Summary
Two major isoforms of human carboxylesterases (CEs) are found in metabolically active tissues, CES1 and CES2. These hydrolytic enzymes are involved in xenobiotic and endobiotic metabolism. CES1 is abundantly expressed in human liver and monocytes/macrophages, including the THP1 cell line; CES2 is expressed in liver but not in monocytes/macrophages. The cholesteryl ester hydrolysis activity in human macrophages has been attributed to CES1. Here, we report the direct inhibitory effects of several endogenous oxysterols and fatty acids on the CE activity of THP1 monocytes/macrophages and recombinant human CES1 and CES2. Using THP1 whole-cell lysates we found: (1) 27-hydroxycholesterol (27-HC) is a potent inhibitor of carboxylesterase activity (IC50=33 nM); (2) 24(S),25-epoxycholesterol had moderate inhibitory activity (IC50=8.1 μM); and (3) cholesterol, 7-ketocholesterol, 22(R)-hydroxycholesterol, 24(S)-hydroxycholesterol, and 25-hydroxycholesterol each had little inhibitory activity. 27-HC was a partially noncompetitive inhibitor of recombinant CES1 (Kiapp=10 nM) and impaired intracellular CES1 activity following treatment of intact THP1 cells. In contrast, recombinant CES2 activity was not inhibited by 27-HC, suggesting isoform-selective inhibition by 27-HC. Furthermore, unsaturated fatty acids were better inhibitors of CES1 activity than saturated fatty acids, while CES2 activity was unaffected by any fatty acid. Arachidonic acid (AA) was the most potent fatty acid inhibitor of recombinant CES1 and acted by a noncompetitive mechanism (Kiapp=1.7 μM); when not complexed to albumin, exogenous AA penetrated intact THP1 cells and inhibited CES1. Inhibition results are discussed in light of recent structural models for CES1 that describe ligand binding sites separate from the active site. In addition, oxysterol-mediated inhibition of CES1 activity was demonstrated by pretreatment of human liver homogenates or intact THP1 cells with exogenous 27-HC, which resulted in significantly reduced hydrolysis of the pyrethroid insecticide bioresmethrin, a CES1-specific xenobiotic substrate. Collectively, these findings suggest that CE activity of recombinant CES1, cell lysates, and intact cells can be impaired by naturally occurring lipids, which may compromise the ability of CES1 to both detoxify environmental pollutants and metabolize endogenous compounds in vivo.
Keywords: 27-hydroxycholesterol, arachidonic acid, oxysterol, fatty acids, cholesteryl ester hydrolase, carboxylesterase, macrophage, xenobiotic biotransformation
Introduction
Several environmental toxicants, pharmaceutical agents, and illicit drugs are ester-containing compounds that are degraded in vivo by hydrolytic metabolism [1]. Examples include pyrethroid insecticides, phthalate esters, chemotherapeutic prodrugs (e.g., irinotecan), and narcotics such as cocaine and heroin. These xenobiotics are cleared from the body, or bioactivated (in the case of irinotecan), in part by the action of hydrolytic enzymes called carboxylesterases (CEs). Pyrethroid insecticides are particularly important substrates of CEs because of their extensive use in agriculture and public health [2]; they are increasingly used worldwide as a consequence of the curtailed usage of organophosphate (OP) insecticides. In addition to xenobiotic substrates, several endogenous substrates have been identified for the CEs including triacylglycerols [3], 2-arachidonoylglycerol [4], and cholesteryl esters [5]. Hydrolytic metabolism of cholesteryl esters found in cytoplasmic lipid droplets is an important regulatory step in macrophage reverse cholesterol transport (mRCT) [6]. One of the best candidate enzymes responsible for the neutral cholesteryl ester hydrolase activity in human macrophages is cholesteryl ester hydrolase (CEH), which was cloned from human THP1 monocytes/macrophages and human liver by Ghosh [5,7]. Macrophage CEH appears to be the same protein as human carboxylesterase 1 (CES1), an enzyme with an important role in xenobiotic metabolism [8] that is the most abundantly expressed CE isoform in human liver (~50–60-fold greater than the CES2 isoform; [9]). We refer to macrophage CEH as CES1 throughout this paper. Over-expression of human CES1 in the macrophages of Ldlr−/− mice was found to reduce atherosclerosis, lesion necrosis, and to increase mRCT [10], thus lending support to the notion that this enzyme plays an important role in mRCT. In addition, treatment of cholesterol-loaded THP1 macrophages with benzil, a specific inhibitor of CES1, increased cholesteryl ester retention under culture conditions that favor cholesterol efflux [11]. However, despite the importance of CES1 in xenobiotic and endobiotic metabolism, very little is known about its regulation at the transcriptional, translational, or post-translational levels; or regulation of its hydrolytic activity by non-covalent binding with small endogenous molecules.
Oxysterols are oxidized forms of cholesterol produced both enzymatically and non-enzymatically via the auto-oxidation of cholesterol [12]. A great deal of attention has focused on the ability of oxysterols to regulate gene transcription. Several oxysterols, including 22(R)-hydroxycholesterol [22(R)-HC], 24(S)-hydroxycholesterol [24(S)-HC], 24(S),25-epoxycholesterol (epoxycholesterol) [13,14], and 27-hydroxycholesterol (27-HC) [15] are ligands for the liver X receptors (LXRα and LXRβ), which regulate the transcription of ABCA1, ABCG1, sterol regulatory element binding protein-1c (SREBP-1c), and apoE genes [16]. These gene products are involved in cholesterol efflux from macrophages. Recently, Ouimet et al. [17] postulated that both 22(R)-HC and epoxycholesterol would increase cholesterol efflux from macrophage foam cells to extracellular apolipoproteinA1 (apoA1) and HDL because each are LXR agonists that would increase the expression of ABCA1 and ABCG1. Paradoxically epoxycholesterol treatment of macrophage foam cells caused the rate of cholesterol efflux to decrease, while treatment of foam cells with 22(R)-HC increased the rate of cholesterol efflux, as expected. As predicted from their LXR-binding actions, both 22(R)-HC and epoxycholesterol increased the expression of ABCA1 and ABCG1 in the foam cells. Thus, it was concluded that epoxycholesterol impaired cholesteryl ester hydrolysis, while 22(R)-HC did not [17]. However, these conclusions were mostly inferred in this report and direct evidence was not provided. Thus, we hypothesized that the differential effects of 22(R)-HC and epoxycholesterol on cholesterol efflux were caused by direct inhibition of CES1 activity by epoxycholesterol, while 22(R)-HC would have no inhibitory effect.
Fatty acids (FAs) also act as ligands for nuclear receptors, such as peroxisome proliferator-activated receptors (PPARs), thus regulating gene expression [18]. Indeed, CES1 promoter activity is downregulated by activated PPARα and PPARγ receptors [19]. However, fatty acids also affect protein function by more direct mechanisms, such as allosteric effects on enzyme activities [20–22]. In particular, arachidonic acid (AA) has potent effects on enzymes involved in drug metabolism [23–25]. While the basal level of intracellular free AA is tightly controlled, it can increase dramatically during states of inflammation and disease [26]. AA is liberated from glycerophospholipids by cytosolic phospholipase A2 and is a substrate for multiple enzyme systems in cells that mediate inflammation and vascular tone, including cyclooxygenase, lipooxygenase, and cytochrome P450 enzymes. While the literature on the metabolism of AA by these enzymes is abundant, information regarding AA as an allosteric effector of enzyme activity is relatively sparse.
Hydrolytic metabolism of many ester-containing lipids and xenobiotics is due to the activity of human CES1 in several tissues, including liver and monocytes/macrophages, underscoring the ubiquitous distribution and broad substrate specificity of this enzyme [27]. Enzymes in cellular contexts are exposed to a large number of both endogenous and exogenous small molecules that can modulate their functional activity via protein-metabolite interactions [28]. Previous studies have shown that exogenous compounds, such as drugs [29] or pollutants [30], can inhibit CES1 activity. Here, we investigated the ability of several endogenous small molecules to inhibit CES1 function. Because oxysterols and fatty acids have important roles in the regulation of lipid metabolism and are produced during normal and pathophysiological conditions, such as inflammation, we examined the inhibitory effects of these compounds on the carboxylesterase activity of THP1 whole-cell lysates, intact THP1 cells, and recombinant human CES1 protein. Further, we determined the mode of inhibition of both 27-HC and AA on CES1 activity because these two compounds had the most potent effects on carboxylesterase activity. We also assessed the biological significance of this inhibition by evaluating how 27-HC and AA can affect the metabolism of ester-containing model substrates (p-nitrophenyl valerate) and xenobiotic substrates (pyrethroid insecticides) by human liver S9 fraction and intact THP1 cells. Finally, we determined the effects of two oxysterols (27-HC and epoxycholesterol) on the distribution of cholesterol between its free and esterified forms in THP1 macrophage foam cells.
Materials and Methods
Materials
Chemicals and reagents
Human THP-1 monocytes, murine J774 macrophages, RPMI-1640 medium, Dulbecco’s Modified Eagle’s Medium (DMEM), gentamicin sulfate solution (50 mg/ml), and Hanks’ balanced salt solution without calcium, magnesium or phenol red were purchased from the American Type Culture Collection (ATCC) (Manassas, VA). Fetal bovine serum (FBS) was purchased from Invitrogen (Carlsbad, CA). p-Nitrophenyl valerate (pNPV), β-mercaptoethanol, 4-methylumbelliferyl acetate (4-MUBA), 4-methylumbelliferyl oleate (4-MUBO), phorbol 12-myristate 13-acetate (PMA), penicillin, gentamicin, streptomycin, and all components of the buffers were purchased from Sigma (St. Louis, MO). Epoxycholesterol and 24(S)-HC were purchased from BioMol Research Laboratories, Inc. (Plymouth Meeting, PA). Cholesterol, 22(R)-HC, 25-hydroxycholesterol (25-HC), and 7-ketocholesterol were purchased from Sigma. 27-HC was purchased from Research Plus Inc. (Barnegat, NJ). All fatty acids and fatty-acid free bovine serum albumin were purchased from Sigma. Bioresmethrin and trans-permethrin were purchased from Chem Service (West Chester, PA). Recombinant CES1 and CES2 were expressed in baculovirus-infected Spodoptera frugiperda cells and purified by methods previously described [31]. The rat ortholog of CES1 (Hydrolase A) was purified to homogeneity from male Sprague-Dawley rat liver by the procedure of Sanghani et al. [32] with slight modification. This entailed removal of an 80-kDa impurity by anion exchange chromatography. Human acetylated LDL was purchased from Intracel (Bethesda, MD). Normal human liver specimens (HL1220 and HL1274) were obtained from the Liver Tissue Cell Distribution System at the University of Minnesota. Human liver S9 subcellular fractions were prepared by homogenization of liver samples as a 20% (w/v) homogenate in ice-cold 0.1 M Tris-acetate buffer (pH 7.4) containing 0.1 M KCl, 1 mM EDTA, and 20 μM butylated hydroxytoluene, followed by centrifugation at 9,000 × g (30 min, 4°C). Liver S9 fractions were stored at −80°C.
Methods
Cell culture conditions
THP-1 monocytes were grown in suspension in RPMI-1640 medium supplemented with 10% FBS, 0.05 mM β-mercaptoethanol, and 50 μg/mL gentamicin (complete growth medium) at 37°C and 5% CO2. Cells were grown at a density between 0.2–1×106 cells/ml as recommended by ATCC. Culture medium was replaced every two-to-three days with fresh growth medium. J774 macrophages were grown as monolayers in DMEM supplemented with 10% FBS, 100 units/ml of penicillin, and 100 μg/ml streptomycin at 37°C and 5% CO2. Culture medium was replaced every two-to-three days with fresh medium.
Preparation of cell lysates
THP-1 monocytes were collected by centrifugation (200 × g, 7 min) and washed once with PBS. The cells were re-suspended in ice-cold 50 mM Tris-HCl (pH 7.4) buffer and lysed by sonication (4 × 15 sec bursts while on ice). Whole-cell lysates were stored at −80°C until use. Likewise, J774 cells were scraped, collected by centrifugation (200 × g, 7 min), and washed once with PBS. The cells were re-suspended in ice-cold 50 mM Tris-HCl (pH 7.4) buffer and lysed by sonication (4 × 15 sec bursts while on ice). Cell lysates were stored at −80°C until use.
Protein assay
Protein concentrations of cell lysates, liver S9 fractions, and purified recombinant CES1 were determined using the BCA reagent according to the manufacturer’s instructions (Pierce, Rockford, IL).
Inhibition of carboxylesterase activity by lipids: Spectrophotometric assay
In general, we used the colorimetric ester substrate pNPV for activity assays because we previously showed that CES1 accounts for ~85% of the hydrolytic activity in THP1 cell lysates that liberates p-nitrophenol from pNPV [11]. A continuous assay that monitors the formation of p-nitrophenol was used to determine the carboxylesterase activity of either THP1 cell lysate or recombinant CES1 and CES2 [33]. Briefly, hydrolysis reactions were performed in a 96-well plate format in a total volume of 300 μL with either cell lysate diluted to a final concentration of 50–125 μg protein/ml or recombinant CES1 diluted to 0.27 μg protein/ml. The oxysterol or fatty acid, dissolved in ethanol, was diluted into 50 mM Tris-HCl (pH 7.4) buffer and added to cell lysate or pure recombinant CES1 to yield the desired concentration. The mixture was pre-incubated for 15 min at 37°C. The hydrolysis reaction was initiated by the addition of pNPV to a final concentration of 500 μM. The final volume of ethanol in each well was 1% (v/v), which had no effect on CES1 activity. Reaction progress was monitored by measuring the absorbance at 405 nm for 5 min to estimate the rate of formation of p-nitrophenol. Slopes of activity curves were recorded and an extinction coefficient of 13 cm−1mM−1 [34] was used to convert values to specific activities (μmol of p-nitrophenol formed/min/mg protein). Data were expressed as either fractional inhibition (i), where i = (enzyme activity without inhibitor enzyme activity with inhibitor)/(enzyme activity without inhibitor), or as percent inhibition (= i×100). Enzyme activity without inhibitor is referred to as control activity. All reactions in an experiment were performed in triplicate; each experiment was performed independently at least three times. Recombinant CES2 activity was determined in a similar manner except the final ethanol concentration was 0.2% (v/v) because higher concentrations were found to inhibit CES2 activity.
The concentration of inhibitor that inhibited 50% of the control activity (IC50 value) was estimated by varying the concentration of the indicated oxysterol or fatty acid (the inhibitor) and measuring the carboxylesterase activity. The fractional inhibition was calculated for each inhibitor concentration and plotted against the concentration of inhibitor. Sigma Plot (San Rafael, CA) was used to fit the best curve through the points and IC50 values were interpolated from the curve. r2 values for the curves were ≥0.95.
Inhibition of carboxylesterase activity by AA in the presence of bovine serum albumin
Pure recombinant CES1 (0.5 μg protein) was mixed with AA (20 μM final concentration) in the presence and absence of fatty-acid free bovine serum albumin (2% w/v, final concentration) in 50 mM Tris-HCl (pH 7.4) buffer. The pyrethroid insecticide trans-permethrin (a CES1 substrate; [35]) was immediately added to give a final concentration of 50 μM (250 μL final reaction volume). The pyrethroid was added from an acetonitrile stock solution. This compound was used as the ester substrate instead of pPNV because it is not hydrolyzed by BSA, while pNPV is (data not shown). Reaction mixtures were incubated for 30 min at 37 °C, at which point an equal volume of acetonitrile was added to terminate the reactions. After brief centrifugation to pellet proteins, samples were analyzed by HPLC-UV as described previously [35]. The relative quantities of 3-phenoxybenzyl alcohol, a hydrolysis product of trans-permethrin, in samples with and without albumin were determined by integrating its chromatographic peak area.
Determination of Ki values for 27-hydroxycholesterol and AA
The mechanism of inhibition of CES1 activity by 27-HC and AA, and Ki values, were determined using the approach of Wadkins et al. [36]. Briefly, carboxylesterase activity of recombinant CES1 was determined using a single concentration of pNPV (500 μM) in the presence of increasing concentrations of 27-HC (1–1000 nM) and AA (0.001–100 μM). Alternatively, varying concentrations of substrate were incubated with fixed amounts of inhibitor. The lipids were preincubated with enzyme for 15 min (37°C) before adding substrate. Ki values for each inhibitor were determined using at least eight different concentrations of inhibitor. Using Sigma Plot, the data were fit to an equation that describes general enzyme inhibition ([37]; Supplementary Fig. 1): i = [I]{[S](1−β)+Ks(α−β)}/[I]{[S]+αKs}+Ki{α[S]+αKs}; where i is fractional inhibition, [I] is inhibitor concentration, [S] is substrate concentration, Ks is the dissociation constant of the enzyme-substrate complex (E·S), α is the change in affinity of substrate for enzyme caused by the presence of inhibitor, β is the change in rate of decomposition of E·S complex to product caused by the presence of inhibitor, and Ki is the inhibitor constant. This general equation can be subdivided into simpler equations to describe competitive, partially competitive, noncompetitive, partially noncompetitive, mixed, and uncompetitive inhibition models [37]. The model that provided the best curve fit (greatest r2 value) and lowest Akaike’s information criterion value was used to assign the mechanism of enzyme inhibition. Since the inhibitor was preincubated with enzyme for 15 min before addition of substrate, we have reported Ki values as apparent inhibition constants (Kiapp). In addition, the data was analyzed using a Dixon plot (1/velocity vs [I]), which was subsequently replotted (slope of each Dixon plot at each inhibitor concentration vs 1/[S]) to determine mode of inhibition [38]. To verify the mode of inhibition established by these models, the data were also transformed into Hanes-Woolf and Eadie-Scatchard plots [38].
Intracellular inhibition of carboxylesterase activity by exogenous 27-hydroxycholesterol and arachidonic acid
Intact THP1 monocytes (1.3 × 106 cells in one ml) were incubated with increasing amounts of 27-HC (0.01–1 μM) or AA (0.2–20 μM) in PBS at 37°C with gentle shaking. Aliquots of the cell suspension (150 μl) were removed at 80 min and added to wells of a clear 96-well plate (2 × 105 cells per well). One-hundred and fifty μl of 1 mM pNPV (prepared in PBS) was immediately added to the same wells to bring the final concentration of pNPV to 500 μM. The extent of intracellular hydrolysis of pNPV was determined by monitoring the absorbance (405 nm) on a plate reader. Negative control reactions contained ethanol vehicle instead of lipid, and positive control reactions contained 10 μM paraoxon or 50 μM benzil. Paraoxon and benzil both effectively inhibit CES1 activity in intact THP1 cells [11]. Data are reported as % inhibition of intracellular carboxylesterase activity. Cell viability was checked at the end of the incubation period by trypan blue exclusion. In a separate experiment, AA was complexed to BSA prior to dilution into PBS and addition to cultured THP1 cells (final concentration of AA, 0.2–20 μM). These incubations were allowed to proceed for 60 min.
Intact THP1 monocytes were also preincubated with 1 μM 27-HC in PBS for 60 min, as above, followed by addition of 50 μM bioresmethrin (pyrethroid insecticide) for another 60 min. The incubation was stopped by adding 3 ml of ethyl acetate (containing 0.5% acetic acid and internal standard) to the medium/cells. Samples were vigorously mixed and briefly centrifuged to separate the two layers. The upper organic layer was collected, evaporated to dryness under nitrogen, and the residues resuspended in 200 μl 1:1 (v/v) acetonitrile/water. Samples were analyzed by HPLC-UV to determine the extent of pyrethroid hydrolysis, as described previously [35].
Inhibition of the hydrolysis activity of human liver subcellular fraction by 27-HC
Two individual human liver S9 fractions (0.5 mg protein/ml, final concentration) were preincubated with 1 μM 27-HC for 15 min in 50 mM Tris-HCl (pH 7.4) buffer followed by the addition of 50 μM bioresmethrin. After 30 min incubation with pyrethroid, reactions were stopped by adding an equal volume of acetonitrile containing internal standard. The samples were placed on ice for 15 min and centrifuged. The supernatant was analyzed by HPLC-UV for pyrethroid hydrolysis product formation [35].
Treatment of cholesterol-loaded THP1 macrophages with exogenous oxysterols
THP1 monocytes were plated into 60-mm dishes (5 × 106 cells/dish) and differentiated into macrophages by incubation for 4 days in complete growth medium that was supplemented with 100 nM phorbol 12-myristate 13-acetate (PMA). Culture medium was replaced on day 3 with fresh complete growth medium containing PMA. Macrophages were loaded with cholesterol by culturing in RPMI-1640 medium supplemented with 50 μg/ml human acetylated LDL, 2 mg/ml BSA, 50 μg/ml gentamicin, and 50 μM mercaptoethanol for 24 h. Following cholesterol loading, macrophages were washed and incubated in RPMI medium supplemented with 2 mg/ml BSA, 50 μg/ml gentamicin, and either epoxycholesterol (10 μM), 27-hydroxycholesterol (10 μM), 22(R)-hydroxycholesterol (10 μM) or ethanol vehicle for another 12 h or 24 h to permit equilibration of the intracellular pools of cholesteryl esters and free cholesterol. Cells were then scraped into 50 mM Tris-HCl (pH 7.4) buffer, lysed by sonication, and cholesterol/cholesteryl esters were extracted and analyzed as described previously [11], with the exception that the saponification step was carried out at 37°C instead of 80°C since it was determined that this temperature was sufficient to hydrolyze cholesteryl esters to free cholesterol in ethanolic KOH. The amount of free and total cholesterol in lipid extracts were determined by gas chromatography and normalized on the amount of cell protein in each dish. Esterified cholesterol levels were calculated as the difference between total and free cholesterol amounts.
Results
Inhibition of carboxylesterase activity in THP1 cell lysates by oxysterols
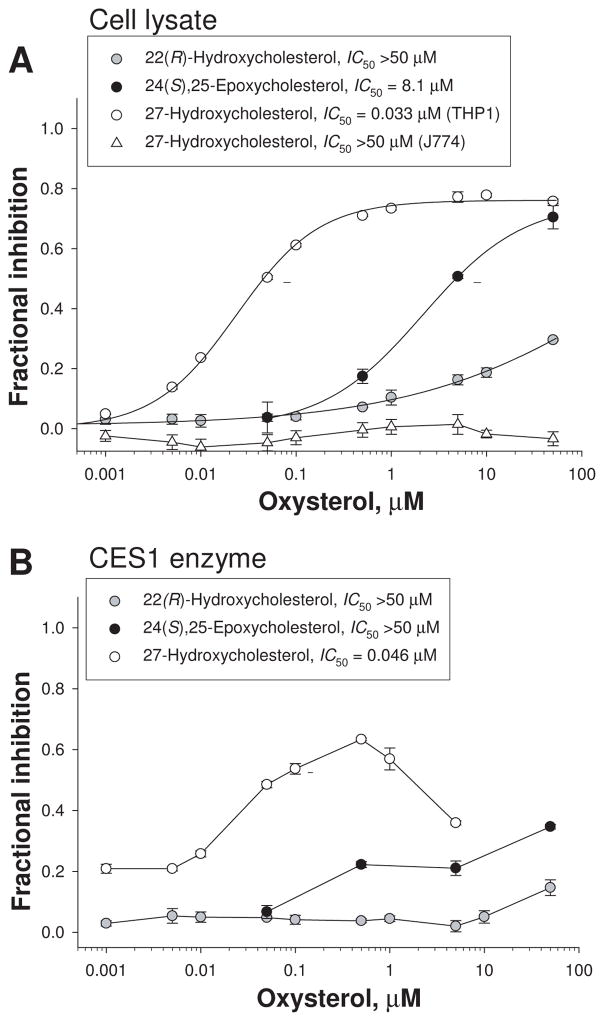
The ability of oxysterols to inhibit carboxylesterase activity in THP1 cell lysates was determined using the model ester substrate pNPV. Oxysterol inhibition data are presented in Fig. 1A. 22(R)-HC had a limited ability to inhibit the carboxylesterase activity of THP1 cell lysate. Maximum inhibition of activity was ~30% at a concentration of 50 μM. In contrast, 50 μM epoxycholesterol inhibited ~70% of the carboxylesterase activity. The IC50 of epoxycholesterol was estimated to be 8.1±4.6 μM. 27-HC was by far the most potent oxysterol inhibitor of carboxylesterase activity in THP1 cell lysates (Fig. 1A). The IC50 of 27-HC was estimated to be 0.033±0.011 μM, with a maximum inhibition of ~78% at 50 μM. In marked contrast, 27-HC did not inhibit the carboxylesterase activity in murine macrophage cell lysate (J774, Fig. 1A). Several other oxysterols, including 24(S)-HC, 25-HC and 7-ketocholesterol, were also examined but found to have minimal inhibitory effect on the carboxylesterase activity of THP1 cell lysates, while cholesterol had no inhibitory effect (see Supplementary Table 1).
Figure 1.
Inhibition of carboxylesterase activity of THP1 and J774 cell lysates (A) and recombinant human CES1 (B) by 22(R)-HC, epoxycholesterol, and 27-HC. Cell lysate from either THP1 or J774 cells (A) and recombinant CES1 protein (B) were preincubated at 37°C for 15 min in the presence or absence of oxysterol prior to addition of pNPV. The rate of formation of p-nitrophenol was measured and carboxylesterase activity determined. The dashed line segments represent 50% inhibition of enzyme activity. Data are representative of at least 3 independent experiments (mean ± SD of triplicate determinations).
Inhibition of the carboxylesterase activity of recombinant human CES1 by oxysterols
Next, we examined the ability of oxysterols to inhibit the carboxylesterase activity of pure recombinant human CES1 protein. The fractional inhibition curves for 22(R)-HC, epoxycholesterol, and 27-HC are shown in Fig. 1B. Once again 27-HC was clearly the most potent inhibitor, with epoxycholesterol being the next most potent and 22(R)-HC having minimal activity (Fig. 1B). The other oxysterols tested (24-HC, 25-HC, and 7-ketocholesterol) inhibited <15% of the carboxylesterase activity of CES1 (Supplementary Table 1). Similar to THP1 cell lysates, cholesterol had almost no effect on the carboxylesterase activity of CES1. Interestingly, when a broad range of concentrations of 27-HC was examined for its effect on CES1 activity, a biphasic curve was seen (Fig. 1B). The maximum inhibition observed was ~67% at 500 nM; however, progressively less inhibition was found at higher concentrations of 27-HC. A nearly identical inhibition curve was observed when the fluorogenic substrate 4-methylumbelliferyl acetate was used instead of the colorimetric substrate pNPV (data not shown), suggesting that this behavior was substrate and assay independent. This biphasic behavior is likely due to exceeding the predicted solubility limit of 27-HC in aqueous solution (0.52 μM; [39]) and the resulting formation of micelles, thus effectively reducing the free monomeric concentration of 27-HC in solution. Turbidity was noticeable in reaction mixtures that contained 10 μM or 50 μM 27-HC and CES1 enzyme; however, turbidity was not observed when the same amounts of 27-HC were added to THP1 cell lysates. Turbidity was not observed in reaction mixtures that contained recombinant CES1 and any other oxysterol, except for 7-ketocholesterol (slight turbidity was sometimes observed at 50 μM 7-ketocholesterol). In contrast to CES1, recombinant CES2 activity was not inhibited to any appreciable extent by 27-HC (IC50 > 5 μM; maximum concentration used for 27-HC), nor by any of the other oxysterols tested (IC50 > 50 μM for each oxysterol).
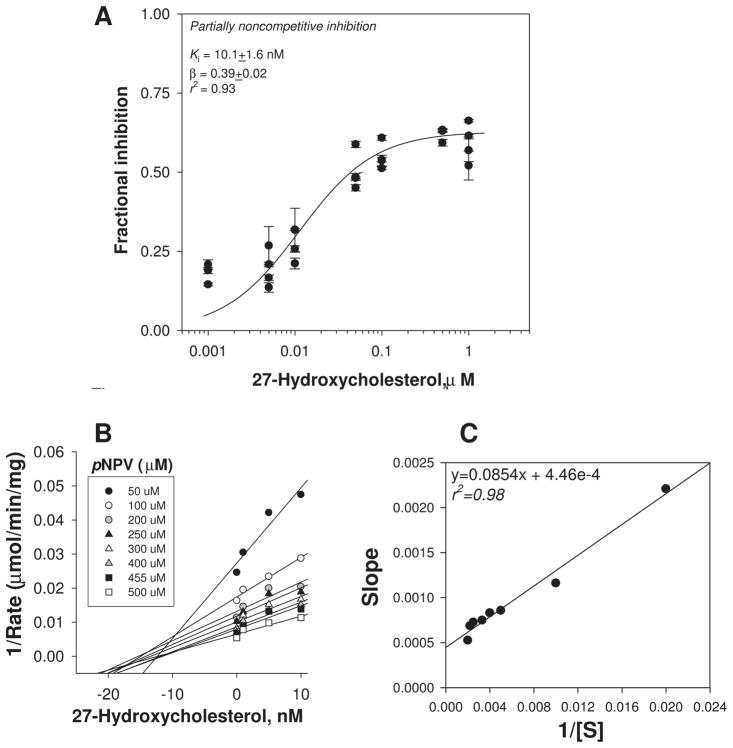
Determination of the mode of inhibition of CES1 by 27-hydroxycholesterol
The mode of inhibition of recombinant CES1 enzyme by 27-HC was determined by plotting the fractional inhibition of CES1 activity (determined using pNPV as substrate) versus increasing concentrations of 27-HC (Fig. 2A). The resulting plot suggested that 27-HC is a partial non-competitive inhibitor of CES1 (r2=0.93), which indicates that 27-HC and pNPV bind to separate sites on the CES1 enzyme. Furthermore, the ternary enzyme-inhibitor-substrate (E I S) complex that is formed remains partly catalytically active (Supplementary Fig. 1; [37]), as evidenced by the incomplete inhibition of enzyme activity at high concentrations of inhibitor. The estimated Kiapp for 27-HC was 10.1±1.6nM following 15 min preincubation with enzyme, which is ~5-fold greater than the concentration of recombinant CES1 used in the experiment. Consistent with 27-HC acting as a partial noncompetitive inhibitor, the Kiapp for 27-HC using the ester substrate 4-MUBA was very similar, 10.4±1.3 nM (data not shown). Similar to results using pNPV, maximum inhibition of CES1-catalyzed 4-MUBA hydrolysis by 27-HC never reached 100%, as would be expected for a partial inhibitor. When 27-HC and ester substrate (pNPV) were added simultaneously to recombinant CES1, a very similar inhibition curve and Ki value as shown in Fig. 2A was obtained (data not shown). This result suggests that equilibrium between 27-HC and CES1 is rapidly attained and preincubation of enzyme with inhibitor for 15 min does not greatly influence the estimated Ki value. When the data in Fig. 2A were fit to an equation that describes competitive inhibition, a poor fit was obtained (r2=0.31), which indicates that 27-HC does not act as a competitive inhibitor. Moreover, when the mode of inhibition of 27-HC was examined using Dixon plots (Fig. 2B,C), the resulting curves had features that were consistent with non-competitive inhibition [28].
Figure 2.
Inhibition of the carboxylesterase activity of recombinant human CES1 by 27-HC. The data were best fit to an equation that describes partially noncompetitive enzyme inhibition (A) [27]: i = (1−β) * [I]/([I] + Ki). For a partially noncompetitive inhibitor it is assumed that the E S I complex is partially catalytically active (i.e., 0<β<1), and the affinity of substrate for enzyme is unchanged by the inhibitor (i.e., α=1). The combined data from 3–4 independent experiments are shown [error bars represent the intra-assay variation (SD) for each independent experiment, which were performed in triplicate]. Analysis of data using a Dixon plot (shown in B) followed by a replot of the slopes of the Dixon plot versus 1/[S] (shown in C) confirmed a non-competitive mode of inhibition [28].
Further evidence that the enzyme activity of CES1 had been altered by 27-HC came from an experiment in which recombinant CES1 was preincubated for 30 min with 27-HC followed by treatment with an activity-based probe, fluorophosphonate (FP)-biotin [52]. The estimated IC50 for 27-HC using this probe (IC50 = 40 ± 20 nM; Supplementary Figure 2) was very similar to the value obtained using the spectrophotometric assay (IC50 = 50 ± 20 nM; Supplementary Table 1).
Inhibition of carboxylesterase activity of THP1 cell lysates and recombinant human CES1 by fatty acids
A series of fatty acids of varying carbon chain length and degrees of saturation were analyzed for their ability to inhibit the carboxylesterase activity of THP1 cell lysates. Their IC50 values are reported in Table 1. AA was the most potent inhibitor of the fatty acids examined. The IC50 for AA was ~4.4 μM, with 100 μM AA able to inhibit ~90% of the carboxylesterase activity. In general, unsaturated fatty acids, e.g. palmitoleic acid and oleic acid, were more potent inhibitors of carboxylesterase activity in cell lysates than saturated fatty acids of the same carbon chain length, namely palmitic acid and stearic acid. However, there was no significant difference between the inhibitory potencies of myristic acid and myristoleic acid, based on IC50 values. Among the saturated fatty acids, a shorter carbon chain length was associated with a greater ability to inhibit carboxylesterase activity.
Table 1.
Inhibition of carboxylesterase activity of THP1 cell lysates and recombinant CES1 by fatty acids
| Fatty acid | Enzyme inhibition | |
|---|---|---|
| Systematic name (common name) | Cell lysate IC50 (μM) | CES1 IC50 (μM) |
| Tetradecanoic acid (myristic acid, C14:0) | 28±4a | 9±3a |
| 9Z-Tetradecenoic acid (myristoleic acid, C14:1 ω5) | 23±6a | 12±2a |
| Hexadecanoic acid (palmitic acid, C16:0) | >100b | 25±13a |
| 10Z-Hexadecenoic acid (palmitoleic acid, C16:1 ω6) | 16±3a | 7±4 |
| Octadecanoic acid (stearic acid, C18:0) | >100c | >100d |
| 9Z-Octadecenoic acid (oleic acid, C18:1 ω9) | 11±4a | 7±3 |
| 9Z,12Z-Octadecadienoic acid (linoleic acid, C18:2 ω6) | 20±3a | 9±6 |
| 6Z,9Z,12Z-Octadecatrienoic acid (γ-linolenic acid, C18:3 ω6) | 23±3a | 19±10a |
| 5Z,8Z,11Z,14Z-Eicosatetraenoic acid (arachidonic acid, C20:4 ω6) | 4±1 | 2±1 |
| 11,12-Epoxy-5Z,8Z,14Z-eicosatrienoic acid (11,12-EET) | -- | 27±1 |
| 14,15-Epoxy-5Z,8Z,14Z-eicosatrienoic acid (14,15-EET) | -- | 38±1 |
| 15-deoxy-Δ12,14-Prostaglandin J2 | -- | 13±3 |
| Prostaglandin F2α | -- | >100 |
| Prostaglandin E2 | -- | >100 |
All values are the mean ± standard deviation of 2–3 independent experiments.
p≤0.05 compared to AA (Student’s t-test)
maximum inhibition was 43±2% at 100 μM fatty acid
maximum inhibition was 12±5% at 100 μM fatty acid
maximum inhibition was 9±9% at 100 μM fatty acid
The ability of fatty acids to inhibit the activity of pure recombinant human CES1 was also determined and IC50 values are reported in Table 1. The rank order of CES1 inhibition by fatty acids was similar to that observed with THP1 cell lysate. However, most fatty acids were more potent inhibitors of the carboxylesterase activity of CES1 than of THP1 cell lysate, as judged by the IC50 values reported in Table 1. Again, AA was the most potent fatty-acid inhibitor of CES1, while stearic acid was the poorest inhibitor whatever the source of enzyme used. AA also inhibited CES1-catalyzed hydrolysis of the ester-containing lipid surrogate 4-methylumbelliferyl oleate (IC50 ~10 μM), which suggests that its inhibitory activity is not substrate specific. We also discovered that the rat CES1 ortholog (termed Hydrolase A; [34]) was far less sensitive to the inhibitory effects of AA than human CES1. In fact, the IC50 (=50μM) for AA toward rat CES1 was 25-fold higher than for the human enzyme. Finally, we found that several metabolites of AA (11,12-EET; 14,15-EET; 15-deoxy-Δ12,14-prostaglandin J2; prostaglandin F2α; and prostaglandin E2) were all much less potent inhibitors of the carboxylesterase activity of human recombinant CES1 than was AA. As observed with 27-HC and CES2, no demonstrable inhibition of CES2 activity was observed with AA (IC50>100μM), nor by the other fatty acids that were tested (IC50>100μM; for each fatty acid shown in Table 1).
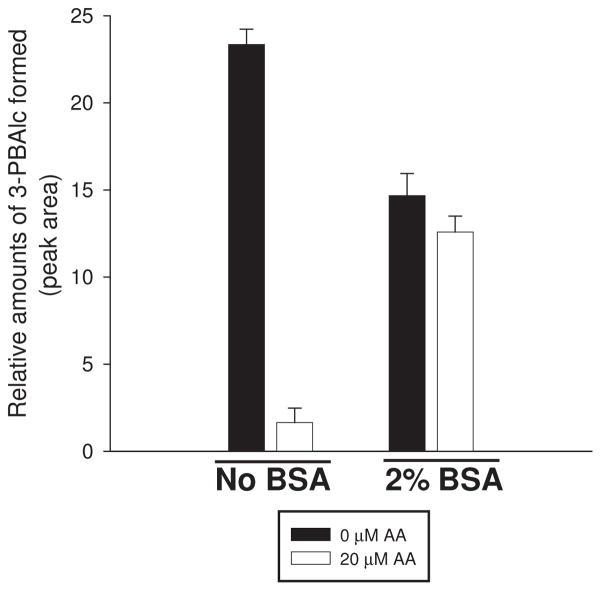
Because previous reports had shown that the inhibition of the enzymatic activity of UDP-glucuronosyl transferases (UDPGTs) and cytochrome P450s (CYPs) by AA was relieved by addition of fatty acid-free albumin [23–25], we also examined whether albumin could reverse the inhibitory effects of AA on recombinant CES1. Indeed, we found that the inhibitory effects of AA could be relieved by the addition of 2% (w/v) fatty acid-free bovine serum albumin to the incubation mixture. As shown in Fig. 3, CES1 hydrolytic activity toward a fixed concentration of trans-permethrin (50 μM) was inhibited ~93% in the presence 20 μM AA when no albumin was present. However, when albumin was added, the extent of inhibition was reduced to only 14%, which suggests that albumin can relieve the inhibitory actions of AA by sequestering the fatty acid from the CES1 enzyme. It was further noted that albumin alone could reduce the amount of hydrolysis product (3-phenoxybenzyl alcohol) formed during a 30 min incubation period (Fig. 3). This is likely because the substrate trans-permethrin is very hydrophobic and its effective free concentration is reduced by addition of albumin due to protein binding of substrate with a concomitant reduction in hydrolysis rate. The rationale for using trans-permethrin was that bovine serum albumin catalyzes the hydrolysis of the colorimetric substrate pNPV, but it has no hydrolytic activity toward trans-permethrin. We have previously observed similar reductions in pyrethroid hydrolysis rates, caused by the presence of albumin, when these compounds were incubated in rat plasma as opposed to incubation in buffer containing only pure CES enzyme [30].
Figure 3.
Addition of bovine serum albumin reverses inhibition of recombinant human CES1 by AA. The rate of hydrolysis of trans-permethrin (a pyrethroid insecticide) by CES1 was determined in the presence or absence of AA (20 μM) in reactions also supplemented (or not) with 2% (w/v) bovine serum albumin. Data are from one experiment and represent the mean ± SD of triplicate determinations.
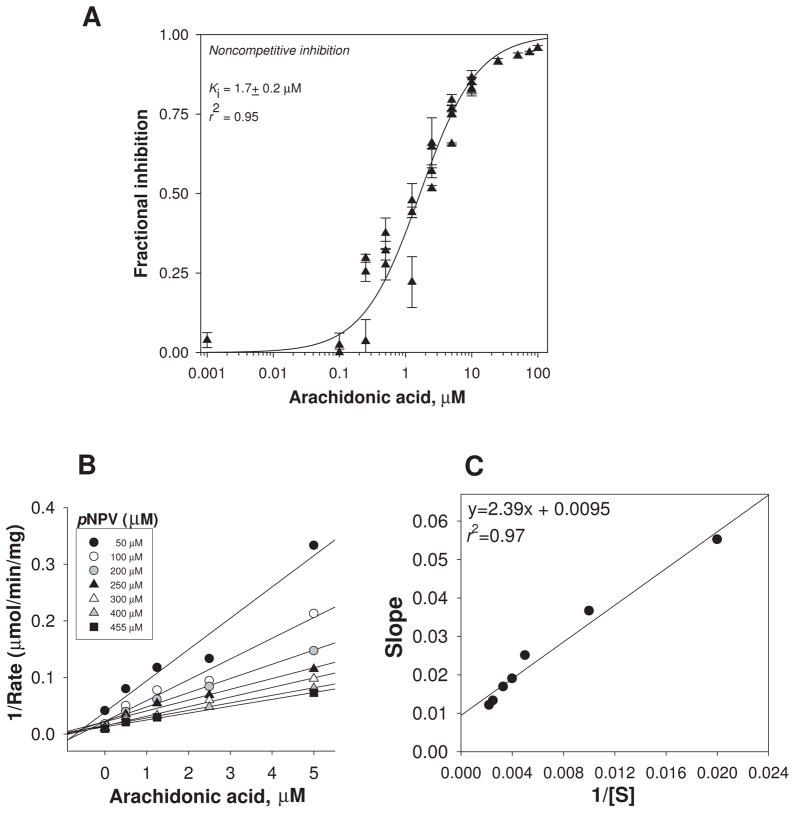
Determination of the mode of inhibition of CES1 by AA
The mode of inhibition of CES1 activity by AA was investigated. Analysis of the fractional inhibition data was best described by a noncompetitive inhibition model (Fig. 4; r2 =0.95). The Kiapp for AA was estimated to be 1.7±0.2 μM following preincubation with enzyme, which is almost the same as its IC50 value. This is as would be expected for a noncompetitive inhibitor [37]. Furthermore, simultaneous addition of AA and pNPV to CES1 enzyme revealed a similar inhibition curve (as shown in Fig. 4A) and Ki value, which suggests that AA equilibrates rapidly with enzyme (data not shown). Analysis of AA’s mode of inhibition of CES1 by Dixon plots (Fig. 4B,C) was also consistent with a noncompetitive mode of inhibition.
Figure 4.
Inhibition of the carboxylesterase activity of recombinant human CES1 by AA. The data were best fit to an equation that describes noncompetitive enzyme inhibition (A) [27]: i = [I]/([I] + Ki). For a noncompetitive inhibitor it is assumed that the E S I complex is catalytically inactive (i.e., β=0), and the affinity of substrate for enzyme is unchanged by the inhibitor (i.e., α=1). The combined data from 3–4 independent experiments are shown [error bars represent the intra-assay variation (SD) for each independent experiment, which were performed in triplicate]. Analysis of data using a Dixon plot (shown in B) followed by a replot of the slopes of the Dixon plot versus 1/[S] (shown in C) confirmed a noncompetitive mode of inhibition [28].
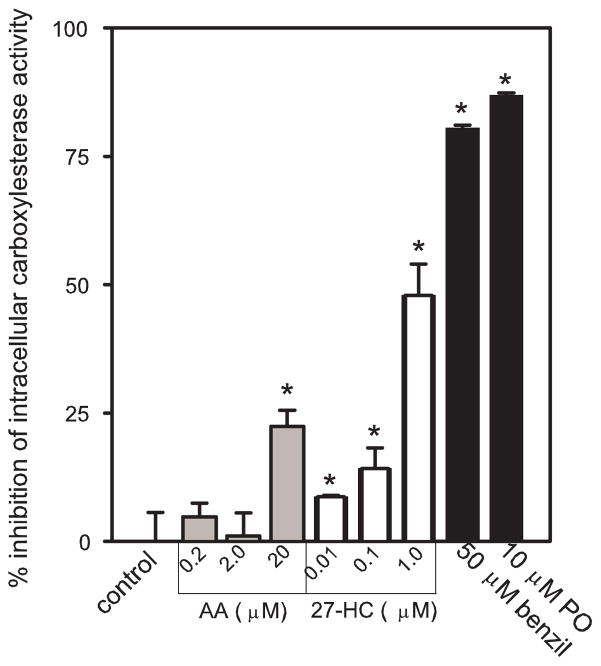
Inhibition of intracellular carboxylesterase activity by 27-hydroxycholesterol and AA
THP1 monocytes were incubated with increasing concentrations of either 27-HC or AA to determine if exogenously added lipids could inhibit CES1 activity within intact cells. Because conventional biochemical assays would cause reversible inhibitors of CES1 to be extensively diluted during lysate preparation and assay performance, it was necessary to perform an intracellular carboxylesterase assay [40] to gauge the inhibitory activity of 27-HC and AA within a cellular context. The results are presented in Fig. 5 and show that 27-HC could inhibit up to ~50% of the intracellular carboxylesterase activity of THP1 cells in a dose-dependent manner, while AA also significantly impaired CES1 activity (~22%) at a concentration of 20 μM. Incubation of cells with 50-μM benzil and 10-μM paraoxon inhibited 80% and 87% of the intracellular carboxylesterase activity, respectively. Importantly, cell viability was >90% at the end of each chemical treatment period. Longer incubation times (up to 270 min) did not enhance the inhibition potency of 27-HC or AA. Furthermore, in contrast to when AA was added directly to THP1 monocytes, inhibition of CES1 activity was completely abrogated when AA was complexed with fatty acid-free BSA prior to addition to THP1 cells (data not shown).
Figure 5.
Inhibition of intracellular carboxylesterase activity by arachidonic acid and 27-hydroxycholesterol. THP1 cells were incubated with the indicated amounts of arachidonic acid, 27-HC, benzil, and paraoxon (PO) for 80 min. The intracellular carboxylesterase activity was then determined in situ using pNPV as the substrate (see Materials and methods). Data are representative of three independent experiments; each experiment was performed in triplicate (mean ± SD). Asterisks indicates significant difference when compared to control (p<0.05, ANOVA and Dunnett’s test).
27-Hydroxycholesterol and AA impair the ability of human liver S9 fractions and intact THP1 cells to metabolize a pyrethroid insecticide
Two individual human liver S9 fractions (HL1220 and HL1274) were preincubated with 1 μM of 27-HC before adding the pyrethroid insecticide bioresmethrin. As a result, both samples exhibited an impaired ability to hydrolyze the insecticide. The ability of HL1220 and HL1274 to metabolize bioresmethrin was inhibited by 59±3% and 82±1%, respectively (n=3 replicates). Similarly, intact THP1 monocytes pretreated with 1-μM 27-HC also exhibited an impaired ability to hydrolyze bioresmethrin (77±9% inhibition; n=2 replicates). We also observed that AA reduces human hepatic carboxylesterase activity, and we are currently investigating if the endogenous levels of AA in individual human livers correlate with their ability to metabolize ester-containing substrates (Xie S. et al., in preparation).
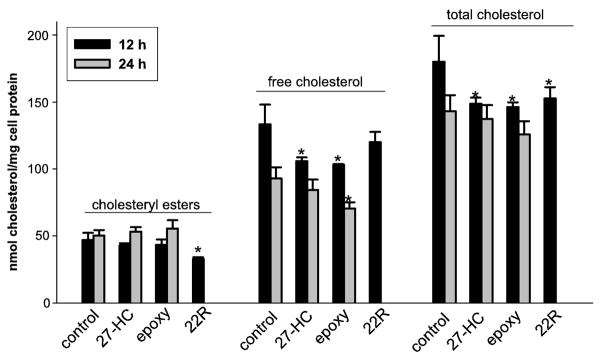
Effect of oxysterols on levels of intracellular cholesteryl esters, free cholesterol, and total cholesterol
When cholesterol-loaded THP1 macrophages were treated with epoxycholesterol and 27-HC (10 μM each for 12 and 24 h) with no cholesterol acceptors present in the culture medium, except bovine serum albumin, no significant changes in intracellular cholesteryl esters were observed when compared to the time-matched vehicle-treated (control) cells (see Fig. 6). However, a significant reduction in cholesteryl esters was observed in the 22(R)-HC-treated cells [at 12 h; only one time point used for 22(R)-HC]. Free cholesterol levels were reduced in the epoxycholesterol-treated THP1 cells at 12 and 24 h, while free cholesterol levels were significantly reduced by the 27-HC treatment only at 12 h. No change in free cholesterol was noted at 12 h in the 22(R)-HC-treated cells. With regard to total cholesterol (FC + EC) amounts, significant reductions were noted in each oxysterol treatment group at 12 h; however, no significant changes were found by 24 h. Taken together, 27-HC and epoxycholesterol appeared to reduce the amount of free cholesterol in the macrophages, but did not affect the levels of cholesteryl esters. In contrast, 22(R)-HC reduced the level of cholesteryl ester but did not alter the amount of free cholesterol.
Figure 6.
Amounts of intracellular cholesterol (cholesteryl esters, free cholesterol, and total cholesterol) following treatment of THP1 macrophage foam cells with oxysterols for 12 or 24 h. THP1 macrophages were loaded for 24 h with acLDL (50 μg/ml) followed by addition of ethanol vehicle (control), epoxycholesterol (epoxy, 10 μM), 27-hydroxycholesterol (27-HC, 10 μM), or 22(R)-hydroxycholesterol (22R, 10 μM) to culture medium containing 2 mg/ml BSA for 12 or 24 h. Only one time point was determined with 22(R)-HC (12 h). Cells were harvested and intracellular levels of free and total cholesterol were determined by gas chromatography. Esterified cholesterol levels were calculated as the difference between total and free cholesterol amounts. Results are representative of two independent experiments; each experiment was performed in triplicate or quadruplicate (mean ± SD). Asterisks indicate significant differences when compared to the appropriate time-matched control group (p<0.05, ANOVA and Dunnett’s test).
Discussion
Protein-endogenous small molecule interactions are increasingly being shown to have important roles in normal and aberrant cell physiology [28]. Here, we have examined the effects of two lipid classes, oxysterols and free fatty acids, on the hydrolytic enzyme CES1 for two reasons. First, these compounds are increasingly characterized as bioactive lipids with protein binding properties that modulate cell function [14,26]. Second, CES1 can metabolize ester-containing xenobiotics and endobiotics that have important roles in health and disease [41]. Despite the functional demonstration in cultured human macrophages [42] and intact mice [10] that CES1 can metabolize cholesteryl esters for eventual cholesterol efflux, we have previously reported that our recombinant CES1 preparation does not catalyze the hydrolysis of cholesteryl [1-14C]oleate in vitro [11]. Consistent with this, Okazaki et al. [43] showed that a recombinant murine ortholog of CES1 also does not hydrolyze cholesteryl esters in vitro. The reason for this lack of in vitro activity is likely due to the absence of appropriate co-factors, post-translational modifications, and/or protein-protein interactions that permit correct presentation of substrate to the enzyme active site. Thus, while it would be ideal to use bona fide physiologic substrates of CES1 for our biochemical studies, we opted to use a surrogate ester-containing substrate (pNPV) to characterize the inhibitory effects of oxysterols and fatty acids on CES1. This was done because pNPV is hydrolyzed nearly exclusively (~85%) by CES1 in THP1 monocytes/macrophages and is a convenient substrate to use.
We initially examined oxysterols because a recent study reported that epoxycholesterol impaired cholesteryl ester hydrolysis in THP1 macrophage foam cells, while 22(R)-HC did not [17]. Thus, we compared the direct inhibitory effects of these two oxysterols on the carboxylesterase activity of THP1 cell lysates and pure recombinant human CES1. Our results showed that epoxycholesterol could inhibit ~60% of the carboxylesterase activity in THP1 cell lysate, suggesting it inhibited a significant portion of CES1’s hydrolytic activity in THP1 cells. We then confirmed that epoxycholesterol inhibited the hydrolytic activity of recombinant human CES1, although not as effectively as it did for cell lysate. In addition, we found that 22(R)-HC was much less effective than epoxycholesterol at inhibiting the hydrolytic activity of either THP1 cell lysate or CES1. Our results are consistent with the observation that epoxycholesterol could retard cholesterol efflux from foam cells but 22(R)-HC could not [17], and provides a possible mechanism by which this may occur. Namely, epoxycholesterol may inhibit CES1-catalyzed hydrolysis of cholesteryl esters in foam cells, while 22(R)-HC does not. Recently, a 45–50 kDa protein termed KIAA 1363 was shown to possess cholesteryl ester hydrolase activity and is expressed in primary human monocyte-derived macrophages, as determined by western blotting [44]. However, KIAA 1363 is not expressed in THP-1 macrophages; only in THP-1 cells transduced with recombinant adenovirus encoding KIAA 1363 was protein detectable by immunoblotting. We confirmed this with our own THP-1 cells; KIAA 1363 protein was not detectable in non-transduced cells (data not shown). Thus, at least in cultured THP1 macrophages, CES1 is the most likely cholesteryl ester hydrolase target of oxysterols, and not KIAA 1363.
We next investigated the effects of several other physiologically important oxysterols on the hydrolytic activity of THP1 cell lysates. Interestingly, we found that 27-HC was a potent inhibitor of the hydrolytic activity of both THP1 cell lysate and pure CES1 enzyme, with IC50 values in the nanomolar range. Further analysis of 27-HC’s ability to inhibit CES1 was most consistent with a partially noncompetitive mode of inhibition, indicating that 27-HC interacts with a binding site separate from the active site and does not completely inhibit enzyme activity. In contrast to 27-HC and epoxycholesterol, all of the remaining oxysterols and cholesterol were poor inhibitors of the hydrolytic activity of THP1 cell lysate and recombinant CES1. Thus, our results demonstrate for the first time that 27-HC is an extremely potent and selective oxysterol inhibitor of CES1 activity in cells and exhibits nanomolar affinity for pure CES1 enzyme. In contrast, 27-HC did not inhibit the CE activity of murine macrophage cell lysate (J774 cell lysate), which also does not appear to express a CES1 ortholog as judged by immunoblotting (data not shown). Also, 27-HC did not inhibit the activity of CES2, a carboxylesterase isoform with ~48% sequence homology to CES1 [45], suggesting that 27-HC exhibits CE isoform specificity. Importantly, we also demonstrated that exogenously added 27-HC could penetrate viable THP1 monocytes and inhibit intracellular carboxylesterase activity (up to ~50% at 1 μM). Since 27-HC is a partially noncompetitive inhibitor, it would not be expected to yield complete inhibition of CES1 enzyme activity. This data suggests that the inhibitory effects of 27-HC are relevant in a cellular context.
How oxysterols, such as 27-HC and epoxycholesterol, bind to CES1 is currently unknown. Crystal structure data of CES1 has identified a solvent accessible binding site in the regulatory domain of the protein termed the ‘Z site’, which permits promiscuous binding of several ligands including the cholesterol derivatives, cholate and taurocholate [27]. This site is in close proximity to the active site and has been proposed to regulate the equilibrium between an active trimer state of CES1 and an inactive hexamer state. We discovered that cholate, which based on co-crystallization studies is known to bind the Z site, weakly inhibited enzyme activity (IC50 ~7 mM; data not shown). Thus, it is tempting to speculate that 27-HC might also bind the Z site, albeit with a markedly higher affinity than cholate, and therefore inhibit CES1 activity. Further, since cholesterol had no inhibitory effect on CES1 function, and 22(R)-, 24(S)-, and 25-HC each had little impact on activity, the position of the C27 hydroxyl group in 27-HC appears to be crucial for its apparent high affinity for CES1 and inhibitory action. However, unambiguous identification of the specific ligand binding site of 27-HC on CES1 awaits determination of a crystal structure of CES1 in complex with 27-HC.
Epoxycholesterol and 27-HC both activate the nuclear receptor LXR with EC50 values between 1–10 μM in gene reporter experiments [13–15]. Epoxycholesterol inhibits carboxylesterase activity in THP1 cell lysate with an IC50 value of 8.1 μM, which is within the range found for LXR activation. More strikingly, 27-HC inhibits THP1 cell lysate carboxylesterase activity in the low nanomolar range, which is two to three orders of magnitude lower than the concentration of 27-HC needed to activate transcription via ligand binding to LXR. Because of their low concentrations in vivo, oxysterols are difficult to quantify. For example, epoxycholesterol production in macrophages was documented but quantitative values were not reported [46]. In vivo levels of total 27-HC (free and esterified) in human serum was reported to be 117±35 ng/mL (or 291±87 nM) [47], 10% of which (or 29 nM) is in the free form [48]. Interestingly, 27-HC is the predominant oxysterol detected in atherosclerotic plaque and is concentrated in foam cells by as much as two orders of magnitude [47,48]. Collectively, these findings suggest that the inhibitory effects of 27-HC and epoxycholesterol on carboxylesterase activity may be physiologically relevant. Indeed, our results demonstrate that 27-HC can impair the metabolism of an ester-containing xenobiotic (pyrethroid insecticide) by both human liver S9 fraction and THP1 monocytes. Moreover, our data show that treatment of cholesterol-loaded macrophages with epoxycholesterol or 27-HC results in a decrease in intracellular free cholesterol but no change in levels of esterified cholesterol. The mechanism responsible for these findings is unclear. However, these findings are consistent with epoxycholesterol and 27-HC increasing the rate of free cholesterol efflux in the presence of BSA while not altering the mobilization of cholesterol from the cholesteryl ester pool as intracellular free cholesterol levels are decreasing. Kobayashi et al. [49] previously reported an ABCG1-dependent cholesterol efflux mechanism in the presence of albumin (when no other cholesterol acceptors were present) from HEK293 cells engineered to overexpress ABCG1, although this has not been shown in macrophages. Thus, it is possible that induction of ABCG1 by oxysterols increases free cholesterol efflux and is responsible for the observed decrease in free cholesterol levels for epoxycholesterol and 27-HC. Interestingly, when cholesterol-loaded macrophages were treated with 22(R)-HC, which induces ABCG1 [50] but does not inhibit CES1 activity efficiently in vitro, intracellular free cholesterol was not significantly decreased but the amount of esterified cholesterol was decreased. These results are consistent with 22(R)-HC inducing ABCG1 expression and increasing free cholesterol efflux while also not inhibiting CES1, so that cholesterol is able to move from the pool of cholesteryl esters to free cholesterol for subsequent efflux. Nevertheless, to prove these are the mechanisms responsible for our observations will require radiolabeling both the free and esterified intracellular cholesterol pools and measuring the effect of oxysterol treatment on the efflux of cholesterol and the flux of cholesterol between the two intracellular cholesterol pools in the presence of a specific acyl CoA cholesterol acyltransferase inhibitor to prevent re-esterification of free cholesterol.
Another goal of this study was to examine the effects of FAs on carboxylesterase activity (Table 1). FAs affect gene transcription by binding to nuclear receptors, such as the PPARs, or by indirectly influencing the abundance of transcription factors such as SREBP and CREBP [51]. PPARα- and PPARγ-specific ligands, including 5E,8E,11E,14E-eicosatetraenoic acid (EYTA), a structural isomer of AA, were previously found to downregulate CES1 promoter activity [19]. In our study, we found that FAs could directly inhibit the hydrolytic activity of THP1 cell lysate and human recombinant CES1 with IC50 values in the micromolar range. AA, the most potent inhibitor of CES1 hydrolytic activity, was found to be a noncompetitive inhibitor of CES1. Consistent with our data, AA was previously shown to be a potent fatty-acid inhibitor of UDPGTs [23,24] and CYPs [25]. Our results demonstrate for the first time that certain FAs are also potent inhibitors of CES1 hydrolytic activity. Experiments using BSA also suggest that levels of FA-binding proteins in cells may affect the inhibitory activity of intracellular FAs and perhaps modulate the enzyme activity of carboxylesterases within cellular and subcellular contexts. Brash [26] estimated that the intracellular free concentration of AA is ~50 μM, and may be even higher within inflamed tissues. Thus, the Kiapp value of 1.7 μM that we estimated for AA on CES1 activity appears to be physiologically relevant; however, the role of AA in modulating CES1 activity in vivo, specifically as it relates to cholesterol metabolism, remains unknown. The molecular mechanism by which FAs inhibit CES1 is not known. However, CES1 is known to bind palmitate following hydrolysis of the endogenous lipid, palmitoyl CoA [27]. Palmitate is bound at the ‘side door’ of CES1 in an X-ray crystal structure, which suggests that the side door may be important in the release of fatty acids following hydrolysis of esterified lipids, e.g. cholesteryl esters [4]. Together, these data suggest that inhibition of CES1 by fatty acids may occur via interactions with the side-door domain.
The ability of oxysterols and FAs to regulate the transcription of genes important in lipid metabolism via activated nuclear receptors has been established. Yet, these bioactive lipids can also interact directly with other proteins and affect their catalytic functions. Indeed, the recent report by Ouimet et al. [17] showed that treatment of human macrophages with epoxycholesterol could impair cholesteryl ester hydrolysis, and suggested that epoxycholesterol might directly inhibit the hydrolytic activity of CES1. We have found that the endogenous lipids, 27-HC and epoxycholesterol, as well as several fatty acids (especially AA), are particularly effective inhibitors of CES1’s hydrolytic activity. The low concentrations of 27-HC and AA that inhibit CES1 in vitro suggest that these bioactive lipids may directly modulate enzyme activity in vivo. Physiological significance of this inhibition is shown by the reduced ability of oxysterol-treated human liver S9 fraction and intact THP1 cells to metabolize added pyrethroid chemical. In conclusion, elevated levels of oxysterols and AA in cells may modulate the ability of CES1 to both detoxify environmental pollutants and metabolize endogenous compounds in vivo.
Supplementary Material
Supplementary Figure 1. General kinetic model for an enzyme inhibitor. See text for definition of abbreviations.
Supplementary Figure 2. Inhibition of recombinant CES1 by 27-hydroxycholesterol—Activity-based probe assay. (A) The extent of FP-biotin–mediated covalent modification of the recombinant CES1 active site is reduced by 30 min preincubation with 27-hydroxycholesterol. (B) Plot of integrated band density of each lane in (A) versus 27-hydroxycholesterol concentration. IC50 value for 27-HC is shown. See supplementary method for experimental details. Experiment is representative of two independent trials.
Acknowledgments
This project was supported by NIH 1R15ES015348 and the American Lebanese Syrian Associated Charities (ALSAC). Analytical support was provided by the Bioanalytical Chemistry Core at Mississippi State University (National Center for Research Resources COBRE grant P20RR017661). Human liver specimens were obtained from the Liver Tissue Cell Distribution System (University of Minnesota), which is supported by NIH #N01-DK-7-0004/HHSN267200700004C. Kate Lightner is gratefully acknowledged for her initial studies on fatty acid-mediated inhibition of recombinant CES1 activity.
Abbreviations
- AA
arachidonic acid
- ABCA1
ATP binding cassette transporter A1
- ABCG1
ATP binding cassette transporter G1
- apoA1
apolipoproteinA1
- apoE
apolipoproteinE
- CES1
carboxylesterase 1
- CES2
carboxylesterase 2
- CEH
cholesteryl ester hydrolase
- CVD
cardiovascular disease
- CYP27A1
cholesterol 27-hydroxylase
- epoxycholesterol
24(S),25-epoxycholesterol
- EYTA
5,8,11,14-eicosatetraenoic acid
- IC50
concentration that inhibits 50% of control enzyme activity
- i
fractional inhibition
- Ki
dissociation constant for an inhibitor
- FA
fatty acid
- LXR
liver X receptor
- mRCT
macrophage reverse cholesterol transport
- 4-MUBA
4-methylumbelliferyl acetate
- 4-MUBO
4-methylumbelliferyl oleate
- PO
paraoxon
- pNPV
p-nitrophenyl valerate
- PPAR
peroxisome proliferater-activated receptor
- SREBP-1c
sterol regulatory element–binding protein-1c
- Vmax
maximum velocity
- 22(R)-HC
22(R)-hydroxycholesterol
- 24(S)-HC
24(S)-hydroxycholesterol
- 25-HC
25-hydroxycholesterol
- 27-HC
27-hydroxycholesterol
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Potter PM, Wadkins RM. Carboxylesterases: detoxifying enzymes and targets for drug therapy. Curr Med Chem. 2006;13:1045–1054. doi: 10.2174/092986706776360969. [DOI] [PubMed] [Google Scholar]
- 2.Casida JE. Pest toxicology: the primary mechanisms of pesticide action. Chem Res Toxicol. 2009;22:609–619. doi: 10.1021/tx8004949. [DOI] [PubMed] [Google Scholar]
- 3.Dolinsky VW, Gilham D, Alam M, Vance DE, Lehner R. Triacylglycerol hydrolase: role in intracellular lipid metabolism. Cell Mol Life Sci. 2004;61:1633–1651. doi: 10.1007/s00018-004-3426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Streit T, Borazjani A, Lentz SE, Wierdl M, Potter PM, Gwaltney SR, Ross MK. Evaluation of the ‘side door’ in carboxylesterase-mediated catalysis and inhibition. Biol Chem. 2008;389:149–162. doi: 10.1515/BC.2008.017. [DOI] [PubMed] [Google Scholar]
- 5.Ghosh S. Cholesteryl ester hydrolase in human monocyte/macrophage: cloning, sequencing and expression of full length cDNA. Physiol Genomics. 2000;2:1–8. doi: 10.1152/physiolgenomics.2000.2.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Rothblat GH, Llera-Moya MDL, Favari E, Yancey PG, Kellner-Weibel G. Cellular cholesterol flux studies: methodological considerations. Atherosclerosis. 2002;163:1–8. doi: 10.1016/s0021-9150(01)00713-4. [DOI] [PubMed] [Google Scholar]
- 7.Zhao B, Natarajan R, Ghosh S. Human liver cholesteryl ester hydrolase: cloning, molecular characterization, and role in cellular cholesterol homeostasis. Physiol Genomics. 2005;23:304–310. doi: 10.1152/physiolgenomics.00187.2005. [DOI] [PubMed] [Google Scholar]
- 8.Satoh T, Hosokawa M. The mammalian carboxylesterases: from molecules to functions. Ann Rev Pharmacol Toxicol. 1998;38:257–288. doi: 10.1146/annurev.pharmtox.38.1.257. [DOI] [PubMed] [Google Scholar]
- 9.Godin SJ, Crow JA, Scollon EJ, Hughes MF, DeVito MJ, Ross MK. Identification of rat and human cytochrome P450 isoforms and a rat serum esterase that metabolize the pyrethroid insecticides deltamethrin and esfenvalerate. Drug Metab Dispos. 2007;35:1664–1671. doi: 10.1124/dmd.107.015388. [DOI] [PubMed] [Google Scholar]
- 10.Zhao B, Song J, Chow WN, St Clair RW, Rudel LL, Ghosh S. Macrophage-specific transgenic expression of cholesteryl ester hydrolase significantly reduces atherosclerosis and lesion necrosis in Ldlr−/− mice. J Clin Invest. 2007;117:2983–2992. doi: 10.1172/JCI30485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crow JA, Middleton BL, Borazjani A, Hatfield MJ, Potter PM, Ross MK. Inhibition of carboxylesterase 1 is associated with cholesteryl ester retention in human THP-1 monocyte/macrophages. Biochim Biophys Acta. 2008;1781:643–654. doi: 10.1016/j.bbalip.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gill SR, Chow R, Brown AJ. Sterol regulators of cholesterol homeostais and beyond: the oxysterol hypothesis revisited and revised. Prog Lipid Res. 2008;47:391–404. doi: 10.1016/j.plipres.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Janowski BA, Willy PJ, Rama Devi T, Falck JR, Mangelsdorf DJ. An oxysterol signaling pathway mediated by the nuclear receptor LXRα. Nature. 1996;383:728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 14.Lehman JM, Kliewer SA, Moore LB, Smith-Oliver TA, Oliver BB, Su JL, Sundseth SS, Winegar DA, Blanchard DE, Spencer TA, Willson TM. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J Biol Chem. 1997;272:3137–3140. doi: 10.1074/jbc.272.6.3137. [DOI] [PubMed] [Google Scholar]
- 15.Fu X, Menke JG, Chen Y, Zhou G, MacNaul KL, Wright SD, Sparrow CP, Lund EG. 27-Hydroxycholesterol is an endogenous ligand for liver X receptor in cholesterol-loaded cells. J Biol Chem. 2001;276:38378–38387. doi: 10.1074/jbc.M105805200. [DOI] [PubMed] [Google Scholar]
- 16.Ory DS. Nuclear receptor signaling in the control of cholesterol homeostasis: have the orphans found a home? Circ Res. 2004;95:660–670. doi: 10.1161/01.RES.0000143422.83209.be. [DOI] [PubMed] [Google Scholar]
- 17.Ouimet M, Wang MD, Cadotte N, Ho K, Marcel YL. Epoxycholesterol impairs cholesteryl ester hydrolysis in macrophage foam cells, resulting in decreased cholesterol efflux. Arterioscler Thromb Vasc Biol. 2008;28:1144–1150. doi: 10.1161/ATVBAHA.107.157115. [DOI] [PubMed] [Google Scholar]
- 18.Desvergne B, Wahli W. Peroxisome prolififerator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh S, Natarajan R. Cloning of the human cholesteryl ester hydrolase promoter: identification of functional peroxisomal proliferator-activated receptor responsive elements. Biochem Biophys Res Comm. 2001;284:1065–1070. doi: 10.1006/bbrc.2001.5078. [DOI] [PubMed] [Google Scholar]
- 20.Stewart JM, Blakely JA. Long chain fatty acids inhibit and medium chain fatty acids activate mammalian cardiac hexokinase. Biochim Biophys Acta. 2000;1484:278–286. doi: 10.1016/s1388-1981(00)00008-1. [DOI] [PubMed] [Google Scholar]
- 21.Elabbadi N, Day CP, Gamouh A, Zyad A, Yeaman SJ. Relationship between the inhibition of phosphatidic acid phosphohydrolase-1 by oleate and oleoyl-CoA ester and its apparent translocation. Biochimie. 2005;87:437–443. doi: 10.1016/j.biochi.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Murthy SN, Chung PH, Lin L, Lomasney JW. Activation of phospholipase C epsilon by free fatty acids and cross talk with phospholipase D and phospholipase A2. Biochemistry. 2006;45:10987–10997. doi: 10.1021/bi060648+. [DOI] [PubMed] [Google Scholar]
- 23.Tsoutsikos P, Miners JO, Stapleton A, Thomas A, Sallustio BC, Knights KM. Evidence that unsaturated fatty acids are potent inhibitors of renal UDP-glucuronosyl transferases (UGT): kinetic studies using human kidney cortical microsomes and recombinant UGT1A9 and UGT2B7. Biochem Pharmacol. 2004;67:191–199. doi: 10.1016/j.bcp.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 24.Rowland A, Gaganis P, Elliot DJ, Mackenzie PI, Knights KM, Miners JO. Binding of inhibitory fatty acids is responsible for the enhancement of UDP-glucuronosyltransferase 2B7 activity by albumin: implications for in vitro-in vivo extrapolation. J Pharmacol Exp Ther. 2007;321:137–147. doi: 10.1124/jpet.106.118216. [DOI] [PubMed] [Google Scholar]
- 25.Rowland A, Elliot DJ, Knights KM, Mackenzie PI, Miners JO. The “albumin effect” and in vitro-in vivo extrapolation: sequestration of long-chain unsaturated fatty acids enhances phenytoin hydroxylation by human liver microsomal and recombinant cytochrome P450 2C9. Drug Metab Dispos. 2008;36:870–877. doi: 10.1124/dmd.107.019885. [DOI] [PubMed] [Google Scholar]
- 26.Brash AR. Arachidonic acid as a bioactive molecule. J Clin Invest. 2001;107:1339–1345. doi: 10.1172/JCI13210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bencharit S, Edwards CC, Morton CL, Howard-Williams EL, Kuhn P, Potter PM, Redinbo MR. Multisite promiscuity in the processing of endogenous substrates by human carboxylesterase 1. J Mol Biol. 2006;363:201–214. doi: 10.1016/j.jmb.2006.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tagore R, Thomas HR, Homan EA, Munawar A, Saghatelian A. A global metabolite profiling approach to identify protein-metabolite interactions. J Am Chem Soc. 2008;130:14111–14113. doi: 10.1021/ja806463c. [DOI] [PubMed] [Google Scholar]
- 29.Wadkins RM, Hyatt JL, Wei X, Yoon KJ, Wierdl M, Edwards CC, Morton CL, Obenauer JC, Damodaran K, Beroza P, Danks MK, Potter PM. Identification and characterization of novel benzil (diphenylethane-1,2-dione)analogues as inhibitors of mammalian carboxylesterases. J Med Chem. 2005;48:2906–2915. doi: 10.1021/jm049011j. [DOI] [PubMed] [Google Scholar]
- 30.Crow JA, Borazjani A, Potter PM, Ross MK. Hydrolysis of pyrethroids by human and rat tissues: examination of intestinal. liver and serum carboxylesterases. Toxicol Appl Pharmacol. 2007;221:1–12. doi: 10.1016/j.taap.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morton CL, Potter PM. Comparison of Escherichia coli, Saccharomyces cerevisiae, Pichia pastoris, Spodoptera frugiperda, and COS7 cells for recombinant gene expression. Application to a rabbit liver carboxylesterase. Mol Biotechnol. 2000;16:193–202. doi: 10.1385/MB:16:3:193. [DOI] [PubMed] [Google Scholar]
- 32.Sanghani SP, Davis WI, Dumaual NG, Mahrenholz A, Bosron WF. Identification of microsomal rat liver carboxylesterases and their activity with retinyl palmitate. Eur J Biochem. 2002;269:4387–4398. doi: 10.1046/j.1432-1033.2002.03121.x. [DOI] [PubMed] [Google Scholar]
- 33.Ross MK, Borazjani A. Enzymatic activity of human carboxylesterases. Curr Protoc Toxicol Unit 14. 2007;24:1–14. doi: 10.1002/0471140856.tx0424s33. [DOI] [PubMed] [Google Scholar]
- 34.Morgan EW, Yan BD, Greenway D, Petersen DR, Parkinson A. Purification and characterization of two rat liver microsomal CEs (hydrolase A and B) Arch Biochem Biophys. 1994;315:495–512. doi: 10.1006/abbi.1994.1531. [DOI] [PubMed] [Google Scholar]
- 35.Ross MK, Borazjani A, Edwards CC, Potter PM. Hydrolytic metabolism of pyrethroids by human and other mammalian carboxylesterases. Biochem Pharmacol. 2006;71:657–669. doi: 10.1016/j.bcp.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 36.Wadkins RM, Hyatt JL, Yoon KJ, Morton CL, Lee RE, Damodaran K, Beroza P, Danks MK, Potter PM. Discovery of novel selective inhibitors of human intestinal carboxylesterase for the amelioration of irinotecan-induced diarrhea: synthesis, quantitative structure-activity relationship analysis, and biological activity. Mol Pharmacol. 2004;65:1336–1343. doi: 10.1124/mol.65.6.1336. [DOI] [PubMed] [Google Scholar]
- 37.Webb JL. General Principles of Inhibition. Vol. 1. New York: Academic Press Inc; 1963. Enzyme and Metabolic Inhibitors. [Google Scholar]
- 38.Segel I. Biochemical Calculations: How to Solve Mathematical Problems in General Biochemistry. 2. John Wiley and Sons; New York: 1976. [Google Scholar]
- 39. [accessed February 6, 2009];The Human Metabolome Database (HMDB) version 2.0. www.hmdb.ca/metabolites/HMDB02103.
- 40.Hyatt JL, Tsurkan L, Wierdl M, Edwards CC, Danks MK, Potter PM. Intracellular inhibition of carboxylesterases by benzil: modulation of CPT-11 cytotoxicity. Mol Cancer Ther. 2006;5:2281–2288. doi: 10.1158/1535-7163.MCT-06-0160. [DOI] [PubMed] [Google Scholar]
- 41.Ross MK, Crow JA. Role of carboxylesterases in xenobiotic and endobiotic metabolism. J Biochem Mol Toxicol. 2007;21:187–196. doi: 10.1002/jbt.20178. [DOI] [PubMed] [Google Scholar]
- 42.Zhao B, Song J, St Clair R, Ghosh S. Stable over-expression of human macrophage cholesteryl ester hydrolase (CEH) results in enhanced free cholesterol efflux from human THP1-macrophages. Am J Physiol Cell Physiol. 2007;292:C405–C412. doi: 10.1152/ajpcell.00306.2006. [DOI] [PubMed] [Google Scholar]
- 43.Okazaki H, Igarashi M, Nishi M, Tajima M, Sekiya M, Okazaki S, Yahagi N, Ohashi K, Tsukamoto K, Amemiya-Kudo M, Matsuzaka T, Shimano H, Yamada N, Aoki J, Morikawa R, Takanezawa Y, Arai H, Nagai R, Kadowaki T, Osuga J, Ishibashi S. Identification of a novel member of the carboxylesterase family that hydrolyzes triacylglycerol: a potential role in adipocyte lipolysis. Diabetes. 2006;55:2091–2097. doi: 10.2337/db05-0585. [DOI] [PubMed] [Google Scholar]
- 44.Okazaki H, Igarashi M, Nishi M, Sekiya M, Tajima M, Takase S, Takanashi M, Ohta K, Tamura Y, Okazaki S, Yahagi N, Ohashi K, Amemiya-Kudo M, Nakagawa Y, Nagai R, Kadowaki T, Osuga J, Ishibashi S. Identification of neutral cholesterol ester hydrolase, a key enzyme removing cholesterol from macrophages. J Biol Chem. 2008;283:33357–33364. doi: 10.1074/jbc.M802686200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwer H, Langmann T, Daig R, Becker A, Aslanidis C, Schmitz G. Molecular cloning and characterization of a novel putative carboxylesterase, present in human intestine and liver. Biochem Biophys Res Commun. 1997;233:117–120. doi: 10.1006/bbrc.1997.6413. [DOI] [PubMed] [Google Scholar]
- 46.Wong J, Quinn CM, Brown AJ. Statins inhibit synthesis of an oxysterol ligand for the liver X receptor in human macrophages with consequences for cholesterol efflux. Arterioscler Thromb Vasc Biol. 2008;24:2365–2371. doi: 10.1161/01.ATV.0000148707.93054.7d. [DOI] [PubMed] [Google Scholar]
- 47.Honda A, Yamashita K, Hara T, Ikegami T, Miyazaki T, Shrai M, Xu G, Numazawa M, Matsuzaki Y. Highly sensitive quantification of key regulatory oxysterols in biological samples by LC-ESI-MS/MS. J Lipid Res. 2009;50:350–357. doi: 10.1194/jlr.D800040-JLR200. [DOI] [PubMed] [Google Scholar]
- 48.Brown AJ, Jessup W. Oxysterols and atherosclerosis. Atherosclerosis. 1999;142:1–28. doi: 10.1016/s0021-9150(98)00196-8. [DOI] [PubMed] [Google Scholar]
- 49.Kobayashi A, Takanezawa Y, Hirata T, Shimizu Y, Misasa K, Kioka N, Arai H, Ueda K, Matsuo M. Efflux of sphingomyelin, cholesterol, and phosphatidylcholine by ABCG1. J Lipid Res. 2006;47:1791–1802. doi: 10.1194/jlr.M500546-JLR200. [DOI] [PubMed] [Google Scholar]
- 50.Venkateswaran A, Repa JJ, Lobaccaro JMA, Bronson A, Mangelsdorf DJ, Edwards P. Human White/murine ABC8 mRNA levels are highly induced in lipid-loaded macrophages. J Biol Chem. 2000;275:14700–14707. doi: 10.1074/jbc.275.19.14700. [DOI] [PubMed] [Google Scholar]
- 51.Pegorier JP, Le May C, Girard J. Control of gene expression by fatty acids. J Nutr. 2004;134:2444S–2449S. doi: 10.1093/jn/134.9.2444S. [DOI] [PubMed] [Google Scholar]
- 52.Liu Y, Patricelli MP, Cravatt BF. Activity-based protein profiling: the serine hydrolases. Proc Natl Acad Sci U S A. 1999;96:14694–14699. doi: 10.1073/pnas.96.26.14694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. General kinetic model for an enzyme inhibitor. See text for definition of abbreviations.
Supplementary Figure 2. Inhibition of recombinant CES1 by 27-hydroxycholesterol—Activity-based probe assay. (A) The extent of FP-biotin–mediated covalent modification of the recombinant CES1 active site is reduced by 30 min preincubation with 27-hydroxycholesterol. (B) Plot of integrated band density of each lane in (A) versus 27-hydroxycholesterol concentration. IC50 value for 27-HC is shown. See supplementary method for experimental details. Experiment is representative of two independent trials.