Abstract
Plant cells are ideal bioreactors for the production and oral delivery of vaccines and biopharmaceuticals, eliminating the need for expensive fermentation, purification, cold storage, transportation and sterile delivery. Plant-made vaccines have been developed for two decades but none has advanced beyond Phase I. However, two plant-made biopharmaceuticals are now advancing through Phase II and Phase III human clinical trials. In this review, we evaluate the advantages and disadvantages of different plant expression systems (stable nuclear and chloroplast or transient viral) and their current limitations or challenges. We provide suggestions for advancing this valuable concept for clinical applications and conclude that greater research emphasis is needed on large-scale production, purification, functional characterization, oral delivery and preclinical evaluation.
Need for a new platform for production of therapeutic proteins
Approximately 15 million (>25%) of 57 million deaths per year worldwide are estimated to be related directly to infectious diseases [1]. Vaccination is considered to be the most efficient and cost-effective means for health intervention to combat infectious diseases. However, the high cost of vaccination makes it unaffordable for most people living in developing countries, as the daily average income for nearly one billion people is <US$1. A 14-fold increase in the cost of vaccines over the past decade [2] makes it necessary to investigate alternate strategies for their production and delivery. Also, with an emphasis on safety, there has been an increasing shift towards the development of subunit vaccines (that use one or two proteins instead of avirulent or killed pathogen strains), with reliance on recombinant expression systems. The high cost of current vaccines and biopharmaceuticals is largely as a result of their complex production and delivery methods, including the significant costs of fermentation and purification systems and additional expenses associated with adjuvant, cold storage, transportation and sterile delivery. No viable alternative to fermentation technology has yet emerged for their mass production, a void that could be addressed through the use of plants as bioreactors for the production and oral delivery of therapeutic proteins. In this review, we evaluate the advantages and disadvantages of different plant expression systems (stable nuclear, transient viral and stable chloroplast) and their current limitations or challenges ahead. We further discuss directions for advancing this valuable concept for clinical applications.
Plant-made therapeutic proteins: current status and challenges ahead
Plant-made vaccines and therapeutics refer to protein products with clinical or veterinary applications produced in recombinant plant systems. These systems can be broadly divided into those using nuclear transgenic technology, chloroplast transplastomic technology and plant viral technology (Figure 1 ). In all cases, the antigens or therapeutics are expressed in plant tissues, from which they can be either purified or the plant tissue can be processed into a form that can be applied topically or, more uniquely, orally. The major advantage of plant expression systems over other vaccine production systems is reduced manufacturing cost. Fermenters and bioreactors can be replaced with contained plant growth rooms or greenhouses or plants can be grown in the field with appropriate biological containment of foreign genes, such as maternal inheritance or male sterility, or expression in vegetative tissues with harvest before appearance of any reproductive structure. This should lead to reduced upstream facility costs. In the case of oral plant-produced vaccines and therapeutics, downstream processing costs are also reduced. Instead of protein purification targeting up to ∼99% purity, plant tissues can be inexpensively processed for oral delivery. The relevant technology has already been developed in the food and feed industries and can be adapted for plant-produced therapeutic proteins.
Figure 1.
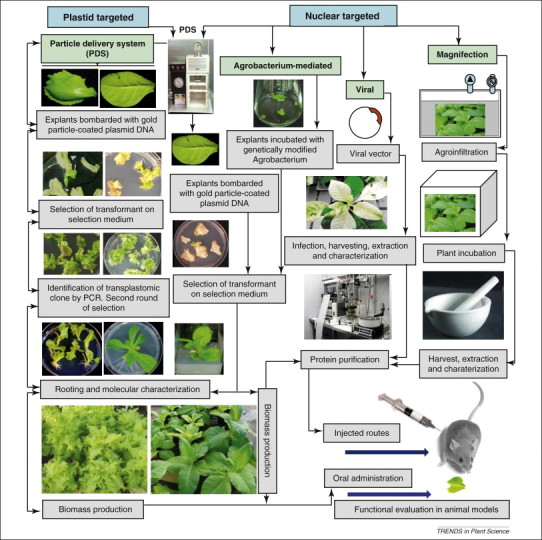
Schematic representation of vaccine antigens and biopharmaceuticals production in plants and their functional evaluation in animal models. Explants are tissues that have the potential for regeneration into mature plants. Here, we provide lettuce and tobacco as examples.
As with other eukaryotic systems, plant-produced vaccines enable the introduction of post-translational modifications. Proper folding and disulfide bond formation occur in chloroplasts [3] or the endoplasmic reticulum (ER) [4]. Proper lipid modifications of vaccine antigens have also been observed in chloroplasts [5]. Therapeutic proteins are not glycosylated in chloroplasts but are glycosylated when targeted to the ER, although glycosylation patterns are not identical to mammalian systems. Although numerous therapeutic proteins have been expressed in plant cells (Table 1, Table 2, Table 3 , Appendix BTables S1-S3 in the supplementary material online), several challenges remain. Transgene silencing in nuclear transgenic plants requires further investigation and improvement. More tools are needed for transgene containment to facilitate field production of vaccines or biopharmaceuticals, when expressed via the nuclear genome. More generally, expression levels are target dependent and are a challenge for all recombinant systems.
Table 1.
Recent vaccine antigens expressed via the plant nuclear genome that report immunogenicity or protection (for full length table see Appendix BTable S1 in the supplementary material online)
| Antigen human vaccines (Stable expression) | Expression system | Expression level | Functional evaluation | Refs |
|---|---|---|---|---|
| Bacterial antigens | ||||
| Enterotoxigenic E. coli Heat-labile toxin B subunit (LTB) | Carrots | 0.3% TSP | Immunogenic and protective against CT challenge | [15] |
| Soybean | 2.4% TSP | Immunogenic and partial protection against LT challenge in mice | [4] | |
| Cholera toxin B subunit (CTB) | Tomato | 0.081% TSP | Immunogenic by oral delivery to mice | [79] |
| Rice | 2.1% TSP | Immunogenic and protective against CT challenge to mice following oral administration | [16] | |
| Viral antigens | ||||
| Hepatitis B virus surface antigen (HBsAg) | Potato | 8.5 μg g−1 FW | Immunogenic response in humans following oral administration | [19] |
| Hepatitis B virus surface antigen fused with preS1 epitope | Rice | 31.5 ng g−1 DW | Immunogenic by intraperitoneal delivery to mice | [80] |
| Human group A rotavirus (VP6) protein | Alfalfa | 0.06–0.28% TSP | Immunogenic in mice and offspring developed less severe diarrhea after challenge with simian rotavirus SA-11, indicating that antibodies generated in the dams provided passive heterotypic protection to the pups | [81] |
| Rotavirus (VP7) | Potato tubers | 0.3–0.4% TSP | Immunogenic in mice following oral delivery. Neutralization activity against rotavirus | [82] |
| Norwalk virus capsid protein (NVCP) | Tomato fruit and potato | 8% and 0.4% TSP | Elicit systemic and mucosal antibody responses in mice following oral administration | [21] |
| SARS–CoV S protein (S1) | Tomato and tobacco leaf | 0.1% TSP | Immunogenic to mice following oral administration | [83] |
| Smallpox recombinant vaccine virus B5 antigenic domain (pB5) | Tobacco and collard leaf | Not reported | Antibody response in mice immunized parenterally and protects against lethal dose of vaccinia virus | [84] |
| Japanese encephalitis virus (JEV) envelope protein (E) | Rice | 1.1–1.9 μg mg–1 TSP | JEV specific neutralizing antibody detected in mice following intraperitoneal or oral administration | [85] |
| Protozoan antigens | ||||
| Plasmodium yoelii merozoite surface protein (PyMSP4/5) | Tobacco | 0.02–0.04% TSP | Induces antigen-specific antibodies in mice following parenteral delivery | [86] |
| Human vaccines (viral/transient expression) Bacterial antigens | ||||
| Yersinia pestis F1 and LcrV antigens | Tobacco leaf tissue | 380 and 120 μg g−1 FW | Immunogenic and protective in monkeys against Y. pestis following subcutaneous injection | [57] |
| Yersinia pestis F1–V antigens | Tobacco leaf | 1 and 2 mg g−1 | Immunogenic and protection in vaccinated guinea pigs against Y. pestis aerosol challenge | [26] |
| Viral antigens | ||||
| Smallpox recombinant vaccine virus B5 antigenic domain (pB5) | Tobacco leaf | Not reported | Antibody response in mice immunized parenterally and protective against lethal dose of vaccinia virus | [84] |
| Encoding domain III of the dengue 2 envelope protein (D2EIII) | Tobacco | 0.28% TSP | Retains antigenicity and immunogenicity as well as inducing neutralizing antibodies in vaccinated animals | [87] |
| HIV entry inhibitors red algal protein griffithsin (GRFT) | Tobacco (TMV) | 1 g kg−1 FW | Active against HIV at picomolar concentrations, directly virucidal via binding to HIV envelope glycoproteins and capable of blocking cell-to-cell HIV transmission | [75] |
| Pathogenic avian influenza virus (H5N1 subtype) | Tobacco | 60 mg kg−1 FW | Immunogenic in mice and ferret and also protects ferrets against challenge infection with virus | [28] |
Table 2.
Biopharmaceutical proteins expressed via the plant nuclear genome (for full length table see Appendix BTable S2 in the supplementary material online)
| Pharmaceutical protein | Expression system | Expression level | Functional evaluation | Refs |
|---|---|---|---|---|
| Human growth hormone (hGH) | Rice suspension cells | 57 mg l−1 | The biological activity of shGH accumulated in the transgenic rice cell suspension culture was similar to that of the E. coli-derived recombinant hGH, as shown by proliferation of Nb2 node lymphoma cells | [88] |
| Mouse interleukin-12 (IL-12) | Tomato leaf and fruit | 2.7–7.3 and 1–3.4 μg g−1 FW | Biologically active in vitro. The plant-produced mIL-12 induced the secretion of IFN-γ by T cells | [89] |
| Human epidermal growth factor (hEGF) | Tobacco | 0.09–0.3% TSP | Plant-produced hEGF significantly stimulated Vero E6 cell expansion and proliferation similar to commercial hEGF products | [90] |
| Human basic fibroblast growth factor (bFGF) | Soybean | 2.3% TSP | Mitogenic assay demonstrated that bFGF stimulated Balb/c 3T3 cells to proliferate in a dose-dependent manner, indicating similar biological activity as native bFGF | [91] |
| Human acid β-glucosidase (GCase) | Tobacco seeds | Not reported | Plant-derived GCase was taken up by fibroblasts of a Gaucher type II patient and lacks potentially immunogenic glycans | [33] |
| Human glucocerebrosidase enzyme (GCD) | Carrot cells | Not reported | Recombinant GCD in transgenic plant cells is biologically active. Phase I clinical trial have shown that no clinical or laboratory evidence of any significant innate or humoral immune reactions. A Phase III clinical trial is currently ongoing | [8] |
| Human cytokine granulocyte macrophage colony stimulating factor (GM-CSF) | Sugarcane | Undetectable–0.02% TSP | Human bone marrow cells (TF-1), which require GM-CSF for cell division, proliferated when growth media was supplemented with transgenic sugarcane extracts and had identical activity levels | [92] |
| Human granulocyte-macrophage colony stimulating factor (hGM-CSF) | Rice | 1.2–1.3% TSP | Rice seed-derived hGM-CSF induces proliferation of TF-1 cells similar to E. coli-derived hGM-CSF | [93] |
| Macrophage colony-stimulating factor (M-CSF) 1 | Tobacco | 0.02–1.92% TSP | Plant-derived M-CSFsR inhibits colony formation of J6-1 cells | [94] |
| Human granulocyte-colony stimulating factor (hG-CSF) | Rice suspension cells | 0.7% TSP | Plant-derived hG-CSF supports proliferation of the AML-193 cells similar to commercial E. coli-derived hG-CSF | [95] |
| Bone morphogenetic protein 2 (BMP2) | Tobacco | 0.02% TSP | Application of hBMP2 to mouse C2C12 cell line significantly increased cell ALP activity but is lower than commercial rhBMP2 | [96] |
| Human a-1-antitrypsin (AAT) | Tomato | 0.44–1.55% TSP | Biologically active, showing high specific activity and efficient inhibition of elastase activity | [97] |
| Human IA-2 (IA-2ic), a diabetes-associated autoantigen | Tobacco (Transient) | 0.5% TSP | Plant-derived IA-2ic protein is specifically recognized by human IA-2ic autoantibodies | [35] |
| Human fibroblast growth factor 8 isoform b (FGF8b) | Tobacco (transient) | 90–150 μg g−1 FW | Plant-expressed FGF8b effectively increased the rate of cell proliferation of NIH3T3 as bacterially expressed mouse FGF8b | [36] |
Table 3.
Functional vaccine antigens and biopharmaceutical proteins expressed via the chloroplast genome (for full length table see Appendix BTable S3 in the supplementary material online)
| Vaccine antigens | Expression system | Expression level | Functional evaluation | Refs |
|---|---|---|---|---|
| Bacterial antigens | ||||
| Cholera toxin B (CtxB) | Tobacco Lettuce | 4.1%, 8% and 12.3% TSP4.8% and 9.4% TSP | GM1 ganglioside-binding assay. Long-term protection (50% mouse lifespan) against CT challenge in both oral (100%) and subcutaneously (89%) immunized mice; protection correlated with CTB-specific IgA and IgG1 titers in oral and IgG1 in subcutaneously immunized mice; increasing numbers of IL-10+T cell but not Foxp3+ regulatory T cells, suppression of IFN-γ and absence of IL-17 were observed in protected mice | 3, 66 |
| Tetanus toxin (TetC) | Tobacco | 18–27% and 7–10% TSP | Mice developed systemic immune response and survived the tetanus toxin challenge | [54] |
| Anthrax protective antigen (Pag) | Tobacco | 4.5–14.2% TSP | Macrophage lysis assay, systemic immune response, toxin neutralization assay, mice survived (100%) challenge with lethal doses of toxin | [55] |
| Lyme disease –OspA (OspA, OspA-T) | Tobacco | 1% and 10% TSP | Systemic immune response in mice. Protected mice against Borrelia burgdorferi | [5] |
| Plague F1–V (CaF1-LcrV) | Tobacco | 14.8% TSP | Immunogenic in mice (IgG1 titers). Oral delivery offered greater protection (88%) and immunity than subcutaneous (33%) injection when challenged with 50-fold lethal dose of aerosolized Y. pestis | [58] |
| E. coli enterotoxin B (LTB) | Tobacco | 2.3% TSP | GM1 ganglioside-binding assay; oral immunization partially protected mice from CT challenge | [53] |
| Viral antigens | ||||
| Canine parvovirus (CTB–2L21, GFP–2L21) | Tobacco | 31.1% and 22.6% TSP | Rabbit sera neutralized CPV in an in vitro assay | [59] |
| Hepatitis E virus (HEV E2) | Tobacco leaves Seeds | 0.63–1.09 ng and 0.015–0.018 ng μg–1 TSP | Immune response in mice | [62] |
| Swine fever virus (CSFV E2) | Tobacco | 1–2% TSP | Immune response in mice | [60] |
| Human Papillomavirus (L1) | Tobacco | 20–26% TSP | Systemic immune response in mice after intraperitoneal injection and neutralizing antibodies were detected | [63] |
| Protozoan antigens | ||||
| Amoebiasis (LecA) | Tobacco | 7% TSP | Systemic immune response in mice | [65] |
| Malaria (CTB–ama1 and CTB–msp1) | Tobacco Lettuce | 12.3% TSP9.4% TSP | Sera of immunized mice completely inhibited proliferation of the malaria parasite and crossreacted with the native parasite proteins/parasites in immunoblots and immunofluorescence studies, at the ring, trophozoite or schizont parasite stages | 3, 66 |
| Tobacco Lettuce | 8% TSP4.8% TSP | |||
| Autoantigens | ||||
| Diabetes – type 1 (CTB–pins) | Tobacco and lettuce | ∼16% TSP2.05–2.5% TSP | CTB–Pins treated mice showed significant decrease in inflammation (insulitis) in non-obese diabetic mice; insulin-producing β-cells in the pancreatic islets of CTB–Pins-treated mice were highly protected, increase in insulin production with lower blood or urine glucose levels; increased expression of immunosuppressive cytokines (IL-4, IL-10) | [49] |
| Diabetes – type 1 (hGAD65) | Chlamydomonas | 0.25–0.3% TSP | Immunoreactivity to diabetic sera | [67] |
| Biopharmaceutical proteins | ||||
| Interferon α2b (IFN-α2b) | Tobacco LAMD Petit Havana | 8.0–21.0% TSP2.0–14.0% TSP | Immunogenic in mice. Transgenic IFN-α2b protected baby hamster kidney cells against cytopathic viral replication in vesicular stomatitis virus cytopathic effect assay, HeLa cells from HIV-1 entry and mice from a highly metastatic tumor line. Also, it increased the expression of major histocompatibility complex class I on splenocytes and the total number of natural killer cells | [41] |
| Insulin-like growth factor (IGF-1n, IGF-1s) | Tobacco | 32.4% TSP32.7% TSP | Growth response in cultured HU-3 cells | [72] |
| Human alpha1-antitrypsin (A1AT) | Tobacco | 2% TSP | Fully active and binds to porcine pancreatic elastase | [71] |
| Antimicrobial peptide (2 lysine-type protein) | Tobacco | ∼30% TSP | Bacteriolytic activity and kills Streptococcus pneumoniae, the causative agent of pneumonia | [43] |
Many functional assays (such as host cell protein) have not yet been developed for injectable products. Despite active research, there is limited published work on highly purified proteins and characterization of contaminants. It is well known that expression level varies depending on the developmental stage of leaves, the time of day and the regulatory sequences used, underscoring the importance of the developmental stage of the plants and the time of harvest. Most importantly, achieving consistency of transgene expression in different batches is an important challenge. Therefore, harvested leaves should be homogenized and level of therapeutic protein must be determined for each harvested batch. There are limited studies on determination of stability of therapeutic proteins after harvest, processing and storage. Removal of Agrobacteria after vacuum infiltration of viral vectors is yet another challenge. All the above concerns need to be addressed with careful and reproducible investigations.
The current focus for most groups is on purified antigens prepared from plants grown in contained conditions. This avoids environmental release and enables controlled plant growth but decreases the cost advantage versus field-grown plants because construction of greenhouses or growth rooms to meet regulatory approval is relatively expensive. In the case of oral delivery, moving forward on the most promising veterinary candidates should lay the foundation for human vaccines. Determining a regulatory pathway for oral products is a major hurdle, given the limited resources of the prominent research groups in the field. Purified vaccines and therapeutic proteins might follow a similar regulatory path to recombinant proteins produced in other systems, with the focus on stability, potency and efficacy of the purified product. As with other purified protein subunit vaccines, adjuvants are needed and must be developed alongside target antigens.
Over the past two decades, vaccine antigens expressed via the plant nuclear genome have elicited appropriate immunoglobulin responses and have conferred protection upon oral delivery 6, 7 but no transgenic plant-based vaccine has yet moved beyond a Phase I clinical trial [7]. However, at least two therapeutics developed by Biolex (http://www.biolex.com) and Protalix (http://www.protalix.com) are moving through Phase II and Phase III 8, 9, 10.
Stably integrated nuclear transgenes
The earliest research using plants for the recombinant expression of vaccine antigens was performed using stable transformation of the nuclear genome of tobacco (Nicotiana tobacum) [11]. The ease of nuclear transgene methodology, started by Agrobacterium-mediated delivery of DNA, facilitated this approach, which was the dominant strategy for several years. Advantages include the ability to scale up production of large amounts of vaccine antigens from transgenic seed stocks and the possibility of expression in fruits or other edible plant organs, enabling oral delivery of minimally processed materials. However, stably integrated nuclear transgenes typically yield relatively low levels of expression (<1% total soluble protein, TSP; Appendix BTable S1 in the supplementary material online), which can vary plant to plant and generation to generation, probably owing to gene silencing or position effect [12].
Many different vaccine antigens have been produced in various plant species, including tobacco and edible plants (e.g. Solanum tuberosum, Solanum lycopersicum, Zea mays, Daucus carota and Glycine max; Table 1, Appendix BTable S1 in the supplementary material online). Oral delivery of several plant-derived antigens has elicited antigen-specific antibodies in mice and, in a few clinical trials, in humans 13, 14. Vaccine antigens derived from enteric pathogens, such as Norwalk virus capsid protein (NVCP), rotavirus capsid protein and the bacterial ganglioside-binding protein cholera toxin B subunit (CTB) and Escherichia coli heat-labile toxin B subunit (LTB), have been extensively evaluated in nuclear transgenic plants (Appendix BTable S1 in the supplementary material online). For example, mice orally immunized with carrot-derived LTB [15] or rice-derived CTB [16] induce protection against cholera toxin (CT) challenge. Several other antigens from non-enteric pathogens such as F protein from respiratory syncytial virus [17] and surface antigen from hepatitis B 18, 19 also stimulate antigen-specific antibodies after oral delivery. However, adjuvants might be required for such stimulation. For example, in the case of hepatitis B surface antigen (HBsAg) fed to mice in transgenic potato tubers [20], it was necessary to use CT, a potent mucosal adjuvant, to stimulate significant levels of anti-HBsAg antibodies, although the same HBsAg potatoes fed to humans without adjuvant provoked serum anti-HBsAg in most of the volunteers.
Given that potatoes and most other plant organs (except seeds) have a limited shelf life in the fresh state, and vaccine dosage must be carefully monitored, it is unlikely that fresh, unprocessed plant materials will be used as vaccines. Thus, some form of processing, such as freeze-drying fruits or other plant organs, must be used to produce a product batch that can be validated for potency and stability. For NVCP, it was demonstrated that either freeze- or air-drying of transgenic tomato fruits yielded material that was at least as orally immunogenic as fresh transgenic tomato [21]. However, freeze-dried potato NVCP produced poor results compared with fresh potato NVCP [21], with oxidation of phenolic compounds in dried potato probably causing the problem. Thus, control of oxidation will be an important issue when tissues rich in phenolics and the enzyme polyphenol oxidase are used. The use of seed tissues is more promising, and such material has demonstrated extended stability for both human and animal vaccine candidates. Seed-based vaccines are particularly attractive for the development of veterinary products because of convenience in storage and delivery and have shown promising results in farmed animal studies [22].
Transient expression with viral vectors
Development of a plant viral vector system for transient expression has enabled rapid expression of recombinant proteins at levels higher than with stably integrated nuclear transgenes (Table 1, Appendix BTable S1 in the supplementary material online). Tobacco mosaic virus, although an RNA virus that replicates in the cytosol, can be delivered as a DNA construct that is transcribed in the nucleus to produce viral RNA. In one iteration, “magnIcon” technology has been used to express hepatitis B core antigen [23], HBsAg [24], NVCP [25] and plague antigens F1 and V [26] at levels of up to 1–2 mg g–1 fresh leaf mass in Nicotiana benthamiana, and in a similar “Launch Vector” technology a range of candidates, including influenza targets, have been expressed at high levels in N. benthamiana 27, 28. Given that tobacco or its close relatives, such as N. benthamiana, are the usual hosts for this system, and vectors are generally introduced by vacuum infiltration of Agrobacteria, the products must be purified for delivery as vaccines. This ensures removal of any noxious host plant compounds and removal of Agrobacteria. However, because purification is necessary, soluble antigens might lose oral immunogenicity owing to the removal of the encapsulation effect that might help protect them from the harsh stomach environment. Nonetheless, NVCP that was partially purified from leaves did induce serum IgG and mucosal IgA responses after oral delivery to mice [25]. One approach that could overcome this limitation is the expression of vaccine antigens as virus-like particles. This has been followed for a non-viral based transient expression system, with promising immunogenicity results following injection of purified influenza antigen [29]. This might be extendable to oral delivery.
A further DNA replicon system was developed using the geminivirus bean yellow dwarf virus (BeYDV) [30]. The construct is delivered by agro-infiltration, similar to the magnIcon and launch vector systems. Similar to the launch vector system [27], it can be used as a single vector, whereas the magnIcon system requires three separate vectors 25, 26. Expression of HBcAg and NVCP in the range of 0.5–0.8 mg g−1 leaf mass was observed with the BeYDV replicon, which is similar to that obtained with the magnIcon system. The magnIcon system has also been adapted for IgG expression but requires the combination of a TMV (tobacco mosaic virus) replicon with a potato virus X replicon [31] and five different Agrobacterium vectors. The BeYDV replicon was also stably incorporated into the nuclear genome, with an inducible promoter driving the viral Rep gene to control gene amplification [32]. Tenfold increases in expression of NVCP in cultured tobacco cells were obtained after induction. This system might enable the use of stable transgenic lines with improved expression.
Nuclear-derived pharmaceutical proteins
Recombinant pharmaceutical proteins are useful for treatment of various conditions such as genetic diseases that result in the production of an insufficient quantity or quality of a particular protein. Given that many of these proteins would be administered parenterally, a high degree of purification under current Good Manufacturing Practice conditions is necessary for human use. Nuclear transgenic plant strategies have been used in many studies to demonstrate the production of many valuable human proteins, including epidermal growth factor, serum albumin, interferons, interleukins, lysozyme, lactoferrin and human acid β-glucosidase (Table 2, Appendix BTable S2 in the supplementary material online). However, as with vaccine antigens, expression levels are generally low. Specific assays demonstrated activity of the recombinant protein in vitro or in cell-based assays, but extensive animal studies have not yet been published. Although plant cells can synthesize and process mammalian proteins, in some cases, plant-specific glycosylation of ER- or cell surface-targeted proteins might produce undesired immunogenicity of the recombinant protein, which could diminish its half-life and utility. β-Glucosidase produced in seeds of tobacco lacks the plant-specific xylose and fucose residues that can be problematic [33]. If seed-based expression of other ER- or cell surface-targeted proteins yields similar results, then targeting of recombinant proteins to seed storage vacuoles will be an advantage. ER retention of proteins through the C-terminal inclusion of a KDEL or KDEL-like tag serves to limit glycosylation of recombinant proteins to core glycan structures, by comparison to cell surface-targeted proteins [4]. Also, recent advances in plant glycan engineering hold the promise of broadly applicable production of pharmaceutical glycoproteins in plants [34]. In addition, a few pharmaceutical proteins (human growth hormone, human fibroblast growth factor, human diabetes-associated autoantigen, etc.) have been produced in plants using the transient expression system 35, 36, 37, where subcellular targeting is again an option.
Chloroplast-derived vaccine antigens and therapeutics
In chloroplast technology, foreign genes are integrated into the chloroplast genome by homologous recombination [38], eliminating variation of expression among independent transgenic lines. Moreover, gene silencing has not yet been reported in chloroplast transgenic (transplastomic) lines. High levels of expression of vaccine antigens are facilitated by >10,000 copies of transgenes in each transformed plant cell. Chloroplast expression minimizes the risk of foreign gene transfer via pollen from genetically modified crops to other related crops or weeds owing to maternal inheritance of transgenes 39, 40. In addition, expression of therapeutic proteins in leaves facilitates their harvest before the development of any reproductive structures and offers additional opportunities for containment. Therefore, several chloroplast-derived vaccine antigens and biopharmaceutical proteins have been grown in field conditions and at least one such study has been published [41]. To date, 23 vaccine antigens against 16 different diseases and 11 biopharmaceutical proteins have been expressed in chloroplasts (Table 3, Appendix BTable S3 in the supplementary material online). The presence of chaperones and post-translational modification enzymes in the chloroplast creates a niche for target processing and assembly with suitable post-translational modifications (assembly of multimers, disulfide bonds, lipid modification, etc.), although glycosylation is not an option because N-glycosylation is not known to occur within chloroplasts. The chloroplast expression system has been used to produce several fully functional vaccine antigens against bacterial, viral and protozoan pathogens (Table 3, Appendix BTable S3 in the supplementary material online). Proteins that are difficult to express in other systems, such as antimicrobial proteins, have also been expressed in chloroplasts 42, 43. In addition to plants, Chlamydomonas chloroplasts have been used for expression of therapeutic proteins, although achieving higher levels of expression was a major challenge until recent improvements in this system [44]. Indeed, adequate levels of expression of therapeutic proteins in chloroplasts are not always possible and only a few proteins have been evaluated for functionality (Table 3, Appendix BTable S3 in the supplementary material online).
One major limitation in this field is that several vaccine antigens and therapeutic proteins have only been expressed in tobacco. Tobacco is not edible and the addictiveness of nicotine also makes it unsuitable for oral delivery of therapeutic proteins. As an alternative, the carrot plastid transformation system was developed with adequate levels of gene expression in edible tissues to facilitate oral delivery [45]. However, carrot regeneration is slow and is not suitable for rapid production of therapeutic proteins. Although adequate expression of human immune deficiency virus (HIV) p24 antigen was observed in tomato leaves [46], there was a >90% reduction in green fruits and no expression was observed in ripe fruits. Lettuce (Lactuca sativa L.) chloroplast transformation has also been developed 47, 48, 49 and there has been success in expressing the first therapeutic protein (proinsulin) in lettuce chloroplasts [49]. Recently, this system has been optimized and several therapeutic proteins have been expressed [3]. The level of expression in lettuce chloroplasts is similar to that in tobacco chloroplasts and regeneration is as rapid as in tobacco, thus opening the door to practical oral delivery of chloroplast expressed proteins.
Another challenge is oral delivery of chloroplast-derived proteins in adequate doses to confer immunity or, in cases of autoantigens, to induce tolerance. In this context, it has been demonstrated that chloroplast-derived therapeutic proteins, delivered orally via plant cells, are protected from degradation in the stomach, presumably because of bioencapsulation of the antigen by the plant cell wall. To facilitate translocation of vaccine antigens or therapeutics from the gut lumen into the circulatory system, target proteins have been fused to the CTB transmucosal carrier protein, which can bind to the epithelial receptor GM1 [50]. This approach has been widely applied to many orally delivered antigens, both for stable nuclear transgenics and for transplastomic approaches.
Chloroplast-derived bacterial antigens
Several vaccine antigens against bacterial diseases have been produced in chloroplasts (Table 3, Appendix BTable S3 in the supplementary material online). CTB subunit 51, 52, the first vaccine expressed in chloroplasts, was shown to be functional by the GM1 binding assay. Subsequently, a fusion of LTB and heat-stable toxin (ST) has been expressed in tobacco chloroplasts, and mice immunized with a chloroplast-derived LTB–ST fusion protein were partly protected against CT challenge [53]. Recently, two CTB fusion proteins were expressed in tobacco and lettuce chloroplasts [3]. Both subcutaneously and orally administered mice with chloroplast-derived CTB fusion proteins were protected against CT challenge. High levels of serum and intestinal CTB–IgA were detected in orally administered mice. Although none of the mice administered antigen subcutaneously had detectable CTB–IgA, high levels of IgG1 titer conferred protection (86%) against the CT challenge. Comparable patterns of IgG1 expression observed in orally administered mice in addition to intestinal and serum IgA conferred 100% protection and confirmed that oral vaccination results in both a mucosal and a systemic immune response [3]. Most importantly, long-term immunity and protection (up to 50% of mouse lifespan) was observed. This is a significant advancement because immunity is lost in the current cholera vaccine in children within 3 years and it is not fully protective in adults. Vaccine antigen against tetanus also conferred complete protection against pathogen challenge [54]. Although chloroplast-derived outer surface lipoprotein A (OspA) generated a lower level of protective antibodies compared with lipidated Escherichia coli-derived OspA, the generated antibody level could protect against Lyme disease [5].
The chloroplast transformation system has been used successfully to develop vaccines against bioterrorism agents. The currently available anthrax vaccine obtained from the culture of Bacillus anthracis has undesirable side effects, such as local pain and edema, and relatively high rates of local and systemic reactions, including inflammation and flu-like symptoms. In an attempt to produce anthrax vaccine in large quantities that is free of extraneous bacterial contaminants, protective antigen (PA) was expressed in transgenic tobacco chloroplasts by inserting the pagA gene into the chloroplast genome 55, 56. High levels of IgG titers have been reported in mice immunized subcutaneously with partially purified chloroplast-derived PA and 100% of animals survived against lethal doses of toxin challenge. It is estimated that up to 360 million doses of fully functional anthrax vaccine could be produced from one acre of tobacco 55, 56.
By contrast, there is no approved vaccine for bubonic or pneumonic plague, which is a potential agent for bioterrorism [57]. Recently, a plague F1–V fusion antigen has been expressed in chloroplasts and its functionality was investigated in mice [58]. Animals immunized either subcutaneously or orally with chloroplast-derived protective antigen F1–V showed significantly higher levels of serum F1–V specific IgG1 titers. Of the subcutaneously immunized mice, 33% survived. By contrast, 88% of the orally immunized mice were protected against a heavy dose of aerosolized Yersinia pestis plague challenge. A comparison of splenic Y. pestis CFU counts showed up to a 10-log reduction in the mean bacterial burden in survivors. This shows that oral delivery offers greater protection and immunity [58] and demonstrates that oral delivery of a chloroplast-derived vaccine can be an effective mode of delivery for plant-derived vaccine antigens.
Chloroplast-derived viral antigens
The first viral antigen capable of eliciting a protective immune response when expressed in chloroplasts was against canine parvovirus (CPV) 52, 59. Mice immunized intraperitoneally with chloroplast-derived 2L21 fused to CTB in leaf extract elicited anti-2L21 antibodies. Furthermore, the antibodies induced in rabbits by CTB–2L21-enriched plant extract efficiently neutralized CPV infection of CRFK cells [59]. The structural protein E2, a neutralizing antigen for classical swine fever virus (CSFV) was expressed in tobacco chloroplasts and subcutaneous immunization induced serum antibody reaction against CSFV; however, no immune response was detected by oral immunization [60], in contrast to studies described above with F1–V.
It has been shown that a chloroplast-derived subunit vaccine for hepatitis E virus ORF2-encoded pE2 peptide induce pE2-specific antibody in subcutaneously immunized mice 61, 62. Also, the major structural protein of human papillomavirus, HPV-16 capsid, L1 was expressed from the tobacco chloroplast genome and the chloroplast-derived L1 protein displayed conformation-specific epitopes and assembled into virus-like particles visible by transmission electron microscopy 63, 64. Furthermore, chloroplast-derived L1 protein showed immunogenic and neutralizing antibodies in mice injected intraperitoneally [63].
Chloroplast-derived protozoan antigens
A potential anti-amoebic candidate, LecA, a surface antigen of Entamoeba histolytica, expressed in tobacco chloroplasts, resulted in high immunogenicity in mice following subcutaneous administration [65]. It has been reported that 29 million doses of vaccine antigen can be produced per acre of transplastomic plants [65]. Recently, malaria vaccine candidates AMA1 and MSP1 fused with CTB were expressed in tobacco and lettuce chloroplasts, with a significant level of antigen-specific titers in both oral and subcutaneously immunized mice 3, 66. Immunoblot and immunofluorescence studies have shown that the chloroplast-derived AMA1 and MSP1 inhibit the proliferation of the malaria parasite and crossreact with the native parasite proteins at the schizont, ring or trophozoite stage. In vitro parasite inhibition studies show that mice with higher anti-MSP1 antibody titer exhibit the highest inhibition 3, 66.
Chloroplast-derived autoantigens
Type 1 diabetes (TD1) results from the autoimmune destruction of insulin-producing cells and current treatments are not only expensive but also provide only transient relief. Recently, human proinsulin fused with CTB was expressed in lettuce and tobacco chloroplasts [49]. It was shown that the pancreas of mice immunized with chloroplast-derived CTB–proinsulin had decreased infiltration of cells characteristic of lymphocytes (insulitis) and significantly preserved insulin-producing B-cells in the pancreatic islets with lower blood and urine glucose levels. Moreover, increased expression of immunosuppressive cytokines, such as interleukin-4 and interleukin-10 (IL-4 and IL-10), was observed in the pancreas of chloroplast-derived CTB–proinsulin treated mice, indicating T-helper 2 (Th2) lymphocyte-mediated oral tolerance [49]. Another autoantigen for the prevention and delayed onset of TD1, glutamic acid decarboxylase 65 (GAD65), has been expressed in Chlamydomonas chloroplasts and recombinant hGAD65 reacted with TD1 sera from non-obese diabetic mice and stimulated the proliferation of spleen lymphocytes from these mice [67].
Chloroplast-derived human blood proteins
Several chloroplast-derived pharmaceutical proteins have been produced (Table 3, Appendix BTable S3 in the supplementary material online). For example, human somatotropin was expressed in tobacco chloroplasts and functionality was shown by using a rat lymphoma cell line, Nb2 proliferation assay [68]. Human serum albumin expressed in tobacco chloroplasts formed inclusion bodies [69]. Human interferon gamma (IFN-γ) expressed in chloroplasts protected a human lung carcinoma cell line against infection with the encephalomyocarditis virus [70]. Chloroplast-derived IFN-α2b had similar activity to commercially produced PEG-Intron™, protecting cells against cytopathic viral replication in the vesicular stomatitis virus cytopathic effect assay, inhibiting early-stage HIV infection, and increasing the expression of major histocompatibility complex class I on splenocytes and the total number of natural killer cells. IFN-α2b purified from transplastomic lines protected mice from a highly metastatic tumor line following intraperitoneal injection [41]. Human alpha1-antitrypsin (A1AT) expressed in tobacco chloroplasts binds to porcine pancreatic elastase [71], although a detailed functional evaluation is needed. Insulin-like growth factor 1 (IGF-1) was expressed in tobacco chloroplast [72]. Mass spectrometry confirmed the identity of purified chloroplast-derived human IGF-1. The biological activity of the chloroplast-derived and the commercially synthesized human IGF-1 have similar levels of mitotic activity, as shown by a mammalian cell (human HU-3 cells) proliferation assay [72]. Proper functionality of several of these human blood proteins show that required disulfide bonds are formed in chloroplasts 41, 68, 70. Therefore, proper post-translational modifications and functionally active chloroplast-derived therapeutic proteins have opened the door for the production of low-cost plant based therapeutic proteins.
Conclusions
When delivered orally, plant-derived vaccines and biopharmaceuticals have the advantage that there is no need for expensive fermentation and purification systems and other expenses associated with cold storage, transportation and sterile delivery. Although plant-made vaccines have been developed and advanced for two decades, none of them have advanced beyond Phase I human clinical trials. Crucial evaluation of various expression systems highlights the advantages and disadvantages of each system. Stable nuclear expression systems have been developed over three decades and are available in a large number of crops, with the ability to facilitate tissue-specific expression and inducible expression. However, as seen in the list of vaccine antigens and biopharmaceuticals expressed via the nuclear genome (Appendix BTables S1 and S2 in the supplementary material online), expression levels are often inadequate for commercial development, although adequately expressed antigens have advanced into clinical trials in a few cases. Chloroplasts generally offer higher levels of expression of vaccine antigens against bacterial, viral and protozoan pathogens and biopharmaceuticals, and transgene containment via maternal inheritance. Chloroplast system for expression of therapeutic proteins has been developed in the past 8 years and recent development of an efficient lettuce chloroplast system for oral delivery is a significant advancement. However, glycoproteins cannot be expressed in chloroplasts in their glycosylated form. Although viral expression systems produce therapeutic proteins quickly, they are generally not suitable for oral delivery [73]. Therefore, costs associated with purification, cold storage, transportation and sterile delivery would be major challenges for the viral systems. The downstream processing cost is typically 80% of the production cost and a significant challenge for the future is to reduce this [7]. However, research into the viral system has made significant progress recently, including the development of an antibody for passive immunity against non-Hodgkin's lymphoma in Phase I clinical trials [74], testing F1–V plague vaccine in monkeys [57] and the development of anti-HIV peptides [75]. The transient expression technology is well suited for pandemic responses, such as that faced with H1N1 influenza strains.
The ultimate success of plant-based production systems for subunit vaccine and protein therapeutic candidates will probably depend on lead commercially viable targets successfully transitioning through clinical trials over the next few years. Plant-based production systems for recombinant proteins have been researched over the past 20 years, with a proliferation of academic groups and biotechnology companies, together with groups from larger agrochemical companies, developing the expression technologies and lead product candidates. This effort needs to be repaid with positive results in later clinical studies for the field to continue to advance. Notable early clinical stage successes have been recorded, advancing several oral (or edible) vaccine candidates into Phase I trials 14, 19, 76, 77, 78. However, none of these candidate products have progressed further, largely because they either represented proof-of-principle targets or impractical oral delivery vehicles. During the past 5 years, insufficient funding and lack of commercial interest in plant-based oral vaccines has led to this approach slowing, and no oral vaccine candidates have recently entered the clinic. The challenges of guiding novel plant-based oral vaccines through the regulatory process currently outweigh available resources. More recently, there has been an increased focus on using plant-based production systems to generate purified recombinant proteins for vaccines and therapeutics to be delivered by injection. Notable recent examples that have had clinical trial success include a personalized therapeutic vaccine for non-Hodgkin's lymphoma assessed in a Phase I clinical trial [74], IFN-α2b to combat hepatitis C assessed in a Phase IIa clinical trial (http://www.biolex.com), and human glucocerebrosidase to combat Gaucher's disease, which has now progressed into a Phase III clinical trial [8]. These and similar purified protein products represent the current best options for making plant-based production systems for human vaccines and therapeutics a reality. In these cases, the emphasis is on demonstrating that the plant production systems can generate antigen or therapeutic protein of comparable safety potency and efficacy to other recombinant systems, without added concerns of an oral recombinant plant delivery vehicle. On a parallel track, a plant-produced vaccine candidate for Newcastle disease in chickens was approved for use in 2006. This again focused on a protein product purified from plant cells and emphasizes that plant-produced targets can progress along the regulatory path.
In summary, the availability of diverse plant-based production systems offers opportunities to produce a wide array of antigens and biopharmaceutical proteins against diverse diseases, and for different means of delivery to be explored. However, greater research emphasis is needed on large-scale production, purification, functional characterization, oral delivery and preclinical evaluation for this field to move forward.
Acknowledgements
Investigations on chloroplast-derived vaccines and biopharmaceuticals in the Daniell lab were supported in part by grants from USDA 3611-21000-021-02S and NIH R01 GM 63879 to H.D.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.tplants.2009.09.009.
Appendix A. Supplementary data
References
- 1.Morens D.M. The challenge of emerging and re-emerging infectious diseases. Nature. 2004;430:242–249. doi: 10.1038/nature02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu K. Protecting households from catastrophic health spending. Health Aff. 2007;26:972–983. doi: 10.1377/hlthaff.26.4.972. [DOI] [PubMed] [Google Scholar]
- 3.Davoodi-Semiromi A. The green vaccine: a global strategy to combat infectious and autoimmune diseases. Hum. Vaccin. 2009;5:488–493. doi: 10.4161/hv.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moravec T. Production of Escherichia coli heat labile toxin (LT) B subunit in soybean seed and analysis of its immunogenicity as an oral vaccine. Vaccine. 2007;25:1647–1657. doi: 10.1016/j.vaccine.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Glenz K. Production of a recombinant bacterial lipoprotein in higher plant chloroplasts. Nat. Biotechnol. 2006;24:76–77. doi: 10.1038/nbt1170. [DOI] [PubMed] [Google Scholar]
- 6.Arntzen C.J. Plant science. Using tobacco to treat cancer. Science. 2008;321:1052–1053. doi: 10.1126/science.1163420. [DOI] [PubMed] [Google Scholar]
- 7.Yusibov V., Rabindran S. Recent progress in the development of plant derived vaccines. Expert Rev. Vaccines. 2008;7:1173–1183. doi: 10.1586/14760584.7.8.1173. [DOI] [PubMed] [Google Scholar]
- 8.Aviezer D. A plant-derived recombinant human glucocerebrosidase enzyme – a preclinical and phase I investigation. PLoS ONE. 2009;4:e4792. doi: 10.1371/journal.pone.0004792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biolex Therapeutics (2009) Biolex therapeutics researchers present Locteron® U.S. phase 2a hepatitis C trial results at EASL conference (http://www.biolex.com)
- 10.Shaaltiel Y. Production of glucocerebrosidase with terminal mannose glycans for enzyme replacement therapy of Gaucher's disease using a plant cell system. Plant Biotechnol. J. 2007;5:579–590. doi: 10.1111/j.1467-7652.2007.00263.x. [DOI] [PubMed] [Google Scholar]
- 11.Mason H.S. Expression of hepatitis B surface antigen in transgenic plants. Proc. Natl. Acad. Sci. U. S. A. 1992;89:11745–11749. doi: 10.1073/pnas.89.24.11745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voinnet O. An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. Plant J. 2003;33:949–956. doi: 10.1046/j.1365-313x.2003.01676.x. [DOI] [PubMed] [Google Scholar]
- 13.Arakawa T. Efficacy of a food plant-based oral cholera toxin B subunit vaccine. Nat. Biotechnol. 1998;16:292–297. doi: 10.1038/nbt0398-292. [DOI] [PubMed] [Google Scholar]
- 14.Tacket C.O. Immunogenicity in humans of a recombinant bacterial antigen delivered in a transgenic potato. Nat. Med. 1998;4:607–609. doi: 10.1038/nm0598-607. [DOI] [PubMed] [Google Scholar]
- 15.Rosales-Mendoza S. Ingestion of transgenic carrots expressing the Escherichia coli heat-labile enterotoxin B subunit protects mice against cholera toxin challenge. Plant Cell Rep. 2008;27:79–84. doi: 10.1007/s00299-007-0439-z. [DOI] [PubMed] [Google Scholar]
- 16.Nochi T. From the cover: rice-based mucosal vaccine as a global strategy for cold-chain- and needle-free vaccination. Proc. Natl. Acad. Sci. U. S. A. 2007;104:10986–10991. doi: 10.1073/pnas.0703766104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sandhu J.S. Oral immunization of mice with transgenic tomato fruit expressing respiratory syncytial virus-F protein induces a systemic immune response. Transgenic Res. 2000;9:127–135. doi: 10.1023/a:1008979525909. [DOI] [PubMed] [Google Scholar]
- 18.Richter L.J. Production of hepatitis B surface antigen in transgenic plants for oral immunization. Nat. Biotechnol. 2000;18:1167–1171. doi: 10.1038/81153. [DOI] [PubMed] [Google Scholar]
- 19.Thanavala Y. Immunogenicity in humans of an edible vaccine for hepatitis B. Proc. Natl. Acad. Sci. U. S. A. 2005;102:3378–3382. doi: 10.1073/pnas.0409899102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong Q. Oral immunization with hepatitis B surface antigen expressed in transgenic plants. Proc. Natl. Acad. Sci. U. S. A. 2001;98:11539–11544. doi: 10.1073/pnas.191617598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X. Tomato is a highly effective vehicle for expression and oral immunization with Norwalk virus capsid protein. Plant Biotechnol. J. 2006;4:419–432. doi: 10.1111/j.1467-7652.2006.00191.x. [DOI] [PubMed] [Google Scholar]
- 22.Lamphear B.J. Delivery of subunit vaccines in maize seed. J. Control Release. 2002;85:169–180. doi: 10.1016/S0168-3659(02)00282-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Z. Rapid, high-level production of hepatitis B core antigen in plant leaf and its immunogenicity in mice. Vaccine. 2006;24:2506–2513. doi: 10.1016/j.vaccine.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 24.Huang Z. High-yield rapid production of hepatitis B surface antigen in plant leaf by a viral expression system. Plant Biotechnol. J. 2008;6:202–209. [Google Scholar]
- 25.Santi L. An efficient plant viral expression system generating orally immunogenic Norwalk virus-like particles. Vaccine. 2008;26:1846–1854. doi: 10.1016/j.vaccine.2008.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santi L. Protection conferred by recombinant Yersinia pestis antigens produced by a rapid and highly scalable plant expression system. Proc. Natl. Acad. Sci. U. S. A. 2006;103:861–866. doi: 10.1073/pnas.0510014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Musiychuk K. A launch vector for the production of vaccine antigens in plants. Influenza Other Respi. Viruses. 2007;1:19–25. doi: 10.1111/j.1750-2659.2006.00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shoji Y. Plant-derived hemagglutinin protects ferrets against challenge infection with the A/Indonesia/05/05 strain of avian influenza. Vaccine. 2009;27:1087–1092. doi: 10.1016/j.vaccine.2008.11.108. [DOI] [PubMed] [Google Scholar]
- 29.D’Aoust M.A. Influenza virus-like particles produced by transient expression in Nicotiana benthamiana induce a protective immune response against a lethal viral challenge in mice. Plant Biotechnol. J. 2008;6:930–940. doi: 10.1111/j.1467-7652.2008.00384.x. [DOI] [PubMed] [Google Scholar]
- 30.Huang Z. A DNA replicon system for rapid high-level production of virus-like particles in plants. Biotechnol. Bioeng. 2009;103:706–714. doi: 10.1002/bit.22299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giritch A. Rapid high-yield expression of full-size IgG antibodies in plants coinfected with noncompeting viral vectors. Proc. Natl. Acad. Sci. U. S. A. 2006;103:14701–14706. doi: 10.1073/pnas.0606631103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X., Mason H. Bean Yellow Dwarf Virus replicons for high-level transgene expression in transgenic plants and cell cultures. Biotechnol. Bioeng. 2006;93:271–279. doi: 10.1002/bit.20695. [DOI] [PubMed] [Google Scholar]
- 33.Reggi S. Recombinant human acid beta-glucosidase stored in tobacco seed is stable, active and taken up by human fibroblasts. Plant Mol. Biol. 2005;57:101–113. doi: 10.1007/s11103-004-6832-x. [DOI] [PubMed] [Google Scholar]
- 34.Strasser R. Generation of glyco-engineered Nicotiana benthamiana for the production of monoclonal antibodies with a homogeneous human-like N-glycan structure. Plant Biotechnol. J. 2008;6:392–402. doi: 10.1111/j.1467-7652.2008.00330.x. [DOI] [PubMed] [Google Scholar]
- 35.Mett V. Engineering and expression of the intracellular domain of insulinoma-associated tyrosine phosphatase (IA-2ic), a type 1 diabetes autoantigen, in plants. Transgenic Res. 2007;16:77–84. doi: 10.1007/s11248-006-9033-3. [DOI] [PubMed] [Google Scholar]
- 36.Potula H.H. Transient expression, purification and characterization of bioactive human fibroblast growth factor 8b in tobacco plants. Transgenic Res. 2008;17:19–32. doi: 10.1007/s11248-007-9072-4. [DOI] [PubMed] [Google Scholar]
- 37.Wirth S. Expression of active human epidermal growth factor (hEGF) in tobacco plants by integrative and non-integrative systems. Mol. Breed. 2004;13:23–35. [Google Scholar]
- 38.Verma D., Daniell H. Chloroplast vector systems for biotechnology applications. Plant Physiol. 2007;145:1129–1143. doi: 10.1104/pp.107.106690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daniell H. Molecular strategies for gene containment in transgenic crops. Nat. Biotechnol. 2002;20:581–586. doi: 10.1038/nbt0602-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daniell H. Transgene containment by maternal inheritance: effective or elusive? Proc. Natl. Acad. Sci. U. S. A. 2007;104:6879–6880. doi: 10.1073/pnas.0702219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arlen P.A. Field production and functional evaluation of chloroplast-derived interferon-alpha2b. Plant Biotechnol. J. 2007;5:511–525. doi: 10.1111/j.1467-7652.2007.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeGray G. Expression of an antimicrobial peptide via the chloroplast genome to control phytopathogenic bacteria and fungi. Plant Physiol. 2001;127:852–862. [PMC free article] [PubMed] [Google Scholar]
- 43.Oey M. Plastid production of protein antibiotics against pneumonia via a new strategy for high-level expression of antimicrobial proteins. Proc. Natl. Acad. Sci. U. S. A. 2009;106:6579–6584. doi: 10.1073/pnas.0813146106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Surzycki R. Factors effecting expression of vaccines in microalgae. Biologicals. 2009;37:133–138. doi: 10.1016/j.biologicals.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 45.Kumar S. Plastid-expressed betaine aldehyde dehydrogenase gene in carrot cultured cells, roots, and leaves confers enhanced salt tolerance. Plant Physiol. 2004;136:2843–2854. doi: 10.1104/pp.104.045187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou F. High-level expression of human immunodeficiency virus antigens from the tobacco and tomato plastid genomes. Plant Biotechnol. J. 2008;6:897–913. doi: 10.1111/j.1467-7652.2008.00356.x. [DOI] [PubMed] [Google Scholar]
- 47.Kanamoto H. Efficient and stable transformation of Lactuca sativa L. cv. Cisco (lettuce) plastids. Transgenic Res. 2006;15:205–217. doi: 10.1007/s11248-005-3997-2. [DOI] [PubMed] [Google Scholar]
- 48.Lelivelt C.L. Stable plastid transformation in lettuce (Lactuca sativa L.) Plant Mol. Biol. 2005;58:763–774. doi: 10.1007/s11103-005-7704-8. [DOI] [PubMed] [Google Scholar]
- 49.Ruhlman T. Expression of cholera toxin B-proinsulin fusion protein in lettuce and tobacco chloroplasts – oral administration protects against development of insulitis in non-obese diabetic mice. Plant Biotechnol. J. 2007;5:495–510. doi: 10.1111/j.1467-7652.2007.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Limaye A. Receptor-mediated oral delivery of a bioencapsulated green fluorescent protein expressed in transgenic chloroplasts into the mouse circulatory system. FASEB J. 2006;20:959–961. doi: 10.1096/fj.05-5134fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daniell H. Expression of the native cholera toxin B subunit gene and assembly as functional oligomers in transgenic tobacco chloroplasts. J. Mol. Biol. 2001;311:1001–1009. doi: 10.1006/jmbi.2001.4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Molina A. High-yield expression of a viral peptide animal vaccine in transgenic tobacco chloroplasts. Plant Biotechnol. J. 2004;2:141–153. doi: 10.1046/j.1467-7652.2004.00057.x. [DOI] [PubMed] [Google Scholar]
- 53.Rosales-Mendoza S. Expression of an Escherichia coli antigenic fusion protein comprising the heat labile toxin B subunit and the heat stable toxin, and its assembly as a functional oligomer in transplastomic tobacco plants. Plant J. 2009;57:45–54. doi: 10.1111/j.1365-313X.2008.03666.x. [DOI] [PubMed] [Google Scholar]
- 54.Tregoning J.S. Protection against tetanus toxin using a plant-based vaccine. Eur. J. Immunol. 2005;35:1320–1326. doi: 10.1002/eji.200425453. [DOI] [PubMed] [Google Scholar]
- 55.Koya V. Plant-based vaccine: mice immunized with chloroplast-derived anthrax protective antigen survive anthrax lethal toxin challenge. Infect. Immun. 2005;73:8266–8274. doi: 10.1128/IAI.73.12.8266-8274.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watson J. Expression of Bacillus anthracis protective antigen in transgenic chloroplasts of tobacco, a non-food/feed crop. Vaccine. 2004;22:4374–4384. doi: 10.1016/j.vaccine.2004.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mett V. A plant-produced plague vaccine candidate confers protection to monkeys. Vaccine. 2007;25:3014–3017. doi: 10.1016/j.vaccine.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 58.Arlen P.A. Effective plague vaccination via oral delivery of plant cells expressing F1-V antigens in chloroplasts. Infect. Immun. 2008;76:3640–3650. doi: 10.1128/IAI.00050-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Molina A. Induction of neutralizing antibodies by a tobacco chloroplast-derived vaccine based on a B cell epitope from canine parvovirus. Virology. 2005;342:266–275. doi: 10.1016/j.virol.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 60.Shao H.B. The expression of classical swine fever virus structural protein E2 gene in tobacco chloroplasts for applying chloroplasts as bioreactors. C. R. Biol. 2008;331:179–184. doi: 10.1016/j.crvi.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 61.Skidmore S. Overview of hepatitis E virus. Curr. Infect. Dis. Rep. 2002;4:118–123. doi: 10.1007/s11908-002-0051-x. [DOI] [PubMed] [Google Scholar]
- 62.Zhou Y.X. A truncated hepatitis E virus ORF2 protein expressed in tobacco plastids is immunogenic in mice. World J. Gastroenterol. 2006;12:306–312. doi: 10.3748/wjg.v12.i2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fernandez-San Millan A. Human papillomavirus L1 protein expressed in tobacco chloroplasts self-assembles into virus-like particles that are highly immunogenic. Plant Biotechnol. J. 2008;6:427–441. doi: 10.1111/j.1467-7652.2008.00338.x. [DOI] [PubMed] [Google Scholar]
- 64.Lenzi P. Translational fusion of chloroplast-expressed human papillomavirus type 16 L1 capsid protein enhances antigen accumulation in transplastomic tobacco. Transgenic Res. 2008;17:1091–1102. doi: 10.1007/s11248-008-9186-3. [DOI] [PubMed] [Google Scholar]
- 65.Chebolu S., Daniell H. Stable expression of Gal/GalNAc lectin of Entamoeba histolytica in transgenic chloroplasts and immunogenicity in mice towards vaccine development for amoebiasis. Plant Biotechnol. J. 2007;5:230–239. doi: 10.1111/j.1467-7652.2006.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Davoodi-Semiromi, A. et al. A green vaccine confers dual immunity against cholera and malaria by oral and injectable immunization. Plant Biotechnol. J. (in press) [DOI] [PMC free article] [PubMed]
- 67.Wang X. A novel expression platform for the production of diabetes-associated autoantigen human glutamic acid decarboxylase (hGAD65) BMC Biotechnol. 2008;8:87. doi: 10.1186/1472-6750-8-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Staub J.M. High-yield production of a human therapeutic protein in tobacco chloroplasts. Nat. Biotechnol. 2000;18:333–338. doi: 10.1038/73796. [DOI] [PubMed] [Google Scholar]
- 69.Fernandez-San Millan A. A chloroplast transgenic approach to hyper-express and purify human serum albumin, a protein highly susceptible to proteolytic degradation. Plant Biotechnol. J. 2003;1:71–79. doi: 10.1046/j.1467-7652.2003.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leelavathi S., Reddy V. Chloroplast expression of His-tagged GUS-fusions: a general strategy to overproduce and purify foreign proteins using transplastomic plants as bioreactors. Mol. Breed. 2003;11:49–58. [Google Scholar]
- 71.Nadai M. High-level expression of active human alpha1-antitrypsin in transgenic tobacco chloroplasts. Transgenic Res. 2008;18:173–183. doi: 10.1007/s11248-008-9209-0. [DOI] [PubMed] [Google Scholar]
- 72.Daniell H. Optimization of codon composition and regulatory elements for expression of human insulin like growth factor-1 in transgenic chloroplasts and evaluation of structural identity and function. BMC Biotechnol. 2009;9:23. doi: 10.1186/1472-6750-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Green B.J. Transient protein expression in three Pisum sativum (green pea) varieties. Biotechnol. J. 2009;4:230–237. doi: 10.1002/biot.200800256. [DOI] [PubMed] [Google Scholar]
- 74.McCormick A.A. Plant-produced idiotype vaccines for the treatment of non-Hodgkin's lymphoma: safety and immunogenicity in a phase I clinical study. Proc. Natl. Acad. Sci. U. S. A. 2008;105:10131–10136. doi: 10.1073/pnas.0803636105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.O’Keefe B.R. Scaleable manufacture of HIV-1 entry inhibitor griffithsin and validation of its safety and efficacy as a topical microbicide component. Proc. Natl. Acad. Sci. U. S. A. 2009;106:6099–6104. doi: 10.1073/pnas.0901506106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tacket C.O. Human immune responses to a novel Norwalk virus vaccine delivered in transgenic potatoes. J. Infect. Dis. 2000;182:302–305. doi: 10.1086/315653. [DOI] [PubMed] [Google Scholar]
- 77.Tacket C.O. Immunogenicity of recombinant LT-B delivered orally to humans in transgenic corn. Vaccine. 2004;22:4385–4389. doi: 10.1016/j.vaccine.2004.01.073. [DOI] [PubMed] [Google Scholar]
- 78.Yusibov V. Expression in plants and immunogenicity of plant virus-based experimental rabies vaccine. Vaccine. 2002;20:3155–3164. doi: 10.1016/s0264-410x(02)00260-8. [DOI] [PubMed] [Google Scholar]
- 79.Jiang X.L. Cholera toxin B protein in transgenic tomato fruit induces systemic immune response in mice. Transgenic Res. 2007;16:169–175. doi: 10.1007/s11248-006-9023-5. [DOI] [PubMed] [Google Scholar]
- 80.Qian B. Immunogenicity of recombinant hepatitis B virus surface antigen fused with preS1 epitopes expressed in rice seeds. Transgenic Res. 2008;17:621–631. doi: 10.1007/s11248-007-9135-6. [DOI] [PubMed] [Google Scholar]
- 81.Dong J.L. Oral immunization with pBsVP6-transgenic alfalfa protects mice against rotavirus infection. Virology. 2005;339:153–163. doi: 10.1016/j.virol.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 82.Li J.T. Immunogenicity of a plant-derived edible rotavirus subunit vaccine transformed over fifty generations. Virology. 2006;356:171–178. doi: 10.1016/j.virol.2006.07.045. [DOI] [PubMed] [Google Scholar]
- 83.Pogrebnyak N. Severe acute respiratory syndrome (SARS) S protein production in plants: development of recombinant vaccine. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9062–9067. doi: 10.1073/pnas.0503760102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Golovkin M. Smallpox subunit vaccine produced in planta confers protection in mice. Proc. Natl. Acad. Sci. U. S. A. 2007;104:6864–6869. doi: 10.1073/pnas.0701451104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Y. Generation and immunogenicity of Japanese encephalitis virus envelope protein expressed in transgenic rice. Biochem. Biophys. Res. Commun. 2009;380:292–297. doi: 10.1016/j.bbrc.2009.01.061. [DOI] [PubMed] [Google Scholar]
- 86.Wang L. Immunogenicity of Plasmodium yoelii merozoite surface protein 4/5 produced in transgenic plants. Int. J. Parasitol. 2008;38:103–110. doi: 10.1016/j.ijpara.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 87.Saejung W. Production of dengue 2 envelope domain III in plant using TMV-based vector system. Vaccine. 2007;25:6646–6654. doi: 10.1016/j.vaccine.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 88.Kim T.G. Expression of human growth hormone in transgenic rice cell suspension culture. Plant Cell Rep. 2008;27:885–891. doi: 10.1007/s00299-008-0514-0. [DOI] [PubMed] [Google Scholar]
- 89.Gutierrez-Ortega A. Expression of functional interleukin-12 from mouse in transgenic tomato plants. Transgenic Res. 2005;14:877–885. doi: 10.1007/s11248-005-1464-8. [DOI] [PubMed] [Google Scholar]
- 90.Bai J.Y. Expression and characteristic of synthetic human epidermal growth factor (hEGF) in transgenic tobacco plants. Biotechnol. Lett. 2007;29:2007–2012. doi: 10.1007/s10529-007-9438-y. [DOI] [PubMed] [Google Scholar]
- 91.Ding S.H. High-level expression of basic fibroblast growth factor in transgenic soybean seeds and characterization of its biological activity. Biotechnol. Lett. 2006;28:869–875. doi: 10.1007/s10529-006-9018-6. [DOI] [PubMed] [Google Scholar]
- 92.Wang M.L. Production of biologically active GM-CSF in sugarcane: a secure biofactory. Transgenic Res. 2005;14:167–178. doi: 10.1007/s11248-004-5415-6. [DOI] [PubMed] [Google Scholar]
- 93.Sardana R. Biologically active human GM-CSF produced in the seeds of transgenic rice plants. Transgenic Res. 2007;16:713–721. doi: 10.1007/s11248-006-9062-y. [DOI] [PubMed] [Google Scholar]
- 94.Zheng G.G. Expression of bioactive human M-CSF soluble receptor in transgenic tobacco plants. Protein Expr. Purif. 2006;46:367–373. doi: 10.1016/j.pep.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 95.Hong S.Y. Production of bioactive human granulocyte-colony stimulating factor in transgenic rice cell suspension cultures. Protein Expr. Purif. 2006;47:68–73. doi: 10.1016/j.pep.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 96.Suo G. Expression of active hBMP2 in transgenic tobacco plants. Plant Cell Rep. 2006;25:1316–1324. doi: 10.1007/s00299-006-0173-y. [DOI] [PubMed] [Google Scholar]
- 97.Agarwal S. Expression of modified gene encoding functional human alpha-1-antitrypsin protein in transgenic tomato plants. Transgenic Res. 2008;17:881–896. doi: 10.1007/s11248-008-9173-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


