Abstract
Objective
Stress and depressive symptoms predict exaggerated inflammatory responses to a biological challenge in nonpregnant humans and animals. The extent to which these findings generalize to pregnancy is unknown because the immune system exhibits substantial changes to support pregnancy. Notably, inflammatory responses to infectious agents play a causal role in the development of gestational hypertension as well as risk for preterm birth. Thus, depressive symptoms may increase susceptibility to these outcomes via sensitization of inflammatory processes. The current study was designed to test the hypothesis that depressive symptoms would predict an exaggerated proinflammatory response to an in vivo antigen challenge, influenza virus vaccination, among pregnant women.
Method
Twenty-two pregnant women completed two study visits: baseline and one week after receiving influenza virus vaccination. Depressive symptoms were measured with the Center for Epidemiological Studies Depression Scale (CES-D) at baseline. Serum levels of macrophage migration inhibitory factor (MIF) were determined using a high sensitivity immunoassay at both study visits.
Outcomes
Analyses demonstrated that, as compared to those in the lowest tertile of CES-D scores, those in the highest tertile exhibited significantly higher levels of MIF one week after influenza virus vaccination (p = .035).
Conclusions
Depressive symptoms predicted exaggerated MIF production following influenza virus vaccination during pregnancy. These data support the hypothesis that depressive symptoms are associated with sensitization of the inflammatory response during pregnancy. Thus, women with greater depressive symptoms may be more vulnerable to negative sequelae of infectious illness during pregnancy.
Conservatively, it is estimated that 25–40% of preterm births are attributable intrauterine infection and associated inflammation (Goldenberg et al., 2008). In addition, infections including periodontal disease and urinary tract infection are associated with increased risk of preeclampsia and preterm delivery (Conde-Agudelo et al., 2008; Jeffcoat et al., 2001). Further, certain maternal infections, including influenza, predict increased risk of negative health outcomes in offspring and inflammatory processes are implicated in this link (Smith et al., 2007).
Stress and depressive symptoms predict exaggerated responses to biological challenges in humans and animals. Rodents exposed to repeated stressors evidence exaggerated inflammatory responses upon antigen exposure, higher levels of circulating interleukin(IL)-6, and enhanced IL-6 and tumor necrosis factor (TNF)-α production from myeloid cells (Avitsur et al., 2005; Johnson et al., 2002; Stark et al., 2002). In human studies, lymphocytes from depressed individuals show greater inflammatory responses upon in vitro exposure to mitogens (Anisman et al., 1999; Maes, 1995, 1999). Similarly, in response to the in vivo antigen challenge of influenza virus vaccination, older adults who reported more depressive symptoms exhibited increases in serum IL-6 two weeks following vaccination while no increase was seen among those with fewer depressive symptoms (Glaser et al., 2003). Providing further evidence that depression primes inflammatory responding, women with a history of major depression showed greater inflammatory responses following childbirth, a physical and psychological stressor (Maes et al., 2001).
The extent to which such effects generalize to pregnancy is of interest. The immune system demonstrates considerable changes to support pregnancy and negative perinatal outcomes have consistently been associated with an exaggerated inflammatory state as compared to healthy pregnancy (Redman et al., 1999; Sargent et al., 2006). Available data from pregnant women show that perceived stress is associated with elevated maternal serum proinflammatory cytokines, lower antiinflammatory cytokines, and exaggerated production of the proinflammatory cytokines by lymphocytes stimulated in vitro (Coussons-Read et al., 2007; Coussons-Read et al., 2005). Recent data from our group demonstrate that current depressive symptoms during pregnancy are associated with elevated maternal serum IL-6 and TNF-α (Christian et al., in press). To our knowledge, no studies have examined effects of distress (e.g., perceived stress or depressive symptoms) on inflammatory responses to in vivo antigen challenge in pregnancy. As described above, inflammatory responses to infectious agents are associated with negative effects for maternal health and pregnancy outcomes. Therefore, exaggerated inflammatory responses may have unique implications during pregnancy.
The role of macrophage migration inhibitory factor (MIF) in inflammatory responses has gained increasing attention. In the 1960s, MIF was one of the first cytokines identified; however, it was not until the mid-1990s that the functional properties of MIF began to be elucidated. Notably, MIF has the unique ability to counteract the anti-inflammatory properties of glucocorticoids in a dose-response fashion (Calandra and Bucala, 1995; Flaster et al., 2007). Due to its broad effects and essential role in both innate and adaptive immune function, antagonism of MIF is now being considered as a therapeutic target for many diseases with an inflammatory component, including rheumatoid arthritis (Morand et al., 2003), vascular diseases (Burger-Kentischer et al., 2006), and multiple sclerosis (Denkinger et al., 2003).
In pregnancy, elevated MIF has been implicated in risk of preeclampsia (Todros et al., 2005) and preterm delivery (Pearce et al., 2008). Moreover, Pearce (2004) reported that, compared to nondepressed pregnant women, clinically depressed pregnant women exhibited significantly higher serum levels of MIF. Due to its unique proinflammatory properties and emerging links with perinatal outcomes, the effects of psychosocial factors on MIF during pregnancy are of great interest.
The current study was designed to examine whether depressive symptoms predict sensitization of MIF responses during pregnancy. To test this hypothesis, influenza virus vaccination was used as an in vivo antigen challenge. It was hypothesized that women reporting greater depressive symptoms would exhibit greater MIF responses, indicating heightened sensitivity of the inflammatory response.
Of note, pregnant women evidence higher rates of influenza-related health complications and deaths than nonpregnant individuals. Given their high risk status, the American College of Obstetricians and Gynecologists, American Academy of Family Physicians, and Centers for Disease Control recommend that all women who are pregnant or will be pregnant during influenza season be vaccinated (Fiore et al., 2008). Despite this recommendation, it is estimated that only 12–13% of pregnant women have been vaccinated in recent years (Fiore et al., 2008). Thus, influenza vaccination provides an excellent model for examining immune responses while promoting vaccination.
Method
Study Design and Participants
Twenty-two pregnant women were recruited from the Ohio State University (OSU) General Perinatal Medical Clinic. Participants completed two study visits: baseline and one week after influenza virus vaccination. Depressive symptoms were assessed at baseline and serum levels of macrophage migration inhibitory factor (MIF) were assessed at both study visits. Women were excluded from participation if they reported recent acute illness, chronic health conditions with implications for immune function, or if fetal anomaly or preeclampsia was indicated per medical records. Participants received compensation for their participation at each study visit. The study was approved by The OSU Biomedical Institutional Review Board. Data were collected from November 2006 to April 2007.
Demographic and Psychosocial Measures
Information regarding height, current weight, pre-pregnancy weight, age, race, education level, marital status, income, and employment status was collected. The following health behaviors were assessed: cigarette use, participation in regular physical activity (i.e., at least one hour per week of vigorous activity), the number of hours of sleep in the previous night, frequency of prenatal vitamin use, and receipt of influenza virus vaccination in the prior influenza season.
Depressive symptoms were assessed prior to vaccination using the Center for Epidemiological Studies Depression Scale (CES-D), which demonstrates good test-retest reliability and excellent construct validity (Radloff, 1977). In addition, women were asked if they had ever been diagnosed with and/or treated for a mood disorder (i.e., major depression, dysthymia or minor depression, or bipolar disorder) by a healthcare provider.
Cytokine Measurements
At both study visits, whole blood was collected into vacutainer tubes between 9:30 am –1:30 pm while subjects were in a seated position. Post-vaccination samples were collected between 6–9 days following vaccination. Samples were immediately centrifuged, aliquoted, and placed in −80 C degree freezer storage until analysis. Serum levels of MIF were assayed in duplicate by using a Quantikine High Sensitivity ELISA kit (R&D Systems Minneapolis, MN) per kit instructions.
Influenza Virus Vaccination
Each woman received Fluarix vaccine (GlaxoSmithKline) during the 2006–2007 influenza season. Vaccines were administered within 30 minutes after blood sampling. Each .05mL dose contained 45 μg hemagglutinin (HA), with 15 μg HA of each of the following three virus strains: A/New Caledonia/20/99 (H1N1), A/Wisconsin/67/2005 (H3N2), and B/Malaysia/2506/2004.
Analytic Strategy
Cytokine data were log-transformed to normalize the data distribution. Data points ≥ 3 standard deviations from the mean were considered to be outliers and were excluded from analyses. One-way ANOVAs were used to examine equivalence of women classified as low, moderate, and high in depressive symptoms in terms of demographic and behavioral factors. A repeated measures general linear model was utilized to examine effects of depressive symptoms on changes in serum MIF over time, with levels of MIF as the dependent variable and tertiles of depressive symptoms (low, moderate, high) as the independent variable. One-way analysis of variance (ANOVA) and post-hoc tests of least significant difference were utilized to test simple main effects post-vaccination. Data analyses were performed using SPSS statistical software (SPSS 16.0).
Results
Demographic and Descriptive Characteristics
After excluding data from one participant with outlying MIF values, data were available from 22 women. Demographic and descriptive data are presented in Table 1. Participants were 25 (SD=4.6) years of age and at an average of 17 (SD=9) weeks gestation. Participants were primarily African-American (n=14), unmarried (n=16), had completed high school or less education (n=17), and reported a total annual family income of less than $15,000 per year (n=15).
Table 1.
Demographic Characteristics
| Age (years) | Mean: 25 (SD=4.6) Range: 18–37 |
| Race | African-American: 14 |
| Caucasian: 7 | |
| Bi-racial: 1 | |
| Marital Status | Unmarried: 16 |
| Married: 6 | |
| Education | Less than high school: 4 |
| High school: 13 | |
| Greater than high school: 5 | |
| Employment Status | Employed: 8 |
| Unemployed: 14 | |
| Income | < $15,000: 15 |
| 15,000–29,999: 5 | |
| >30,000: 2 | |
| Body Mass Index (kg/m2) | |
| Pre-pregnancy: | Mean: 26.8 (SD=6.0) Range: 18.2–38.7 |
| Per scale: | Mean: 28.9 (SD=6.3) Range: 20.2–42.9 |
| Weeks Gestation | Mean: 17 (SD=9) Range: 5–36 |
| Gravidity | Mean: 3.2 (SD=1.5) Range: 1–7 |
| Parity | Mean: 1.9 (SD=1.3) Range: 0–5 |
Depressive Symptoms
Scores on the CES-D ranged from 2–37 with an average score of 16 (SD=10). For the purposes of analyses, CES-D scores were split into tertiles. Women were classified as having no/minimal depressive symptoms (scores ranging 0–10; n=8), mild/moderate depressive symptoms (11–21; n=8), and significant depressive symptoms (≥ 22; n=6). Three women reported that they had previously been diagnosed with major depression (n=2) or bipolar disorder (n=1) by a healthcare provider. All three were in the highest tertile in terms of current depressive symptoms and had previously been treated with antidepressant or mood stabilizing medications. No women in the study were currently taking psychotropic medications.
Group Characteristics
One-way ANOVAs demonstrated that women in the three tertiles of depressive symptoms did not differ in terms of age (p=.31), pre-pregnancy BMI (p=.73), education (p=.23), income (p=.67), gravidity (p=.64), parity (p=.46), or weeks gestation at the time of vaccination (p=.67). Chi-square analyses demonstrated no group differences marital status (χ2 (2)=3.3 p=.19) or race (χ2 (2)=1.9, p=.38). As further confirmation of lack of racial difference in depressive symptoms, t-tests comparing CES-D scores among African-American versus Caucasian women demonstrated no significant difference (p=.67). In terms of health behaviors, groups did not differ in terms of hours of sleep the night prior to vaccination (p=.32) or post-vaccination session (p=.70). In addition, chi-square analyses showed that groups did not differ in terms of cigarette smoking (χ2 (2)=1.7, p=.92) or endorsement of regular vigorous physical activity ( 1 hour per week (χ2 (2)= 1.7, p=.44). One participant reported receiving influenza virus vaccination during the prior influenza season. Repeated measures ANOVA and tests for simple main effects indicated no significant differences as compared to participants who had not been vaccinated in the previous season in terms of MIF levels at baseline or post-vaccination (ps ≥ .13).
Analyses revealed a statistically significant difference in the days from vaccination to post-vaccination follow-up between women with low depressive symptoms versus moderate depressive symptoms (p =.03). The post-vaccination visit was completed at an average of 7.13 (SD = .64) days among those with low depressive symptoms, 8.13 (SD = .99) among those with moderate depressive symptoms and 7.33 (.82) among those with high depressive symptoms. Based on these analyses, days to post-vaccination visit was used as a covariate in subsequent analyses.
Depressive Symptoms and MIF Responses
Next, analyses were conducted to examine relationships between depressive symptoms and MIF levels. Repeated measures analyses controlling for days to post-vaccination visit demonstrated no main effect for change in MIF from pre to post-vaccination (F(1,18) = 1.25, p=.28). Post-hoc univariate ANOVAs were conducted to test for simple main effects at baseline and post-vaccination. Results demonstrated that, controlling for days to post-vaccination visit, women who scored in the top tertile on the CES-D (scores ≥ 22) exhibited significantly higher levels of macrophage MIF at one week post-vaccination as compared to those scoring in the bottom tertile (scores 0–10) (p=.035; Figure 1).
Figure 1. Macrophage migration inhibitory factor (MIF) levels prior to and one week post-influenza virus vaccination.
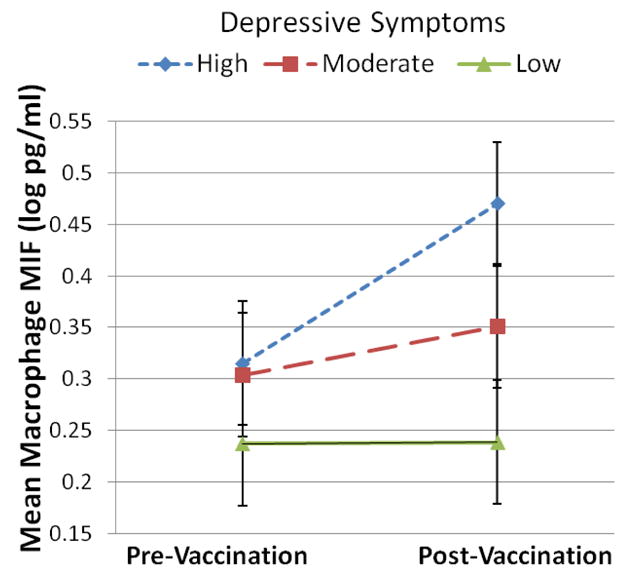
As compared to women in the lowest tertile of CES-D scores (n=8), those in the highest tertile (n=6) exhibited significantly higher levels macrophage MIF at one week after vaccination (p=.035).
Discussion
The current data provide evidence that depressive symptoms predict sensitization of inflammatory responses to an in vivo immune challenge during pregnancy. Women scoring in the highest tertile of depressive symptoms as measured by the CES-D exhibited significantly higher MIF at one week following influenza virus vaccination as compared to women in the lowest tertile. Groups did not differ in demographic characteristics (e.g., age, BMI, race, income) or health behaviors (e.g., sleep, smoking, regular exercise) assessed, suggesting that these factors did not account for the effects of depressive symptoms.
The absence of an increase in MIF levels at one week post-vaccination among women with lower depressive symptoms is consistent with previous evidence that influenza virus vaccination does not generally cause an extended inflammatory response (Glaser et al., 2003; Posthouwer et al., 2004; Tsai et al., 2005). Thus, we suggest that the inflammatory response seen among the more depressed individuals at one week post-vaccination indicates dysregulation of normal inflammatory processes.
These data have important implications for understanding risk of negative perinatal outcomes. As described previously, inflammatory responses to infection during pregnancy are implicated in outcomes relevant to maternal health, preterm delivery, and fetal development. Women who experience greater depressive symptoms during pregnancy may experience greater inflammatory responses upon exposure to other immune challenges including infectious agents, putting them at greater risk for negative sequelae. Relatedly, inflammatory responses to influenza vaccination are mild as compared to responses to influenza infection (e.g., Hayden et al., 1998; Tsai et al., 2005), supporting the clinical utility of vaccination.
Evidence also suggests that individuals experiencing greater stress are more susceptible to infection. For example, chronic stress among pregnant women has been associated with approximately two times greater risk of bacterial vaginosis (BV) (Culhane et al., 2001). Therefore distress may contribute to inflammation during pregnancy by increasing both susceptibility and response to infectious agents.
To our knowledge, there are no prior studies examining whether distress predicts priming of MIF responses to biological challenge. However, data from animal models indicates that MIF rises in response to acute behavioral stress (Calandra et al., 1995) and elevations in MIF have previously been associated with depressive symptoms among young men (Hawkley et al., 2006) and pregnant women (Pearce, 2004).
In the current investigation, depressive symptoms did not predict differences in MIF prior to vaccination, although a trend towards this effect equivalent to a medium effect size was suggested. Thus, additional research with a larger sample size is needed to confirm the present results and to further examine associations of depressive symptoms with MIF prior to vaccination.
Although the CES-D is a valid and reliable measure of depressive symptomatology, it does not allow for diagnosis of clinical depression. It is estimated that 40–50% of those scoring at or above the commonly used cut-off of 16 on the CES-D meet criteria for major depression as determined by clinical interview (Weissmann et al., 1977). A variety of higher cut-offs have been suggested to provide greater specificity and to identify more severe cases (e.g., Radloff, 1991). In the current sample, exaggerated inflammatory responses were seen among those scoring in the top third (≥ 22). Research in which diagnostic clinical interviews are utilized in conjunction with questionnaire measures would allow for better determination of the predictive validity of assessing depressive symptoms versus clinical depression. Also, in the current study, three women classified as the highest tertile of depressive symptoms reported that they had previously been diagnosed with and treated for a mood disorder. The study sample size did not allow for analyses of effects of current versus recurrent depressive symptoms which could be addressed in a larger sample with more comprehensive assessment of psychiatric history.
Of note, our ability to detect effects of interest within a small sample was largely a function of the notably high rate of depressive symptoms in our study population. This rate was similar to other studies of pregnant women from lower socioeconomic backgrounds, highlighting the importance of delineating effects of depressive symptoms during pregnancy. For example, Seguin and colleagues (1995) found that 47% of pregnant women whose incomes were below the poverty line scored at or above a clinical cut-off for depressive symptoms as compared to 20% of pregnant women from higher socioeconomic backgrounds.
The current study does not address the dynamics of change in MIF over time. The follow-up timepoint of one week post-vaccination was selected to capture relatively extended inflammatory responses; because prolonged responses are not generally expected in the context of vaccination, such responses may provide an indication of immune dysregulation. However, research examining the dynamics of inflammatory responses over time, including timepoints more immediately post-vaccination, would be highly informative. In addition, the extent to which inflammatory responses to vaccination predict health outcomes (e.g., preterm delivery, preeclampsia, antibody response to vaccination) should be addressed in future studies.
These data do not allow for examination of the effects of stage of pregnancy on inflammatory responses. In addition, because effects of depressive symptoms during pregnancy were of primary interest, a nonpregnant comparison group was not included in this study. Future research utilizing a larger sample size and more comprehensive assessment of health behaviors will allow for more thorough analysis of demographic and behavioral factors which may mediate and/or moderate effects noted in the current investigation.
In sum, the current data provide evidence that depressive symptoms predict exaggerated inflammatory responses to an in vivo antigen challenge during pregnancy. These findings implicate a novel and plausible pathway by which depressive symptoms may affect maternal health (e.g., preeclampsia), pregnancy outcomes (e.g., preterm delivery), and offspring health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anisman H, Ravindran AV, Griffiths J, Merali Z. Endocrine and cytokine correlates of major depression and dysthymia with typical or atypical features. Molecular Psychiatry. 1999;4:182–188. doi: 10.1038/sj.mp.4000436. [DOI] [PubMed] [Google Scholar]
- Avitsur R, Kavelaars A, Heijnen C, Sheridan JF. Social stress and the regulation of tumor necrosis factor-alpha secretion. Brain Behav Immun. 2005;19:311–317. doi: 10.1016/j.bbi.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Burger-Kentischer A, Gobel H, Kleemann R, Zernecke A, Bucala R, Leng L, Finkelmeier D, Geiger G, Schaefer HE, Schober A, Weber C, Brunner H, Rutten H, Ihling C, Bernhagen J. Reduction of the aortic inflammatory response in spontaneous atherosclerosis by blockade of macrophage migration inhibitory factor (MIF) Atherosclerosis. 2006;184:28–38. doi: 10.1016/j.atherosclerosis.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Calandra T, Bernhagen J, Metz CN, Spiegel LA, Bacher M, Donnelly T, Cerami A, Bucala R. Mif as a Glucocorticoid-Induced Modulator of Cytokine Production. Nature. 1995;377:68–71. doi: 10.1038/377068a0. [DOI] [PubMed] [Google Scholar]
- Calandra T, Bucala R. Macrophage migration inhibitory factor: a counter-regulator of glucocorticoid action and critical mediator of septic shock. Journal of Inflammation. 1995;47:39–51. [PubMed] [Google Scholar]
- Christian LM, Franco A, Glaser R, Iams J. Depressive symptoms are associated with elevated serum proinflammatory cytokines among pregnant women. Brain Behav Immun. doi: 10.1016/j.bbi.2009.02.012. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde-Agudelo A, Villar J, Lindheimer M. Maternal infection and risk of preeclampsia: Systematic review and metaanalysis. American Journal of Obstetrics and Gynecology. 2008;198:7–22. doi: 10.1016/j.ajog.2007.07.040. [DOI] [PubMed] [Google Scholar]
- Coussons-Read ME, Okun ML, Nettles CD. Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain, Behavior, and Immunity. 2007;21:343–350. doi: 10.1016/j.bbi.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Coussons-Read ME, Okun ML, Schmitt MP, Giese S. Prenatal stress alters cytokine levels in a manner that may endanger human pregnancy. Psychosomatic Medicine. 2005;67:625–631. doi: 10.1097/01.psy.0000170331.74960.ad. [DOI] [PubMed] [Google Scholar]
- Culhane JF, Rauh V, McCollum KF, Hogan VK, Agnew K, Wadhwa PD. Maternal stress is associated with bacterial vaginosis in human pregnancy. Maternal and Child Health Journal. 2001;5:127–134. doi: 10.1023/a:1011305300690. [DOI] [PubMed] [Google Scholar]
- Denkinger CM, Denkinger M, Kort JJ, Metz C, Forsthuber TG. In vivo blockade of macrophage migration inhibitory factor ameliorates acute experimental autoimmune encephalomyelitis by impairing the homing of encephalitogenic T cells to the central nervous system. Journal of Immunology. 2003;170:1274–1282. doi: 10.4049/jimmunol.170.3.1274. [DOI] [PubMed] [Google Scholar]
- Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G, Bresee JS, Cox NJ. Prevention and Control of Influenza: Recommendations of the Advisory Commitee on Immunization Practices, 2008. MMWR Recomm Rep. 2008;57:1–60. [PubMed] [Google Scholar]
- Flaster H, Bernhagen J, Calandra T, Bucala R. The macrophage migration inhibitory factor-glucocorticoid dyad: regulation of inflammation and immunity. Molecular Endocrinology. 2007;21:1267–1280. doi: 10.1210/me.2007-0065. [DOI] [PubMed] [Google Scholar]
- Glaser R, Robles TF, Sheridan J, Malarkey WB, Kiecolt-Glaser JK. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Archives of General Psychiatry. 2003;60:1009–1014. doi: 10.1001/archpsyc.60.10.1009. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkley LC, Bosch JA, Engeland CG, Marucha PT, Cacioppo JT. Loneliness, dysphoria, stress and immunity: A role for cytokines. In: Potnikoff NP, Faith RE, Murgo AJ, Good RA, editors. Cytokines: Stress and Immunity. CRC Press; Boca Raton, FL: 2006. [Google Scholar]
- Hayden FG, Fritz RS, Lobo MC, Alvord WG, Strober W, Straus SE. Local and systemic cytokine responses during experimental human influenza A virus infection -Relation to symptom formation and host defense. Journal of Clinical Investigation. 1998;101:643–649. doi: 10.1172/JCI1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffcoat MK, Geurs NC, Reddy MS, Cliver SP, Goldenberg RL, Hauth JC. Periodontal infection and preterm birth - Results of a prospective study. Journal of the American Dental Association. 2001;132:875–880. doi: 10.14219/jada.archive.2001.0299. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O’Connor KA, Deak T, Stark M, Watkins LR, Maier SF. Prior stressor exposure sensitizes LPS-induced cytokine production. Brain Behav Immun. 2002;16:461–476. doi: 10.1006/brbi.2001.0638. [DOI] [PubMed] [Google Scholar]
- Maes M. Evidence for an immune response in major depression: a review and hypothesis. Progress in Neuropharmacology and Biological Psychiatry. 1995;19:11–38. doi: 10.1016/0278-5846(94)00101-m. [DOI] [PubMed] [Google Scholar]
- Maes M. Major depression and activation of the inflammatory response system. Advances in Experimental Medicine and Biology. 1999;461:25–46. doi: 10.1007/978-0-585-37970-8_2. [DOI] [PubMed] [Google Scholar]
- Maes M, Ombelet W, De Jongh R, Kenis G, Bosmans E. The inflammatory response following delivery is amplified in women who previously suffered from major depression, suggesting that major depression is accompanied by a sensitization of the inflammatory response system. J Affect Disord. 2001;63:85–92. doi: 10.1016/s0165-0327(00)00156-7. [DOI] [PubMed] [Google Scholar]
- Morand EF, Bucala R, Leech M. Macrophage migration inhibitory factor - An emerging therapeutic target in rheumatoid arthritis. Arthritis and Rheumatism. 2003;48:291–299. doi: 10.1002/art.10728. [DOI] [PubMed] [Google Scholar]
- Pearce BD. The role of a unique immunohormonal molecule (MIF) in depression during pregnancy. NARSAD’s 16th annual scientific symposium; New York City. 2004. [Google Scholar]
- Pearce BD, Garvin SE, Grove J, Bonney EA, Dudley DJ, Schendel DE, Thorsen P. Serum macrophage migration inhibitory factor in the prediction of preterm delivery. American Journal of Obstetrics and Gynecology. 2008;199(46):e41–46. doi: 10.1016/j.ajog.2007.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posthouwer D, Voorbij HAM, Grobbee DE, Numans ME, van der Bom JG. Influenza and pneumococcal vaccination as a model to assess C-reactive protein response to mild inflammation. Vaccine. 2004;23:362–365. doi: 10.1016/j.vaccine.2004.05.035. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Radloff LS. The Use of the Center for Epidemiologic Studies Depression Scale in Adolescents and Young-Adults. Journal of Youth and Adolescence. 1991;20:149–166. doi: 10.1007/BF01537606. [DOI] [PubMed] [Google Scholar]
- Redman CWG, Sacks GP, Sargent IL. Preeclampsia: An excessive maternal inflammatory response to pregnancy. American Journal of Obstetrics and Gynecology. 1999;180:499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- Sargent IL, Borzychowski AM, Redman CWG. NK cells and human pregnancy - an inflammatory view. Trends in Immunology. 2006;27:399–404. doi: 10.1016/j.it.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Seguin L, Potvin L, St-Denis M, Loiselle J. Chronic stressors, social support, and depression during pregnancy. Obstetrics and Gynecology. 1995;85:583–589. doi: 10.1016/0029-7844(94)00449-N. [DOI] [PubMed] [Google Scholar]
- Smith SEP, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. Journal of Neuroscience. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark JL, Avitsur R, Hunzeker J, Padgett DA, Sheridan JF. Interleukin-6 and the development of social disruption-induced glucocorticoid resistance. J Neuroimmunol. 2002;124:9–15. doi: 10.1016/s0165-5728(02)00004-8. [DOI] [PubMed] [Google Scholar]
- Todros T, Bontempo S, Piccoli E, Ietta F, Romagnoli R, Biolcati M, Castellucci M, Paulesu L. Increased levels of macrophage migration inhibitory factor (MIF) in preeclampsia. European Journal of Obstetrics Gynecology and Reproductive Biology. 2005;123:162–166. doi: 10.1016/j.ejogrb.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Tsai M, Hanson N, Straka R, Hoke T, Ordovas J, Peacock J, Arends V, Arnett D. Effect of influenza vaccine on markers of inflammation and lipid profile. Journal of Laboratory and Clinical Medicine. 2005:145. doi: 10.1016/j.lab.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Weissmann MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. American Journal of Epidemiology. 1977;106:203–214. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]


