve function.
Action tremor may respond to beta-blockers, primidone, and topiramate, at the doses used to treat essential tremor.(66, 67) Benzodiazepines such as alprazolam may also be effective in some patients,(68) since tremor is aggravated by anxiety and stress. Other options with less published support are gabapentin, levetiracetam, clonazepam, clozapine, nadolol, nimodipine and botulinum toxin.(69) Regarding the latter, injection of just the flexor muscles and not the extensors reduces the occurrence of excessive weakness (personal communication, J Jankovic). Occupational therapy may offer techniques to improve function, such as wrist weights or use of the Assistive Mouse Adapter (IBM Watson Research Center), which is designed to assist persons with tremor use a keyboard.(70) Thalamic deep brain stimulation is an option for severe, medically intractable tremor,(9, 71) and perhaps should be limited to unilateral treatment since bilateral surgery is more likely to worsen cognition(72) and gait.(71)
While minimal use of psychoactive medication is indicated in elderly persons with relatively advanced stage FXTAS, judicious treatment of psychiatric symptoms and cognitive decline is generally rewarding. Regarding depression, selective serotonin reuptake inhibitors (SSRIs) with minimal drug-drug interaction (e.g. citalopram, sertraline, escitalopram) are preferred for use in the elderly, rather than paroxetine, fluvoxamine, are fluoxetine. Anecdotal experience suggests that the selective serotonin norepinephrine reuptake inhibitors (SNRIs; venlafaxine and duloxetine) are especially effective,(60) not only for depression but also for anxiety, agitation, hostility and irritability.(60)
Cognitive decline has been reported to slow in a few persons with FXTAS with use of acetylcholinesterase inhibitors.(66) This may be because pathological studies in FXTAS show marked pathology in the hippocampus, (73), although the clinical profile of cognitive deficits in Alzheimer's disease and FXTAS are distinct.(23) Addition of memantine is indicated in Alzheimer's disease to treat the memory deficit, and is suspected to reduce glutamate-associated excitotoxicity in neurodegenerative disorders. Anecdotal reports suggest memantine may be helpful in FXTAS at the same dose used in Alzheimer's disease.(60)
Pain from peripheral neuropathy may be reduced with anticonvulsants (especially gabapentin and pregabilin), antidepressants (especially cymbalta), and topical analgesics (such as lidocaine cream). Pain from fibromyalgia in women with FXTAS may respond to pregabalin(74) and standard therapy.(75) Standard therapy includes exercise, psychotherapy, and antidepressants.
Although parkinsonism is usually mild in FXTAS, some patients improve with dopaminergic medications and anticholinergics, at standard doses used in Parkinson disease. In the later stages of FXTAS, when patients are more fragile, levodopa is expected to be better tolerated than dopamine agonists, and anticholinergics need monitoring for exacerbation of memory and urinary problems. Physical therapy, especially step and gait training, has been shown to improve function in Parkinson disease,(76, 77) so may be beneficial in carriers with parkinsonism.
Autonomic dysfunction in FXTAS may include impotence, orthostatic hypotension, urinary frequency or incontinence, and bowel incontinence (in the later stages). Urological referral will dictate which types of medication may be helpful for the urinary symptoms. Hyperactive detrusor activity responds to small doses of tricyclic antidepressants, to muscarinic receptor antagonists, and to botulinum toxin injections. Initial treatment of orthostatic hypotension consists of increased salt and fluid intake, eating frequent small meals, use of Jobst stockings, and elevation of the head of the bed at night. Useful medications include mineralocorticoids (fludrocortisones) and midodrine, an alpha-1 adrenergic agonist which increases blood pressure. Regarding midodrin, patients need to be warned not to lie flat for four hours after a dose, as dangerously high blood pressure can occur.(78) Some patients benefit from use of both midodrine and fludrocortisones. Orthostasis also responds to pyridostigmine.(60, 79)
A few other points regarding the management of FXTAS are worth noting. Caretakers are often in need of support, emotional and practical, as the patient's disease progresses. Depression and/or anxiety are common in spouses, and treatment with an SSRI can be quite helpful. Appropriate referrals are needed to assist the caretaker in obtaining help in the household, legal guardianship, and, eventually, placement of the patient in a specialized chronic care facility. Another important point is that persons with FXTAS will sometimes be misdiagnosed as having normal pressure hydrocephalus. However, surgery for this condition in persons with FXTAS has resulted in deterioration.(12, 60)
Conclusion
Mutation of the FMR1 gene is associated with FXS, POI, and FXTAS. The premutation, which causes POI and FXTAS, is relatively common in the general population, and thus an important source of morbidity. To date most persons with FXTAS have been identified only after a grandchild is diagnosed with FXS. FXTAS is under-recognized and misdiagnosed, since it has only recently been described, and because its' presentation is variable and mainly consists a combination of signs that are common in the elderly. However, accurate diagnosis is important, not only for the patient, but so that family members can be educated about serious genetic and health risks.
Many important questions about FMR1 premutation related disease remain. For example, prospective studies of disease progression and modifying factors would provide valuable information needed for life planning. Most importantly, the basic mechanisms by which the FMR1 premutation cause symptoms need further study, so that effective treatment can be developed to slow and prevent disease.
Acknowledgments
The author wishes to thank Ms. Leah Gaspari for her administrative assistance in preparation of this manuscript. This work was supported by a National Institutes of Health Interdisciplinary Research Consortium (Roadmap) grant (UL1DE19583, PJ Hagerman; RL1AG032115, RJ Hagerman) and the symposium was supported in part by a grant from the National Center for Research Resources.
References
- 1.Hagerman RJ, Leehey M, Heinrichs W, et al. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology. 2001;57:127–130. doi: 10.1212/wnl.57.1.127. [DOI] [PubMed] [Google Scholar]
- 2.Tassone F, Hagerman RJ, Taylor AK, Gane LW, Godfrey TE, Hagerman PJ. Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in the fragile-X syndrome. American Journal of Human Genetics. 2000;66:6–15. doi: 10.1086/302720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tassone F, Hagerman RJ, Garcia-Arocena D, Khandjian EW, Greco CM, Hagerman PJ. Intranuclear inclusions in neural cells with premutation alleles in fragile X associated tremor/ataxia syndrome. J Med Genet. 2004;41:e43. doi: 10.1136/jmg.2003.012518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brussino A, Gellera C, Saluto A, et al. FMR1 gene premutation is a frequent genetic cause of late-onset sporadic cerebellar ataxia. Neurology. 2005;64:145–147. doi: 10.1212/01.WNL.0000148723.37489.3F. [DOI] [PubMed] [Google Scholar]
- 5.Van Esch H, Dom R, Bex D, et al. Screening for FMR-1 premutations in 122 older Flemish males presenting with ataxia. Eur J Hum Genet. 2005;13:121–123. doi: 10.1038/sj.ejhg.5201312. [DOI] [PubMed] [Google Scholar]
- 6.Tassone F, Adams J, Berry-Kravis EM, et al. CGG repeat length correlates with age of onset of motor signs of the fragile X-associated tremor/ataxia syndrome (FXTAS) Am J Med Genet B Neuropsychiatr Genet. 2007;144:566–569. doi: 10.1002/ajmg.b.30482. [DOI] [PubMed] [Google Scholar]
- 7.Leehey M, Brunberg J, Lang A, et al. MRI increased T2 signal in the cerebellar peduncles: Specific for the fragile X premutation tremor/ataxia syndrome? Neurology. 2002;58:A481. [Google Scholar]
- 8.Jacquemont S, Hagerman RJ, Leehey M, et al. Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. American Journal of Human Genetics. 2003;72:869–878. doi: 10.1086/374321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters N, Kamm C, Asmus F, et al. Intrafamilial variability in fragile X-associated tremor/ataxia syndrome. Mov Disord. 2006;21:98–102. doi: 10.1002/mds.20673. [DOI] [PubMed] [Google Scholar]
- 10.Dombrowski C, Levesque S, Morel ML, Rouillard P, Morgan K, Rousseau F. Premutation and intermediate-size FMR1 alleles in 10572 males from the general population: loss of an AGG interruption is a late event in the generation of fragile X syndrome alleles. Hum Mol Genet. 2002;11:371–378. doi: 10.1093/hmg/11.4.371. [DOI] [PubMed] [Google Scholar]
- 11.Rousseau F, Rouillard P, Morel ML, Khandjian EW, Morgan K. Prevalence of carriers of premutation-size alleles of the FMRI gene--and implications for the population genetics of the fragile X syndrome. Am J Hum Genet. 1995;57:1006–1018. [PMC free article] [PubMed] [Google Scholar]
- 12.Jacquemont S, Hagerman RJ, Leehey MA, et al. Penetrance of the fragile X-associated tremor/ataxia syndrome in a premutation carrier population. Journal of the American Medical Association. 2004;291:460–469. doi: 10.1001/jama.291.4.460. [DOI] [PubMed] [Google Scholar]
- 13.Coffey SM, Cook K, Tartaglia N, et al. Expanded clinical phenotype of women with the FMR1 premutation. Am J Med Genet A. 2008;146A:1009–1016. doi: 10.1002/ajmg.a.32060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacquemont S, Leehey MA, Hagerman RJ, Beckett LA, Hagerman PJ. Size bias of fragile X premutation alleles in late-onset movement disorders. J Med Genet. 2006 doi: 10.1136/jmg.2006.042374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leehey M, Hagerman P. Fragile X-associated Tremor/Ataxia Syndrome (FXTAS) In: Subramoney S, Durr A, editors. Handbook of Clinical Neurology. Amsterdam, New York: Elsevier; in press. [Google Scholar]
- 16.Leehey MA, Berry-Kravis E, Goetz CG, et al. FMR1 CGG repeat length predicts motor dysfunction in premutation carriers. Neurology. 2008;70:1397–1402. doi: 10.1212/01.wnl.0000281692.98200.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bacalman S, Farzin F, Bourgeois JA, et al. Psychiatric phenotype of the fragile X-associated tremor/ataxia syndrome (FXTAS) in males: newly described fronto-subcortical dementia. J Clin Psychiatry. 2006;67:87–94. doi: 10.4088/jcp.v67n0112. [DOI] [PubMed] [Google Scholar]
- 18.Moore CJ, Daly EM, Schmitz N, et al. A neuropsychological investigation of male premutation carriers of fragile X syndrome. Neuropsychologia. 2004;42:1934–1947. doi: 10.1016/j.neuropsychologia.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Loesch DZ, Churchyard A, Brotchie P, Marot M, Tassone F. Evidence for, and a spectrum of, neurological involvement in carriers of the fragile X pre-mutation: FXTAS and beyond. Clin Genet. 2005;67:412–417. doi: 10.1111/j.1399-0004.2005.00425.x. [DOI] [PubMed] [Google Scholar]
- 20.Grigsby J, Brega AG, Jacquemont S, et al. Impairment in the cognitive functioning of men with fragile X-associated tremor/ataxia syndrome (FXTAS) J Neurol Sci. 2006;248:227–233. doi: 10.1016/j.jns.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 21.Grigsby J, Brega AG, Engle K, et al. Cognitive profile of fragile X premutation carriers with and without fragile X-associated tremor/ataxia syndrome. Neuropsychology. 2008;22:48–60. doi: 10.1037/0894-4105.22.1.48. [DOI] [PubMed] [Google Scholar]
- 22.Grigsby J, Brega AG, Leehey MA, et al. Impairment of executive cognitive functioning in males with fragile X-associated tremor/ataxia syndrome. Mov Disord. 2007;22:645–650. doi: 10.1002/mds.21359. [DOI] [PubMed] [Google Scholar]
- 23.Seritan AL, Nguyen DV, Farias ST, et al. Dementia in fragile X-associated tremor/ataxia syndrome (FXTAS): Comparison with Alzheimer's disease. Am J Med Genet B Neuropsychiatr Genet. 2008 doi: 10.1002/ajmg.b.30732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bourgeois JA, Cogswell JB, Hessl D, et al. Cognitive, anxiety and mood disorders in the fragile X-associated tremor/ataxia syndrome. Gen Hosp Psychiatry. 2007;29:349–356. doi: 10.1016/j.genhosppsych.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hessl D, Tassone F, Loesch DZ, et al. Abnormal elevation of FMR1 mRNA is associated with psychological symptoms in individuals with the fragile X premutation. Am J Med Genet B Neuropsychiatr Genet. 2005;139:115–121. doi: 10.1002/ajmg.b.30241. [DOI] [PubMed] [Google Scholar]
- 26.Hagerman RJ, Coffey SM, Maselli R, et al. Neuropathy as a presenting feature in fragile X-associated tremor/ataxia syndrome. Am J Med Genet A. 2007;143A:2256–2260. doi: 10.1002/ajmg.a.31920. [DOI] [PubMed] [Google Scholar]
- 27.Soontarapornchai K, Maselli R, Fenton-Farrell G, et al. Abnormal nerve conduction features in fragile X premutation carriers. Arch Neurol. 2008;65:495–498. doi: 10.1001/archneur.65.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berry-Kravis E, Goetz CG, Leehey MA, et al. Neuropathic features in fragile X premutation carriers. American Journal of Medical Genetics Part A. 2007;143:19–26. doi: 10.1002/ajmg.a.31559. [DOI] [PubMed] [Google Scholar]
- 29.Leehey MA, Berry-Kravis E, Min SJ, et al. Progression of tremor and ataxia in male carriers of the FMR1 premutation. Mov Disord. 2007;22:203–206. doi: 10.1002/mds.21252. [DOI] [PubMed] [Google Scholar]
- 30.Gokden M, Al-Hinti JT, Harik SI. Peripheral nervous system pathology in fragile X tremor/ataxia syndrome (FXTAS) Neuropathology. 2008 doi: 10.1111/j.1440-1789.2008.00948.x. [DOI] [PubMed] [Google Scholar]
- 31.Pugliese P, Annesi G, Cutuli N, et al. The fragile X premutation presenting as postprandial hypotension. Neurology. 2004;63:2188–2189. doi: 10.1212/01.wnl.0000145709.61117.08. [DOI] [PubMed] [Google Scholar]
- 32.Kamm C, Healy DG, Quinn NP, et al. The fragile X tremor ataxia syndrome in the differential diagnosis of multiple system atrophy: data from the EMSA Study Group. Brain. 2005;128:1855–1860. doi: 10.1093/brain/awh535. [DOI] [PubMed] [Google Scholar]
- 33.Louis E, Moskowitz C, Friez M, Amaya M, Vonsattel JP. Parkinsonism, dysautonomia, and intranuclear inclusions in a fragile X carrier: a clinical-pathological study. Mov Disord. 2006;21:420–425. doi: 10.1002/mds.20753. [DOI] [PubMed] [Google Scholar]
- 34.Greco CM, Berman RF, Martin RM, et al. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS) Brain. 2006;129:243–255. doi: 10.1093/brain/awh683. [DOI] [PubMed] [Google Scholar]
- 35.Cronister A, Schreiner R, Wittenberger M, Amiri K, Harris K, Hagerman RJ. Heterozygous fragile X female: historical, physical, cognitive, and cytogenetic features. Am J Med Genet. 1991;38:269–274. doi: 10.1002/ajmg.1320380221. [DOI] [PubMed] [Google Scholar]
- 36.Sullivan AK, Marcus M, Epstein MP, et al. Association of FMR1 repeat size with ovarian dysfunction. Hum Reprod. 2005;20:402–412. doi: 10.1093/humrep/deh635. [DOI] [PubMed] [Google Scholar]
- 37.Wittenberger MD, Hagerman RJ, Sherman SL, et al. The FMR1 premutation and reproduction. Fertil Steril. 2007;87:456–465. doi: 10.1016/j.fertnstert.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 38.Franke P, Maier W, Hautzinger M, et al. Fragile-X carrier females: evidence for a distinct psychopathological phenotype? Am J Med Genet. 1996;64:334–339. doi: 10.1002/(SICI)1096-8628(19960809)64:2<334::AID-AJMG20>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 39.Sobesky WE, Taylor AK, Pennington BF, et al. Molecular/clinical correlations in females with fragile X. Am J Med Genet. 1996;64:340–345. doi: 10.1002/(SICI)1096-8628(19960809)64:2<340::AID-AJMG21>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 40.Franke P, Leboyer M, Gansicke M, et al. Genotype-phenotype relationship in female carriers of the premutation and full mutation of FMR-1. Psychiatry Res. 1998;80:113–127. doi: 10.1016/s0165-1781(98)00055-9. [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez-Revenga L, Madrigal I, Alegret M, Santos M, Mila M. Evidence of depressive symptoms in fragile-X syndrome premutated females. Psychiatr Genet. 2008;18:153–155. doi: 10.1097/YPG.0b013e3282f97e0b. [DOI] [PubMed] [Google Scholar]
- 42.Brouwer JR, Severijnen E, de Jong FH, et al. Altered hypothalamus-pituitary-adrenal gland axis regulation in the expanded CGG-repeat mouse model for fragile X-associated tremor/ataxia syndrome. Psychoneuroendocrinology. 2008;33:863–873. doi: 10.1016/j.psyneuen.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berry-Kravis E, Potanos K, Weinberg D, Zhou L, Goetz CG. Fragile X-associated tremor/ataxia syndrome in sisters related to X-inactivation. Ann Neurol. 2005;57:144–147. doi: 10.1002/ana.20360. [DOI] [PubMed] [Google Scholar]
- 44.Jacquemont S, Orrico A, Galli L, et al. Spastic paraparesis, cerebellar ataxia, and intention tremor: a severe variant of FXTAS? J Med Genet. 2005;42:e14. doi: 10.1136/jmg.2004.024190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hagerman RJ, Leavitt BR, Farzin F, et al. Fragile-X-associated tremor/ataxia syndrome (FXTAS) in females with the FMR1 premutation. Am J Hum Genet. 2004;74:1051–1056. doi: 10.1086/420700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berry-Kravis E, Lewin F, Wuu J, et al. Tremor and ataxia in fragile X premutation carriers: blinded videotape study. Annals of Neurology. 2003;53:616–623. doi: 10.1002/ana.10522. [DOI] [PubMed] [Google Scholar]
- 47.Biancalana V, Toft M, Le Ber I, et al. FMR1 premutations associated with fragile X-associated tremor/ataxia syndrome in multiple system atrophy. Arch Neurol. 2005;62:962–966. doi: 10.1001/archneur.62.6.962. [DOI] [PubMed] [Google Scholar]
- 48.Zuhlke C, Budnik A, Gehlken U, et al. FMR1 premutation as a rare cause of late onset ataxia--evidence for FXTAS in female carriers. Journal of Neurology. 2004;251:1418–1419. doi: 10.1007/s00415-004-0558-1. [DOI] [PubMed] [Google Scholar]
- 49.Al-Hinti JT, Nagan N, Harik SI. Fragile X premutation in a woman with cognitive impairment, tremor, and history of premature ovarian failure. Alzheimer Dis Assoc Disord. 2007;21:262–264. doi: 10.1097/WAD.0b013e31811ec130. [DOI] [PubMed] [Google Scholar]
- 50.Karmon Y, Gadoth N. Fragile X associated tremor/ataxia syndrome (FXTAS) with dementia in a female harbouring FMR1 premutation. J Neurol Neurosurg Psychiatry. 2008;79:738–739. doi: 10.1136/jnnp.2007.139642. [DOI] [PubMed] [Google Scholar]
- 51.Adams JS, Adams PE, Nguyen D, et al. Volumetric brain changes in females with fragile X-associated tremor/ataxia syndrome (FXTAS) Neurology. 2007;69:851–859. doi: 10.1212/01.wnl.0000269781.10417.7b. [DOI] [PubMed] [Google Scholar]
- 52.Brunberg JA, Jacquemont S, Hagerman RJ, et al. Fragile X premutation carriers: characteristic MR imaging findings of adult male patients with progressive cerebellar and cognitive dysfunction. American Journal of Neuroradiology. 2002;23:1757–1766. [PMC free article] [PubMed] [Google Scholar]
- 53.Schrag A, Kingsley D, Phatouros C, et al. Clinical usefulness of magnetic resonance imaging in multiple system atrophy. J Neurol Neurosurg Psychiatry. 1998;65:65–71. doi: 10.1136/jnnp.65.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Storey E, Billimoria P. Increased T2 signal in the middle cerebellar peduncles on MRI is not specific for fragile X premutation syndrome. J Clin Neurosci. 2005;12:42–43. doi: 10.1016/j.jocn.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 55.Lee J, Lacomis D, Comu S, Jacobsohn J, Kanal E. Acquired hepatocerebral degeneration: MR and pathologic findings. AJNR Am J Neuroradiol. 1998;19:485–487. [PMC free article] [PubMed] [Google Scholar]
- 56.Cohen S, Masyn K, Adams J, et al. Molecular and imaging correlates of the fragile X-associated tremor/ataxia syndrome. Neurology. 2006;67:1426–1431. doi: 10.1212/01.wnl.0000239837.57475.3a. [DOI] [PubMed] [Google Scholar]
- 57.Moore CJ, Daly EM, Tassone F, et al. The effect of pre-mutation of X chromosome CGG trinucleotide repeats on brain anatomy. Brain. 2004;127:2672–2681. doi: 10.1093/brain/awh256. [DOI] [PubMed] [Google Scholar]
- 58.Loesch DZ, Litewka L, Brotchie P, Huggins RM, Tassone F, Cook M. Magnetic resonance imaging study in older fragile X premutation male carriers. Ann Neurol. 2005;58:326–330. doi: 10.1002/ana.20542. [DOI] [PubMed] [Google Scholar]
- 59.O'Dwyer JP, Clabby C, Crown J, Barton DE, Hutchinson M. Fragile X-associated tremor/ataxia syndrome presenting in a woman after chemotherapy. Neurology. 2005;65:331–332. doi: 10.1212/01.wnl.0000168865.36352.53. [DOI] [PubMed] [Google Scholar]
- 60.Hagerman RJ, Hall DA, Coffey S, et al. Treatment of fragile X-associated tremor ataxia syndrome (FXTAS) and related neurological problems. Clin Interv Aging. 2008;3:251–262. doi: 10.2147/cia.s1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perlman SL. Cerebellar Ataxia. Curr Treat Options Neurol. 2000;2:215–224. doi: 10.1007/s11940-000-0004-3. [DOI] [PubMed] [Google Scholar]
- 62.Botez MI, Botez-Marquard T, Elie R, Pedraza OL, Goyette K, Lalonde R. Amantadine hydrochloride treatment in heredodegenerative ataxias: a double blind study. J Neurol Neurosurg Psychiatry. 1996;61:259–264. doi: 10.1136/jnnp.61.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jacquemont S, Farzin F, Hall D, et al. Aging in individuals with the FMR1 mutation. Am J Ment Retard. 2004;109:154–164. doi: 10.1352/0895-8017(2004)109<154:AIIWTF>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zesiewicz TA, Sullivan KL. Treatment of ataxia and imbalance with varenicline (chantix): report of 2 patients with spinocerebellar ataxia (types 3 and 14) Clin Neuropharmacol. 2008;31:363–365. doi: 10.1097/WNF.0b013e31818736a9. [DOI] [PubMed] [Google Scholar]
- 65.Zesiewicz TA, Sullivan KL, Freeman A, Juncos JL. Treatment of imbalance with varenicline Chantix(R): report of a patient with fragile X tremor/ataxia syndrome. Acta Neurol Scand. 2009;119:135–138. doi: 10.1111/j.1600-0404.2008.01070.x. [DOI] [PubMed] [Google Scholar]
- 66.Hall DA, Berry-Kravis E, Hagerman RJ, Hagerman PJ, Rice CD, Leehey MA. Symptomatic treatment in the fragile X-associated tremor/ataxia syndrome. Mov Disord. 2006;21:1741–1744. doi: 10.1002/mds.21001. [DOI] [PubMed] [Google Scholar]
- 67.Wasielewski PG, Burns JM, Koller WC. Pharmacologic treatment of tremor. Mov Disord. 1998;13 3:90–100. doi: 10.1002/mds.870131316. [DOI] [PubMed] [Google Scholar]
- 68.Gunal DI, Afsar N, Bekiroglu N, Aktan S. New alternative agents in essential tremor therapy: double-blind placebo-controlled study of alprazolam and acetazolamide. Neurol Sci. 2000;21:315–317. doi: 10.1007/s100720070069. [DOI] [PubMed] [Google Scholar]
- 69.Zesiewicz TA, Elble R, Louis ED, et al. Practice parameter: therapies for essential tremor: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2005;64:2008–2020. doi: 10.1212/01.WNL.0000163769.28552.CD. [DOI] [PubMed] [Google Scholar]
- 70.Bodine C, J L, Lindstrum J, Meyer L. Effects of mouse tremor smoothing adapter on ease of computer mouse use by individuals with essential tremor. Universal Access in Human Computer Interaction. Coping with Diversity. 4th International Conference on Universal Access in Human-Computer Interaction, HCI International; 2007; Beijing, China. 2007. pp. 632–636. [Google Scholar]
- 71.Leehey MA, Munhoz RP, Lang AE, et al. The fragile X premutation presenting as essential tremor. Arch Neurol. 2003;60:117–121. doi: 10.1001/archneur.60.1.117. [DOI] [PubMed] [Google Scholar]
- 72.Aybek S, Vingerhoets FJ. Does deep brain stimulation of the subthalamic nucleus in Parkinson's disease affect cognition and behavior? Nat Clin Pract Neurol. 2007;3:70–71. doi: 10.1038/ncpneuro0379. [DOI] [PubMed] [Google Scholar]
- 73.Greco CM, Hagerman RJ, Tassone F, et al. Neuronal intranuclear inclusions in a new cerebellar tremor/ataxia syndrome among fragile X carriers. Brain. 2002;125:1760–1771. doi: 10.1093/brain/awf184. [DOI] [PubMed] [Google Scholar]
- 74.Zareba G. New treatment options in the management of fibromyalgia: role of pregabalin. Neuropsychiatr Dis Treat. 2008;4:1193–1201. doi: 10.2147/ndt.s3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hauser W, Thieme K, Turk DC. Guidelines on the management of fibromyalgia syndrome - A systematic review. Eur J Pain. 2009 doi: 10.1016/j.ejpain.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 76.Miyai I, Fujimoto Y, Yamamoto H, et al. Long-term effect of body weight-supported treadmill training in Parkinson's disease: a randomized controlled trial. Arch Phys Med Rehabil. 2002;83:1370–1373. doi: 10.1053/apmr.2002.34603. [DOI] [PubMed] [Google Scholar]
- 77.Protas EJ, Mitchell K, Williams A, Qureshy H, Caroline K, Lai EC. Gait and step training to reduce falls in Parkinson's disease. NeuroRehabilitation. 2005;20:183–190. [PubMed] [Google Scholar]
- 78.Wright RA, Kaufmann HC, Perera R, et al. A double-blind, dose-response study of midodrine in neurogenic orthostatic hypotension. Neurology. 1998;51:120–124. doi: 10.1212/wnl.51.1.120. [DOI] [PubMed] [Google Scholar]
- 79.Gales BJ, Gales MA. Phosphodiesterase-5 inhibitors for lower urinary tract symptoms in men. Ann Pharmacother. 2008;42:111–115. doi: 10.1345/aph.1K422. [DOI] [PubMed] [Google Scholar]
- 80.Sulkowski GM, Kaufman LM. Oculomotor abnormalities in a patient with fragile X-associated tremor/ataxia syndrome. J Aapos. 2008;12:195–196. doi: 10.1016/j.jaapos.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 81.Macpherson J, Waghorn A, Hammans S, Jacobs P. Observation of an excess of fragile-X premutations in a population of males referred with spinocerebellar ataxia. Hum Genet. 2003;112:619–620. doi: 10.1007/s00439-003-0939-z. [DOI] [PubMed] [Google Scholar]
- 82.Horvath J, Burkhard PR, Morris M, Bottani A, Moix I, Delavelle J. Expanding the phenotype of fragile X-associated tremor/ataxia syndrome: a new female case. Mov Disord. 2007;22:1677–1678. doi: 10.1002/mds.21571. [DOI] [PubMed] [Google Scholar]
- 83.Goncalves MR, Capelli LP, Nitrini R, et al. Atypical clinical course of FXTAS: rapidly progressive dementia as the major symptom. Neurology. 2007;68:1864–1866. doi: 10.1212/01.wnl.0000262058.68100.ea. [DOI] [PubMed] [Google Scholar]
- 84.Capelli LP, Goncalves MR, Kok F, et al. Fragile X-associated tremor/ataxia syndrome: intrafamilial variability and the size of the FMR1 premutation CGG repeat. Mov Disord. 2007;22:866–870. doi: 10.1002/mds.21347. [DOI] [PubMed] [Google Scholar]


