Abstract
Purpose
To investigate the impact of an intervention program to improve adherence with topical, once daily therapy for glaucoma.
Design
Randomized controlled clinical trial.
Participants
Sixty-six patients with glaucoma being treated with a prostaglandin analog in one or both eyes at the Scheie or Wilmer Eye Institutes between November 2006 and June 2007.
Methods
Participants in an observational study, who took 75% or fewer doses (as measured using the travoprost Dosing Aid [DA]) during an initial 3-month period, were randomized into two groups. The intervention group watched an educational video, reviewed current barriers to drop taking and possible solutions with a study coordinator, received regular phone call reminders, and had audible and visible reminders activated on their DA devices. The control group was told to take drops as prescribed and received no additional intervention.
Main Outcome Measures
Change in drop usage adherence as determined by the DA device.
Results
In the 3-month observation period prior to randomization, intervention group patients had used a mean of 54 ± 17% of scheduled doses and this increased to 73 ± 22% during the following 3-month period (p < 0.001, n = 35). The control mean adherence rate of 46 ± 23% at baseline was statistically unchanged during the follow up observation period (51 ± 30%, p = 0.16, n = 31). In a multivariate analysis, intervention, baseline compliance rate of less than 50%, and White ethnicity were predictors of improved adherence during the 3 months of intervention. The intraocular pressure (IOP) of intervention and control groups did not change between months 3 to 6 after intervention (p = 0.96, 0.34 respectively), and there was no correlation of IOP change with adherence rate change among both groups (Pearson Correlation r = 0.06, p = 0.51).
Conclusion
A multifaceted intervention significantly increased adherence with glaucoma medications. Those with improved adherence were in the intervention group, had very low adherence rates at baseline and were White. IOP did not correlate with adherence. Further research is needed to determine which components of this intervention were most effective.
Introduction
Adherence to chronic therapy in asymptomatic disease, both for systemic conditions1 and for glaucoma2,3 is less than ideal; repeated documentation has demonstrated that patients take 70% or less of prescribed treatment. multiple clinical trials have proven that the lowering of intraocular pressure (IOP) slows glaucoma progression. 4, 5 Alternatively, higher IOP is associated with greater incidence and prevalence of open angle glaucoma, as reported in the Barbados6 and the Baltimore Eye Surveys.7 Hence, the authors believe it is logical to assume that poor adherence would be associated with less benefit and worse outcome since IOP is elevated at times when adherence is poor. Thus, developing strategies to improve adherence is an important clinical goal.
Adherence with glaucoma eyedrops is suboptimal for many reasons including: situational and environmental factors (e.g., major life events, travel, competing activities, change in routine); medication regimen factors (e.g., refill, cost, complexity, change, adverse events); patient-related factors (e.g., knowledge, memory, motivation and health beliefs, comorbidity); and provider-related factors (e.g., satisfaction with and communication by physicians).8 Friedman et al.9 identified associations with adherence as measured using a large claims database among patients who also were extensively interviewed. Cost of medication and forgetting while away from home were clear risk factors for poor adherence. In addition, doctor-patient communication and health-related beliefs of patients contributed to patient adherence. Patients who were less concerned about the future effects of glaucoma and the risks of not taking medications had lower adherence. These findings suggest that educational efforts in the office, along with reminder systems, might improve adherence to glaucoma therapy.
There have been many different strategies tested, alone and in combination, to increase patient adherence to medical therapy for chronic conditions. McDonald and colleagues10 summarized the results of randomized controlled trials (RCTs) on interventions to enhance patient adherence to self-administered medication, finding that successful interventions included more instruction for patients, simplified dosing regimens, added reminders, increased convenience and accessibility to healthcare, provided rewards for improvement and counseling, and aimed to improve patient-provider communication. A more recent major review by Haynes and colleagues summarized RCTs for interventions to improve adherence in several chronic conditions, including hypertension, diabetes and asthma.11 Haynes reported that effective interventions for chronic conditions almost always addressed multiple potential barriers simultaneously. Many RCTs failed to demonstrate a benefit from intervention.
No RCTs studying adherence to glaucoma medications have been published using medications currently in wide use for glaucoma patients. In 1979, Norell12 randomized patients to receive an educational intervention to improve adherence among 82 patients taking pilocarpine eyedrops. Laster and colleagues13 reported a crossover trial in 13 glaucoma patients on pilocarpine using an electronic monitor with an audible reminder. Both studies showed significant improvement with a single intervention over short intervals. We used electronic monitoring of drop taking to assess a multifaceted program of interventions to improve adherence with topical, once daily glaucoma medication in a randomized, controlled clinical trial.
Methods
Study Design
Patients were recruited from the Glaucoma Services of the Wilmer Eye Institute and the Scheie Eye Institute. Institutional Review Boards at both centers approved the study protocol and written informed consent was obtained from all study patients.
The study had two phases. Phase one was a prospective, observational cohort study of patient adherence to travoprost therapy for a 3-month interval.14 Since no other bottles for glaucoma medications fit within the dosing aid (DA), it can provide data on use of travoprost only. Travoprost bottles were supplied to those already taking prostaglandin medication, or those newly prescribed this class of drug, and patients were instructed in using the dosing aid (DA) to administer the drops. The DA both squeezes the drop from the bottle and records the time and date of delivery on an internal, battery-operated chip. We have previously reported on the acceptable accuracy of this device for monitoring drop taking.15 Patients were aware that the devices recorded their drop taking.
In phase two, participants with 75% or fewer administered doses were randomized to either intervention or to usual care. The data used for this determination included values obtained during the 8 weeks starting 2 weeks after enrollment and ending 2 weeks before the follow-up visit. Only these data were used because we detected that there was significantly greater adherence just after a visit and just before a visit.14 A dose was considered taken if it the lever of the DA was depressed and recorded ± 4 hours from that patient's median dosing hour (as determined from the DA data). Since we recognized from our previous study15 that the device has the potential to make extra recordings when the lever is depressed erroneously, we did not count more than one dose taken per eye per day in our adherence rate calculation. When the lever was depressed outside the time window it was assumed that a dose was not taken, and when the lever was depressed multiple times in the time window only a single dose for one or both eyes was assumed to have been delivered.
The intervention consisted of: 1) a 10-minute educational video created through Alcon, Inc. marketing branch for the DA device, that stressed the importance of regular drop-taking, its rationale and expected effects, alternatives to eyedrops, and methods to maximize cooperation, such as linking drops to a daily activity, keeping a drop-taking calendar diary, and using family members to help in reminding them; 2) a structured discussion with the study coordinator in order to develop a strategy for improving adherence that included finding the best time of day to take the medication, distributing a blank calendar diary and going over details of how to keep it, and discussing individual patient barriers to taking the medication; 3) reminder telephone calls from the coordinator, including administration of a questionnaire about drop-taking behavior, difficulty with drops, side-effects, and eliciting questions about therapy. This call was made once a week for the first follow-up month and then every other week for the next two months; and 4) activation of the audible and visible alarms on the DA. Those in the usual care arm (“controls”) were told that it is important to take their eyedrops as prescribed, but had no other intervention. Participants were randomized using random numbers placed in serially marked, sealed envelopes that were opened at the time of randomization. To perform the randomization procedure, a string of random numbers was selected from a random numbers table. The numbers were placed into envelopes and then sealed and initialed across the seal. The envelopes were numbered consecutively starting with 1. When an eligible patient was identified, an envelope was opened; if the envelope contained an even number then the participant received the intervention.
The target sample size was calculated assuming a mean adherence rate at 75% before intervention. In order to have 80% power to identify the intervention compliance rate improvement of 20% with a Type 1 error of 5%, the target sample size was 49 persons per arm to complete the three months of the intervention.
Eligibility Criteria (both phases)
Patients had one of the following diagnoses: open angle glaucoma, angle-closure glaucoma, glaucoma suspect, or ocular hypertension. Patients were 18 years of age or older, were using or were prescribed a topical prostaglandin analog, and were able to return for 3 and 6-month follow-up visits. Some participants had undergone past laser or surgical glaucoma therapy, but not within the 3 months prior to study enrollment. Patients were excluded if they were unable to understand the study, if they did not instill their own drops, or if they were incapable of using the DA after a brief demonstration.
Patient Recruitment and Follow-Up
Consenting patients were given sufficient travoprost for the study free of charge and were instructed in using the DA by a study coordinator using an instructional video. All patients were instructed on how to place a bottle of travoprost in the DA and how to depress the lever arm to deliver a drop. Patients practiced using the DAs under supervision prior to starting the study. Each patient received one DA device and was instructed to administer the drops in either one or both eyes, depending on his or her ocular diagnosis. Patients were enrolled on days when the study coordinator was available in the clinic.
Patients who were receiving latanoprost or bimatoprost prior to the study were switched to travoprost. Baseline demographic and medical information was obtained, including age, sex, self-reported ethnicity, home address zip code (to estimate income), presence of co-morbid diseases, ocular medications and dosage, systemic medications and dosage, family history of glaucoma, baseline untreated IOP of each eye (if available), length of past glaucoma treatment and types of past ocular medication, including allergies and severe adverse events, and current target IOP of each eye. In addition, data of medications for each eye for the preceding two years, the most recent visual fields and most recent evaluation of the optic disc by clinical assessment, laser imaging, or photography were also recorded.
Phase one patients were instructed to use the devices to deliver their travoprost each night until the 3-month follow-up visit. Patients brought their DA devices to the 3-month visit, the information was downloaded onto computer-based software, the battery was changed, and a questionnaire was administered to evaluate self-reported adherence and satisfaction with the devices. At the 3-month visit, eligible patients for phase two were randomized, as outlined above. Also, visual acuity and applanation IOP were measured.
At the 6-month visit, the data from the devices were downloaded, and the intervention and control groups answered a questionnaire about adherence to therapy, side-effects, and any change in satisfaction with the devices. Visual acuity and IOP were recorded at the 6-month visit.
Statistical Analysis
Baseline characteristics were compared between the intervention group and the control group. Comparisons for patient-level characteristics were made by Fisher's exact test for the comparison of proportions and by the Student's t test for the comparison of means. The identification of factors for improved adherence was performed using univariate linear regression models of adherence rate as a continuous variable. The factors associated with p-value < 0.10 (treatment group, length of time on glaucoma medication, bilateral use of medication, and Institute) or factors of clinical importance (age, race, education, baseline compliance rate, use of travoprost without using the device) were included in the multivariate models. Institute was initially considered, but was eventually excluded from the final multivariate model because of its collinearity with the race and education, and the complex interpretation of the resulting results. Patients with missing data in one specific variable were excluded from the analysis of this specific variable, but were still included in the analysis of other variables without missing data. Statistical analysis was performed by using SAS v9.1 (SAS Institute, Inc, Cary, NC).
Results
Study recruitment for the phase two randomized trial began in November, 2006 and ended June, 2007. Of the 66 persons who were adherent < 75% of the time, 35 patients (53%) were randomized to the intervention group and 31 (47%) were randomized to the control group. The intervention and control groups were similar in mean age, race, sex, education level and income based on zip code (p > 0.05 for all, Table 1). The two groups also had generally similar ocular characteristics, though the controls were significantly more likely to have used glaucoma drops for one year or less (p < 0.01, Table 2).
Table 1. Baseline Demographic Characteristics of Intervention and Control Groups.
| Baseline Characteristics | Intervention (N=35) n (%) |
Control (N=31) n (%) |
P-value |
|---|---|---|---|
| Age (yrs) | 0.45* | ||
| <50 | 3 (8.57) | 4 (12.9) | |
| 50 -59 | 5 (14.3) | 7 (22.6) | |
| 60-69 | 13 (37.1) | 8 (25.8) | |
| 70-79 | 8 (22.9) | 10 32.3) | |
| >=80 | 6 (17.1) | 2 (6.45) | |
| Mean ± SD | 66.2 ± 13.1 | 63.8 ± 13.4 | 0.70§ |
| Gender | 0.59* | ||
| Female | 17 (48.6) | 13 (41.9) | |
| Male | 18 (51.4) | 18 (58.1) | |
| Race | 0.43* | ||
| Black | 23 (65.7) | 17 (54.8) | |
| White | 12 (34.3) | 13 (41.9) | |
| Asian | 0 (0.00) | 1 (3.23) | |
| Education | 0.06* | ||
| < High School | 4 (11.4) | 5 (16.7) | |
| High School | 6 (17.1) | 10 (33.3) | |
| College | 18 (51.4) | 6 (20.0) | |
| Graduate School | 6 (17.1) | 9 (30.0) | |
| Unknown | 1 (2.86) | 1 (3.13) | |
| General Health | 0.91* | ||
| Excellent | 7 (20.0) | 5 (16.1) | |
| Good | 22 (62.9) | 20 (64.5) | |
| Fair/poor | 6 (17.1) | 6 (19.4) | |
| Depression score | 0.70* | ||
| <=0.1 | 11 (31.4) | 11 (35.5) | |
| (0.1, 0.3] | 7 (20.0) | 9 (29.0) | |
| (0.3, 0.7] | 9 (25.7) | 5 (16.1) | |
| (0.7, 2.5] | 8 (22.9) | 6 (19.4) | |
| Mean (SD) | 0.47 ± 0.46 | 0.42 ± 0.54 | 0.65§ |
| Family income based on zip code | 0.06* | ||
| <=35 K | 12 (34.3) | 8 (25.8) | |
| (35, 50K] | 8 (22.9) | 5 (16.1) | |
| (50, 75K] | 4 (11.4) | 12 (38.7) | |
| >75K | 11 (31.4) | 5 (16.1) | |
| Unknown | 1 (3.23) | ||
| Glaucoma family history | 0.11* | ||
| None | 16 (45.7) | 18 (58.1) | |
| 1 | 17 (48.6) | 8 (25.8) | |
| ≥ 2 | 2 (5.71) | 5 (16.1) |
SD = standard deviation; yrs = years; K=thousand dollars.
Fisher exact test for the comparison of proportions between intervention and non-adherent control groups.
t test for the comparison of means between intervention and non-adherent control groups.
Note: Unknowns are excluded from the calculation of p-value.
Table 2. Baseline Ocular Characteristics of Intervention and Control Groups.
| Ocular Characteristics | Intervention (N=35) n (%) |
Control (N=31) n (%) |
P-value* |
|---|---|---|---|
| Cup Disk ratio of worse eye | 0.11 | ||
| <=0.7 | 7 (20.0) | 13 (41.9) | |
| (0.7, 0.8] | 11 (31.4) | 9 (26.0) | |
| (0.8, 0.9] | 11 (31.4) | 7 (20.0) | |
| >0.9 | 6 (17.1) | 1 (3.23) | |
| Unknown | 1 (3.23) | ||
| Mean Deviation of worse eye | 0.27 | ||
| <=5 db | 14 (40.0) | 14 (45.2) | |
| (5, 15] db | 5 (14.3) | 8 (25.8) | |
| >15 db | 15 (42.9) | 8 (25.8) | |
| Unknown | 1 (2.86) | 1 (3.23) | |
| IOP of worse eye | 0.14 | ||
| <=15 mmHg | 10 (28.6) | 11 (35.5) | |
| (15, 17] mmHg | 10 (28.6) | 2 (6.45) | |
| (17, 20] mmHg | 5 (14.3) | 7 (22.6) | |
| >20 mmHg | 10 (28.6) | 11 (35.5) | |
| Length of time on glaucoma medication | 0.01 | ||
| <= 1 year | 2 (5.71) | 9 (29.0) | |
| > 1 year | 33 (94.3) | 22 (71.0) | |
| Use of medicine | 0.76 | ||
| Unilateral | 9 (25.7) | 9 (29.0) | |
| Bilateral | 26 (74.3) | 22 (71.0) | |
| Use of other glaucoma medications | 0.11 | ||
| Only on travoprost | 14 (40.0) | 15 (48.4) | |
| On a second agent | 12 (34.3) | 14 (45.2) | |
| On three or more agents | 9 (25.7) | 2 (6.45) | |
| Institute | 0.39 | ||
| JHU | 28 (80.0) | 22 (71.0) | |
| PENN | 7 (20.0) | 9 (29.0) |
db=decibels; IOP= intraocular pressure; mmHg= millimeters of mercury; JHU = Wilmer Eye Institute; PENN = Scheie Eye Institute
Fisher exact test for the comparison of proportions between intervention and non-adherent control groups.
Note: Unknowns are excluded from the calculation of p-value.
Adherence to Therapy
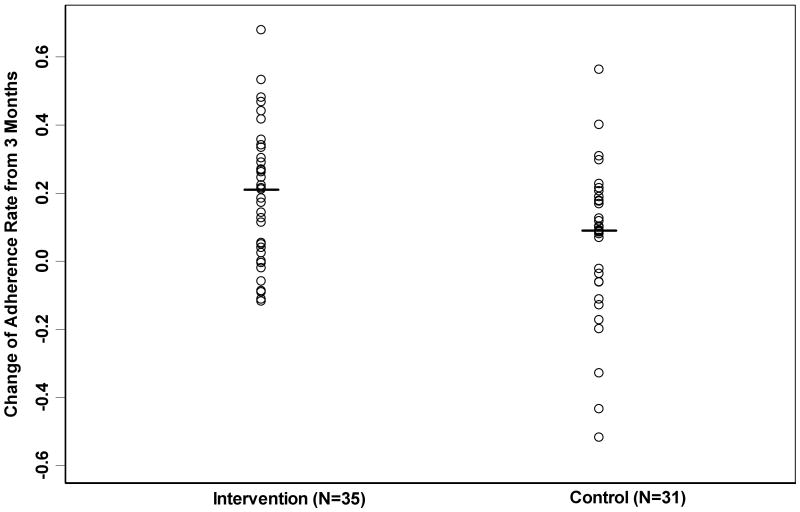
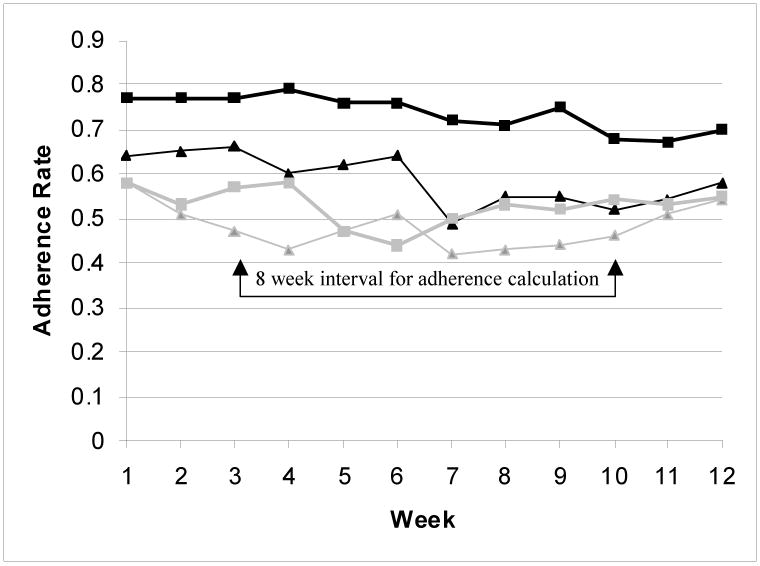
The baseline mean adherence in the intervention group was higher than in the control group (54% versus 46%) but this was not statistically significant (p = 0.10). The mean (± SD) adherence rate for the intervention group improved to 73 ± 22% after intervention in phase two (p < 0.01, Table 3). By contrast, the control group had no significant change, with 51 ± 30% in phase two (p = 0.19, Table 3). The mean adherence rate improvement was 19 ± 20% in intervention group, and 6% ± 23% in control group (p=0.01 for the difference between the two groups, Figure 1). The mean adherence was higher for every week of follow up in the intervention group compared to the controls (p < 0.01, Figure 2).
Table 3. The Adherence Rate by Randomization Group of Intervention at 3 and 6 Months (N=66).
| Intervention (n=35) | Control (n=31) | P-value† | |
|---|---|---|---|
| 3 months before intervention | |||
| Mean (SD) | 0.54 (0.17) | 0.46 (0.23) | 0.10 |
| Median (Min - Max) | 0.60 (0.06 – 0.74) | 0.53 (0.03 – 0.75) | |
| 3 months after intervention | |||
| Mean (SD) | 0.73 (0.22) | 0.51 (0.30) | 0.001 |
| Median (Min - Max) | 0.82 (0.13 – 0.97) | 0.52 (0.04– 0.95) | |
| Change between 3 and 6 months | |||
| Mean (SD) | 0.19 (0.20) | 0.06 (0.23) | 0.01 |
| Median (Min - Max) | 0.21 (-0.12 – 0.68) | 0.09 (-0.52 – 0.56) | |
| P-value* | <0.0001 | 0.19 |
SD = standard deviation
For the test whether the change in compliance rate is different from 0, using paired t-test.
From two group t-test for the comparison of means between intervention and non-adherent control groups.
Figure 1.
Scatterplot demonstrating distribution of change of adherence rate from 3 months in the non-adherent control group and intervention group. Circles indicate adherence rate change for each individual patient; lines indicate the median change of adherence rate in each group.
Figure 2.
Line graph demonstrating comparison of adherence rate by group at 0-3 (phase 1 prior to intervention) and 3-6 months (phase 2 after the intervention). From bottom to top: Gray thin line with triangles indicates the mean adherence rate for the non-adherent control group over 12 weeks during phase one from 0 to 3 months. Gray heavy line with squares indicates the mean adherence rate for the non-adherent control group over 12 weeks during phase two from 3 to 6 months. Black thin line with triangles indicates the mean adherence rate for the intervention group over 12 weeks during phase one from 0-3 months. Black heavy line with squares indicates the mean adherence rate for the intervention group over 12 weeks during phase two from 3-6 months.
The distribution of change in adherence for all 66 patients ranged from an absolute decrease in compliance of 52% to an increase of 68% (Figure 1). In the intervention group, the adherence rate improved by at least 10% in 66% of patients (n = 23/35), a significantly higher proportion than the 45% (n=14/31) in the control group (p = 0.05).
Factors Associated with Improved Adherence
In a univariate analysis, factors associated with an improved adherence rate included intervention group (p=0.01), bilateral use of medicine (p=0.04), and institution (p=0.03) (Table 4). Patient's attitudes and knowledge of glaucoma and their self-reported use of topical ocular hypotensive agents were not associated with improved adherence in univariate analysis (data not shown). In multivariate analysis that included the treatment, length of time on glaucoma medication, bilateral use of medication, age, race, education, baseline compliance rate and use of travoprost without using the devise as predictors, with adherence rate change as a continuous variable, the factors that were significantly associated with improved adherence were intervention (p < 0.01), having a low baseline adherence rate (< 50%, p < 0.01), and being White (p = 0.02, Table 5). African-Americans were less likely to have any improvement in adherence rate (60%) compared with Whites (88%, p=0.02).
Table 4. Univariate Analysis of Factors Associated with Change of Adherence Rate between 3 and 6 months for Intervention and Control Groups (N=66).
| Factors | N | Adherence rate change from 3 months Mean (SE) | P-value§ |
|---|---|---|---|
| Age (yrs) | 0.46 | ||
| <50 | 7 | 0.15 (0.08) | |
| 50 -59 | 12 | 0.08 (0.06) | |
| 60-69 | 21 | 0.19 (0.05) | |
| 70-79 | 18 | 0.11 (0.05) | |
| >=80 | 8 | 0.05 (0.08) | |
| Sex | 0.61 | ||
| Female | 30 | 0.14 (0.04) | |
| Male | 36 | 0.12 (0.04) | |
| Race | 0.32 | ||
| Black | 40 | 0.10 (0.03) | |
| White | 25 | 0.18 (0.04) | |
| Asian | 1 | 0.18 (0.22) | |
| Education | 0.67 | ||
| < High School | 9 | 0.09 (0.07) | |
| High School | 16 | 0.16 (0.06) | |
| College | 24 | 0.11 (0.05) | |
| Graduate School | 15 | 0.18 (0.06) | |
| General Health | 0.99 | ||
| Excellent | 12 | 0.13 (0.06) | |
| Good | 42 | 0.13 (0.03) | |
| Fair/poor | 12 | 0.13 (0.06) | |
| Depression score | 0.10 | ||
| <=0.1 | 22 | 0.13 (0.05) | |
| (0.1, 0.3] | 16 | 0.02 (0.05) | |
| (0.3, 0.7] | 14 | 0.18 (0.06) | |
| (0.7, 2.5] | 14 | 0.20 (0.06) | |
| Family income based on zip code | 0.11 | ||
| <=35 K | 20 | 0.07 (0.05) | |
| (35, 50]K | 13 | 0.16 (0.06) | |
| (50, 75]K | 16 | 0.07 (0.05) | |
| >75K | 16 | 0.23 (0.05) | |
| Glaucoma family history | 0.68 | ||
| None | 34 | 0.13 (0.04) | |
| 1 | 25 | 0.15 (0.04) | |
| ≥ 2 | 7 | 0.06 (0.08) | |
| Cup Disk ratio of worse eye | 0.42 | ||
| <=0.7 | 20 | 0.06 (0.05) | |
| (0.7, 0.8] | 20 | 0.18 (0.05) | |
| (0.8, 0.9] | 18 | 0.13 (0.05) | |
| >0.9 | 7 | 0.14 (0.08) | |
| Mean Deviation of worse eye | 0.25 | ||
| <=5 db | 28 | 0.12 (0.04) | |
| (5, 15] db | 13 | 0.22 (0.06) | |
| >15 db | 23 | 0.09 (0.05) | |
| IOP of worse eye | 0.32 | ||
| <=15 mmHg | 21 | 0.17 (0.05) | |
| (15, 17] mmHg | 12 | 0.18 (0.06) | |
| (17, 20] mmHg | 12 | 0.04 (0.06) | |
| >20 mmHg | 21 | 0.11 (0.05) | |
| Length of time on glaucoma medication | 0.08 | ||
| <= 1 year | 11 | 0.02 (0.07) | |
| > 1 year | 55 | 0.15 (0.03) | |
| Use of medicine | 0.04 | ||
| Unilateral | 18 | 0.04 (0.05) | |
| Bilateral | 48 | 0.16 (0.03) | |
| Use of other glaucoma medications | 0.20 | ||
| Only on travoprost | 29 | 0.08 (0.04) | |
| On a second agent | 26 | 0.18 (0.04) | |
| On three or more agents | 11 | 0.13 (0.07) | |
| Institute | 0.03 | ||
| JHU | 50 | 0.16 (0.03) | |
| PENN | 16 | 0.02 (0.05) | |
| Compliance rate at 3 months | 0.27 | ||
| <=0.50 | 28 | 0.16 (0.04) | |
| 0.501-0.75 | 38 | 0.10 (0.04) | |
| Treatment Group | 0.01 | ||
| Control | 31 | 0.06 (0.04) | |
| Intervention | 35 | 0.19 (0.04) |
SE= standard error; SD = standard deviation; yrs = years; K=thousand dollars. db=decibels; IOP= intraocular pressure; mmHg= millimeters of mercury; JHU = Wilmer Eye Institute; PENN = Scheie Eye Institute
From one-way analysis of variance.
Adjusted by treatment group assignment.
Table 5. Multivariate Analysis for Factors Associated with Change of Adherence Rate between 3 and 6 Months for Randomized Intervention and Control Groups (N=62*).
| Factors | N | Adherence rate change from 3 months Adjusted Mean (SE) | P-value§ |
|---|---|---|---|
| Treatment Group | 0.0001 | ||
| Control | 28 | -0.002 (0.04) | |
| Intervention | 34 | 0.21 (0.05) | |
| Adherence rate at Month 3 | 0.003 | ||
| <=0.50 | 26 | 0.18 (0.04) | |
| 0.501-0.75 | 36 | 0.03 (0.05) | |
| Race | 0.02 | ||
| Black | 37 | 0.04 (0.04) | |
| White | 25 | 0.17 (0.05) | |
| Education | 0.051 | ||
| < High School | 8 | 0.09 (0.07) | |
| High School | 16 | 0.18 (0.05) | |
| College | 24 | 0.01 (0.05) | |
| Graduate School | 14 | 0.14 (0.06) | |
| I have used travoprost without using the device during the study | 0.12 | ||
| Never | 43 | 0.14 (0.04) | |
| Ever | 19 | 0.06 (0.05) | |
| Use of medicine | 0.16 | ||
| Unilateral | 17 | 0.04 (0.05) | |
| Bilateral | 45 | 0.16 (0.03) | |
| Age (yrs) | 0.65 | ||
| <50 | 7 | 0.11 (0.08) | |
| 50 -59 | 11 | 0.07 (0.06) | |
| 60-69 | 21 | 0.16 (0.05) | |
| 70-79 | 16 | 0.13 (0.05) | |
| >=80 | 7 | 0.05 (0.07) | |
| Length of time on glaucoma medication | 0.67 | ||
| <= 1 year | 9 | 0.09 (0.07) | |
| > 1 year | 53 | 0.12 (0.03) |
3 patients were excluded from the multivariate analysis due to missing value in education status (n=2) and use of travoprost without using the device during the study (n=1), Asian was also excluded due to small number (n=1).
SE = standard error; yrs = years
From one-way analysis of variance
Note: All the independent variables included in the multivariate model are listed above.
IOP and Adherence Patterns
The mean IOP did not change significantly from baseline to the end of phase one at 3 months, nor was it significantly different between months 3 to 6 after intervention, whether all patients were considered together or were split into study groups (p = 0.81). Likewise, there was no correlation between IOP change from phase one to phase two and the adherence rate change for all study eyes taken together (n = 114 eyes, Pearson Correlation r = 0.06, p = 0.51).
Intervention Assessment
For the 35 patients randomized to the intervention group, telephone calls were made at weeks 1-5, 7, 9 and 11. The number of patients contacted was highest at week 1 (100%) and over the remaining weeks there was a decline in the number successfully contacted (week 11, 63%). Reasons for the decline included early drop out from study, inability to contact patients, and an early final visit. During weeks 6, 8, 10 and 12 the intervention patients were not called and this did not appear to adversely affect the adherence rate for the group.
Discussion
We found that a multifaceted program for enhancing glaucoma eyedrop usage improved the adherence rate from 54% to 73% (p < 0.001) in persons whose baseline drop-taking was less than 75%. The intervention was administered completely by study staff, and did not include physician input with the patient. The intervention was designed to maximize the chance that the adherence with medication use would improve. Our findings suggest that using several approaches at once likely did increase the probability that the interventions changed eyedrop use. While the strategy used in this trial clearly was effective, we cannot determine which aspects of the intervention were most valuable and which individual elements can pragmatically be implemented in clinical practice. We did not record the actual time required for the video and structured interview or determine costs for implementation of the intervention. However, our demonstration that adherence can be improved should stimulate further research into the individual components of our intervention.
While better adherence should produce lower IOP in general, improvement in adherence was not matched by lower IOP levels as measured in the clinic. This was not surprising, since we had only 3 IOP measurements, one at each study visit—compared to daily values for adherence. In addition, our phase one data14 showed that poorly adherent patients increase drop taking during the two weeks prior to the office visit. Hence, IOP taken during the office visit was an inadequate surrogate for estimating adherence. These findings are not unique to ophthalmology. Studies of interventions in patients with hypertension and asthma also have found improved adherence, but not necessarily improved clinical measurements at the time of office visits.10,11
We found previously that physicians have used the IOP level as an important measure of poor adherence.9 A patient who is failing to achieve the IOP target needs either a change in medication or an improvement in adherence. But, the current findings clearly showed that many non-adherent patients had satisfactory IOP at routine visits. Thus, better tools are needed to distinguish poor adherence from poor efficacy in patients not at target in order to avoid overmedication (or over-prescription with continued poor adherence to multiple drugs).
It is likely that educational efforts to improve patient drop taking played an important role in improving patient adherence in the intervention arm. These included instruction on proper administration of eye drops, correct dosing schedules, minimization of waste of medication, and a clear discussion that vision can be lost if the medications are not used properly. Further research on the most effective methods to communicate with patients, through better physician communication, educational programs administered by office staff, video presentations, or combinations of these, is needed.
We showed that the effect of education and reminder systems could be sustained for at least 3 months. Norell12 found a significant decrease in adherence with pilocarpine drops over the interval between visits when the education effort occurred only in the office. Laster's use of a device alarm showed a more continuous effect over the interval in between office visits.13 With the availability of cell phones and internet communication, there are several potential avenues that deserve exploration to improve adherence using continuous reminder systems. 16,17,18
Past studies have shown that the cost of medication and access to care are significant barriers to adherence.19,20 Our study eliminated both of these obstacles by providing free medication and by assuring minimal loss to follow-up among persons already able to access care. The authors speculate that adherence would be even lower among patients for whom these barriers remain in place.
Among our patients, there were 3 factors associated with greater improvement in adherence in univariate analysis: intervention, bilateral use of medicine, and attendance at the Wilmer Eye Institute Glaucoma service. Among these 3 factors, the multivariate analysis showed that only the intervention remained significantly associated with improved adherence, while institution and bilateral use of medicine were no longer significant and ethnicity and extremely low baseline adherence became significant. There may be substantial correlation among these variables. For example, nearly all our Scheie Institute patients were African-derived, while the majority of Wilmer patients were White. Patients from Wilmer had taken drops longer than those at Scheie. Other factors that may play a role in associations between ethnicity and adherence include patient-physician interaction,21 perceived personal dissimilarity of the patient with the doctor,22 and having had experiences with discrimination23 may also contribute to a patient's decreased intent to adhere. Further research is needed to understand more clearly what factors led African-American patients to have both lower baseline adherence14 and lower improvement in adherence with intervention. It is possible that interventions for adherence must be tailored to the beliefs and situation of major ethnic groups.
A baseline adherence rate < 50% was associated with improved adherence. It is possible that this finding is in part due to regression to the mean. However, we previously found an association between less knowledge about glaucoma treatment and low adherence(Friedman DS. Risk factors for poor adherence with eyedrops in electronically monitored glaucoma patients. Poster presented at American Glaucoma Society, March 2008, Washington, D.C.) This has been demonstrated in Korean hypertension patients whose adherence was higher in those more informed about the disease.24 It is logical that our educational efforts about the disease in the intervention eliminated some of the lack of adherence due to this factor.
Our study had some limitations. Though we used a standard randomization process, the intervention group had somewhat more veteran eyedrop takers. This could have increased the magnitude of the intervention effect, since our univariate analysis showed lower adherence among less experienced drop takers. The adherence rate of the controls rose slightly, which was most likely due to regression to the mean. This effect was small in comparison to the treatment effect in the intervention group, but if we assume the intervention group would have had a similar rise, the treatment effect is likely smaller than measured. We informed patients that they were being monitored, and provided drugs at no cost. It is likely that the adherence of patients who are not in a study under these conditions would be lower at baseline, and perhaps might exhibit a different intervention effect.
We used an electronic device to measure adherence as the primary outcome variable. Electronic monitoring of drug-taking behavior is the most accurate method for identifying nonadherence.14,25,26 Research with the DA has limitations, however, as shown by patients in this study who took their drops without placing the bottle in the devices, which in fact lowered the measured adherence rate in the intervention group, but not significantly. When excluding those who took drops without the DA, the measured adherence improved slightly in the control group, but still the difference in magnitude of improved adherence between the intervention and control groups remained large. In addition, the findings here were limited to the use of one prostaglandin analogue, since only its bottle fits in the device.
In conclusion, adherence with glaucoma drop usage improved over a 3-month period with an intervention strategy consisting of education and reminder systems. Additionally, improvement was immediate and sustained over 3 months. There was greater improvement in adherence among those with the lowest baseline adherence and among White patients. IOP was a poor surrogate for monitoring adherence, probably due to increased adherence just before the visit. Further research is needed to determine which components of this intervention were most effective.
Acknowledgments
This research was supported in part by the National Institutes of Health, Bethesda, Maryland (Clinician—Scientist Training award K12 EY015398 [Dr Okeke]), by a grant from The Paul & Evanina Bell Mackall Foundation Trust, New York, NY (Drs. Okeke and Ying), by the Glaucoma Division, Wilmer Institute research program, Baltimore, MD, and by unrestricted funds and material support from Alcon, Inc., Ft. Worth, Texas.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rudd P, Ahmed S, Zachary V, et al. Improved compliance measures: applications in an ambulatory hypertensive drug trial. Clin Pharmacol Ther. 1990;48:676–85. doi: 10.1038/clpt.1990.211. [DOI] [PubMed] [Google Scholar]
- 2.Gordon ME, Kass MA. Validity of standard compliance measures in glaucoma compared with an electronic eye drop monitor. In: Cramer JA, Spiker B, editors. Patient Compliance in Medical Practice and Clinical Trials. New York: Raven Press; 1991. pp. 163–73. [Google Scholar]
- 3.Kass MA, Meltzer DW, Gordon M, et al. Compliance with topical pilocarpine treatment. Am J Ophthalmol. 1986;101:515–23. doi: 10.1016/0002-9394(86)90939-6. [DOI] [PubMed] [Google Scholar]
- 4.Leske MC, Heijl A, Hussein M, et al. Early Manifest Glaucoma Trial Group. Factors for glaucoma progression and the effect of treatment: the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2003;121:48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- 5.Musch DC, Gillespie BW, Lichter PR, et al. CIGTS Study Investigators. Visual field progression in the Collaborative Initial Glaucoma Treatment Study: the impact of treatment and other baseline factors. Ophthalmology. 2009;116:200–7. doi: 10.1016/j.ophtha.2008.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leske MC, Wu SY, Hyman L, et al. Barbados Eye Studies Group. Four-year incidence of visual impairment: Barbados Incidence Study of Eye Diseases. Ophthalmology. 2004;111:118–24. doi: 10.1016/j.ophtha.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Sommer A, Tielsch JM, Katz J, et al. Baltimore Eye Survey Research Group. Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans: the Baltimore Eye Survey. Arch Ophthalmol. 1991;109:1090–5. doi: 10.1001/archopht.1991.01080080050026. [DOI] [PubMed] [Google Scholar]
- 8.Tsai JC, McClure CA, Ramos SE, et al. Compliance barriers in glaucoma: a systematic classification. J Glaucoma. 2003;12:393–8. doi: 10.1097/00061198-200310000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Friedman DS, Hahn SR, Gelb L, et al. Doctor-patient communication, health-related beliefs, and adherence in glaucoma: results from the Glaucoma Adherence and Persistency Study. Ophthalmology. 2008;115:1320–7. doi: 10.1016/j.ophtha.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 10.McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: scientific review. JAMA. 2002;288:2868–79. doi: 10.1001/jama.288.22.2868. [DOI] [PubMed] [Google Scholar]
- 11.Haynes RB, Ackloo E, Sahota N, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;(2) doi: 10.1002/14651858.CD000011.pub3. CD000011. [DOI] [PubMed] [Google Scholar]
- 12.Norell SE. Improving medication compliance: a randomised clinical trial. Br Med J. 1979;2:1031–3. doi: 10.1136/bmj.2.6197.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laster SF, Martin JL, Fleming JB. The effect of a medication alarm device on patient compliance with topical pilocarpine. J Am Optom Assoc. 1996;67:654–8. [PubMed] [Google Scholar]
- 14.Okeke CO, Quigley HA, Jampel HD, et al. Adherence with topical glaucoma medication monitored electronically: the Travatan Dosing AidStudy. Ophthalmology. 2009;116:191–9. doi: 10.1016/j.ophtha.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Friedman DS, Jampel HD, Congdon NG, et al. The TRAVATAN Dosing Aid accurately records when drops are taken. Am J Ophthalmol. 2007;143:699–701. doi: 10.1016/j.ajo.2006.11.036. [DOI] [PubMed] [Google Scholar]
- 16.Downer SR, Meara JG, Da Costa AC, et al. SMS text messaging improves outpatient attendance. Aust Health Rev. 2006;30:389–96. doi: 10.1071/ah060389. [DOI] [PubMed] [Google Scholar]
- 17.Chen ZW, Fang LZ, Chen LY, Dai HL. Comparison of an SMS text messaging and phone reminder to improve attendance at a health promotion center: a randomized controlled trial. J Zhejiang Univ Sci B. 2008;9:34–8. doi: 10.1631/jzus.B071464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koshy E, Car J, Majeed A. Effectiveness of mobile-phone short message service (SMS) reminders for ophthalmology outpatient appointments: observational study. [April 7, 2009];BMC Ophthalmol. 2008 :8–9. doi: 10.1186/1471-2415-8-9. serial online. Available at: http://www.biomedcentral.com/1471-2415/8/9. [DOI] [PMC free article] [PubMed]
- 19.Kripalani S, Henderson LE, Jacobson TA, Vaccarino V. Medication use among inner-city patients after hospital discharge: patient-reported barriers and solutions. Mayo Clin Proc. 2008;83:529–35. doi: 10.4065/83.5.529. [DOI] [PubMed] [Google Scholar]
- 20.Bender BG, Bender SE. Patient-identified barriers to asthma treatment adherence: responses to interviews, focus groups, and questionnaires. Immunol Allergy Clin North Am. 2005;25:107–30. doi: 10.1016/j.iac.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Saha S, Arbelaez JJ, Cooper LA. Patient-physician relationships and racial disparities in the quality of health care. Am J Public Health. 2003;93:1713–9. doi: 10.2105/ajph.93.10.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Street RL, Jr, O'Malley KJ, Cooper LA, Haidet P. Understanding concordance in patient-physician relationships: personal and ethnic dimensions of shared identity. Ann Fam Med. 2008;6:198–205. doi: 10.1370/afm.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Casagrande SS, Gary TL, LaVeist TA, et al. Perceived discrimination and adherence to medical care in a racially integrated community. J Gen Intern Med. 2007;22:389–95. doi: 10.1007/s11606-006-0057-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim EY, Han HR, Jeong S, et al. Does knowledge matter?: intentional medication nonadherence among middle-aged Korean Americans with high blood pressure. J Cardiovasc Nurs. 2007;22:397–404. doi: 10.1097/01.JCN.0000287038.23186.bd. [DOI] [PubMed] [Google Scholar]
- 25.Kass MA, Gordon M, Meltzer DW. Can ophthalmologists correctly identify patients defaulting from pilocarpine therapy? Am J Ophthalmol. 1986;101:524–30. doi: 10.1016/0002-9394(86)90940-2. [DOI] [PubMed] [Google Scholar]
- 26.Norell SE. Accuracy of patient interviews and estimates by clinical staff in determining medication compliance. Soc Sci Med [E] 1981;15:57–61. doi: 10.1016/0271-5384(81)90063-6. [DOI] [PubMed] [Google Scholar]