Abstract
Entorhinal cortex (ERC) volume in adults with mild cognitive impairment has been shown to predict prodromal Alzheimer's disease (AD). Likewise, neuronal loss in ERC has been associated with AD, but not with normal aging. Because ERC is part of a major pathway modulating input to the hippocampus, structural changes there may result in changes to cognitive performance and functional brain activity during memory tasks. In 32 cognitively intact older adults, we examined the relationship between left ERC thickness and functional magnetic resonance imaging (fMRI) activity during an associative verbal memory task. This task has been shown previously to activate regions that are sensitive to aging and AD risk. ERC was manually defined on native space, high resolution, oblique coronal MRI scans. Subjects having thicker left ERC showed greater activation in anterior cingulate and medial frontal regions during memory retrieval, but not encoding. This result was independent of hippocampal volume. Anterior cingulate cortex is directly connected to ERC, and is, along with medial frontal cortex, implicated in error detection, which is impaired in AD. Our results suggest that in healthy older adults, processes that engage frontal regions during memory retrieval are related to ERC structure. Hum Brain Mapp, 2009. © 2009 Wiley‐Liss, Inc.
Keywords: aging, medial temporal lobe, cingulate gyrus, cognition, frontal lobe, Alzheimer's disease
INTRODUCTION
Entorhinal cortex (ERC) plays a critical role in communications between the hippocampus and cortical association and limbic areas. As shown in studies of non‐human primates, ERC receives afferents from widespread cortical regions [Insausti et al., 1987]. It projects to other cortical regions, including anterior cingulate cortex (Brodmann areas (BAs) 24 and 32), orbitofrontal regions, and parts of the medial temporal lobe [Munoz and Insausti, 2005]. Additionally, because projections from layers II and III of ERC give rise to a major pathway into the hippocampus [Witter and Amaral, 1991], damage to this region may have a profound impact on memory [Gomez‐Isla et al., 1996].
Alzheimer's disease (AD), which ultimately results in severe memory and executive function deficits [American Psychiatric Association, 2000], is characterized by accumulations of amyloid plaques and neurofibrillary tangles in the brain [Braak and Braak, 1991]. These accumulations may occur in cognitively intact adults years before clinical symptoms of AD are evident [Bouras et al., 1994; Braak and Braak, 1991, 1997; Price and Morris, 1999]. By the time adults are between 56 and 60 years old, 67% have neurofibrillary tangles in at least the entorhinal/transentorhinal region of the brain [Braak and Braak, 1997], where the tangles are deposited first [Braak and Braak, 1995].
Many studies of neuronal loss have found ERC degeneration with AD, but not with normal aging [Fukutani et al., 2000; Giannakopoulos et al., 2003; Gomez‐Isla et al., 1996; Hof et al., 2003; Kordower et al., 2001; von Gunten et al., 2006]. Additionally, low ERC volume has been shown in a number of studies to be a good predictor of future AD onset or cognitive decline [deToledo‐Morrell et al., 2004; Dickerson et al., 2001; Killiany et al., 2002; Stoub et al., 2005]. In contrast, hippocampal volume decreases with age, and that decrease becomes greater with the onset of AD [Jack et al., 1998, 2000]. Despite the known relationship between these brain structures and memory, few studies have examined the relationship between structure size and fMRI activity during memory performance. The current study accomplishes this in older, cognitively intact adults by investigating the relationships between left ERC thickness, left hippocampal volume, and fMRI activity during a verbal paired associates task shown previously to activate regions sensitive to AD risk [Bookheimer et al., 2000]. We focused on left hemisphere structures because several prior studies have emphasized the role that left medial temporal lobe plays in performance of verbal memory tasks [Rosen et al., 2003; Tranel, 1991], including in early AD [deToledo‐Morrell et al., 2000; Eustache et al., 2001].
One study to date has examined the relationship between ERC structure and fMRI activation during memory encoding. Rosen et al. [ 2005] used an incidental memory encoding task in older adults, and found that larger ERC volumes were associated with greater fMRI activity in frontal lobe. Our study instead measured ERC cortical thickness, which may be a more sensitive measure of ERC integrity than volume is [Burggren et al., 2008], and is unrelated to head size, precluding a need to adjust for intracranial volume. Additionally, in the present study, we examined both memory encoding and retrieval, using a verbal task that required explicit associative memory encoding; in contrast, the previous study's task required incidental encoding of individual items [Rosen et al., 2005]. In normal aging and amnestic mild cognitive impairment, performance on tests of associative memory have been shown to be affected [Wolk et al., 2008], sometimes even more than single item recall [Naveh‐Benjamin, 2000; Troyer et al., 2008; Westerberg et al., 2006]. Therefore, exploring contributions to brain function during an associative memory task is important to understanding cognitive aging. Although some past studies suggest that the hippocampus mediates associative memory more than does the neighboring cortex in medial temporal lobe [Davachi and Wagner, 2002], the dichotomy of functions may not be complete. The hippocampus has been implicated in recognition and single‐item memory [Squire et al., 2007; Stark and Squire, 2001, 2003], while entorhinal and perirhinal cortices both have been shown to be recruited during tests of associative memory [Ekstrom et al., 2007; Jackson and Schacter, 2004; Kirwan and Stark, 2004; Klingberg et al., 1994]. In order to further explore the relative contributions of the hippocampus and ERC to functional brain activity during associative memory, we compared measures of their structural integrity with fMRI activity during a task that required the encoding and retrieval of verbal paired associates.
Past fMRI connectivity research collected during performance of a verbal recognition task indicated that, compared with younger adults, older adults showed a more closely related pattern of activity between rhinal regions and prefrontal cortex [Daselaar et al., 2006]. Those results suggested a compensatory reliance on the ERC‐frontal lobe network, possibly in response to age‐related deficits elsewhere in the brain. In keeping with previous research, we hypothesized that, for our associative memory task, thicker left ERC would be related to greater fMRI activity in frontal regions, specifically in medial frontal or orbitofrontal regions, which receive efferents from ERC [Munoz and Insausti, 2005]. This hypothesis is consistent with a model in which those having atrophied ERC are less able to engage ERC‐frontal lobe circuits. Because hippocampal volume is known to decrease with increased age [Jack et al., 1998, 2000], and because hippocampal activity has been implicated in many previous studies of associative memory, we further hypothesized that the relationship between ERC thickness and fMRI activity would be modulated by hippocampal volume. Specifically, we hypothesized that the relationship would be stronger in those having a smaller hippocampal volume.
MATERIALS AND METHODS
Subjects
Participants were 32 cognitively intact older volunteers (mean age, 60; range, 42–77) selected from a larger pool of 62 adults. Participants were recruited through advertisements and seminars, and selected without regard to ethnicity or race. Volunteers were given neurological and neuropsychological testing and underwent structural and functional MRI scanning. From the larger pool of 62 subjects, 4 were excluded for left‐handedness, 9 were excluded for cholinergic drug use, 8 were excluded for neurological or psychological diseases or disorders that could affect cognition, and 9 were excluded for technological problems, including failure of the stimulus presentation software and excessive subject motion (functional scans in which total subject motion was >2 mm or significantly correlated with task paradigm) (Table I).
Table I.
Demographic characteristics of participants
| No. of participants | 32 |
| Males/females | 10/22 |
| Agea | 60 (42–77) |
| Education (years)a | 16 (12–24) |
| No. with family history of ADb | 18 |
| Mini Mental State Exam (MMSE)a [Folstein et al., 1975] | 29 (27–30) |
| National adult reading test—Revised full IQa [Nelson and Willison, 1991] | 113 (93–123) |
| Verbal paired associates pretest (max = 42)a | 30 (13–42) |
| Left ERC thickness (mm)a | 2.7 (2.2–3.3) |
| Left hippocampal volume (% of intracranial volume)a | 0.24% (0.15–0.32%) |
Demographic and clinical features are listed as mean (range).
Family history is defined as parent, grandparent, or sibling known to be afflicted with AD.
The research was completed in accordance with the Helsinki Declaration. We obtained informed written consent from all subjects, and the study was approved by the Institutional Review Board of the University of California, Los Angeles (UCLA).
Memory Activation Task
Participants were tested using a verbal paired associates task known to preferentially activate prefrontal, superior temporal, and parietal regions in those with a genetic risk for AD [Bookheimer et al., 2000]. This task required subjects to learn pairs of words, and to recall the second words in the pairs given the first as cues.
We created alternate forms of the task using established normative data [Nelson et al., 2004] to match for average word frequency, length, and concreteness, with the exception that no concreteness norms were available for two words in each list. Each list contained 4 two‐syllable words and 10 one‐syllable words combined into 7 word pairs. Eleven of the 14 words in each list were nouns and 3 were adjectives. No word in any list was a common associate of any other word in that list, based on word association norms [Nelson et al., 2004]. To obtain behavioral data for each subject, one version of the task was administered verbally as a pretest prior to scanning. Total correct retrievals for each subject were summed across their 6 trials to arrive at a total score out of a possible 42.
During the scan, subjects encoded seven pairs of unrelated words presented one at a time both visually and auditorily using MacStim presentation software (WhiteAnt Occasional Publishing). Each encoding block was followed by a distracter task (control task), included to discourage rehearsal. In the control task, participants pressed a button any time a symbol on the screen changed between a fixation cross and a circle. During recall blocks, participants saw and heard the first word of each pair presented briefly, and attempted to recall the second word silently. Subjects pressed buttons to indicate perceived success or failure at recalling the second word, and responses were recorded. After completion of the scan, subjects received a verbal posttest to assess learning of the stimuli. Word pair order was counterbalanced across trials. Score on the verbal paired associates pretest significantly correlated with raw score on the widely used Wechsler Memory Scale Verbal Paired Associates test [Wechsler, 1987] raw score (R 2 = 0.48; P = 0.00002) (Fig. 1).
Figure 1.
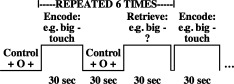
Verbal paired associates paradigm. During encoding, each printed word was presented visually for 0.18 sec, followed by 0.82 sec of blank screen. An additional 2 sec separated the 7 word pairs from one another. A distracter task followed each 30‐sec encoding block. During retrieval blocks, the first word of each word pair was presented visually for 0.18 sec. During the 3.82 sec of blank screen that followed, subjects attempted to recall the second word. All visual word presentations were accompanied by simultaneous auditory presentations.
Imaging Procedures
We performed whole brain structural Magnetization Prepared RApid Gradient Echo (MPRAGE) T1 weighted volumetric scans (TR = 2300 ms; TE = 2.93 ms; 1 mm slices/0.5 mm gap; 256 × 256 [1.3 × 1.3 mm] in‐plane resolution) for use in calculating hippocampal volumes, and proton density/T2‐weighted double‐echo structural scans (Siemens Allegra 3T) to aid in assessing general brain health. Fast spin echo structural scans were administered with 19 oblique coronal slices cut perpendicular to the long axis of the hippocampus to aid in viewing ERC cross‐sections (TR = 5200 ms; TE = 105 ms; 3 mm slices/0 mm gap; 512 × 512 [0.39 × 0.39] in‐plane resolution; FOV = 200 × 200; flip angle = 139°; averages = 2). Functional MRI was performed using a gradient echo, echo‐planar scan sequence while the subjects performed a verbal paired associates task (TR = 2500 ms; TE = 35 ms; 3 mm slices/1 mm gap; 64 × 64 [3.1 × 3.1 mm] in‐plane resolution; FOV = 200 × 200; flip angle = 90°). We acquired high resolution spin echo scans (TR = 5000 ms; TE = 33 ms; 128 × 128 [1.6 × 1.6 mm] in‐plane resolution; FOV = 200 × 200; flip angle = 90°; averages = 4) coplanar to the functional scans to aid in registration to a standard Montreal Neurologic Institute (MNI) brain, allowing fMRI analysis to take place in standard space.
Functional Data Analysis
We performed all functional data analyses using the “Analysis Group at the Oxford Centre for Functional MRI of the Brain” (FMRIB) software library (FSL) tools. Skulls were first stripped automatically from each high resolution spin echo coplanar scan using FSL “Brain Extraction Tool” (BET) [Smith, 2002]. Next we used FSL “FMRI Expert Analysis Tool” (FEAT) for individual fMRI scan preprocessing and for individual and group statistical analyses. Preprocessing of functional scans included brain extraction using BET, and motion correction with FSL's “Motion Correction using FMRIB's Linear Image Registration Tool” (MCFLIRT) [Jenkinson et al., 2002]. Each functional scan was coregistered to its corresponding coplanar high resolution image (using rigid body transformations) and to the MNI standard brain. We applied high‐pass temporal filtering of 120 sec to the fMRI images, and images were spatially smoothed using a Gaussian smoothing kernel of 6 mm.
Statistical Comparisons
We performed statistical analyses on individual scans within FEAT using “FMRIB's Improved Linear Model” (FILM) [Woolrich et al., 2001]. For each individual, we contrasted regional blood oxygenation levels during the control task with levels during both encoding and retrieval of verbal paired associates.
We next performed group analysis using FSL “FMRIB's Local Analysis of Mixed Effects” (FLAME) [Beckmann et al., 2003] to generate Z statistic images (Z > 2.3, with cluster threshold of 765 voxels to adjust the imagewise threshold to P < 0.01) of the combined individual fMRI activation results across subjects for encoding versus control and retrieval versus control. Next, in order to test our primary hypothesis that functional activity during the memory task would be significantly correlated with left ERC thicknesses, we used those values as covariates in the group analysis. Lastly, because we hypothesized that decreased hippocampal volume would result in a functional deficit that may be compensated for through increased activity in an ERC‐frontal lobe network, we used hippocampal volume as a covariate in a separate fMRI analysis.
Secondary Analyses
To generate plots of fMRI activity versus ERC thickness, we created a mask of regions showing preferential fMRI activation in subjects having thicker ERC. We then separated that mask into anatomic components (anterior cingulate and medial frontal cortices). Anterior cingulate was identified on the MNI brain based on guidelines described previously although, unlike in the previous paper, our analysis did not separate anterior cingulate into subregions [Zetzsche et al., 2007]. Medial frontal cortex was anterior to anterior cingulate and inferior to the Talairach coordinate z = 28 mm. We then applied each component mask to individual fMRI scans and used FSL FEAT query to calculate percent signal change in each region.
We found that ERC thickness correlated with fMRI activity during retrieval, but not during encoding. In order to determine whether our results were truly specific to retrieval or were simply subthreshold during encoding, we contrasted retrieval blocks with encoding blocks and again added ERC thickness as a covariate of interest. We limited this analysis to regions that during retrieval were significantly correlated with ERC thickness.
Structural Data Analysis
We used “FSL view” to manually outline left ERC on each high resolution oblique coronal scan using detailed guidelines derived from Amaral and Insausti's histologic anatomic atlas [Amaral and Insausti, 1990]. Specifically, the most anterior slice on which ERC was delineated was the first in which both the alveus was visible and the uncal fissure cut through the hippocampus. The most posterior slice was the slice immediately anterior to the one in which the cerebral spinal fluid fully separated the hippocampus from the remainder of the brain medially. On the most anterior slices in which the subiculum tilted superiorly at its medial aspect rather than curving inferiorly into ERC, the superior ERC boundary was the continuation of the subiculum medially at the angle followed by subicular cortex on that slice. On the more posterior slices, the superior ERC boundary was set perpendicular to the cortical strip two voxels inferior to the lowest, most medial point of the horizontal portion of the subicular cortical strip. In all slices containing ERC, the inferior ERC boundary was drawn from the gray matter point nearest the center of base of the collateral sulcus to the deepest curve of the white matter immediately superior to it. Visible cerebral spinal fluid was excluded from the region of interest (ROI) (Fig. 2A).
Figure 2.
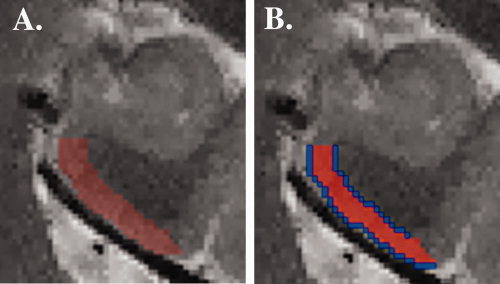
Image (A) is of one anterior slice of the fast spin echo high resolution scan displaying left ERC (red). Image (B) shows the left ERC drawn in Figure 2A. Blue lines 1‐voxel thick border the ROI on either side. Their area is averaged to approximate region length.
In order to determine the cortical thickness for left ERC, we next created a 1‐voxel thick border along each length of the ROI (Fig. 2B). We calculated the volume of left ERC using FSL. We then divided that volume by an average length of the 1‐voxel thick borders on each side (which, within each coronal slice, approximates the length through the center of the entorhinal cortical strip). This calculation yields an average left ERC thickness (in voxels). Because the borders are 1‐voxel thick lines, they are one‐dimensional. Therefore, the volume of the borders will be equal to the length of the lines. Finally, we multiplied the resulting thickness by 0.39 mm (in‐plane resolution) to determine thickness in mm. This method assumes that MRI slices are taken perpendicular to the ERC cortical strip. If not, thickness will be overestimated by tan θ, where θ is the angle the cortical sheet makes with the slices. Previously in our lab, we compared ERC thickness in a sample of 14 young (average age = 29), cognitively normal adults with their intracranial volume. ERC thickness was not significantly correlated with intracranial volume (R 2 = 0.03; P = 0.58).
We also calculated hippocampal volume as a percentage of intracranial volume. To do this, we automatically created a brain matter mask of the MPRAGE using FSL BET [Smith, 2002]. The masks were then manually refined using “FSL view,” and the completed masks were applied to exclude non‐brain matter. Hippocampal ROIs were defined by a single rater in native space, using a modification of guidelines outlined previously [Pruessner et al., 2000]. Specifically, we included hippocampal head, body, and tail in one ROI. Also, because our subjects were older and the size of their lateral ventricles varied considerably, we did not use the lateral ventricles as a landmark to exclude the Andreas‐Retzius gyrus as described previously [Pruessner et al., 2000]. Instead, in the sagittal slice we excluded the apparent hippocampal tail on all slices medial to the last one in which the parahippocampal gyrus inferior to the hippocampus was unbroken. Volumes were determined using FSL.
Reliability
All ERC thickness measures in the current study were performed by one rater. We tested the reliability of our measures by re‐tracing and calculating ERC thickness and hippocampal volume in older adults, randomly selected from our sample. The researcher was blind to the previous measures of thickness and volume when performing the re‐testing. Reliability was high for both measures. The intraclass correlation (ICC) for ERC thicknesses was 0.87, with an average percent error of 2.2%. The ICC for hippocampal volumes was 0.93, with an average percent error of 2.5%.
RESULTS
In simple separate comparisons of encoding and retrieval versus control, participants displayed increased fMRI signal during both encoding and retrieval. Activations occurred throughout the brain, including in sensory input areas, anterior and posterior language areas, episodic memory regions such as the hippocampus and parahippocampal gyrus, and working memory and executive function regions including anterior cingulate cortex, dorsal lateral prefrontal cortex, and parietal cortex (Table II).
Table II.
Functional MRI activations during encoding and retrieval compared to control blocks
| Sample MNI coordinates encodinga | Maximum Z scores encoding | Sample MNI coordinates retrievala | Maximum Z scores retrieval | |
|---|---|---|---|---|
| Cingulate | ||||
| Anterior cingulate cortex | −6, 18, 40 | 5.16 | −4, 18, 40 | 6.48 |
| Posterior cingulate cortex | −2, −34, 28 | 3.41 | −2, −32, 26 | 5.22 |
| Parietal | ||||
| Precuneus | −24, −74, 34 | 7.30 | −28, −64, 36 | 6.75 |
| Inferior parietal lobule | −28, −56, 42 | 6.76 | −28, −60, 34 | 6.38 |
| Superior parietal lobe | −26, −62, 46 | 6.80 | −30, −64, 48 | 6.45 |
| Temporal | ||||
| Superior temporal gyrus | −64, −22, 2 | 12.02 | 60, −18, 2 | 6.79 |
| Middle temporal gyrus | −64, −32, 8 | 8.72 | −62, −32, 0 | 7.54 |
| Parahippocampal gyrus | −30, −30, −22 | 5.46 | −28, −28, −28 | 5.64 |
| Hippocampus | −30, −22, −16 | 4.35 | −18, −28, −10 | 5.29 |
| Frontal | ||||
| DLPFC | −44, 6, 34 | 7.57 | −46, 12, 22 | 8.19 |
| Inferior frontal gyrus | −46, 32, 0 | 6.58 | −34, 26, −4 | 6.67 |
| Precentral gyrus | −50, −6, 50 | 7.47 | −50, −8, 52 | 6.88 |
| Occipital lobe | 0, −76, 8 | 4.46 | 2, −84, 32 | 6.62 |
| Brainstem | −8, −26, −14 | 6.47 | ||
| Striatum | −10, 0, 18 | 6.11 | ||
| Thalamus | −6, −20, 12 | 5.95 | ||
| Cerebellum | −8, −60, −14 | 6.12 | ||
Coordinates for statistically significant activations were identified based on the MNI atlas (Z = 2.3; cluster thresholding corrected for multiple comparisons to adjust the imagewise threshold to P < 0.01). The sample coordinates represent the voxel with the highest Z value in each anatomical region listed.
We next included left ERC thickness as a covariate in the fMRI analysis. During retrieval, those having thicker left ERC showed greater fMRI activity in anterior cingulate and medial frontal (BAs 9 and 10) cortices compared with those having thinner left ERC (Table III; Fig. 3). There was no significant relationship between left ERC thickness and fMRI activity during encoding.
Table III.
Correlations with left ERC thickness
| Brodmann areas | Sample MNI coordinatesa | Maximum Z scores | |
|---|---|---|---|
| Anterior cingulate | 24, 32 | 6, 26, 30 | 3.89 |
| Medial frontal cortex | 9, 10 | −10, 66, 14 | 3.75 |
MNI coordinates represent regions in which activation was greater during retrieval in those with thicker left ERC (Z = 2.3; cluster thresholding adjusted the imagewise threshold to P < 0.01). All activations were bilateral. The sample coordinates represent the voxel with the highest Z value in each anatomical region listed.
Figure 3.
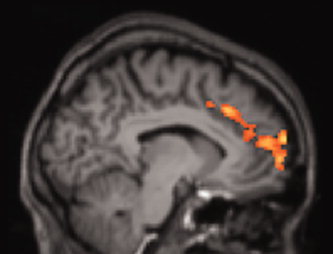
Older adults with thicker left ERC showed greater fMRI activation during retrieval in anterior cingulate and medial frontal cortices compared with those having thinner left ERC (Z = 2.3; cluster thresholding corrected for multiple comparisons to adjust the imagewise threshold to P < 0.01).
We likewise covaried left hippocampal volume with fMRI activity. During attempted retrieval, those having larger hippocampi as a percentage of intracranial volume had greater fMRI activation in bilateral retrosplenial cortex (BA 30) and occipital cortex (BA 17). However, hippocampal volume was significantly correlated with age. When age was added as a covariate of no interest to the fMRI analysis, no region showed a significant correlation between fMRI activity and left hippocampal volume.
In order to determine whether the relationship between left ERC thickness and fMRI activity in these regions was similar in those having thinner or thicker ERC, and was not being driven by outlying data points, we plotted percent signal change versus left ERC thickness in only the areas preferentially activated in those with thicker ERC. The relationships between left ERC thickness and fMRI activity in anterior cingulate (R 2 = 0.26; P = 0.0029) and medial frontal cortex (R 2 = 0.36; P = 0.0003) were significant and consistent throughout the range of values (Fig. 4A,B).
Figure 4.
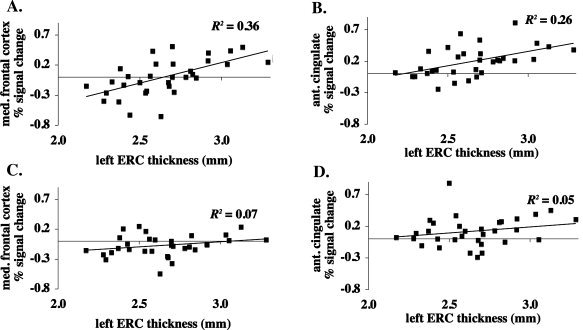
Graphs represent percent signal change versus cortical thickness in left ERC in regions of (A) medial frontal cortex (BAs 9 and 10) and (B) anterior cingulate cortex (BAs 24 and 32) that were preferentially activated during memory retrieval in those having thicker left ERC. Percent signal change in the same regions during memory encoding is shown versus left ERC thickness in (C) and (D) for comparison purposes. R 2 values are for Pearson's correlations.
ERC thickness was not significantly correlated with age, sex, score on the memory task, hippocampal volume, MMSE score, family history of AD, or education level. Likewise, preferential fMRI activation in anterior cingulate and medial frontal cortices was not significantly associated with any of these measures. In order to test the hypothesis that the relationships between ERC thickness and fMRI activity in anterior cingulate and medial frontal cortices were modulated by hippocampal volume, we separated the data into those having large or small hippocampal volumes (as a percentage of intracranial volume) based on a simple median split (0.24%). Using ANCOVA, we determined that in those with large versus small hippocampal volumes, there was no significant difference in the relationship between ERC thickness and preferential fMRI activity in anterior cingulate (P = 0.98) or medial frontal (P = 0.14) cortices.
To determine the extent to which our results were specific to processes used during retrieval, but not encoding, we next covaried fMRI activity with ERC thickness in a contrast that explored where retrieval activity was significantly greater than encoding activity. The resulting preferential activity overlapped 35% with the original functional region (where ERC thickness was significantly correlated with activation during retrieval alone) and included portions of both anterior cingulate and medial frontal cortices. These results suggest that the relationship between ERC thickness and fMRI activity was at least partly specific to retrieval.
As expected, hippocampal volume, but not ERC thickness, was significantly smaller with increasing age (R 2 = 0.22; P = 0.007). Although the verbal paired associates scores were also significantly lower with increased age (R 2 = 0.19; P = 0.01), hippocampal volume was not significantly correlated with task score.
DISCUSSION
Reduced ERC volume has been shown in numerous studies to be associated with AD [Pennanen et al., 2004; Xu et al., 2000] and risk for AD [deToledo‐Morrell et al., 2004; Dickerson et al., 2001; Killiany et al., 2002; Stoub et al., 2005]. In the current study, we used left ERC thickness as an indicator of increased AD risk, and examined its relationship with fMRI activity during encoding and retrieval in an associative memory task. During attempted memory retrieval, older adults having thicker left ERC showed greater fMRI activity in anterior cingulate (BAs 24 and 32) and medial frontal (BAs 9 and 10) cortices than those having thinner left ERC. These results were independent of age, task performance, and hippocampal volume. Our results suggest that there are functional consequences to structural thinning of ERC and that processes specific to attempted memory retrieval are particularly sensitive to ERC integrity.
Layers V and VI of ERC are strongly connected to anterior cingulate cortex in non‐human primates [Arikuni et al., 1994; Munoz and Insausti, 2005]. Furthermore, in the rhesus monkey there are direct connections between anterior cingulate (BAs 24 and 32) and medial frontal (BAs 9 and 10) cortices [Barbas and Pandya, 1989]. Therefore, the significant correlation between ERC structure and activity in anterior cingulate and medial frontal cortices is consistent with the known anatomical connectivity of these regions.
On average, across subjects, anterior cingulate cortex showed increased activation during attempted memory retrieval, which is consistent with several past studies [Buckner et al., 1996; Cabeza et al., 2003; Gould et al., 2003]. The portion of medial frontal cortex that was preferentially activated in those with thicker left ERC was not active on average during memory retrieval, however, because approximately half of the participants showed a negative percent signal change in that region, diluting the overall percent signal change across the group. During both episodic memory encoding and recognition, previous researchers found that compared with younger adults, older adults showed less task‐induced deactivation in medial frontal cortex [Grady et al., 2006]. The question arises then, if older adults with thicker ERC (in the current study) are presumably more like younger adults, why do they have greater activation during task, while young adults previously showed greater deactivation during task? One possible explanation for our results is that those older adults having thicker ERC were better able to recruit medial frontal cortex to compensate for unspecified age‐related deficits in the brain (such as changes in neurotransmitter systems). Because we included only cognitively intact older adults in this study, all subjects must be successfully compensating in some way for any age‐related cognitive deficits that they may have, so it is not surprising that the behavioral effect of such compensation is not evident in our sample. Our results are consistent with those of a recent study finding that during an episodic recognition task, cognitively intact middle‐aged adults with lower AD risk showed greater fMRI activation in anterior cingulate and medial frontal cortices compared with those having increased AD risk [Xu et al., 2008].
Many past studies of cognitively intact older adults have examined the relationship between increased AD risk and fMRI activity during a memory or novelty encoding task, primarily using increased genetic risk for AD [Bondi et al., 2005; Bookheimer et al., 2000; Dickerson et al., 2005; Fleisher et al., 2005; Han et al., 2006; Trivedi et al., 2006; Wishart et al., 2006] or family history of AD [Bassett et al., 2006] as indicators of increased AD risk. Most of these studies did not report the individual relationship during memory retrieval (versus control period) between increased AD risk and whole brain fMRI activation. As in our study, one study by Bassett et al. [ 2006] reported that during retrieval of word pairs, those who had lower risk for AD activated more in regions that included anterior cingulate cortex (BAs 24 and 32) and middle frontal gyrus (BA 9) (although more laterally than in the current study).
In a study similar to ours, Rosen et al. [ 2005] examined the relationship in older adults between left ERC volume and functional activity during incidental encoding of individual words. They found that frontal activity during encoding was greater in those with greater left ERC volume, but this increased activation was in right BA 47/insula rather than in anterior cingulate and medial frontal cortices as in our study. Additionally, we found a significant relationship between ERC thickness and fMRI activity during attempted retrieval, but not encoding. However, our studies differ methodologically in several ways, including task, measure of ERC atrophy, age, and sample size. Specifically, during scanning, subjects in the study by Rosen et al. [ 2005] were instructed to make semantic judgments about words presented to them, but were not instructed to remember those words. They were later tested on their memory for the words outside the scanner. In contrast, our subjects were instructed to encode pairs of words and were later scanned while being cued with the first word in order to recall its mate. It is therefore not surprising that such different tasks resulted in variations of activation. Additionally, our subjects were on average nearly 10 years younger and spanned a wider age range than in the Rosen and colleagues study. Finally, the previous study contained only 13 subjects, while ours examined 32; some of the differences between our study and theirs may be due to normal variations associated with subject selection for a small sample size [Rosen et al., 2005].
As in our study, previous research using a verbal paired associates task found a more pronounced relationship between fMRI activity and AD risk during retrieval than during encoding [Bookheimer et al., 2000]. This is to be expected because, although recollection shares many attributes of memory encoding (including encoding itself), it also involves additional processes that memory encoding lacks, such as using a cue to derive the associated word, response selection and monitoring, and blocking of distracting information in order to arrive at the correct answer.
Our data provide evidence that the structural integrity of ERC contributes to functional brain activity during an associative recollection task. The same words were presented six times throughout the encoding task, but we did not see a significant correlation between ERC thickness and fMRI activity during encoding. This suggests that our results were not solely due to the familiarity of the cue words presented at retrieval. Our results support our hypothesis that even during an associative task, retrieval attempts in older adults were mediated by a circuit that includes ERC and frontal lobe, and that ERC integrity played a role in this relationship.
Anterior cingulate cortex is believed to be associated with response monitoring, in particular for evaluating possible errors. Such monitoring may be beneficial in updating memory strategies [Rushworth, 2008]. Functional activity in anterior cingulate cortex and in BAs 9 and 10 has been implicated in recognition success [Konishi et al., 2000; Rugg et al., 1996]. Additionally, activity in anterior prefrontal regions during recognition (success and failure) has a late onset and sustained duration, suggesting that like anterior cingulate cortex, this region is involved in post‐retrieval monitoring [Schacter et al., 1997]. The fact that both anterior cingulate cortex and BA 10 have been shown to activate more during memory retrieval when a participant is less confident in his or her response offers further support for their role in response monitoring [Fleck et al., 2006]. Although response monitoring is important for adjusting strategy to meet the needs of a given task, it is only one aspect of the processes involved in encoding and recalling information. Additionally, in older adults, factors other than ERC thickness (such as education or age‐related deficits in neurotransmitter systems) may affect memory ability [Volkow et al., 1998]. This may explain why ERC thickness and fMRI function were not related to score on the paired associates memory task. Had we not limited our study to cognitively intact adults, which limits the range of cognition to those who are by definition successfully compensating for cognitive stressors, the relationship between ERC thickness and cognition may have been detectable.
Although ERC provides major inputs to the hippocampus, we did not find a significant relationship between ERC thickness and hippocampal fMRI activity. However, the hippocampus is composed of subregions that may be recruited differently during a given memory task [Zeineh et al., 2003], and that are differently susceptible to AD‐related pathology [Braak and Braak, 1991] and degeneration [Bobinski et al., 1998]. Accurate identification and coregistration of these small subregions across subjects is difficult with standard fMRI scans. Using scans optimized for detecting fMRI activity in specific hippocampal subregions may have facilitated exposing such a relationship, if one existed.
The brain's response to error detection has been shown to change with aging [Falkenstein et al., 2001], and error detection is impaired with AD [Bettcher et al., 2008]. The current study provides a mechanism by which changes to medial temporal lobe may be linked to changes in frontal lobe function in older adults at risk for AD. Specifically, ERC thickness in older adults appears to modify the ability to engage anterior cingulate and frontal regions believed to be important in error detection and other post‐retrieval processing. ERC volume is smaller in those having AD, but not in those who are aging normally [Fukutani et al., 2000; Giannakopoulos et al., 2003; Gomez‐Isla et al., 1996; Hof et al., 2003; Kordower et al., 2001; von Gunten et al., 2006]. However, AD risk also increases with age. Therefore, particularly in older adults, thinner ERC may suggest cortical degeneration even in some cognitively intact adults. We do not suggest that all people having thin ERC are preclinical for AD, but rather that thin ERC in older adults increases the likelihood of preclinical AD, including in some older adults believed to be aging normally. This study is cross‐sectional rather than longitudinal, so we do not know which of the subjects in this study will eventually develop AD. Because those participants with thinner ERC are at higher risk for AD, however, these data provide a framework with which to examine current and future studies of AD risk.
The relationship between ERC thickness and fMRI activity was not different in those having larger versus smaller hippocampal volumes. Additionally, after adjusting for age, hippocampal volume was not significantly associated with fMRI activity during encoding or retrieval. This suggests that if the greater fMRI activity we saw in those with thicker ERC was in compensation for deficits elsewhere in the brain, those deficits were not related to age‐associated overall hippocampal atrophy. It is possible that measurements of selected hippocampal regions would be a more sensitive grouping mechanism when evaluating these relationships. It is also possible that the increased fMRI activity in those with thicker ERC instead compensates for deficits in frontal lobe function, which is known to decline with age [Cohen et al., 1987; De Luca et al., 2003; Gazzaley et al., 2005]. Such changes may relate to changes in neurotransmitter synthesis and processing, which are believed to occur in older adults [Adolfsson et al., 1979; Cruz‐Muros et al., 2007; Goldberg et al., 2004].
CONCLUSION
During attempted memory retrieval, older adults having thicker left ERC showed more fMRI activation in anterior cingulate and medial frontal cortices compared with those having thinner left ERC, independent of hippocampal volume. Our results suggest that structural atrophy of ERC was associated with functional changes during memory performance in regions distal but connected to ERC. Thus, the data provide evidence linking ERC structure to the cognitive and functional deficits seen in aging generally and with AD risk in particular.
Acknowledgements
We thank Ms. Andrea Kaplan, Ms. Deborah Dorsey, and Ms. Teresann Crowe‐Lear for their help in recruiting volunteers and coordinating the study, Ms. Gwendolyn Byrd for her help in scheduling volunteers and collecting their personal data, and Dr. Karen Miller for her administration and coordination of neuropsychological testing.
Disclosure: The authors have no actual or potential conflicts of interest. However, they disclose the following: The University of California, Los Angeles, owns a U.S. patent (6,274,119) entitled “Methods for Labeling Beta‐Amyloid Plaques and Neurofibrillary Tangles,” which has been licensed to Siemens. Dr. Small is among the inventors, has received royalties, and will receive royalties on future sales. Dr. Small reports having served as a consultant and/or having received lecture fees from Abbott, Dakim, Eisai, Forest, Mattel, Myriad Genetics, Novartis, Ortho‐McNeil, Pfizer, Radica, Siemens, and Medivation. Dr. Small also reports having received stock options from Dakim. M.N.B. was supported by an Individual National Research Service Award (F31 NS45425) from the National Institutes of Health/National Institute of Neurological Disorders and Stroke, and by a scholarship from ARCS Foundation, Inc./The John Douglas French Alzheimer's Foundation (with the Erteszek Foundation)
M.N.B. was supported by an Individual National Research Service Award (F31 NS45425) from the National Institutes of Health/National Institute of Neurological Disorders and Stroke, and by a scholarship from ARCS Foundation, Inc./The John Douglas French Alzheimer's Foundation (with the Erteszek Foundation).
REFERENCES
- Adolfsson R, Gottfries CG, Roos BE, Winblad B ( 1979): Post‐mortem distribution of dopamine and homovanillic acid in human brain, variations related to age, and a review of the literature. J Neural Transm 45: 81–105. [DOI] [PubMed] [Google Scholar]
- Amaral D, Insausti R ( 1990): Hippocampal formation In: Paxinos G, editor. The Human Nervous System. San Diego: Academic Press; pp 711–755. [Google Scholar]
- American Psychiatric Association ( 2000): Diagnosis and Statistical Manual of Mental Disorders DSM‐IV‐TR (Text Revision). Washington, DC: American Psychiatric Association. [Google Scholar]
- Arikuni T, Sako H, Murata A ( 1994): Ipsilateral connections of the anterior cingulate cortex with the frontal and medial temporal cortices in the macaque monkey. Neurosci Res 21: 19–39. [DOI] [PubMed] [Google Scholar]
- Barbas H, Pandya DN ( 1989): Architecture and intrinsic connections of the prefrontal cortex in the rhesus monkey. J Comp Neurol 286: 353–375. [DOI] [PubMed] [Google Scholar]
- Bassett SS, Yousem DM, Cristinzio C, Kusevic I, Yassa MA, Caffo BS, Zeger SL ( 2006): Familial risk for Alzheimer's disease alters fMRI activation patterns. Brain 129( Part 5): 1229–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM ( 2003): General multilevel linear modeling for group analysis in FMRI. Neuroimage 20: 1052–1063. [DOI] [PubMed] [Google Scholar]
- Bettcher BM, Giovannetti T, Macmullen L, Libon DJ ( 2008): Error detection and correction patterns in dementia: A breakdown of error monitoring processes and their neuropsychological correlates. J Int Neuropsychol Soc 14: 199–208. [DOI] [PubMed] [Google Scholar]
- Bobinski M, de Leon MJ, Tarnawski M, Wegiel J, Reisberg B, Miller DC, Wisniewski HM ( 1998): Neuronal and volume loss in CA1 of the hippocampal formation uniquely predicts duration and severity of Alzheimer disease. Brain Res 805: 267–269. [DOI] [PubMed] [Google Scholar]
- Bondi MW, Houston WS, Eyler LT, Brown GG ( 2005): fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology 64: 501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak‐Vance MA, Mazziotta JC, Small GW ( 2000): Patterns of brain activation in people at risk for Alzheimer's disease. N Engl J Med 343: 450–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouras C, Hof PR, Giannakopoulos P, Michel JP, Morrison JH ( 1994): Regional distribution of neurofibrillary tangles and senile plaques in the cerebral cortex of elderly patients: A quantitative evaluation of a one‐year autopsy population from a geriatric hospital. Cereb Cortex 4: 138–150. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E ( 1991): Neuropathological stageing of Alzheimer‐related changes. Acta Neuropathol (Berl) 82: 239–259. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E ( 1995): Staging of Alzheimer's disease‐related neurofibrillary changes. Neurobiol Aging 16: 271–278, discussion 278–284. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E ( 1997): Frequency of stages of Alzheimer‐related lesions in different age categories. Neurobiol Aging 18: 351–357. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Raichle ME, Miezin FM, Petersen SE ( 1996): Functional anatomic studies of memory retrieval for auditory words and visual pictures. J Neurosci 16: 6219–6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burggren AC, Zeineh MM, Ekstrom AD, Braskie MN, Thompson PM, Small GW, Bookheimer SY ( 2008): Reduced cortical thickness in hippocampal subregions among cognitively normal apolipoprotein E e4 carriers. Neuroimage 41: 1177–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Locantore JK, Anderson ND ( 2003): Lateralization of prefrontal activity during episodic memory retrieval: Evidence for the production‐monitoring hypothesis. J Cogn Neurosci 15: 249–259. [DOI] [PubMed] [Google Scholar]
- Cohen RL, Sandler SP, Schroeder K ( 1987): Aging and memory for words and action events: Effects of item repetition and list length. Psychol Aging 2: 280–285. [DOI] [PubMed] [Google Scholar]
- Cruz‐Muros I, Afonso‐Oramas D, Abreu P, Barroso‐Chinea P, Rodriguez M, Gonzalez MC, Hernandez TG ( 2007): Aging of the rat mesostriatal system: Differences between the nigrostriatal and the mesolimbic compartments. Exp Neurol 204: 147–161. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R ( 2006): Effects of healthy aging on hippocampal and rhinal memory functions: An event‐related fMRI study. Cereb Cortex 16: 1771–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L, Wagner AD ( 2002): Hippocampal contributions to episodic encoding: Insights from relational and item‐based learning. J Neurophysiol 88: 982–990. [DOI] [PubMed] [Google Scholar]
- De Luca CR, Wood SJ, Anderson V, Buchanan JA, Proffitt TM, Mahony K, Pantelis C ( 2003): Normative data from the CANTAB. I. development of executive function over the lifespan. J Clin Exp Neuropsychol 25: 242–254. [DOI] [PubMed] [Google Scholar]
- DeToledo‐Morrell L, Dickerson B, Sullivan MP, Spanovic C, Wilson R, Bennett DA ( 2000): Hemispheric differences in hippocampal volume predict verbal and spatial memory performance in patients with Alzheimer's disease. Hippocampus 10: 136–142. [DOI] [PubMed] [Google Scholar]
- DeToledo‐Morrell L, Stoub TR, Bulgakova M, Wilson RS, Bennett DA, Leurgans S, Wuu J, Turner DA ( 2004): MRI‐derived entorhinal volume is a good predictor of conversion from MCI to AD. Neurobiol Aging 25: 1197–1203. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Goncharova I, Sullivan MP, Forchetti C, Wilson RS, Bennett DA, Beckett LA, DeToledo‐Morrell L ( 2001): MRI‐derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer's disease. Neurobiol Aging 22: 747–754. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Greve DN, Chua EF, Rand‐Giovannetti E, Rentz DM, Bertram L, Mullin K, Tanzi RE, Blacker D, Albert MS, Sperling RA ( 2005): Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology 65: 404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom A, Viskontas I, Kahana M, Jacobs J, Upchurch K, Bookheimer S, Fried I ( 2007): Contrasting roles of neural firing rate and local field potentials in human memory. Hippocampus 17: 606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eustache F, Desgranges B, Giffard B, de la Sayette V, Baron JC ( 2001): Entorhinal cortex disruption causes memory deficit in early Alzheimer's disease as shown by PET. Neuroreport 12: 683–685. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J ( 2001): Changes of error‐related ERPs with age. Exp Brain Res 138: 258–262. [DOI] [PubMed] [Google Scholar]
- Fleck MS, Daselaar SM, Dobbins IG, Cabeza R ( 2006): Role of prefrontal and anterior cingulate regions in decision‐making processes shared by memory and nonmemory tasks. Cereb Cortex 16: 1623–1630. [DOI] [PubMed] [Google Scholar]
- Fleisher AS, Houston WS, Eyler LT, Frye S, Jenkins C, Thal LJ, Bondi MW ( 2005): Identification of Alzheimer disease risk by functional magnetic resonance imaging. Arch Neurol 62: 1881–1888. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR ( 1975): “Mini‐mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 189–198. [DOI] [PubMed] [Google Scholar]
- Fukutani Y, Cairns NJ, Shiozawa M, Sasaki K, Sudo S, Isaki K, Lantos PL ( 2000): Neuronal loss and neurofibrillary degeneration in the hippocampal cortex in late‐onset sporadic Alzheimer's disease. Psychiatry Clin Neurosci 54: 523–529. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D'Esposito M ( 2005): Top‐down suppression deficit underlies working memory impairment in normal aging. Nat Neurosci 8: 1298–1300. [DOI] [PubMed] [Google Scholar]
- Giannakopoulos P, Herrmann FR, Bussiere T, Bouras C, Kovari E, Perl DP, Morrison JH, Gold G, Hof PR ( 2003): Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer's disease. Neurology 60: 1495–1500. [DOI] [PubMed] [Google Scholar]
- Goldberg S, Smith GS, Barnes A, Ma Y, Kramer E, Robeson K, Kirshner M, Pollock BG, Eidelberg D ( 2004): Serotonin modulation of cerebral glucose metabolism in normal aging. Neurobiol Aging 25: 167–174. [DOI] [PubMed] [Google Scholar]
- Gomez‐Isla T, Price JL, McKeel DW Jr, Morris JC, Growdon JH, Hyman BT ( 1996): Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer's disease. J Neurosci 16: 4491–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould RL, Brown RG, Owen AM, ffytche DH, Howard RJ ( 2003): fMRI BOLD response to increasing task difficulty during successful paired associates learning. Neuroimage 20: 1006–1019. [DOI] [PubMed] [Google Scholar]
- Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur G ( 2006): Age‐related changes in brain activity across the adult lifespan. J Cogn Neurosci 18: 227–241. [DOI] [PubMed] [Google Scholar]
- Han SD, Houston WS, Jak AJ, Eyler LT, Nagel BJ, Fleisher AS, Brown GG, Corey‐Bloom J, Salmon DP, Thal LJ, et al. ( 2006): Verbal paired‐associate learning by APOE genotype in non‐demented older adults: fMRI evidence of a right hemispheric compensatory response. Neurobiol Aging 28: 238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof PR, Bussiere T, Gold G, Kovari E, Giannakopoulos P, Bouras C, Perl DP, Morrison JH ( 2003): Stereologic evidence for persistence of viable neurons in layer II of the entorhinal cortex and the CA1 field in Alzheimer disease. J Neuropathol Exp Neurol 62: 55–67. [DOI] [PubMed] [Google Scholar]
- Insausti R, Amaral DG, Cowan WM ( 1987): The entorhinal cortex of the monkey. II. Cortical afferents. J Comp Neurol 264: 356–395. [DOI] [PubMed] [Google Scholar]
- Jack CR Jr, Petersen RC, Xu Y, O'Brien PC, Smith GE, Ivnik RJ, Tangalos EG, Kokmen E ( 1998): Rate of medial temporal lobe atrophy in typical aging and Alzheimer's disease. Neurology 51: 993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr, Petersen RC, Xu Y, O'Brien PC, Smith GE, Ivnik RJ, Boeve BF, Tangalos EG, Kokmen E ( 2000): Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology 55: 484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson O III, Schacter DL ( 2004): Encoding activity in anterior medial temporal lobe supports subsequent associative recognition. Neuroimage 21: 456–462. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S ( 2002): Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17: 825–841. [DOI] [PubMed] [Google Scholar]
- Killiany RJ, Hyman BT, Gomez‐Isla T, Moss MB, Kikinis R, Jolesz F, Tanzi R, Jones K, Albert MS ( 2002): MRI measures of entorhinal cortex vs hippocampus in preclinical AD. Neurology 58: 1188–1196. [DOI] [PubMed] [Google Scholar]
- Kirwan CB, Stark CE ( 2004): Medial temporal lobe activation during encoding and retrieval of novel face‐name pairs. Hippocampus 14: 919–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg T, Roland PE, Kawashima R ( 1994): The human entorhinal cortex participates in associative memory. Neuroreport 6: 57–60. [DOI] [PubMed] [Google Scholar]
- Konishi S, Wheeler ME, Donaldson DI, Buckner RL ( 2000): Neural correlates of episodic retrieval success. Neuroimage 12: 276–286. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Stebbins GT, DeKosky ST, Cochran EJ, Bennett D, Mufson EJ ( 2001): Loss and atrophy of layer II entorhinal cortex neurons in elderly people with mild cognitive impairment. Ann Neurol 49: 202–213. [PubMed] [Google Scholar]
- Munoz M, Insausti R ( 2005): Cortical efferents of the entorhinal cortex and the adjacent parahippocampal region in the monkey (Macaca fascicularis). Eur J Neurosci 22: 1368–1388. [DOI] [PubMed] [Google Scholar]
- Naveh‐Benjamin M ( 2000): Adult age differences in memory performance: Tests of an associative deficit hypothesis. J Exp Psychol Learn Mem Cogn 26: 1170–1187. [DOI] [PubMed] [Google Scholar]
- Nelson D, McEvoy CL, Schreiber TA ( 2004): The University of South Florida word association, rhyme, and word fragment norms. Behav Res Methods Instrum Comput 36: 402–407. [DOI] [PubMed] [Google Scholar]
- Nelson HE, Willison J ( 1991): National Adult Reading Test (NART): Test Manual, 2nd ed Windsor: NFER‐Nelson. [Google Scholar]
- Pennanen C, Kivipelto M, Tuomainen S, Hartikainen P, Hanninen T, Laakso MP, Hallikainen M, Vanhanen M, Nissinen A, Helkala EL, et al. ( 2004): Hippocampus and entorhinal cortex in mild cognitive impairment and early AD. Neurobiol Aging 25: 303–310. [DOI] [PubMed] [Google Scholar]
- Price JL, Morris JC ( 1999): Tangles and plaques in nondemented aging and “preclinical” Alzheimer's disease Ann Neurol 45: 358–368. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N, Lupien S, Evans AC ( 2000): Volumetry of hippocampus and amygdala with high‐resolution MRI and three‐dimensional analysis software: Minimizing the discrepancies between laboratories. Cereb Cortex 10: 433–442. [DOI] [PubMed] [Google Scholar]
- Rosen AC, Prull MW, Gabrieli JD, Stoub T, O'Hara R, Friedman L, Yesavage JA, DeToledo‐Morrell L ( 2003): Differential associations between entorhinal and hippocampal volumes and memory performance in older adults. Behav Neurosci 117: 1150–1160. [DOI] [PubMed] [Google Scholar]
- Rosen AC, Gabrieli JD, Stoub T, Prull MW, O'Hara R, Yesavage J, DeToledo‐Morrell L ( 2005): Relating medial temporal lobe volume to frontal fMRI activation for memory encoding in older adults. Cortex 41: 595–602. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Fletcher PC, Frith CD, Frackowiak RS, Dolan RJ ( 1996): Differential activation of the prefrontal cortex in successful and unsuccessful memory retrieval. Brain 119( Part 6): 2073–2083. [DOI] [PubMed] [Google Scholar]
- Rushworth MF ( 2008): Intention, choice, and the medial frontal cortex. Ann N Y Acad Sci 1124: 181–207. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Buckner RL, Koutstaal W, Dale AM, Rosen BR ( 1997): Late onset of anterior prefrontal activity during true and false recognition: An event‐related fMRI study. Neuroimage 6: 259–269. [DOI] [PubMed] [Google Scholar]
- Smith SM ( 2002): Fast robust automated brain extraction. Hum Brain Mapp 17: 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE ( 2007): Recognition memory and the medial temporal lobe: A new perspective. Nat Rev Neurosci 8: 872–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CE, Squire LR ( 2001): Simple and associative recognition memory in the hippocampal region. Learn Mem 8: 190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CE, Squire LR ( 2003): Hippocampal damage equally impairs memory for single items and memory for conjunctions. Hippocampus 13: 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoub TR, Bulgakova M, Leurgans S, Bennett DA, Fleischman D, Turner DA, DeToledo‐Morrell L ( 2005): MRI predictors of risk of incident Alzheimer disease: A longitudinal study. Neurology 64: 1520–1524. [DOI] [PubMed] [Google Scholar]
- Tranel D ( 1991): Dissociated verbal and nonverbal retrieval and learning following left anterior temporal damage. Brain Cogn 15: 187–200. [DOI] [PubMed] [Google Scholar]
- Trivedi MA, Schmitz TW, Ries ML, Torgerson BM, Sager MA, Hermann BP, Asthana S, Johnson SC ( 2006): Reduced hippocampal activation during episodic encoding in middle‐aged individuals at genetic risk of Alzheimer's disease: A cross‐sectional study. BMC Med 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer AK, Murphy KJ, Anderson ND, Hayman‐Abello BA, Craik FI, Moscovitch M ( 2008): Item and associative memory in amnestic mild cognitive impairment: Performance on standardized memory tests. Neuropsychology 22: 10–16. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Gur RC, Wang GJ, Fowler JS, Moberg PJ, Ding YS, Hitzemann R, Smith G, Logan J ( 1998): Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry 155: 344–349. [DOI] [PubMed] [Google Scholar]
- von Gunten A, Kovari E, Bussiere T, Rivara CB, Gold G, Bouras C, Hof PR, Giannakopoulos P ( 2006): Cognitive impact of neuronal pathology in the entorhinal cortex and CA1 field in Alzheimer's disease. Neurobiol Aging 27: 270–277. [DOI] [PubMed] [Google Scholar]
- Wechsler D. 1987. Wechsler Memory Scale—Revised Manual. San Antonio: The Psychological Corporation. [Google Scholar]
- Westerberg CE, Paller KA, Weintraub S, Mesulam MM, Holdstock JS, Mayes AR, Reber PJ ( 2006): When memory does not fail: Familiarity‐based recognition in mild cognitive impairment and Alzheimer's disease. Neuropsychology 20: 193–205. [DOI] [PubMed] [Google Scholar]
- Wishart HA, Saykin AJ, Rabin LA, Santulli RB, Flashman LA, Guerin SJ, Mamourian AC, Belloni DR, Rhodes CH, McAllister TW ( 2006): Increased brain activation during working memory in cognitively intact adults with the APOE {epsilon}4 Allele. Am J Psychiatry 163: 1603–1610. [DOI] [PubMed] [Google Scholar]
- Witter MP, Amaral DG ( 1991): Entorhinal cortex of the monkey. V. Projections to the dentate gyrus, hippocampus, and subicular complex. J Comp Neurol 307: 437–459. [DOI] [PubMed] [Google Scholar]
- Wolk DA, Signoff ED, Dekosky ST ( 2008): Recollection and familiarity in amnestic mild cognitive impairment: A global decline in recognition memory. Neuropsychologia 46: 1965–1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM ( 2001): Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage 14: 1370–1386. [DOI] [PubMed] [Google Scholar]
- Xu G, McLaren DG, Ries ML, Fitzgerald ME, Bendlin BB, Rowley HA, Sager MA, Atwood C, Asthana S, Johnson SC ( 2008): The influence of parental history of Alzheimer's disease and apolipoprotein E {varepsilon}4 on the BOLD signal during recognition memory. Brain 132( Part 2): 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Jack CR Jr, O'Brien PC, Kokmen E, Smith GE, Ivnik RJ, Boeve BF, Tangalos RG, Petersen RC ( 2000): Usefulness of MRI measures of entorhinal cortex versus hippocampus in AD. Neurology 54: 1760–1767. [DOI] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Thompson PM, Bookheimer SY ( 2003): Dynamics of the hippocampus during encoding and retrieval of face‐name pairs. Science 299: 577–580. [DOI] [PubMed] [Google Scholar]
- Zetzsche T, Preuss U, Frodl T, Watz D, Schmitt G, Koutsouleris N, Born C, Reiser M, Moller HJ, Meisenzahl EM ( 2007): In‐vivo topography of structural alterations of the anterior cingulate in patients with schizophrenia: New findings and comparison with the literature. Schizophr Res 96: 34–45. [DOI] [PubMed] [Google Scholar]


