Abstract
Identifying and counseling individuals with Acute HIV Infection (AHI) offers a critical opportunity to avert preventable HIV transmission, however opportunities to recognize these individuals may be missed. We surveyed 32 adults diagnosed with AHI during voluntary HIV testing from 1/1/03 to 2/28/05 in publicly funded testing sites in NC to describe their clinical, social, and behavioral characteristics. Eighty-one percent of participants were men; 59% were African American. Seventy-five percent experienced symptoms consistent with acute retroviral syndrome; although 83% sought medical care for these symptoms, only 15% were appropriately diagnosed at that initial medical visit, suggesting opportunities to diagnose these individuals earlier were missed. Eighty-five percent of the men engaged in sex with men. More than 50% of the participants thought they were infected with HIV by a steady partner. This study yields important information to assist in identifying populations at risk for or infected with AHI and designing both primary and secondary prevention interventions.
Keywords: Acute HIV Infection (AHI), North Carolina (NC), HIV/AHI screening, AHI Epidemiology, HIV Risk Factors
Introduction
Despite ongoing human immunodeficiency virus (HIV) treatment and prevention efforts, the HIV incidence in the United States (US) in 2006 was estimated at 56,300 new infections (Hall et al., 2008). Efforts to stem the US epidemic have been hindered, partly by difficulties accurately identifying and understanding populations at highest risk of transmitting HIV, such as those with acute HIV Infection (AHI) (Weintrob et al., 2003).
AHI, the period from initial transmission until HIV antibodies develop (approximately 22 days after infection) (Fiebig et al., 2003; Lindback et al., 2000), is the most infectious stage of HIV (Hollingsworth, Anderson and Fraser, 2008; Jacquez et al., 1994; Leynaert, Downs and de Vincenzi, 1998; Pilcher et al., 2004; Wawer et al., 2005) because HIV viral replication and shedding peak during this time, resulting in extremely high viral loads (sometimes >1,000,000 copies/ml) (Quinn, 1997; Schacker et al., 1996; Schacker et al., 1998). There are estimates that 43% of all new HIV infections in the US may be transmitted by persons with AHI (Wawer et al., 2005). This is particularly concerning because people with AHI may be engaging in the same high-risk sexual behaviors that led to their recent acquisition of HIV, particularly if they do not yet know they are infected. (Colfax et al., 2002; Hecht et al., 2002; Jacquez et al., 1994; Koopman et al., 1997; Patel et al., 2006; Pilcher et al., 2001; Pilcher et al., 2004). Identifying and counseling individuals with AHI offers a critical opportunity to avert a significant number of HIV transmission events (Koopman et al., 1997; Patel et al., 2006; Pilcher et al., 2005; Pilcher et al., 2001), however little is known about these individuals to inform the development of prevention interventions for people in this stage of infection. Because diagnosis of AHI usually depends upon the clinical recognition of acute retroviral syndrome (ARS) (Kahn and Walker, 1998), which is characterized by non-specific symptoms (Hecht et al., 2002; Schacker, 1996), it is rarely diagnosed, which significantly hinders entry into care and assessment of AHI (Bollinger et al., 1997; Kahn and Walker, 1998; Schacker et al., 1996; Vanhems et al., 1997). However, in 2002, the state of North Carolina (NC) began universal screening for AHI, testing all HIV-antibody negative specimens submitted for voluntary HIV testing at any publicly-funded testing site in NC, using a cost-effective screening system (Daar et al., 2001; Pilcher et al., 2005; Pincus et al., 2003). To gain an understanding of the health care seeking behaviors, risk perceptions and transmission risk behaviors of people with AHI, we assessed the demographic characteristics, HIV testing histories, risk perceptions, behaviors and health care access of 32 people diagnosed with AHI using this system in NC. We compared these factors among this group by gender and sexual orientation. Our ultimate goal in understanding factors associated with AHI is to promote recognition of early HIV infection so that care and prevention services-including partner services and risk reduction counseling may be provided.
Methods
Study Design
We conducted a cross-sectional observational interview study from September 1, 2003 to March 31, 2005 to characterize individuals diagnosed with AHI during routine HIV testing between January 1, 2003 and February 28, 2005.
Study Participant Identification
The NC Department of Health and Human Services (DHHS) HIV/STD Prevention and Care Branch’s current Screening and Tracing for Active HIV Transmission (STAT) Program screens all specimens with a negative or intermediate antibody test for AHI by nucleic acid amplification tests (NAAT). The STAT Program uses an algorithm that pools specimens into groups of nine which are then pooled into 10 groups of nine enabling 90 specimens at a time to be screened by one NAAT (methods more fully described elsewhere) (Pilcher et al., 2005). At the time of HIV test results delivery, clinical staff asked permission of all those who were AHI positive to be contacted regarding potential study participation. Eligibility criteria for the study reported here were as follows: at least 18 years of age, proficient in English, a positive pooled NAAT test after an initial negative HIV antibody test, and consent to be contacted for an interview.
Study Procedures/Measures
All eligible participants were interviewed for approximately 60 minutes face-to-face by trained study staff, using a standardized questionnaire that included closed and open-ended questions developed by researchers from the University of North Carolina. We collected information on the following topic areas: demographic characteristics, HIV-testing history, lifetime HIV risk factors, risk behavior during the year prior to HIV diagnosis, health care access, use of health services, health care delivery, and any symptoms of acute illness consistent with ARS. Written informed consent for participation was obtained at the time of the interview.
Data Analyses
De-identified data from the interviews were entered into a secure database for analysis. A quality control investigator confirmed accurate data entry. We conducted comparative statistics to assess differences between the demographic characteristics of persons diagnosed with AHI during the study period that did participate and did not participate. Descriptive, univariate statistics, as well as comparative analyses assessing differences in age and number of sexual partners by gender and self-reported sexual identity, were generated using statistical software STATA version 9.2. For highly skewed data, medians were reported. Participants were excluded from analyses for which they had information missing. Regarding sexual orientation, all participants were included in the group to which they self-identified. The group referred to as “MSM” included all men who had at least one male sexual partner in the previous year regardless of sexual orientation. Each analysis either specifies sexual orientation (gay, heterosexual, bisexual) or reported sexual behavior (MSM). Differences of statistical significance were not examined among the subgroup characteristics of gender, age, race, or sexual orientation due to the small sample size resulting in inadequate power to detect true differences.
Results
Study Participants
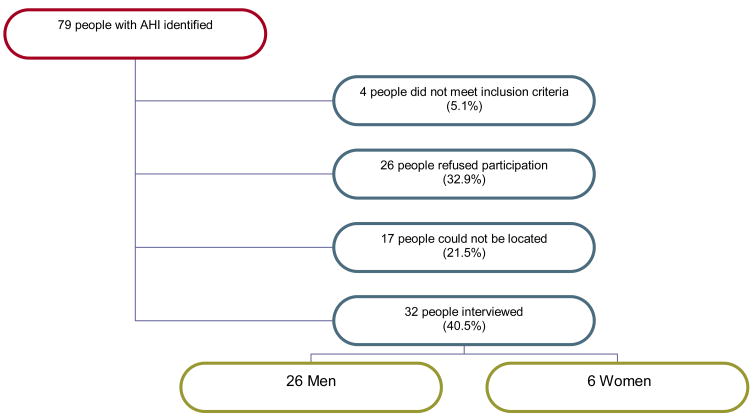
Seventy-nine people were diagnosed with AHI HIV between January 1, 2003 and February 28, 2005 (see Figure 1). Of these, 26 people declined to be contacted for an interview and 17 could not be located by study staff. Of the remaining 36 participants, 32 met the eligibility criteria. General demographic characteristics were not statistically significant between those who agreed to participate in the study and those who refused or could not be located. Reasons for declining participation included concerns about confidentiality (n=5), not wanting to talk about their diagnosis (n=2), not interested in study participation (n=6) and no reason given (n=13). All interviews took place within 12 months of AHI diagnosis (Median = 61 days, Range = 10–365 days).
Figure 1.
Selection of Study Participants from All People Identified with Acute HIV Infection (AHI) at All Publicly-Funded Testing Sites in NC Diagnosed Between 1/1/03–2/28/05
Demographic Characteristics
Demographic features of the study participants are displayed in Table 1. Of note, 81% were men, 59% African American, mean age was 29 with a much narrower age range for women (21–28 years) compared with men (19–63 years).
Table I.
Characteristics of People with Acute HIV Infection in NC Diagnosed Between 1/1/03–2/28/05
| Characteristics | Total No. | Women | Men | |||
|---|---|---|---|---|---|---|
| All men | Heterosexual | Bisexual | Homosexual | |||
| n=32 (%) | n=6 | n=26 | n=6 | n=7 | n=13 | |
| Age, mean years [SD] (range) | 29 [12] (19–63) | 27 [6] (21–28) | 30 [13] (19–63) | 38 [20] (19–63) | 22 [2] (19–25) | 30 [10] (19–51) |
| Race/Ethnicity | ||||||
| African American | 19 (59%) | 4 | 15 | 4 | 7 | 4 |
| White | 9 (28%) | 2 | 7 | 1 | 0 | 6 |
| Latino | 2 (6%) | 0 | 2 | 0 | 0 | 2 |
| Other | 2 (6%) | 0 | 2 | 1 | 0 | 1 |
| Relationship status | ||||||
| Married | 3 (9%) | 1 | 2 | 2 | 0 | 0 |
| Single & never been married | 20 (62%) | 3 | 17 | 2 | 5 | 10 |
| Long-term relationship with a partner | 5 (16%) | 1 | 4 | 1 | 1 | 2 |
| Divorced | 3 (9%) | 1 | 2 | 1 | 0 | 1 |
| Education | ||||||
| Less than high school | 5 (16%) | 2 | 3 | 1 | 0 | 2 |
| High school or obtained equivalency | 10 (31%) | 2 | 8 | 3 | 2 | 3 |
| Some college or technical trade school | 11 (34%) | 1 | 10 | 2 | 5 | 3 |
| 4 year college degree | 3 (9%) | 1 | 2 | 0 | 0 | 2 |
| Graduate or professional school | 3 (9%) | 0 | 3 | 0 | 0 | 3 |
| Employed | 23 (72%) | 2 | 21 | 5 | 6 | 10 |
| Has male sexual partners | 28 (87%) | 6 | 22 | 2 | 7 | 13 |
| Has female sexual partners | 11 (34%) | 0 | 11 | 6 | 5 | 0 |
| No. of lifetime sexual partners, [median], (range) | [20] (1–1000) | [9] (1–19) | [31] (1–1000) | [20] (1–72) | [12] (1–54) | [50] (2–1000) |
| No. of sexual partners in yr prior to AHI diagnosis, [median], (range) | [6] (1–100) | [1] (1–8) | [7] (1–100) | [2] (1–8) | [5] (1–8) | [22] (3–100) |
| Any health care coverage in year prior to HIV positive test | 22 (69%) | 4 | 18 | 5 | 3 | 10 |
| Private health insurance plan from employer or workplace | 13 (59%) | 3 | 10 | 2 | 1 | 7 |
| Private health insurance plan purchased directly | 1 (3%) | 0 | 1 | 1 | 0 | 0 |
| Medicare | 1 (3%) | 1 | 0 | 0 | 0 | 0 |
| Medicaid/medical assistance/other state program | 3 (9%) | 2 | 1 | 1 | 0 | 0 |
| On parents insurance plan | 7 (22%) | 0 | 7 | 2 | 2 | 3 |
Sexual Identity and Gender of Sexual Partners
All women (n=6) identified as heterosexual and reported only male sexual partners (see Table I). Twenty-two men (85%) reported having male sexual partners, and 42% reported having female sexual partners in their lifetime. Of the six men who identified as heterosexual, all had female sexual partners, and two also reported at least one male sexual partner in his lifetime. Of the seven men who identified as bisexual, all reported having had male sexual partners and five reported having had female sexual partners. None of the 13 men who identified as gay reported having any lifetime female partners.
Number of Sexual Partners and HIV Transmission Risk Behaviors
The median number of lifetime sexual partners of all participants was 20 (range, 1–1,000). Heterosexual men had a median of 20 partners (range, 1–72 partners), bisexual men 12 partners (range, 1–54 partners), and gay men 50 partners (range, 2-1,000 partners) (see Table I).
The median number of sexual partners in the year prior to diagnosis for all participants was six (range, 1–100 partners) (see Table II). Among the men, heterosexual men had a median of two partners (range, 1–8 partners), bisexual men had a median of five partners (range, 1–8 partners), and gay men had a median of 22 partners (range, 3–100 partners) in the year prior to being diagnosed with AHI. In the year prior to diagnosis, women had a median of one partner (range, 1–8).
Table II.
HIV Risk Factors in Year Prior to HIV Diagnosis by Gender and Sexual Orientation of People with Acute HIV Infection in NC Diagnosed Between 1/1/03–2/28/05
| Risk Factor | Women | Men | |||
|---|---|---|---|---|---|
| All men | Heterosexual | Bisexual | Homosexual | ||
| n=6 (%) | n=26 (%) | n=6 | n=7 | n=13 | |
| Intravenous drug use (IVDU) | 0 | 0 | 0 | 0 | 0 |
| With a steady female partner | |||||
| Oral sex | 0 | 4 (15%) | 3 | 1 | 0 |
| Protected vaginal sex | NA | 4 (15%) | 2 | 2 | 0 |
| Unprotected vaginal sex | NA | 6 (23%) | 4 | 2 | 0 |
| Protected or unprotected anal sex | NA | 0 | 0 | 0 | 0 |
| With a casual female partner | |||||
| Oral sex | 0 | 3 (12%) | 2 | 1 | 0 |
| Protected vaginal sex | NA | 3 (12%) | 2 | 1 | 0 |
| Unprotected vaginal sex | NA | 3 (12%) | 3 | 0 | 0 |
| Protected or unprotected anal sex | NA | 0 | 0 | 0 | 0 |
| With a steady male partner | |||||
| Protected oral sex | 0 | 2 (8%) | 0 | 2 | 0 |
| Unprotected oral sex | 5 (83%) | 20 (77%) | 2 | 5 | 13 |
| Protected vaginal sex | 2 (33%) | NA | NA | NA | NA |
| Unprotected vaginal sex | 4 (67%) | NA | NA | NA | NA |
| Protected receptive anal sex | 0 | 15 (58%) | 2 | 4 | 9 |
| Unprotected receptive anal sex | 1 (17%) | 16 (62%) | 1 | 4 | 11 |
| Protected insertive anal sex | NA | 12 (46%) | 0 | 4 | 8 |
| Unprotected insertive anal sex | NA | 12 (46%) | 0 | 4 | 8 |
| With a casual male partner | |||||
| Protected oral sex | 0 | 3 (12%) | 0 | 3 | 0 |
| Unprotected oral sex | 2 (33%) | 16 (62%) | 2 | 4 | 10 |
| Protected receptive anal sex | 0 | 15 (58%) | 2 | 4 | 9 |
| Unprotected receptive anal sex | 0 | 10 (38%) | 1 | 3 | 6 |
| Protected insertive anal sex | NA | 11 (42%) | 0 | 3 | 8 |
| Unprotected insertive anal sex | NA | 9 (35%) | 0 | 3 | 6 |
In the year before diagnosis, no heterosexually-identified men reported engaging in any acts of insertive anal sex with another man or with a woman, but 2 reported receptive (1 unprotected) anal sex, and 4 reported unprotected vaginal sex. In their pre-diagnosis year, 85% of the gay-identified men reported unprotected receptive anal sex with a steady male partner and 62% reported unprotected insertive anal sex with a steady male partner. Almost half of the gay-identified men (n=6) reported unprotected insertive and unprotected receptive anal sex. None of the women and only 8% of the men reported having protected oral sex. No one reported using injection drugs in the year before AHI diagnosis.
Perception of Risk
When asked to describe what their perceptions had been of their risk of contracting HIV during the year before receiving their AHI diagnosis, 78% of participants reported that they had perceived their risk to have been “unlikely” or “very unlikely”. Six percent had believed, during that year before infection, that their risk of HIV infection was “likely”. A majority of the participants (53% of the total; 83% of women, 57% of MSM, 17% of heterosexual men) thought that they acquired their HIV infection from a steady partner, (defined as someone they had known for more than two months, who they had an emotional bond with, and with whom they had regular sex, such as a boyfriend, spouse, significant other or life partner). More than half (n=17) of the sample reported that before they were diagnosed with HIV, they had thought the chances their steady partner could have been infected with an STI (Sexually Transmitted Infection) or HIV was “unlikely” or “very unlikely”. In contrast, when asked about their thoughts regarding their casual partners before diagnosis, only 30% (n=7) felt their casual partners were “unlikely” or “very unlikely” to have an STI or HIV among individuals (n=23) reporting causal partners.
Lifetime History of Sexually Transmitted Infections
One quarter of the study participants (n = 8) reported a history of Neisseria gonorrhoeae during their lifetime. In the past, five participants (16%) had been diagnosed with Chlamydia trachomatis, five (16%) with hepatitis B, and four (13%) with syphilis.
Lifetime History of HIV Testing (Table III) and Reasons for Testing
Table III.
Number and Pattern of Lifetime HIV Testing by Demographic Characteristics of People with Acute HIV Infection in NC Diagnosed Between 1/1/03–2/28/05
| Lifetime Number of Tests | Pattern of Testing | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Once | Twice | 3–4 tests | 5–10 tests | >10 tests | Every 3 months | Every 6 months | Yearly | <Once per year | Othera | |
| All (n=32) | 4 | 5 | 11 | 8 | 4 | 2 | 11 | 2 | 8 | 9 |
| Race | ||||||||||
| Black (n=19) | 2 | 4 | 8 | 4 | 1 | 0 | 6 | 1 | 5 | 7 |
| White (n=9) | 1 | 1 | 2 | 3 | 2 | 1 | 4 | 1 | 2 | 1 |
| Other (n=4) | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| Gender | ||||||||||
| Male (n=26) | 4 | 4 | 9 | 5 | 4 | 2 | 10 | 2 | 7 | 5 |
| Female (n=6) | 0 | 1 | 2 | 3 | 0 | 0 | 1 | 0 | 1 | 4 |
| Sexual Identity Reported by Male Participants | ||||||||||
| Heterosexual (n=6) | 3 | 1 | 1 | 1 | 0 | 0 | 2 | 0 | 4 | 0 |
| Bisexual (n=7) | 0 | 2 | 4 | 0 | 1 | 0 | 3 | 1 | 1 | 2 |
| Gay (n=13) | 1 | 1 | 4 | 4 | 3 | 2 | 5 | 1 | 2 | 3 |
Other includes: Pregnancy (3), based on start or end of relationships (3), no specific pattern (3)
Overall, more than one third of the study participants (38%) had tested 5 or more times for HIV infection (see Table IV). Only four people (12%) reported that their positive AHI test was their first HIV test. About half (54%) of the gay-identified men reported testing for HIV at least every 6 months, compared to 43% of the bisexual men, 33% of heterosexual men and 17% of women.
Table IV.
Reported Reasons for Voluntarily Seeking an HIV Test of People with Acute HIV Infection in NC Diagnosed Between 1/1/03–2/28/05a
| Reasons for HIV testing at time of + test | Number of people (%) (n=32) |
|---|---|
| Wanted Reassurance, Make Sure You Were Negative | 18 (56%) |
| Had Unprotected Anal Sex With a Man | 18 (56%) |
| Symptoms of Acute Retroviral Syndrome (ARS) | 10 (31%) |
| Part of a Routine Physical Examination or Medical Check-Up | 8 (25%) |
| Sex Partner is HIV-Positive | 5 (16%) |
| Symptoms of Other STI | 5 (16%) |
| Sex Partner Asked You To | 4 (12%) |
| Had Unprotected Vaginal Sex with a Woman (n=26) | 3 (9%) |
| Routine Practice | 3 (9%) |
| Sex Partner may be HIV-Positive | 2 (6%) |
| Pregnancy | 1 (3%) |
| Undefined High-Risk Exposure | 1 (3%) |
| Contacted By DISb Regarding Exposure | 1 (3%) |
| Shared Needles or Syringes | 0 |
| Any Other Reasons | 24 (75%) |
Reasons for seeking an HIV test were not mutually exclusive. Most participants gave more than one reason for testing and so individuals are represented several times in the table.
Disease Intervention Specialists (DIS) contact people who have been reported as exposed to HIV by someone newly diagnosed with the disease.
Most individuals (78%) reported multiple reasons for seeking testing at the time of their positive AHI diagnosis (see Table IV), with the majority reporting being tested because they “had unprotected anal sex with a man” (56%), and/or because they “wanted the reassurance that they were HIV negative” (56%). Ten people (31%) had an HIV test because they felt sick at that time. Only six participants (19%) reported ever hearing at all about AHI before their diagnosis.
Acute Retroviral Syndrome and Medical Evaluation
Three quarters of participants (n = 24) reported feeling ill some time during the three months before their positive test for AHI. Twenty had sought medical attention for their illness. The reasons that four people did not seek medical attention despite having ARS symptoms included: the cost of medical care or lack of insurance (n=3); the symptoms were not severe enough to warrant seeking medical care (n=3); and/or the symptoms resolved on their own (n=2).
Of the 24 individuals who experienced ARS symptoms, the most commonly reported symptoms were fever (80%), loss of appetite/weight loss (76%), gastrointestinal upset (68%), sore throat (64%). and skin rashes (31%).
Of the 20 people who sought medical care for ARS-like symptoms in the three months prior to their AHI diagnosis, 12 people (60%) sought care in an emergency department or urgent care, six people sought care from a primary care provider, one in a student health clinic and one at an infectious disease clinic.
Ten of those who sought medical care were diagnosed with a viral syndrome (such as an upper respiratory infection, hepatitis, influenza, and gastroenteritis). Five were diagnosed with bacterial infections (such as streptococcal pharyngitis, Rocky Mountain Spotted Fever, and pneumonia), one person was told that he was experiencing a stress reaction and another was evaluated for a myocardial infarction. Three individuals were accurately diagnosed with AHI at their initial sick visit. Seven individuals were prescribed unnecessary antibiotics. Of the 17 misdiagnosed individuals, none were tested for HIV at their initial visit, nine sought later testing at either an HIV counseling and testing site or an STD (Sexually Transmitted Disease) clinic, seven were tested during subsequent medical evaluation at either a hospital, specialty clinic or their primary provider, and one was tested by State Partner Counseling and Referral Officers due to being named as a contact of a HIV positive partner.
Discussion
In this study, we identified several areas of missed opportunities in the care of individuals with AHI, which, if improved upon, could reduce HIV transmission domestically. First, although participants reported risky activities, high rates of previous STI infections, and relatively high rates of HIV testing, few perceived themselves to be at high risk of acquiring HIV during the period when they acquired it. Furthermore, few were aware of AHI or that ARS symptoms could suggest an HIV infection. The vast majority of persons with AHI in NC were relatively young MSM. Taken together, our findings suggest that while educating young MSM about their actual risks of HIV acquisition is a critical first step, providing additional guidance around HIV testing frequency and supporting more programs that reinforce peer norms around condom use may be needed. Second, a majority of persons with AHI sought medical care for symptoms of ARS but were misdiagnosed. Thus, programs are critically needed to increase awareness of AHI among providers, especially those caring for MSM. Additionally, community and patient education programs focusing on ARS symptoms could help to increase awareness of the need for HIV testing at the time of symptoms among vulnerable populations. Finally, a substantial minority of persons with AHI symptoms did not seek medical attention, some due to the cost of care, suggesting that inability to pay for medical care may hinder health care and public health practitioners’ capacity to identify individuals with AHI.
In this study the percentage of people identified with AHI who were MSM (85%) was nearly double the percentage of MSM (46% and 49%) diagnosed with any stage of HIV in similar years in NC and as reported by the CDC, respectively (NC HIV Surveillance Report, 2007; CDC, 2007). Two of the heterosexually identified men in this study, both of whom were young, college educated African Americans, reported having sex with other men and suspecting that their steady male partner had transmitted HIV to them. These findings are consistent with other studies indicating that substantial proportions of heterosexually identified Black men report having sex with men (Doll et al., 1992; Goldbaum et al., 1998; McKirnan et al., 2001; Millett et al., 2005; Ross et al., 2003; Wohl et al., 2002) and are less likely to disclose their sexual orientation or behaviors (CDC, 2003b; Kennamer et al., 2000; Ostrow et al., 1991). These findings also support the notion that patients and providers need to discuss the patient’s sexual activities and perceptions of HIV acquisition risk.
The lack of women identified with AHI in this study, a trend frequently seen in other AHI studies (Celum et al., 2001; Hecht et al., 2002; Pao et al., 2005; Patel et al., 2006; Pilcher et al., 2005; Pincus et al., 2003; Sabundayo et al., 2006; Schacker et al., 1996; Weintrob et al., 2003) could be confounded by women receiving less testing than men, which could be due to either less HIV testing or providers routinely offering it less to women (Siegel, Karus and Raveis, 1997). In our study all of the women had received health care in the year prior to their diagnosis (median, 5 times (range 2–20)), but none reported being asked about or given advice regarding HIV prevention and/or testing. Again, these findings suggest the need to educate providers to ask female patients about risk factors for HIV and to be more poised to encourage testing women for HIV.
Unprotected oral-genital contact, which has been shown to less efficiently transmit HIV, was more frequent in our study participants known to have just acquired HIV than unprotected anal-genital contact (Kingsley et al., 1987). Other studies have shown similar findings (Schacker et al., 1996; Schwarcz et al., 1995) and suggest that the rates of transmission for oral genital contact may be higher than originally suspected. While such findings may reflect underreporting of improper condom use during non-oral sex, another potential missed opportunity may be to direct increased attention to oral-genital contact as a risk factor for HIV (Schacker et al., 1996 Velum et al., 2001).
This study has some limitations. The cross-sectional design limits our ability to determine temporality and thus to address causality. However, because little is known about those who have AHI, descriptive prevalence studies that attempt to test the universe of patients diagnosed with AHI are informative and useful. Second, the fact that the majority of men in this study identified as either gay or bisexual may reflect a detection bias as they tested for HIV more often than men who have sex with women only. Third, because of our use of self-reported information and that the diagnosis of HIV preceded the interview, recall bias may have affected participants’ perceptions of their risk behaviors prior to diagnosis (Pao et al., 2005). Fourth, the power to detect statistically significant differences was limited in our study due to the small sample size. A strength of this study is that, rather than using a convenience sample as has been implemented in most other AHI studies, we were able to identify all the AHI diagnosed in one state in a given time period and interview all those who could be located and agreed to participate. While our findings are useful in similar settings, they may not be generalizable to other states or regions that may have different demographic characteristics.
Throughout the history of the AIDS epidemic, it has often proven difficult to identify these high-risk groups until an outbreak is well underway. It is our hope that this research might reduce the number of missed opportunities by better facilitating early diagnosis (Cates, Chesney and Cohen, 1997; CDC, 2004; Koopman et al., 1997), therapeutic intervention, and behavioral counseling during AHI (Colfax et al., 2002; Schacker et al., 1996). An early and accurate assessment and diagnosis of AHI could eliminate much of the extensive clinical evaluation of serological tests, blood cultures, imaging studies, and hospitalization that individuals with ARS often go through before arriving at the correct diagnosis (Weintrob et al., 2003). Public health programs may be able to decrease the infectiousness of individuals with AHI through the timely initiation of antiretroviral therapy, which can control genital tract shedding and decrease the level of plasma viremia during acute infection (Pilcher et al., 2001; Yerly et al., 2001).
Acknowledgments
This study was supported by a Developmental Grant from the University of North Carolina Center for AIDS Research (CFAR) and NIMH K23 MH075718-01
References
- Bollinger RC, Brookmeyer RS, Mehendale SM, Paranjape RS, Shepherd ME, Gadkari DA, Quinn TC. Risk factors and clinical presentation of acute primary HIV infection in India. Journal of the American Medical Association. 1997;278:2085–2089. [PubMed] [Google Scholar]
- Cates W, Jr, Chesney MA, Cohen MS. Primary HIV infection--a public health opportunity. American Journal of Public Health. 1997;87:1928–1930. doi: 10.2105/ajph.87.12.1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Advancing HIV Prevention: New Strategies for a Changing Epidemic - United States, 2003. Morbidity and Mortality Weekly Report. 2003a;52:329 – 332. [PubMed] [Google Scholar]
- CDC. HIV/STD Risks in Young Men Who Have Sex with Men Who Do Not Disclose Their Sexual Orientation: Six U.S. Cities, 1994–2000. Atlanta: Centers for Disease Control and Prevention; 2003b. [PubMed] [Google Scholar]
- CDC. HIV transmission among black college student and non-student men who have sex with men--North Carolina, 2003. Morbidity and Mortality Weekly Report. 2004;53:731–734. [PubMed] [Google Scholar]
- Celum CL, Buchbinder SP, Donnell D, Douglas JM, Jr, Mayer K, Koblin B, Marmor M, Bozeman S, Grant RM, Flores J, Sheppard HW. Early human immunodeficiency virus (HIV) infection in the HIV Network for Prevention Trials Vaccine Preparedness Cohort: risk behaviors, symptoms, and early plasma and genital tract virus load. Journal of Infectious Diseases. 2001;183:23–35. doi: 10.1086/317658. [DOI] [PubMed] [Google Scholar]
- Colfax GN, Buchbinder SP, Cornelisse PGA, Vittinghoff E, Mayer K, Celum C. Sexual risk behaviors and implications for secondary HIV transmission during and after HIV seroconversion. AIDS. 2002;16:1529 – 1535. doi: 10.1097/00002030-200207260-00010. [DOI] [PubMed] [Google Scholar]
- Daar ES, Little S, Pitt J, Santangelo J, Ho P, Harawa N, Kerndt P, Glorgi JV, Bal J, Gaut P, Richman DD, Mandel S, Nichols S. Diagnosis of Primary HIV-1 Infection. Annals of Internal Medicine. 2001;134:25 – 29. doi: 10.7326/0003-4819-134-1-200101020-00010. [DOI] [PubMed] [Google Scholar]
- Doll LS, Petersen LR, White CR, Johnson ES, Ward JS. Homosexually and Nonhomosexually Identified Men Who have Sex with Men: A Behavioral Comparison. Journal of Sex Research. 1992;29:1–14. [Google Scholar]
- Fiebig EW, Wright DJ, Rawal BD, Garrett PE, Schumacher RT, Peddada L, Heldebrant C, Smith R, Conrad A, Kleinman SH, Busch MP. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS. 2003;17:1871 – 1879. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- Goldbaum G, Perdue T, Wolitski R, Rietmeijer C, Hedrich A, Wood R, Fishbein M, Cohn D, Corby N, Freeman A, Guenther-Grey C, Sheridan J, Tross S. Differences in Risk Behavior and Sources of AIDS Information among Gay, Bisexual, and Straight-Identified Men Who have Sex with Men. AIDS and Behavior. 1998;2:13–21. [Google Scholar]
- Hall HI, Song R, Rhodes P, Prejean J, An Q, Lee LM, Karon J, Brookmeyer R, Kaplan EH, McKenna MT, Janssen RS. Estimation of HIV Incidence in the United States. Journal of the American Medical Association. 2008;300:520–29. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht FM, Busch MP, Rawal BD, Webb M, Rosenberg E, Swanson M, Chesney M, Anderson J, Levy J, Kahn JO. Use of laboratory tests and clinical symptoms for identification of primary HIV infection. AIDS. 2002;16:1119 – 1129. doi: 10.1097/00002030-200205240-00005. [DOI] [PubMed] [Google Scholar]
- Hollingsworth TD, Anderson RM, Fraser C. HIV-1 Transmission, by Stage of Infection. Journal of Infectious Diseases. 2008;198:687–93. doi: 10.1086/590501. [DOI] [PubMed] [Google Scholar]
- Jacquez JA, Koopman JS, Simon CP, Longini LM. Role of the Primary Infection in Epidemics of HIV Infection in Gay Cohorts. Journal of Acquired Immune Deficiency Syndromes. 1994;7:1169 – 1184. [PubMed] [Google Scholar]
- Kahn JO, Walker BD. Acute human immunodeficiency virus type 1 infection. New England Journal of Medicine. 1998;339:33–39. doi: 10.1056/NEJM199807023390107. [DOI] [PubMed] [Google Scholar]
- Kennamer JD, Honnold J, Bradford J, Hendricks M. Differences in disclosure of sexuality among African American and White gay/bisexual men: Implications for HIV/AIDS prevention. AIDS Education and Prevention. 2000;12:519–531. [PubMed] [Google Scholar]
- Kingsley L, Kaslow R, Rinaldo C, Detre K, Odaka N, Vanraden M, Detels R, Polk BF, Chmiel J, Kelsey SF, Ostrow D, Visscher B. Risk Factors For Seroconversion to Human Immunodeficiency Virus Among Male Homosexuals: Results from the Multicenter AIDS Cohort Study. The Lancet. 1987;329:345–349. doi: 10.1016/s0140-6736(87)91725-9. [DOI] [PubMed] [Google Scholar]
- Koopman JS, Jacquez JA, Welch GW, Simon CP, Foxman B, Pollock SM, Barth-Jones D, Adams AL, Lange K. The Role of Early HIV Infection in the Spread of HIV Through Populations. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology. 1997;14:249 – 258. doi: 10.1097/00042560-199703010-00009. [DOI] [PubMed] [Google Scholar]
- Leynaert B, Downs AM, de Vincenzi I. Heterosexual Transmission of Human Immunodeficiency Virus: Variability of Infectivity throughout the Course of Infection. American Journal of Epidemiology. 1998;148:88–96. doi: 10.1093/oxfordjournals.aje.a009564. [DOI] [PubMed] [Google Scholar]
- Lindback S, Thorstensson R, Karlsson AC, Sydow M, Flamholc L, Blaxhult A, Sonnerborg A, Biberfeld G, Gaines H. Diagnosis of primary HIV-1 infection and duration of follow-up after HIV exposure. AIDS. 2000;14:2333 – 2339. doi: 10.1097/00002030-200010200-00014. [DOI] [PubMed] [Google Scholar]
- McKirnan DJ, Stokes JP, Doll L, Burzette RG. Bisexually active men: Social characteristics and sexual behavior. Journal of Sex Research. 1995;3(2):65–76. [Google Scholar]
- McKirnan DJ, Vanable PA, Ostrow DG, Hope B. Expectancies of sexual “escape” and sexual risk among drug and alcohol-involved gay and bisexual men. Journal of Substance Abuse. 2001;13:137–154. doi: 10.1016/s0899-3289(01)00063-3. [DOI] [PubMed] [Google Scholar]
- Millett G, Malebranche D, Mason B, Spikes P. Focusing “down low”: Bisexual black men, heterosexual black women, and HIV risk. Journal of the National Medical Association. 2005;97:52S–59S. [PMC free article] [PubMed] [Google Scholar]
- North Carolina HIV/STD Surveillance Report. 2007 Available at: http://www.epi.state.nc.us/epi/hiv/stats.html.
- Ostrow DG, Whitaker RE, Frasier K, Cohen C, Wan J, Frank C, Fisher E. Racial differences in social support and mental health in men with HIV infection: A pilot study. AIDS Care. 1991;3:55–62. doi: 10.1080/09540129108253047. [DOI] [PubMed] [Google Scholar]
- Pao D, Fisher M, Hue S, Dean G, Murphy G, Cane PA, Sabin CA, Pillay D. Transmission of HIV-1 during primary infection: relationship to sexual risk and sexually transmitted infections. AIDS. 2005;19:85–90. doi: 10.1097/00002030-200501030-00010. [DOI] [PubMed] [Google Scholar]
- Patel P, Klausner JD, Bacon OM, Liska S, Taylor M, Gonzalez A, Kohn RP, Wong W, Harvey S, Kerndt PR, Holmberg SD. Detection of acute HIV infections in high-risk patients in California. Journal of Acquired Immune Deficiency Syndromes. 2006;42:75 – 79. doi: 10.1097/01.qai.0000218363.21088.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilcher CD, Eron JJ, Vemazza PL, Battegay M, Harr T, Yerly S, Vom S, Perrin L. Sexual Transmission During the Incubation Period of Primary HIV Infection. Journal of the American Medical Association. 2001;286:1713 – 1714. doi: 10.1001/jama.286.14.1713. [DOI] [PubMed] [Google Scholar]
- Pilcher CD, Fiscus SA, Nguyen TQ, Foust E, Wolf L, Williams D, Ashby R, O’Dowd JO, McPherson JT, Stalzer B, Hightow L, Miller WC, Eron JJ, Jr, Cohen MS, Leone PA. Detection of acute infections during HIV testing in North Carolina. New England Journal of Medicine. 2005;352:1873–1883. doi: 10.1056/NEJMoa042291. [DOI] [PubMed] [Google Scholar]
- Pilcher CD, Shugars DC, Fiscus SA, Miller WC, Menezes P, Giner J, Dean B, Robertson K, Hart CE, Lennox JL, Eron JJ, Hicks CB. HIV in body fluids during primary HIV infection: implications for pathogenesis, treatment and public health. AIDS. 2001;15:837 – 845. doi: 10.1097/00002030-200105040-00004. [DOI] [PubMed] [Google Scholar]
- Pilcher CD, Tien CH, Eron JJ, Vernazza PL, Leu SY, Stewer PW, Goh LE, Cohen MS. Brief but efficient: acute HIV infection and the sexual transmission of HIV. Journal of Infectious Diseases. 2004;18(9):1785–1792. doi: 10.1086/386333. [DOI] [PubMed] [Google Scholar]
- Pincus JM, Crosby SS, Losina E, King ER, LaBelle C, Freedberg KA. Acute Human Immunodeficiency Virus Infection in Patients Presenting to an Urban Urgent Care Center. Clinical Infectious Diseases. 2003;37:1699 – 1704. doi: 10.1086/379772. [DOI] [PubMed] [Google Scholar]
- Quinn TC. Acute primary HIV infection. Journal of the American Medical Association. 1997;278:58–62. [PubMed] [Google Scholar]
- Ross MW, Essien EJ, Williams ML, Fernandez-Esquer ME. Concordance between sexual behavior and sexual identity in street outreach samples of four racial/ethnic groups. Sexually Transmitted Diseases. 2003;30:110–113. doi: 10.1097/00007435-200302000-00003. [DOI] [PubMed] [Google Scholar]
- Sabundayo BP, McArthur JH, Langan SJ, Gallant JE, Margolick JB. High frequency of highly active antiretroviral therapy modifications in patients with acute or early human immunodeficiency virus infection. Pharmacotherapy. 2006;26:674–681. doi: 10.1592/phco.26.5.674. [DOI] [PubMed] [Google Scholar]
- Schacker T, Collier AC, Hughes J, Shea T, Corey L. Clinical and epidemiologic features of primary HIV infection. Annals of Internal Medicine. 1996;125:257–264. doi: 10.7326/0003-4819-125-4-199608150-00001. [DOI] [PubMed] [Google Scholar]
- Schacker TW, Hughes JP, Shea T, Coombs RW, Corey L. Biological and virologic characteristics of primary HIV infection. Annals of Internal Medicine. 1998;128:613 – 620. doi: 10.7326/0003-4819-128-8-199804150-00001. [DOI] [PubMed] [Google Scholar]
- Schwarcz SK, Kellogg TA, Kohn RP, Katz MH, Lemp GF, Bolan GA. Temporal Trends in Human Immunodeficiency Virus Seroprevalence and Sexual Behavior at the San Francisco Municipal Sexually Transmitted Disease Clinic, 1989–1992. American Journal of Epidemiology. 1995;142:314–322. doi: 10.1093/oxfordjournals.aje.a117637. [DOI] [PubMed] [Google Scholar]
- Siegel K, Karus D, Raveis VH. Testing and treatment behaviour of HIV-infected women: White, African-American, Puerto Rican comparisons. AIDS Care. 1997;9:297–309. doi: 10.1080/713613154. [DOI] [PubMed] [Google Scholar]
- Vanhems P, Allard R, Cooper DA, Perrin L, Vizzard J, Hirschel B, Kinloch-de Loes S, Carr A, Lambert J. Acute human immunodeficiency virus type 1 disease as a mononucleosis-like illness: is the diagnosis too restrictive? Clinical Infectious Diseases. 1997;24:965–970. doi: 10.1093/clinids/24.5.965. [DOI] [PubMed] [Google Scholar]
- Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Li X, Laeyendecker O, Kiwanuka N, Kigozi G, Kiddugavu M, Lutalo T, Nalugoda F, Wabwire-Mangen F, Meehan MP, Quinn TC. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. Journal of Infectious Diseases. 2005;191:1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- Weintrob AC, Giner J, Menezes P, Patrick E, Benjamin DK, Lennox J, Pilcher CD, Eron JJ, Hicks CB. Infrequent Diagnosis of Primary Human Immunodeficiency Virus Infection. Archives of Internal Medicine. 2003;163:2097 – 2100. doi: 10.1001/archinte.163.17.2097. [DOI] [PubMed] [Google Scholar]
- Wohl AR, Johnson DF, Lu S, Jordan W, Beall G, Currier J, Simon PA. HIV risk behaviors among African American men in Los Angeles county who self-identify as heterosexual. Journal of Acquired Immune Deficiency Syndromes. 2002;31:354–360. doi: 10.1097/00126334-200211010-00013. [DOI] [PubMed] [Google Scholar]
- Yerly S, Vora S, Rizzardi P, Chave JP, Vernazza PL, Flepp M, Telenti A, Battegay M, Veuthey AL, Bru JP, Rickenbach M, Hirschel B, Perrin L Swiss HIV Cohort Study. Acute HIV infection: impact on the spread of HIV and transmission of drug resistance. AIDS. 2001;15:2287–92. doi: 10.1097/00002030-200111230-00010. [DOI] [PubMed] [Google Scholar]