Abstract
Background
CF patients often demonstrate hypersensitivity to one or multiple antibiotics due to frequent and repeated exposures. Attempts at antibiotic desensitization in this population are historically complicated by higher reaction rates, failure to complete the procedure and consequent withholding of first-line therapy. This study evaluates the outcomes of a rapid desensitization protocol developed at our institution.
Methods
We retrospectively reviewed the medical records of 15 patients undergoing 52 rapid antibiotic desensitizations at Brigham and Women’s Hospital and Children’s Hospital Boston utilizing our protocol.
Results
Mean FEV1 % predicted was 44.1 (SD 16.5), with two patients at <30% and one patient desensitized during bilateral lung transplantation. Adverse reactions during desensitization occurred in 13.4%, and most were mild. 100% of patients completed the protocol and ultimately tolerated subsequent full-strength antibiotic courses.
Conclusions
CF patients with antibiotic hypersensitivity can safely receive first-line antibiotics via our rapid desensitization protocol, including those with severe obstructive lung disease.
Keywords: cystic fibrosis, antibiotic, allergy, hypersensitivity, desensitization, protocol
Introduction
Patients with cystic fibrosis (CF) have a higher prevalence of allergic reactions to one or multiple antibiotics, especially beta-lactams, thought to be due in part to multiple and repeated exposures. The worldwide prevalence of beta-lactam allergy in CF patients has been reported as high as 36% [1], with as many as 60% of Danish CF patients affected [2]. The high incidence of pseudomonal infections, the consequent need to treat with specific anti-pseudomonal antibiotics and the risk of potentially life-threatening allergic reactions to these medications often complicate management in these patients.
Antibiotic desensitizations have been performed by allergists to tolerize these patients to first-line therapy. However, several factors have impeded their widespread utilization. The concept of reintroducing highly allergenic medications into sensitized individuals with significantly impaired lung function and, thus, at a presumed increased risk for anaphylaxis [3] causes great concern among pulmonologists regarding the safety of rapid desensitization for their patients. This apprehension has been supported by desensitization failure rates of approximately 25% in previously published case series [4,5] characterized by severe reactions during desensitizations, institution of life-saving measures, discontinuation of the desensitization protocol and switching to second-line therapy.
The primary aim of our study was to determine the efficacy of a rapid intravenous antibiotic desensitization protocol originally developed at our institution for chemotherapeutic agents [6–8]. We also wanted to assess the safety of our protocol in a high-risk group of patients with moderate-to-severe obstructive lung disease.
Methods
After obtaining IRB approval, we retrospectively reviewed the medical records of all CF patients who underwent antibiotic desensitization at Brigham and Women’s Hospital (BWH) and Children’s Hospital Boston (CHB) from 1998 to 2009 using the BWH protocol. Patients were determined to be amenable to rapid desensitization based on a clinical history consistent with a type I hypersensitivity reaction (HSR) and skin testing results when available. Type I HSRs were defined as reactions occurring during or shortly after an antibiotic infusion, and characterized by the following signs and symptoms: cutaneous (flushing, pruritus, urticaria, angioedema), cardiovascular (chest pain, tachycardia, sense of impending doom, presyncope, syncope, hypertension, hypotension), respiratory (sneezing, nasal congestion, dyspnea, coughing, wheezing, oxygen desaturation), throat tightness, gastrointestinal (nausea, vomiting, abdominal pain, diarrhea, bloating), and neuromuscular (disorientation, hallucination, vision disturbances, ringing/pounding in ears, unusual taste, back pain, numbness/weakness). Patients who presented with a maculopapular rash, delayed-type hypersensitivity, serum sickness, erythema multiforme, Stevens-Johnson syndrome or toxic epidermal necrolysis were excluded from consideration for desensitization. Atopy was defined as the presence of allergic rhinoconjunctivitis, food allergy, urticaria/angioedema, eczema and/or latex allergy. Asthma was not included in the definition, as the symptoms and objective findings in this pulmonary syndrome are often difficult to distinguish from those of CF. Forced expiratory volume at one second (FEV1) was obtained at the time closest to that of the desensitization to determine the degree of obstructive lung disease. The choice of antibiotic was determined by the consulting pulmonologist based on first-line sensitivity to the bacterial pathogen. The desensitization procedure was deemed successful if the full antibiotic dose was administered.
After written consent was obtained prior to each procedure, rapid intravenous desensitization was performed to the selected antibiotic using the standard 12-step or 16-step protocol developed for chemotherapeutic agents [6–8], subject to modification based on reactions during prior desensitizations. Diphenhydramine (25 mg oral or intravenous) and either famotidine (20 mg intravenous) or ranitidine (50 mg intravenous) were administered 20 minutes before the initiation of the protocol. Patients 12 and 14 received lorazepam 0.5–1 mg for anxiety. Prior to the second desensitization course to ceftazidime for Patient 4, both aspirin (325 mg oral) and montelukast (10 mg oral) were administered 60 minutes beforehand, as this medication regimen has proven to be useful in patients with refractory mast cell mediator-related symptoms [9]. Emergency resuscitation equipment and standard medications (epinephrine, methylprednisolone, albuterol, antihistamines and glucagon) were at the bedside. All initial desensitizations and 94% of subsequent desensitizations were performed in the medical intensive care unit (MICU) with 1:1 nursing. Following successful desensitization, the antibiotic was continued in full doses and at regularly-scheduled intervals for a treatment period typically lasting 14 to 21 days.
Results
As described in Table 1, we performed a total of 52 desensitizations in 15 patients, ranging from 1 to 12 desensitization courses per patient (mean 3.5), mean age 32.2 (21–49, SD 10.7), female to male ratio 3:2. Nine of the 15 patients (60%) were atopic and 12 (80%) were allergic to multiple antibiotics. Nine different antibiotics were administered, with piperacillin and ceftazidime being most common. Bilateral lung transplantation was performed in six patients (40%), with one undergoing desensitization intraoperatively. Desensitizations were performed in patients with FEV1s ranging from 11 to 77% predicted (0.47–2.83L), with two patients consistently below 30% and a mean FEV1 of 44.1% predicted (SD 16.5).
Table 1.
Patient characteristics and outcomes of desensitization
| Patient | Age,Sex | Initial Reaction | FEV1 (L) | (% predicted) | Lung Transplant | Antibiotic | Skin Test | Desensitization |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protocol | # of courses (52) | Step/Reaction | Treatment | Completed | ||||||||
| 1 | 46,F | Urticaria,angioedema | 0.50–0.60 1 | (22–26) 1 | Yes | Meropenem | nd | 12 Step, 250 mL bag | 3 | No | — | Yes |
| 2 | 51,M | Urticaria | 2.19 | (66) | Yes | TMP/SMX | nd | 12 Step, 250 mL bag | 1 | No | — | Yes |
| 3 | 29,F | Urticaria | 1.40 | (43) | Yes | TMP/SMX | nd | 12 Step, 250 mL bag | 2 | No | — | Yes |
| 4 | 40,M | Uricaria,tongue swelling | 0.47–0.90 | (11–25) | Yes | Piperacillin/tazobactam | - | 12 Step, 250 mL bag | 1 | No | — | Yes |
| 12 Step, 250 mL bag | 1 | Step 3: Urticaria,sore throat | Diphenhydramine | Yes | ||||||||
| 12 Step, 250 mL bag | 1 | Step 12: Urticaria | Diphenhydramine | Yes | ||||||||
| 12 Step, 250 mL bag | 1 | Step 12: Urticaria | Diphenhydramine, methylprednisolone | Yes | ||||||||
| 13 Step, 250 mL bag | 1 | No | — | Yes | ||||||||
| Uricaria,angioedema | 0.79 | (20) | Ceftazidime | nd | 12 Step, 250 mL bag | 1 | No | — | Yes | |||
| 16 Step, 100 mL bag | 1 | No | — | Yes | ||||||||
| 5 | 22,F | Urticaria,throat swelling | 1.17 | (31) | Yes | Ceftazidime | nd | 16 Step, 100 mL bag | 2 | No | — | Yes |
| Uricaria | N/A | TMP/SMX | nd | 16 Step, 100 mL bag | 1 | No | — | Yes | ||||
| 6 | 35,F | Urticaria,throat pruritus | 2.32 | (77) | No | Ceftazidime | + | 12 Step, 250 mL bag | 1 | No | — | Yes |
| Uricaria,lip swelling | 1.66–2.10 | (56–72) | Cefepime | nd | 16 Step, 20 mL bag | 3 | No | — | Yes | |||
| 12 Step, 50 mL bag | 3 | No | — | Yes | ||||||||
| 7 | 21,F | Urticaria | 1.55 | (49) | No | Cefoxitin | nd | 12 Step, 250 mL bag | 1 | No | — | Yes |
| 8 | 42,M | Urticaria | 1.52 | (38) | No | Ceftazidime | - | 12 Step, 100 mL bag | 1 | No | — | Yes |
| 9 | 21,F | Urticaria | 1.72–1.84 | (54–58) | No | Piperacillin/tazobactam | + | 16 Step, 100 mL bag | 1 | Nausea,diarrhea flushing,erythema 2 | Diphenhydramine | Yes |
| 12 Step, 250 mL bag | 1 | No | — | Yes | ||||||||
| 16 Step, 100 mL bag | 1 | Urticaria,flushing 2 | Diphenhydramine | Yes | ||||||||
| 10 | 26,M | Urticaria | 1.11–2.02 | (27–47) | Yes | Ceftazidime | nd | 12 Step, 100 mL bag | 4 | No | — | Yes |
| 12 Step, 100 mL bag | 1 | Acute respiratory failure 2 | Intubation | Yes | ||||||||
| 12 Step, 250 mL bag | 2 | No | — | Yes | ||||||||
| 12 Step, 100 mL bag | 1 | No | — | Yes | ||||||||
| Urticaria,dyspnea,wheezing | 1.11–2.02 | (27–47) | Piperacillin/tazobactam | - | 12 Step, 250 mL bag | 2 | No | — | Yes | |||
| 12 Step, 100 mL bag | 2 | No | — | Yes | ||||||||
| 11 | 25,F | Urticaria | 1.81 | (51) | No | Tobramycin | nd | 12 Step, 200mL bag | 1 | No | — | Yes |
| Urticaria | 1.81 | (51) | Meropenem | nd | 12 Step, 100 mL bag | 1 | No | — | Yes | |||
| 12 | 26,M | Urticaria | 1.50–2.80 | (32–61) | No | Ceftazidime | + | 12 Step, 250 mL bag | 2 | No | — | Yes |
| 12 Step, 250 mL bag | 1 | Step 12: Urticaria | Diphenhydramine | Yes | ||||||||
| 13 | 29,M | Urticaria | 2.83 | (65) | No | Ciprofloxacin | nd | 12 Step, 250 mL bag | 2 | No | — | Yes |
| 14 | 22,F | Urticaria | 1.70 | (60) | No | Piperacillin/tazobactam | - | 12 Step, 250 mL bag | 1 | No | — | Yes |
| 15 3 | 49,F | Urticaria, scratchy throat | 1.50 | (49–50) | No | Ceftazidime | nd | 12 Step, 100 mL bag | 3 | No | — | Yes |
| Urticaria,wheezing | 1.50 | (49) | TMP/SMX | nd | 12 Step, 500mL bag | 1 | No | — | Yes | |||
N/A : Not available (immediately post-transplant)
nd : Not done
Desensitized once during lung transplant
Reaction after step 12 and prior to first full dose
Anaphylactic reaction to Amoxicillin : sudden onset of rash and throat swelling requiring a visit to the emergency room and epinephrine
Fifty of 52 desensitizations (96.2%) were completed without severe adverse events. Patient 9 developed nausea, diarrhea, flushing and a generalized erythematous rash 15 minutes after completing desensitization and was successfully re-desensitized with a modified protocol. Patient 10 developed acute respiratory failure requiring intubation shortly after the procedure, an event retrospectively attributed to worsening pulmonary infection. Minor reactions in Patients 4, 9 and 12 (9.6%) consisted of flushing, hives, nausea or diarrhea and were treated with antihistamines and/or systemic corticosteroids. There were no severe reactions that prevented completion of desensitization.
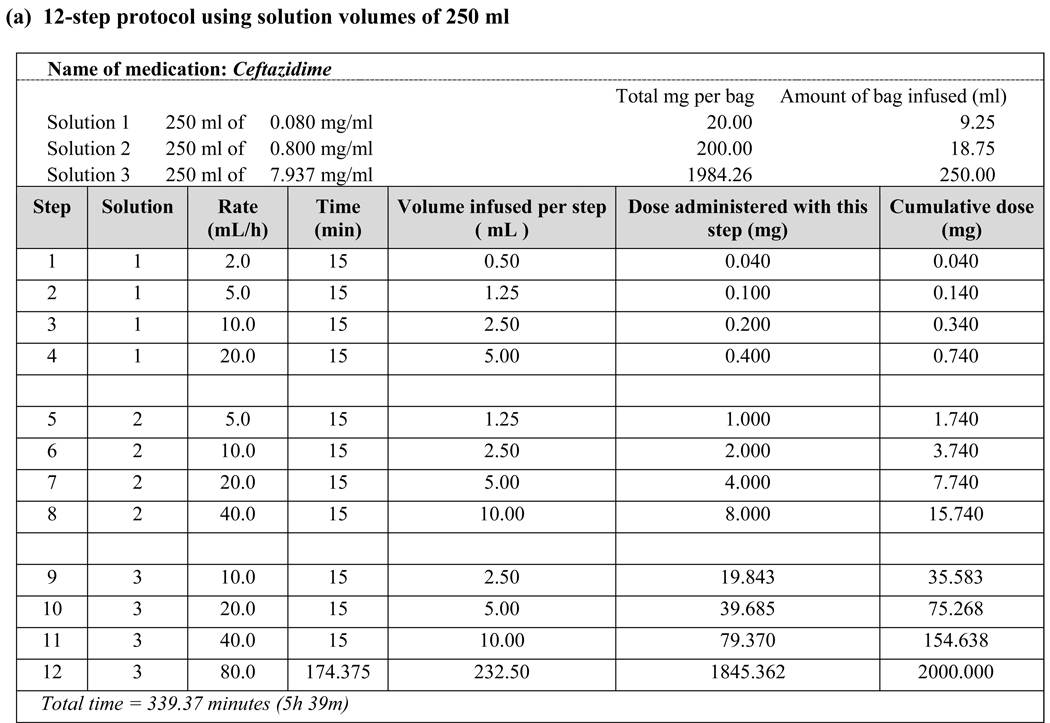
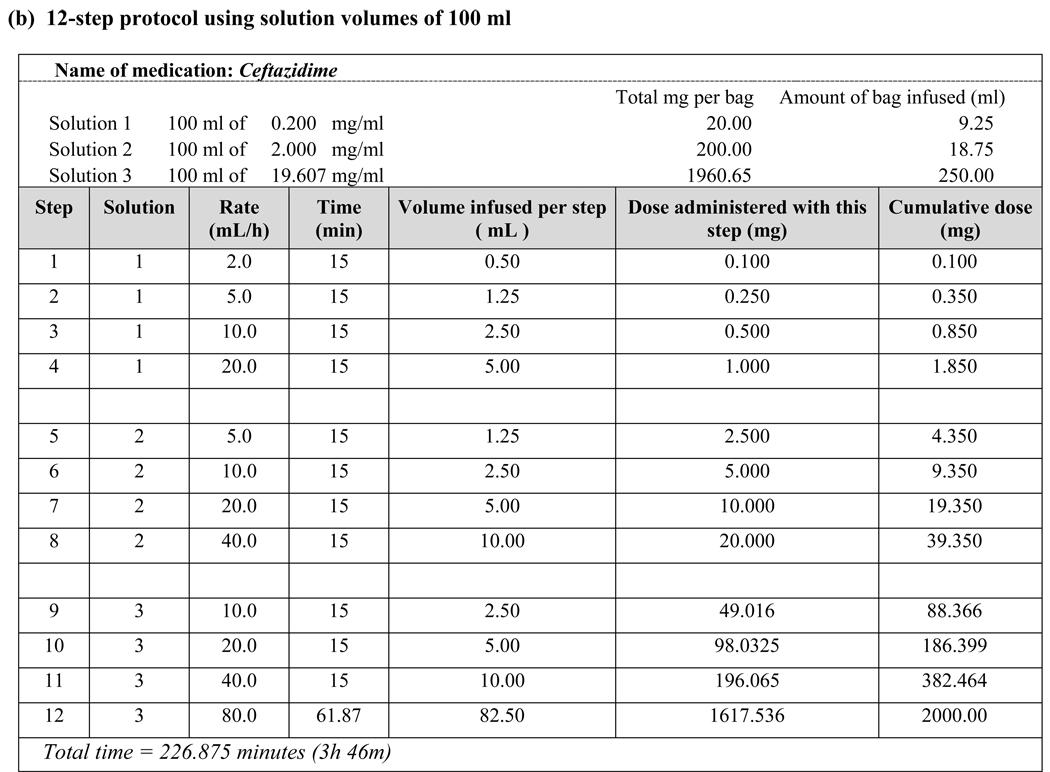
All patients received the full dose of the desired antibiotic, regardless of FEV1 or transplant status. There was a slightly higher rate of reactions during desensitization of patients with lower FEV1 but they were mild, well-tolerated and easily treated. Patients 4 and 8 completed desensitization using the original 12-step protocol [6–8] but subsequently developed flushing, urticaria and/or angioedema during administration of the full-dose antibiotic. Patient 10 developed isolated dyspnea during full-dose treatments that followed uncomplicated desensitizations on two separate admissions. Matching the final concentration of Solution 3 to the antibiotic concentration used in non-desensitized patients allowed these patients to tolerate the full antibiotic course (Fig. 1b).
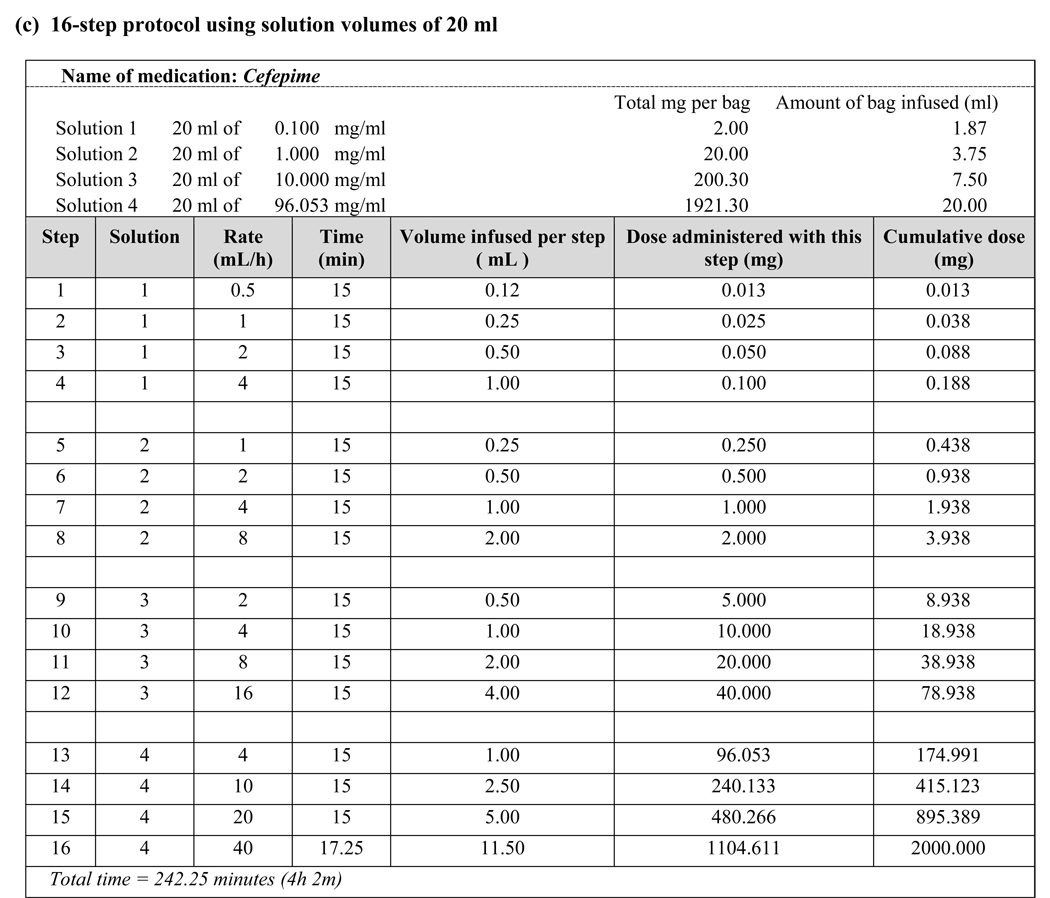
Figure 1.
Examples of desensitization protocols using various target antibiotic concentrations
Discussion
As patients with CF live longer—due to improved therapeutics, social support, availability of lung transplants—and have more frequent exposures to antibiotics (some of which, such as piperacillin, may be more immunogenic [2]) the prevalence of allergy to antibiotics has increased and will continue to increase. Drug desensitization is a safe and effective method to keep patients on first-line, preferred therapy, even in those patients with severely decreased lung function [10].
While unsuccessful desensitizations have been reported in 24% [5] and 25% [4] of other series using empiric protocols, we report a 100% desensitization completion rate using our protocol with two systemic reactions that were amenable to re-desensitization. Our results can be explained by the use of a safe protocol modeled after an in vitro experimental system [11], the exclusion of patients with reactions not typical of type I HSRs and perseverance through reactions during desensitization procedures once the appropriate treatments and/or protocol modifications have been instituted. Although the evaluation of this protocol is ongoing, we have previously demonstrated its success for both chemotherapeutic agents and monoclonal antibodies in the largest reported case series [8].
Medications that require dosing intervals greater than 24 hours, such as chemotherapy and monoclonal antibodies, need to be administered by desensitization in sensitized individuals with each treatment. Antibiotics, however, are dosed more frequently–often several times a day–and, thus, need only be administered by desensitization once per treatment course if the desensitization is successful and subsequent doses are administered in a timely fashion. In contrast to desensitization to chemotherapeutic agents, where a final target dose must be reached, our experience with CF patients who tolerated their desensitization but subsequently reacted to a full-strength antibiotic dose led us to modify our original protocol to a final target dose and concentration. For example, Patients 4 and 10 were successfully desensitized to their HSR-inducing antibiotic in a lower concentration solution (250-mL volume) than the typical concentration for that antibiotic (50- or 100-mL volume) and subsequently developed breakthrough reactions when receiving their scheduled doses at the typical concentration. We consequently modified their protocols to adjust the concentration of each bag during desensitization to match the typical concentration of the antibiotic and successfully reduced breakthrough reactions with subsequent antibiotic doses given at the typical concentration and schedule. We have since adopted this strategy for desensitization of our non-CF patients with antibiotic hypersensitivity, which has resulted in even greater success [unpublished data]. We have also implemented this approach to allow us to perform desensitizations in very high-concentration (20-mL volume) home infusion pumps (Fig. 1c).
The major limitation of this study is that clinical history has almost exclusively guided our decision to perform desensitization, as skin testing data was not routinely available due to the lack of commercially-available testing reagents in the United States since 2005. Without standardized reagents, the positive and negative predictive values of skin testing for penicillin and beta-lactams remains unknown and, thus, no patient seen after 2005 underwent skin testing. It should also be noted that the decision on whether or not to modify the desensitization protocol based on concentration often occurred in the setting of an urgent or emergent consultation, when a detailed review of a patient’s prior history of desensitization protocols and reactions was not always available. Since the establishment of our desensitization program and the introduction of a longitudinal electronic medical record system at our institution, we can now access all prior desensitization records and, thus, be able to evaluate and manage patients with previous histories of reactions during desensitizations in a more standardized fashion. Our ongoing studies seek to determine the clinically relevant concentrations of various antibiotics in patients with CF and to evaluate the implementation of our protocol as the standard of care in order to safely provide patients with first-line therapy.
Conclusions
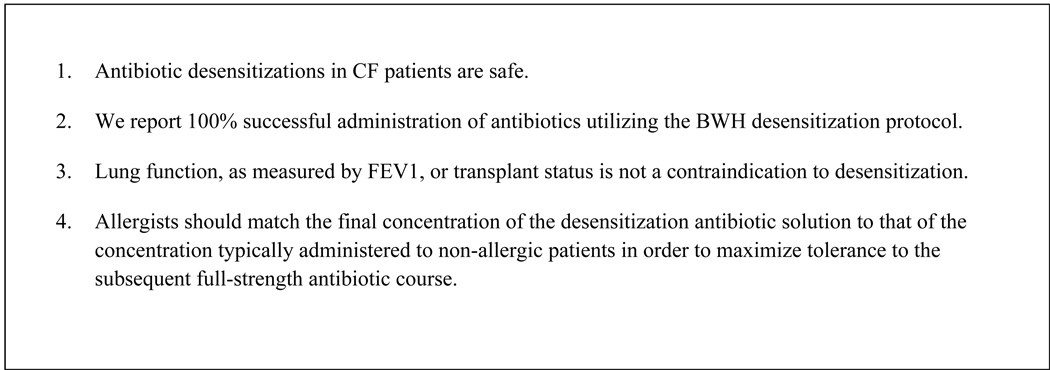
Desensitizations to antibiotics in patients with CF are safe using our protocol. We have had a 100% successful delivery of the desired antibiotic in our patient population. We decreased the percentage of reactions occurring during desensitization and during subsequent doses by adjusting the final concentration of the desensitization infusion to match the concentration that is typically administered for non-allergic patients. Even the most critically ill patients with extremely low FEV1 and/or imminent lung transplant safely tolerated the procedure. Therefore, neither lung function nor lung transplant status should be viewed as a contraindication to performing desensitization. Rather, clinicians should focus on treating their patients’ underlying infections with first-line agents utilizing this procedure.
Figure 2.
Pearls from our experience
Acknowledgments
We would like to thank the nurses in the MICU and the fellows in Allergy and Immunology at BWH for their participation in our drug desensitization program. We acknowledge the support of our ACGME (Accreditation Council for Graduate Medical Education)-accredited training program, operating under an NIH (National Institutes of Health)-funded T32 grant. Most importantly, we would not have a program without the courage and willingness of CF patients to undergo desensitization.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burrows JA, Nissen LM, Kirkpatrick CM, et al. Beta-lactam allergy in adults with cystic fibrosis. J Cyst Fibros. 2007;6:297–303. doi: 10.1016/j.jcf.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Koch C, Hjelt K, Pedersen SS, et al. Retrospective clinical study of hypersensitivity reactions to aztreonam and six other beta-lactam antibiotics in cystic fibrosis patients receiving multiple treatment courses. Rev Infect Dis. 1991;13 Suppl 7:S608–S611. doi: 10.1093/clinids/13.supplement_7.s608. [DOI] [PubMed] [Google Scholar]
- 3.Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001;107:191–193. doi: 10.1067/mai.2001.112031. [DOI] [PubMed] [Google Scholar]
- 4.Turvey SE, Cronin B, Arnold AD, et al. Antibiotic desensitization for the allergic patient: 5 years of experience and practice. Ann Allergy Asthma Immunol. 2004;92:426–432. doi: 10.1016/S1081-1206(10)61778-4. [DOI] [PubMed] [Google Scholar]
- 5.Burrows JA, Toon M, Bell SC. Antibiotic desensitization in adults with cystic fibrosis. Respirology. 2003;8:359–364. doi: 10.1046/j.1440-1843.2003.00461.x. [DOI] [PubMed] [Google Scholar]
- 6.Lee CW, Matulonis UA, Castells MC. Rapid inpatient/outpatient desensitization for chemotherapy hypersensitivity: standard protocol effective in 57 patients for 255 courses. Gynecol Oncol. 2005;99:393–399. doi: 10.1016/j.ygyno.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 7.Feldweg AM, Lee CW, Matulonis UA, et al. Rapid desensitization for hypersensitivity reactions to paclitaxel and docetaxel: a new standard protocol used in 77 successful treatments. Gynecol Oncol. 2005;96:824–829. doi: 10.1016/j.ygyno.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 8.Castells MC, Tennant NM, Sloane DE, et al. Hypersensitivity reactions to chemotherapy: outcomes and safety of rapid desensitization in 413 cases. J Allergy Clin Immunol. 2008;122:574–580. doi: 10.1016/j.jaci.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 9.Breslow RG, Caiado J, Castells MC. Acetylsalicylic acid and montelukast block mast cell mediator-related symptoms during rapid desensitization. Ann Allergy Asthma Immunol. 2009;102:155–160. doi: 10.1016/S1081-1206(10)60247-5. [DOI] [PubMed] [Google Scholar]
- 10.Kerem E, Reisman J, Corey M, et al. Prediction of mortality in patients with cystic fibrosis. N Engl J Med. 1992;326:1187–1191. doi: 10.1056/NEJM199204303261804. [DOI] [PubMed] [Google Scholar]
- 11.Morales AR, Shah N, Castells M. Antigen-IgE desensitization in signal transducer and activator of transcription 6-deficient mast cells by suboptimal doses of antigen. Ann Allergy Asthma Immunol. 2005;94:575–580. doi: 10.1016/S1081-1206(10)61136-2. [DOI] [PubMed] [Google Scholar]