Abstract
Vaccines currently licensed for the prevention of seasonal influenza induce antibodies against the influenza hemagglutinin (HA) and neuraminidase (NA) contained in the vaccine preparation but require at least two weeks after immunization for the development of protective immunity. These vaccines do not induce protective responses quickly enough to blunt the effects of infection when administered after exposure. We have developed a novel vaccine based on recombinant vesicular stomatitis virus which expresses the influenza hemagglutinin (rVSV HA) and protects mice from lethal influenza challenge when the vaccine is administered intramuscularly at least 24 hours after delivery of the influenza challenge virus. To our knowledge this is the first vaccine that effectively protects animals from lethal influenza challenge when delivered by a systemic route after influenza exposure has occurred. The induction of HA-specific immune responses by the vaccine is necessary for full protection from challenge, because animals immunized with an empty rVSV vector were not protected equally. Our results are consistent with a model in which vaccination induces an immediate antiviral cytokine response, followed by development of humoral and cellular immune responses which act to reduce pulmonary viral loads and accelerate recovery. Consistent with this model, mice vaccinated with the specific vaccine rVSV HA had high levels of IFN-α in the serum by 24 hours after challenge/vaccination, developed serum neutralizing Ab to influenza two days prior to control animals, and had detectable anti-HA CD8 T cells present in the peripheral blood three days prior to control mice.
Keywords: rVSV, influenza, post-exposure vaccine
1. INTRODUCTION
Vaccines against infectious microorganisms are usually administered prophylactically, and protective immunity is generated in the vaccinated individual before the vaccinee encounters the pathogen against which the vaccine protects. In emergent circumstances however, prophylactic vaccination is often impractical or ineffective. Vaccines against smallpox [1], rabies [2], and hepatitis B [3] can generate protective immunity in humans even when the vaccines are administered after exposure to these viruses has occurred. The mechanism(s) of post-exposure protection have not been defined for these vaccines, but innate and adaptive immune responses are probably induced by the vaccine before the infecting virus can establish fulminant infection or cause significant pathology. There are currently two licensed vaccines for the prevention of seasonal influenza in humans, both of which are administered prophylactically. The conventional inactivated vaccine is administered by injection and is a trivalent cocktail of circulating influenza strains (2 influenza A strains and 1 influenza B strain) which are formalin-inactivated and “lipid-split” to enrich for the viral hemagglutinin (HA) and neuraminidase (NA). The alternate vaccine is a live cold-adapted virus preparation (FluMist™) which is administered intranasally [4]. FluMist also contains two influenza A and one influenza B strains. The influenza strains included in the vaccines are selected based upon the recommendation of the World Health Organization, which samples emerging and circulating influenza viruses to determine those most likely to cause disease in humans in a given year. Both vaccine preparations induce anti-influenza neutralizing antibodies in vaccinees, and protection is assumed to depend upon the action of those antibodies. Both types of influenza vaccine are administered prior to the circulation of influenza virus in a community, allowing time for protective immunity to develop before individuals are likely to encounter influenza virus naturally. The timing of seasonal influenza epidemics is generally predictable, and existing vaccines protect well as long as the identity of the predominant circulating strains is predicted correctly. However, a significant limitation of existing influenza vaccines is that they require a minimum of two weeks to establish protective immunity in the vaccinee [5], making them unsuitable for use as a post-exposure therapeutic. In addition, existing vaccines do not protect at all against highly pathogenic avian influenzas (influenza A/H5N1, HPAI). Human infection with recently emerged HPAI has a mortality of up to 81% [6, 7]. Current avian influenza strains are not efficiently transmitted from human to human but the high mutation rate of the virus, the increasing incidence of human infection, and the isolated reports of human to human transmission [8–10] suggest that the virus will eventually acquire that ability. Because it is not possible to predict precisely when HPAI will acquire the ability to transmit between humans, or what the pandemic strain might be, the development of an influenza vaccine that could generate protective immunity in the shortest time possible after immunization would be highly desirable. In May of this year, a novel H1N1 virus (H1N1 swine flu) with the potential for pandemic spread [11, 12] emerged in the human population. The vaccine that has been developed against the novel H1N1 induces antibody titers predictive of protection by 2–3 weeks after immunization [13, 14], but because the vaccine strain did not grow well in eggs, thereby limiting vaccine production efficiency, it is likely that a large portion of the population will not be vaccinated during the height of the natural flu season. We have developed a novel rVSV-based vaccine that addresses some of the weaknesses of existing influenza vaccines. Recombinant vesicular stomatitis virus (VSV)-based vaccines rapidly induce cellular and humoral responses when administered systemically or mucosally, and are protective against a wide range of infectious challenges when administered prophylactically [15–20]. In addition, a recent series of reports demonstrated that an rVSV expressing the Ebola virus (EBOV) glycoprotein could protect macaques from lethal EBOV challenge when administered within 20 minutes after EBOV challenge and when the challenge and vaccine viruses were delivered by the same route [21–24]. The mechanism by which the VSV-based EBOV vaccine protects has not been determined, and the inherent challenges in working with EBOV in non-human primates make mechanistic experiments difficult to complete. Because the vaccine and challenge viruses were delivered by the same route, it is possible that the VSV-EBOV vaccine virus competed directly for host cell binding, and/or that the vaccine induced innate and adaptive responses which blunted the effects of EBOV infection. We undertook the present study to determine whether a recombinant vesicular stomatitis virus expressing the hemagglutinin (rVSV HA) of influenza A/PR/8/34 (PR8) could protect animals from influenza challenge when administered after exposure to influenza had occurred. We report here that 100% of Balb/C mice immunized intramuscularly with rVSV HA within 24 hours of high dose influenza challenge were protected from mortality. Mice immunized with an empty rVSV vector were partially but not equally protected, suggesting that innate immune responses to rVSV contributed to protection but that full protection and recovery from challenge required development of antigen specific responses. Protection was likely dependent upon the combined action of innate, cellular, and humoral factors, as mice vaccinated with rVSV HA had high levels of IFN-α in the serum and lung within 24 hours after challenge, and had detectable anti-influenza antibody and CD8 T cell responses prior to those detected in control animals. These results demonstrate that therapeutic immunization with an rVSV based vaccine can rescue animals from death and significant morbidity after high dose influenza challenge, and may represent an alternative to the use of current influenza vaccines in emergency situations where conventional immunization is not practical or effective. Because our model makes use of a well characterized virus and uses inbred mice as the host organism, further mechanistic studies to determine the precise mechanism(s) of action of this effective post-exposure vaccine will be feasible.
2. MATERIALS AND METHODS
2.1. Construction of plasmids and recovery of recombinant viruses
To obtain plasmids that could be used to recover rVSV expressing influenza genes from the first or fifth position in the VSV genome, influenza gene sequences (influenza strain A/PR/8/34) were PCR-amplified from plasmids generously provided by Dr. Peter Palese (Mt. Sinai School of Medicine). The forward primer introduced a Sal I site upstream of the coding sequence and the reverse primer introduced an Nhe I site. PCR products were digested with Xho I and Nhe I, purified, and ligated into the pVSVXN2 vector that had been digested with the same enzymes (VSV cloning vectors provided by Dr. John Rose, Yale University). pVSVXN2 allows insertion of the foreign gene in the fifth position of the VSV genome. Plasmids were recovered after transformation of E. coli and purified using a Maxi kit (QIAGEN) and the insert sequences verified (Duke Sequencing Facility). Recombinant virus was recovered from the pVSVXN2 HA plasmid as described previously [25]. Briefly, BHK-21 cells were grown to 50% confluency and infected at a multiplicity of infection (MOI) of 10 with vTF7-3, vaccinia virus expressing T7 RNA polymerase. One hour after infection, cells were transfected with 10 μg of the plasmid encoding the full length VSV genome plus foreign gene of interest along with 3 μg of pBluescript-N (pBS-N), 5 μg of pBS-P, 1 μg of pBS-L, and 4 μg of pBS-G (VSV Indiana serotype). While pBS-G is not required for recovery of recombinants, it was included to enhance efficiency. After 48 hours, cell supernatants were passaged onto BHK-21 cells through a 0.2μM filter, and medium containing virus was collected about 24 h after cytopathic effect was seen. Virus grown from individual plaques was used to prepare stocks that were grown on BHK-21 cells and stored at −80°C.
2.2. Metabolic labeling and SDS-PAGE of cells infected with recombinants
BHK cells (106) were infected at an MOI of 20 with VSV recombinant or control virus. After 5 hrs, medium was removed and cells were washed twice with methionine-free Dulbecco’s modified Eagle’s medium (DMEM). Methionine-free DMEM (1 ml) containing 100 μCi of [35S]-methionine was added to each plate for two additional hours. Medium was removed, cells were washed with phosphate-buffered saline (PBS), lysed with 500 μl of detergent solution (1%Nonidet P-40, 0.4% deoxycholate, 50 mM Tris-HCl [pH 8], 62.5 mM EDTA) on ice for 5 min, and collected into 1.5-ml Eppendorf tubes. The protein extracts were centrifuged for 2 min at 16,000 × g to remove the nuclei and stored at −20°C. Protein extracts were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 15% acrylamide), and proteins were visualized by autoradiography.
2.3. Inoculation of mice
Eight to ten-week-old female Balb/C mice were obtained from Charles River Laboratories and housed for at least 1 week before experiments were initiated. Mice were housed in microisolator cages in a biosafety level 2-equipped animal facility. Viral stocks were diluted to appropriate titers in serum-free DMEM. For intramuscular vaccination (i.m.), mice were injected with the indicated amount of virus(es) in 50μl total volume. For intranasal (i.n.) challenge with influenza, mice were lightly anesthetized with isoflurane using a vaporizer and administered the indicated amount of virus in 40μl total volume. The Institutional Animal Care and Use Committee of Duke University approved all animal experiments. After influenza challenge mice were monitored daily for weight loss and change in body temperature (Physitemp rodent thermometer, Physitemp Inc., Clifton, NJ).
Depletion of CD8 T cells
Mice depleted of CD8 T cells were intraperitoneally injected with 1mg/mouse of a monoclonal antibody (Clone YTS 169AG 101HL) reactive against CD8α. Mice received injections on day −3, day −1, and day +6 after challenge. Depletion of CD8 T cells was confirmed (in the blood and peripheral blood) by flow cytometry on Day 0 and Day 14 of the study.
2.4. Tetramer assay
To obtain peripheral blood lymphocytes blood was collected into serum free medium (DMEM) containing heparin. Blood was layered onto a Ficoll gradient and spun, after which lymphocytes were collected from the interface. Cells were washed and resuspended in DMEM containing 5% FCS. To obtain lymphocytes from the lung, mice were sacrificed via anesthetic overdose and lungs aseptically removed. Lungs were chopped into fine pieces, and digested for 2hrs at 37 in DMEM containing 5% fetal bovine serum, 150U/ml collagenase (Worthington Labs), and 20μg/ml DNase (Sigma). After digestion the cells were pushed through a metal sieve, filtered through a 70 micron filter and layered onto a Ficoll gradient (Lympholyte M, Cedarlane Labs). Lymphocytes were collected from the interface and washed 2x before staining. Staining was performed on freshly isolated lymphocytes as previously described [26]. Briefly, approximately 5×106 cells were added to the wells of a 96-well V-bottom plate and were blocked with unconjugated streptavidin (Molecular Probes) and Fc block (Pharmingen) for 15 min at room temperature (RT). Following a 5-min centrifugation at 500 × g, lymphocytes were labeled with a FITC-conjugated anti-CD62L antibody, (Pharmingen), an allophycocyanin-conjugated anti-CD8 antibody (Pharmingen), and tetramer for 30 min at RT. The tetramer was a PE-conjugated major histocompatibility complex (MHC) class I Kd tetramer (NIH Tetramer Facility) containing the H-2Kd restricted peptide HA533–541 (N-IYSTVASSL-C). Sham-inoculated control animals were used to determine background levels of tetramer binding. Background was routinely less than 0.1% and was subtracted from all reported percentages.
2.5. Microneutralization assay for anti-influenza Ab
A microneutralization assay was performed as described in [27]. Heat-inactivated serum from immunized or control animals was serially diluted and incubated with virus for one hour at RT. Residual infectivity was detected on MDCK cells after a 4 day culture period. Neutralizing titer was defined as the highest dilution of serum that completely neutralized infectivity of 105 TCID50 of PR8 influenza virus. Infectivity was defined by presence of cpe on day four post infection.
2.6. Determination of viral titers by plaque assay
Mice were sacrificed via anesthetic overdose and lungs removed aseptically. After dissection lungs were, weighed, and homogenized in sterile buffer (100μl buffer per 0.1g organ weight). Homogenates were titered by standard plaque assay on MDCK cells in the presence of 1μg/ml TPCK-trypsin (Sigma) and using a solid agar overlay to detect infectious influenza virus. Another aliquot was titered on BHK-21 cells to detect infectious VSV. After 72 hrs (influenza) or 48 hrs (VSV) the overlay was removed and the cell layer stained with crystal violet to visualize plaques. No infectious VSV was detected in the lungs of mice tested in these studies.
2.7. Statistical Analysis
All statistical tests were performed using Graph Pad Prism statistical analysis software. Results were considered significant when P<0.05 was reached. Specific analyses used are indicated in the text and figure legends.
3. RESULTS
3.1. Expression of influenza genes from recombinant VSV
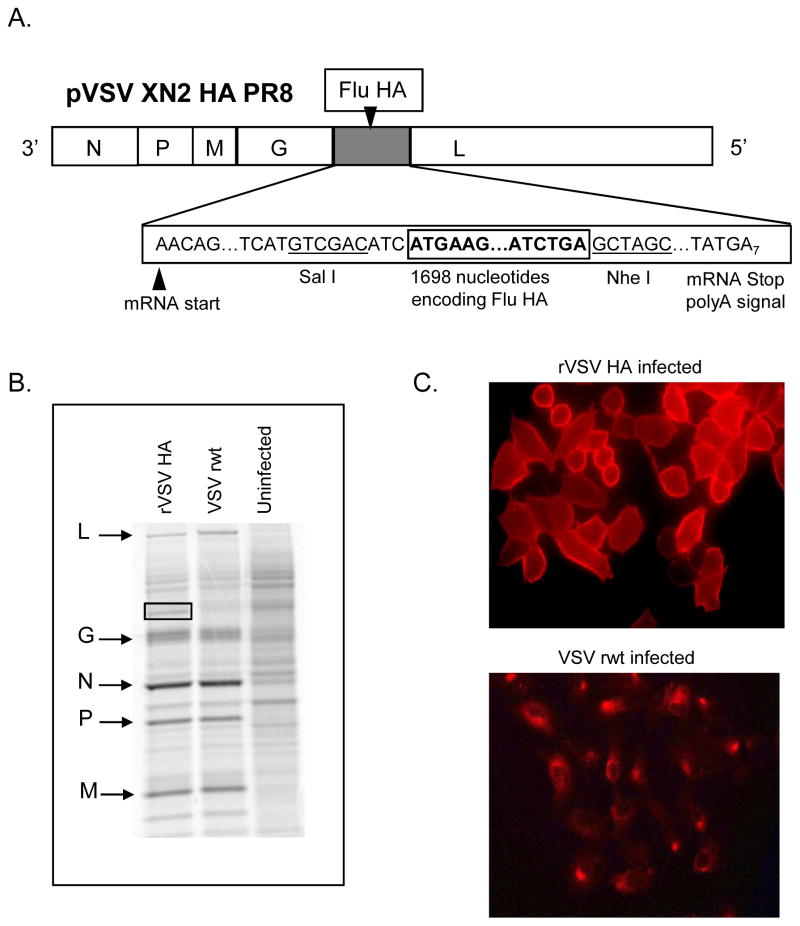
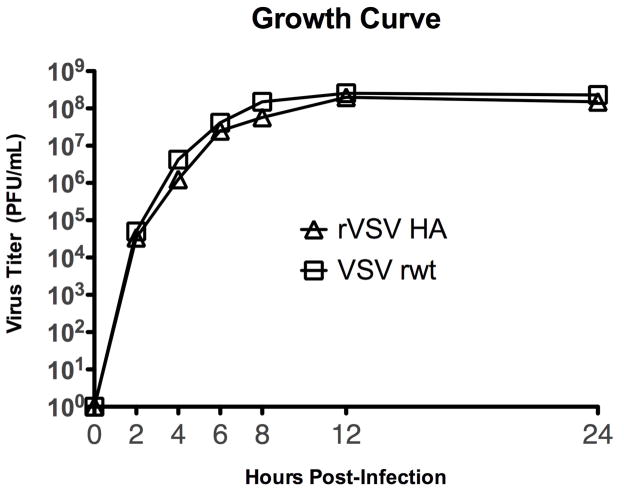
To obtain a replication competent rVSV expressing the influenza hemagglutinin (HA) we inserted DNA encoding the influenza hemagglutinin from the pathogenic mouse-adapted influenza A/PR/8/34 (provided by Dr. Peter Palese) into plasmid vector pVSV XN2 (provided by Dr. John Rose). This allowed recovery of rVSV expressing influenza hemagglutinin from the fifth transcribed gene in the VSV genome (diagrammed in Figure 1A). Expression of influenza HA in cells infected with the recombinant was verified by metabolic labeling with 35S-methionine, SDS-PAGE, and autoradiography (Figure 1B). In cells infected with the VSV recombinants we detected the indicated VSV proteins as well as a new protein band with the mobility (size) expected for the influenza HA. Surface expression of HA on the surface of infected cells was confirmed by immunofluorescent staining of cells infected with the recombinant (Figure 1C). For foreign proteins to be efficiently incorporated into the VSV envelope, the cytoplasmic tail of the foreign protein must be replaced with the VSV G tail [28]. We elected not to attach the VSV tail to HA. Therefore, since this virus retains the VSV G, and does not efficiently incorporate HA into the VSV envelope, it is likely that infection of host cells is mediated almost entirely by VSV G and not by the inserted HA. Expression of foreign genes in rVSVs can, but does not always, attenuate the rVSV [29]. To determine whether expression of hemagglutinin reduced the ability of the rVSV HA to replicate in vitro we performed a one-step growth curve. As shown in Figure 2 the recombinant virus (rVSV HA) grew to equivalent titers as the parent virus (VSV rwt), indicating that the expression of influenza HA does not attenuate rVSV HA in vitro.
Figure 1. Recombinant VSV genome and protein expression.
Panel A is a diagram showing insertion of the influenza HA gene at Position 5 in the VSV genome. The gene order is shown in the 3′-5′ direction of transcription on the negative-strand RNA genome. The influenza gene insertion sites and flanking nucleotides including the transcription and translation start and stop sites are indicated. Restriction enzyme sites used for cloning the influenza genes at the DNA stage are indicated also. All sequences are shown in the positive (antigenome) sense for clarity. Panel B shows SDS-PAGE (10% acrylamide) of lysates of BHK cells infected with the recombinant virus (rVSV HA) and labeled with [35S]-methionine. The gel image was collected on a phosphorimager. Positions of VSV proteins are indicated by arrows on the left side of the gel image. Positions of influenza HA is indicated by the box on the gel image. Panel C shows immunofluorescent staining of BHK cell infected with rVSV HA. Cells were stained with post-immune serum from mice infected with a sublethal dose of A/PR8 influenza.
Figure 2. One-step growth curve for rVSV HA and parent virus VSV rwt.
BHK cells (2×106 cells) were infected with rVSV HA (open triangles) or with the parent virus VSV rwt (open squares) at an MOI of 10. Viruses were adsorbed to the cells for 30 min, then the cells were washed to remove input virus and returned to complete culture medium (DMEM containing 5% fetal bovine serum). At the indicated timepoints post-infection supernatant samples were collected, kept on ice, and titered by plaque assay on BHK cells.
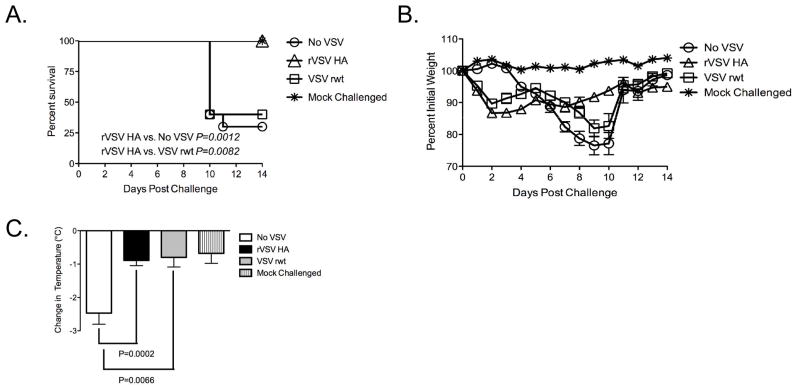
3.2. 100% of Balb/C mice immunized with rVSV HA immediately after challenge are protected from mortality
We have shown previously that rVSV HA expressing the HA from influenza A/PR/8/34 (PR8) protects mice from morbidity and mortality when mice are immunized with the rVSV HA 6–8 weeks before influenza challenge [20]. To determine whether rVSV HA protected mice from morbidity and mortality when rVSV HA was delivered after influenza challenge we performed the experiment shown in Figure 3. Adult Balb/C mice were challenged intranasally with 100MLD50 PR8 influenza in a total volume of 40μl. Immediately (within 1–2 minutes) after influenza challenge, mice were either intramuscularly mock-immunized with diluent alone (n=10, No VSV), immunized intramuscularly with 5×108 PFU rVSV empty vector (VSV rwt, n=5), or immunized intramuscularly with 5×108 PFU rVSV HA (rVSV HA, n=10). As shown in Figure 3A, all (10/10) rVSV HA vaccinated Balb/C mice survived, and the rate of survival of mice immunized with rVSV HA was significantly higher than the rate of survival of mock-immunized mice (no VSV, P=0.0012, Mantel-Cox test) or of mice immunized with empty vector VSV rwt (VSV rwt, P=0.0082, Mantel Cox test). Two of the five mice immunized with the empty vector VSV rwt survived, but the rate of survival in VSV rwt immunized animals was not significantly different from that of mock-immunized mice (P=0.748, Mantel-Cox test). This suggested that the VSV rwt empty vector conferred partial protection, but that specific responses were ultimately required for complete protection. Consistent with this idea, the weight loss pattern of mice vaccinated with rVSV HA diverged significantly from that of mice vaccinated with empty vector VSV rwt from the seventh day after challenge onward, with rVSV HA immunized mice recovering more rapidly (Figure 3B) than those immunized with VSV rwt. Between days 0 and 2 after challenge/immunization all rVSV immunized mice had a transient weight loss of approximately 10% pre-infection body weight. Weight loss in mice immunized with high doses of VSV correlates with host production of TNF-α and typically resolves by 3–4 days post immunization [30]. Flu-challenged mice not receiving VSV did not lose weight during this time (No VSV group, days 0–2 post challenge, Figure 3B) which supported the idea that the initial weight loss was due to VSV and not to influenza pathogenesis. In combination with weight loss, measurement of temperature depression during the acute phase of murine influenza infection is a sensitive indicator of pathology [31]. A decrease in the magnitude of temperature loss indicates a better clinical outcome and enhanced likelihood of survival. Consistent with the observation that immunization with rVSV induces systemic production of TNF-α [30], mice immunized with both VSV rwt and rVSV HA had transiently raised temperatures (of up to 1 C) during the first 24 hours after challenge, which returned to normal by 48 hours after challenge (data not shown). From the second day after challenge onward the temperature of all except the mock-challenged control mice began to decline. When we compared the magnitude of temperature depression from days 2–6 after challenge (during which time temperature change was due to influenza rather than VSV pathogenesis), the difference in temperature depression between mice immunized with rVSV HA and with VSV rwt was not statistically significant (Figure 3C). This supported the notion that mice immunized with the empty vector VSV rwt controlled infection as well as mice immunized with rVSV HA until the seventh day after challenge, but that after the seventh day antigen specific responses were required for survival and recovery. Mice vaccinated with either rVSV HA or VSV rwt lost significantly less temperature than those receiving diluent alone (P=0.0002 for rVSV HA and P=0.0066 for VSV rwt respectively, two-tailed Student t test, Figure 3C), consistent with the idea that a non-specific anti-viral response induced by rVSV vaccination protected mice from pathology up until the seventh day after challenge.
Figure 3. Balb/C mice immunized with rVSV HA immediately after influenza challenge are completely protected from mortality.
Adult female Balb/C mice were challenged intranasally with 100MLD50 PR8. Immediately (within 1–2 minutes) after influenza challenge, mice were either intramuscularly sham-inoculated with diluent alone (n=10), immunized intramuscularly with 5×108 PFU rVSV empty vector (VSV rwt, n=5), or immunized intramuscularly with 5×108 PFU rVSV HA (n=10). Mice were monitored for survival and weight loss (Panels A and B), and change in body temperature (Panel C). Mice were humanely euthanized when their body weight reached 75% of pre-infection body weight for two consecutive days. rVSV HA immunized mice had a significantly higher rate of survival than mice receiving no VSV (P=0.0012, Mantel-Cox test) or vaccinated with the empty vector VSV rwt (P=0.0082, Mantel-Cox test). This experiment has been performed a total of four times with consistent results.
3.3. Immunization with rVSV induces IFN-α in the serum by 24 hours after challenge
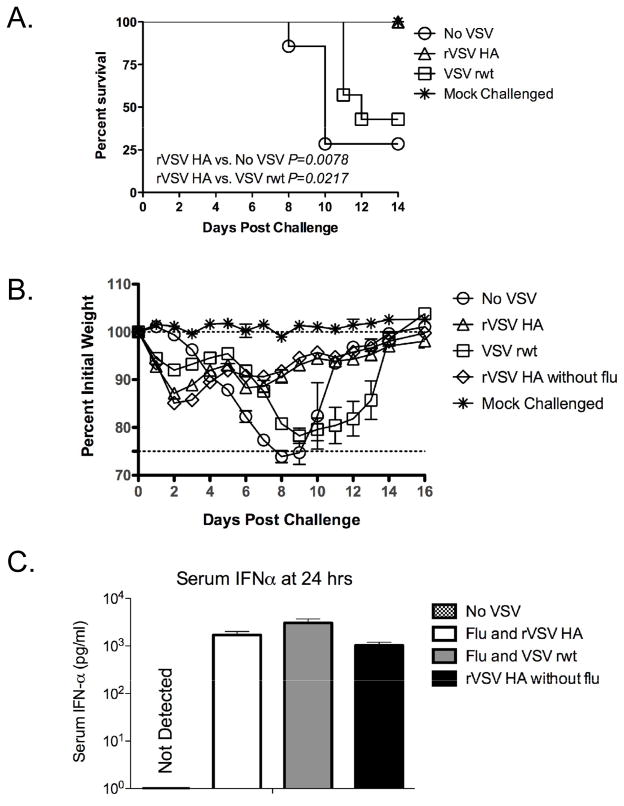
To confirm the results of the first study, and to begin to elucidate the mechanism(s) responsible for protection elicited by vaccination, we challenged and immunized a second larger cohort of mice (n=30 per group) as shown in Figure 4. We also included a cohort of mice (n=5) which were immunized with rVSV HA alone, and were not challenged with influenza, to allow us to measure the immune responses to both viruses alone. Seven mice from each group were followed for 14 days to monitor weight loss and survival, and the remainder of each group was sacrificed to perform immune response assays. As shown in Figure 4A, all (7/7) rVSV HA vaccinated mice survived, and the rate of survival of mice immunized with rVSV HA was significantly higher than the rate of survival of mock-immunized mice (no VSV, P=0.0078, Mantel-Cox test) or of mice immunized with empty vector VSV rwt (VSV rwt, P=0.0217, Mantel Cox test). Three of the seven mice immunized with the empty vector VSV rwt survived, but as in the first experiment the rate of survival in VSV rwt immunized animals was not significantly different from that of mock-immunized mice (P=0.192, Mantel-Cox test). Weight loss of mice challenged with influenza and immunized with rVSV HA was similar to that of mice challenged with influenza and immunized with the empty vector until the seventh day after challenge, after which time mice immunized with the empty vector lost weight rapidly and mice immunized with rVSV HA recovered (Figure 4B). Mice immunized with rVSV HA but not challenged with influenza (n=5, open diamonds, Figure 4B) had an initial weight loss of approximately 10–15% (between days 1 and 2) which was not observed in mice not receiving VSV. This confirmed that initial weight loss was due to VSV and not influenza pathogenesis. Interferon-α (IFN-α) reduces pathology during acute influenza infection in humans and some laboratory animals, when produced naturally as a result of viral infection, or provided exogenously [32–34]. The role of IFN-α in protection of inbred mice from influenza is indirect, since many mouse strains (including Balb/C) express a non-functional allele of the interferon responsive gene Mx [35]. Functional Mx directly inhibits transcription of the influenza genome, reducing viral titers in Mx-intact mice [36]. In Mx-deficient inbred mice, IFN-α by moderates the severe pulmonary immunopathology resulting from influenza infection, and promotes antibody class-switching to IgG2a isotype [37]. To determine whether rVSV immunization induced IFN-α production in immunized mice we collected serum from vaccinees (4 mice per group) at 12, 24, and 48 hours after challenge/vaccination and measured the amount of IFN-α by ELISA. We also measured IFN-α in the bronchoalveolar lavage (BAL) fluid and in whole lung homogenates at 24 hours after challenge/vaccination. No IFN-α was detected in the BAL or lung homogenates in any of the challenged/vaccinated mice. In the serum, IFN-α was detected by 12 hours after challenge in all rVSV vaccinated mice (data not shown), peaked at 24 hours, and was no longer detectable by 48 hours after challenge. As shown in Figure 4C, IFN-α was detected in the serum of mice immunized with rVSV HA or with VSV rwt, but not in the serum of mice challenged with influenza and immunized with diluent alone (No VSV group, Figure 4C). The amount of IFN-α in the serum of mice immunized with rVSV HA was not significantly different than the amount of IFN-α in the serum of mice immunized with the empty vector VSV rwt (P=0.0558, Student t test). This result supported the idea that both rVSV vectors induced non-specific antiviral responses which were able to protect vaccinees until the seventh day after challenge/immunization. The amount of IFN-α in the serum of mice immunized with the rVSV HA vector alone, in the absence of flu challenge (rVSV HA only group, Figure 4C) was not significantly different to that induced by rVSV HA in the presence of flu, which further supported the idea that IFN-α was produced in response to the rVSV rather than influenza.
Figure 4. Immunization with rVSV induces IFN-α production.
Adult female Balb/C mice were challenged intranasally with 100MLD50 PR8. Immediately (within 1–2 minutes) after influenza challenge, mice were either intramuscularly sham-inoculated with diluent alone (n=7), immunized intramuscularly with 5×108 PFU rVSV empty vector (VSV rwt, n=7), or immunized intramuscularly with 5×108 PFU rVSV HA (n=7). Five additional mice were mock-challenged with PBS alone and then immunized intramuscularly with 5×108 PFU rVSV HA. Mice were monitored for survival (Panel A) and weight loss (Panel B), and induction of IFN-α in the serum (Panel C). Mice were humanely euthanized when their body weight reached 75% of pre-infection body weight for two consecutive days. Mice challenged with flu and immunized with rVSV HA had a significantly higher rate of survival than mice receiving no VSV (P=0.0078, Mantel-Cox test) or vaccinated with the empty vector VSV rwt (P=0.0217, Mantel-Cox test). Graph in Panel C shows concentration of IFN-α in pg/ml in the individually assayed 24 hr timepoint sera of vaccinated and control mice (n=4–5 per group) as measured by ELISA. The amount of IFN-α induced in mice immunized with the empty vector VSV rwt was not significantly different than the amount of IFN-α induced in mice immunized with the specific vector rVSV HA.
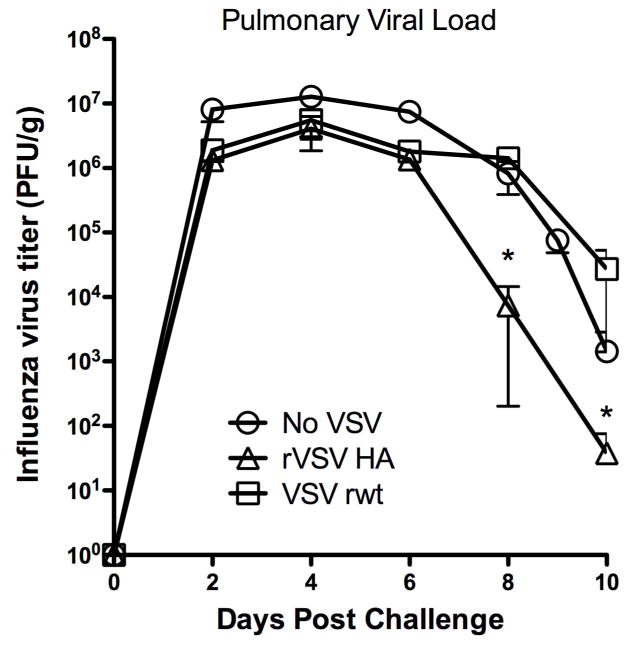
3.4. Pulmonary viral loads of rVSV HA vaccinated animals are reduced in rVSV HA immunized mice
Using the same cohorts of mice shown in Figure 4, we performed the assay shown in Figure 5 to begin to determine the mechanism of vaccine action. We sacrificed four mice per group per day at the timepoints shown in Figure 5 and determined the titer of influenza and VSV (where applicable) by plaque assay on MDCK and BHK-21 cells respectively. No infectious VSV was recovered from the lung of any VSV-immunized animal at any timepoint tested (day 2- day 10 post challenge). This demonstrated that rVSV delivered intramuscularly did not disseminate to the lung and meant that the VSV vaccine viruses probably did not interfere directly with the ability of influenza to infect host cells. Pulmonary influenza titers peaked in all mice by four days after challenge (Figure 5). Viral loads of mice immunized with either of the rVSV vectors were similar to the viral loads of mice receiving no VSV for the first six days after challenge, suggesting that the non-specific immune responses induced by rVSV immunization in the first six days after challenge did not directly limit viral replication. The pulmonary viral loads of mice immunized with rVSV HA and VSV rwt were not significantly different until the sixth day after challenge, after which time the viral loads of rVSV HA immunized mice declined rapidly while the viral loads of VSV rwt immunized mice remained significantly higher. Specifically, beginning on the eighth day after challenge (indicated by asterisks on the graph, Figure 5) the average pulmonary viral load of mice vaccinated with rVSV HA was significantly lower than the average viral load of control mice vaccinated with the empty vector VSV rwt (P<0.05 via one-way ANOVA with Tukey multiple comparison test). This result was consistent with the idea that non-specific responses provided equal protection to mice immunized with the specific and empty vector until the sixth day after challenge, and suggested that the specific response required for vaccine efficacy from the seventh day onward directly lowered the pulmonary viral load (induction of CTL, e.g.) of mice vaccinated with rVSV HA.
Figure 5. Pulmonary viral loads are reduced in mice vaccinated with rVSV HA.
Adult female Balb/C mice were challenged intranasally with 100MLD50 PR8. Immediately (within 1–2 minutes) after influenza challenge, were either intramuscularly sham-inoculated with diluent alone, immunized intramuscularly with 5×108 PFU rVSV empty vector, or immunized intramuscularly with 5×108 PFU rVSV HA. At the timepoints shown four mice per group were sacrificed and the amount of influenza and/or rVSV in the lung determined by plaque assay. No infectious VSV was detected in the lungs of any mice at any time. Graph shows average pulmonary viral load (influenza) for each group in PFU/g. Beginning on the eighth day after challenge (indicated by asterisk on the graph) the average pulmonary viral load of mice vaccinated with rVSV HA was significantly lower than the average viral load of control mice vaccinated with the empty vector VSV rwt (P<0.05 via one-way ANOVA with Tukey multiple comparison test).
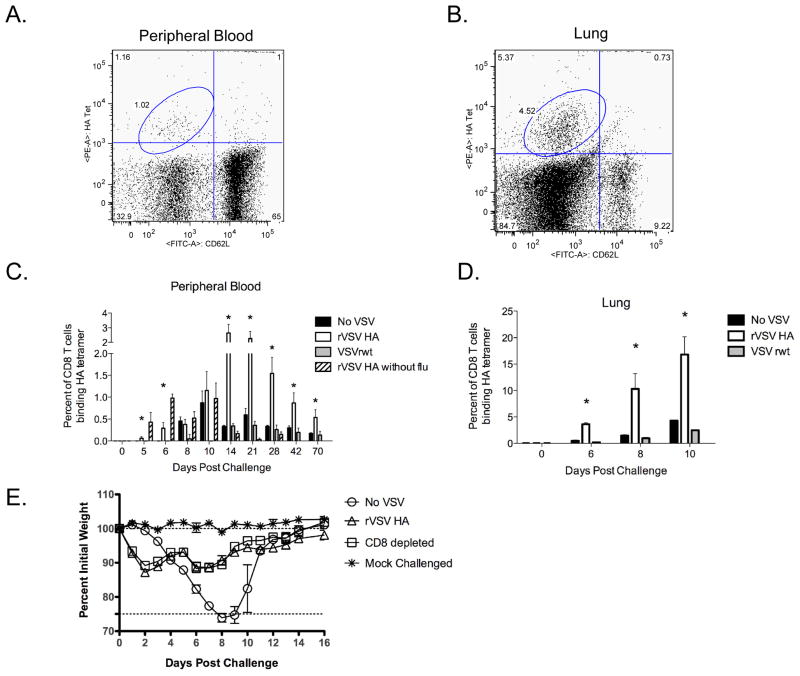
3.5. CD8 T cells recognizing influenza HA are present two days earlier in rVSV HA immunized mice
When mice are intranasally infected with influenza CD8 T cells are primed and activated in the mediastinal lymph node 3–4 days later [38]. By 5–7 days after infection antigen-specific CD8 T cells are present in the lung and airway epithelium [39] where they facilitate viral clearance via perforin and granzyme mediated killing of infected epithelial cells [40]. To determine whether the kinetics or magnitude of CD8 T cell induction differed between vaccinated and non-vaccinated mice in our system we used an MHC Class I tetramer recognizing the H-2Kd restricted epitope HA533–541 (N-IYSTVASSL-C) to quantitate HA-specific CD8 T cells in the peripheral blood and lung of vaccinated and control mice. Representative FACS plots from the blood and lung of flu challenged mice are shown in Figure 6A and 6B respectively. In mice challenged with influenza and immunized with rVSV HA, we detected HA-specific CD8 T cells in the blood three days earlier (day 5 vs. day 8, Figure 6C) in rVSV HA vaccinated mice vs. sham-vaccinated or VSV rwt vaccinated animals. When we measured anti-HA T cell responses in the lung we observed a similar pattern, with rVSV HA immunized mice having significantly greater percentages of HA-specific CD8 T cells than sham inoculated mice or mice vaccinated with the empty vector VSV rwt at all time points tested (P<0.05 for all comparisons via one-way ANOVA with Tukey multiple comparison test, Figure 6D). In the peripheral blood the induction of anti-HA CD8 T cells was synergistic in mice challenged with influenza and immunized with rVSV HA, with CD8 T cell responses in rVSV HA vaccinated animals being significantly greater from day 14 after challenge/vaccination onward compared to that of mice challenged with influenza or vaccinated with the rVSV HA alone. The induction of anti-HA CD8 T cells immediately preceded recovery from challenge in vaccinees. To test whether CD8 T cells were necessary for vaccine mediated protection we used a monoclonal antibody reactive against murine CD8α to deplete a cohort of vaccinees (n=10, open squares, Figure 6E) of CD8 T cells prior to challenge/vaccination with rVSV HA. The depletion protocol resulted in complete (>95%) removal of CD8 T cells from the lung, spleen, and peripheral blood which was confirmed by flow cytometry on day 0 and day 14 of the experiment (data not shown). All CD8-depleted mice survived challenge, and weight loss in CD8 depleted animals vaccinated with rVSV HA was not significantly different than weight loss in CD8-intact animals vaccinated with rVSV HA. This demonstrated that CD8 T cells were not necessary for vaccine mediated protection in this challenge and vaccination model, although it remains possible that CD8 T cells could enhance protection against heterologous challenge.
Figure 6. CD8 T cell responses induced by rVSV HA vaccination are earlier and of greater magnitude.
Adult female Balb/C mice were challenged intranasally with 100MLD50 PR8. Immediately (within 1–2 minutes) after influenza challenge, mice were either intramuscularly sham-inoculated with diluent alone (n=7), immunized intramuscularly with 5×108 PFU rVSV empty vector (VSVrwt, n=7), or immunized intramuscularly with 5×108 PFU rVSV HA (n=7). Five additional mice were mock-challenged with PBS alone and then immunized intramuscularly with 5×108 PFU rVSV HA. Panels A and B show representative FACS plots of tetramer staining of lymphocytes isolated from the peripheral blood (Panel A) or whole lung (Panel B) of challenged and rVSV HA vaccinated mice isolated on the sixth day after immunization/challenge. Panels C and D show a timecourse of anti-HA CD8 T cell responses in the peripheral blood (Panel C) and whole lung (Panel D) of vaccinated animals (n=at least 4 mice per group per day analyzed individually). Bars on the graph represent average percent of CD8 T cells binding an MHC Class I tetramer specific for the Kd restricted HA533–541 epitope ±SEM for each immunization group. Asterisks above the bars represent days on which the mice challenged with influenza and vaccinated with rVSV HA had significantly more anti-HA specific CD8 T cells than mice challenged with influenza and vaccinated with the empty vector or challenged with influenza and sham vaccinated with diluent alone (P<0.05 via ANOVA with Tukey multiple comparison test). Panel E shows the outcome of challenge vaccination in mice depleted of CD8 T cells (n=10, open squares). All CD8 T cell depleted mice survived, and the course of weight loss was not significantly different than that observed in CD8 T cell intact mice. This experiment (CD8 depletion) has been performed three times with consistent results.
3.6. Serum neutralizing antibody against influenza is generated two days earlier in rVSV HA vaccinated versus control animals
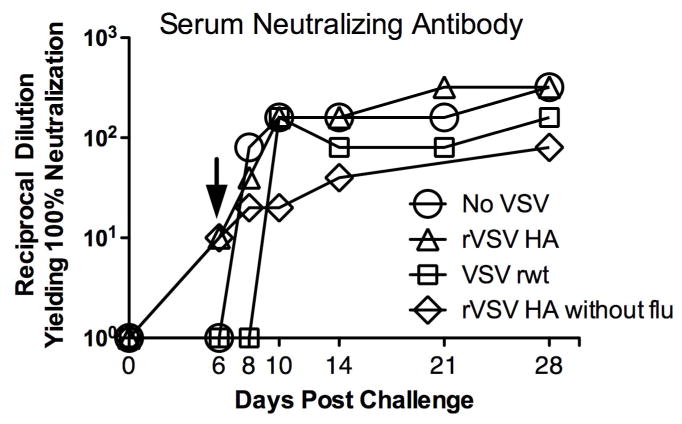
Neutralizing antibody against influenza prevents attachment of the viral HA to the host cell, and the induction of neutralizing antibody is a major correlate of protection against human influenza infection. To determine whether immunization with rVSV HA was generating anti-influenza neutralizing antibody in vaccinated mice we collected serum from rVSV HA vaccinated and control animals over a timecourse and detected neutralizing antibodies against the PR8 challenge virus via microneutralization assay. We collected serum from 3 animals per group on days 4,6, 8, and 10 after challenge/vaccination. As shown in Figure 7, mice challenged with influenza and vaccinated with rVSV HA (open triangles) developed anti-influenza neutralizing antibodies by the sixth day after challenge/immunization. Mice vaccinated with rVSV HA in the absence of flu challenge (Figure 7, open diamonds) also developed neutralizing antibody by six days after immunization, which demonstrated that co-infection with flu did not positively or negatively affect the speed with which serum neutralizing antibody was induced by the rVSV HA. Sham-immunized (No VSV) and empty vector immunized mice did not have detectable serum neutralizing antibody until day 8 and 10 (respectively) after challenge. This result was consistent with the decline in viral titers beginning on day 8, in mice immunized with rVSV HA, and suggested that the induction of neutralizing antibodies may have contributed to protection. In surviving animals neutralizing titers increased during the recovery phase of infection to average titers of 1:80 by three months after challenge (data not shown). These results were consistent with the finding that viral loads were reduced in rVSV HA vaccinated versus control mice, and suggested that since rVSV HA vaccinated animals developed neutralizing antibody more quickly than mock or empty vector immunized mice, it is likely that the induction of serum neutralizing Ab contributed to protection induced by rVSV HA vaccination.
Figure 7. Mice immunized with rVSV HA develop serum neutralizing antibody against influenza two days before control animals.
Adult female Balb/C mice were challenged intranasally with 100MLD50 PR8. Immediately (within 1–2 minutes) after influenza challenge, mice were either intramuscularly sham-inoculated with diluent alone (n=7), immunized intramuscularly with 5×108 PFU rVSV empty vector (VSV rwt, n=7), or immunized intramuscularly with 5×108 PFU rVSV HA (n=7). Five additional mice were mock-challenged with PBS alone and then immunized intramuscularly with 5×108 PFU rVSV HA. Graph shows serum neutralizing titers measured by microneutralization assay using pooled serum samples from at least 4 vaccinated or control animals for each timepoint. At day six after challenge only mice vaccinated with rVSV HA had detectable titers (indicated by arrow on graph). Data shown is from a single experiment which has been performed a total of three times with consistent results.
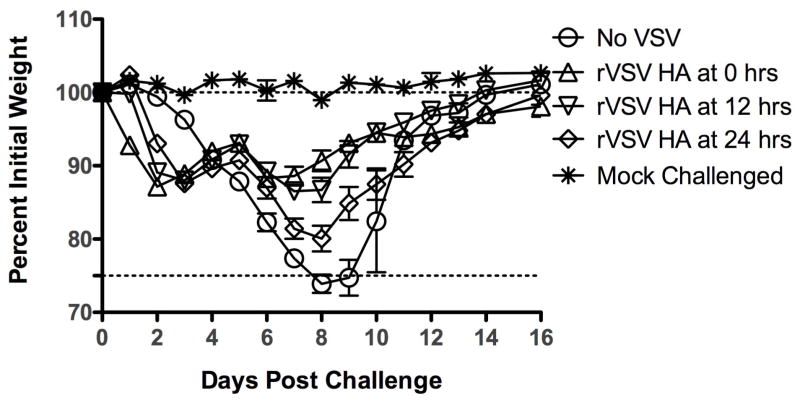
3.7. Mice immunized with rVSV HA by at least 24 hours after challenge are fully protected from mortality
To determine how long after challenge we could delay rVSV HA immunization and retain protection we performed the experiment shown in Figure 8. As in the first experiment, Balb/C mice (n=7–10 mice per group) were challenged with 100MLD50 PR8 influenza in a total volume of 40μl. Mice were then immunized immediately with either rVSV HA or with VSV rwt (n=7 per group) as in Figure 3. We included additional groups (n=10 per group) of mice which were immunized with rVSV HA at either 12 or 24 hours after challenge. All mice immunized with rVSV HA at 0, 12, or 24 hours after challenge survived, although weight loss in the mice immunized at 24 hours was more severe and prolonged than in mice receiving the vaccine at earlier timepoints (Figure 8). This demonstrated that rVSV HA vaccination could be delayed by up to 24 hours after challenge and retain efficacy.
Figure 8. Mice immunized with rVSV HA by at least 24 hours after influenza challenge are fully protected from mortality.
Adult female Balb/C mice were challenged intranasally with 100MLD50 PR8. At the indicated time after influenza challenge, mice were either intramuscularly sham-inoculated with diluent alone (n=7), immunized intramuscularly with 5×108 PFU rVSV empty vector (VSV rwt, n=7), or immunized intramuscularly with 5×108 PFU rVSV HA (n=7). All mice immunized with rVSV HA at 12 hours (n=10, inverted triangles) and 24 hours (n=10, open diamonds) after challenge survived.
4. DISCUSSION
We undertook this study to determine whether immunization with rVSV HA could protect mice from lethal influenza challenge when the vaccine virus was delivered systemically after lethal intranasal influenza challenge. We demonstrated that 100% of mice immunized with the rVSV HA specific vector, and up to 40% of mice receiving the control empty vector VSV rwt were protected from mortality. Administration of the rVSV HA vector could be delayed for up to 24 hours after challenge with full retention of protection, showing that post-exposure vaccination against influenza was feasible and potentially translatable to use in emergent situations. The main question arising from these results is what the mechanism(s) of vaccine induced protection were. Up to 40% of mice immunized with the empty vector survived challenge, which suggested that protection was partially mediated by non-specific factors. However, the rate of survival was significantly higher in mice vaccinated with the specific vector (rVSV HA, 100% survival), which showed that specific responses were required for complete and optimal protection. With this in mind, several mechanisms of vaccine action can be considered, either alone or in combination. First, the rVSV vaccine virus could have directly interfered with the ability of influenza to infect host cells. Our data did not support this possibility for two reasons. First, rVSV HA and VSV rwt would be expected to compete equally well with influenza for host cell binding, since both VSV viruses infect via the VSV G attachment protein. Mice immunized with VSV rwt had a significantly lower rate of survival than rVSV HA immunized mice, which demonstrated that the mechanism of protection was not induced equally by the two rVSVs. Second, the influenza challenge virus was delivered intranasally, and the rVSV viruses were delivered intramuscularly at a distal site (rear quadriceps). When we titered infectious virus from lung homogenates of co-infected/vaccinated mice, we confirmed that no infectious VSV was present in the lung of mice challenged with influenza and immunized with either rVSV HA or VSV rwt. This showed that VSV did not disseminate to the lung of vaccinated mice, and since influenza virus was restricted to the lung in our model, it was therefore not likely that the VSV vaccine viruses interfered directly with the ability of influenza to infect host cells. Consistent with these results, viral loads of control and rVSV vaccinated mice did not differ substantially at the earliest timepoints examined (days 2–4 after challenge) which suggested that the influenza virus was able to replicate equally well in vaccinated and control mice until the adaptive response was mounted, and added further evidence that direct viral interference was not an important mechanism of protection in our model.
A second possible mechanism of vaccine action was that the rVSV vaccine could have induced non-specific innate antiviral responses which promoted the induction of adaptive responses and/or inhibited the ability of influenza to replicate and/or cause pathology. Non-specific innate immune responses theoretically would be induced equally by the rVSV HA specific vector and the VSV rwt empty vector. The induction of type-I IFNs such as IFN-α/β is part of the earliest innate host response to viral infection. Because the Balb/c mice used in these studies are naturally deficient in the IFN-inducible gene Mx [35, 36], which inhibits primary transcription of the influenza A virus genome [41], the role of IFN-α in our model is likely to be indirect, rather than direct. Consistent with this idea, we did not detect IFN-α in the lung of vaccinated/challenged mice, and viral loads were not significantly lower in rVSV versus control mice during the time that IFN was detected. These results supported the idea that the IFN-α induced by VSV did not inhibit influenza virus replication directly but rather that systemic IFN-α enhanced the development of the adaptive immune responses and/or moderated immunopathology induced by influenza infection. In support of this idea, it has been reported that provision of exogenous IFN-α can protect inbred Mx-deficient mice from influenza infection, even when delivered parenterally [32]. Stat1 −/− mice, which are unable to respond to IFN-α, are significantly more susceptible to influenza infection than wild type control mice [42]. Stat1 −/− mice control influenza virus replication as well as wild type mice, but have uncontrolled pulmonary immunopathology and reduced anti-influenza humoral responses which lead to a higher rate of mortality relative Stat-intact animals [37]. Finally, it has been reported that the IFN-α/β induced by rVSV infection significantly augments the development of antiviral neutralizing antibody by plasma cells expressing the IFN-α/β receptor (IFNAR) [43]. Directly confirming that IFN-α contributes to protection in our model will not be straightforward, since mice that are unable to respond to interferon (IFNAR−/−) are highly susceptible to low dose VSV infection [44] and would undoubtedly be killed by the high doses of rVSV vaccine viruses used in our studies. Nonetheless, the observation that mice immunized with rVSV HA had more rapid induction of neutralizing antibody than control animals, and that mice immunized with either rVSV HA or VSV rwt had significantly less morbidity (weight loss, temperature depression) than control mice during the first six days after infection are consistent with the idea that innate immune responses made by both groups were superior to those mounted by control mice during the early phase of infection. It is very likely that multiple cytokines were induced by rVSV immunization (TNF-α and IL-1β e.g.) and contributed to protection in our model. Further studies to define the specific cytokine responses induced by rVSV vaccination and which are required for vaccine-mediated protection are necessary.
It was also possible that rVSV vectors could have induced specific cellular or humoral responses against influenza which “outcompeted” the influenza virus. Our results showed a clear temporal separation in adaptive immune responses induced by influenza infection alone (No VSV mock vaccinated control mice) versus those induced by rVSV HA vaccination in the presence or absence of influenza challenge. Mice challenged with influenza and vaccinated with rVSV HA developed anti-HA CD8 T cells two to three days earlier (in the peripheral blood), and had anti-HA CD8 T cell responses of significantly greater magnitude in the lung, than did control mock-vaccinated mice, or those vaccinated with the empty vector. Mice receiving rVSV HA alone (without flu) also had detectable anti-HA T cells in the peripheral blood by 5 days after immunization. These results demonstrated that vaccination with rVSV HA resulted in more rapid induction of anti-HA CD8 T cells, and that the presence of influenza did not affect (either positively or negatively) the speed with which the T cells were induced in response to rVSV HA, although the magnitude of the response was slightly reduced in co-infected mice in the early stage of infection, versus those immunized with rVSV HA in the absence of flu. Despite the vigorous induction of HA-specific CD8 T cells in the lung and periphery of rVSV HA vaccinated mice, depletion of CD8 T cells in these animals did not increase morbidity, delay recovery, or reduce survival. This demonstrated that CD8 T cells were not essential to protection in our model, although it does not preclude the possibility that CD8 T cells (especially those directed against conserved antigens) might contribute to protection against heterologous challenge viruses. Interestingly, the induction of anti-HA CD8 T cells in mice challenged with influenza and vaccinated with rVSV HA was markedly synergistic from two weeks after challenge onward (through day 70 after challenge), with co-infected mice having responses of significantly higher magnitude than mice infected with either virus alone. The synergistic effect was not present in mice challenged with flu and immunized with the rVSV empty vector, suggesting that the synergy was not due simply to a non-specific “adjuvant” effect of VSV. Dissecting the reason for the observed synergy and determining the effect of co-infection with rVSV HA and flu on long term memory T cell responses will be an important area for further investigation. Mice challenged with influenza and vaccinated with rVSV HA had detectable serum neutralizing antibody against influenza two days earlier (Day 6 vs. Day 8) than control mice. Neutralizing antibody correlates with protection from influenza in humans and animals, and is assumed to be the primary mechanism by which existing influenza vaccines mediate protection in human vaccinees. We predict that the induction of neutralizing antibody is crucial to protection by our vaccine, and further studies in which B cells are depleted at specific timepoints after vaccination/challenge will be required to confirm that hypothesis. Our data do not exclude the possibility that vaccination with rVSV HA or VSV rwt altered the overall pulmonary or systemic cytokine milieu in challenged and vaccinated animals. The elaboration of inflammatory cytokines is a major source of pathology in influenza infection, and it is possible that the presence of the vaccine virus reduced or altered the inflammatory response in a beneficial manner. Detailed analysis of the pulmonary and systemic cytokine response to rVSV immunization in the context of pre-existing flu infection is an important future direction.
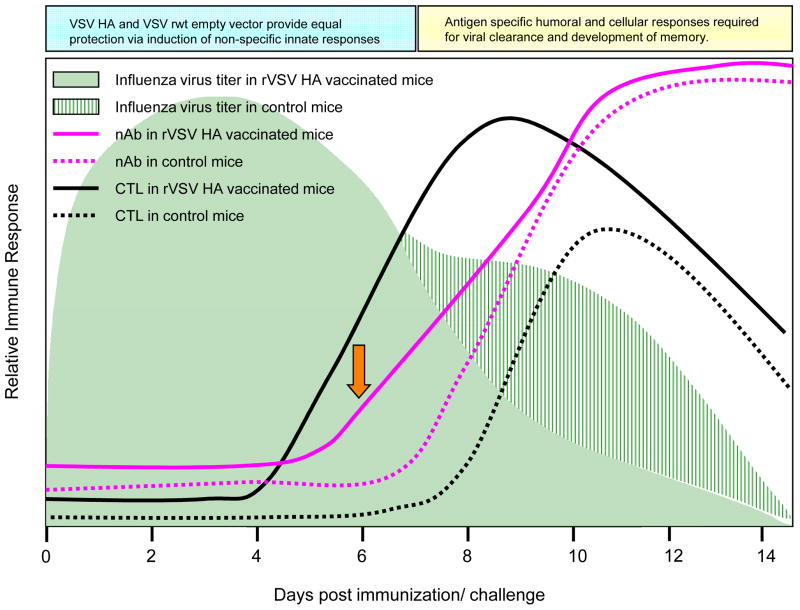
Our results are consistent with a model (diagrammed in Figure 9) in which both rVSV HA and VSV rwt rapidly induce non-specific antiviral responses which protect vaccinees equally until approximately day 6–7 after challenge. We predict that the reason mice receiving the empty vector are not equally protected from day 6–7 after challenge onward is because they do not make antigen specific responses to influenza. Our data further suggest that neutralizing antibody reduces pulmonary viral loads in mice receiving the rVSV HA, and that the clearance of influenza virus infected cells may be aided by, but is not absolutely dependent upon, the action of HA-specific CTL. It will be important to establish, in future studies, the maximum length of time that rVSV HA vaccination can be delayed while retaining full protection. In these studies, when the rVSV vaccine was delivered immediately after challenge the mock immunized control mice died between days 8–10 after challenge. In rVSV PR8 HA vaccinated mice we detected CD8 T cell responses at Day 5 and neutralizing antibody responses at Day 6 after challenge. If delaying the rVSV immunization delays development of immune responses proportionally, then we do not anticipate being able to delay rVSV PR8 HA immunization by more than 2 days after challenge to retain protection.
Figure 9. Proposed Model for rVSV HA induced protection.
Model shows relative induction of adaptive immune responses in rVSV HA versus control mice (immunized with VSV rwt, and those receiving no VSV immunization). Orange arrow highlights earlier induction of serum neutralizing Ab in rVSV HA immunized mice.
In summary, these results demonstrate that vaccination with rVSV HA can protect mice from lethal influenza challenge when the vaccine virus is delivered systemically up to 24 hours after challenge. Further experiments to precisely define the mechanism(s), both specific and non-specific, which contribute to vaccine efficacy are necessary, and will further not only optimization of the vaccine design, but our understanding of the fine differences between the host response to viral antigens in their natural context, versus the response to a viral vectored vaccine. It will also be important to compare, in “head to head” studies, the efficacy of existing influenza vaccines, used therapeutically, to our rVSV HA vaccine used therapeutically. Finally, the addition of rVSV expressing conserved influenza antigens to our vaccine may broaden efficacy such that protection against highly pathogenic avian or other and newly emerging influenza strains could be achieved.
Acknowledgments
This work was supported by NIH grant number AI51445. Work was conducted in the Global Health Research Building at Duke University which receives support from grant number UC6 AI058607.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Massoudi MS, Barker L, Schwartz B. Effectiveness of postexposure vaccination for the prevention of smallpox: results of a delphi analysis. J Infect Dis. 2003;188(7):973–6. doi: 10.1086/378357. [DOI] [PubMed] [Google Scholar]
- 2.Rupprecht CE, Gibbons RV. Clinical practice. Prophylaxis against rabies. N Engl J Med. 2004;351(25):2626–35. doi: 10.1056/NEJMcp042140. [DOI] [PubMed] [Google Scholar]
- 3.Yu AS, Cheung RC, Keeffe EB. Hepatitis B vaccines. Clin Liver Dis. 2004;8(2):283–300. doi: 10.1016/j.cld.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Ohmit SE, Victor JC, Teich ER, Truscon RK, Rotthoff JR, Newton DW, et al. Prevention of symptomatic seasonal influenza in 2005–2006 by inactivated and live attenuated vaccines. J Infect Dis. 2008;198(3):312–7. doi: 10.1086/589885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clements ML, Murphy BR. Development and persistence of local and systemic antibody responses in adults given live attenuated or inactivated influenza A virus vaccine. J Clin Microbiol. 1986;23(1):66–72. doi: 10.1128/jcm.23.1.66-72.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuen KY, Chan PK, Peiris M, Tsang DN, Que TL, Shortridge KF, et al. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet. 1998;351(9101):467–71. doi: 10.1016/s0140-6736(98)01182-9. [DOI] [PubMed] [Google Scholar]
- 7.Beigel JH, Farrar J, Han AM, Hayden FG, Hyer R, de Jong MD, et al. Avian influenza A (H5N1) infection in humans. N Engl J Med. 2005;353(13):1374–85. doi: 10.1056/NEJMra052211. [DOI] [PubMed] [Google Scholar]
- 8.Buxton Bridges C, Katz JM, Seto WH, Chan PK, Tsang D, Ho W, et al. Risk of influenza A (H5N1) infection among health care workers exposed to patients with influenza A (H5N1), Hong Kong. J Infect Dis. 2000;181(1):344–8. doi: 10.1086/315213. [DOI] [PubMed] [Google Scholar]
- 9.Katz JM, Lim W, Bridges CB, Rowe T, Hu-Primmer J, Lu X, et al. Antibody response in individuals infected with avian influenza A (H5N1) viruses and detection of anti-H5 antibody among household and social contacts. J Infect Dis. 1999;180(6):1763–70. doi: 10.1086/315137. [DOI] [PubMed] [Google Scholar]
- 10.Ungchusak K, Auewarakul P, Dowell SF, Kitphati R, Auwanit W, Puthavathana P, et al. Probable person-to-person transmission of avian influenza A (H5N1) N Engl J Med. 2005;352(4):333–40. doi: 10.1056/NEJMoa044021. [DOI] [PubMed] [Google Scholar]
- 11.Cohen J. Swine flu outbreak. Past pandemics provide mixed clues to H1N1’s next moves. Science. 2009;324(5930):996–7. doi: 10.1126/science.324_996. [DOI] [PubMed] [Google Scholar]
- 12.Human infection with new influenza A (H1N1) virus: clinical observations from Mexico and other affected countries, May 2009. Wkly Epidemiol Rec. 2009;84(21):185–9. [PubMed] [Google Scholar]
- 13.Greenberg ME, Lai MH, Hartel GF, Wichems CH, Gittleson C, Bennet J, et al. Response after One Dose of a Monovalent Influenza A (H1N1) 2009 Vaccine -- Preliminary Report. N Engl J Med. 2009 doi: 10.1056/NEJMoa0907413. [DOI] [PubMed] [Google Scholar]
- 14.Clark TW, Pareek M, Hoschler K, Dillon H, Nicholson KG, Groth N, et al. Trial of Influenza A (H1N1) 2009 Monovalent MF59-Adjuvanted Vaccine -- Preliminary Report. N Engl J Med. 2009 doi: 10.1056/NEJMoa0907650. [DOI] [PubMed] [Google Scholar]
- 15.Kapadia SU, Rose JK, Lamirande E, Vogel L, Subbarao K, Roberts A. Long-term protection from SARS coronavirus infection conferred by a single immunization with an attenuated VSV-based vaccine. Virology. 2005;340(2):174–82. doi: 10.1016/j.virol.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahn JS, Roberts A, Weibel C, Buonocore L, Rose JK. Replication-competent or attenuated, nonpropagating vesicular stomatitis viruses expressing respiratory syncytial virus (RSV) antigens protect mice against RSV challenge. J Virol. 2001;75(22):11079–87. doi: 10.1128/JVI.75.22.11079-11087.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Natuk RJ, Cooper D, Guo M, Calderon P, Wright KJ, Nasar F, et al. Recombinant vesicular stomatitis virus vectors expressing herpes simplex virus type 2 gD elicit robust CD4+ Th1 immune responses and are protective in mouse and guinea pig models of vaginal challenge. J Virol. 2006;80(9):4447–57. doi: 10.1128/JVI.80.9.4447-4457.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramsburg E, Rose NF, Marx PA, Mefford M, Nixon DF, Moretto WJ, et al. Highly effective control of an AIDS virus challenge in macaques by using vesicular stomatitis virus and modified vaccinia virus Ankara vaccine vectors in a single-boost protocol. J Virol. 2004;78(8):3930–40. doi: 10.1128/JVI.78.8.3930-3940.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rose NF, Marx PA, Luckay A, Nixon DF, Moretto WJ, Donahoe SM, et al. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell. 2001;106(5):539–49. doi: 10.1016/s0092-8674(01)00482-2. [DOI] [PubMed] [Google Scholar]
- 20.Barefoot BE, Sample CJ, Ramsburg EA. A vesicular stomatitis virus recombinant expressing influenza nucleoprotein induces CD8 T cell responses which enhance antibody mediated protection after lethal influenza challenge. Clin Vaccine Immunol. 2009 doi: 10.1128/CVI.00451-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daddario-DiCaprio KM, Geisbert TW, Geisbert JB, Stroher U, Hensley LE, Grolla A, et al. Cross-protection against Marburg virus strains by using a live, attenuated recombinant vaccine. J Virol. 2006;80(19):9659–66. doi: 10.1128/JVI.00959-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daddario-DiCaprio KM, Geisbert TW, Stroher U, Geisbert JB, Grolla A, Fritz EA, et al. Postexposure protection against Marburg haemorrhagic fever with recombinant vesicular stomatitis virus vectors in non-human primates: an efficacy assessment. Lancet. 2006;367(9520):1399–404. doi: 10.1016/S0140-6736(06)68546-2. [DOI] [PubMed] [Google Scholar]
- 23.Feldmann H, Jones SM, Daddario-DiCaprio KM, Geisbert JB, Stroher U, Grolla A, et al. Effective post-exposure treatment of Ebola infection. PLoS Pathog. 2007;3(1):e2. doi: 10.1371/journal.ppat.0030002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geisbert TW, Daddario-DiCaprio KM, Williams KJ, Geisbert JB, Leung A, Feldmann F, et al. Recombinant vesicular stomatitis virus vector mediates postexposure protection against Sudan Ebola hemorrhagic fever in nonhuman primates. J Virol. 2008;82(11):5664–8. doi: 10.1128/JVI.00456-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawson ND, Stillman EA, Whitt MA, Rose JK. Recombinant vesicular stomatitis viruses from DNA. Proc Natl Acad Sci U S A. 1995;92(10):4477–81. doi: 10.1073/pnas.92.10.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haglund K, Leiner I, Kerksiek K, Buonocore L, Pamer E, Rose JK. High-level primary CD8(+) T-cell response to human immunodeficiency virus type 1 gag and env generated by vaccination with recombinant vesicular stomatitis viruses. J Virol. 2002;76(6):2730–8. doi: 10.1128/JVI.76.6.2730-2738.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suguitan AL, Jr, McAuliffe J, Mills KL, Jin H, Duke G, Lu B, et al. Live, attenuated influenza A H5N1 candidate vaccines provide broad cross-protection in mice and ferrets. PLoS Med. 2006;3(9):e360. doi: 10.1371/journal.pmed.0030360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schnell MJ, Buonocore L, Kretzschmar E, Johnson E, Rose JK. Foreign glycoproteins expressed from recombinant vesicular stomatitis viruses are incorporated efficiently into virus particles. Proc Natl Acad Sci U S A. 1996;93(21):11359–65. doi: 10.1073/pnas.93.21.11359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramsburg E, Publicover J, Buonocore L, Poholek A, Robek M, Palin A, et al. A vesicular stomatitis virus recombinant expressing granulocyte-macrophage colony-stimulating factor induces enhanced T-cell responses and is highly attenuated for replication in animals. J Virol. 2005;79(24):15043–53. doi: 10.1128/JVI.79.24.15043-15053.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Publicover J, Ramsburg E, Robek M, Rose JK. Rapid pathogenesis induced by a vesicular stomatitis virus matrix protein mutant: viral pathogenesis is linked to induction of tumor necrosis factor alpha. J Virol. 2006;80(14):7028–36. doi: 10.1128/JVI.00478-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toth LA, Rehg JE, Webster RG. Strain differences in sleep and other pathophysiological sequelae of influenza virus infection in naive and immunized mice. J Neuroimmunol. 1995;58(1):89–99. doi: 10.1016/0165-5728(94)00193-r. [DOI] [PubMed] [Google Scholar]
- 32.Beilharz MW, Cummins JM, Bennett AL. Protection from lethal influenza virus challenge by oral type 1 interferon. Biochem Biophys Res Commun. 2007;355(3):740–4. doi: 10.1016/j.bbrc.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 33.Isomura S, Ichikawa T, Miyazu M, Naruse H, Shibata M, Imanishi J, et al. The preventive effect of human interferon-alpha on influenza infection; modification of clinical manifestations of influenza in children in a closed community. Biken J. 1982;25(3):131–7. [PubMed] [Google Scholar]
- 34.Van Hoeven N, Belser JA, Szretter KJ, Zeng H, Staeheli P, Swayne DE, et al. Pathogenesis of the 1918 pandemic and H5N1 influenza virus infection in a guinea pig model: The antiviral potential of exogenous alpha-interferon to reduce virus shedding. J Virol. 2009 doi: 10.1128/JVI.02174-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Staeheli P, Grob R, Meier E, Sutcliffe JG, Haller O. Influenza virus-susceptible mice carry Mx genes with a large deletion or a nonsense mutation. Mol Cell Biol. 1988;8(10):4518–23. doi: 10.1128/mcb.8.10.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Staeheli P, Haller O, Boll W, Lindenmann J, Weissmann C. Mx protein: constitutive expression in 3T3 cells transformed with cloned Mx cDNA confers selective resistance to influenza virus. Cell. 1986;44(1):147–58. doi: 10.1016/0092-8674(86)90493-9. [DOI] [PubMed] [Google Scholar]
- 37.Durbin JE, Fernandez-Sesma A, Lee CK, Rao TD, Frey AB, Moran TM, et al. Type I IFN modulates innate and specific antiviral immunity. J Immunol. 2000;164(8):4220–8. doi: 10.4049/jimmunol.164.8.4220. [DOI] [PubMed] [Google Scholar]
- 38.Lawrence CW, Braciale TJ. Activation, differentiation, and migration of naive virus-specific CD8+ T cells during pulmonary influenza virus infection. J Immunol. 2004;173(2):1209–18. doi: 10.4049/jimmunol.173.2.1209. [DOI] [PubMed] [Google Scholar]
- 39.Lawrence CW, Ream RM, Braciale TJ. Frequency, specificity, and sites of expansion of CD8+ T cells during primary pulmonary influenza virus infection. J Immunol. 2005;174(9):5332–40. doi: 10.4049/jimmunol.174.9.5332. [DOI] [PubMed] [Google Scholar]
- 40.Topham DJ, Tripp RA, Doherty PC. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol. 1997;159(11):5197–200. [PubMed] [Google Scholar]
- 41.Pavlovic J, Haller O, Staeheli P. Human and mouse Mx proteins inhibit different steps of the influenza virus multiplication cycle. J Virol. 1992;66(4):2564–9. doi: 10.1128/jvi.66.4.2564-2569.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia-Sastre A, Durbin RK, Zheng H, Palese P, Gertner R, Levy DE, et al. The role of interferon in influenza virus tissue tropism. J Virol. 1998;72(11):8550–8. doi: 10.1128/jvi.72.11.8550-8558.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fink K, Lang KS, Manjarrez-Orduno N, Junt T, Senn BM, Holdener M, et al. Early type I interferon-mediated signals on B cells specifically enhance antiviral humoral responses. Eur J Immunol. 2006;36(8):2094–105. doi: 10.1002/eji.200635993. [DOI] [PubMed] [Google Scholar]
- 44.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, et al. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264(5167):1918–21. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]