Abstract
Purpose
Induction of oxidative stress has been implicated in UV-induced melanoma. We sought to determine whether the antioxidant N-acetylcysteine (NAC) could be safely administered to protect melanocytic nevi from the oxidative stress resulting from acute UV exposure.
Experimental design
Patients at increased risk for melanoma were recruited from a screening clinic. Induction and detection of oxidative stress (reactive oxygen species and glutathione depletion) was optimized in nevi following ex-vivo UV-irradiation. Nevi were removed from patients prior to, and following, oral ingestion of a single (1200 mg) dose of NAC, and then these nevi were UV-irradiated (4000 J/m2).
Results
Oxidative stress was induced in nevi 24-48 h following ex-vivo UV-irradiation. A single oral dose of NAC was well-tolerated in all patients (n=72). Basal levels of reduced glutathione and the NAC metabolite cysteine were well-correlated between similar-appearing nevi from the same patient, and were significantly increased in nevi removed 3 h after NAC ingestion compared to nevi removed prior to drug ingestion. In approximately half (9/19) of patients tested, UV-induced glutathione depletion was attenuated in the post-drug (compared to pre-drug) nevus.
Conclusions
NAC can be safely administered to patients for the purpose of modulating UV-induced oxidative stress in nevi. This study suggests the feasibility of patients taking NAC prophylactically prior to acute UV exposure, to prevent pro-oncogenic oxidative stress in nevi and ultimately reduce long-term melanoma risk.
Keywords: chemoprevention, melanoma, antioxidant, nevi, UV
Introduction
Melanoma is a potentially lethal form of skin cancer, which unfortunately in recent years has seen a dramatic increase in incidence that has not been matched by the development of effective therapies for patients with advanced disease (1). Although melanoma may arise directly from isolated melanocytes, a significant fraction of melanomas develop from nevi (or moles) (2), which represent congenital or acquired clonal neoplasms of melanocytes (3). Nevi are far less prevalent on sun-protected skin and their development is related to sun exposure (4), which also is the major environmental risk factor for melanoma (5). Patients can reduce the potentially harmful effects of UV by limiting sun exposure and/or using sunscreen, although some studies suggest that sunscreen use may increase melanoma risk (6), perhaps due to increased sun exposure in sunscreen users (7). While sunscreens are designed to prevent sunburn, it is unclear whether they protect against all possible carcinogenic effects of UV exposure. Given these considerations, and the fact that most patients do not apply sunscreens properly (8), reliance on sunscreen alone may be inadequate and there is need for additional preventive strategies.
UV in sunlight is a potent inducer of reactive oxygen species (ROS) in the skin (9), which may damage intracellular constituents and deplete vital reductants such as glutathione (10). Sustained oxidative stress can cause oxidative DNA lesions that may result in oncogenic mutations if not repaired by the DNA glycosylase OGG1 prior to DNA replication (11). There is a good deal of correlative evidence suggesting that one link between UV radiation and melanoma may lie in the generation of oxidative damage (12). Interestingly, endogenous antioxidants are reduced in melanocytes isolated from melanoma patients (13), and increased ROS in melanoma cell lines was found to correlate with more aggressive behavior (14). Increased ROS has been linked to activation of Akt in melanoma cell invasion (15) and viability (16). Mutations in the melanocortin-1 receptor (MC1R), which regulates UV-induced ROS production and metabolism in melanocytes (17, 18), are associated with melanoma predisposition (19). In the Xiphophorus fish model, the UV action spectrum for melanin-dependent oxidant production is identical to that for melanoma induction (20). In addition, mutation or loss of OGG-1 has been associated with melanoma progression (21, 22). Finally, we recently reported that the antioxidant N-acetylcysteine (NAC) delays tumor development in an animal model of UV-induced melanoma (23).
NAC is a highly potent, orally bioavailable cell-permeable antioxidant that is deacetylated to L-cysteine (Cys) and then converted to glutathione (24). It is commercially available, inexpensive, Food and Drug Administration-approved for acetaminophen toxicity (25), and more recently has been used to preserve lung function in patients with idiopathic pulmonary fibrosis (26) and to prevent contrast-medium-induced nephropathy in patients undergoing angioplasty (27). Additionally, topically-applied NAC was reported to reduce UV-mediated glutathione depletion and ROS induction in human skin (28).
Here, we show that NAC can be safely administered to patients for the purpose of modulating acute UV-induced oxidative stress in nevi.
Materials and Methods
Patients
This study was approved by the Institutional Review Board of the University of Utah. Patients were recruited from our pigmented lesion clinic, in which patients with history of numerous or atypical nevi, and/or personal or family history of melanoma are regularly monitored. Seventy-two patients (ages 24-73, 57% males) were given NAC as part of this study. Patients that were under age 18, critically ill or mentally handicapped, prisoners, pregnant, non-English speaking, or having history of asthma or allergic reaction to NAC, were excluded. All patients signed a consent form to participate. Patients were not charged for nevus removal or histological examination, and each was compensated $100 following their completion of the study.
Nevus tissues
Nevi (usually ≥ 6mm in diameter to provide sufficient tissue for analysis) that were not suspicious for melanoma were selected. In cases where two nevi were removed from the same patient, nevi were selected with similar morphologies (color, shape, size) and from similar body location to control for history of sun exposure. Nevi were removed by shave technique, and then a representative 1 mm central slice was incised and submitted for routine histological analysis; thus the tissue section examined by the dermatopathologist (S.R.F.) was comparable to that which is normally viewed in a bisected specimen (Supplementary Fig. 1). We have shown previously that the diagnostic information present in one section of nevus could be extrapolated to the remainder of the specimen without adverse consequences from a diagnostic or therapeutic perspective (29). The remaining nevus fragments were grossly macrodissected from normal surrounding skin and either untreated or immediately UV-irradiated (described below), and then placed in 6-well dishes containing DMEM (supplemented with 10% fetal calf serum, glutamine, and antibiotics) and cultured at 37 °C in a humidified atmosphere containing 5% CO2. We found that neither UV irradiation nor the culture system disturbs the nevus architecture – if the central portion of the nevus later proved histologically suspicious for melanoma and the dermatopathologist requested to examine the entire nevus, then these tissue fragments were retrieved from culture, formalin-fixed and processed for histology, and not used for further study.
UV, ROS, and oxidative damage
Nevus fragments were placed on saline-soaked gauze, dermal side down, and either untreated or UV-irradiated (4 J/m2 per s) in a fan-cooled box containing unfiltered sun lamps (FS20T12-UVB, National Biological Corp.) emitting wavelengths of 250-420 nm (72.6% UVB, 27.4% UVA, 0.01% UVC) as previously described (23). Endogenous ROS were quantified by addition of 20 μM 2′,7′-dichlorodihydrofluorescein diacetate (DCFDA, Invitrogen) at 37 °C. After 30 min, tissue fragments were rinsed in PBS, blotted dry, and then homogenized for approximately 2 min in a disposable eppendorf tube with plastic pestle (Fisher Scientific) containing 100-200 μL lysis buffer (2% SDS, 50 mM Tris and 7.5% glycerol), with more buffer used for larger fragments. After centrifugation, 2 μL supernatant was subjected to bicinchoninic acid reaction (Thermo Fisher Scientific) to quantify protein content, and fluorimetric analysis of 30 μg cell lysate (brought to 50 μL with lysis buffer) was performed in triplicate using a CytoFluor II plate reader (Perseptive Biosystems) with excitation and emission wavelengths set at 485 and 535 nm, respectively. Staining of formalin-fixed paraffin-embedded nevus sections for nuclei (with 4,6-diamidino-2-phenylindole) and apoptotic cells (Tdt-mediated dUTP nick end labeling, TUNEL) was performed as we have described previously (30).
Determination of thiols
Following ex-vivo culture, nevus fragments were rinsed in PBS, blotted dry, weighed, and then stored at -80 °C. Tissue thiols were derivatized with monobromobimane and analyzed by reverse-phase chromatography using a protocol modified from one described previously (31), and reagents obtained from Sigma Chemical Co. Nevus samples were transferred to disposable eppendorf tubes with plastic pestles as above, and manually homogenized in ice-cold 200 mM methanesulfonic acid (6.7 μL per mg tissue) for 1 min. One volume 4 M sodium methane sulfonate was added, gently mixed, and then centrifuged (×15,000 g) for 10 min at 4 °C. Fifty μL supernatant was transferred to a fresh tube, to which was added 150 μL of a freshly-prepared solution consisting of 200 mM N-2-hydroxyethylpiperazine-N′-3-propanesulfonic acid / methane sulfonate, 5 mM diethylenetriaminepentaacetic acid, and 3 mM monobromobimane. After incubation for 10 min at room temperature in the dark, 14 μL of 1.5 M methanesulfonic acid was added, and aliquots were stored at -80 °C.
Samples were diluted 1:10 and subjected to reverse-phase chromatography using a Luna 5u C18 100A column (Phenomenex). To separate reduced glutathione (GSH), Cys and NAC from the matrix peaks, the following gradient program with 100% methanol (mobile phase B) and 0.25% acetic acid pH 3.5 (mobile phase A) was used: 0-5 min: 15% B; 5-15 min: 15-23% B; 15-45 min: 23-42% B; 45-65 min: 42-65 % B, 65-67 min: 65-100% B; 67-70 min: 100% B; 70-85 min: 100-15% B. The column was equilibrated 20 min at 15% B between runs. Fluorescent-labeled thiols were detected using a multi-wavelength fluorescence detector (model 2475, Waters) set at excitation and emission wavelengths of 360 and 475 nm, respectively.
Using this protocol, the thiols (NAC, GSH, and Cys) could easily be resolved from one another, but GSH eluted near another peak in the nevus matrix (Supplementary Fig. 2). Its identity was confirmed by comparing the chromatograms of derivatized lysates to those of samples spiked with derivatized chemically-pure NAC and GSH (Sigma), which revealed a symmetrical increase in these thiol peaks (Supplementary Fig. 2). As a control, derivatization of a nevus lysate pre-treated with iodacetamide (which alkylates thiol residues but is not detectable by fluorescence analysis) reduced the thiol peaks by 94% (not shown). The stability of the derivatized thiols was determined at room temperature in the auto sampler. No significant change was seen in the peak area values of the standards or thiols in samples that were re-run 24 h later.
NAC
N-acetylcysteine solution (NAC, 200 mg per mL solution, American Regent) obtained from our hospital pharmacy was diluted approximately 1:4 in tomato juice (to mask salty taste) immediately prior to use.
Statistics
Statistical analysis was performed using Prism 3.0 (Graphpad) and R 2.8.0 (R Foundation for Statistical Computing) software. P values ≤ 0.05 were considered statistically significant.
Results
UV-induced oxidative responses in nevi
To avoid any potential risks of exposing patients to UV radiation, we developed an ex-vivo experimental system for evaluating UV-induced oxidative stress in nevi. Following removal from the patient, nevi were dissected into representative fragments (Supplementary Fig. 1) which were either untreated or UV-irradiated, and then cultured in an incubator. Under our culturing conditions, nevi remained viable for up to 72 h (Fig. 1A). Following UV irradiation at 4000 J/m2, no inflammatory cells were present at 6h but by 24 h a marked inflammatory response was evident (Fig. 1A). Interestingly, these inflammatory cells must have arisen from blood and lymphatic vessels in the dermal component of the specimen. By 48 h, cells with hyperchromatic and pyknotic nuclei could be seen (Fig. 1A), and the presence of apoptotic cells could be detected by TUNEL staining (Supplementary Figure 3). Despite these changes, tissue architecture in UV-irradiated nevi was preserved for up to 48 h, allowing sufficient time either to obtain a histologic diagnosis on the originally submitted portion, or to submit the UV-irradiated tissue so this remainder of the lesion could also be examined. In this way, we were able to initiate our studies of UV responses in nevi immediately following removal of the lesion. Following treatment of nevi with 4000 J/m2, ROS levels were slightly decreased at 24 h but significantly elevated at 48 h compared to fragments from the same nevi that were not irradiated (Fig. 1B). Under these same conditions, GSH content was significantly lower at both 24 h and 48 h compared to that measured in corresponding unirradiated nevus fragments (Fig. 1C). We initially evaluated a range of UV doses (400-4000 J/m2) for the capacity to induce oxidative stress, but found that the highest dose appeared to be optimal and that lower doses were not sufficient to induce detectable ROS accumulation and GSH depletion in this experimental system (not shown). This UV dose is not out of the range of physiologic relevance, as we determined that a single dose of 4000 J/m2 is comparable to 4-5 h of summertime sun exposure based on the average UV index in Utah1. Thus we have developed a convenient system for assessing modulation of oxidative stress in human nevi induced by UV exposure. Our intention was to use this ex-vivo system to evaluate NAC-mediated protection against acute UV-induced oxidative stress in nevi.
Fig. 1.
Inflammatory and oxidative responses in human nevi following ex-vivo UV irradiation. A, Fragments from an individual nevus were untreated or UV-irradiated (4000 J/m2), then at the indicated time points were formalin-fixed and paraffin-embedded sections were stained with hematoxylin and eosin (original magnification ×400). Inflammatory cells are recognized by their small size and dark nuclei. B, Fragments from individual nevi were untreated or UV-irradiated (4000 J/m2), then ROS levels in protein equivalent lysates were measured by DCFDA fluorescence either 24 h (n=5 nevi) or 48 h (n=7 nevi) later. For each nevus, fluorescence value for the UV-treated fragment was normalized to the untreated fragment which was set at 1. *P<0.001 (two-tailed one sample t test). C, Fragments from individual nevi were untreated or UV-irradiated (4000 J/m2), then GSH content (normalized to nevus weight) was measured by chromatography coupled with fluorescence detection either 24 h (n=6 nevi) or 48 h (n=4 nevi) later. For each nevus, GSH content for the UV-treated fragment was normalized to the untreated fragment which was set at 100. *P<0.001, **P=0.008 (two-tailed one sample t tests).
Safety of oral NAC and detection in nevi
In the course of these studies, 72 patients were administered a single oral dose of NAC. The first two patients received 600 mg, and all 70 subsequent patients received 1200 mg. Since the 600 mg dose was well-tolerated in the first two patients, the remaining patients were given the higher dose of 1200 mg to increase likelihood of observing a pharmacologic effect. All patients were surveyed by telephone following drug ingestion to assess potential side effects such as nausea and itching (25), and no adverse reactions were reported. In all cases, the drug was well-tolerated, confirming the safety of single oral high-dose NAC administration.
We were unable, however, to detect significant levels of NAC in nevi 1.5-24 h following drug ingestion (not shown). This was likely due to its rapid metabolism and ease of oxidation prior to analysis, and is consistent with a previous study in mice in which NAC could not be detected in animals given a comparable dose (32). Since NAC is metabolized to Cys and incorporated into GSH (24), we reasoned that both Cys and GSH may be informative surrogate markers for NAC delivery to nevus tissues.
Inter-nevus variability in oxidative biomarkers
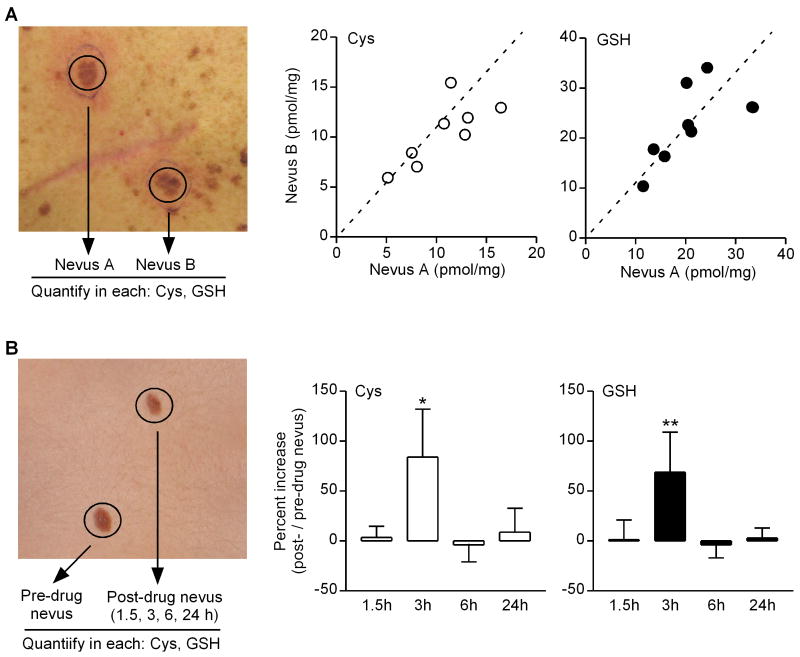
We felt it was important, in determining parameters for suitable drug delivery to nevi, to use a ‘pre-drug’ nevus as a reference to control for inter-patient variability. We realized such an approach, however, could be flawed if there were significant variation between nevi in individual patients. Thus our next step was to assess inter-nevus variability with respect to particular biomarkers that would be used to assess drug delivery (Cys, GSH content). We measured basal Cys and GSH levels in pairs of nevi from eight patients (Fig. 2A, left panel), and found that while content of these markers varied considerably between nevi from patient to patient, there was very good correlation of both Cys and GSH levels among similar-appearing nevi in each patient (Fig. 2A, right panels).
Fig. 2.
Inter-nevus correlation of oxidative biomarkers, and NAC-mediated oxidative modulation in nevi. A, Two similar-appearing nevi were removed from individual patients (n=8), and Cys and GSH content (normalized to nevus weight) was determined (left panel). For each patient, values for Cys (left plot, open circles) and GSH (right plot, filled circles) are expressed as a single data point reflecting each pair of nevi (nevus A, abscissa; nevus B, ordinate). Dotted lines represent theoretical correlation where data points should fall for pairs of nevi with identical values. For Cys measurements, correlation coefficient (r)=0.77 (95% confidence interval 0.14-0.95, P=0.03). For GSH measurements, correlation coefficient (r)=0.69 (95% confidence interval -0.03 – 0.94, P=0.06). B, Two similar-appearing nevi were removed from individual patients immediately prior to, and either 1.5 h, 3 h, 6 h, or 24 h (n=5-6 at each time point) following ingestion of 1200 mg NAC (left panel). Cys and GSH content (normalized to nevus weight) was determined for each nevus, and data expressed as percent increase in post-drug vs. pre-drug nevus (right panels). *P=0.047; **P=0.016 (Wilcoxon Signed Rank tests).
NAC delivery to nevus tissues
To investigate NAC delivery to nevus tissues in these patients, we removed a nevus just prior to drug ingestion and a second nevus either 1.5, 3, 6, or 24 h later (Fig. 2B, left panel). These time points were chosen given the reported half-life of NAC in humans of 5.6 h (25). We analyzed ‘pre-drug’ and ‘post-drug’ nevi from a group of patients for content of Cys and GSH, and found significant increases in both of these thiols in ‘post-drug’ nevi 3 h after NAC ingestion, but by 6 h these biomarkers had returned to levels in the ‘pre-drug’ nevi (Fig. 2B, right panels). Thus following a single dose of 1200 mg NAC, we were able to detect increased levels of drug metabolites (Cys, GSH) in nevus tissues.
NAC-mediated protection against UV-induced oxidative stress
Having optimized the parameters for inducing oxidative stress (UV dose, incubation time) and delivering NAC to nevi (drug dose, time), we used this experimental system to examine whether oral NAC could confer protection against UV-induced oxidative stress. Two similar-appearing nevi were removed from patients, one immediately prior to, and another 3 h following ingestion of 1200 mg NAC (Fig. 3A). Fragments from each nevus were either untreated or UV-irradiated (4000 J/m2), then incubated for 24 h (n=8 patients) or 48 h (n=11 patients). We chose to examine nevi both 24 h and 48 h following UV treatment since we had observed UV-induced GSH depletion at both time points (Fig. 1C). At the end of the culture period, GSH content was measured in each fragment, and percent UV-induced GSH depletion was determined for each nevus (Fig. 3A). We found protection against UV-induced oxidative stress (less GSH depletion) in approximately 50% of patients (Fig. 3B).
Fig. 3.
NAC-mediated protection against UV-induced oxidative stress suggests potential utility of novel paradigm for melanoma chemoprevention. A, Two similar-appearing nevi were removed from 19 patients immediately prior to, and 3 h following ingestion of 1200 mg NAC. Fragments of each nevus were either untreated or UV-irradiated (4000 J/m2), then cultured for either 24 h (8 patients) or 48 h (11 patients). GSH content (normalized to nevus weight) was determined for each nevus fragment. B, Data expressed as percent UV-induced GSH depletion (UV-treated vs. untreated) in fragments of pre-drug nevi (open bars) and post-drug nevi (filled bars) from each patient. For nevi cultured 24 h, there was protection (ie. less GSH depletion) in 3/8 patients (left panel); for nevi cultured 48 h, there was protection in 6/11 patients. C, Schematic illustrating proposed novel paradigm for melanoma chemoprevention based on these pilot studies. Patients could take NAC in anticipation of sun exposure, to protect their nevi and other skin melanocytes from UV-induced oxidative stress (lower panel), and reduce their long-term risk of melanoma if applied over many years and sun exposures. This approach would circumvent the major pitfalls of conventional chemoprevention (upper panel) by timing drug administration with activation of a relevant oncogenic pathway, and episodic drug ingestion would avoid potential deleterious effects that may be associated with chronic use of any drug.
Discussion
Our previous studies implicated UV-induced oxidative stress as a causative oncogenic insult in an animal model of melanoma, and suggested that the antioxidant NAC may be useful as a chemopreventive agent in human melanoma (23). Here we describe an ex-vivo system for studying UV-induced oxidative stress in human nevi, and have used this system to test whether NAC orally-administered to patients can protect their nevi against UV-induced oxidative stress. We found in approximately 50% of patients that nevi removed 3 h following a single 1200 mg dose of NAC, compared to matched nevi removed just prior to drug ingestion, were less susceptible to UV-induced GSH depletion. Our results demonstrate the potential utility of NAC in protecting against pro-carcinogenic oxidative stress induced by UV exposure, and further suggest and support a novel paradigm for melanoma chemoprevention, as discussed below.
Given the potent antioxidant capacity of NAC and our ability to optimize its delivery to nevi in vivo, one might have expected a higher rate of efficacy in our model system. There are several variables, however, which we did not account for that may have affected either UV-induced oxidative stress or NAC-mediated protection. First, we do not have genotyping information our patients for MC1R, which regulates both UV-induced ROS production and metabolism in melanocytes (17, 18). Although we attempted to control for the degree of UV-induced oxidative stress by incorporating a ‘pre-drug’ nevus from the same patient, it is possible that MC1R polymorphisms in some patients may have resulted in a different spectrum and/or levels of ROS or antioxidant response that may be more refractory to NAC protection. In addition, we did not alter drug dosage based on patient weight, nor account for whether the drug was taken immediately prior to or following eating, which may have affected drug efficacy. It is also important to note that we assumed that optimal conditions for drug delivery to nevi were similarly optimal for restoration of depleted GSH. Although we may not have fully optimized the experimental conditions, the fact that we did observe a protective effect in a number of patients does satisfy a proof-of-principle that NAC may protect nevi against oxidative stress if taken in advance of UV exposure.
According to recent U.S.-based epidemiologic studies, melanoma is more common and increasing at a faster rate in men then women (33). Although the basis for these trends is unclear, we note that markers of systemic oxidative stress such as γ-glutamyltransferase (34) are increased in males compared to females (35). Moreover, animal studies revealed that females appear to be protected against oxidative stress due to endogenous estrogens (36). Interestingly, we found that 3 of 10 (30%) non-responders and 1 of 9 (11%) responders (Fig. 3B) were female, suggesting the possibility that female sex may be a predictor of non-responsiveness to NAC in our patients. However, the higher rate of male responders (odds ratio 3.2, 95% confidence interval 0.20-202) was not statistically significant (p=.58). We will be able to pursue this question further in a larger future study (see below).
There are many obstacles associated with a conventional chemoprevention approach for cancer, which usually involves chronic drug administration (Fig. 3C). First, it is difficult to maintain and monitor patient compliance for an extended period of time. In addition, there may be unpredictable toxicities associated with chronic ingestion of any agent. Even if informative biomarkers are available and assessed at intermediate time points to monitor drug delivery and action, it is generally not possible to assess clinical benefit until the end of the trial (i.e. did the patients in the experimental/intervention group develop less cancer?). Another potential problematic consideration is that the chemopreventive agent may not be administered in conjunction with the specific oncogenic stimulus, which for many cancers is unknown (Fig. 3C). For melanoma, the long latency time and low (annual) risk of tumor development are such that large numbers of patients would need to be treated and monitored for many years to determine whether a given preventive agent is effective. Finally, a combination of these factors may yield unanticipated adverse (or paradoxical) results as observed in various prevention trials of antioxidants (37). For example, patients who took β-carotene and retinol for two years exhibited an increased risk (relative risk 1.38) of lung cancer (38), and a French study recently reported that mixed antioxidant supplementation over seven years was associated with increased risk (hazard ratio 1.68) of skin cancer in women (39).
Several agents targeting oxidative stress have been considered for melanoma chemoprevention (40). These include epigallocatechin-3-gallate (EGCG), a polyphenol antioxidant present in green and black teas, which decreases inflammation and induces cell cycle arrest and apoptosis in vitro (41, 42). Orally-delivered EGCG has also been shown to inhibit tumor promotion and metastasis in a mouse melanoma model (43). Though these data for EGCG are encouraging, a prospective cohort study in postmenopausal women found no association between tea drinking and melanoma incidence (44). Other proposed antioxidants for melanoma chemoprevention include Vitamin E, β-carotene, lycopene, flavonoids, resveratrol and selenium, but as with EGCG, there is lack of compelling data in humans (40). Moreover, the proposed use of these agents would be subject to all the obstacles associated with conventional chemoprevention regimens noted above.
The antioxidant NAC has not been formally tested as a chemopreventive agent in melanoma. Our data, however, suggest that NAC may be useful – not in a conventional chemoprevention protocol, but rather as a chemopreventive agent in the context of acute UV exposure. A chemoprevention strategy in which NAC is taken episodically, rather than chronically, in anticipation of sun exposure, would bypass most of the obstacles associated with conventional chemoprevention. We envision that patients with nevi, who are at increased risk for melanoma (45), could take NAC as a prophylactic ‘sunburn pill’ in anticipation of sun exposure to protect their nevi against UV-induced oxidative stress (Fig. 3C). If successfully implemented, this strategy would have a presumed benefit, since a reduction in pro-oncogenic oxidative stress in nevi over the course of many UV exposures would be predicted to decrease long-term (lifetime) melanoma risk. In addition, there would be less oxidative damage over time in isolated melanocytes, from which the majority of melanomas arise (2).
This study paves the way for a formal trial in which NAC-mediated protection could be assessed in the context of incident (in vivo) UV exposure, which would better represent the setting in which most people acquire UV-induced oxidative stress in their skin and nevi over time. Finally, we note that this novel chemopreventive strategy may be useful for other cancers triggered by known environmental stimuli.
Supplementary Material
Fig. S1. Schematic showing protocol for obtaining nevus tissues for these studies. A, Nevus specimens biopsied and submitted for routine histological analysis are usually bisected, then embedded for sectioning. A section corresponding to the central portion of the nevus is prepared for viewing, and usually is sufficient for histological diagnosis (left panel). B, For nevi used in this study, a central section was removed from the specimen following biopsy and submitted for routine processing while the remaining tissues were held back. The section generated from this submitted specimen would resemble that initially obtained if the entire specimen had been submitted (right panel). If a clear diagnosis could not be made from the submitted specimen, then the remaining fragments could also be submitted for analysis (and not used for research).
Fig. S2. Efficient separation of thiols in nevus specimens by reverse-phase chromatography. Thiols in nevus lysates were derivatized with monobromobimane, loaded onto a C-18 column, and their elution monitored using a fluorescence detector. Overlay of histograms showing elution patterns of thiols from mixture of standards (Cys, GSH, NAC; black tracing), nevus lysate (red tracing), and nevus lysate spiked with standards (blue tracing).
Fig. S3. UV-induced apoptosis in nevi. Fragments from an individual nevus were untreated or UV-irradiated (4000 J/m2), then 48 h later stained for nuclei (4,6-diamidino-2-phenylindole, blue) and apoptotic cells (TUNEL, red). Overlay fluorescent images are shown (original magnification ×400).
Acknowledgments
We acknowledge the use of the Tissue Resource and Applications core facility, supported by P30 CA042014 awarded to Huntsman Cancer Institute.
Grant support: NIH grants R21 AR056797 (D. Grossman) and T32 CA093247 (M.A. Cotter), the Department of Dermatology, and the Huntsman Cancer Foundation.
Footnotes
Note: A.G. Goodson and M.A. Cotter contributed equally to this work.
Conflicts of interest: None declared.
Statement of Translational Relevance: We demonstrate that the antioxidant NAC can be safely administered to patients for the purpose of modulating UV-induced oxidative responses in nevi. This work establishes the feasibility of a future in vivo trial to test whether NAC can be taken prophylactically prior to acute UV exposure to reduce pro-carcinogenic oxidative stress in nevi and skin. For patients at increased risk for melanoma, this strategy of episodic drug administration, targeting a known oncogenic pathway, would be predicted to reduce their long-term risk if applied over many years and sun exposures. If successfully implemented, such a strategy would obviate many of the pitfalls inherent in most conventional chemoprevention approaches. In addition to nevi, this strategy may confer oxidative protection to isolated melanocytes from which melanoma may also arise. Finally, this novel chemopreventive strategy may be useful for other cancers triggered by known environmental stimuli.
References
- 1.Tsao H, Atkins MB, Sober AJ. Management of cutaneous melanoma. N Engl J Med. 2004;351:998–1012. doi: 10.1056/NEJMra041245. [DOI] [PubMed] [Google Scholar]
- 2.Crucioli V, Stilwell J. The histogenesis of malignant melanoma in relation to pre-existing pigmented lesions. J Cutan Pathol. 1982;9:396–404. doi: 10.1111/j.1600-0560.1982.tb01078.x. [DOI] [PubMed] [Google Scholar]
- 3.Robinson WA, et al. Human acquired nevi are clonal. Melanoma Res. 1998;8:499–503. doi: 10.1097/00008390-199812000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Kelly JW, et al. Sunlight: a major factor associated with the development of melanocytic nevi in Australian schoolchildren. J Am Acad Dermatol. 1994;30:40–8. doi: 10.1016/s0190-9622(94)70005-2. [DOI] [PubMed] [Google Scholar]
- 5.Gilchrest BA, Eller MS, Geller AC, Yaar M. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med. 1999;340:1341–48. doi: 10.1056/NEJM199904293401707. [DOI] [PubMed] [Google Scholar]
- 6.Bigby M. The sunscreen and melanoma controversy. Arch Dermatol. 1999;135:1526–27. doi: 10.1001/archderm.135.12.1526. [DOI] [PubMed] [Google Scholar]
- 7.Autier P, Boniol M, Dore JF. Sunscreen use and increased duration of intentional sun exposure: still a burning issue. Int J Cancer. 2007;121:1–5. doi: 10.1002/ijc.22745. [DOI] [PubMed] [Google Scholar]
- 8.Lademann J, et al. Sunscreen application at the beach. J Cosmet Dermatol. 2004;3:62–68. doi: 10.1111/j.1473-2130.2004.00107.x. [DOI] [PubMed] [Google Scholar]
- 9.Herrling T, Jung K, Fuchs J. Measurements of UV-generated free radicals/reactive oxygen species (ROS) in skin. Spectrochim Acta A Mol Biomol Spectrosc. 2006;63:840–45. doi: 10.1016/j.saa.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Farmer PJ, et al. Melanin as a target for melanoma chemotherapy: pro-oxidant effect of oxygen and metals on melanoma viability. Pigment Cell Res. 2003;16:273–79. doi: 10.1034/j.1600-0749.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- 11.Bruner SD, Norman DP, Verdine GL. Structural basis for recognition and repair of the endogenous mutagen 8-oxoguanine in DNA. Nature. 2000;403:859–66. doi: 10.1038/35002510. [DOI] [PubMed] [Google Scholar]
- 12.Meyskens FL, Jr, Farmer P, Fruehauf JP. Redox regulation in human melanocytes and melanoma. Pigment Cell Res. 2001;14:148–54. doi: 10.1034/j.1600-0749.2001.140303.x. [DOI] [PubMed] [Google Scholar]
- 13.Grammatico P, et al. Increased sensitivity to peroxidizing agents is correlated with an imbalance of antioxidants in normal melanocytes from melanoma patients. Exp Dermatol. 1998;7:205–12. doi: 10.1111/j.1600-0625.1998.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 14.de Souza GA, et al. Proteomic and SAGE profiling of murine melanoma progression indicates the reduction of proteins responsible for ROS degradation. Proteomics. 2006;6:1460–70. doi: 10.1002/pmic.200500243. [DOI] [PubMed] [Google Scholar]
- 15.Govindarajan B, et al. Overexpression of Akt converts radial growth melanoma to vertical growth melanoma. J Clin Invest. 2007;117:719–29. doi: 10.1172/JCI30102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy KK, et al. The antidepressant sertraline downregulates Akt and has activity against melanoma cells. Pigment Cell Melanoma Res. 2008;21:451–56. doi: 10.1111/j.1755-148X.2008.00481.x. [DOI] [PubMed] [Google Scholar]
- 17.Samokhvalov A, et al. Oxidation potentials of human eumelanosomes and pheomelanosomes. Photochem Photobiol. 2005;81:145–48. doi: 10.1562/2004-07-23-RC-245. [DOI] [PubMed] [Google Scholar]
- 18.Kadekaro AL, et al. alpha-Melanocortin and endothelin-1 activate antiapoptotic pathways and reduce DNA damage in human melanocytes. Cancer Res. 2005;65:4292–99. doi: 10.1158/0008-5472.CAN-04-4535. [DOI] [PubMed] [Google Scholar]
- 19.Landi MT, et al. MC1R germline variants confer risk for BRAF-mutant melanoma. Science. 2006;313:521–22. doi: 10.1126/science.1127515. [DOI] [PubMed] [Google Scholar]
- 20.Wood SR, et al. UV causation of melanoma in Xiphophorus is dominated by melanin photosensitized oxidant production. Proc Natl Acad Sci U S A. 2006;103:4111–15. doi: 10.1073/pnas.0511248103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zyrek-Betts J, et al. Malignant blue nevus with lymph node metastases. J Cutan Pathol. 2008;35:651–57. doi: 10.1111/j.1600-0560.2007.00878.x. [DOI] [PubMed] [Google Scholar]
- 22.Pashaei S, et al. Concordant loss of heterozygosity of DNA repair gene, hOGG1, in melanoma in situ and atypical melanocytic hyperplasia. J Cutan Pathol. 2008;35:525–31. doi: 10.1111/j.1600-0560.2007.00865.x. [DOI] [PubMed] [Google Scholar]
- 23.Cotter MA, et al. N-acetylcysteine protects melanocytes against oxidative stress/damage and delays onset of ultraviolet-induced melanoma in mice. Clin Cancer Res. 2007;13:5952–58. doi: 10.1158/1078-0432.CCR-07-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maxwell SR. Prospects for the use of antioxidant therapies. Drugs. 1995;49:345–61. doi: 10.2165/00003495-199549030-00003. [DOI] [PubMed] [Google Scholar]
- 25.Physician's Desk Reference (PDR) 2007:1031–34. [Google Scholar]
- 26.Demedts M, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353:2229–42. doi: 10.1056/NEJMoa042976. [DOI] [PubMed] [Google Scholar]
- 27.Marenzi G, et al. N-acetylcysteine and contrast-induced nephropathy in primary angioplasty. N Engl J Med. 2006;354:2773–82. doi: 10.1056/NEJMoa054209. [DOI] [PubMed] [Google Scholar]
- 28.Kang S, et al. Topical N-acetyl cysteine and genistein prevent ultraviolet-light-induced signaling that leads to photoaging in human skin in vivo. J Invest Dermatol. 2003;120:835–41. doi: 10.1046/j.1523-1747.2003.12122.x. [DOI] [PubMed] [Google Scholar]
- 29.Florell SR, et al. Sampling of melanocytic nevi for research purposes: a prospective, pilot study to determine effect on diagnosis. J Am Acad Dermatol. 2008;59:814–21. doi: 10.1016/j.jaad.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 30.Yan H, et al. Induction of melanoma cell apoptosis and inhibition of tumor growth using a cell-permeable Survivin antagonist. Oncogene. 2006;25:6968–74. doi: 10.1038/sj.onc.1209676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fahey RC, Newton GL. Determination of low-molecular-weight thiols using monobromobimane fluorescent labeling and high-performance liquid chromatography. Methods Enzymol. 1987;143:85–96. doi: 10.1016/0076-6879(87)43016-4. [DOI] [PubMed] [Google Scholar]
- 32.Yim CY, Hibbs JB, Jr, McGregor JR, Galinsky RE, Samlowski WE. Use of N-acetyl cysteine to increase intracellular glutathione during the induction of antitumor responses by IL-2. J Immunol. 1994;152:5796–805. [PubMed] [Google Scholar]
- 33.Linos E, Swetter SM, Cockburn MG, Colditz GA, Clarke CA. Increasing burden of melanoma in the United States. J Invest Dermatol. 2009;129:1666–74. doi: 10.1038/jid.2008.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emdin M, Pompella A, Paolicchi A. Gamma-glutamyltransferase, atherosclerosis, and cardiovascular disease: triggering oxidative stress within the plaque. Circulation. 2005;112:2078–80. doi: 10.1161/CIRCULATIONAHA.105.571919. [DOI] [PubMed] [Google Scholar]
- 35.Turgut O, Yilmaz A, Yalta K, Karadas F, Birhan Yilmaz M. gamma-Glutamyltransferase is a promising biomarker for cardiovascular risk. Med Hypotheses. 2006;67:1060–64. doi: 10.1016/j.mehy.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Bureau I, et al. Female rats are protected against oxidative stress during copper deficiency. J Am Coll Nutr. 2003;22:239–46. doi: 10.1080/07315724.2003.10719299. [DOI] [PubMed] [Google Scholar]
- 37.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. Jama. 2007;297:842–57. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 38.Omenn GS, et al. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J Natl Cancer Inst. 1996;88:1550–59. doi: 10.1093/jnci/88.21.1550. [DOI] [PubMed] [Google Scholar]
- 39.Hercberg S, et al. Antioxidant supplementation increases the risk of skin cancers in women but not in men. J Nutr. 2007;137:2098–105. doi: 10.1093/jn/137.9.2098. [DOI] [PubMed] [Google Scholar]
- 40.Francis SO, Mahlberg MJ, Johnson KR, Ming ME, Dellavalle RP. Melanoma chemoprevention. J Am Acad Dermatol. 2006;55:849–61. doi: 10.1016/j.jaad.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 41.Hsu S. Green tea and the skin. J Am Acad Dermatol. 2005;52:1049–59. doi: 10.1016/j.jaad.2004.12.044. [DOI] [PubMed] [Google Scholar]
- 42.Nihal M, Ahmad N, Mukhtar H, Wood GS. Anti-proliferative and proapoptotic effects of (-)-epigallocatechin-3-gallate on human melanoma: possible implications for the chemoprevention of melanoma. Int J Cancer. 2005;114:513–21. doi: 10.1002/ijc.20785. [DOI] [PubMed] [Google Scholar]
- 43.Taniguchi S, et al. Effect of (-)-epigallocatechin gallate, the main constituent of green tea, on lung metastasis with mouse B16 melanoma cell lines. Cancer Lett. 1992;65:51–54. doi: 10.1016/0304-3835(92)90212-e. [DOI] [PubMed] [Google Scholar]
- 44.Zheng W, et al. Tea consumption and cancer incidence in a prospective cohort study of postmenopausal women. Am J Epidemiol. 1996;144:175–82. doi: 10.1093/oxfordjournals.aje.a008905. [DOI] [PubMed] [Google Scholar]
- 45.Nordlund JJ, et al. Demographic study of clinically atypical (dysplastic) nevi in patients with melanoma and comparison subjects. Cancer Res. 1985;45:1855–61. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Schematic showing protocol for obtaining nevus tissues for these studies. A, Nevus specimens biopsied and submitted for routine histological analysis are usually bisected, then embedded for sectioning. A section corresponding to the central portion of the nevus is prepared for viewing, and usually is sufficient for histological diagnosis (left panel). B, For nevi used in this study, a central section was removed from the specimen following biopsy and submitted for routine processing while the remaining tissues were held back. The section generated from this submitted specimen would resemble that initially obtained if the entire specimen had been submitted (right panel). If a clear diagnosis could not be made from the submitted specimen, then the remaining fragments could also be submitted for analysis (and not used for research).
Fig. S2. Efficient separation of thiols in nevus specimens by reverse-phase chromatography. Thiols in nevus lysates were derivatized with monobromobimane, loaded onto a C-18 column, and their elution monitored using a fluorescence detector. Overlay of histograms showing elution patterns of thiols from mixture of standards (Cys, GSH, NAC; black tracing), nevus lysate (red tracing), and nevus lysate spiked with standards (blue tracing).
Fig. S3. UV-induced apoptosis in nevi. Fragments from an individual nevus were untreated or UV-irradiated (4000 J/m2), then 48 h later stained for nuclei (4,6-diamidino-2-phenylindole, blue) and apoptotic cells (TUNEL, red). Overlay fluorescent images are shown (original magnification ×400).