Abstract
Mice defective in the Polk gene (which encodes DNA polymerase kappa) are viable and do not manifest obvious phenotypes. The present studies document a spontaneous mutator phenotype in Polk−/− mice. The initial indication of enhanced spontaneous mutations in these mice came from the serendipitous observation of a postulated founder mutation that manifested in multiple disease states among a cohort of mice comprising all three possible Polk genotypes. Polk−/− and isogenic wild type controls carrying a reporter transgene (the λ-phage cII gene) were used for subsequent quantitative and qualitative studies on mutagenesis in various tissues. We observed significantly increased mutation frequencies in the kidney, liver, and lung of Polk−/− mice, but not in the spleen or testis. G:C base pairs dominated the mutation spectra of the kidney, liver, and lung. These results are consistent with the notion that Polκ is required for accurate translesion DNA synthesis past naturally occurring polycyclic guanine adducts, possibly generated by cholesterol and/or its metabolites.
1. Introduction
Vertebrate cells are endowed with at least nine specialized DNA polymerases, all of which copy native DNA with markedly reduced fidelity and are devoid of 3′->5′ proofreading exonuclease activity [1]. In vitro, these enzymes have been shown to support replicative bypass [translesion DNA synthesis (TLS)] past numerous types of base damage that can arrest high fidelity DNA replication in living cells. One of these DNA polymerases, Polη, supports replication past thymine-thymine (and presumably other) cyclobutane pyrimidine dimers (CPD) [2]. Regardless of the fact that Polη displays extremely low fidelity when copying native DNA, TLS past thymine-containing CPD is remarkably accurate in vitro [2]. This is apparently also the case in living cells, since humans and mice defective in Polη activity display typical clinical features of xeroderma pigmentosum (XP), including enhanced UV radiation-dependent mutagenesis and increased predisposition to skin cancer [3,4].
These observations suggest that some, if not all, specialized DNA polymerases evolved to relieve arrested DNA replication associated with specific types of naturally occurring base damage of either environmental (such as that caused by UV radiation from the sun in the case of Polη) or spontaneous origin [1]. This notion embraces the nuance that while TLS by an appropriate specialized polymerase is largely error-free, its absence invokes one or more other such enzymes to subserve this function. Cells are thereby rescued from the lethal consequences of arrested DNA replication, but in a manner that generates an increased mutational burden [1].
Cognate substrates for specialized DNA polymerases other than Polη have not yet been identified. However, a number of reported observations suggest that DNA polymerase kappa (Polκ) may have evolved to support error-free bypass of polycyclic N2-guanine adducts in DNA. Significantly, the promoter region of the mouse (and human) Polk gene (but not the promoter regions of other specialized polymerases) contains two canonical arylhydrocarbon receptor-binding sites [5]. Such sites bind polycyclic aromatic ligands with high affinity and the ensuing receptor-ligand complex ultimately promotes the transcriptional activation of genes required for the catabolism of polycyclic hydrocarbons [5]. Consistent with this observation, cells derived from a Polk−/− mouse strain are hypersensitive to killing in the presence of BPDE [6], and Polκ (but not Polη) is specifically required for recovery from checkpoint arrest associated with exposure of mouse cells to this polycyclic aromatic compound [7]. Additionally, chicken DT40 Polk−/− cells manifest increased sensitivity to killing and increased chromosomal abnormalities associated with exposure to polycyclic estrogen analogs such as tamoxifen and 4-hydroxyestradiol [8]. Finally, in vitro [6,9–11,12] and in vivo [13] studies indicate that Polκ efficiently bypasses various polycyclic N2-guanine adducts in a largely error-free manner, supporting the preferential incorporation of dC [10].
Volcanoes and natural fires are well known spontaneous sources of benzo[a]pyrene and other polycyclic compounds [14], and may have conceivably promoted selection of Polκ during evolution. However, plants and microorganisms are endowed with numerous polycyclic aromatic phenolic compounds, glycosides, and alkaloids [15]. Additionally, though not planar in its native configuration, cholesterol and a plethora of metabolic derivatives of this compound, including sex hormones and steroids, can be aromatized to polycyclic derivatives that can covalently interact with DNA [16]. In this regard it is intriguing that Polk mRNA (but not Poli or Poll mRNA) is particularly highly expressed in the adrenal cortex of embryonic and adult mice, the site of steroid biosynthesis [17].
These considerations, coupled with the knowledge that many polycyclic aromatic compounds bind in the minor groove of DNA and covalently attach to the N2-position of guanine, anticipate that Polk−/− mice may carry an increased spontaneous mutational burden, with a spectrum consistent with error-prone bypass (TLS) of N2-guanine adducts.
Here we document that cells from an independently-derived Polk mutant mouse [18] are also distinctly sensitive to exposure to benzo[a]pyrene diol epoxide. Significantly, we also document increased levels of spontaneous mutations in various (but not all) tissues from these Polk−/− mice. We further demonstrate that the spectra of enhanced mutagenesis is indeed consistent with mutations primarily involving G:C base pairs, potentially implicating guanine as a source of spontaneous DNA damage.
2. Materials and Methods
2.1. Polk−/− Mice
Mice carrying the Polktm1.1Esp allele have been previously described [18]. Mice were screened for a naturally occurring mutation in the Poli gene of 129sv mice and were found to be wild type for the gene. Mice were in a mixed 129 x C57BL/6 background and were housed in either a conventional mouse facility that was not specific-pathogen-free (SPF) or in an SPF facility. Food, water and housing were the same between the facilities. Food (6% fat mouse chow) and water were provided ad libitum.
2.2 Big Blue Polk−/− and Big Blue Polh−/− mice
Big Blue mice (named for the lacI color-based plaque screening) were obtained from Stratagene (C57BL/6 strain background). These mice carry 80 copies of the chromosomally integrated LIZ shuttle vector, which harbors the lambda cII reporter gene. Our Polk−/+ mice (129/Ola backcrossed twice into C57BL/6; ~75% C57BL/6) were mated with Big Blue mice to obtain BB-Polk+/+ or BB-Polk−/− animals carrying 40 or 80 copies of the λ-LIZ shuttle vector. Similarly, Polh−/+ (129/Ola/C57BL/6) mice from the laboratory of Dr. Raju Kucherlapati [4] were mated with Big Blue mice from Stratagene to obtain BB-Polh−/− animals carrying 40 or 80 copies of the λ-LIZ shuttle vector. Male and female mice for each genotype were housed in a specific pathogen-free facility.
2.3 Genotyping
DNA was isolated from tails with a tissue DNA kit (formerly Gentra Systems now Qiagen, Valencia, CA.). Genotyping for Polk was performed as previously reported [18]. For Polh genotyping, primers XPV-F7 (5′AAGGGACAAGCGAACAGAGA3′), XPV-R14 (5′AGCAATATCACAGGC-CCAAC3′), and XPV-R1 (TCACTTCAACACTAGCTTCCC3′) were used in combination at a 1:1:1 concentration at a 58°C annealing temperature to amplify either a 500bp fragment (mutant), a 370bp fragment (WT), or both (heterozygous). For detection of the λ-LIZ shuttle vector, primers CII-F (5′CCACACCTATGGTGTATG3′) and CII-R (5′CCTCTGCCGAAGTTGAGTAT3′) were used to PCR-amplify a 432-bp band containing the cII gene using a 52°C annealing temperature with 5% DMSO. Sequencing reactions were carried out according to the manufacturer’s protocol using the ABI 3100 Genetic Analyzer (ABI, Foster City, CA). To further determine whether mice were hemizygous (40 copies) or homozygous (80 copies) for the λ-LIZ shuttle vector, Q-PCR was used to quantify relative cII copy numbers using primers CII-F1 (5′CTGCTTGCTGTTCTTGAATGGG3′) and CII-R1 (5′CGCTCGGTTGCCGCC3′) with Stratagene’s Brilliant Q-PCR Mastermix. Primers were used at an optimized concentration of 0.5mM.
2.4 Isolation of DNA and packaging into λ phage
Tissues harvested at the time of sacrifice (at 3,9, or 12 months of age) from BB-Polk−/− or BB-Polk+/+ mice (relatively half male, half female) were flash frozen and stored at −80°C. DNA was isolated from kidney, liver, lung, or spleen using the RecoverEase DNA isolation kit (Stratagene) as directed. 12μL of DNA was used for packaging into λ-phage using Transpack packaging extract (Stratagene).
2.5 Transformation into E. coli
Packaging extracts were diluted in 966mL of SM Buffer. Triplicates of 100x dilutions were generated and 100μL or 20μl of each triplicate was transformed into G1250 E. coli culture (in MgSO4, OD=0.5) for phage titering. The remaining packaged DNA was used to transform G1250 E. coli cells for the selection of cII− mutants. Cells were plated onto TB1 plates using heated TB1 top agar cooled to 55°C. Titer plates were grown at 37°C overnight and screening plates were grown at room temperature (24°C) for 48h.
2.6 Verification of putative λcII− mutants
Putative mutant plaques were cored, transferred to a 96 well plate containing 250μL of SM buffer per well, and stored at 4°C. Putative plaques were individually transformed and plated (1μL per transformation) at low density on TB1 media, and grown at the selective temperature (24°C) for 48h. Plaques visible by 48h were cored and transferred to a new 96 well plate containing 250μL of SM buffer per well and stored at 4°C indefinitely.
2.7 PCR amplification and sequence analysis of cII− mutants
Verified mutant plaques immersed in SM buffer were directly used as PCR templates. CII-F and CII-R (Stratagene) primers were used to amplify the promotor region immediately upstream of the cII gene and the cII open reading frame. A total of 5μL of each PCR reaction was treated with 2μL ExoSap-It enzyme (GE Healthcare) and incubated at 37°C for 30 min., followed by a heat-shock at 80°C for 15 min. Each sample was sequenced with the CII-R primer using the ABI Big Dye Terminator Cycle Sequencing Kit on an automated ABI Prism 3100 Genetic Analyzer.
2.8 Mutation Frequencies
Raw mutation frequencies were corrected for “jackpot” mutations and wild-type sequences as previously described [19]. The corrected mutation frequency was determined as the total number of independent mutations per sample divided by the total number of plaque forming units screened (PFUs) per sample. For each experimental group, mutation frequencies from 4–7 experiments were averaged to represent the median mutation frequency. Mutation spectrum data was combined for each experimental group (in order to obtain a full distribution) by taking the total number of each mutation type per group divided by the total number of PFUs per group.
2.9 Statistical Analysis of Mutation Frequencies
The standard error of the mean (SEM) of the mutation frequency for each age and genotype was calculated by standard methods. To determine statistical significance for differences observed between different experimental groups, the non-parametric Wilcoxon rank-sum test was used to obtain a two-tailed p-value (http://elegans.swmed.edu/~leon/stats/utest.cgi). To determine the statistical significance of mutation-type frequencies Fisher’s Exact test was used to obtain a two-tailed p-value (http://www.langsrud.com/fisher.htm).
2.10 Sensitivity of Polk−/− cells to benzo[a]pyrene diol epoxide
Benzo[a]pyrene-dihydrodiol epoxide (BPDE) (NCI carcinogen repository) was dissolved in DMSO to make 1mM stocks. Polk−/− and wild type control cells were plated the day before treatment and grown to 50–60% confluence. BPDE was added directly to the growth medium with a final concentration of 0, 100, 200, or 400nM. Twenty-four hours later cells were washed with PBS and split into triplicate 10-cm dishes at a density of 1000–2000 cells/dish. After 10 days, colonies on dishes were fixed with ethanol, stained with crystal violet, and counted. Average survival and SEM was calculated and plotted for each data point.
2.11 Survival of Polk−/− mice
Survival of wild type, heterozygous, and Polk−/− mice was determined over a course of 2 years. Survival analysis was performed by generating Kaplan-Meier survival curves, which were compared by using a log-rank test.
3. Results
3.1 Phenotypes observed in an inbred colony of mice derived from a Polk+/− breeding pair
A cohort of mice derived from breeding a male and two female Polk+/− animals was maintained in a non-pathogen-free animal facility and progeny were monitored for multiple generations. Beginning with the second generation, mice were identified with multiple autosomal recessive disease states, notably diabetes insipidus (DI), vitiligo, neurological abnormalities, and skeletal malformations (data not shown). Affected mice had any of the three possible Polk genotypes in approximately equal numbers, indicating that these disease states were not a direct consequence of defective Polk gene function. Conceivably, they arose from a random spontaneous mutation(s) in a single founder mouse. Consistent with this notion, numerous attempts to recapitulate these phenotypes in other Polk cohorts were unsuccessful. In view of the serendipitous nature of these observations, subsequent studies were devoted to the direct analysis of spontaneous mutagenesis in Polk−/− mice using a reporter gene.
3.2 Spontaneous mutations in mice carrying a reporter gene
BigBlue mice carrying multiple integrated copies of bacteriophage λ in which the cII gene serves as a reporter for qualitatively and quantitatively monitoring mutations, have been previously described [20–23]. Rederived Polk−/− mice carrying the cII gene were generated by standard genetic crosses and were maintained in a strictly pathogen-free facility. Various organs from 4 or 5 such animals were harvested at 3, 9, or 12 months of age and genomic DNA was isolated and packaged into λ-phage for the λ-select cII mutation detection assay, as previously described [24]. A minimum of ~300,000 plaques were screened per DNA sample and a total of at least 1.7 million cII plaques were screened for each tissue/genotype. The λ-cII gene was sequenced for each verified plaque, and mutation frequencies were calculated and corrected for “jackpot” mutations that can arise from the clonal expansion of cells carrying a single mutation, as previously described [19].
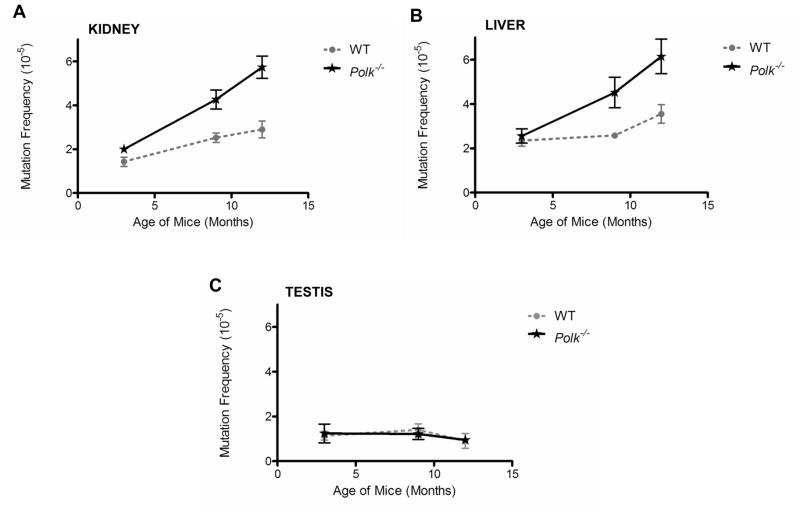
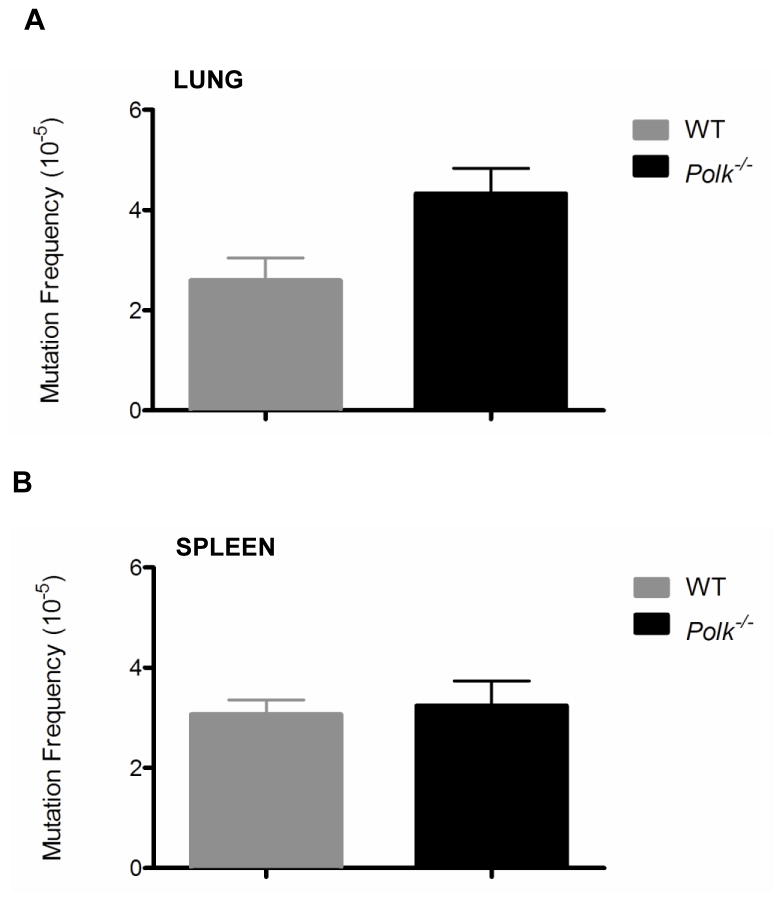
The liver and kidney are major sites of metabolism of polycyclic aromatic hydrocarbons [14,25]. At the age of 3-months the frequency of mutations in cells from these organs was not significantly different from that observed in wild-type littermates (Figs. 1A and 1B.) However, by 9 months of age a statistically significant increase in spontaneous mutations was noted in these tissues (Figs. 1A and 1B)(liver: p=0.006; kidney: p=0.008). This trend persisted at 12 months of age (Figs. 1A and 1B)(liver: p=0.027; kidney p=0.008). A significant increase in mutations was also observed in the lung of Polk−/− animals at 12 months of age (Fig. 2A; p=0.009). However, enhanced mutagenesis above wild-type levels was not observed in the spleen of 12-month-old Polk mutant animals (Fig. 2B; p=0.806). Furthermore, examination of DNA from the testis revealed no age-dependent increase in mutation frequency in wild-type animals or enhanced mutagenesis in Polk−/− mice (Fig. 1C).
Figure 1. Spontaneous mutagenesis is enhanced in an age-dependent manner in Polk−/− kidney and liver, but not the testis.
Spontaneous mutation frequencies with respect to age in Polk−/− and Polk+/+ mice in the (A) kidney, (B) liver, and (C) testis. At 9 and 12 months of age the enhanced mutation frequency observed in the kidney and liver of Polk−/− mice are statistically significant. Spontaneous mutagenesis in the testis was not enhanced in an age-dependent manner in Polk+/+ animals nor was enhanced mutagenesis observed in Polk−/− mice. Error bars represent standard error of the mean (SEM): N=4–7.
Figure 2. Spontaneous mutagenesis is enhanced in 12-month-old Polk−/− lung tissue, but not in the spleen.
Spontaneous mutation frequencies in the lung of (A) 12-month-old Polk−/− mice were elevated with respect to Polk+/+ animals. No statistically significant increases were observed in the (B) spleen. Error bars represent standard error of the mean (SEM): N=4–7.
In light of the enhanced expression of Polk mRNA in the adrenal cortex of embryonic and adult mice, it was of obvious interest to examine mutation frequencies in this tissue. However, we were unable to isolate sufficient amounts of high molecular weight DNA from the adrenal glands of any single animal due to the limited amount of starting tissue, and elected not to pool adrenal glands from multiple animals to avoid unpredictable bias.
3.3 The mutation spectrum in Polk−/− mice primarily involves G:C base pairs
The mutation spectrum was determined for each tissue investigated. Because integrated λ-bacteriophage DNA necessarily undergoes multiple rounds of replication in the course of the experiments, it is not possible to determine which DNA strand a particular mutation arose in. Sequenced mutations were therefore placed in one of the following categories: G:C>T:A, G:C>A:T, G:C>C:G, T:A>G:C, T:A>C:G, T:A>A:T, (−1) frameshifts, (+1) frameshifts, insertion/deletion(s) >2bp, and tandem base mutations.
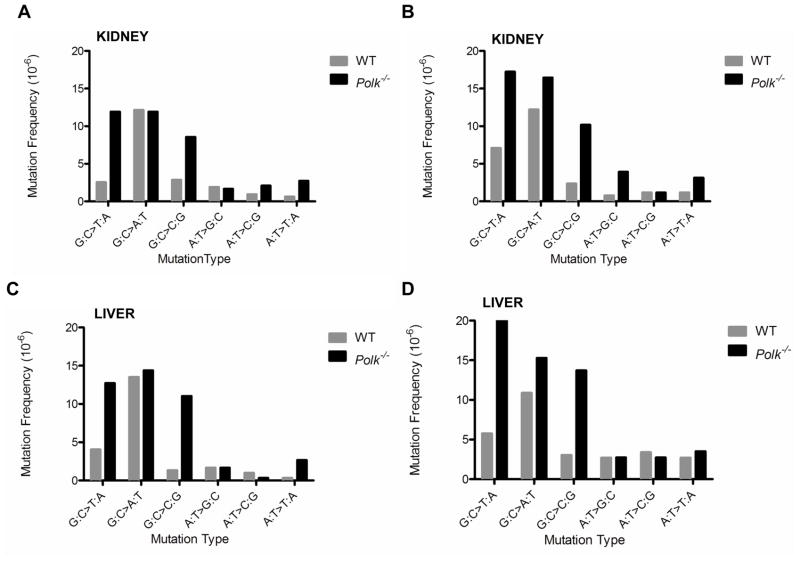
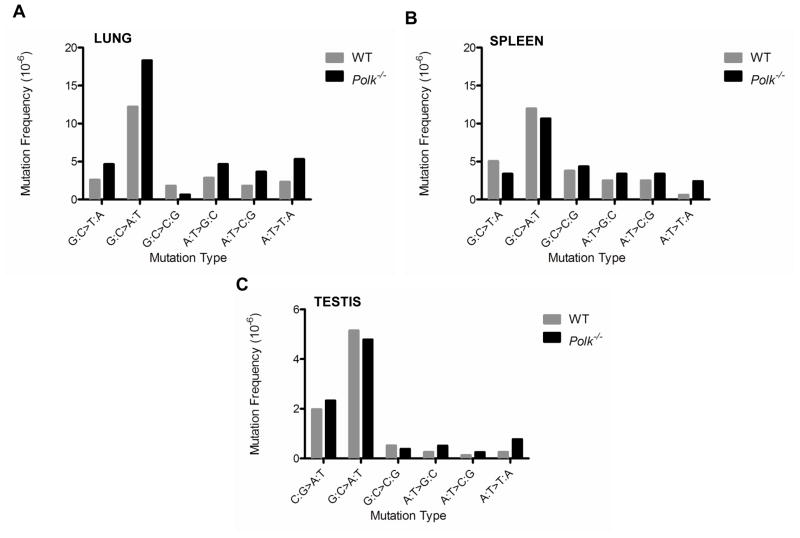
The spectra of mutations observed in tissues from Polk+/+ mice were similar to those previously reported for wild-type mice (data not shown) [26,27]. However, we observed a predominant increase in G:C>T:A mutations in the kidney of 9-month-old (Fig. 3A; p=3×e−6) and 12-month-old (Fig. 3B; p=7×e−5;) Polk−/− mice, as well as in the liver at 9 months (Fig. 3C; p=3×e−4;) and at 12 months (Fig. 3D; p=1.2×e−7;) of age. We also observed an increased frequency of G:C>C:G mutations in these tissues from Polk−/− animals (Fig. 3A, kidney at 9 months, p=1×e−3; Fig. 3B, kidney at 12 months, p=6×e−6; Fig. 3C, liver at 9 months, p=1×e−6; Fig. 3D liver at 12 months, p=1×e−5). In the lung tissue of 12-month-old Polk−/− mice, a moderate but statistically significant increase of G:C>A:T mutations was observed (Fig. 4A; p=0.045). A moderate increase in A:T>T:A transversion mutations was also seen in lung tissue at 12 months and liver at 9 months (liver, p=0.04, lung, p=0.046), although this was not the case in the liver at 12 months, nor the kidney at 9 or 12 months (liver, p=0.47; kidney at 9 months, p=0.06; kidney at 12 months, p=0.09). Consistent with the failure to observe an increased mutation frequency in the spleen or testis of Polk−/− animals, no significant differences in the mutation spectrum were noted (Fig. 4B and 4C). Enhanced insertions, deletions, and frame-shift mutations were not observed in any tissues examined from Polk−/− mice.
Figure 3. Mutation spectra in the liver and kidney of 9- and 12-month-old Polk−/− mice.
The mutation spectrum in the kidney of Polk−/− mice reveals a significant increase of G:C>T:A and G:C>C:G mutations at both (A) 9 months and (B) 12 months of age relative to that observed in Polk+/+ animals. Similar increases were observed in the liver at (C) 9 months and (D) 12 months of age.
Figure 4. Mutation spectra in (A) lung, (B) spleen, and (C) testis of Polk−/− mice.
The mutation spectrum in (A) lung tissue from 12-month-old Polk−/− mice reveals a significant increase in G:C>A:T mutations. No increases were noted in the spectrum of mutations in either the spleen of (B) 12-month-old mice or the (C) testis of pooled 3-, 9- and 12-month-old animals.
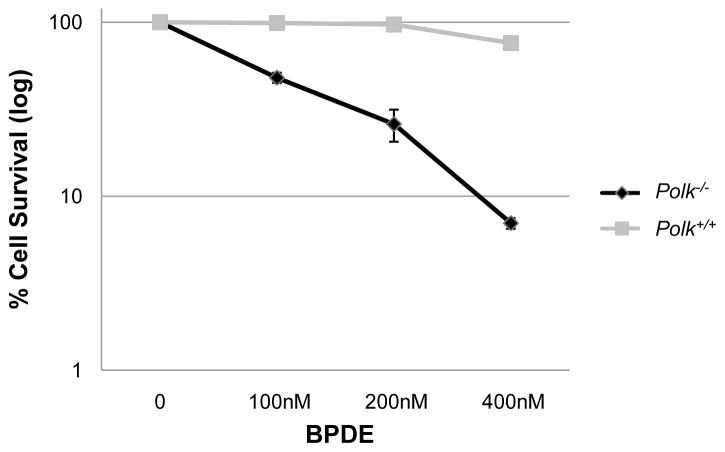
3.4 MEF cells from a second Polk mutant strain are sensitive to killing by benzo[a]pyrene diol epoxide
Strains of Polk−/− knockout mice were independently generated in Japan [6] and in Germany [18]. Cells from the Japanese strain were previously reported to be markedly sensitive to killing by BPDE [6]. In the present studies, we demonstrate that cells from the German strain also exhibit this phenotype (Fig. 5).
Figure 5. Polk−/− cells are sensitive to killing by BPDE.
Cells from the German strain of Polk−/− mice manifest enhanced sensitivity to killing following exposure to BPDE. Cells were treated with 0, 100, 200, or 400nM BPDE. Average survival and SEM are shown.
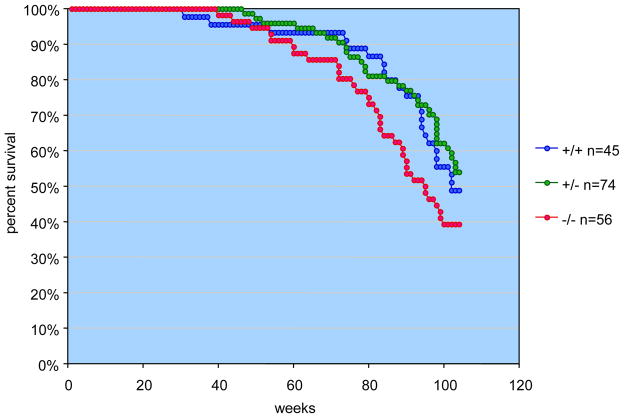
3.5 Survival of Polk−/− mice is reduced as a function of age
Polk−/− mice manifested slightly reduced survival over a period of about 2 years, compared to wild type and heterozygous littermates (Fig. 6). Autopsy examination of some of these mice did not reveal any specific abnormalities. The difference in survival between the populations of Polk−/−, Polk−/+, and Polk+/+ mice was determined to be significant by the log-rank test(p=0.0041).
Figure 6. Survival of mice as a function of age.
Beginning at about one year of age the survival of Polk−/− mice was reduced relative to that of Polk+/− and Polk−/− mice.
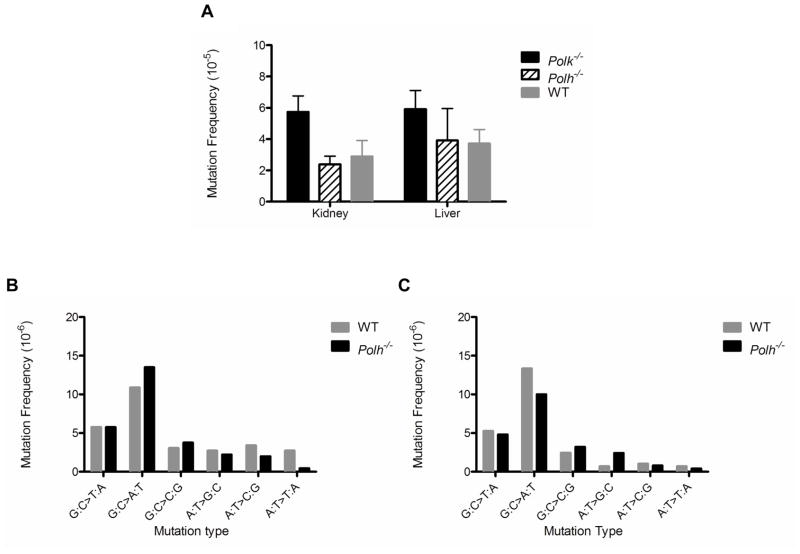
3.6 Polh−/− mice do not show a spontaneous mutator phenotype
As discussed earlier, thymine-thymine CPD (and presumably other CPD) are the cognate substrate for TLS by Polη [2]. We therefore anticipated that cells from tissues of Polh−/− mice would not manifest enhanced spontaneous mutagenesis. Such was indeed the case in the liver and kidney of these animals (Fig. 7A), providing a cogent negative control for the results obtained with Polk−/− mice. Furthermore, no significant differences between the Polh−/− mutation spectra in these tissues compared to wild-type were observed (Fig. 7B and 7C).
Figure 7. Spontaneous mutagenesis and mutation spectra in Polh−/− mice.
The spontaneous mutation frequency was not enhanced in the (A) kidney or liver of 12-month-old Polh−/− mice relative to that in Polk+/+ animals (N=4–7). The mutation spectra in the (B) liver or (C) kidney of Polh−/− mice were also comparable to those observed in Polk+/+ animals.
4. Discussion
Specialized DNA polymerases have been implicated in somatic hypermutation and class switching of immunoglobulin genes [28–33]. However, members of the Y-family (Polη, Polκ and Polι), as well as Polζ are represented in organisms that predate the evolution of an immune system [1]. These polymerases may be reasonably considered to have evolved primarily, if not exclusively, for TLS past naturally occurring types or classes of base damage. This notion is supported by the well documented fact that human and mouse cells defective for Polη activity manifest an enhanced mutational burden following exposure of the shaved dorsal skin and the ears to UV radiation [3,4]. While the precise mechanism of origin of these mutations remains to be formally established, it is likely that in the absence of functional Polη TLS past CPD by one or more other specialized polymerases supports cellular viability at the expense of reduced fidelity.
Two mouse strains defective for Polκ have been independently generated by the deletion of exons encoding regions of the Polκ polypeptide essential for polymerase activity [6,18]. Other than the observation that the Polk−/− mice used in the present studies died sooner than their heterozygous mutant and wild-type littermates after about a year of life (Fig. 6), neither of the Polk−/− strains manifest obvious phenotypes and to date no human disease has been associated with defective Polκ activity. These limitations notwithstanding, the phenotypes of cells derived from Polk mutant mice and of chicken DT40 cells and some biochemical properties of purified Polκ are provocative:
The mouse and human Polk genes contain canonical arylhydrocarbon binding sites in the promoter of the wild-type Polk gene [5]. These are not present in the promoter regions of the Polh or Poli genes.
As was previously demonstrated in a different Polk mutant mouse strain [6], we observed significantly enhanced sensitivity of Polk−/− mouse cells to BPDE.
Polk−/− chicken DT40 cells manifest increased sensitivity to killing and/or chromosomal aberrations in response to polycyclic estrogen derivatives [8].
When Polk−/− mouse cells in culture are transfected with gapped circular plasmid DNA containing benzo[a]pyrene adducts opposite the gaps, the efficiency of TLS past these adducts is markedly reduced and the mutation frequency is correspondingly increased [13].
Polk−/− mouse cells fail to activate an intra-S phase checkpoint in response to treatment with BPDE [7].
Purified mouse Polk is considerably more efficient at incorporating dC opposite N2-furfuryl-dG than opposite undamaged dG [34].
It is of course impossible to establish whether or not atmospheric benzo[a]pyrene from spontaneous fires and volcanic eruptions was a primary driver of the evolution of Polκ. Hence, the observation that vertebrate cells defective in this specialized DNA polymerase manifest phenotypes in response to exposure to other polycyclic compounds prompts the consideration that one or more of these compounds constitutes the true cognate substrate(s) for this enzyme. Under such circumstances, one anticipates that cells from Polk−/− mice would manifest enhanced spontaneous mutagenesis and a mutation spectrum consistent with the formation of cyclic N2-guanine adducts. Such was indeed the case in the kidney, liver, and lung. Indeed, the spectrum of spontaneous mutations in the liver (and kidney) are remarkably concordant with a previously published study on benzo[a]pyrene-induced mutagenesis in mice [35]. Generalized enhanced spontaneous mutagenesis may also explain the reduced longevity observed in Polk−/− mice relative to their wild-type or heterozygous mutant littermates.
The absence of enhanced mutagenesis in the spleen, as well as the unique mutation spectrum observed in the lung, suggest that in the absence of Polk, mutagenesis is tissue-specific. This phenomenon has been reported in previous studies [36,37] and conceivably reflects the deployment of different specialized polymerases in various cell types defective for Polκ.
Previous studies reported an increased frequency of mutations in the germ line of Polk−/− mice [38]. However, these were detected by Southern hybridization that only reflects base additions and/or deletions. Regardless, the failure to observe enhanced base substitutions in the testis of Polk−/− mice in the present study begs an explanation. The observation, both in our studies and those previously reported [26,39] that demonstrate in wild-type mice that the testis is relatively immune to age-dependent mutagenesis, may lie at the heart of such an explanation. It may also be relevant that multiple Polk transcripts are expressed in mouse testis, possibly reflecting the existence of multiple functional Polκ isoforms [40]. Current studies are addressing whether or not such protein isoforms are in fact detectable in mouse testis and what their functional role(s) may be.
The mutation spectra observed in the kidney, liver, and lung of Polk−/− mice are consistent with error-prone TLS past dG adducts in DNA, supporting the notion that these organs accumulate DNA base damage caused by naturally occurring polycyclic planar compounds. Mammalian cells contain numerous such compounds, many of which are derived from cholesterol. Notably, both the liver and kidney are important sites of cholesterol metabolism. Studies are in progress to determine whether hypercholesterolemia in Polk−/− mice promotes further enhanced mutagenesis.
The supposition that each of the multiple specialized DNA polymerases in vertebrates evolved for error-free TLS past naturally occurring base damage predicts that mice defective for Polη (which evolved to relieve arrested DNA replication by CPD) do not manifest enhanced mutagenesis without exposure to ultraviolet light. Our observations indicate that such is indeed the case, confirming recent studies to this effect [41].
In conclusion, our results offer the interesting possibility that demonstrating spontaneous mutagenesis in mice defective for other specialized DNA polymerases, such as Polι, may provide clues about naturally occurring base damage that provides cognate substrates for these enzymes.
Acknowledgments
The authors thank Ana Doughty and Jeanetta Washburn for skilled technical assistance and Elizabeth McClellan for statistical help. Studies supported by grant R01-ES011344 from the NIH, USPHS.
Footnotes
Conflict of interest statement
None declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Friedberg EC. Suffering in silence: the tolerance of DNA damage. Nat Rev Mol Cell Biol. 2005;6:943–953. doi: 10.1038/nrm1781. [DOI] [PubMed] [Google Scholar]
- 2.Masutani C, Araki M, Yamada A, Kusumoto R, Nogimori T, Maekawa T, Iwai S, Hanaoka F. Xeroderma pigmentosum variant (XP-V) correcting protein from HeLa cells has a thymine dimer bypass DNA polymerase activity. EMBO J. 1999;18:3491–3501. doi: 10.1093/emboj/18.12.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohkumo T, Kondo Y, Yokoi M, Tsukamoto T, Yamada A, Sugimoto T, Kanao R, Higashi Y, Kondoh H, Tatematsu M, Masutani C, Hanaoka F. UV-B radiation induces epithelial tumors in mice lacking DNA polymerase eta and mesenchymal tumors in mice deficient for DNA polymerase iota. Mol Cell Biol. 2006;26:7696–7706. doi: 10.1128/MCB.01076-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin Q, Clark AB, McCulloch SD, Yuan T, Bronson RT, Kunkel TA, Kucherlapati R. Increased susceptibility to UV-induced skin carcinogenesis in polymerase eta-deficient mice. Cancer Res. 2006;66:87–94. doi: 10.1158/0008-5472.CAN-05-1862. [DOI] [PubMed] [Google Scholar]
- 5.Ogi T, Mimura J, Hikida M, Fujimoto H, Fujii-Kuriyama Y, Ohmori H. Expression of human and mouse genes encoding polkappa: testis-specific developmental regulation and AhR-dependent inducible transcription. Genes Cells. 2001;6:943–953. doi: 10.1046/j.1365-2443.2001.00478.x. [DOI] [PubMed] [Google Scholar]
- 6.Ogi T, Shinkai Y, Tanaka K, Ohmori H. Polkappa protects mammalian cells against the lethal and mutagenic effects of benzo [a]pyrene. Proc Natl Acad Sci U S A. 2002;99:15548–15553. doi: 10.1073/pnas.222377899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bi X, Slater DM, Ohmori H, Vaziri C. DNA polymerase kappa is specifically required for recovery from the benzo [a]pyrene-dihydrodiol epoxide (BPDE)-induced S-phase checkpoint. J Biol Chem. 2005;280:22343–22355. doi: 10.1074/jbc.M501562200. [DOI] [PubMed] [Google Scholar]
- 8.Mizutani A, Okada T, Shibutani S, Sonoda E, Hochegger H, Nishigori C, Miyachi Y, Takeda S, Yamazoe M. Extensive chromosomal breaks are induced by tamoxifen and estrogen in DNA repair-deficient cells. Cancer Res. 2004;64:3144–3147. doi: 10.1158/0008-5472.can-03-3489. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Wu X, Guo D, Rechkoblit O, Wang Z. Activities of human DNA polymerase kappa in response to the major benzo [a]pyrene DNA adduct: error-free lesion bypass and extension synthesis from opposite the lesion. DNA Repair (Amst) 2002;1:559–569. doi: 10.1016/s1568-7864(02)00055-1. [DOI] [PubMed] [Google Scholar]
- 10.Rechkoblit O, Zhang Y, Guo D, Wang Z, Amin S, Krzeminsky J, Louneva N, Geacintov NE. trans-Lesion synthesis past bulky benzo [a]pyrene diol epoxide N2-dG and N6-dA lesions catalyzed by DNA bypass polymerases. J Biol Chem. 2002;277:30488–30494. doi: 10.1074/jbc.M201167200. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki N, Itoh S, Poon K, Masutani C, Hanaoka F, Ohmori H, Yoshizawa I, Shibutani S. Translesion synthesis past estrogen-derived DNA adducts by human DNA polymerases eta and kappa. Biochemistry. 2004;43:6304–6311. doi: 10.1021/bi0360298. [DOI] [PubMed] [Google Scholar]
- 12.Choi JY, Angel KC, Guengerich FP. Translesion synthesis across bulky N2-alkyl guanine DNA adducts by human DNA polymerase kappa. J Biol Chem. 2006;281:21062–21072. doi: 10.1074/jbc.M602246200. [DOI] [PubMed] [Google Scholar]
- 13.Avkin S, Goldsmith M, Velasco-Miguel S, Geacintov N, Friedberg EC, Livneh Z. Quantitative analysis of translesion DNA synthesis across a benzo [a]pyrene-guanine adduct in mammalian cells: the role of DNA polymerase kappa. J Biol Chem. 2004;279:53298–53305. doi: 10.1074/jbc.M409155200. [DOI] [PubMed] [Google Scholar]
- 14.Zedeck MS. Polycyclic aromatic hydrocarbons: a review. J Environ Pathol Toxicol. 1980;3:537–567. [PubMed] [Google Scholar]
- 15.Polya G. Biochemical targets of plant bioactive compounds: a pharmacological reference guide to sites of action and biological effects. CRC Press; 2003. [Google Scholar]
- 16.Zhdanov RI, D’yachkov EP, Strazhevskaya NB, Shmyrina AS, Krylov AS, D’yachkov PN, Lorenz W, Kubatiev AA. Cholesterol and its fatty acid esters in native DNA preparations: lipid analysis, computer simulation of their interaction with DNA and cholesterol binding to immobilized oligodeoxyribonucleotides. Russ Chem Bull. 2005;54:2204–2210. [Google Scholar]
- 17.Velasco-Miguel S, Richardson JA, Gerlach VL, Lai WC, Gao T, Russell LD, Hladik CL, White CL, Friedberg EC. Constitutive and regulated expression of the mouse Dinb (Polkappa) gene encoding DNA polymerase kappa. DNA Repair (Amst) 2003;2:91–106. doi: 10.1016/s1568-7864(02)00189-1. [DOI] [PubMed] [Google Scholar]
- 18.Schenten D, Gerlach VL, Guo C, Velasco-Miguel S, Hladik CL, White CL, Friedberg EC, Rajewsky K, Esposito G. DNA polymerase kappa deficiency does not affect somatic hypermutation in mice. Eur J Immunol. 2002;32:3152–3160. doi: 10.1002/1521-4141(200211)32:11<3152::AID-IMMU3152>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 19.Nishino H, Schaid DJ, Buettner VL, Haavik J, Sommer SS. Mutation frequencies but not mutant frequencies in Big Blue mice fit a Poisson distribution. Environ Mol Mutagen. 1996;28:414–417. doi: 10.1002/(SICI)1098-2280(1996)28:4<414::AID-EM16>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 20.Monroe JJ, Kort KL, Miller JE, Marino DR, Skopek TR. A comparative study of in vivo mutation assays: analysis of hprt, lacI, cII/cI and as mutational targets for N-nitroso-N-methylurea and benzo [a]pyrene in Big Blue mice. Mutat Res. 1998;421:121–136. doi: 10.1016/s0027-5107(98)00171-7. [DOI] [PubMed] [Google Scholar]
- 21.Harbach PR, Zimmer DM, Filipunas AL, Mattes WB, Aaron CS. Spontaneous mutation spectrum at the lambda cII locus in liver, lung, and spleen tissue of Big Blue transgenic mice. Environ Mol Mutagen. 1999;33:132–143. doi: 10.1002/(sici)1098-2280(1999)33:2<132::aid-em5>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 22.Mei N, Heflich RH, Moore MM, Chen T. Age-dependent sensitivity of Big Blue transgenic mice to the mutagenicity of N-ethyl-N-nitrosourea (ENU) in liver. Mutat Res. 2005;572:14–26. doi: 10.1016/j.mrfmmm.2004.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slikker W, 3rd, Mei N, Chen T. N-ethyl-N-nitrosourea (ENU) increased brain mutations in prenatal and neonatal mice but not in the adults. Toxicol Sci. 2004;81:112–120. doi: 10.1093/toxsci/kfh177. [DOI] [PubMed] [Google Scholar]
- 24.Zimmer DM, Harbach PR, Mattes WB, Aaron CS. Effect of plating medium and phage storage on mutant frequency and titer in the lambda cII transgenic mutation assay. Environ Mol Mutagen. 1998;32:325–330. [PubMed] [Google Scholar]
- 25.Wall KL, Gao WS, te Koppele JM, Kwei GY, Kauffman FC, Thurman RG. The liver plays a central role in the mechanism of chemical carcinogenesis due to polycyclic aromatic hydrocarbons. Carcinogenesis. 1991;12:783–786. doi: 10.1093/carcin/12.5.783. [DOI] [PubMed] [Google Scholar]
- 26.Hill KA, Buettner VL, Halangoda A, Kunishige M, Moore SR, Longmate J, Scaringe WA, Sommer SS. Spontaneous mutation in Big Blue mice from fetus to old age: tissue-specific time courses of mutation frequency but similar mutation types. Environ Mol Mutagen. 2004;43:110–120. doi: 10.1002/em.20004. [DOI] [PubMed] [Google Scholar]
- 27.Stuart GR, Oda Y, de Boer JG, Glickman BW. Mutation frequency and specificity with age in liver, bladder and brain of lacI transgenic mice. Genetics. 2000;154:1291–1300. doi: 10.1093/genetics/154.3.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masuda K, Ouchida R, Yokoi M, Hanaoka F, Azuma T, Wang JY. DNA polymerase eta is a limiting factor for A:T mutations in Ig genes and contributes to antibody affinity maturation. Eur J Immunol. 2008;38:2796–2805. doi: 10.1002/eji.200838502. [DOI] [PubMed] [Google Scholar]
- 29.Delbos F, Aoufouchi S, Faili A, Weill JC, Reynaud CA. DNA polymerase eta is the sole contributor of A/T modifications during immunoglobulin gene hypermutation in the mouse. J Exp Med. 2007;204:17–23. doi: 10.1084/jem.20062131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jansen JG, Langerak P, Tsaalbi-Shtylik A, van den Berk P, Jacobs H, de Wind N. Strand-biased defect in C/G transversions in hypermutating immunoglobulin genes in Rev1-deficient mice. J Exp Med. 2006;203:319–323. doi: 10.1084/jem.20052227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ross AL, Sale JE. The catalytic activity of REV1 is employed during immunoglobulin gene diversification in DT40. Mol Immunol. 2006;43:1587–1594. doi: 10.1016/j.molimm.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 32.Schenten D, Kracker S, Esposito G, Franco S, Klein U, Murphy M, Alt FW, Rajewsky K. Pol zeta ablation in B cells impairs the germinal center reaction, class switch recombination, DNA break repair, and genome stability. J Exp Med. 2009;206:477–490. doi: 10.1084/jem.20080669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faili A, Aoufouchi S, Flatter E, Gueranger Q, Reynaud CA, Weill JC. Induction of somatic hypermutation in immunoglobulin genes is dependent on DNA polymerase iota. Nature. 2002;419:944–947. doi: 10.1038/nature01117. [DOI] [PubMed] [Google Scholar]
- 34.Jarosz DF, Godoy VG, Delaney JC, Essigmann JM, Walker GC. A single amino acid governs enhanced activity of DinB DNA polymerases on damaged templates. Nature. 2006;439:225–228. doi: 10.1038/nature04318. [DOI] [PubMed] [Google Scholar]
- 35.Shane BS, de Boer J, Watson DE, Haseman JK, Glickman BW, Tindall KR. LacI mutation spectra following benzo [a]pyrene treatment of Big Blue mice. Carcinogenesis. 2000;21:715–725. doi: 10.1093/carcin/21.4.715. [DOI] [PubMed] [Google Scholar]
- 36.Jiao J, Douglas GR, Gingerich JD, Soper LM. Analysis of tissue-specific lacZ mutations induced by N-nitrosodibenzylamine in transgenic mice. Carcinogenesis. 1997;18:2239–2245. doi: 10.1093/carcin/18.11.2239. [DOI] [PubMed] [Google Scholar]
- 37.Ushijima T, Hosoya Y, Ochiai M, Kushida H, Wakabayashi K, Suzuki T, Hayashi M, Sofuni T, Sugimura T, Nagao M. Tissue-specific mutational spectra of 2-amino-3,4-dimethylimidazo [4,5-f]quinoline in the liver and bone marrow of lacI transgenic mice. Carcinogenesis. 1994;15:2805–2809. doi: 10.1093/carcin/15.12.2805. [DOI] [PubMed] [Google Scholar]
- 38.Burr KL, Velasco-Miguel S, Duvvuri VS, McDaniel LD, Friedberg EC, Dubrova YE. Elevated mutation rates in the germline of Polkappa mutant male mice. DNA Repair (Amst) 2006;5:860–862. doi: 10.1016/j.dnarep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 39.Hill KA, Halangoda A, Heinmoeller PW, Gonzalez K, Chitaphan C, Longmate J, Scaringe WA, Wang JC, Sommer SS. Tissue-specific time courses of spontaneous mutation frequency and deviations in mutation pattern are observed in middle to late adulthood in Big Blue mice. Environ Mol Mutagen. 2005;45:442–454. doi: 10.1002/em.20119. [DOI] [PubMed] [Google Scholar]
- 40.Guo C, Gao T, Confer N, Velasco-Miguel S, Friedberg EC. Multiple PolK (POLK) transcripts in mammalian testis. DNA Repair (Amst) 2005;4:397–402. doi: 10.1016/j.dnarep.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 41.Busuttil RA, Lin Q, Stambrook PJ, Kucherlapati R, Vijg J. Mutation frequencies and spectra in DNA polymerase eta-deficient mice. Cancer Res. 2008;68:2081–2084. doi: 10.1158/0008-5472.CAN-07-6274. [DOI] [PubMed] [Google Scholar]