Abstract
Reactive astrogliosis, whereby astrocytes undergo varying molecular and morphological changes, is a ubiquitous but poorly understood hallmark of all central nervous system pathologies. Now genetic tools are enabling the molecular dissection of the functions and mechanisms of reactive astrogliosis in vivo. Recent studies provide compelling evidence that reactive astrogliosis may exert both beneficial and detrimental effects in a context-dependent manner determined by specific molecular signaling cascades. Reactive astrocytes may have both loss of normal functions and gain of abnormal effects that can feature prominently in a variety of disease processes. This article reviews developments in the signaling mechanisms that regulate specific aspects of reactive astrogliosis and highlights the potential to identify novel therapeutic molecular targets for diverse neurological disorders.
Introduction
Astrocytes (Fig. 1A) are complex, highly differentiated cells that tile the entire central nervous system (CNS) in a contiguous fashion and that make numerous essential contributions to normal function in the healthy CNS, including regulation of blood flow, provision of energy metabolites to neurons, participation in synaptic function and plasticity, and maintenance of the extracellular balance of ions, fluid balance and transmitters 1-4. In addition, astrocytes respond to all forms of CNS insults such as infection, trauma, ischemia and neurodegenerative disease by a process commonly referred to as reactive astrogliosis, which involves changes in their molecular expression and morphology (Fig. 1B), and in severe cases, scar formation (Fig. 1C) 5-9. In spite of the long-standing recognition that astrocytes have the potential to undergo these changes after CNS insults, and in spite of the ubiquitous presence of reactive astrocytes at all sites of CNS pathology, the functions and effects of reactive astrocytes are surprisingly poorly understood and their roles in specific disease processes are largely uncertain.
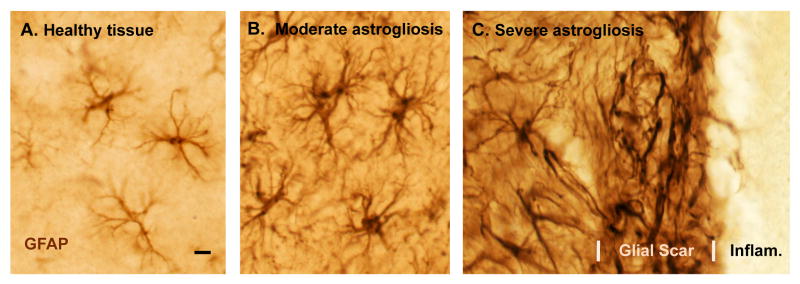
Figure 1. Photomicrographs of astrocytes in healthy tissue and of different gradations of reactive astrogliosis and glial scar formation after tissue insults of different types and different severity.
(A-C) Immunohistochemical staining of glial fibrillary protein (GFAP) in wild-type mice. Note that GFAP staining demonstrates the main stem processes and general appearance of the astrocytes, but does not reveal all of the fine branches and ramifications. (A) Appearance of ‘normal’ astrocytes in healthy cerebral cortex of an untreated mouse. Note that the territories of astrocyte processes do not overlap. (B) Moderately reactive astrogliosis in mouse cerebral cortex in response to intracerebral injection of the bacterial antigen, lipopolysaccharide (LPS). Note that the territories of moderately reactive astrocyte processes also do not overlap. (C) Severely reactive astrogliosis and glial scar formation adjacent to a region of severe traumatic injury and inflammation (Infam.) in the cerebral cortex. Note the extensive overlap and inter-digitations of processes of severely reactive and scar-forming astrocytes. Scale bar = 8μm.
Perhaps the most well known aspect of reactive astrogliosis is that of scar formation. The ability of astrocytes to form scars that inhibit axon regeneration has been recognized for well over 100 years and has led to an overall negative connotation that has long dominated concepts about the ramifications of reactive astrogliosis. Nevertheless, a growing body of information indicates that reactive astrocytes exert a number of essential beneficial effects and that astrocytes have a wide spectrum of potential, and often subtle, responses to CNS insults, of which scar formation is only one and lies at the extreme end in terms of its severity. This article summarizes recent advances in the molecular dissection of the functions and mechanisms of reactive astrogliosis, with a main focus on deletion experiments using transgenic mouse models that allow either cellular ablation or molecular deletion in combination with different types of injury or disease paradigms in vivo. The article begins with a definition and model of astrogliosis that includes surveys of molecules produced by reactive astrocytes and of triggering mechanisms and signaling pathways that regulate astrogliosis. It concludes with surveys of the functions of astrogliosis, the potential for dysfunction to contribute to disease mechanisms, and the identification of novel therapeutic targets. Space constraints prevent exhaustive review of all topics and limit discussion to a cross-section of recent advances.
Defining reactive astrogliosis
What is astrogliosis? What features distinguish a reactive astrocyte from one that is non-reactive? Is astrogliosis an all-or-none process or a gradated one? Is it a good thing, or bad? What are its molecular triggers, or its functional consequences? Is astrogliosis synonymous with scar-formation? Perhaps a majority of well-informed neurobiologists would be hard pressed to answer such questions. In spite of the increasing recognition that astrocytes play central roles in normal CNS function and that reactive astrocytes are primary responders to injury and disease, the concept of reactive astrogliosis seems generally elusive, with no commonly agreed upon definition or model. It is therefore useful to begin a review of recent studies into the functions and mechanisms of reactive astrogliosis by presenting a working model that defines various aspects of astrogliosis in a manner parsimonious with currently available information, while recognizing that any such model will require updating as new information accrues.
This article proposes a definition and model of ‘reactive astrogliosis’ that integrates four interdependent key features: (i) reactive astrogliosis is a spectrum of changes in astrocytes that occur in response to all forms and severities of CNS injury and disease including subtle perturbations; (ii) the changes undergone by reactive astrocytes vary with the nature and severity of the insult along a gradated continuum of progressive alterations in molecular expression, progressive cellular hypertrophy and, in severe cases, proliferation and scar formation (Figs. 1,2), (iii) the changes of astrogliosis are regulated in a context-specific manner by specific signaling events that have the potential to modify both the nature and degree of those changes; (iv) the changes undergone during reactive astrogliosis have the potential to alter astrocyte activities both through gain and loss of functions that can impact both beneficially and detrimentally on surrounding neural and non-neural cells.
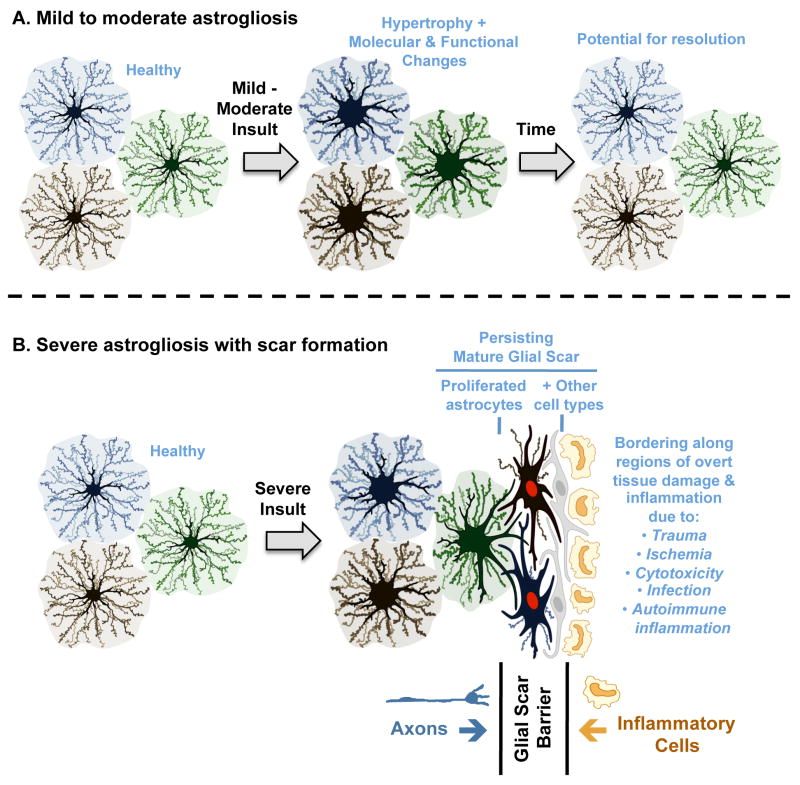
Figure 2. Schematic representations of different gradations of reactive astrogliosis that vary with insult severity.
(A) Mild to moderate reactive astrogliosis comprises variable changes in molecular expression and functional activity together with variable degrees of cellular hypertrophy. Such changes occur after mild trauma or at sites distant from a more severe injury, or after moderate metabolic or molecular insults or milder infections or inflammatory activation. These changes vary with insult severity, involve little anatomical overlap of the processes of neighboring astrocytes and exhibit the potential for structural resolution if the triggering insult is removed or resolves. (B) Severe reactive astrogliosis with persisting scar formation generally occurs along borders to areas of overt cell and tissue damage and inflammation. Glial scar formation includes newly proliferated astrocytes (with red nuclei in figure) and other cell types (with grey nuclei in figure) such as fibromeningeal cells and other glia. In the mature glial scar, astrocytes no longer occupy discrete domains and instead have overlapping processes. Mature glial scars tend to persist for long periods and act as barriers not only to axon regeneration but also to inflammatory cells in a manner that protects healthy tissue from nearby areas of intense inflammation.
According to this definition and model, reactive astrogliosis is not an all-or-none response, nor is it a single uniform process, nor is it ubiquitously synonymous with scar formation. Instead, reactive astrogliosis is a finely gradated continuum of progressive changes in gene expression and cellular changes (Figs. 1,2) that are subtly regulated by complex inter- and intra-cellular signaling as discussed in detail below. In its mild and moderate forms, reactive astrogliosis exhibits the potential for resolution if the triggering mechanism has resolved, in which the cells return to an appearance similar to that in healthy tissue (Fig. 2A). The extent to which various molecular changes resolve or persist is not well known. In this context, it is interesting to note that, in healthy tissue, the extensive network of finely branched processes of individual astrocytes occupy contiguous non-overlapping domains 10. In mild-to-moderate reactive astrogliosis, there appears to be preservation of the individual non-overlapping domains of reactive astrocytes in spite of the hypertrophy of the cell body and processes 11 (Fig. 1B, 2A). At its extreme level of activation in response to overt tissue damage and inflammation, reactive astrogliosis involves scar formation that incorporates newly proliferated cells and in which astrocyte processes overlap in manners not seen in healthy tissue 12-14 (Fig. 1C,2B). It deserves mention that astrocytes interact with other cell types, in particular fibromeningeal and other glial cells (such as NG2-positive glia) to form complex glial scars in the CNS 14, 15 (Fig. 2B). The structural changes associated with scar formation are long lasting and persist long after the triggering insult may have resolved (Fig. 1C,2B). The striking potential differences along the continuum of reactive astrocyte responses to insults of different kinds are likely to be of importance when considering the functions and impact of reactive astrogliosis on CNS functions, as discussed below. In other words, reactive astrogliosis of different kinds will have different consequences.
Extensive molecular repertoire of reactive astrocytes
A detailed catalogue of information is now available regarding the many different molecules that can be produced by astrocytes under different stimulation conditions, enabling the development of hypotheses regarding potential functions or effects of reactive astrogliosis. This information is available from several decades of in vitro studies looking at biochemical measures of molecular expression in primary astrocyte cell cultures and from recent studies using gene array and proteomic analyses 5, 16-20. Such studies show that astrocytes have the potential under different conditions of stimulation to produce intercellular effector molecules of many different categories, or to alter their expression of molecules involved in all aspects of cellular activity including cell structure, energy metabolism, intracellular signaling and membrane transporters and pumps (Table 1). Such changes in molecular expression undergone by astrocytes as they become reactive have the potential to have pronounced effects on surrounding neural cells and these studies provide much-needed information for functional analyses. A caveat regarding biochemical analyses of large batches of astrocytes grown in vitro is the potential to miss or mask cellular heterogeneity. Increasing evidence points towards heterogeneity of astrocytes that derive from different CNS regions 20, and even so-called primary astrocyte cultures derived from single regions are comprised of heterogeneous cell populations that exhibit heterogeneity of molecular phenotype and functional characteristics among individual cells within the same cultures 21. This topic is not yet well studied and single-cell evaluations will be needed to probe astrocyte heterogeneity and the possibility that different types of astrocytes may exert different roles during reactive astrogliosis.
Table 1. Different functional categories of genes and molecules modulated by reactive astrocytes in vivo or in vitro.
| Aspects of Reactive astrocytes | Modulatory Effect in astrocytes |
|---|---|
| Structural | GFAP ↑; Vimentin ↑; Nestin ↑5,6 |
| Transcriptional regulators | NFκB↑; STAT3↑; cAMP↑; Olig2 ↑; mTor↑; SOX9↑; etc. 14, 52, 83-85 |
| Extracellular matrix and cell-cell interactions | Chondroitin sulfate proteoglycans ↑↓; collagens ↑↓; laminins ↑; integrins↑; cadherins↑↓; Ephrins↑; metalloproteases ↑; 65, 84, 86, 87 |
| Inflammatory cell regulators | Cytokines & growth factors ↑↓; Glutathione ↑↓ 5, 43,44, 49, 84 |
| Fluid and ion homeostasis | AQP4 ↑↓; Na/K transporters ↑↓ 39, 56 |
| Extracellular transmitter clearance | Glutamate transporter ↑↓ 8, 38 |
| Vascular regulators | PGE ↑↓ NO↑↓; 54, 55 |
| Purines & receptors | ATP↑↓; P2Y↑↓ 25 |
| Oxidative stress & protection | NO ↑↓; NOS ↑↓; SOD ↑↓; Glutathione ↑↓; 43, 44, 69 |
| Gap junction proteins | C×43↑↓ 88 |
| Energy provision | Lactate ↑↓ 3 |
| Synapse formation & remodeling | Thrombospondin ↑↓; Complement C1q ↑↓; 58, 59 |
↑↓ arrows indicate up or down regulation. Note that some families of molecules (e.g. cytokines) simultaneously can have some members that are ↑ and some that are ↓ regulated. In addition, some individual molecules (e.g. glutathione) can be either ↑or↓ regulated by different triggers of astrogliosis or at different times after insults.
Triggers and signaling mechanisms of reactive astrogliosis
Many different types of molecules that can be generated through a wide variety of different mechanisms are able to trigger aspects of reactive astrogliosis as summarized in Table 2. Molecular mediators of reactive astrogliosis can be released by any cell type in CNS tissue, including neurons, microglia, oligodendrocyte lineage cells, endothelia, leukocytes and other astrocytes, in response to CNS insults ranging from subtle cellular perturbations to intense tissue injury and cell death. Although we are in the early stages of dissecting the signaling routes between specific forms of CNS damage or disease and specific aspects of reactive astrogliosis, there is now considerable evidence, as summarized in Table 3, that different specific signaling mechanisms trigger different molecular, morphological and functional changes in reactive astrocytes in a manner that reflects the gradated responses of reactive astrogliosis (Figs. 1,2). It deserves emphasis that the responses of reactive astrocytes can differ in response to different signals in a context-dependent manner and have the potential to impact on essentially all aspects of neural function, from regulation of blood flow to provision of energy to synaptic function and plasticity (Table 3). Moreover, in some cases different specific signaling mechanisms have the potential to induce reactive astrocytes to have different and sometimes opposite effects, for example by stimulating production of molecules that might be pro- or anti-inflammatory, or that might increase or reduce oxidative stress (Table 3). These seemingly paradoxical effects need not be viewed as being contradictory, but rather that reactive astrocytes can exert different effects in response to different challenges or during different phases of the response to insults as the response progresses over time.
Table 2. Molecular triggers and modulators of reactive astrogliosis.
| Process in astrogliosis | Implicated factors |
|---|---|
| Cytokines & growth factors | Il6, LIF, CNTF, IL1, Il10, TGFβ, TNFα, INFγ, etc. 49, 67 |
| Mediators of innate immunity | LPS & other Toll-like receptor ligands 51 |
| Neurotransmitters & modulators | Glutamate, Noradrenalin 89 |
| Small molecules released by cell injury | ATP 90 |
| Molecules of oxidative stress | Nitric oxide (NO), reactive oxygen species (ROS) 43 |
| Ischemia associated | Hypoxia and glucose deprivation 43 |
| Neurodegeneration associated | Amyloid-beta 91 |
| Systemic metabolic toxicity | NH4+ 92 |
Table 3. Signaling pathways and molecules implicated in mediating specific aspects of reactive astrogliosis.
| Specific aspect of astrogliosis | Signaling pathways and molecules |
|---|---|
| Structural molecules: GFAP ↑; Vimentin ↑; Nestin ↑ | STAT3 14; NFκB 52; cAMP, Lipocalin-Rho-kinase 93 |
| Astrocyte hypertrophy↑ | STAT3 14 |
| Astrocyte proliferation↑ | Olig2 85; Endothelin-1 26; JNK/c-Jun 26 |
| Astrocyte migration↑ | CEPB1 94 |
| Astrocyte scar formation↑ | STAT3 14; EphB2 15 |
| Scar related axon growth inhibitors↑ | Egr-1 95; TGFβ 84; SOX9 84 |
| Pro-inflammatory | NFκB 42, 52; P2X7 96; MAP-kinase 96 |
| Anti-inflammatory | STAT3 14; Nrf2 45, 46; SOCS1 & 3 97 |
| Limiting infection | gp130 22 |
| Extracellular homeostasis & neuroprotection via glutamate transporters↑ | cAmp, PI-3K/Akt, 98; EGF, FGF 99 |
| Neuroprotection via glutathione ↑ & Oxidative stress ↓ | Nrf2 45,46 |
| Bacteriocidal and cytotoxic molecules: NO ↑; NOS ↑ | IFγ, Toll-like receptor ligands, TGFβ 69 |
Reactive astrocyte proliferation and scar formation
In its extreme form, reactive astrogliosis can lead to the appearance of newly proliferated astrocytes and scar formation (Fig. 2B) in response to severe tissue damage or inflammation after severe trauma, stroke, infection, autoimmune responses or degenerative disease 13, 22, 23. Molecular triggers that lead to proliferation of reactive astrocytes in vivo are incompletely characterized but include EGF, FGF, endothelin 1 and ATP 24-26. The source of newly divided scar-forming astrocytes is not well established, and there are several potential possibilities. Perhaps the strongest evidence thus far is that mature astrocytes can re-enter the cell cycle and proliferate during scar formation 12, 26, 27.
Evidence has also been presented that some proliferating reactive astrocytes may derive from NG2 progenitor cells in the local parenchyma 28 or from ependymal cell progenitors after injury or stroke 29, 30. Another potential source may be multipotent progenitors in subependymal tissue that express glial fibrillary acidic protein (GFAP) 31 and generate progeny cells that migrate towards sites of injury after trauma or stroke 32, but thus far no evidence has been presented that these particular progenitors give rise to newly generated scar-forming reactive astrocytes. Additional studies will be required to establish the relative contributions of these potential sources.
Dissecting the functions and mechanisms of reactive astrogliosis
Transgenic manipulations and other molecular techniques provide powerful tools with which to test hypotheses regarding functions and effects of reactive astrogliosis in vivo using experimental models of specific CNS insults. Techniques that are able to target genetic manipulations specifically to astrocytes (Box 1), in combination with techniques that enable either ablating specific cell types or the knockout or knockdown of specific molecules 7 are enabling the molecular dissection of reactive astrocyte biology in vivo.
Deletion experiments: Ablation of proliferating scar-forming astrocytes
Scar formation is a prominent and widely recognized aspect of reactive astrogliosis. To look for potential functions of scar-formation, an experimental deletion model was developed that allowed the ablation of proliferating scar-forming reactive astrocytes 12, 33. The model is based on the transgenically targeted expression of herpes simplex virus thymidine kinase (HSV-TK) specifically to astrocytes, which renders dividing astrocytes vulnerable to the antiviral drug ganciclovir. This model, which allows both cell-type-specific and temporal control of cell ablation, has now been applied in combination with various forms of experimental CNS insults, including forebrain stab injury 12, spinal cord injury 13, traumatic brain injury 34 and experimental autoimmune encephalomyelitis (EAE) 23. The results can be summarized as follows. Proliferating reactive scar-forming astrocytes (Fig. 2) were consistently found along borders between healthy tissue and pockets of damaged tissue and inflammatory cells after (i) the rapid, locally triggered, innate inflammatory response to acute traumatic injury in spinal cord and brain 7, 12, 13, 34, as well as (ii) during the chronic, peripherally initiated, adaptive autoimmune inflammation associated with EAE 23. In all of these models, ablation of proliferating reactive astrocytes disrupted scar formation, which in turn resulted in increased spread and persistence of inflammatory cells, failure of repair of the blood-brain barrier (BBB) to serum proteins, increased tissue damage and lesion size, increased neuronal loss and demyelination, and exacerbation of clinical signs or impaired recovery of function 7, 12, 13, 23, 34, providing evidence that scar-forming astrocytes play essential roles in neural protection and repair.
Deletion experiments: Knockout or knockdown of molecules produced by reactive astrocytes
A number of different strategies have been employed to study the effects of deletion or knockdown of molecules produced by reactive astrocytes. The first used has been global gene deletion or knockout (KO) of molecules preferentially expressed by astrocytes such as GFAP 35, GFAP plus vimentin 36, 37, glutamate transporter 38, aquaporin 4 (AQP4) 39 and connexin 43 (C×43) 40. These studies have provided insights regarding the roles of specific molecules in reactive astrogliosis. For example, KO of the intermediate filaments GFAP and vimentin reduce astrogliosis and increase axon regeneration 6, 37, but exacerbate EAE 35 and stroke 36 by increasing lesion size and worsening outcome. KO of astrocyte glutamate transporters is associated with seizures and neurodegeneration 38. KO of the astrocyte water channel AQP4 reduces cytotoxic edema and improves outcome after stroke 39. KO of the astrocyte gap junction protein C×43 disrupts the neuroprotective effect of pre-conditioning to hypoxia 40.
Another approach has been to delete preferentially from astrocytes molecules that are active in many cell types by using the Cre-loxP system, which allows for conditional knockout (CKO) of molecules from specific cell types 7. CKO from astrocytes of the signaling molecule STAT3, which is an intracellular signal transducer for various cytokines such as interleukin 6 (Il6), ciliary neurotrophic factor (CNTF) and leukemia inhibitory factor (LIF), markedly attenuates various aspects of reactive astrogliosis, including cell hypertrophy, upregulation of GFAP and scar formation, leading to increased inflammation, increased lesion size and demyelination and impaired functional recovery 14, 41. Interestingly, CKO of Gp130, the extracellular receptor for the cytokines Il6, CNTF and LIF, exacerbates the spread and severity of CNS infection 22. In contrast, conditional deletion or functional knockdown in astrocytes of the signaling molecules suppressor of cytokine signaling 3 (SOCS3) or NF-κB, reduce inflammation and lesion size after spinal cord injury or EAE 41, 42. Taken together, these findings, point towards multiple, complex functions of reactive astrocytes, which may differ in response to different signaling mechanisms in a context-specific manner, for example at different times after insults or in accordance with different combinations of circumstances.
‘Big picture’ functions of reactive astrogliosis and glial scar formation
Our understanding about the functions and effects of reactive astrogliosis and scar formation and their impact on neural function is at an early stage. The over 100 year long emphasis on glial scar formation as an inhibitor of axon regeneration has led to a widespread negative view of reactive astrogliosis per se. In this light, it is important to emphasize that many new lines of evidence point towards numerous essential beneficial functions of reactive astrogliosis and scar formation, particularly as regards neural protection and repair, and regulation of CNS inflammation.
Reactive astrogliosis and neural protection and repair
A large number of studies provide different kinds of evidence in vivo and in vitro that reactive astrocytes can protect CNS cells and tissue in various ways, including by (i) uptake of potentially excitotoxic glutamate 12, 38, 43, (ii) protection from oxidative stress via glutathione production 43-46, (iii) protection via adenosine release 40, (iv) protection from NH4+ toxicity 47, (v) protection by degradation of amyloid-beta peptides 48 (vi) facilitating blood brain barrier repair 12, (vii) reducing vasogenic edema after trauma, stroke or obstructive hydrocephalus 12, 39, and (viii) stabilizing extracellular fluid and ion balance and reducing seizure threshold 39. In addition, several different types of transgenic models from different laboratories show that either ablation or attenuation of reactive astrogliosis causes increased lesion size, increased neuronal loss, demyelination and exacerbated loss of function after traumatic injury, stroke or infection 12-14, 22, 34, 36, 41, as discussed in detail above. Together, these findings indicate that reactive astrocytes can exert a range of essential neuroprotective and repair-related functions in response to CNS insults of various kinds, including trauma, infection, stroke, and degenerative disease.
Reactive astrogliosis, scar formation and regulation of inflammation
Of particular interest regarding functions of reactive astrogliosis and glial scar formation is recent new in vivo evidence that they play important roles in regulating CNS inflammation. It has been known for some time that astrocytes in vitro have the capacity to make many different kinds of molecules with either pro- or anti-inflammatory potential in response to different kinds of stimulation 5, 49. In addition, astrocytes interact extensively with microglia, key players in CNS inflammation, and can exert both pro- and anti-inflammatory effects on microglia 50, 51. In agreement with these findings, recent transgenic deletion studies indicate that reactive astrocytes can exert both pro- and anti-inflammatory regulatory functions in vivo. As regards anti-inflammatory effects, several different transgenic models used in different laboratories indicate that either ablation or attenuation of reactive scar-forming astrocytes can exacerbate the spread of inflammatory cells during (i) locally initiated innate inflammatory responses to traumatic injury 12-14, 36, 41, or (ii) during peripherally initiated adaptive immune responses such as EAE 23, 35. In this regard it is also noteworthy that attenuation of reactive astrocytes also leads to increased spread of infection 22. Nevertheless, reactive astrocytes also have pro-inflammatory potential, and other studies provide evidence that deletion or knockdown of certain molecules in astrocytes is associated with a reduction in inflammation after traumatic injury or EAE 41, 42, 52. Together these findings indicate that astrocyte involvement in the regulation of CNS inflammation is complex and likely to be context dependent and regulated by multimodal extra- and intra-cellular signaling events that may differ at different time points after insults. A functional model that may reconcile the apparent paradox of the potential to exert both pro- and anti-inflammatory activities might be that reactive astrocytes function not only to activate inflammation, in particular at early times after insults, but also over time form potent cell migration barriers that demarcate areas where intense inflammation is needed and restrict the spread of inflammatory cells and infectious agents into nearby healthy tissue 7 (Fig. 2B). This model is congruent with ideas that evolutionary pressures shaping CNS injury responses are likely to have favored mechanisms to keep small injuries small and uninfected, and that the inhibition of the migration of inflammatory cells and infectious agents and other cells might have led to the accidental by-product of inhibiting axon regeneration owing to the redundancy between migration cues across cell types 7, 12 (Fig. 2B). These notions might be of relevance when considering how best to develop therapeutic strategies to promote axon regeneration through scar tissue without producing unwanted potential side effects such as exacerbated spread of inflammatory cells.
Dysfunctions or effects of reactive astrogliosis as potential disease mechanisms
The studies described above provide compelling evidence that reactive astrogliosis is a ubiquitous, complex and essential part of the response to all CNS insults. In addition, there is also a growing realization that dysfunctions or effects of reactive astrogliosis may contribute to, or to be primary sources of, CNS disease mechanisms either through loss of essential functions performed by astrocytes or by reactive astrocytes, or through gain of detrimental effects.
Potential for loss of essential functions by reactive astrocytes
In healthy neural tissue, astrocytes play critical roles in many functions, including energy provision 3, 53, regulation of blood flow 54, 55, homeostasis of extracellular fluid 39, 56, homeostasis of ions 56 and transmitters 8, and regulation of synapse function 4, 57 and synaptic remodeling 58, 59. In addition, different types of transgenic deletion studies show that reactive astrocytes exert a number of essential beneficial functions in response to CNS insults, including BBB repair, neural protection and restricting the spread of inflammatory cells and infection as discussed in detail above. A growing awareness is emerging from both experimental and clinical studies 1, 7, 60, 61 that loss or disturbance of functions normally performed by astrocytes or reactive astrocytes during the process of reactive astrogliosis has the potential to underlie neural dysfunction and pathology in various conditions including trauma, stroke, multiple sclerosis and others. Genetic animal models are providing evidence for ways in which loss or attenuation of reactive astrocyte functions might worsen outcome after various kinds of CNS insults for example through excitotoxic neurodegeneration due to failure or attenuation of glutamate uptake 12, 38, 43 or increased inflammation or infection due to loss or failure of astrocyte functions 12-14, 22, 23. In addition, clinical studies are beginning to identify conditions precipitated by loss of astrocyte functions, for example, autoimmune destruction of astrocyte endfeet that contact and envelop blood vessels is associated with CNS inflammation and a form of multiple sclerosis 62, and in Rasmussen's disease autoantibody destruction of astrocytes causes seizures 63, 64.
Potential for gain of detrimental effects by reactive astrocytes
Much of the attention paid to possible involvement of reactive astrocytes in disease has focused on the potential for gain of detrimental effects, the classical example of which is the inhibition of axon regeneration by reactive scar-forming astrocytes that was first proposed over 100 years ago and is now supported by extensive molecular and cell-biological evidence 65. Following on from this early negative ‘branding’ of reactive astrocytes, there has been a tendency among certain authors to typecast the entire process of reactive astrogliosis as a uniformly negative and maladaptive phenomenon that unavoidably causes neurotoxicity, inflammation or chronic pain, and that total inhibition of reactive astrogliosis can be regarded as a therapeutic strategy. This simplistic and uniformly negative viewpoint is no longer tenable and it is clear that there is a normal process of reactive astrogliosis that exerts many beneficial effects and does not do harm. Nevertheless, there is also evidence from both clinical and experimental studies that, under specific circumstances, astrocytes or reactive astrocytes have the potential to exert detrimental effects. For example, clinical studies have identified at least two single gene mutations that cause gain of detrimental effects by astrocytes and/or reactive astrocytes. In Alexander's disease, a dominant, gain-of-function mutation of the gene encoding GFAP is associated with macroencephalopathy, seizures, psychomotor disturbances and premature death 66. In a familial form of amyotrophic lateral sclerosis (ALS) or motor neuron disease, a dominant gain-of-function mutation of the gene encoding superoxide dismutase (SOD) leads to production by reactive astrocytes of molecules that are toxic to motor neurons 67, 68. It is important to emphasize that, in both of these examples, the neuronal toxicity and damage to neural tissue is due to genetically mutated and abnormal astrocytes rather than to the generic process of reactive astrogliosis per se. It is also interesting that transgenic animals and other experimental models are providing evidence that reactive astrocytes might be stimulated by specific signaling cascades to gain detrimental effects such as (i) exacerbation of inflammation via cytokine production 42, 52, (ii) production and release of neurotoxic levels of reactive oxygen species 43, 69, (iii) release of potentially excitotoxic glutamate 70, (iv) potential contribution to seizure genesis 71, 72, (v) compromise of BBB function due to VEGF-production 73, (vi) cytotoxic edema during trauma and stroke through AQP4 over activity 39 and (vii) potential for chronic cytokine activation of astrocytes to contribute to chronic pain 74. In these cases where there is no obvious genetic mutation, the gain of detrimental effects by reactive astrocytes is incompletely understood but may result from specific signaling cascades that result from complex interactions with other cell types such as microglia and other inflammatory cells 75, or for example, by simultaneous exposure to microbial antigens such as lipopolysaccharide (LPS), which can markedly increase the levels of potentially toxic NO produced by astrocytes in response to cytokines 69. Context-dependent acute or chronic combinatorial signaling events involving different cell types might thus stimulate reactive astrocytes to produce potentially cytotoxic levels of molecules or might lead to chronic inflammation or neuropathic pain. A greater understanding of such combinatorial signaling potential could facilitate targeted therapeutic strategies that preserve the beneficial and attenuate the potentially detrimental aspects of reactive astrogliosis. In this regard, it will also be interesting in future studies to look for genetic polymorphisms that may alter reactive astrocyte functions in certain individuals and thereby predispose them to certain disease processes.
Identifying novel therapeutic targets
Molecular dissection of reactive astrocytes is beginning to identify molecules whose functions might be enhanced or blocked in specific disease contexts as potential therapeutic strategies. For example, augmenting the function of the astrocyte glutamate transporter EAAT2 with parawexin 1, a molecule isolated from spider venom, has been shown to protect retinal neurons from ischemic degeneration by enhancing glutamate uptake and thereby reducing the potential for glutamate excitotoxicity 76. A high-throughput screen of small molecules has identified that certain β-lactam antibiotics can enhance astrocyte-mediated glutamate uptake sufficiently to provide neuroprotection in models of stroke and ALS by stimulating the expression of astrocyte glutamate transporters and thereby reducing excitotoxicity 77. Other potential targets for improving outcome by manipulating functions and effects related to reactive astrogliosis might include manipulation of AQP4 channels during different forms of cerebral edema 39, attenuation of NF-κB 42, 52 or augmentation of STAT3 14 signaling mechanisms to reduce inflammation in specific contexts. Additional potential molecular targets associated with reactive astrocyte functions are summarized in Tables 1–3. Understanding the molecular mechanisms of reactive astrogliosis has the potential to identify novel therapeutic targets and strategies for a wide variety of CNS disorders. For example, the β-lactam antibiotic, Ceftriaxone, began stage 3 clinical trials in May 2009 to determine efficacy in reducing excitotoxicity and neurodegeneration in ALS.
Concluding remarks
Studies using molecular dissection techniques provide compelling evidence that reactive astrogliosis is not a single all-or-none response but is a complex and multifaceted process that can involve a finely gradated continuum of changes ranging from subtle and reversible alterations in gene expression and morphology up to the pronounced and long-lasting changes associated with scar formation. Accumulating evidence shows that the responses of reactive astrocytes to CNS insults are controlled in a context-dependent manner by complex and combinatorial inter- and intra-cellular signaling mechanisms that mediate numerous essential beneficial functions, but that under certain circumstances can also lead to harmful effects. Big-picture functions of reactive astrogliosis and scar formation are being identified that include protecting neural cells, tissue and function, and restricting the spread of inflammation and infection. Increasing evidence indicates that dysfunctions of the process of reactive astrogliosis and scar formation have the potential to contribute to, or to be primary causes of, CNS disease mechanisms either through loss of normal functions or through gain of detrimental effects. Future directions include identifying specific factors that might alter or perturb the process of reactive astrogliosis through (i) genetic mutations or polymorphisms, (ii) autoimmune events, or (iii) simultaneous exposure to combinatorial activators. The simplistic but widely held notions that reactive astrogliosis and scar formation are maladaptive responses, and that complete blockade of reactive astrogliosis per se will be beneficial, are no longer tenable. Therapeutic strategies need to be directed at specific aspects of reactive astrogliosis that might be augmented or attenuated for specific purposes. In this regard, molecular dissection of specific aspects of reactive astrogliosis represents a powerful means of identifying novel potential therapeutic targets that could be of interest in a wide range of neurological disorders.
Box 1.
Selectivity of promoters for targeting genetic manipulations to astrocytes
Several different promoters are now in use for targeting transgenes to astrocytes and their differences in spatial and temporal regulation warrant brief discussion. A small fragment of the human GFAP promoter (hGFAP) in common use is expressed robustly not only by mature astrocytes but also by early developmental radial progenitors and consequently targets Cre-mediated gene deletion to a substantial proportion of forebrain neurons 78. While useful to study the lineage of neurons derived from these progenitors 79, this technique is not suitable for targeting conditional gene deletion selectively to astrocytes. Endogenous mouse GFAP does not appear to be expressed by radial cell progenitors until the peri- and postnatal periods, and large promoter constructs of the mouse GFAP gene (mGFAP) can target Cre-mediated gene deletion exclusively to astrocytes in the spinal cord 14 and most brain regions other than the restricted sites of postnatal neurogenesis 31. Temporal regulation of gene expression can be achieved using tamoxifen systems and these have been applied to astrocyte targeting using various promoters including hGFAP and mGFAP 80, 81 and the astrocyte glutamate transporter GLAST 82. For all transgenic models there are caveats. Targeting specificity and efficiency must be confirmed at the single-cell level for every region studied using multiple labeling techniques 14. With regard to tamoxifen-induced expression, variability of efficacy among individual mice can complicate quantitative comparisons. As regards injury studies, tamoxifen has potent anti-estrogenic effects that may impact on the response to traumatic injury, stroke or inflammation. Each of the systems has pros and cons and it is important to understand their appropriate uses and limitations.
Acknowledgments
The author's work is supported by NIH NINDS (NS057624) and the Roman Reed Spinal Cord Injury Initiative of California. The author thanks Donna Crandal for artwork.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60:430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Nedergaard M, et al. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Pellerin L, et al. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. 2007;55:1251–1262. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- 4.Seifert G, et al. Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci. 2006;7:194–206. doi: 10.1038/nrn1870. [DOI] [PubMed] [Google Scholar]
- 5.Eddleston M, Mucke L. Molecular profile of reactive astrocytes -implications for their role in neurological disease. Neuroscience. 1993;54:15–36. doi: 10.1016/0306-4522(93)90380-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 7.Sofroniew MV. Reactive astrocytes in neural repair and protection. Neuroscientist. 2005;5:400–407. doi: 10.1177/1073858405278321. [DOI] [PubMed] [Google Scholar]
- 8.Maragakis NJ, Rothstein JD. Mechanisms of Disease: astrocytes in neurodegenerative disease. Nat Clin Pract Neurol. 2006;2:679–689. doi: 10.1038/ncpneuro0355. [DOI] [PubMed] [Google Scholar]
- 9.Correa-Cerro LS, Mandell JW. Molecular mechanisms of astrogliosis: new approaches with mouse genetics. J Neuropathol Exp Neurol. 2007;66:169–176. doi: 10.1097/01.jnen.0000248555.53079.d5. [DOI] [PubMed] [Google Scholar]
- 10.Bushong EA, et al. Protoplasmic astrocytes in CA1 atratum radiatum occupy separate anatomical domains. J Neurosci. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilhelmsson U, et al. Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc Natl Acad Sci USA. 2006;103:17513–17518. doi: 10.1073/pnas.0602841103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bush TG, et al. Leukocyte infiltration, neuronal degeneration and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. doi: 10.1016/s0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- 13.Faulkner JR, et al. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrmann JE, et al. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci. 2008;28:7231–7243. doi: 10.1523/JNEUROSCI.1709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bundesen LQ, et al. Ephrin-B2 and EphB2 regulation of astrocyte-meningeal fibroblast interactions in response to spinal cord lesions in adult rats. J Neurosci. 2003;23:7789–7800. doi: 10.1523/JNEUROSCI.23-21-07789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.John GR, et al. IL-1-regulated responses in astrocytes: relevance to injury and recovery. Glia. 2005;49:161–176. doi: 10.1002/glia.20109. [DOI] [PubMed] [Google Scholar]
- 17.Cahoy JD, et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lovatt D, et al. The transcriptome and metabolic gene signature of protoplasmic astrocytes in the adult murine cortex. J Neurosci. 2007;27:12255–12266. doi: 10.1523/JNEUROSCI.3404-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daginakatte GC, et al. Expression profiling identifies a molecular signature of reactive astrocytes stimulated by cyclic AMP or proinflammatory cytokines. Exp Neurol. 2008;210:261–267. doi: 10.1016/j.expneurol.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 20.Yeh TH, et al. Microarray analyses reveal regional astrocyte heterogeneity with implications for neurofibromatosis type 1 (NF1)-regulated glial proliferation. Glia. 2009;57:1239–1249. doi: 10.1002/glia.20845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Imura T, et al. Phenotypic and functional heterogeneity of GFAP-expressing cells in vitro: Differential expression of LeX/CD15 by GFAP-expressing multipotent neural stem cells and non-neurogenic astrocytes. Glia. 2006;53:277–293. doi: 10.1002/glia.20281. [DOI] [PubMed] [Google Scholar]
- 22.Drogemuller K, et al. Astrocyte gp130 expression is critical for the control of Toxoplasma encephalitis. J Immunol. 2008;181:2683–2693. doi: 10.4049/jimmunol.181.4.2683. [DOI] [PubMed] [Google Scholar]
- 23.Voskuhl RR, et al. Reactive astrocytes form scar-like barriers to leukocytes during adaptive immune inflammation of the central nervous system. J Neurosci. 2009 doi: 10.1523/JNEUROSCI.1514-09.2009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levison SW, et al. IL-6-type cytokines enhance epidermal growth factor-stimulated astrocyte proliferation. Glia. 2000;32:328–337. doi: 10.1002/1098-1136(200012)32:3<328::aid-glia110>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 25.Neary JT, Zimmermann H. Trophic functions of nucleotides in the central nervous system. Trends Neurosci. 2009;32:189–198. doi: 10.1016/j.tins.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Gadea A, et al. Endothelin-1 regulates astrocyte proliferation and reactive gliosis via a JNK/c-Jun signaling pathway. J Neurosci. 2008;28:2394–2408. doi: 10.1523/JNEUROSCI.5652-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buffo A, et al. Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. Proc Natl Acad Sci USA. 2008;105:3581–3586. doi: 10.1073/pnas.0709002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magnus T, et al. Adult glial precursor proliferation in mutant SOD1G93A mice. Glia. 2008;56:200–208. doi: 10.1002/glia.20604. [DOI] [PubMed] [Google Scholar]
- 29.Meletis K, et al. Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol. 2008;6:e182. doi: 10.1371/journal.pbio.0060182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlen M, et al. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nat Neurosci. 2009;12:259–267. doi: 10.1038/nn.2268. [DOI] [PubMed] [Google Scholar]
- 31.Garcia ADR, et al. GFAP-expressing progenitors are the principle source of constitutive neurogenesis in adult mouse forebrain. Nature Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- 32.Ohab JJ, Carmichael ST. Poststroke neurogenesis: emerging principles of migration and localization of immature neurons. Neuroscientist. 2008;14:369–380. doi: 10.1177/1073858407309545. [DOI] [PubMed] [Google Scholar]
- 33.Bush TG, et al. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189–201. doi: 10.1016/s0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- 34.Myer DJ, et al. Essential protective roles of reactive astrocytes in traumatic brain injury. Brain. 2006;129:2761–2772. doi: 10.1093/brain/awl165. [DOI] [PubMed] [Google Scholar]
- 35.Liedtke W, et al. Experimental autoimmune encephalomyelitis in mice lacking glial fibrillary acidic protein is characterized by a more severe clinical course and an infiltrative central nervous system lesion. Am J Pathol. 1998;152:251–259. [PMC free article] [PubMed] [Google Scholar]
- 36.Li L, et al. Protective role of reactive astrocytes in brain ischemia. J Cereb Blood Flow Metab. 2008;28:468–481. doi: 10.1038/sj.jcbfm.9600546. [DOI] [PubMed] [Google Scholar]
- 37.Wilhelmsson U, et al. Absence of glial fibrillary acidic protein and vimentin prevents hypertrophy of astrocytic processes and improves post-traumatic regeneration. J Neurosci. 2004;24:5016–5021. doi: 10.1523/JNEUROSCI.0820-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rothstein JD, et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 39.Zador Z, et al. Role of aquaporin-4 in cerebral edema and stroke. Handb Exp Pharmacol. 2009:159–170. doi: 10.1007/978-3-540-79885-9_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin JH, et al. A central role of connexin 43 in hypoxic preconditioning. J Neurosci. 2008;28:681–695. doi: 10.1523/JNEUROSCI.3827-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okada S, et al. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nature Med. 2006;12:829–834. doi: 10.1038/nm1425. [DOI] [PubMed] [Google Scholar]
- 42.Brambilla R, et al. Transgenic inhibition of astroglial NF-kappaB improves functional outcome in experimental autoimmune encephalomyelitis by suppressing chronic central nervous system inflammation. J Immunol. 2009;182:2628–2640. doi: 10.4049/jimmunol.0802954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swanson RA, et al. Astrocyte influences on ischemic neuronal death. Curr Mol Med. 2004;4:193–205. doi: 10.2174/1566524043479185. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y, et al. Astrocytes protect neurons from nitric oxide toxicity by a glutathione-dependent mechanism. J Neurochem. 2001;77:1601–1610. doi: 10.1046/j.1471-4159.2001.00374.x. [DOI] [PubMed] [Google Scholar]
- 45.Shih AY, et al. Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J Neurosci. 2003;23:3394–3406. doi: 10.1523/JNEUROSCI.23-08-03394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vargas MR, et al. Nrf2 activation in astrocytes protects against neurodegeneration in mouse models of familial amyotrophic lateral sclerosis. J Neurosci. 2008;28:13574–13581. doi: 10.1523/JNEUROSCI.4099-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rao KV, et al. Astrocytes protect neurons from ammonia toxicity. Neurochem Res. 2005;30:1311–1318. doi: 10.1007/s11064-005-8803-2. [DOI] [PubMed] [Google Scholar]
- 48.Koistinaho M, et al. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat Med. 2004;10:719–726. doi: 10.1038/nm1058. [DOI] [PubMed] [Google Scholar]
- 49.John GR, et al. Cytokines: Powerful regulators of glial cell activation. Neuroscientist. 2003;9:10–22. doi: 10.1177/1073858402239587. [DOI] [PubMed] [Google Scholar]
- 50.Min KJ, et al. Astrocytes induce hemeoxygenase-1 expression in microglia: a feasible mechanism for preventing excessive brain inflammation. J Neurosci. 2006;26:1880–1887. doi: 10.1523/JNEUROSCI.3696-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Farina C, et al. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 52.Brambilla R, et al. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med. 2005;202:145–156. doi: 10.1084/jem.20041918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suh SW, et al. Astrocyte glycogen sustains neuronal activity during hypoglycemia: studies with the glycogen phosphorylase inhibitor CP-316,819 ([R-R*,S*]-5-chloro-N-[2-hydroxy-3-(methoxymethylamino)-3-oxo-1-(phenylmet hyl)propyl]-1H-indole-2-carboxamide) J Pharmacol Exp Ther. 2007;321:45–50. doi: 10.1124/jpet.106.115550. [DOI] [PubMed] [Google Scholar]
- 54.Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- 55.Gordon GR, et al. Astrocyte control of the cerebrovasculature. Glia. 2007;55:1214–1221. doi: 10.1002/glia.20543. [DOI] [PubMed] [Google Scholar]
- 56.Simard M, Nedergaard M. The neurobiology of glia in the context of water and ion homeostasis. Neuroscience. 2004;129:877–896. doi: 10.1016/j.neuroscience.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 57.Rouach N, et al. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322:1551–1555. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- 58.Christopherson KS, et al. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 59.Stevens B, et al. The classical complement cascade mediates CNS synapse elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 60.Sofroniew MV. Astrocyte failure as a cause of CNS dysfunction. Mol Psychiatry. 2000;5:230–232. doi: 10.1038/sj.mp.4000753. [DOI] [PubMed] [Google Scholar]
- 61.De Keyser J, et al. Dysfunctional astrocytes as key players in the pathogenesis of central nervous system disorders. J Neurol Sci. 2008;267:3–16. doi: 10.1016/j.jns.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 62.Lennon VA, et al. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202:473–477. doi: 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Whitney KD, McNamara JO. GluR3 autoantibodies destroy neural cells in a complement-dependent manner modulated by complement regulatory proteins. J Neurosci. 2000;20:7307–7316. doi: 10.1523/JNEUROSCI.20-19-07307.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bauer J, et al. Astrocytes are a specific immunological target in Rasmussen's encephalitis. Ann Neurol. 2007;62:67–80. doi: 10.1002/ana.21148. [DOI] [PubMed] [Google Scholar]
- 65.Silver J, Miller JH. Regeneration beyond the glial scar. Nature Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 66.Brenner M, et al. Mutations in GFAP, encoding glial fibrillary acidic protein, are associated with Alexander disease. Nat Genet. 2001;27:117–120. doi: 10.1038/83679. [DOI] [PubMed] [Google Scholar]
- 67.Di Giorgio FP, et al. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci. 2007;10:608–614. doi: 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nagai M, et al. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10:615–622. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hamby ME, et al. TGF-beta1 potentiates astrocytic nitric oxide production by expanding the population of astrocytes that express NOS-2. Glia. 2006;54:566–577. doi: 10.1002/glia.20411. [DOI] [PubMed] [Google Scholar]
- 70.Takano T, et al. Receptor-mediated glutamate release from volume sensitive channels in astrocytes. Proc Natl Acad Sci USA. 2005;102:16466–16471. doi: 10.1073/pnas.0506382102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tian GF, et al. An astrocytic basis of epilepsy. Nat Med. 2005;11:973–981. doi: 10.1038/nm1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jansen LA, et al. Epileptogenesis and reduced inward rectifier potassium current in tuberous sclerosis complex-1-deficient astrocytes. Epilepsia. 2005;46:1871–1880. doi: 10.1111/j.1528-1167.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- 73.Argaw AT, et al. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc Natl Acad Sci USA. 2009;106:1977–1982. doi: 10.1073/pnas.0808698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bezzi P, et al. CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–710. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- 76.Fontana AC, et al. Enhancing glutamate transport: mechanism of action of Parawixin1, a neuroprotective compound from Parawixia bistriata spider venom. Mol Pharmacol. 2007;72:1228–1237. doi: 10.1124/mol.107.037127. [DOI] [PubMed] [Google Scholar]
- 77.Rothstein JD, et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 78.Zhuo L, et al. hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. 85-94. 2001;31:85–94. doi: 10.1002/gene.10008. [DOI] [PubMed] [Google Scholar]
- 79.Malatesta P, et al. Neuronal or glial progeny: Regional differences in radial glia fate. Neuron. 2003;37:751–764. doi: 10.1016/s0896-6273(03)00116-8. [DOI] [PubMed] [Google Scholar]
- 80.Casper KB, et al. Characterization of astrocyte-specific conditional knockouts. 85-94. 2007;45:292–299. doi: 10.1002/dvg.20287. [DOI] [PubMed] [Google Scholar]
- 81.Ganat YM, et al. Early postnatal astroglial cells produce multilineage precursors and neural stem cells in vivo. J Neurosci. 2006;26:8609–8621. doi: 10.1523/JNEUROSCI.2532-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Slezak M, et al. Transgenic mice for conditional gene manipulation in astroglial cells. Glia. 2007;55:1565–1576. doi: 10.1002/glia.20570. [DOI] [PubMed] [Google Scholar]
- 83.Codeluppi S, et al. The Rheb-mTOR pathway is upregulated in reactive astrocytes of the injured spinal cord. J Neurosci. 2009;29:1093–1104. doi: 10.1523/JNEUROSCI.4103-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gris P, et al. Transcriptional regulation of scar gene expression in primary astrocytes. Glia. 2007;55:1145–1155. doi: 10.1002/glia.20537. [DOI] [PubMed] [Google Scholar]
- 85.Chen Y, et al. The basic helix-loop-helix transcription factor olig2 is critical for reactive astrocyte proliferation after cortical injury. J Neurosci. 2008;28:10983–10989. doi: 10.1523/JNEUROSCI.3545-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wanner IB, et al. A new in vitro model of the glial scar inhibits axon growth. Glia. 2008;56:1691–1709. doi: 10.1002/glia.20721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hsu JY, et al. Matrix metalloproteinase-9 facilitates glial scar formation in the injured spinal cord. J Neurosci. 2008;28:13467–13477. doi: 10.1523/JNEUROSCI.2287-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Retamal MA, et al. C×43 hemichannels and gap junction channels in astrocytes are regulated oppositely by proinflammatory cytokines released from activated microglia. J Neurosci. 2007;27:13781–13792. doi: 10.1523/JNEUROSCI.2042-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bekar LK, et al. Locus coeruleus alpha-adrenergic-mediated activation of cortical astrocytes in vivo. Cereb Cortex. 2008;18:2789–2795. doi: 10.1093/cercor/bhn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Neary JT, et al. Activation of extracellular signal-regulated kinase by stretch-induced injury in astrocytes involves extracellular ATP and P2 purinergic receptors. J Neurosci. 2003;23:2348–2356. doi: 10.1523/JNEUROSCI.23-06-02348.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Simpson JE, et al. Astrocyte phenotype in relation to Alzheimer-type pathology in the ageing brain. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 92.Norenberg MD, et al. Signaling factors in the mechanism of ammonia neurotoxicity. Metab Brain Dis. 2009;24:103–117. doi: 10.1007/s11011-008-9113-6. [DOI] [PubMed] [Google Scholar]
- 93.Lee S, et al. Lipocalin-2 is an autocrine mediator of reactive astrocytosis. J Neurosci. 2009;29:234–249. doi: 10.1523/JNEUROSCI.5273-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jones KJ, et al. CPEB1 regulates beta-catenin mRNA translation and cell migration in astrocytes. Glia. 2008;56:1401–1413. doi: 10.1002/glia.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Beck H, et al. Egr-1 regulates expression of the glial scar component phosphacan in astrocytes after experimental stroke. Am J Pathol. 2008;173:77–92. doi: 10.2353/ajpath.2008.070648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Panenka W, et al. P2X7-like receptor activation in astrocytes increases chemokine monocyte chemoattractant protein-1 expression via mitogen-activated protein kinase. J Neurosci. 2001;21:7135–7142. doi: 10.1523/JNEUROSCI.21-18-07135.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Qin H, et al. Expression and functional significance of SOCS-1 and SOCS-3 in astrocytes. J Immunol. 2008;181:3167–3176. doi: 10.4049/jimmunol.181.5.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li LB, et al. Regulation of astrocytic glutamate transporter expression by Akt: evidence for a selective transcriptional effect on the GLT-1/EAAT2 subtype. J Neurochem. 2006;97:759–771. doi: 10.1111/j.1471-4159.2006.03743.x. [DOI] [PubMed] [Google Scholar]
- 99.Figiel M, et al. Regulation of glial glutamate transporter expression by growth factors. Exp Neurol. 2003;183:124–135. doi: 10.1016/s0014-4886(03)00134-1. [DOI] [PubMed] [Google Scholar]