Abstract
Photosensitive caged compounds have enhanced our ability to address the complexity of biological systems by generating effectors with remarkable spatial/temporal resolutions1-3. The caging effect is typically removed by photolysis with ultraviolet light to liberate the bioactive species. Although this technique has been successfully applied to many biological problems, it suffers from a number of intrinsic drawbacks. For example, it requires dedicated efforts to design and synthesize a precursor compound to the effector. The ultraviolet light may cause damage to biological samples and is only suitable for in vitro studies because of its quick attenuation in tissue4. Here we address these issues by developing a platform based on the photothermal effect of gold nanocages. Gold nanocages represent a class of nanostructures with hollow interiors and porous walls5. They can have strong absorption (for the photothermal effect) in the near-infrared (NIR) while maintaining a compact size. When the surface of a gold nanocage is covered with a smart polymer, the pre-loaded effector can be released in a controllable fashion using a NIR laser. This system works well with various effectors without involving sophiscated syntheses, and is well-suited for in vivo studies due to the high transparency of soft tissue in NIR6.
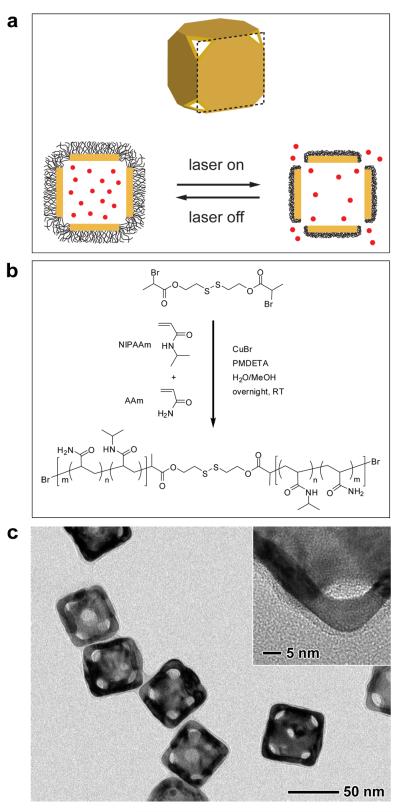
Figure 1a shows a schematic of the controlled release system. The Au nanocages are typically prepared via the galvanic replacement reaction between Ag nanocubes and HAuCl4 or HAuCl2 in water7. The polymer is based on poly(N-isopropylacrylamide) (pNIPAAm) and its derivatives, which can change conformation in response to small variations in temperature8. Upon exposure to a laser beam whose wavelength matches with the absorption peak of the Au nanocage, the light will be absorbed and converted into heat through the photothermal effect9,10. The heat will dissipate into the surroundings, and the rise in temperature will cause the polymer chains to collapse (see the supplementary information for a detailed analysis), exposing the pores on the nanocage and thereby releasing the pre-loaded effector. When the laser is turned off, heating will immediately cease and the drop in temperature will bring the polymer back to its original, extended conformation, closing the pores and stopping the release. We can control the release dosage by manipulating the power density and/or irradiation time.
Figure 1. Schematic illustration and characterization of the controlled release system.
a, Schematic illustrating how the system works. A side view of the Au nanocage is used for the illustration. Upon exposure to a NIR laser, the light is absorbed by the nanocage and converted into heat, triggering the smart polymer to collapse and thus release the pre-loaded effector. When the laser is turned off, the polymer chains will relax back to the extended confrmation and terminate the release. b, Atom transfer radical polymerization of NIPAAm and AAm monomers (at a molar ratio of m/n) as initiated by a disulfide initiator and in the presence of a Cu(I) catalyst. c, TEM images of Au nanocages whose surface was covered by a pNIPAAm-co-pAAm copolymer with an LSCT at 39 °C. The inset shows a magnified TEM image of the corner of such a nanocage.
For pure pNIPAAm, its low critical solution temperature (LCST) is around 32 °C. Below 32 °C, the polymer is hydrophilic and soluble in water. When the temperature is raised above 32 °C, the polymer undergoes a phase transition to a hydrophobic state, generating turbidity due to aggregation. Typically, the LCST is defined as the temperature at which the light transmission of the polymer solution drops to 90% of the original value8. By incorporating acrylamide (AAm) into the polymer chain, we obtained pNIPAAm-co-pAAm copolymers with LCSTs being tuned to anywhere in the range of 32 to 50 °C (Figure S1 and Table S1)8. For in vivo applications, the LCST should be tuned to a value above the body temperature (37 °C) but below the hyperthermia temperature (42 °C). In this work, we have focused on two types of polymers: pNIPAAm and a pNIPAAm-co-pAAm copolymer with an LCST at 32 and 39 °C, respectively. Both of them were prepared using atom transfer radical polymerization (ATRP)11,12.
We covalently anchored the smart polymer to the surface of Au nanocages via gold-thiolate linkage. One way to achieve this is to include a disulfide bond in the middle of the polymer chain by employing a disulfide initiator (Figure 1b)12. Because thiolate has a stronger binding towards Au surface than the C=O group of poly(vinyl pyrrolidone) (PVP), we could replace the PVP on nanocages with the smart polymer. After the displacement, the absorption peak of the nanocages red-shifted by ~13 nm, which could be offset during the nanocage synthesis. As shown in Figure 1c by TEM imaging, the pNIPAAm-co-pAAm coating had a relatively uniform thickness of ~3 nm in the dry state. This result is in reasonable agreement with the value (~5 nm) estimated from the TGA and GPC data shown in Figure S2 and Table S1. By dynamic light scattering, the mean hydrodynamic diameter of the copolymer-covered nanocages was observed to oscillate in response to temperature variation (Figure S3): the diameter increased by 13% upon cooling to 37 °C and shrank to its original value upon heating to 41 °C. These changes with temperature were reversible. During cooling/heating, the polydispersity index of the sample remained less than 0.12, suggesting that no agglomeration occurred in the solution due to the use of dilute samples.
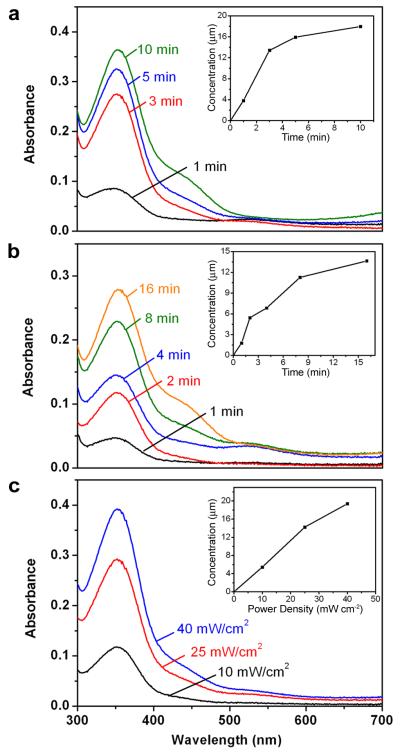
To demonstrate the controlled release of medium-sized effectors from the copolymer-covered nanocages, we prepared alizarin-PEG by coupling the hydroxyl end group of PEG (MW≈5,000) with the carboxylic group of alizarin yellow acid13. The nanocages were added to an aqueous solution of alizarin-PEG (23 mM) and shaken at 42 °C to load the dye. After 12 h, the suspension was quickly cooled with an ice bath to trigger conformational change for the copolymer, closing the pores and keeping the loaded dye inside the nanocages. If the loaded sample was kept under ambient conditions of a lab, the dye remained in the nanocages with negligible release (Figure S4). However, when the sample was heated above the LCST (39 °C) of the copolymer, the dye would come out through the opened pores. Figure 2a shows the release profile when the sample was heated at 42 °C for different periods of time. Since alizarin-PEG has an absorption peak at 354 nm, its release could be easily monitored by recording UV-Vis spectra of the supernatants at different times after the nanocages had been centrifuged down. As heating was prolonged, the amount of alizarin-PEG released into the solution kept increasing, and eventually leveled off. By referring to a calibration curve separately prepared for the same dye, we determined the exact concentration of alizarin-PEG released from the nanocages at different times, as shown in the inset of Figure 2a.
Figure 2. Controlled release of a dye from the Au nanocages covered by a copolymer with an LCST at 39 °C.
Absorption spectra of alizarin-PEG released from the copolymer-covered Au nanocages a, by heating at 42 °C for 1, 3, 5, and 10 min; b, by exposure to a pulsed NIR laser at a power density of 10 mW/cm2 for 1, 2, 4, 8, and 16 min; and c, by exposure to the NIR laser for 2 min at 10, 25, and 40 mW/cm2. The insets show the concentrations of alizarin-PEG released from the nanocages under different conditions.
We also controlled the release with a NIR laser via the photothermal effect. For this purpose, the suspension of dye-loaded nanocages was exposed to a Ti:sapphire laser at 10 mW/cm2 for different periods of time. Under this condition, most of the dye molecules were released within 16 min (Figure 2b). We also tested the dependence of dye release on the power density when the time was kept at 2 min. As shown in Figure 2c, because more heat was generated at a higher power density, the pores were forced to open for a longer period of time. Therefore, higher power density caused more release of alizarin-PEG from the nanocages. Note that the Au nanocages started to melt at a power density of 40 mW/cm2 (Figure S5), a phenomenon that was also observed in previous studies14-16, indicating that a laser power density below 40 mW/cm2 should be used if one wants to reload the Au nanocages with chemical species. We did demonstrate the capability to reuse the smart capsules that were used for Figure 2b after laser-triggered release. In this case, the used nanocages were washed with warm water (42 °C) three times to remove all possibly trapped dye. We then reloaded the nanocages with alizarin-PEG, followed by release with heating. As shown in Figure S6, we obtained a total amount of release essentially the same as the first round of release experiment. This result confirms that the Au nanocages were not melted nor the polymer chains were desorbed from the nanocage surface during a laser-triggered release experiment (see the supplementary information for a detailed discussion).
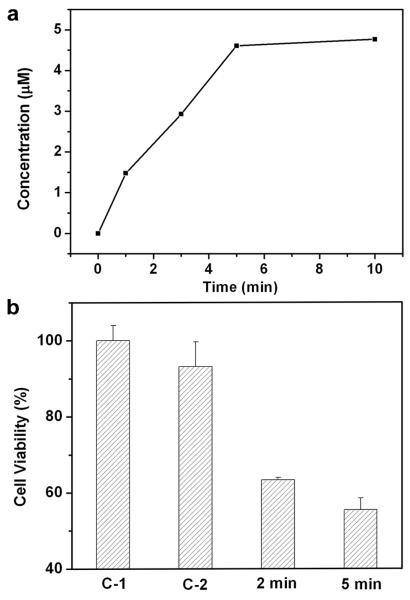
We further extended the controlled release to an in vitro study that involved killing of breast cancer cells with doxorubicin (Dox), a commercial chemotherapeutic drug for breast cancer17. The Dox release profile (Figure 3a) from the copolymer-covered nanocages was similar to what was observed for the dye (Figure 2a). We observed a fast release of Dox when the sample was subjected to heating. It has been shown that the presence of Dox at a micromolar level (μM) can cause breast cancer cells to die and thus provide a simple readout. In a typical study, the cancer cells were seeded in a 24-well plate and allowed to proliferate until reaching 80% confluence. The wells containing cancer cells and Dox-loaded nanocages were irradiated with the Ti:sapphire laser at a power density of 20 mW/cm2 for 2 and 5 min, respectively, and the data are plotted in Figure 3b. As the irradiation time increased, more cancer cells were observed to die because more Dox was released. In comparison, laser irradiation on sample “C-1” in the absence of Au nanocages had essentially no effect on cell viability. Laser irradiation on sample “C-2” in the presence of Au nanocages alone resulted in slight reduction in viability, probably due to the photothermal effect of Au nanocages9,18. These results are consistent with the drug release data shown in Figure 3a. In practice, parameters including cage concentration, drug loading, laser irradiation time, and power density all need to be optimized to further improve the efficacy of killing cancer cells.
Figure 3. Controlled release of an anticancer drug from the Au nanocages covered by a copolymer with an LCST at 39 °C.
a, A plot of the concentrations of Dox released from the Au nanocages upon heating at 45 °C for different periods of time. b, Cell viability for samples after going through different treatments: (C-1) cells irradiated with a pulsed NIR laser for 2 min in the absence of Au nanocages; (C-2) cells irradiated with the laser for 2 min in the presence of Dox-free Au nanocages; and (2/5 min) cells irradiated with the laser for 2 and 5 min in the presence of Dox-loaded Au nanocages. A power density of 20 mW/cm2 was employed for all these studies.
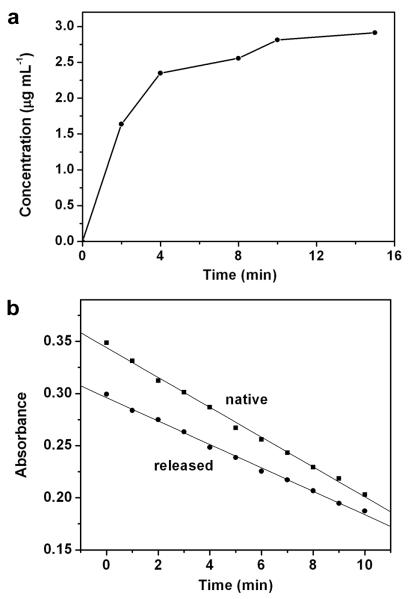
An enzyme was also employed to test encapsulation and controlled release. In this case, we used lysozyme that can damage the cell walls of bacteria by catalyzing the hydrolysis process19. To maintain the enzyme activity, we used pNIPAAm with an LCST of 32 °C. The lysozyme was released by heating the system to 37 °C, as was for the dye. Concentrations of the released lysozyme, shown in Figure 4a, were quantified using the Micro BCA protein assay20. The maximum concentration released (2.91 μg/mL) from the Au nanocages was close to the value (3.88 μg/mL) calculated from the initial concentration of the loading solution and the volume ratio of Au nanocages to the suspension medium. The bioactivity of the released lysozyme was determined from the rate of lysis for Micrococcus Lysodeikticus cells. As shown in Figure 4b, the released lysozyme was able to maintain ~80% of the bioactivity after going through the encapsulation and release processes. This bioactivity is relatively high compared to the typical values (30-80%) reported for other encapsulation schemes21-23.
Figure 4. Controlled release of an enzyme from the Au nanocages covered by pNIPAAm with an LCST at 32 °C.
a, A plot of the concentrations of lysozyme released from the Au nanocages upon heating at 37 °C for different periods of time. b, Lysozyme bioactivity test. Linear fit (y: absorbance, x: time) for native lysozyme: y = −0.01432x + 0.3441, R2 = 0.9964 and linear fit for the released lysozyme: y = −0.01124x + 0.2961, R2 = 0.9966.
In summary, we have demonstrated a platform based on Au nanocages covered with smart polymers for controlled release with NIR light. When combined with optical manipulation (e.g., trapping)24, this platform offers many additional advantages such as high spatial/temporal resolutions. In addition, Au nanocages are bio-inert and the surface can be readily functionalized with targeting ligands such as antibodies using the gold-thiolate chemistry25,26.
Methods
Polymerization of NIPAAm and AAm
NIPAAm (2.0 g), PMDETA (35 μL), BHEDS(BP)2 (0.015 g), deionized water (18 mL), and methanol (12 mL) were mixed in a Schlenk flask and degassed by freeze-pump-thaw cycles. While the mixture was frozen, CuBr (0.010 g) was added. The flask was then filled with argon and left to melt at room temperature. The reaction solution was magnetically stirred overnight at room temperature. After evaporation of the solvent, the crude product was dissolved in water and purified by dialysis to yield poly(N-isopropylacrylamide) (pNIPAAm). For copolymers, the same procedure was used except that the initial monomers were added as the following: i) NIPAAm (95 %, 1.93 g) and AAm (5 %, 0.64 g) for an LCST at 35 °C; ii) NIPAAm (90 %, 1.83 g) and AAm (10 %, 0.13 g) for an LCST at 39 °C; iii) NIPAAm (87.5 %, 1.78 g) and AAm (12.5 %, 0.16 g) for an LCST at 41 °C; and iv) NIPAAm (75 %, 1.53 g) and AAm (25 %, 0.32 g) for an LCST at 49 °C.
Preparation of gold nanocages
Gold nanocages were synthesized via the galvanic replacement reaction between truncated Ag nanocubes and chloroauric acid (HAuCl4). The synthetic procedures were described in detail in a recently published protocol27. For all the experiments, we used Au nanocages of 50 nm in edge length together with a pore size of 5-10 nm. Sulfide-mediated polyol reduction was used to synthesize the Ag nanocubes and the procedure can be found in our previous publications27,28.
Exchange of PVP with pNIPAAm or copolymer
The as-synthesized PVP-covered Au nanocages were dispersed in 2 mL of diionized water and added to a 20 mL aqueous solution of pNIPAAm (0.20 g) or a copolymer (0.20 g). The mixture was shaken at 800 RPM for five days. The solution was then centrifuged at 14,000 RPM and the supernatant was discarded. The polymer-covered Au nanocages were washed three more times with 2 mL (each time) of deionized water.
Loading the nanocages with alizarin-PEG, Dox, or lysozyme
The alizarin-PEG, Dox and lysozyme solutions were freshly prepared and directly used with the nanocages. The polymer-covered nanocages were added to an aqueous solution (1 mL) containing alizarin-PEG (0.12 g), Dox (0.020 g), or lysozyme (0.25 g). The mixture was shaken overnight at 1,000 RPM at 37 °C for pNIPAAm-covered nanocages and at 42 °C for copolymer-covered nanocages, respectively. The mixture was then cooled with an ice bath for 1 h, and centrifuged at 14,000 RPM for 20 min. Finally, the supernatant was decanted and the loaded samples were washed eight more times with 1.5 mL (each time) of distilled water.
Releasing alizarin-PEG from gold nanocages by heating
Prior to alizarin-PEG release, the loaded nanocages were centrifuged down and the supernatant was decanted. 0.5 mL of warm (42 °C) water was added to the sample, followed by incubation under vortexing in a 42 °C water bath for different periods of time. Then, the solution was cooled with an ice bath for 5 min, followed by centrifugation at 14,000 RPM and 20 °C for 10 min. The supernatant was then taken for UV-Vis spectral measurement.
Releasing alizarin-PEG from gold nanocages by laser
0.5 mL of the alizarin-PEG loaded nanocages was prepared and transferred to 1.5-mL centrifuge tubes. The sample was pre-heated in a shaking incubator at 37 °C to mimic the body temperature prior to laser irradiation. The sample was exposed to the Ti:sapphire laser for different periods of time at a fixed power density of 10 mW/cm2, or for 2 min at power densities of 10, 25, and 40 mW/cm2. After exposure, the solution was centrifuged at 14,000 RPM for 20 min, and the supernatant was collected for UV-Vis spectral measurement.
Releasing lysozyme from gold nanocages by heating
We used the same procedure described for the release of alizarin-PEG except that the release temperature was set to 37 °C.
Calculating the concentration of released lysozyme
Native lysozyme was dissolved in DPBS to obtain solutions of 0.5, 1, 2.5, 5, 10, 20, 40, and 200 μg/mL in concentration. 1 mL of Micro BCA Protein Assay was added to 1 mL of the lysozyme sample with a known (native) or an unknown (released from the nanocages) concentration. The culture tubes were incubated at 60 °C for 1 h and then examined with a spectrometer. For the known samples, we could obtain a calibration curve by plotting the absorbance at 562 nm against the concentration of lysozyme. The unknown concentrations of the released lysozyme were then determined from the calibration curve.
Supplementary Material
Acknowledgments
This work was supported by a 2006 Director’s Pioneer Award from the NIH (DP1 OD000798). Part of the work was performed at the Nano Research Facility (NRF), a member of the National Nanotechnology Infrastructure Network (NNIN), which is supported by the NSF under award no. ECS-0335765. NRF is part of School of Engineering and Applied Science at Washington University in St. Louis.
Footnotes
Additional information Supplementary information accompanies this paper on www.nature.com/naturematerials.
References
- 1.Adams SR, Tsien RY. Controlling cell chemistry with caged compounds. Annu. Rev. Physiol. 1993;55:755–784. doi: 10.1146/annurev.ph.55.030193.003543. [DOI] [PubMed] [Google Scholar]
- 2.Kramer RH, Chambers JJ, Trauner D. Photochemical tools for remote control of ion channels in excitable cells. Nat. Chem. Bio. 2005;7:360–365. doi: 10.1038/nchembio750. [DOI] [PubMed] [Google Scholar]
- 3.Mayer G, Heckel A. Biologically active molecules with a light switch. Angew. Chem. Int. Ed. 2006;45:4900–4921. doi: 10.1002/anie.200600387. [DOI] [PubMed] [Google Scholar]
- 4.Schwarz A, et al. Interleukin-12 suppresses ultraviolet radiation-induced apoptosis by inducing DNA repair. Nature Cell Bio. 2002;4:26–31. doi: 10.1038/ncb717. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, et al. Facile synthesis of gold-silver nanocages with controllable pores on the surface. J. Am. Chem. Soc. 2006;128:14776–14777. doi: 10.1021/ja066023g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weissleder R. A clear vision for in vivo imaging. Nat. Biotechnol. 2001;19:316–317. doi: 10.1038/86684. [DOI] [PubMed] [Google Scholar]
- 7.Skrabalak SE, et al. Gold nanocages: Synthesis, properties, and applications. Acc. Chem. Res. 2008;41:1587–1595. doi: 10.1021/ar800018v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffman AS. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002;43:3–12. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 9.Au L, et al. A quantitative study on the photothermal effect of immuno gold nanocages targeted to breast cancer cells. ACS Nano. 2008;2:1645–1652. doi: 10.1021/nn800370j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu GL, Kim J, Lu Y, Lee LP. Optofluidic control using photothermal nanoparticles. Nat. Mater. 2005;5:27–32. doi: 10.1038/nmat1528. [DOI] [PubMed] [Google Scholar]
- 11.Jonas AM, Hu Z, Gline K, Huck WTS. Effect of nanoconfinement on the collapse transition of responsive polymer brushes. Nano Lett. 2008;8:3819–3824. doi: 10.1021/nl802152q. [DOI] [PubMed] [Google Scholar]
- 12.Tsarevsky NV, Matyjaszewski K. Combining atom transfer radical polymerization and disulfide/thiol redox chemistry: A route to well-defined (bio)degradable polymeric materials. Macromolecules. 2005;38:3087–3092. [Google Scholar]
- 13.Li H, Jerome R, Lecomte P. Amphiphilic sun-shaped polymers by grafting macrocyclic copolyesters with PEO. Macromolecules. 2008;41:650–654. [Google Scholar]
- 14.Hu M, et al. Ultrafast laser studies of the photothermal properties of gold nanocages. J. Phys. Chem. B. 2006;110:1520–1524. doi: 10.1021/jp0571628. [DOI] [PubMed] [Google Scholar]
- 15.Link S, Wang ZL, El-Syaed MA. How does a gold nanorod melt? J. Phys. Chem. B. 2000;104:7867–7870. [Google Scholar]
- 16.Wu G, et al. Remotely triggered liposome release by near-infrared light absorption via hollow gold nanoshells. J. Am. Chem. Soc. 2008;130:8175–8177. doi: 10.1021/ja802656d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu EC, et al. Oxidation-triggered release of fluorescent molecules or drugs from mesoporous Si microparticles. ACS Nano. 2008;2:2401–2409. doi: 10.1021/nn800592q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J, et al. Immuno gold nanocages with tailored optical properties for targeted photothermal destruction of cancer cells. Nano Lett. 2007;7:1318–1322. doi: 10.1021/nl070345g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghaderi A, Carlfors J. Biological activity of lysozyme after entrapment in poly(d,l-lactide-co-glycolide) microspheres. Pharm. Res. 1997;14:1556–1562. doi: 10.1023/a:1012122200381. [DOI] [PubMed] [Google Scholar]
- 20.Xie J, Wang C-H. Encapsulation of proteins in biodegradable polymeric microparticles using electrospray in the taylor cone-jet mode. Biotechnol. & Bioeng. 2007;97:1278–1290. doi: 10.1002/bit.21334. [DOI] [PubMed] [Google Scholar]
- 21.Kang F, Jiang G, Hinderliter A, Deluca PP, Singh J. Lysozyme stability in primary emulsion for PLGA microsphere preparation: Effect of recovery methods and stabilizing excipients. Pharm. Res. 2002;19:629–633. doi: 10.1023/a:1015354028908. [DOI] [PubMed] [Google Scholar]
- 22.Srinivasan C, Katare YK, Muthukumaran T, Panda AK. Effect of additives on encapsulation efficiency, stability, and bioactivity of entrapped lysozyme from biodegradable polymer particles. J. Microencapsul. 2005;22:127–138. doi: 10.1080/02652040400026400. [DOI] [PubMed] [Google Scholar]
- 23.Weert MVD, Hoechstetter J, Hennink WE, Crommelin DJA. The effect of a water/organic solvent interface on the structural stability of lysozyme. J. Controlled Release. 2000;68:351–359. doi: 10.1016/s0168-3659(00)00277-7. [DOI] [PubMed] [Google Scholar]
- 24.Hansen PM, Bhatia VK, Harrit N, Oddershede L. Expanding the optical trapping range of gold nanoparticles. Nano Lett. 2005;5:1937–1942. doi: 10.1021/nl051289r. [DOI] [PubMed] [Google Scholar]
- 25.Love JC, Estroff LA, Kriebel JK, Nuzzo RG, Whitesides GM. Self-assembled monolayers of thiolates on metals as a form of nanotechnology. Chem. Rev. 2005;105:1103–1170. doi: 10.1021/cr0300789. [DOI] [PubMed] [Google Scholar]
- 26.Chen J, et al. Gold nanocages: Bioconjugation and their potential use as optical imaging contrast agents. Nano Lett. 2005;5:473–477. doi: 10.1021/nl047950t. [DOI] [PubMed] [Google Scholar]
- 27.Skrabalak SE, Au L, Li X, Xia Y. Facile synthesis of Ag nanocubes and Au nanocages. Nature Protocols. 2007;2:2182–2190. doi: 10.1038/nprot.2007.326. [DOI] [PubMed] [Google Scholar]
- 28.Siekkinen AR, McLellan JM, Chen J, Xia Y. Rapid synthesis of small silver nanocubes by mediating polyol reduction with a trace amount of sodium sulfide or sodium hydrosulfide. Chem. Phys. Lett. 2006;432:491–496. doi: 10.1016/j.cplett.2006.10.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.