Summary
Although T helper 17 (Th17) cells have been found in human tumor tissues, their function in cancer immunity is unclear. Here we show that IL-17-deficient mice were more susceptible to the development of lung melanoma. Conversely, adoptive T cell therapy with tumor-specific Th17 cells prevented tumor development. Importantly, the donor Th17 cells retained their cytokine expression phenotype and exhibited stronger therapeutic efficacy than Th1 cells. Unexpectedly, therapy using Th17 but not Th1 cells elicited a remarkable activation of tumor-specific CD8+ T cells, which were necessary for the anti-tumor effect. Th17 cells promoted dendritic cell recruitment into the tumor tissues and greatly increased the numbers of CD8α+ dendritic cells containing tumor materials in draining lymph nodes. Moreover, compared to Th1 cells, Th17 cells promoted CCL20 chemokine production in tumor tissues and tumor-bearing CCR6-deficient mice were completely impaired in responding to Th17 therapy. Our data indicate that Th17 cells elicit a protective inflammation that ultimately promotes the activation of tumor-specific CD8+ T cells. These findings have important implications in anti-tumor immunotherapies.
Introduction
CD4+ T cells, upon activation by antigen-presenting cells (APCs), differentiate into cytokine-expressing effector helper T (Th) cells, which are classified as Th1, Th2, Th17 and T follicular helper cells (Tfh) subsets based on their cytokine secretion and immune regulatory function. Th17 cells produce the pro-inflammatory cytokines IL-17, IL-17F and IL-22 (Dong, 2008). As the signature cytokine of Th17 cells, IL-17 induces the expression of several chemokines (CCL2, CCL7, CXCL1, and CCL20) and matrix metalloproteinases (MMP3 and MMP13); transgenic overexpression of IL-17 in the lung provokes the induction of pro-inflammatory gene expression and tissue infiltration by leukocytes (Park et al., 2005). Conversely, inhibition of IL-17 signaling leads to impaired host defense against bacterial infection (Ye et al., 2001) and resistance to autoimmune diseases (Langrish et al., 2005; Nakae et al., 2003; Park et al., 2005; Yang et al., 2008).
Th17 cells and IL-17 expression have been found in various human tumors (Kryczek et al., 2007; Langowski et al., 2006; Miyahara et al., 2008; Sfanos et al., 2008; Zhang et al., 2008); however, their function in cancer immunity is unclear. IL-17 over-expression in tumor cell lines promotes angiogenesis and tumor growth when the tumors are implanted in immunodeficient mice, therefore suggesting a pro-tumor activity (Numasaki et al., 2003). In contrast, the expression of IL-17 in a hematopoietically-derived tumor was reported to promote tumor protection in immuno-competent hosts (Benchetrit et al., 2002). The basis for this discrepancy has not been understood, and the presence or absence of the adaptive immune system has been suggested to account for it (Martin-Orozco, 2009). Th17 cells highly express IL-23R; IL-23 is required for the late stage of Th17 development and also functions to expand Th17 cells and promote their function (Langrish et al., 2005; McGeachy et al., 2009). IL-23p19 mRNA expression has been found in several human carcinomas (Langowski et al., 2006). Moreover, IL-23-deficient mice (p19−/− and p40−/−) have been reported to be resistant to chemically induced tumors (Langowski et al., 2006). Paradoxically, the expression of IL-23 at the tumor site or therapy with dendritic cells expressing IL-23 can induce potent tumor-specific immunity against melanoma and glioma (Hu et al., 2006; Overwijk et al., 2006). More recently, it was shown that Th17 cells could protect against skin melanoma in a lymphopenic environment (Muranski et al., 2008); however, since the protection was dependent on IFN-γ, presumably due to conversion of Th17 to Th1 cells, the exact function of Th17 cells remains unclear.
In the current study, we first analyzed tumor development in IL-17-deficient mice using poorly immunogenic B16/F10 melanoma that colonizes to the lung and further used adoptive transfer of Th17 cells in several tumor prevention and treatment models. Our results indicate that IL-17 and Th17 cells play a protective role against tumors. Unexpectedly, tumor-specific Th17 cells triggered a strong CD8+ T cell response against the tumor. Th17 cell therapy promoted dendritic cell (DC) infiltration into tumor tissues and presentation of tumor antigens in the tumor-draining lymph nodes. Compared to Th1 cells, Th17 cells strongly induced CCL20 expression in the tumor tissues and CCR6 deficiency abrogates anti-tumor effects of Th17 cells. Our results thus reveal a protective function of Th17 cells in tumor immunity by eliciting cytotoxic T cell activation.
Results
Enhanced tumor growth in the absence of IL-17
To investigate the role of IL-17 in tumor development in vivo, we challenged IL-17-deficient mice (Yang et al., 2008) and wild-type (WT) age-matched controls on 129×B6 mixed background with B16/F10 melanoma injected intravenously. On days 14 and 16 after the challenge, IL-17−/− mice exhibited increased numbers of tumor foci and larger tumors in size when compared to WT mice (Figure 1A). Consistently, IL-17−/− mice that had been backcrossed to the C57BL/6 background also exhibit increased tumor burdens when compared to WT C57BL/6 mice (Supplementary Figure 1).
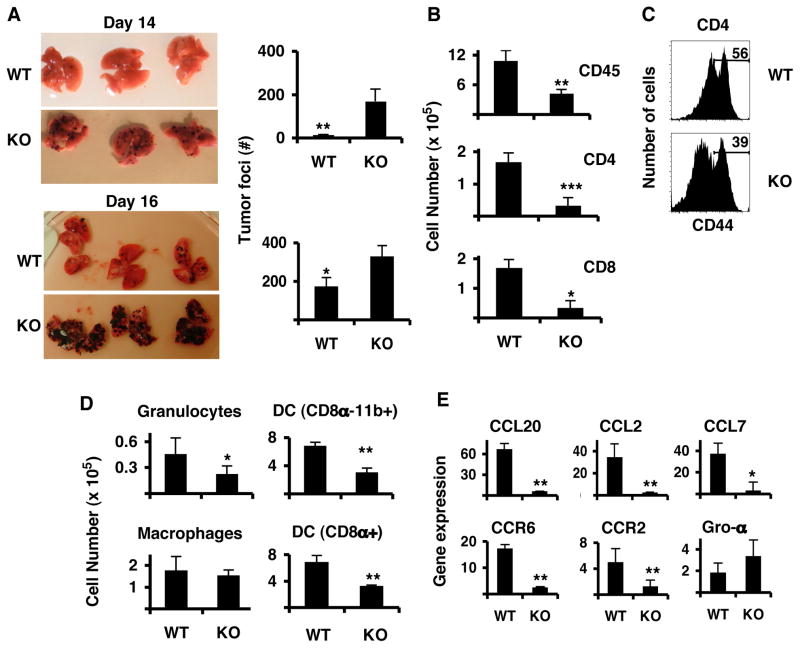
Figure 1. IL-17-deficient mice are more susceptible to B16/F10 melanoma development in the lung.
IL-17−/− (KO) and wild-type (WT) mice were challenged i.v. with B16/F10 melanoma and lungs were analyzed on days 14 and day 16. A. Photographs of the lungs. Graphs on the right show total number of tumor colonies present in the lung lobes (n=4, avg. +/− s.d). B–E. At day 16, lung leukocyte and lung cell fractions were isolated and processed for FACS analysis or RNA extraction. B. Total leukocyte cell numbers from lungs. The numbers were calculated from the percentages of total live cells that were gated on CD45+ cells (n=4, avg. +/− s.d.). C. Expression of CD44 on CD45+CD4+ T cells from the leukocyte fraction. D. Myeloid populations (n=4, avg. +/− s.d). E. Chemokine and chemokine receptor gene expression analyses from lung fractions, which were free of leukocytes. Shown are the averages of 4 mice after duplicate analysis per sample (n=4, avg. +/− s.d). Results shown are from a representative experiment of three, each using 4–5 mice per group.
Since IL-17 is involved in regulation of tissue inflammation, lungs from all mice were analyzed by flow cytometry 16 days after tumor implantation. Compared with WT mice, IL-17−/− mice had significantly reduced numbers of total CD45+ leukocytes (Figure 1B). All subsets of leukocytes including CD4+ and CD8+ T cells, granulocytes and CD11c+CD11b+ and CD11c+CD8α+ DC were all significantly reduced (Figure 1B, 1D). In contrast, CD11b+ macrophage numbers were not significantly different between the two groups. We also examined the activation status of T cells from the leukocyte fractions and found that CD4+ T cells from lungs of IL-17−/− mice showed reduced CD44 expression (54.13%+/−2.29 of WT vs. 40.30%+/−1.9 of KO, p=0.0036) (Figure 1C). Therefore, IL-17-deficient mice had fewer leukocytes to the tumor tissue and CD4+ T cells in the lung were also less activated.
To study how IL-17 deficiency affected leukocyte infiltration and favored tumor development, we analyzed the mRNA expression of IL-17-regulated chemokines and their receptors by lung cells (free of leukocytes) collected from WT and IL-17−/− mice that had B16/F10 tumors for 16 days. Lung cells from IL-17−/− mice had significantly reduced mRNA levels for chemokines CCL20, CCL2 and CCL7 when compared to WT mice (Figure 1E). Interestingly, mRNA levels for CCR2 (the receptor for CCL2) and CCR6 (the receptor for CCL20) were also found significantly reduced in lung cells of IL-17−/− mice (Figure 1E). CCR6 protein expression on lung DCs from IL-17−/− mice was present, but CD8α+ DC from lung lymph nodes (LLN) of WT mice expressed higher levels of CCR6 on their surface compared to those from IL-17−/− mice (MFI 1360+/− 86 for WT and 980+/− 105, p<0.05). Our results indicate tumor protection by IL-17, possibly by regulating chemokine-mediated leukocyte migration into tissues.
Anti-tumor Th17 cells reduce tumor growth in prevention models
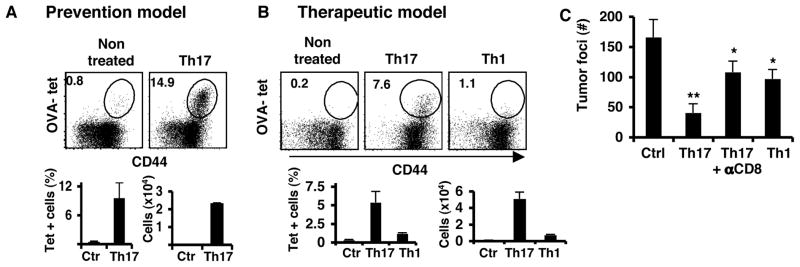
To further understand the function of Th17 cells in tumor immunity, we used a B16/F10 line that expresses chicken ovalbumin (B16-OVA) and performed adopted transfer experiments with OVA-specific Th17 cells. CD4+ T cells purified from CD45.1 OT-II transgenic mice were differentiated into Th17 effector cells in vitro (Chung et al., 2009; Nurieva et al., 2009). In each experiment, the differentiated Th17 cells typically contained >35% IL-17- and/or IL-17F-expressing T cells, with <2% IFN-γ-producing cells as evaluated by intracellular cytokine staining (Figure 2A). First, we transferred Th17 cells on the same day as B16-OVA tumor challenge. Mice treated with Th17 cells contained significantly reduced numbers of tumor colonies in the lung on day 16 compared to control mice that had not received any T cells (Figure 2B). The donor cells were detected in significant percentages in the lung and secondary lymphoid organs at the end point of the experiment, suggesting that Th17 cells did not suffer deletion and were circulating in the mice harboring tumors (Supplementary figure 2A). To substantiate the above finding, we used the BW TRP-1 TCR transgenic mouse, in which CD4+ T cells recognize the tyrosinase-related protein 1 (TRP-1), a melanocyte differentiation antigen present in normal melanocytes and in melanomas (Muranski et al., 2008). Transfer of TRP-1 Th17 cells at the time of the B16 tumor implantation completely inhibited the tumor growth in the lung (Figure 2C). From these results, we conclude that tumor-specific Th17 cells, regardless of their antigenic specificities, can protect mice from developing B16/F10 lung melanoma.
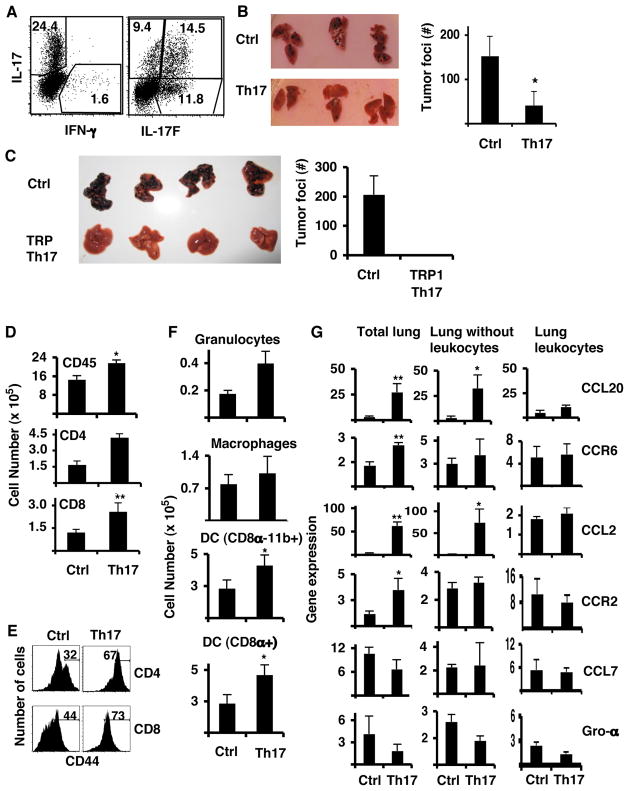
Figure 2. Anti-tumor Th17 cells prevent B16/F10 melanoma development.
A. Cytokine profiles of CD45.1+ OT-II cells polarized to Th17 that were used for the transfer. B,D,E and F. C57BL/6 mice were injected i.v. with 1×105 B16-OVA cells and 5 million OT-II Th17 cells. B. Photographs of lungs from untreated control mice (Ctrl) and mice treated with Th17 cells (Th17). The graph shows the average of tumor colony numbers from 5 mice (avg. +/− s.d.). C. C57BL/6 mice were injected i.v. with 1×105 B16/F10 cells and 5 million of TRP-1 Th17 cells. Shown are photographs of the lungs and the numbers of colonies in the lung (n=5, avg.+/− s.d). D. Numbers of total CD45+, or CD4+ or CD8+ T cells (n=5, avg. +/− s.d.) from the leukocyte fraction. E. Expression of CD44 on gated CD45.2+ CD4+ (upper panel) or CD45.2+ CD8+ T cells (lower panel). Numbers represent the percentages of CD44hi cells. F. Numbers of myeloid cell populations from leukocyte lung fraction were calculated from the percentages of live cells gated on CD45.2+CD11c+ CD11b+ (n=5, avg. +/− s.d.). G. Gene expression analysis of total lung derived cells with leukocytes, lung cells with tumor cells and no leukocytes and leukocyte fractions. Graphs represent the average values of four mice after duplicate analysis per sample (n=4, avg. +/− s.d). (* = p <0.05, ** = p< 0.01). Results shown are from one representative experiment of three using 4–5 mice per group.
To understand how Th17 cells mediate tumor protection, we analyzed the cellular composition of the lungs from the mice described above. OVA-specific Th17 cell-treated tumor-bearing mice had higher numbers of CD45+ leukocytes as well as CD4+ and CD8+ T cells in the lungs compared to the control mice (Figure 2D and Supplementary Figure 2B) and mice receiving TRP-1-specific Th17 cells had five times more CD8+ T cells over the control animals (Supplementary Figure 3A–B). Interestingly, CD4+ and CD8+ T cells from the mice receiving Th17 cells showed higher levels of CD44 expression than control mice (Figure 2E, Supplementary Figures 2C and 3C). When we analyzed the antigen-presenting cells from leukocyte fractions, we found that Th17-recipient mice also had increased numbers of CD11c+CD11b+ and CD11c+CD8α+ DCs and granulocytes while the numbers of macrophages were not altered (Figure 2F and Supplementary Figure 3D). Therefore, transferred Th17 cells induced the recruitment of DC as well as activated CD4+ and CD8+ T cells to the lung.
To understand the inflammatory regulation by Th17 cells, we further analyzed the expression of several chemokines and chemokine receptor genes by lung cells using real-time PCR. We analyzed non-fractionated total lung cells (total lung), leukocytes and leukocyte-depleted lung cells. We found that the expression of CCL20 and CCL2 was greatly increased in lungs from Th17-treated mice (Figure 2G), while CCL7 and Gro- levels were not increased. Further analysis after cellular fractionation revealed that the leukocyte fraction from both Th17-treated and control mice expressed similar levels of chemokines and chemokine receptors (Figure 2G); however, the leukocyte-free lung cells of Th17 cell-treated mice showed greatly increased expression of CCL20 and CCL2 than those from control animals (Figure 2G). These results suggest that Th17 cells in the lung might activate lung and/or tumor cells to produce chemokines CCL20 and CCL2 that promoted the recruitment of DCs and activated T cells.
To determine whether tumor-specific Th17 cells protect against tumors in different tissues other than lung and also to test a different tumor model, we applied a subcutaneous melanoma model and a subcutaneous fibrosarcoma model. Equal numbers of either Th1 or Th17 OT-II cells were transferred into C57BL/6 mice on the same day when B16-OVA cells or MCA205-OVA were implanted subcutaneously. Th17 cells greatly reduced growth of both B16 and MCA 205 in the skin, but Th1 cells had very minimal effects when compared with mice receiving no T cells (Supplementary Figure 4A and 4C). In MCA model, there was no survival advantage of Th17 over Th1 cells, since similar numbers of CD45.1 donor cells were recovered from draining lymph nodes on day 24 (Supplementary Figure 4D–4F).
Therapeutic effects of Th17 cells in mice with established tumors
We then tested whether the transfer of Th17 cells could also help eliminate established tumors. We compared Th17 vs Th1 cells in their protection against 5-day established pulmonary melanomas. Both Th17 and Th1 cytokine production were confirmed by ICS (Supplementary Figure 5) before transfer and equal numbers of cells were injected in C57BL/6 mice harboring B16-OVA. Compared with untreated control mice, those treated with Th1 cells had 40% fewer tumor foci whereas Th17-treated ones showed 75% reduction (Figure 3A). Therefore, both Th1 and Th17 cell therapies helped control tumor growth, with Th17 cells showing greater potency. When we analyzed cytokine production by ICS after activation with OVA323–339 peptide, we found that both Th1 and Th17 cells maintained their cytokine profiles throughout the experiment (data not shown).
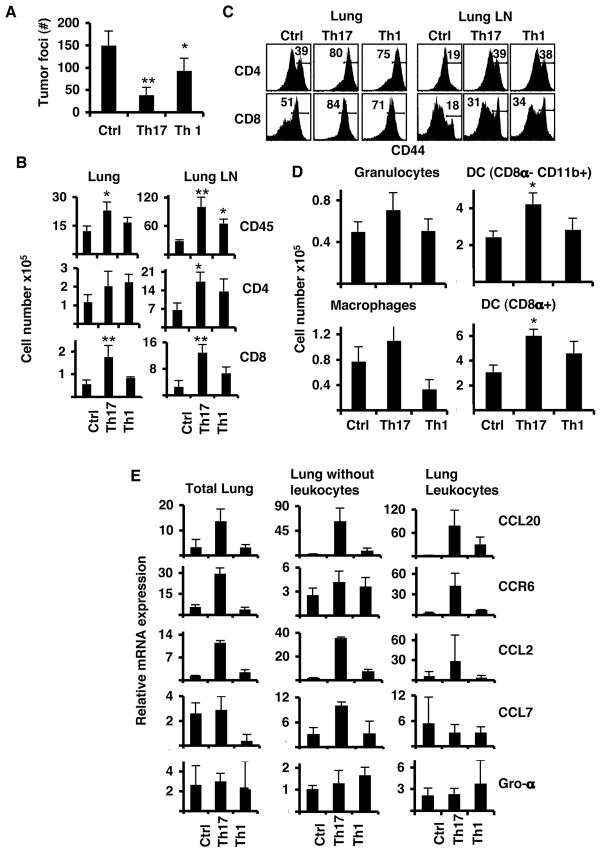
Figure 3. Anti-tumor Th17 cells control established B16/F10 melanoma.
A. Mice were inoculated i.v. with B16-OVA and on day 5, these mice received 3×106 Th1 or Th17 cells i.v. Mice were euthanized on day 14 after tumor challenge for analysis. Shown are the tumor colonies present in the lung lobes of each group of mice (n=4, avg. +/− s.d). B. Cell numbers from leukocyte lung fractions of each group of mice, Control (Ctr), Th1 and Th17, were calculated from the percentages of live cells gated on CD45.2 (n=4, avg. +/− s.d). C. Expression of CD44 on T cells obtained from the lung leukocyte fraction (Lung) or from lung lymph nodes (Lung LN). Cells were gated on CD45.2 and CD4 (middle panel) or CD8 (lower panel). Numbers represent the percentages of CD44hi cells. D. Total numbers of myeloid cell populations from leukocyte lung fraction. Numbers of cells were calculated from the percentages of live cells gated on CD45.2 (n=4, avg. +/− s.d.). E. Gene expression analysis of total lung derived cells with leukocytes and leukocyte fractions. Graphs represent the average values of four mice after duplicate analysis per sample (n=4, avg. +/− s.d). Results shown are from one representative experiment of three using 4–5 mice per group. (* = p< 0.05, ** = p< 0.01).
To compare the inflammatory responses caused by Th1 and Th17 cell therapy, we analyzed the lung infiltrates of all experimental mice. There was increased recruitment of CD4+ T cells in the lungs of mice receiving Th1 or Th17 cells (Figure 3B). These mice also had increased numbers of CD4+ T cells in the lung lymph nodes (LLN). Both Th1- and Th17-treated mice had activated CD4+ and CD8+ T cells in lung and LLN with increased expression of CD44 (Figure 3C). However, only Th17 cell-treated mice had significantly more CD45+ leukocytes and CD8+ T cells in the lungs and LLN (Figure 3B). Further analysis of the myeloid populations showed that Th17 cell treatment elicited the greatest infiltration of granulocytes, macrophages and DC, while Th1 cell treatment showed slightly increased DC but reduced macrophage numbers when compared to the control mice (Figure 3D). In addition, mice receiving Th17 cells had increased numbers of leukocytes in LLN, particularly CD8α+ DC (data not shown and Supplementary Figure 6).
Our data suggested that Th17 and Th1 cells induced different anti-tumor inflammatory responses in the lung. We thus analyzed the chemokine and chemokine receptor expression of the lungs and leukocytes from lungs of mice treated with Th17 or Th1 cells. Elevated expression of CCL20 and CCL2 by the lungs was present only in Th17 treated mice but not in Th1 or control treated mice (Figure 3E). CCL20 and CCL2 expression was predominant in lung cells depleted of leukocytes, which indicated a direct effect of Th17 cells on lung cell production of chemokines. Th1 cell-treated lungs showed a reduced expression of CCL7. CCL20 was highly expressed in the leukocyte fractions from both Th17 and Th1 treated mice but it was dominant in Th17 cell-treated mice (Figure 3E). CCR6 expression was slightly higher in leukocytes from Th1 treated mice compared to control mice but was highly increased in Th17 cell-treated mice.
Th17 cells maintain their phenotypes in vivo
We recently demonstrated that adoptive cell therapy with Th17 cells together with total body irradiation in mice was protective against subcutaneous melanoma; this effect of Th17 cells was dependent on IFN-γ but independent of IL-17 and IL-23 for their protective effects (Muranski et al., 2008). This study suggests a possible conversion of Th17 to Th1 cells upon transfer into the irradiated hosts. Our above data on Th17 vs. Th1 cell function in non-irradiated tumor-bearing mice suggested that Th17 cells might not require conversion to Th1 cells to exert their anti-tumor function in a normal, non-lymphopenic environment. To determine whether Th17 cells were the direct cause of the tumor protection, we further purified Th17 cells using our IL-17F-RFP reporter mice (Yang et al., 2008) and tested their capacity to protect mice with established B16 lung melanoma. CD4+ T cells from OT-II+ IL-17F-RFP mice were cultured in Th17 polarizing conditions and on day 4, live CD4+RFP+ T cells were sorted (Figure 4A) and transferred into mice bearing lung tumors. We found that both total and sorted RFP+ Th17 cells significantly reduced the number of tumor foci to similar extents at day 16 (Figure 4B).
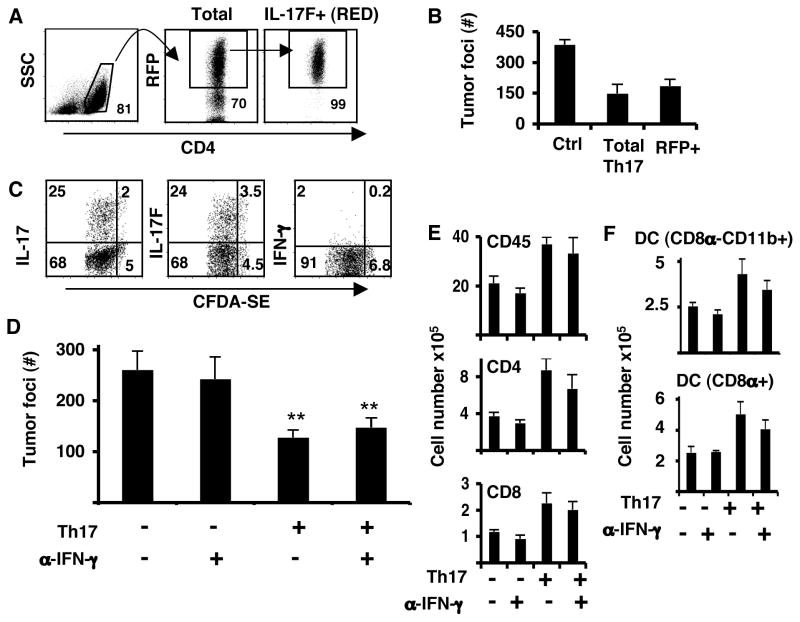
Figure 4. Th17 cells maintain their cytokine expression in tumor-bearing mice.
Purified CD4+ T cells from OT-II.IL-17F-RFP reporter mice were cultured in Th17 conditions. On day 4, CD4+RFP+ cells were sorted and injected into C57BL/6 mice bearing 5-day established pulmonary B16-OVA tumors. A. Sorting strategy for RFP+, IL-17F producing cells. B. Tumor foci from lungs of C57BL/6 mice that had B16-OVA melanoma and received either no treatment (C), unsorted Th17 (total Th17) or RFP+ sorted (IL17F+ RED). C. Purified CD4+ T cells from Rag1−/− OT-II mice were cultured in Th17 conditions and on day 4, cells were labeled with CFDA-SE and transferred into C57BL/6 mice bearing 5-day established pulmonary B16-OVA tumors. LLN from these mice were analyzed for proliferation and IL-17, IL-17F and IFN-γ production on day 4 after transfer. D. Mice were inoculated with B16-OVA tumor and received on the same day Th17 (Th17) cells i.v or no T cells. A set of mice from each group was treated with anti-IFN-γ blocking antibodies (α-IFN-γ) on day -1 and every other day until day 14 after tumor challenge. Shown are the tumor colonies present in the lung lobes of each group of mice (n=4, avg. +/− s.d). (**P<0.01). E. Total cell numbers from leukocyte lung fractions. Number were calculated from the percentages of live cells gated on CD45.2 (n=4, avg. +/− s.d). F. Number of myeloid cell populations from leukocyte lung fraction was calculated from the percentage of live cells gated on CD45.2 (n=4, avg. +/− s.d.).
To directly evaluate the phenotypic characteristics of transferred Th17 cells, we labeled Th17 cells generated from OT-II mice on Rag2−/− background with carboxy-fluorescein diaceate, succinimidyl ester (CFDA-SE) and transferred them into mice bearing lung tumors. On day 4 after the transfer, cell division and cytokine production were evaluated in LLN. We found that Th17 cells had proliferated (more than 90% of the cells were CFDA-SE low) and continued to produce IL-17 and IL-17F but not IFN-γ (Figure 4C). We also analyzed cytokine production from LLN cells recovered on day 14 from mice treated with TRP-1 Th17 cells by ELISPOT. We found that in response to TRP-1 peptide restimulation, the predominant response was IL-17 secretion with very low levels of IFN-γ production (Supplementary Figure 3E). Therefore, we conclude that in non-irradiated tumor-bearing mice, transferred Th17 cells maintained their Th17 cytokine production after encounter of tumor antigens and did not convert to a Th1 phenotype.
Moreover, we injected anti-IFN-γ neutralizing antibodies one day before the transfer of Th17 cells and every other subsequent day until the endpoint. The same anti-IFN-γ antibody regime has been tested and reported by us to inhibit Th1 cell-mediated type I diabetes (Martin-Orozco, 2009). We found that blockade of IFN-γ did not alter the tumor protective effect of Th17 cells (Figure 4D). Moreover, the anti-IFN-γ treatment did not reduce the numbers of total CD45+leukocytes or CD4+ or CD8+ T cells in the lung (Figure 4E). There was also no change in the DC populations in the lungs of Th17-treated mice as a result of the anti-IFN-γ blocking antibody (Figure 4F). Therefore, IFN-γ seems not involved in the recruitment of leukocytes to the lung nor plays a role in the tumor protective effect elicited by Th17 cells. Our results indicate that the Th17 tumor protection effect was not dependent on these cells converting to Th1 phenotype.
Th17 cells enhance the activation of tumor-specific CD8+ T cells
Although Th17 cells have potent protective effect against B16 melanoma in vivo, we did not find any pro-apoptotic effect of IL-17 on B16 cells cultured in vitro (data not shown), suggesting that Th17 cells do not directly kill melanoma cells. In Th17- but not Th1-treated tumor-bearing mice, we observed increased numbers of CD8+ T cells in the lung, suggesting that Th17 cells may promote the activation or recruitment of tumor antigen-specific CD8+ T cells. In B16-OVA tumor model, we searched for endogenous CD8+ T cells reactive against the SIINFEKL peptide derived from OVA protein using a Kb tetramer (OVA-tet). In the lungs of mice that received OT-II- Th17 cells, there was a distinct population of CD8+ OVA-tet+ T cells, approximately 10% and 5% of total CD8+ T cells in the prevention or therapeutic tumor model, respectively. Such OVA-tet+ T cells were absent in untreated control mice (Figure 5A–B). In contrast, Th1 cells did not elicit a significant population of OVA-tet+ T cells in the lung (Figure 5B). We also detected an increased number of OVA-tet+ T cells in mice with flank B16-OVA tumors that were also treated with Th17 cells (Supplementary Figure 4B). Altogether, these results suggest that Th17 cells “help” the activation of endogenous anti-tumor CD8+ cells, and also promote their subsequent localization to the tumor sites.
Figure 5. Th17 cells elicit tumor-specific CD8+ T cell responses.
A–B. Dot plots show the frequencies of OVA tetramer-staining (Kb-SIINFEKL) cells in CD45.2+ CD8+ gated T cells obtained from the leukocyte fraction in mice that received either preventive or therapeutic treatment with Th17 cells. Lower graphs represent total number of cells from the average of 4 mice per group. C. Mice were inoculated i.v. with B16-OVA and on day 5 mice received either PBS or 3×106 of Th1 or Th17 OT-II cells i.v. Depleting antibodies against CD8 were administered i.p on day 4 after the tumor inoculation and every 4 days until the endpoint. Shown are the average numbers of tumor colonies present in the lung lobes of each group of mice (n=4, avg. +/− s.d). (**p<0.01, * p<0.05).
To analyze whether CD8+ T cells mediated the observed tumor protection by Th17 cells, we depleted CD8+ T cells in tumor-bearing mice before they were treated with Th17 cells. Anti-CD8 antibody was injected on day 4 after B16-OVA tumor challenge and every 4 days until the end point. The CD8+ T cell depletion efficiency was confirmed in blood- and lung-derived cells and reached 95% and 86%, respectively. Depletion of CD8+ T cells reduced tumor protection mediated by Th17 (from 75% to 40% reduction of tumor foci), resulting at a level similar to that of Th1 cells without CD8 depletion (Figure 5C). Therefore, CD8+ T cells in large part mediate the tumor protection conferred by Th17 cells.
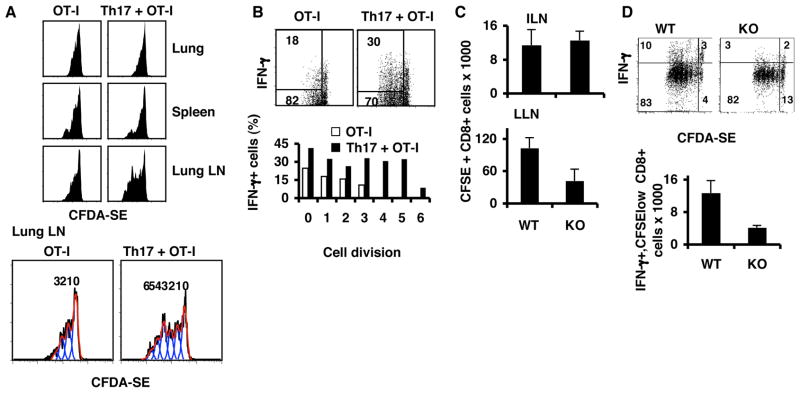
To further assess CD8+ T cell regulation by Th17 cells, we labeled CD8+ OT-I T cells with CFDA-SE and transferred them alone or together with Th17 OT-II cells into mice bearing lung tumors for 5 days. OT-I T cells, when co-transferred with Th17 cells, proliferated more extensively in LLN with at least three more cell divisions than those transferred alone (Figure 6A). However, OT-I cells proliferated similarly with or without Th17 partners in lung or spleen, suggesting that Th17 cell transfer enhances CD8+ T cell priming only in LLN. In addition, in the LLN from mice receiving Th17 cells, there were increased numbers of OT-I T cells producing IFN-γ accompanying their cell division, with approximately 30% cells in the fifth division producing IFN-γ. OT-I T cells transferred alone lost their cytokine expression during their minimal divisions with only 10% IFN-γ+ T cells in the third division (Figure 6B). In contrast, co-transfer of Th1 cells did not influence OT-I cell division or cytokine production of OT-I cells in LLN (Supplementary Figure 7A). These data demonstrate that Th17 cells promote CD8+ T cell proliferation while sustaining their cytokine expression capacities.
Figure 6. Th17 T cell treatment promotes CD8+ effector T cell differentiation.
A–B. Purified CD8+ T cells from OT-I mice were labeled with CFDA-SE and transferred into C57BL/6 mice bearing 5-day established pulmonary B16 nodules (OT-I). On the day of the transfer, one group of mice also received OT-II Th17 (OT-I+ Th17). On day 3 after transfer, LLN, lungs and spleens from these mice were analyzed for T cell proliferation, IFN-γ and IL-2 production. A. Histograms show cell division. Numbers in the lower panel indicates numbers of division. B. Cytokine production. Numbers inside the plots represent the percentages of dividing OT-I cells that also produced the cytokine. Bar graphs indicate the percentages of cells that produced cytokines per cell division. C–D. CD8+ T cells from OT-I mice were labeled with CFDA-SE and transferred into C57BL/6 mice or IL17−/− (KO) bearing 5-day established pulmonary B16-OVA. LLN and inguinal lymph node (ILN) cells from these mice were analyzed for proliferation and cytokine production on day 4 after transfer. C. Total numbers of CD8+CFSE+ T cells recovered from ILN and LLN. D. Cytokine profiles of CD8+ CFSE+ T cells from LLN. E. Total number of CD8+CFSElow IFN-γ+ T cells recovered from LLN.
To confirm that IL-17 can influence CD8+ T cell priming, we transferred OT-I cells labeled with CFDA-SE into WT or IL-17−/− mice that had been inoculated with B16-OVA tumors. We found that on day 4 after transfer, IL-17−/− mice had reduced numbers of CFSE-labeled OT-I cells in LLN (Figure 6C) with lower production of IFN-γ when compared to WT mice (Figure 6D). This result confirms that IL-17 influences the priming of CD8+ T cells to generate IFN-γ-producing effector cells.
Th17 cells regulate DC function in a CCR6-dependent manner
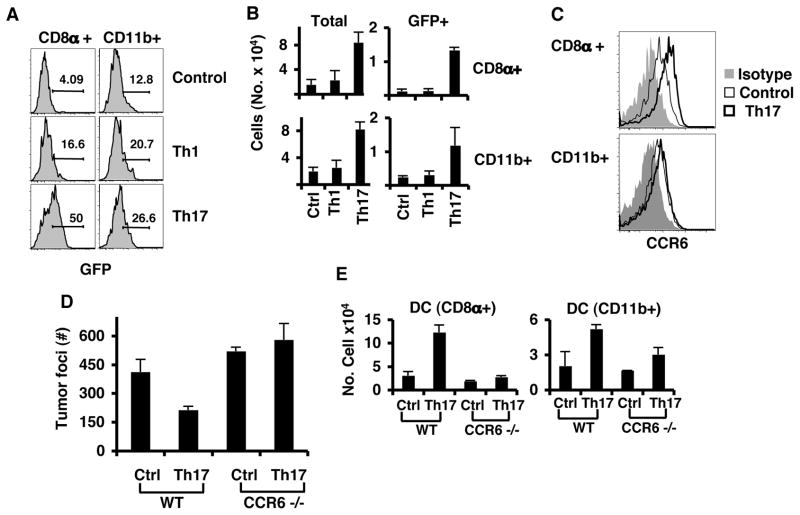
We further investigated the basis of Th17 cell “help” to CD8+ T cells. Th17 cytokines could possibly act on CD8+ T cells directly during priming. However, we did not observe an effect on CD8+ T cell proliferation and cytokine production when they were activated in vitro in the presence of IL-17 or Th17 cell supernatants (data not shown). Alternatively, Th17 cells could indirectly influence CD8+ T cell priming by improving presentation of tumor antigens by DCs. Previous studies have reported that therapy with DC loaded with class I peptides or dead tumor cells protected mice against established B16 solid tumors (Goldszmid et al., 2003; Lou et al., 2004). Consistent with this idea, we have found that CD8α+ DC doubled in percentages in the lung as early as 3 days after Th17 transfer which did not occur with Th1 cells (Supplementary figure 7B). Therefore, we decide to assess this hypothesis by using B16 melanoma that co-expresses OVA and GFP and identify DC uptake of tumor materials and migration to LLN. Mice were injected with B16-OVA-GFP and Th17 or Th1 cells and 72 hour later, LLN were harvested for flow cytometry analysis of DCs uptake of GFP. High levels of GFP were found in CD8α+DCs from mice that received Th17 cells and this signal was higher than Th1 cell-treated mice or controls (Figure 7A). GFP in CD11b+ DCs from mice treated with either Th1 or Th17 cells was also higher than in control mice (Figure 7A). Moreover, since the total numbers of DCs present in LLN were 4 times more in mice treated with Th17 cells compared to those treated with Th1 or control mice, the total number of CD8α+ DCs containing GFP was 10 times more in mice treated with Th17 cells than control or Th1-treated mice (Figure 7B). Therefore, Th17 cells provoked inflammation in the lung that resulted in increased numbers of DCs in the lung and DC containing tumor materials in LLN, resulting in improved anti-tumor CD8+ T cell priming.
Figure 7. Characterization of DCs from LN of mice treated with Th17 cells.
A. C57BL/6 mice were injected i.v. with B16/F10 cells expressing OVA-GFP together with Th1 or Th17 OT-II cells. LLNs were harvested on day 3 for DC analysis by flow cytometry. Histograms show gated CD11bhi CD11c+ (CD11b+) and CD11bint CD11c+ CD8α+ (CD8α+) DCs. B. Lung lymph node cells from A were counted, and total number of cells per DC population calculated from live gate, CD11c, CD11b (TOTAL) or live gate, CD11c, CD11b and GFP (GFP). C. C57BL/6 mice were injected i.v. with B16-OVA and Th17 OT-II cells. LLN were harvested on day 16 for DC analysis. Cells were labeled with antibodies against CCR6 or Isotype Rat IgG. Shown are overlay histograms of either CD8α+ DC or CD11b+ cells. Rat IgG (gray filled histograms), CCR6 from control mice (thin line) and mice that were treated with Th17 cells (bold line). D. C57BL/6 mice or CCR6-deficient mice (CCR6−/−) were injected i.v. with B16-OVA and Th17 OT-II cells. Graph shows the average number of tumor colonies from lung lobes from WT (n=5) and KO (n=3) mice (avg. +/− s.d). E. LLN cells from D were analyzed for DC populations. Graphs show the total number of cells from WT (n=5) and KO (n=3) mice (avg. +/− s.d).
Interestingly, we also found that both CD11b+ and CD8α+ DCs expressed CCR6 on their surface; however, there was only increased CCR6 expression on CD8α+ but not CD11b+ DCs from mice treated with Th17 cells compared to those form the control mice (Figure 7C). Given that CCL20 expression was enhanced by Th17 cells in tumor tissues, we implanted B16-OVA tumor in CCR6-deficient mice and treated them with OT-II Th17 cells. Tumor colonies in the lung of CCR6-deficient mice grew similarly to those in WT mice, whereas CCR6-deficient mice did not respond to treatment with Th17 cells and developed similar numbers of tumor colonies as the untreated mice (Figure 7D). Furthermore, CD8α+ DC numbers were only increased in the LLN of WT mice treated with Th17 but not in CCR6-deficient mice (Figure 7E). The total CD4+ and CD8+ T cells and CD8α+ DCs in the lung of CCR6−/− mice were not increased by Th17 treatment (Supplementary Figure 8A–B). However, there was an increased number of GR1+, CD11b+ DCs and macrophages in CCR6−/− mice treated with Th17 cells. Therefore, we conclude that CCR6 is required for the response to Th17 therapy; the signaling through CCL20 may allow CD8α+ DCs loaded with tumor antigens to prime anti-tumor CD8+ T cells in LLN.
Discussion
Although Th17 cells have been found in human tumors, their physiological function in tumor development has been poorly defined. By using IL-17-deficient mice in a model of lung melanoma, we have provided direct evidence for a protective role of IL-17 in anti-tumor responses. Moreover, we show that the adoptive transfer of tumor-specific Th17 cells protect against various tumors and in the lung melanoma model, promote tumor-specific cytotoxic T cell responses.
The function of IL-17 in tumor immunity has been a controversial subject. The effects of IL-17 on tumor development are directly influenced by the existence of an adaptive immune system- in the presence of lymphocytes, IL-17 promotes tumor rejection, whereas in the absence of them, IL-17 favors tumor growth and agiogenesis (Martin-Orozco, 2009). This notion is consistent with our current data on IL-17−/− mice and adoptive transfer of Th17 cells. Our current data also indicate an active role of IL-17 in tumor immunosurveillance; IL-17 deficiency resulted in reduced leukocyte infiltration into the target tissue and these mice were more susceptible to the tumor development. Also anti-tumor CD8+ effector T cell differentiation was compromised in IL-17-deficient mice. In contrast to our results, IL-23, important in Th17 regulation, has been shown to promote tumor growth and prevent immunosurveillance (Langowski et al., 2006). There are two possibilities to account for these discrepancies. First, other cells or factors regulated by IL-23 may have a distinct function in cancer immunity. It has been recently described that IL-23 produced by macrophages activate STAT-3 in the macrophages and Treg cells and promotes tumor growth (Kortylewski et al., 2009). Second, it is also possible that the IL-23/IL-17 axis of inflammatory responses may have differential functions in different settings; its regulation of chronic inflammation may provide a supportive role for certain types of tumors. Short acute inflammation associated with anti-tumor response may help the fight against the tumor (Overwijk et al., 2006). Further studies on different tumor models may reveal the complex relationships between IL-17/IL-23, inflammation and cancer.
In our current study, we found that Th17 cells provide better protection to tumors than Th1 cells, and this difference was largely due to their unique ability to promote CD8+ T cell priming. Since anti-IFNγ did not influence the protective immunity mediated by Th17 cells against tumors, CD8+ T cells may kill tumors independent of this cytokine, possibly by utilizing the cytolytic enzymes. It was also recently shown in subcutaneous B16 melanoma that transfer of anti-tumor CD4+ Th17 cells together with whole-body irradiation, vaccination and IL-2 treatment could control tumor growth. However, the effect of Th17 vaccination was dependent on IFN-γ and independent of IL-17 and IL-23 (Muranski et al., 2008). We believe that in a lymphopenic environment, conversion of Th17 to Th1 cells occurs; however, Th17 cells maintain their phenotypes in normal hosts in the presence or absence of inflammation (Martin-Orozco et al., 2009; Nurieva et al., 2009). Although large numbers of Th1 cells did provide some degree of tumor protection in our model as well, our Th17 donor cells were more effective in several tumor models tested while maintaining their Th17 cytokine expression profiles. Additionally, when Th17 cells were purified using the RFP reporter, they efficiently provided tumor protection comparable to the total Th17 cells. Thus, our experimental settings including a normal, nonlymphopenic environment allow us to more directly address the physiological function of Th17 cells in cancer.
The protective function of Th17 cells against tumors is likely due to their ability to enhance inflammatory responses, which results in increased antigen presentation by DCs. Leukocyte homing to tumors, usually inhibited by the tumor cells (Gajewski, 2007), was reduced in IL-17−/− mice and promoted by transfer of tumor-specific Th17 cells. Increased numbers of DCs infiltrating the lung after Th17 cell transfer may lead to increased capture of tumor antigens, which are then presented to tumor-reactive CD8+ T cells in the draining lymph nodes. It has been reported that the presence of increased numbers of mature DCs within solid tumor masses induces more effective anti-tumor immune responses in animal models (Furumoto et al., 2004) and is associated with improved prognosis in clinical patients (Lotze, 1997). Moreover, patients with advanced melanoma that have been treated with GM-CSF showed a generally increased of mature DCs, which has been associated with disease remission and delayed tumor recurrence (Daud et al., 2008). At this point, our report is the first to show that Th17 cells influence the generation of CD8+ effector T cells in vivo. Since CD4+ T cell help is essential for the generation of CD8+ memory T cells during the priming phase of an acute viral infection (Shedlock and Shen, 2003), it is possible that Th17 cells may participate in this process via the generation of CD8+ anti-tumor effector/memory T cells. Th17 cells, on one hand, promote proliferation of tumor-specific CD8+ T cells. On the other, they also allow for sustained IFN-γ expression by dividing CD8+ T cells, thus preventing them from becoming “exhausted”.
We did not found a direct effect of Th17 cells on CD8+ T cells effector differentiation however, we observed increased numbers of DCs carrying tumor-derived material in the lymph nodes of Th17 cell-treated mice. The predominant DCs recruited were CD8α+, which are indispensable for cross-presentation of self antigens in several tissues and in the lung (den Haan and Bevan, 2002; Dudziak et al., 2007; Schnorrer et al., 2006). In comparison to Th1-mediated effects, we found that Th17 cells selectively induced the expression of CCL20 and CCR6 deficiency selectively impairs the recruitment of CD8α+ DC, which may account for the difference of Th1 and Th17 cells in activation of tumor-specific CD8+ T cells and in their potency of tumor immunity. Previously Furumoto et al. reported that over expression of CCL20 by B16 cells, though increased intra-tumor DC numbers, did not promote tumor regression, but helped the regression of CT26 colon adenocarcinomas (Furumoto et al., 2004), suggesting that CCL20/CCR might be necessary but not sufficient in the induction of tumor immunity.
Our data altogether suggest the following model: Th17 cells go to the tumor site and by secreting IL-17, activates residential cells to produce CCL2 and CCL20, which provokes the mobilization of DC and other leukocytes to the tumor site. DCs uptake tumor antigens in the lung and migrate to the lymph nodes where they activate CD8+ T cells against the tumor. The new wave of effector CD8+ T cells migrates back to the lung and kills established tumors (Supplementary figure 9).
Our data suggest that tumor immunosurveillance in the lung in the steady state is dependent, in part, on IL-17, but more importantly that tumor-specific Th17 cells may be used in adoptive T cell therapy. To date, adoptive cell therapy for cancer with in vitro-expanded, tumor-infiltrating CD8+ lymphocytes (TIL) has achieved some degree of success in cancer therapy (Dudley and Rosenberg, 2007; Rosenberg and Dudley, 2004). Together with the current literature on the crucial function of Th17 cells in autoimmunity, our results suggest that Th17 cells directed against tumor antigens may be employed in cancer patients. In addition, Th17 and CD8+ T cells derived from the tumors may be expanded ex-vivo and employed together to enhance cancer immunity. Therefore, our data demonstrating that Th17 cells and IL17 participate in anti-tumor immunity by facilitating T cell recruitment to the tumor site and CD8+ T cell priming and effector differentiation suggests a new avenue for developing Th17 cell-based therapy for tumors or chronic viral infections and as an adjuvant for vaccinations.
Experimental Procedures
Mice
C57BL/6 mice were purchased from the NCI, and OT-II (C57BL/6-Tg (TcraTcrb)425Cbn/J), OT-I (C57BL/6-Tg(TcraTcrb)1100Mjb/J), CD45.1 (B6.SJL-Ptprca Pepcb/BoyJ) and CCR6 KO (B6.129P2-Ccr6tm1Dgen/J) mice from The Jackson Laboratory (Yamazaki et al., 2008). OT-II.2 RAG2 (C57BL/6-RAG2tm1Alt-TgNOT-II.2) mice were from Taconic Farms. IL-17 KO mice and IL-17F-RFP mice were generated in our lab (Yang et al., 2008). Homozygous KO and WT animals on the same 129 × C57BL/6 F1 mixed background were bred and used in some experiments. For some experiments, IL-17 KO mice that had been backcrossed to C57BL/6 mice for six generations were used. IL-17F-RFP mice and CD45.1 were crossed to OT-II mice to generate OT-II.IL-17F-RFP and OT-II- CD45.1 mice respectively. Mice were maintained in the MD Anderson Animal Facility and the MD Anderson Cancer Center Institutional Animal Care and Use Committee approved all animal studies. Rag1−/−BW TRP-1 TCR transgenic mice were bred at the National Institutes of Health (NIH).
Induction and assessment of B16/F10 lung melanoma
Six-week-old mice were injected i.v. with 1×105 wild-type B16/F10 cells or with B16/F10 transfected with OVA (B16-OVA). For adoptive transfer experiments, the mice were injected i.v. on the same day with 1–3 ×106 in vitro differentiated Th17 OT-II cells in the prevention model or 5 days after the tumor injection in the therapeutic model. At day 14 or 16 after tumor introduction, mice were sacrificed for enumeration of metastatic lung foci. All lung lobes were evaluated under a tissue microscope (Leica MZFLIII). In the experiments where anti-IFN-γ blocking antibody (XMG1.2 clone, BioXcell, West Lebanon, NH) or anti-CD8 (53.6.72 clone, BioXcell) were used, 100 μg/mouse was injected in 100 μl of PBS i.p every 4 days.
Subcutaneous tumor induction
Mice were injected s.c. in the front of the abdomen with 2×105 B16-OVA or 5×105 MCA205-OVA cells And on the same day, some mice received 3–5 ×106 Th1 or Th17 OT-II cells. Tumor area was measured with digital calipers. Mice were sacrificed once tumors reached 200m2.
In vitro Th17 and Th1 polarization
CD4+ T cells from spleens and lymph nodes of OT-II mice were differentiated into Th1 or Th17 cells as described previously (Chung et al., 2006; Nurieva et al., 2007). When Rag1−/− BW TRP-1 TCR-transgenic cells were used, splenocytes and lymph node cells were cultured with 0.01 μg/ml of TRP-1(106–130) peptide and irradiated splenocytes and the same polarizing conditions. Cells were harvested at day 4 for cytokine analysis and for transfer. Intracellular cytokine analysis was performed on cells that were stimulated with specific peptide (5 μg/ml OVA or 1 μg/ml or TRP-1) for 5 hours and Golgistop and further stained for IL-17, IL-17F and IFN-γ using BD Cytoperm/wash kit according to manufacturer directions (BD Biosciences, San Jose CA). Cytokines were purchased from Peprotec (Rocky Hill, NJ) and blocking antibodies were from Bioxcell (West Lebanon, NH).
Lung fractionation and cell analysis
Lungs were digested with 1 mg/ml collagenase D for 30 minutes at 37°C and 5 minutes with 0.01 EDTA to prevent DC-T cells aggregates (Vremec et al., 2000). The cells were further suspended in LSM lymphocyte separation medium (MP Biomedicals, LLC), followed by centrifugation according to manufacturer instructions. The middle section of the gradient was enriched for leucocytes and the bottom fraction for tumor and lung cells. Leucocyte fractions were counted and used for flow cytometry analysis or RNA extraction.
Flow cytometry
Antibodies against CD45.2, CD4, CD8, CD44, CD62L, CD11c, CD11b, Gr1, IL-17, IFN-γ, and IL-2 were all purchased from BD (BD Biosciences, San Jose CA). Polyclonal anti-IL-17F (Yang et al., 2008) was labeled with Alexa647 (Invitrogen). Anti-CD45.1 was purchased from Biolegend (San Diego, CA). Kb tetramer carrying the OVA257–264 peptide (SIINFEKL) and Db tetramer carrying human gp10025–33 (KVPRNQDWL) were both labeled with streptavidin-PE and were made in-house. Immunofluorescence staining was performed at 4°C except for the tetramer staining at room temperature for 1 hour. Samples were analyzed in a FACS Canto II, LSR II or FACScalibur equipped with DIVA software or CellQuest software respectively. RFP+ cells were sorted in a MoFlow sorter (Cytomation). Files were analyzed using TreeStar Flowjo.
Real-time PCR
The expression of CCL20, CCL2, CCL7, CCR6, CCR2, and Gro-α was performed using specific primers reported previously (Chang and Dong, 2007; Chang et al., 2006) and the BioRad SYBRgreen system. Expression was normalized to the expression of the housekeeping gene actin.
CFDA-SE labeling and transfer experiments
Th17 cells generated from naïve cells in Rag.OT-II mice were labeled with 5 μM CFDA-SE (Invitrogen). 5×106 cells were injected i.v. into mice bearing 5-day established B16-OVA lung tumors. After 4 days, recipient mice were sacrificed and lung, LLN and spleen were harvested and cells were re-stimulated with 5 μg/ml OVA323–339 peptide in the presence of Golgi-stop for 6 hours. ICS for IL-17, IL-17F and IFN-γ was performed using BD Cytoperm/wash kit.
CD8+ T cells from OT-I mice were purified using anti-CD8 beads (Miltenyi) and the AutoMacs system. After purification, the cells were labeled with CFDA-SE and 5×106 cells were injected i.v. into mice bearing 5-day established B16-OVA lung tumors. The same day the mice received i.v. injections of 3–5 million Th17 cells or PBS. Lung, LLN and spleen were harvested on day 3 after transfer and re-stimulated with 5 μg/ml of OVA257–264 peptide in the presence of Golgi-plug for 6 hours. ICS staining for IFN-γ and IL-2 was performed using BD Cytoperm/wash kit.
Similar experiments were performed transferring purified OT-I cells labeled with CFDA-SE into IL17 KO or WT mice in the C57BL/6 strain.
Dendritic cell analysis
Mice were injected i.v. with 1×105 B16/F10 expressing OVA-GFP and 1×106 of either Th1 or Th17 polarized OT-II cells. LLN were harvested at 72 hours and were digested with 1 mg/ml of collagenase and with 0.01 EDTA to prevent DC-T cells aggregates. Cells were counted and were incubated with anti CD16/CD32 (FcBlock) for 20 minutes and further label with mAb against CD11b, CD11c, GR1, CD8α, or Isotype Rat IgG, followed by flow cytometric analysis.
Leukocytes from lung fractionation were analyzed similarly to determined DC, macrophage and granulocyte populations.
ELISPOT
On day 16 after tumor injection, inguinal lymph node cells from mice that were treated with TRP-1 Th17 cells were counted and plated in HA plates (Millipore) previously coated with anti IFN-γ or IL-17 (BD Biosciences). Cells were pulsed with TRP-1(106–130) peptide and cultured for 18 hours. Plates were blotted with biotinylated anti-IFN-γ or IL-17 and developed with avidin-alkaline phosphatase and 5-bromo-4 chloro-3indolyl phosphate and nitro blue tetrazolium chloride (BCIP/NBT). ELISPOT dots were counted on a C.T.L. ImmunoSpot using Image Acquisition 4.4 software for image capture and Immunospot 3 for analysis.
Statistical Methods
All statistical comparisons were analyzed using the Student’s t test.
Supplementary Material
Acknowledgments
We thank the entire Dong lab for their help and discussion. This work is supported in part by grants from the National Institute of Health (to WWO & CD) and a grant from the Center for Targeted Therapy of MD Anderson Cancer Center (to CD and NMO). CD receives a Leukemia and Lymphoma Society Scholar award and a Trust Fellowship of the MD Anderson Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benchetrit F, Ciree A, Vives V, Warnier G, Gey A, Sautes-Fridman C, Fossiez F, Haicheur N, Fridman WH, Tartour E. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood. 2002;99:2114–2121. doi: 10.1182/blood.v99.6.2114. [DOI] [PubMed] [Google Scholar]
- Chang SH, Dong C. A novel heterodimeric cytokine consisting of IL-17 and IL-17F regulates inflammatory responses. Cell Res. 2007;17:435–440. doi: 10.1038/cr.2007.35. [DOI] [PubMed] [Google Scholar]
- Chang SH, Park H, Dong C. Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. J Biol Chem. 2006;281:35603–35607. doi: 10.1074/jbc.C600256200. [DOI] [PubMed] [Google Scholar]
- Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, Dong C. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y, Yang X, Chang SH, Ma L, Tian Q, Dong C. Expression and regulation of IL-22 in the IL-17-producing CD4+ T lymphocytes. Cell Res. 2006;16:902–907. doi: 10.1038/sj.cr.7310106. [DOI] [PubMed] [Google Scholar]
- Daud AI, Mirza N, Lenox B, Andrews S, Urbas P, Gao GX, Lee JH, Sondak VK, Riker AI, Deconti RC, Gabrilovich D. Phenotypic and functional analysis of dendritic cells and clinical outcome in patients with high-risk melanoma treated with adjuvant granulocyte macrophage colony-stimulating factor. J Clin Oncol. 2008;26:3235–3241. doi: 10.1200/JCO.2007.13.9048. [DOI] [PubMed] [Google Scholar]
- den Haan JM, Bevan MJ. Constitutive versus activation-dependent cross-presentation of immune complexes by CD8(+) and CD8(−) dendritic cells in vivo. J Exp Med. 2002;196:817–827. doi: 10.1084/jem.20020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- Dudley ME, Rosenberg SA. Adoptive cell transfer therapy. Semin Oncol. 2007;34:524–531. doi: 10.1053/j.seminoncol.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- Furumoto K, Soares L, Engleman EG, Merad M. Induction of potent antitumor immunity by in situ targeting of intratumoral DCs. J Clin Invest. 2004;113:774–783. doi: 10.1172/JCI19762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski TF. Failure at the effector phase: immune barriers at the level of the melanoma tumor microenvironment. Clin Cancer Res. 2007;13:5256–5261. doi: 10.1158/1078-0432.CCR-07-0892. [DOI] [PubMed] [Google Scholar]
- Goldszmid RS, Idoyaga J, Bravo AI, Steinman R, Mordoh J, Wainstok R. Dendritic cells charged with apoptotic tumor cells induce long-lived protective CD4+ and CD8+ T cell immunity against B16 melanoma. J Immunol. 2003;171:5940–5947. doi: 10.4049/jimmunol.171.11.5940. [DOI] [PubMed] [Google Scholar]
- Hu J, Yuan X, Belladonna ML, Ong JM, Wachsmann-Hogiu S, Farkas DL, Black KL, Yu JS. Induction of potent antitumor immunity by intratumoral injection of interleukin 23-transduced dendritic cells. Cancer Res. 2006;66:8887–8896. doi: 10.1158/0008-5472.CAN-05-3448. [DOI] [PubMed] [Google Scholar]
- Kortylewski M, Xin H, Kujawski M, Lee H, Liu Y, Harris T, Drake C, Pardoll D, Yu H. Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment. Cancer Cell. 2009;15:114–123. doi: 10.1016/j.ccr.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I, Wei S, Zou L, Altuwaijri S, Szeliga W, Kolls J, Chang A, Zou W. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol. 2007;178:6730–6733. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, Basham B, McClanahan T, Kastelein RA, Oft M. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze MT. Getting to the source: dendritic cells as therapeutic reagents for the treatment of patients with cancer. Ann Surg. 1997;226:1–5. doi: 10.1097/00000658-199707000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou Y, Wang G, Lizee G, Kim GJ, Finkelstein SE, Feng C, Restifo NP, Hwu P. Dendritic cells strongly boost the antitumor activity of adoptively transferred T cells in vivo. Cancer Res. 2004;64:6783–6790. doi: 10.1158/0008-5472.CAN-04-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Orozco N, Chen Dong. The IL-17/IL-23 axis of inflammation in cancer: Friend or foe? Current Opinion in Investigational Drugs. 2009:10. [PubMed] [Google Scholar]
- Martin-Orozco N, Chung Y, Chang SH, Wang YH, Dong C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur J Immunol. 2009;39:216–224. doi: 10.1002/eji.200838475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O’Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyahara Y, Odunsi K, Chen W, Peng G, Matsuzaki J, Wang RF. Generation and regulation of human CD4+ IL-17-producing T cells in ovarian cancer. Proc Natl Acad Sci U S A. 2008;105:15505–15510. doi: 10.1073/pnas.0710686105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008 doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of Immune Induction of Collagen-Induced Arthritis in IL-17-Deficient Mice. J Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- Nurieva R, Yang XO, Chung Y, Dong C. Cutting edge: in vitro generated Th17 cells maintain their cytokine expression program in normal but not lymphopenic hosts. J Immunol. 2009;182:2565–2568. doi: 10.4049/jimmunol.0803931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- Overwijk WW, de Visser KE, Tirion FH, de Jong LA, Pols TW, van der Velden YU, van den Boorn JG, Keller AM, Buurman WA, Theoret MR, et al. Immunological and antitumor effects of IL-23 as a cancer vaccine adjuvant. J Immunol. 2006;176:5213–5222. doi: 10.4049/jimmunol.176.9.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Dudley ME. Cancer regression in patients with metastatic melanoma after the transfer of autologous antitumor lymphocytes. Proc Natl Acad Sci U S A. 2004;101(Suppl 2):14639–14645. doi: 10.1073/pnas.0405730101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnorrer P, Behrens GM, Wilson NS, Pooley JL, Smith CM, El-Sukkari D, Davey G, Kupresanin F, Li M, Maraskovsky E, et al. The dominant role of CD8+ dendritic cells in cross-presentation is not dictated by antigen capture. Proc Natl Acad Sci U S A. 2006;103:10729–10734. doi: 10.1073/pnas.0601956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfanos KS, Bruno TC, Maris CH, Xu L, Thoburn CJ, DeMarzo AM, Meeker AK, Isaacs WB, Drake CG. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;14:3254–3261. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- Vremec D, Pooley J, Hochrein H, Wu L, Shortman K. CD4 and CD8 expression by dendritic cell subtypes in mouse thymus and spleen. J Immunol. 2000;164:2978–2986. doi: 10.4049/jimmunol.164.6.2978. [DOI] [PubMed] [Google Scholar]
- Yamazaki T, Yang XO, Chung Y, Fukunaga A, Nurieva R, Pappu B, Martin-Orozco N, Kang HS, Ma L, Panopoulos AD, et al. CCR6 regulates the migration of inflammatory and regulatory T cells. J Immunol. 2008;181:8391–8401. doi: 10.4049/jimmunol.181.12.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, Schwarzenberger P, Shellito JE, Kolls JK. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol. 2001;25:335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- Zhang B, Rong G, Wei H, Zhang M, Bi J, Ma L, Xue X, Wei G, Liu X, Fang G. The prevalence of Th17 cells in patients with gastric cancer. Biochem Biophys Res Commun. 2008;374:533–537. doi: 10.1016/j.bbrc.2008.07.060. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.