Nanoscale science focuses attention on how bulk properties are altered when substances are confined to small spaces and reduced dimensionality. For example, theory suggests that water takes on novel “ice” structures when confined inside carbon nanotubes.1 Calculations also support the idea that confinement of Haq+ to narrow channels creates a proton wire2 having up to 40 times faster proton transport than in bulk.3 These findings are relevant to proton mobility in nanoscale channels of fuel cell membranes and also to biological proton transport where chains of water molecules are found in channels of trans-membrane proteins involved in proton pumping and cellular pH control.4 Counterintuitively, these protein channels are often lined with non-polar residues suggesting that nature may be exploiting special properties of hydrophobically-confined chains of water molecules.5
Structurally well-defined channels containing Haq+ are rare so experimental opportunities to investigate these hypotheses are few.6 Herein, we report several novel structural features of Haq+ when it is confined to a hydrophobic nanotube.
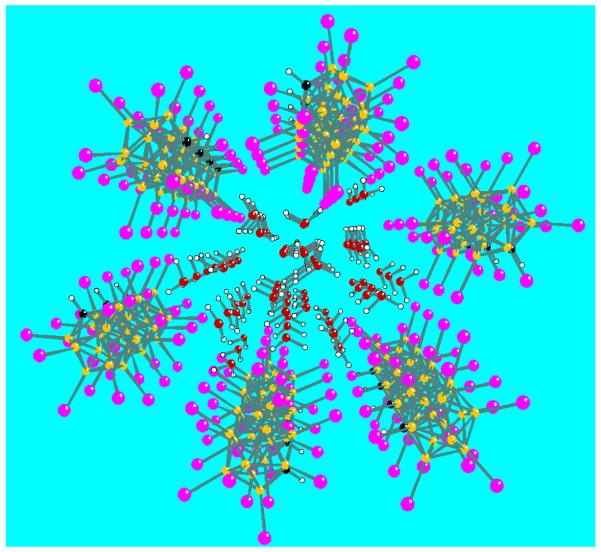
Saturated aqueous solutions of the carborane acid H(CHB11I11)7 deposit crystals of the nominally octahydrated acid H(CHB11I11)·8H2O whose X-ray structure reveals 1.1 × 0.5 nm diameter elliptical channels of Haq+ encased by walls of carborane anions (Fig. 1). This structural motif contrasts with the discrete ionic lattice structures of all other [H(H2O)n+][carborane−] salts having smaller, less polarizable anions e.g., [H9O4]−[CHB11H5Br6],8 [H5O2][CHB11Cl11]9 and [H3O][CHB11Cl11].10 Multiple inter-anion I⋯·I distances (3.68-3.96 Å) are less than the sum of the van der Waals separation suggesting that I⋯·I dispersion forces are important in creating the tubular structure.
Figure 1.
Four unit cells of the tubular structure of H(H2O)n+ cations (red, white) enclosed by CHB11I11− anions (black, yellow, magenta) in tube A of H(CHB11I11)·8H2O.
The unit cell contains six H+, six CHB11I11− anions and 48 water molecules. There are two crystallographically distinct water-filled tubes containing Haq+ cations, labeled A and B. The water structure in tube A is crystallographically ordered (a so-called “ice”) while that in tube B is partially disordered, indicating weaker organizational forces and suggesting unequal H+ distribution between the two tubes.
Direct X-ray determination of the locations of H+ and the H atoms of water is not possible because of their low electron density. In addition, the standard procedures built into crystallographic software for modeling their locations fail because the dimensional constraints of normal H-bonding do not apply to Haq+ clusters. Nevertheless, the locations of the excess protons are revealed by the shortening of O⋯·O separations. When shorter than those in hexagonal ice (2.76 Å)11 or liquid water (2.85 Å)12 they are candidates for bridging H+. The presently known range of O⋯·O distances for O–H⋯O bonds considered to carry positive charge in H(H2O)n+ cations is 2.39-2.59 Å.7,13 Short O⋯·O separations (≤2.42 Å) are associated with symmetrical H-bonding in Zundel-type H5O2+ ions9,14 while longer separations (2.52-2.55 Å) are typical of standard unsymmetrical H-bonds such as those in the H3O+·3H2O Eigen-type ion.8
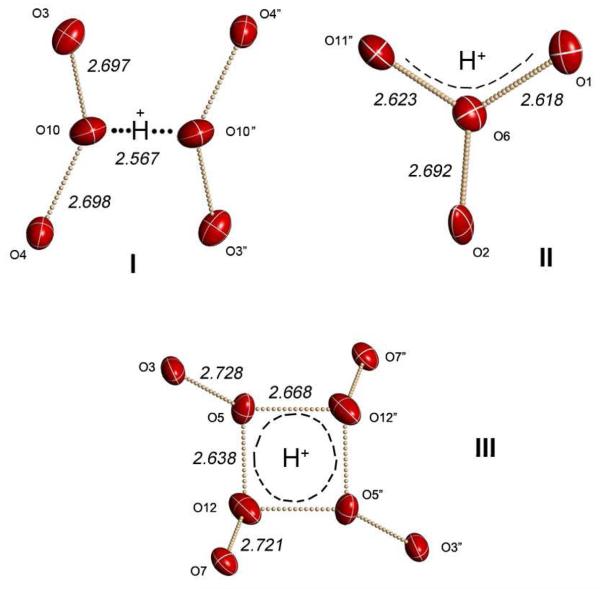
A survey of short O⋯·O separations in the unit cell of tube A (Fig. 2) indicates three structurally different types of Haq+ clusters: cluster I with a single short O10–O10″ distance of 2.567(12) Å, two sites of cluster II with a pair of adjacent short O⋯O distances of 2.623(8) and 2.618(7) Å indicating an H7O3+ core formulation, and an unprecedented cluster III with a nearly square O5–O12–O5″–O12″ arrangement having 2.636(8) and 2.668(8) Å rectangular sides. This stoichiometry leaves only 2 Haq+ sites for tube B and two symmetry-related H7O3+ ions of type II with O⋯O separations of 2.582(13) and 2.573(9) Å are found amongst the ordered water molecules. There are no other short O⋯·O separations. The 4:2 partitioning of H+ between channels A and B corresponds to an average composition of 6 H2O per H+ in A and 12 H2O per H+ in B and is consistent with the disorder (and partial occupancy) of water molecules observed in channel B.
Figure 2.
Structures of the three H(H2O)n+ clusters I, II and III. Thermal ellipsoids are at 50% probability level.
Cluster I is topologically a tetrahydrated H5O2+ Zundel ion, but the O⋯·O distances are quite anomalous. Sitting on a crystallographic center of symmetry, its central O⋯·O distance of 2.57 Å is very long compared to those in other H5O2+ cations (2.39-2.42 Å).9,13,14 It is even longer than the O⋯·O distances in the H3O+·3H2O Eigen ion (2.52-2.55 Å).8 The four O⋯·O distances to the second sphere water molecules in I (2.70 Å) are also large compared to those in the H5O2+·4H2O ion (2.52 Å)14 and only slightly below those in hexagonal ice (2.76 Å). This means that the positive charge of H+ is significantly more delocalized in I than in classical Zundel-type H5O2+ ions.
Cluster II is formulated as a hydrated H7O3+ ion with the two central O⋯·O distances that are equal within experimental error. It typically occurs when H(H2O)n+ cations are ion paired7 and is consistent with location of these clusters near the anion walls, removed from sites of crystallographic symmetry. One water molecule (O2) solvates the central O atom of the H7O3+ core with a somewhat short O⋯·O distance of 2.69 Å. This reflects delocalization of the positive charge onto this water molecule and illustrates the close relationship between monohydrated C2v H7O3+ ions and C3v H9O4+ Eigen-type ions. Four other water molecules (O4, O7, O8″ and O12″) solvate cluster II at O⋯·O distances 2.77-3.03 Å.
Cluster III is unprecedented for a proton hydrate. Four O-atoms share H+ in a nearly square arrangement whose center has crystallographic inversion symmetry. Four solvating water molecules are close enough to qualify for inclusion in the cluster (O7, O7″, O3, O3″ with O⋯·O distances all close to 2.72 Å) leading to an H17O8+ formulation. The next closest are O11 and O11″ at 3.03 Å but since these distances are even greater than that of liquid water (2.85 Å) they are not considered to carry positive charge from H+. The novel structure of this ion must be the result of the electrostatic field peculiar to this inversion symmetry site within the tube. A related square core structure of eight water molecules, but having much longer O⋯·O distances (2.91-3.02 Å) because an excess proton is absent, has been observed in the crystal structure of a hydrated lanthanide chelate.15 The diversity of static Haq+ cluster structures trapped in the tubes is a reflection the highly dynamic nature of the excess proton in water.16
Clusters I, II and III all have much longer O⋯·O separations than would be expected from the existing knowledge base of X-ray structures of crystalline acid hydrates7-10,13,14 or the computed structures of gas phase ions, even those having greater than 20 water molecules.17 This is compelling evidence for greater delocalization of positive charge in these clusters. It suggests that Haq+ in the present extended tubular environment (and presumably in liquid water) is uniquely different from the discrete H(H2O)n+ ions in crystalline salts or the gas phase.
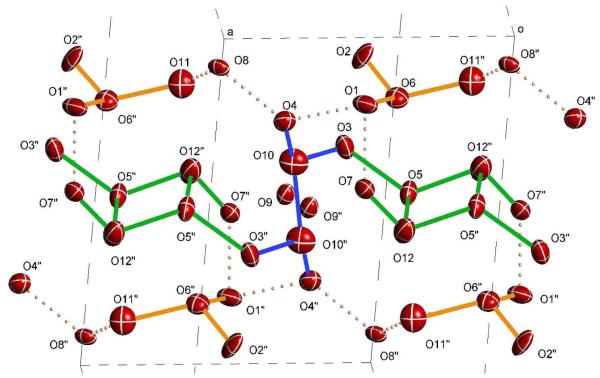
The delocalization of positive charge has another interesting consequence. Clusters I and III in tube A form an infinite chain of short O⋯·O separations i.e., a true proton wire (Fig. 3). These clusters lie along the central axis of the tube and contain individual centers of symmetry. They are linked by common atoms (O3 and O3″) whose O⋯·O distances of 2.70 Å and 2.72 Å to the core O atoms of the clusters are shorter than those of liquid water or hexagonal ice. This indicates an infinite 1D delocalization of positive charge. It is tempting to speculate that H+ mobility would be especially fast along this chain.
Figure 3.
Portion of a proton wire comprised of type I (blue) and III (green) H(H2O)n+ clusters in tube A with O⋯·O separations < 2.72 Å. Dotted bonds represent O⋯·O separations > 2.72 Å. Cluster II, depicted in orange, is not part of the proton wire. Thermal ellipsoids at 50%.
In summary, three structural types of Haq+ have been identified in nanotubular crystals of H(CHB11I11)·8H2O. One is without precedent and all have unexpectedly long O⋯·O separations compared to those in discrete H(H2O)n+ moieties, reflecting more extensive positive charge delocalization. The confined centrosymmetric H(H2O)n+ cations aggregate into infinite proton wires whose short inter-cation distances suggest a mechanism for enhanced one-dimensional H+ mobility.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by NIH grant GM023851.
Footnotes
Supporting Information Available: X-ray crystallographic data for H(CHB11I11)·8H2O with tables and CIF file. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Koga K, Gao GT, Tanaka H, Zeng XC. Nature. 2001;412:802–805. doi: 10.1038/35090532. [DOI] [PubMed] [Google Scholar]
- 2.Nagle JF, Morowitz HJ. Proc. Natl. Acad. Sci. USA. 1978;75:298–302. doi: 10.1073/pnas.75.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Pomès R, Roux B. Biophys. J. 1998;75:33–40. doi: 10.1016/S0006-3495(98)77492-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Brewer ML, Schmitt UW, Voth GA. Biophys. J. 2001;80:1691–1702. doi: 10.1016/S0006-3495(01)76140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Dellago C, Naor MM, Hummer G. Phys. Rev. Lett. 2003;90:105902. doi: 10.1103/PhysRevLett.90.105902. [DOI] [PubMed] [Google Scholar]
- 4.(a) DeCoursey TE. Physiol. Rev. 2003;83:475–579. doi: 10.1152/physrev.00028.2002. [DOI] [PubMed] [Google Scholar]; (b) Mathias G, Marx D. Proc. Natl. Acad. Sci. USA. 2007;104:6980–6985. doi: 10.1073/pnas.0609229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hummer G. Molec. Phys. 2007;105:201–207. [Google Scholar]
- 6.(a) Maniva Y, Kataura H, Abe M, Suzuki S, Achiba Y, Kira H, Matsuda K. J. Phys. Soc. Japan. 2002;71:2863–2866. [Google Scholar]; (b) Fromm KM, Gueneau ED, Goesmann H, Bochet CGZ. Anorg. Allg. Chem. 2003;629:597–600. [Google Scholar]; (c) Dułak M, Bergougnant R, Fromm KM, Hagemann HR, Robin AY, Wesołowski TA. Spectrochim. Acta A. 2006;64:532–548. doi: 10.1016/j.saa.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 7.Stoyanov ES, Stoyanova IV, Tham FS, Reed CA. J. Am. Chem. Soc. 2008;130:12128–12138. doi: 10.1021/ja803535s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zie Z, Bau R, Reed CA. Inorg. Chem. 1995;34:5403–5404. [Google Scholar]
- 9.Stoyanov ES, Reed CA. J. Phys. Chem . A. 2006;110:12992–13002. doi: 10.1021/jp062879w. [DOI] [PubMed] [Google Scholar]
- 10.Stoyanov ES, Hoffmann SP, Kim K-C, Tham FS, Reed CA. J. Am. Chem. Soc. 2005;127:7664–7665. doi: 10.1021/ja050401k. [DOI] [PubMed] [Google Scholar]
- 11.Kuhs WF, Lehmann MS. J. Phys. Chem. 1983;87:4312–4313. [Google Scholar]
- 12.Narten AH, Thiessen WE, Blum L. Science. 1982;217:1033. doi: 10.1126/science.217.4564.1033. [DOI] [PubMed] [Google Scholar]
- 13.Lungdren J-O, Ollovsson I. In: The Hydrogen Bond: II. Structure and Spectroscopy. Schuster P, Zundel G, Sandorfy C, editors. Amsterdam; North-Holland: 1976. Chap. 10. [Google Scholar]
- 14.Bell RA, Christoph GG, Fronczek FR, Marsh RE. Science. 1975;190:151–152. [Google Scholar]
- 15.Neogi S, Savitha G, Bharadwaj PK. Inorg. Chem. 2004;43:3771–3773. doi: 10.1021/ic0495618. [DOI] [PubMed] [Google Scholar]
- 16.Marx D, Tuckerman ME, Hutter J, Parrinello M. Nature. 1999;397:601–604. [Google Scholar]
- 17.Iyengar SS, Day TJF, Voth GA. Intl. J. Mass. Spect. 2005;241:197–204. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.