Abstract
Contraceptive vaccines targeting sperm are an exciting proposition. This review is focused on anti-sperm contraceptive vaccines and genetically engineered human antibodies that can be used as immunocontraceptives. Various methods of vaccinology and antibody engineering have been used to obtain multi-epitope contraceptive vaccines and human single chain variable fragment (scFv) antibodies from immunoinfertile and vasectomized men. Contraceptive vaccines comprised of various sperm antigens, peptide epitopes or DNA have shown various degrees of reversible contraceptive effect in the mouse model and their efficacy is enhanced with the multi-epitope combination vaccine. Failure to achieve a complete fertility block is probably due to variability in the host immune response. Using phage display technology, our laboratory has synthesized in vitro at least four novel scFv antibodies with unique complementarity determining regions (CDRs) that react with specific fertility-related sperm antigens employing cDNA from immunoinfertile and vasectomized men. These antibodies inhibit human sperm function in vitro, and their immunocontraceptive effect in vivo is being investigated. If these human scFv antibodies block fertility in vivo they may provide unique and novel immunocontraceptives, a first-in-kind for human use. The multi-epitope contraceptive vaccines and preformed engineered antibodies of defined specificity may eliminate concern related to inter-individual variability of the immune response.
Keywords: Immunocontraception, Contraceptive vaccine, Antisperm antibodies, scFv antibodies, Immuno infertility
1. Introduction
The population explosion and unintended pregnancies are two major health concerns worldwide. The world population has exceeded 6.73 billion (http://www.census.gov/main/www/popclock.html), and at the current rate will increase by one billion every 12 years. Despite availability of contraception, there are over one million elective abortions each year in the US alone due to unintended pregnancies (Henshaw, 1998; Grow and Ahmed, 2000). Therefore, better methods of contraception are needed (http://contraceptiononline.org). Immunocontraception could be a valuable alternative. This article will review the current status of contraceptive vaccines, with special emphasis on anti-sperm immunocontraception.
Contraceptive vaccines can target gamete production, gamete function, or gamete outcome (Naz et al., 2005). Contraceptive vaccines for gamete production primarily target luteinizing hormone-releasing hormone (LHRH/GnRH), and show only partial effect in inhibiting gametogenesis and affect sex steroids. These vaccines may be useful in clinical situations that require suppression of sex steroid secretion, such as in uterine fibroids, polycystic ovary syndrome (PCOS), endometriosis, and precocious puberty.
Contraceptive vaccines for gamete function target sperm antigens and oocyte zona pellucida (ZP). Vaccines based on ZP proteins are very effective in producing contraception, but invariably induce oophoritis affecting sex steroids in several species (Naz et al., 2005). However, they have been successfully used in controlling feral populations of deer, horses, elephants, and several species of zoo animals (Naz et al., 2005). The current research for human applicability is focused on delineating infertility-related epitopes (B-cell epitopes) from oophoritis-inducing epitopes (T-cell epitopes), and using adjuvants that may avoid oophoritis.
Contraceptive vaccines targeting gamete outcome primarily focus on the human chorionic gonadotropin (hCG) molecule. The hCG vaccine is the first to undergo Phase I and II clinical trials in women (Talwar et al., 1994). Efficacy and non-toxicity have been reasonably well demonstrated for this vaccine. Current research is focused on increasing its immunogenicity and efficacy and examining its clinical application in various hCG-producing cancers.
2. Development of anti-sperm contraceptive vaccines
2.1. Rationale
There is a strong rationale for the development of anti-sperm contraceptive vaccines. The feasibility of anti-sperm contraception is provided by human and animal data. Sperm have auto- as well as iso-antigenic potential and thus can stimulate formation of antibodies in both men and women involuntarily. Up to 70% of vasectomized men produce antisperm antibodies (ASA) and 2-30% of cases of infertility may be associated with the presence of ASA in the male and/or female of an infertile couple (Ohl and Naz, 1995). These ASA affect fertilization and fertility in vitro and in vivo. Active immunization with sperm antigens of male or female animals of various species, including humans (Baskin, 1932; Mancini et al., 1965), produces ASA which leads to infertility. In a classic study Baskin injected 20 fertile women, who had at least one prior pregnancy, with their husband's semen (Baskin, 1932). The women developed antibodies preventing conception for up to one year of the study period. These findings indicate that spermatozoa can generate an immune response capable of inducing contraception. A US patent was issued for this spermatoxic vaccine in 1937 (US patent number 2103240).
Only sperm-specific antigens can be used for contraceptive vaccines development because sperm share several antigens with various somatic cells (Naz, 1999). The function of a sperm antigen in contraceptive vaccines is contingent upon its sperm specificity, surface expression, involvement in fertility, and ability to raise high titer antibodies to be capable of intercepting fertility. The ideal antigen should also be involved in human immune infertility. The presence of antibodies to a sperm antigen in immunoinfertile men and women indicate that it is: a) immunogenic in humans, b) relevant to fertility/infertility, and c) probably sperm specific since these individuals are healthy without any immunopathology concomitant with infertility. The sperm-ZP binding site constitutes the preferred target for immunocontraception.
2.2. Delineating sperm-specific antigens for vaccine development
The next stage in anti-sperm contraceptive vaccine development focused on delineating antigens that are sperm-specific and have a role in fertility. Several approaches of genomics, proteomics, gene knockout, and hybridoma technology were applied in various laboratories to delineate sperm antigens that affect fertilization or fertility and can be used for contraceptive vaccine development. Several sperm genes and antigens have been delineated, cloned, and sequenced. Although the antibodies to a number of these antigens affect sperm function or fertilization in vitro, only immunization with a few of them (either native or recombinant protein, or peptide) causes a contraceptive effect in vivo in animals. Notable among these are FA-1 (Naz and Zho, 1998), YLP12 (Naz and Chuhan, 2002), P10G (O'Rand et al., 1993), A9D (Lea et al., 1998), and SP56 (Hardy and Mobbs, 1999). Most of these active immunization studies were carried out in the mouse model. Thus far, no study has achieved 100% infertility after immunization with any of the antigens; the maximum reduction in fertility observed is approximately 75%. The female mouse ovulates numerous (approximately 20-50) oocytes every cycle and a woman ovulates typically one ovum every cycle. Therefore, it is unclear whether the 75% decrease in fertility in the mouse model translates to a 100% reduction in humans. This may be due to the inherent nature of the mouse model where it is difficult to achieve complete infertility. However, after active immunization or deleting a single gene, one does find a few mice that are totally infertile. The circulating and local antibody titers show a significant linear correlation with the fertility reduction, with the animals that have higher reduction in fertility showing higher circulating and local antibody titers. However, in all these studies, the local titers were measured in the vagina and not in the uterus or fallopian tubes where fertilization occurs.
2.3. Active immunization studies in non-human primates
No sperm antigen has undergone a Phase I or II clinical trial in humans, but to our knowledge three studies have examined the effect of vaccination with a sperm antigen in a non-human primate model. One study reported reduced fertility of female baboons after immunization with LDH-C4 (O'Hearn et al., 1997), whereas a study by another group found no effect on fertility in female monkeys after vaccination with LDH-C4 (Tollner et al., 2002). The reason for this discrepancy is unclear. The third study was carried out in male monkeys. They were immunized with an epididymal protein designated as epididymal protein inhibitor (Eppin) (O'Rand et al., 2004). Seventy-eight percent of male monkeys who developed high antibody titers became infertile, and 71% of those monkeys regained fertility after the titers declined. The booster injections with Eppin in Freund's adjuvant were given every three weeks for the entire duration (691 days) of the study to maintain the high antibody titers. The potential immunopathological effects of immunization were not investigated. This interesting study suggests that anti-sperm contraceptive vaccines can also be developed for males.
2.4. Lessons learned from gene knockout mice to develop anti-sperm contraceptive vaccines
Using gene knockout technology, more than 100 novel testis or sperm genes or proteins have been identified that have a vital role in various aspects of fertility (Naz and Rajesh, 2005a; Naz and Rajesh, 2005b; Naz et al., 2009). These gene knockouts cause different defects such as testis development and endocrine milieu, spermatogenesis, mating behavior, sperm structure/function/motility, and fertilization. The majority of these knockouts also showed an effect on non-reproductive organs associated with fertility. An extensive database analysis was performed to examine the number of these genes and proteins that have the characteristics needed for contraceptive vaccines development. The findings revealed that only a few are expressed on the sperm surface, and thus are amenable to antibody binding. Although the proteins that are not expressed on the surface can provide ideal targets for pharmacological inhibition for contraception, they are not suitable for contraceptive vaccine development. Very few, if any, null mutations in a single gene have made mice totally infertile. The molecules involved in sperm-oocyte membrane fusion are interesting and are being examined (Cho et al., 1998; Le Naour et al., 2000; Inoue et al., 2003). Izumo, named after a Japanese shrine dedicated to marriage, is an exciting molecule that is involved in sperm-oocyte membrane fusion. Mice with a null mutation in the Izumo gene are rendered almost totally infertile (Inoue et al., 2005). The male mice produce normal-appearing sperm that bind to and penetrate the ZP but are incapable of fusing with the oocyte membrane. Human sperm also express Izumo protein. Izumo protein is not detectable on ejaculated sperm but becomes detectable after the sperm cell undergoes acrosome reaction.
2.5. Enhancement of contraceptive efficacy by employing cocktail of sperm antigens
Recently, we conducted a fertility trial by immunizing female mice with various peptides based upon several sperm antigens (Naz, 2008). Immunization with the Izumo-based peptides caused a significant reduction in fertility. The contraceptive effect was enhanced by including peptides based upon other sperm antigens (FA-1, YLP12, and SP56), resulting in an overall 73.3% reduction in fertility. All the animals regained fertility when the circulating/local antibodies against all the peptides disappeared after >9-10 months. The data indicate that the proteins involved in sperm-egg fusion can also be used for contraceptive vaccine development. The contraceptive effect is enhanced by immunizing with multi-peptide vaccines. Enhancement of contraceptive effect after combination vaccination was also observed using sperm DNA vaccines (Naz, 2006a; Naz 2006b). However, even after using multi-epitope vaccines, there was up to 73.3% reduction in fertility rather than a complete block, which may be due to the variability of the immune response among the vaccinated animals.
2.6. Passive immunization approach to obtain fully effective immunocontraceptives
Besides specific concerns associated with each vaccine, the progress of the development of contraceptive vaccines has been hindered by the following facts: 1) variability of the immune response, 2) attainment and maintenance of high antibody titers, 3) time lag from the first injection to the time to achieve reasonably good antibody titers which generally takes three months, and 4) uncertainty regarding how long the antibody titer will remain in circulation to exercise the contraceptive effects. It is envisaged that these concerns may be diminished by using the passive immunization approach. Antibody therapies have been successful against various infectious diseases, both in animals and humans, and several of these antibodies have become treatment modalities in clinics (Casadevall, 1999; Dunman and Nessin, 2003). These therapies have been approved by the Food and Drug Administration (FDA) for clinical use. The mouse monoclonal antibody can elicit strong anti-mouse antibody reaction, chimeric antibody can cause an anti-chimeric response, and xenogenic complementarity-determining regions (CDRs) of humanized antibodies can also evoke an anti-idiotypic response when injected into humans (Koren et al., 2002; Mirick et al., 2004; Sidhu and Fellouse, 2006). Antibodies must therefore be of human origin to be used in humans. Ethical issues and the potentially poor immunogenicity and toxicity of an antigen limit immunization of humans to obtain human antibodies. However, phage display technology can be used to obtain these antibodies against target antigens if they exist involuntarily in humans, such as ASA in vasectomized men and immunoinfertile men and women.
Phage display technology has been widely used to obtain a variety of engineered antibodies, including single chain variable fragment (scFv) antibodies against several antigens (Yokota et al., 1992; Zhou et al., 2007). ScFv is an antibody fragment that plays a major role in the antigen-binding activity and is composed of variable heavy (VH) and variable light (VL) chains connected by a peptide linker. The most widely used peptide linker is a repeat of a 15-residue sequence of glycine and serine (Gly4Ser)3. The affinity and stability of the scFv antibodies produced in bacteria are comparable with those of native antibodies and are maintained by a strong disulfide bond. ScFv antibodies can be produced on a large scale using specially modified bacterial hosts, have an advantage over the whole Ig molecule, lack the Fc portion that eliminates unwanted secondary effects, and due to their small size, can easily be absorbed into tissues and gene manipulated (Yokota et al., 1992).
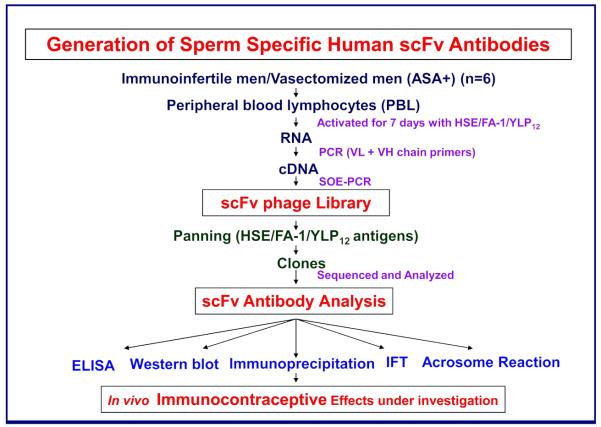
Recently, using phage display technology, we obtained several fertility-related scFv human antibodies that can be used for passive immunization, as well as diagnosis and treatment of immunoinfertility. Peripheral blood leukocytes (PBL) were obtained from ASA-positive immunoinfertile and vasectomized men, activated with human sperm antigens in vitro, and cDNA was prepared from their RNA and PCR-amplified using several primers based on all the available variable regions of VH and VL chains (Samuel and Naz, 2008) (Fig. 1). The amplified VH and VL chains were ligated using the splicing by overlapping extension PCR (SOE-PCR) procedure and the scFv repertoire was cloned into pCANTAB5E vector (Amersham Biosciences AB) to create a human scFv antibody library.
Figure 1.
Peripheral blood lymphocytes (PBL) were collected from immunoinfertile men and vasectomized men and were activated for seven days in vitro with human sperm extract (HSE), purified human fertilization antigen-1 (FA-1), and synthetic YLP12 peptide. RNA was isolated and reverse transcribed to cDNA by PCR using various VL and VH chain primers as described in detail elsewhere (Samuel and Naz, 2008). The scFv assembly of VH and VL chains, with a (G4S)3 linker between the chains, was performed using the splicing by overlapping extension PCR (SOE-PCR) procedure. The final assembled PCR product was ligated into pCANTAB5E vector to make the scFv phage library. The library was panned against various antigens (HSE/FA-1/YLP12) and the isolated clones were sequenced and analyzed for antibody activity using enzyme-linked immunosorbent assay (ELISA), Western blot procedure, immunoprecipitation procedure, indirect immunofluoroescence technique (IFT), and sperm acrosome reaction.
Panning of the library against specific antigens yielded several clones, and the four strongest reactive (designated as AFA-1, FAB-7, YLP20, and AS16) were selected for further analysis. These clones were shown to have novel sequences with unique complementarity determining regions (CDRs) when a search was performed in the immunogenetic database. ScFv antibodies were expressed, purified, and analyzed for human sperm reactivity and effects on human sperm function (Table 1). AFA-1 and FAB-7 scFv antibodies, having IgG3 heavy and IgΚ3 light chains, recognized a specific single sperm protein of 50 ± 4 kD and reacted with the purified and well characterized human sperm FA-1 antigen, which is involved in human sperm function and fertilization. These antibodies bound to post-acrosomal, mid-piece, and tail regions of human sperm and inhibited sperm capacitation/acrosome reaction. Although both of these antibodies reacted with FA-1 antigen, they were directed against different epitopes of the molecule. AFA-1 was directed against an antigenic determinant FA-1a (human FA-1200-219 aa/mouse FA-1117-136 aa) and FAB-7 was directed against the determinant FA-1b (human FA-187-97 aa/mouse FA12-19 aa) of the FA-1 antigen. The third, YLP20 scFv antibody of IgG1 subclass, reacted with a sperm protein of 48 ± 5 kD, which contains the dodecamer sequence, YLPVGGLRIGG. This sequence is present on acrosomal, mid-piece and tail regions of human sperm and is involved in human sperm function and fertilization. The fourth antibody, AS16, reacted with a 18 kD sperm protein (major band) and was found to be a human homolog of the mouse monoclonal recombinant anti-sperm antibody (RASA) (Norton et al., 2001).
Table 1.
Effect of human scFv antibodies on human sperm acrosome reaction
| scFv antibody | Sperm antigen recognized |
Subcellular site reactivity |
Acrosome-reacted sperm |
|---|---|---|---|
| AFA-1 | FA-1(~50 kD) | Post-acrosome, midpiece, and tail |
36 ± 7a |
| FAB-7 | FA-1(~50 kD) | Post-acrosome, midpiece, and tail |
44 ± 6a |
| YLP20 | YLP12 (~48 kD) | Acrosome, midpiece, and tail |
42 ± 3a |
| AS16 | SAGA (18 kD, major band) |
-- | Sperm agglutinated |
| CAB-3 control | None | None | 74 ± 5 |
Various human scFv antibody clones (AFA-1, FAB-7, YLP20, AS16) were examined for sperm reactivity in western blot procedure, immunoprecipitation procedure, indirect immunofluorescence technique (IFT), and for their effect on sperm capacitation and acrosome reaction. AFA-1 and FAB-7 reacted with FA-1 antigen (~50 kD), and YLP20 reacted with YLP12 antigen (~48 kD) in the western blot and immunoprecipitation procedures involving HSE. AS16 scFv antibody recognized several antigens corresponding to sperm agglutinating antigen-1 (SAGA-1) against which murine monoclonal RASA is directed (Norton et al., 2001). AS16 scFv antibody was obtained by activating PBL with HSE. *AFA-1, FAB-7, and YLP20 significantly (p<0.01 to p<0.001) affected capacitation/acrosome reaction when incubated with sperm, as seen by the percent of acrosome-reacted sperm. AS16 agglutinated sperm so it was not examined in the acrosome reaction assay. In these assays, CAB-3 scFv antibody, which does not react with any human sperm antigen, was used as a control (Samuel and Naz, 2008).
All of these antibodies inhibited human sperm capacitation/acrosome reaction in a concentration-dependent manner. This is the first study to report the use of phage display technology to obtain human antisperm scFv antibodies of defined antigen specificities from immunoinfertile/vasectomized men. These antibodies will find clinical applications in the development of novel immunocontraceptives, and specific diagnostics for immunoinfertility in humans. The contraceptive effect of these antibodies in vivo is being investigated.
3. Conclusions
The development of contraceptive vaccines targeting sperm is an exciting proposition, and may provide a valuable alternative to the currently available methods. Like other vaccines, progress in contraceptive vaccine development has been delayed due to the issue of variability of immune response after vaccination. The multi-epitope vaccines may enhance the efficacy and eliminate the concern of the inter-individual variability of the immune response. Also, this concern may be addressed by the passive immunization approach using preformed genetically engineered human antibodies. Several antibodies against various antigens (bacterial/viral/mycoplasmic/fungal/cytokines/oncogenes) are being tried as therapeutic agents. At the present time, >100 antibodies are in clinical trials and ~20 FDA-approved monoclonal antibodies are available in the market for various clinical conditions, including cancer and infectious diseases. Over 80% of these antibodies are genetically engineered (Hollinger and Hudson, 2005). The scFv antibodies that we have synthesized in vitro using cDNAs from ASA-positive immunoinfertile and vasectomized men may provide a useful, once-a-month immunocontraceptive. These human antibodies are sperm-specific and inhibit sperm function in vitro. Their immunocontraceptive potential in vivo is being investigated. Various methods such as PEGylation (Chapman, 2002), fusion with human serum albumin (Smith et al., 2001), and multimerization (Hudson and Kortt, 1999) are being examined to increase the efficacy and half-life of these genetically engineered recombinant antibodies.
Acknowledgements
This work was supported by NIH grant (HD24425). We sincerely thank Sarah Davis and Sarah Wesson for excellent typing assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baskin MJ. Temporary sterilization by injection of human spermatozoa: a preliminary report. Am. J. Obstet. Gynecol. 1932;24:892–897. [Google Scholar]
- Casadevall A. Passive antibody therapies: progress and continuing challenges. Clin. Immunol. 1999;93:5–15. doi: 10.1006/clim.1999.4768. [DOI] [PubMed] [Google Scholar]
- Chapman AP. PEGylated antibodies and antibody fragments for improved therapy: a review. Adv. Drug Deliv. Rev. 2002;54:531–545. doi: 10.1016/s0169-409x(02)00026-1. [DOI] [PubMed] [Google Scholar]
- Cho C, Bunch DO, Faure JE, Goulding EH, Eddy EM, Primakoff P, Myles DG. Fertilization defects in sperm from mice lacking fertilin beta. Science. 1998;281:1857–1859. doi: 10.1126/science.281.5384.1857. [DOI] [PubMed] [Google Scholar]
- Contraception Online, 2004. Baylor College of Medicine, Houston Texas [online] available from URL: http://contraceptiononline.org/
- Dunman PM, Nessin M. Passive immunization as prophylaxis: When and where will this work? Curr. Opin. Pharmacol. 2003;20:351–360. doi: 10.1016/j.coph.2003.05.005. 2003. [DOI] [PubMed] [Google Scholar]
- Grow DR, Ahmed S. New Contraceptive Methods. Obstet. Gynecol. Clin. North Am. 2000;27:909–916. doi: 10.1016/s0889-8545(05)70176-5. [DOI] [PubMed] [Google Scholar]
- Hardy CM, Mobbs JK. Expression of recombinant mouse sperm protein sp56 and assessment of its potential for use as an antigen in an immunocontraceptive vaccine. Mol. Reprod. Dev. 1999;52:527–536. doi: 10.1002/(SICI)1098-2795(199902)52:2<216::AID-MRD13>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Henshaw SK. Unintended pregnancy in the United States. Fam. Plann. Perspect. 1998;30:24–29. [PubMed] [Google Scholar]
- Holliger P, Hudson PJ. Engineered antibody fragments and the rise of single domains. Nat. Biotech. 2005;23:1126–1136. doi: 10.1038/nbt1142. [DOI] [PubMed] [Google Scholar]
- Hudson PJ, Kortt AA. High avidity scFv multimers; diabodies and triabodies. J. Immunol. Methods. 1999;231:177–189. doi: 10.1016/s0022-1759(99)00157-x. [DOI] [PubMed] [Google Scholar]
- Inoue N, Ikawa M, Isotani A, Okabe M. The immunoglobin superfamily protein izumo is required for sperm to fuse with eggs. Nature. 2005;434:234–238. doi: 10.1038/nature03362. [DOI] [PubMed] [Google Scholar]
- Inoue N, Ikawa M, Nakanishi T, Matsumoto M, Nomura M, Seya T, Okabe M. Disruption of mouse CD46 causes an accelerated spontaneous acrosome reaction in sperm. Mol. Cell. Biol. 2003;23:2614–2622. doi: 10.1128/MCB.23.7.2614-2622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren E, Zuckerman LA, Mire-Sluis AR. Immune response to therapeutic proteins in humans-clinical significance, assessment and prediction. Curr. Pharm. Biotechnol. 2002;3:349–360. doi: 10.2174/1389201023378175. [DOI] [PubMed] [Google Scholar]
- Lea IA, van Lierop MJC, Widgren EE, Grootenhuis A, Wen Y, van Duin M, O'Rand MG. A chimeric sperm peptide induced antibodies and strain-specific reversible infertility in mice. Biol. Reprod. 1998;59:527–536. doi: 10.1095/biolreprod59.3.527. [DOI] [PubMed] [Google Scholar]
- Le Naour F, Rubinstein E, Jasmin C, Prenant M, Boucheix C. Severely reduced female fertility in CD9-deficient mice. Science. 2000;287:319–321. doi: 10.1126/science.287.5451.319. [DOI] [PubMed] [Google Scholar]
- Mancini RE, Andrana JA, Saracine D, Bachmann AE, Lavieri JC, Nemirovsky M. Immunological and testicular response in men sensitized with human testicular homogenate. J. Clin. Endocrinol. Metab. 1965;25:859–875. doi: 10.1210/jcem-25-7-859. [DOI] [PubMed] [Google Scholar]
- Mirick GR, Bradt BM, Denardo SJ, Denardo GL. A review of human anti-globulin antibody (HAGA, HAMA, HACA, HAHA) responses to monoclonal antibodies. Not four letter words. Q. J. Nucl. Med. Mol. Imaging. 2004;48:251–257. [PubMed] [Google Scholar]
- Naz RK. Vaccine for contraception targeting sperm. Immunol. Rev. 1999;171:193–202. doi: 10.1111/j.1600-065x.1999.tb01349.x. [DOI] [PubMed] [Google Scholar]
- Naz RK. Effect of sperm DNA vaccine on fertility of female mice. Mol. Reprod. Dev. 2006a;73:918–928. doi: 10.1002/mrd.20487. [DOI] [PubMed] [Google Scholar]
- Naz RK. Effect of fertilization antigen (FA-1) DNA vaccine on fertility of female mice. Mol. Reprod. Dev. 2006b;73:473–1479. doi: 10.1002/mrd.20591. [DOI] [PubMed] [Google Scholar]
- Naz RK, Engle A, Rajnee Gene knockouts that affect male fertility: novel targets for contraception. Front. Biosci. 2009;14:3994–4007. doi: 10.2741/3507. [DOI] [PubMed] [Google Scholar]
- Naz RK. Immunocontraceptive effect of Izumo and enhancement by combination vaccination. Mol. Reprod. Dev. 2008;75:336–344. doi: 10.1002/mrd.20783. [DOI] [PubMed] [Google Scholar]
- Naz RK, Chauhan S. Human sperm-specific peptide vaccine that causes long-term reversible contraception. Biol. Reprod. 2002;64:674–680. doi: 10.1095/biolreprod67.2.674. [DOI] [PubMed] [Google Scholar]
- Naz RK, Gupta SK, Gupta JC, Vyas HK, Talwar GP. Recent advances in contraceptive vaccine development: a mini-review. Hum. Reprod. 2005;20:3271–3283. doi: 10.1093/humrep/dei256. [DOI] [PubMed] [Google Scholar]
- Naz RK, Rajesh C. Novel testis/sperm specific contraceptive targets identified using gene knockout studies. Front. Biosci. 2005a;10:2430–2446. doi: 10.2741/1708. [DOI] [PubMed] [Google Scholar]
- Naz RK, Rajesh P. Gene knockouts that cause female infertility: search for novel contraceptive targets. Front. Biosci. 2005b;10:2447–2459. doi: 10.2741/1709. [DOI] [PubMed] [Google Scholar]
- Naz RK, Zhu X. Recombinant fertilization antigen-1 causes a contraceptive effect in actively immunized mice. Biol. Reprod. 1998;59:1095–1100. doi: 10.1095/biolreprod59.5.1095. [DOI] [PubMed] [Google Scholar]
- Norton EJ, Diekman AB, Westbrook VA, Flickinger CJ, Herr JC. RASA, a recombinant single-chain variable fragment (scFv) antibody directed against the human sperm surface: implications for novel contraceptives. Hum. Reprod. 2001;16:1854–1860. doi: 10.1093/humrep/16.9.1854. [DOI] [PubMed] [Google Scholar]
- O'Hearn PA, Liang ZG, Bambra CS, Goldberg E. Colinear synthesis of an antigen-specific B-cell epitope with a promiscuous tetanus toxin T-cell epitope: a synthetic peptide immunocontraceptive. Vaccine. 1997;15:1761–1766. doi: 10.1016/s0264-410x(97)00105-9. [DOI] [PubMed] [Google Scholar]
- Ohl D, Naz RK. Infertility due to antisperm antibodies. J. Urol. 1995;46:591–602. doi: 10.1016/S0090-4295(99)80282-9. [DOI] [PubMed] [Google Scholar]
- O'Rand MG, Beavers J, Widgren E, Tung K. Inhibition of fertility in female mice by immunization with a B-cell epitope, the synthetic sperm peptide, P10G. Reprod. Immunol. 1993;25:89–102. doi: 10.1016/0165-0378(93)90051-i. [DOI] [PubMed] [Google Scholar]
- O'Rand MG, Widgren EE, Sivashanmugam P, Richardson RT, Hall SH, French FS, Vande Voort CA, Ramachandra SG, Ramesh V, Jagannadha Roa A. Reversible immunocontraception in male monkeys immunized with eppin. Science. 2004;306:1189. doi: 10.1126/science.1099743. [DOI] [PubMed] [Google Scholar]
- Samuel AS, Naz RK. Isolation of human single chain variable fragment antibodies against specific sperm antigens for immunocontraceptive development. Hum. Reprod. 2008;23:1324–1337. doi: 10.1093/humrep/den088. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu SS, Fellouse FA. Synthetic therapeutic antibodies. Nat. Chem. Biol. 2006;2:682–688. doi: 10.1038/nchembio843. [DOI] [PubMed] [Google Scholar]
- Smith BJ, Popplewell A, Athwal D, Chapman AP, Heywood S, West SM, Carrington B, Nesbit A, Lawson AD, Antoniw P, et al. Prolonged in vivo residence times of antibody fragments associated with albumin. Bioconjug. Chem. 2001;12:750–756. doi: 10.1021/bc010003g. [DOI] [PubMed] [Google Scholar]
- Talwar GP, Singh O, Pal R, Chatterjee N, Sahai P, Dhall K, Kaur K, Das SK, Suri S, Bukshee K, Saraya L, Saxena BN. A vaccine that prevents pregnancy in women. Proc. Natl. Acad. Sci. 1994;91:8532–8536. doi: 10.1073/pnas.91.18.8532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollner TL, Overstreet JW, Branciforte D, Primakoff PD. Immunization of female cynomolgus macaques with a synthetic epitope of sperm-specific lactate dehydrogenase results in high antibody titers but does not reduce fertility. Mol. Reprod. Dev. 2002;62:257–264. doi: 10.1002/mrd.10063. [DOI] [PubMed] [Google Scholar]
- World POPClock Projection (Accessed on February 8, 2009) US Census Bureau [online] available from URL:http://www.census.gov/main/www/popclock.html/
- Yokota T, Milenic DE, Whitlow M, Schlom J. Rapid tumor penetration of a single-chain Fv and comparison with other immunoglobulin forms. Cancer Res. 1992;52:3402–3408. [PubMed] [Google Scholar]
- Zhou Y, Drummond DC, Zou H, Hayes ME, Adams GP, Kirpotin DB, Marks JD. Impact of single-chain Fv antibody fragment affinity on nanoparticle targeting of epidermal growth factor receptor expressing tumor cells. J. Mol. Biol. 2007;371:934–947. doi: 10.1016/j.jmb.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]