Abstract
Chronic obstructive pulmonary disease (COPD) is characterized by the degradation of elastin, the major insoluble protein of lung tissues. The degradation of elastin gives rise to desmosine (DES) and isodesmosine (IDES), two major urinary products typified by a hydrophilic pyridinium- based cross-linker structure. A high sensitivity method based on nanoflow liquid chromatography tandem mass spectrometry with multiple reaction monitoring was developed for the analysis of urinary DES and IDES. The analytes were derivatized with propionic anhydride and deuterated DES (D4-DES) was used as an internal standard. This method enables the quantification of DES and IDES in as little as 50 µL of urine and provides a detection limit of 0.10 ng/mL (0.95 fmol on-column). We report the analysis of DES and IDES in a cohort of 40 urine specimens from four groups of individuals: (a) COPD rapid decliners (11.8 ± 3.7 ng/mg creatine (crea)), (b) COPD slow decliners (16.0 ± 3.1 ng/mg crea), (c) healthy smokers (13.2 ± 1.9 ng/mg crea), and (d) healthy nonsmokers (14.9 ± 2.9 ng/mg crea). Our analysis reveals a statistically significant decrease in the level of urinary DES and IDES in COPD rapid decliner patients compared to healthy nonsmoker controls and COPD slow decliner patients. This methodology may be useful for monitoring DES and IDES levels in well controlled animal models for COPD or for longitudinal studies in COPD patients.
Chronic obstructive pulmonary disease, or COPD, is a respiratory disorder generally caused by smoking.1–3 Two major forms of COPD are emphysema and chronic bronchitis.3 COPD is characterized by impaired lung function, inflammation and scarring of bronchial tubes, and degradation of elastin-containing tissues. The elastin is a major protein component of insoluble fibers providing elastic properties to tissues such as lung, skin, and blood vessels.4 Also, elastin peptides were reported to be chemotactic for neutrophils and macrophages and could play a role in the progression of pulmonary emphysema once elastin degradation is initiated.5,6 Most of the COPD biomarkers reported in the literature are cross-linkers1,7,8 released during the elastin breakdown. The two most abundant and studied elastin crosslinkers are desmosine (DES) and isodesmosine (IDES)9,10 (Figure 1). For simplicity, in this report DESs refer to both DES and IDES. DESs are pyridinium-based structural isomers arising from the condensation of four lysine residues by lysyl-oxidase.4,11,12 Only a small proportion of DESs is found as a free form in biological fluids. The larger proportion of these compounds is bound to peptides and require acid digestion for complete release.1,7,8,13–22 For example, the free DES concentration measured by Ma et al.21 in the urine of seven healthy subjects was 1.42 ± 1.16 µg/g of creatinine (crea), whereas a concentration of 8.67 ± 3.75 µg/g crea was obtained after acid digestion. Numerous reports have documented the level of DESs in different biological fluids including urine,1,7,13–15,18,21–24 plasma,1,13,16,22 and sputum.1,16,21
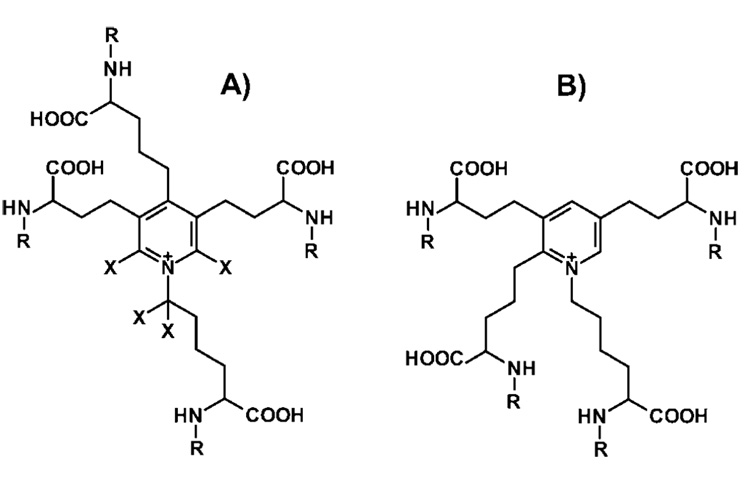
Figure 1.
Structure of (A) desmosine (DES) (X,R = H); deuterated desmosine (D4-DES) (X = d, R = H); propionylated desmosine (DES(prop)) (X = H, R = COCH2CH3), and (B) isodesmosine (IDES) (R = H); propionylated isodesmosine (IDES(prop)) (R = COCH2CH3).
Cocci et al.7 observed a urinary excretion of total DESs that was significantly higher for patients with COPD than for the controls. However, no significant difference in DESs excretion was observed between the smokers and nonsmokers controls. Similarly, there was no difference in DESs levels between patients with COPD who were ex-smokers compared to the active smokers group. In another study, Viglio et al.8 reported that patients with stable COPD generate DESs levels significantly higher than those of control patients but lower than subjects with an exacerbation of COPD. In contrast to Cocci et al.,7 they found a significantly higher DESs level in the smoking controls compared to the nonsmoking controls. Gottlieb et al.25 observed a greater excretion of urinary DES for the COPD rapid decliners group than for the COPD slow decliners group. Recently, Ma et al.1 analyzed the free and total DESs in urine samples from COPD patients and observed a statistically significant increase of the free DESs levels but not of the total DESs levels. Bode et al.15 observed an increase of the variability in total urinary DESs levels from COPD patients compared to the controls. DESs were also used as biomarkers for other diseases causing the degradation of elastin such as cystic fibrosis,8,15 pseudoxanthoma elasticum,13 bronchiectasis,8 acute lung injury,26 and aneurysm.27
Viglio and al. recently reviewed different approaches for the determination of DESs in biological fluids and tissues.28 The most commonly used techniques to analyze DESs in biological fluids are immunochemical methods,7,18,20,26,27 LC—UV,15,17 LC—MS,1,19,21,29 and capillary electrophoresis (CE).8,13,14,16,22–24 Among the nonimmunological methods, the lowest DESs detection limits were obtained using LC—MS and CE. For LC—MS,1 heptafluorobutyric acid was used as an ion pairing reagent and the detection limit obtained was 1 ng/mL (38 fmol on-column). In the case of CE,13,14 a detection limit of 5 ng/mL (0.1 fmol on-column) was observed by laser-induced fluorescence when DESs were derivatized with fluoresceine isothiocyanate.
In the present study, we describe a high-sensitivity nanoflow liquid chromatography–tandem mass spectrometry (nanoLC—MS/MS) method for sensitive detection of DESs in urine. The approach involves the formation of stable N-propionyl DESs derivatives together with D4-DES as an internal standard to compensate for sample losses and instrumental variability. The increased sensitivity of the present method enables the analysis of DESs from urine samples as small as 50 µL with subnanograms per milliliter detection limits. We demonstrate the application of this approach for the analysis of a cohort of 40 human urine samples from four groups of individuals: (1) COPD rapid decliners, (2) COPD slow decliners, (3) healthy smokers, and (4) healthy nonsmokers.
EXPERIMENTAL SECTION
Chemicals
DES (99.5%) was purchased from Elastin Products Company Inc. (Owensville, MO). HPLC grade acetonitrile (ACN), methanol (MeOH), toluene, and molecular biology grade isopropanol (i-Pr) were from Fisher Scientific Company (Ottawa, Canada). ACS grade hydrochloric acid (HCl) and 98% formic acid were from EMD Chemicals Inc. (Darmstadt, Germany). Finally, ACS grade ammonium hydroxide, 99+% propionic anhydride, 99.0% ammonium bicarbonate (NH4HCO3), 99.0% trifluoroacetic acid (TFA), 98% 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), 99.96% deuterium oxide (D2O) were from Sigma-Aldrich Canada Ltd. (Oakville, Canada).
Deuterated Desmosine Synthesis and Purification
We modified a previous deuteration protocol30 for the synthesis of deuterated DES (D4-DES) (Figure 1). Briefly, 250 µg of DES were dissolved in 0.5 mL of D2O, and 25 µL of DBU was added. The solution was stirred at 70 °C for 36 h in a closed vessel. Thereafter, the BDU was removed from the reaction mixture using four consecutive extractions with 1 mL of CHCl3. The reaction mixture was then evaporated to dryness in a SPD-series SpeedVac concentrator (Thermo Fisher Scientific Inc., Waltham, MA). The reaction product was redissolved in 1 mL of H2O and evaporated to dryness three times to remove unreacted deuterated water. The purified D4-DES was dissolved and stored in 1 mL of 0.2% formic acid to inactivate the remaining traces of DBU and thus prevent the deuterium exchange. D4-DES was subsequently analyzed by infusion and nanoLC-MS on a ABI/Sciex (Foster City, CA) Q-trap 4000 mass spectrometer to confirm its purity. To determine the D4-DES concentration, an aliquot of the D4-DES solution was propionylated and analyzed as described in Sample Preparation and NanoLC—MS/MS Analysis. The total D4-DES amount recovered following preparative HPLC was 2.1 µg.
Urine Samples
Urine samples were collected from subjects who were participants in a study of genetic risk factors for nicotine dependence and COPD recruited in Salt Lake City, Utah (UT). The UT COPD cohort is made up of individuals with COPD who were rerecruited after their participation in the Lung Health Study (LHS) beginning in 1987–1988. A description of LHS participant recruitment process is reported by Durkin et al.31 Those who had been participants in the LHS were recruited specifically for the diagnosis of COPD as defined by a forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) ratio less than 70%. Serial spirometry testing done from their entry into the LHS study until their rerecruitment into the UT genetic study was evaluated. On the basis of the 15 year longitudinal information on lung function, COPD patients were distributed in two distinct groups: 9 rapid decliners (losing on average 40 mL of FEV1 or more per year) and 11 slow decliners (losing on average less than 40 mL of FEV1 per year).
Controls without COPD were recruited from a group of respondents to community advertising for nonsmokers and for persons who had smoked more than 100 cigarettes lifetime. Pulmonary function testing was done on all controls to confirm that they did not demonstrate obstruction. The samples analyzed consist of 9 smokers without COPD and 11 healthy nonsmokers. Urine was collected after 2 h of fasting and frozen at −80 °C until analysis. The creatinine levels was measured for each urine sample using a Creatinine Assay Kit from BioVision Inc. (Mountain View, CA) to account for the variability in urine dilution. Creatinine levels varied from 0.13 to 2.86 mg crea/mL of urine across the different samples examined.
Sample Preparation
An aliquot of 50 µL of each urine sample was digested in 500 µL of 6 N HCl (24 h, 110 °C), under N2 atmosphere, using an AccuBlock Digital Dry Bath from Labnet International Inc. (Woodbridge, NJ) to liberate DESs covalently bound to proteins. The samples were evaporated to dryness in the SpeedVac, and 1.5 ng of D4-DES was added as an internal standard to compensate for any loss during the subsequent steps of sample preparation and to increase the precision of the mass spectrometry analysis. The sample residues were then resuspended in 500 µL of 0.05 N HCl and purified using 1 mL (30 mg, 60 µm) of mixed-mode strong cation exchange (MCX) cartridges (Oasis, Waters Corporation, Milford, MA).1,21 The samples were loaded on the MCX cartridges previously conditioned with 1 mL of MeOH and 1 mL of deionized water. The cartridges were washed with 1 mL of deionized water, 1 mL of MeOH, and 1 mL of 0.1 N HCl prior to sample elution with 1.5 mL of 2 N HCl. The eluates were dried in the SpeedVac and were propionylated (Figure 1) to facilitate the analysis of DESs and D4-DES with C18 reversed-phase chromatography. For sample derivatization, 50 µL of 0.1 N NH4HCO3 was added to each sample followed by 50 µL of a freshly prepared solution containing propionic anhydride (23% v/v) and NH4OH (9% v/v) in i-Pr. After 30 min of reaction, the samples containing the DESs propionyl derivatives (DESs(prop)) were evaporated to dryness in the SpeedVac and the residues were resuspended in 50 µL of 5% ACN. The sample preparation method described above can also be applied to the analysis of free DESs in urine. In this case, the acid digestion step is omitted and 500 µL of urine is loaded onto the MCX cartridge. However, the analysis of the free DESs was not performed as part of this study due to the limited volume of urine samples available (~200 µL).
NanoLC—MS/MS Analysis
The samples were separated with an Agilent (Santa Clara, CA) nanoLC (1100 series) system using an homemade precolumn (0.3 i.d. × 4 mm) and analytical column (150 µm i.d. × 10 cm) packed with a C18 (3 µm, 300 Å) Jupiter (Phenomenex Inc., Torrance, CA) stationary phase. The injection volume was 5 µL, and sample loading was performed at a flow rate of 10 µL/min for 4 min. Sample elution on the analytical column was achieved under isocratic conditions (20% ACN, 0.2% formic acid) at a flow rate of 0.6 µL/min. Under these chromatographic conditions, DES and IDES coelute. According to the literature,1 the ratio between these two biomarkers appears to be constant across different sample sets suggesting that there is no inherent advantage in resolving them to the detriment of the sensitivity, the peaks shape, and the analysis time. Three replicate analyses were performed for each sample. For the calibration curve, individual DES standards ranging from 0.005 to 5 ng were added to 1.5 ng of D4-DES, propionylated (Sample Preparation), and dried with a SpeedVac. Each DES standard was redissolved in 50 µL of urine submitted to acid digestion and to MCX purification (Sample Preparation) as the matrix. The DES naturally present in the matrix was not propionylated and consequently does not interfere with the propionylated DES used for the calibration curve.
NanoLC—MS/MS analyses were performed on the Q-trap 4000 mass spectrometer using the following conditions: source temperature, 160 °C; curtain gas, 10; nebulizer gas, 8; source voltage, 3800 V; and declustering potential, 120 V. DESs(prop) (m/z 750.4) and D4-DES(prop) (m/z 754.4) were analyzed simultaneously using two alternative MS/MS experiments. Transitions corresponding to the loss of one propionyl group (m/z 694.4 for DESs(prop) and m/z 698.4 for D4-DES(prop)) were selected for both precursor ions. The highest sensitivity was obtained with Ecoll = 58 eV, Coll gas = 12, and a peak width of ±1 amu.
Reproducibility and Recovery from Solid Phase Extraction
To evaluate the reproducibility of the analytical method, 14 urine aliquots of 50 µL from a healthy volunteer were prepared and analyzed as described in Sample Preparation and NanoLC—MS/MS Analysis. Similarly, for the recovery of the MCX solid phase extraction, 12 aliquots of 50 µL of urine from the healthy volunteers were prepared and analyzed as described in Sample Preparation and NanoLC—MS/MS Analysis, except that the internal standard was added only after the MCX extraction for 6 of these aliquots.
RESULTS AND DISCUSSION
Nano-LC—MS Analysis of the DESs Propionyl Derivatives
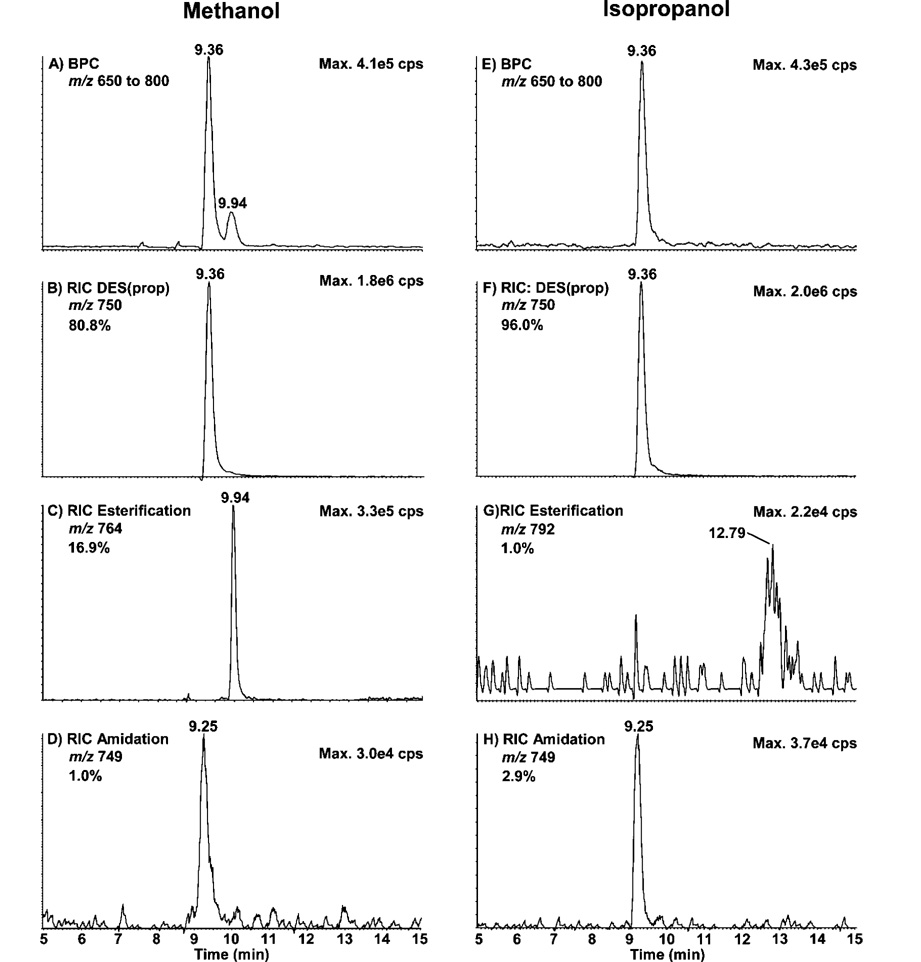
Separation of DES and IDES by reversed-phase chromatography is challenging owing to their highly hydrophilic nature. Separation of these compounds on reversed-phase chromatography requires a high concentration of ion pairing modifiers that adversely affect mass spectrometry sensitivity when using electrospray ionization. To circumvent these limitations, we evaluated the formation of N-propionyl derivatives previously used to improve reversed-phase separation and electrospray responses of bases, ribosides, intact nucleotides, and lysine-rich peptides from histones.32–34 Parts A-D of Figure 2 show the nanoLC—MS results obtained after the propionylation (Sample Preparation) of 25 ng of DES standard performed in MeOH, the solvent generally used for this derivatization.32,34 Figure 2A corresponds to the base peak chromatogram (BPC) obtained using the enhanced MS mode (EMS) (m/z 650–800) and the linear ion trap of the Q-trap instrument. Under these conditions, the derivatization yield was 80.8% assuming equal response factor for all species observed, as shown by the reconstructed ion chromatogram (RIC) of DES(prop) (m/z 750) (Figure 2B). Side reaction products came from the esterification of one of the carboxylic acids of DES with MeOH to generate a methyl ester (16.9%) (m/z 764) (Figure 2C) or with NH4OH to generate an amide (1.0%) (m/z 749) (Figure 2D). A small fraction of DES(prop) with two carboxylic acids esterified by MeOH (1.3%) (m/z 778) was also observed (results not shown). The N-propionylation quantitavely labeled all four NH2 groups of DES with no significant heterogeneity in mono, di-, tri-, or tetra substitution. To increase the propionylation yield, MeOH was replaced by i-Pr as a solvent and the same nanoLC—MS analysis was repeated (Figure 2E–H). i-Pr can efficiently dissolve the propionic anhydride without causing the precipitation of the NH4HCO3 contained in the buffer. Figure 2E shows the BPC obtained using i-Pr as the solvent. The DES(prop) recovery increased to 96.0% as shown by its RIC (Figure 2F). The esterification generated by i-Pr (1.0%) (m/z 792) (Figure 2G) was considerably lower than that observed using MeOH (16.9%). However, with i-Pr as a solvent, the amidation side-reaction slightly increased to 2.9% (m/z 749) (Figure 2H). As for MeOH, the propionylation of the NH2 groups of DES was complete with i-Pr. While the use of i-Pr minimizes the production of side-reaction products, the use of a suitable internal standard such as D4-DES will account for any further variation during the quantification of these compounds in the urine samples.
Figure 2.
NanoLC—MS analysis of 25 ng of DES standards propionylated in methanol (MeOH) (A–D) and in isopropanol (i-Pr) (E–H). (A and E) Base peak chromatogram (BPC) (m/z 650–800). (B and F) Reconstructed ion chromatogram (RIC) of DES(prop) (m/z 750). (C and G) RIC of DES(prop) esterified with MeOH (m/z 764) and i-Pr (m/z 792), respectively. (D and H) RIC of DES(prop) amidated (m/z 749).
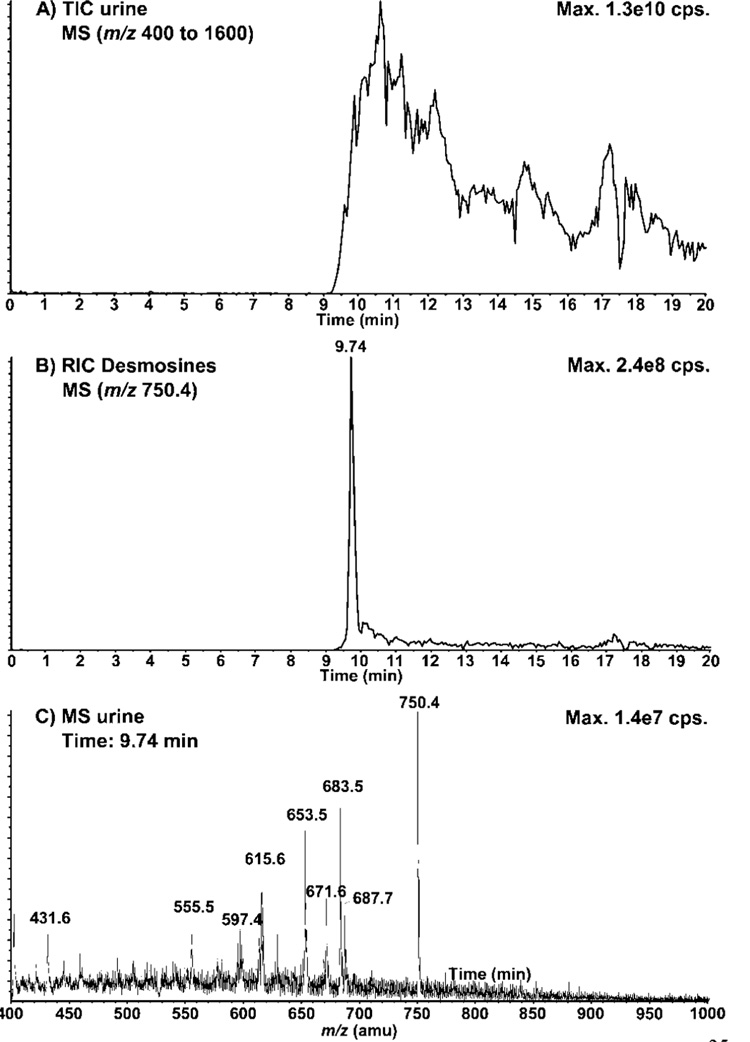
Next, we evaluated the application of the propionyl derivative for the analysis of total DESs from urine samples. A 50 µL aliquot of urine from a healthy volunteer was analyzed by nanoLC—MS using the EMS mode (Figure 3A). Under these conditions, DESs(prop) elutes at 9.7 min along with other significant urinary compounds. Figure 3B shows the RIC for m/z 750.4 corresponding to DESs(prop) where many interferences were noted on the tailing edge of the DESs(prop) peak. The extracted MS spectrum for the peak eluting at 9.7 min (Figure 2C) also shows many interferences coeluting with DESs(prop). These results illustrate the complexity of the urine samples, even after the MCX fractionation. To improve the selectivity and quantitation of DESs in urine samples we evaluated the use of the deuterated DES standard together with multiple reaction monitoring (MRM) in nanoLC—MS/MS experiments.
Figure 3.
NanoLC—MS analysis of DES(prop) in urine. (A) Total ion chromatogram (TIC) of the urine sample using the enhanced MS (EMS) mode (m/z 400–1600), part B) Reconstructed ion chromatogram (RIC) corresponding to DESs(prop) (m/z 750.4) in the urine sample, (C) MS spectrum of urine at the elution time of DESs(prop) (9.74 min). A 50 µL urine sample was digested in HCl, purified on an MCX cartridge, and propionylated. See Experimental Section for conditions.
NanoLC—MS/MS Analyses of DESs in Urine Samples
The purity and suitability of the deuterated DES as an internal standard was first determined using infusion and nanoLC—MS. These analyses revealed that the purified sample of D4-DES contained no major interference. These analyses also enabled the profiling of deuterium isotopomers on the DES molecule and revealed the following distribution: D0-DES, 0.3%; D1-DES, 4.1%; D2-DES, 33.4%; D3-DES, 36.1%; and D4-DES, 26.1%. Although the synthesis of the D4-DES did not yield a single-isotopically enriched compound, only minor correction of the D0-DES was required when this compound was used as an internal standard (IS). The proportion of D0-DES corresponded to 1.1% of that of the D4-DES and was subtracted from the total DESs concentration measured in the urine samples to prevent any overestimation. Also, no statistically significant changes (<0.5%) in the isotopomer distribution were noted upon derivatization with propionic anhydride.
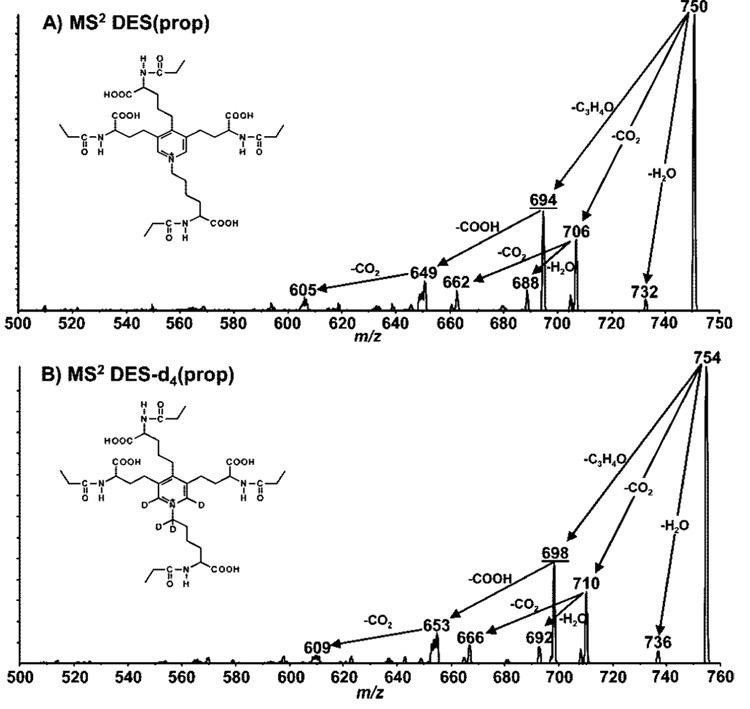
The MS/MS spectra of DES(prop) and D4-DES(prop) were obtained to rationalize the fragmentation characteristics of these analytes and to identify specific fragment ions to be used in subsequent nanoLC—MS/MS experiments using MRM. The tandem mass spectra of DES(prop) and D4-DES(prop) acquired at a collision energy of 58 eV are presented in parts A and B of Figure 4, respectively. Under the present conditions, the MS/MS spectra of both compounds were dominated by prominent losses of methyl ketene (CH3−CH=C=O), formyl (HCO2), CO2, and H2O from the singly charged precursor ion. One of the most significant and characteristic fragmentations observed for both DES(prop) and D4-DES(prop) was the loss of methyl ketene from the N-propionylated moiety giving rise to fragment ions m/z 694.4 for DES(prop) and m/z 698.4 for D4-DES(prop). The relative abundance of these fragments is identical in both spectra indicating that D4-DES is a good internal standard accounting for any instrumental variability.
Figure 4.
Tandem mass spectrometry analyses of (A) DES(prop) and (B) D4-DES(prop). Product ion spectra were optimized for the transitions corresponding to the lost of one propionyl group (m/z 694 and 698, respectively). Coll. gas, 12; Ecoll, 58 eV.
In subsequent experiments, nanoLC—MS/MS with the MRM acquisition mode for the transitions highlighted above (m/z 750.4 → 694.4) and D4-DES(prop) (m/z 754.4 → 698.4) was used to unambiguously quantitate the levels of DESs in extracts from 50 µL urine samples. With the use of this analysis mode, no interference was noted. We evaluated the linearity of response for the analysis of DES present at levels of 0.1–100 ng/mL in urine for a constant D4-DES(prop) concentration of 30 ng/mL. Accordingly, we obtained a linear correlation coefficient (R2) of 0.9999 and a slope of 0.0409 mL/ng of DES over the range examined. The limit of detection (LOD) for DESs is 0.10 ng/mL (0.95 fmol on-column). To our knowledge, this represents the lowest LOD for the analysis of DESs in urine achieved using a nonimmunological method.
The reproducibility of the present analytical method was evaluated using 14 urine aliquots from a healthy volunteer. A relative standard deviation (RSD) of 0.9% was observed for the DESs(prop) concentrations using internal standardization compared to 4.7% when using external calibration. Significant improvement in reproducibility was obtained using D4-DES which is chemically equivalent to DESs and thus compensates for losses during the MCX purification and the propionylation reaction. Moreover, the chromatographic retention time, the ionization efficiency, and the fragmentation behavior of D4-DES(prop) are very similar to those of DESs(prop), thus compensating for any fluctuations in mass spectrometry response. It is noteworthy that preliminary experiments using trilysine as an IS35 provided RSD values of 6.4% which is significantly higher than that obtained for D4-DES. The D4-DES deuterated standard is stable and its isotopic profile was not affected by the sample preparation.
We also evaluated the DES recoveries after the MCX solid phase extraction, using urine aliquots spiked with D4-DES before the extraction, which were then compared to urine samples spiked with the same amount of D4-DES after the extraction. The recovery for D4-DES (and thus for DESs) measured after the extraction was 96 ± 3%. However, during samples analysis, the loss of DESs during the extraction is compensated for by an equivalent loss of the internal standard (D4-DES), while the measured DESs concentration remained unaffected.
Application of NanoLC—MS/MS for the Quantitation of DESs Levels in Urine for Individuals with COPD
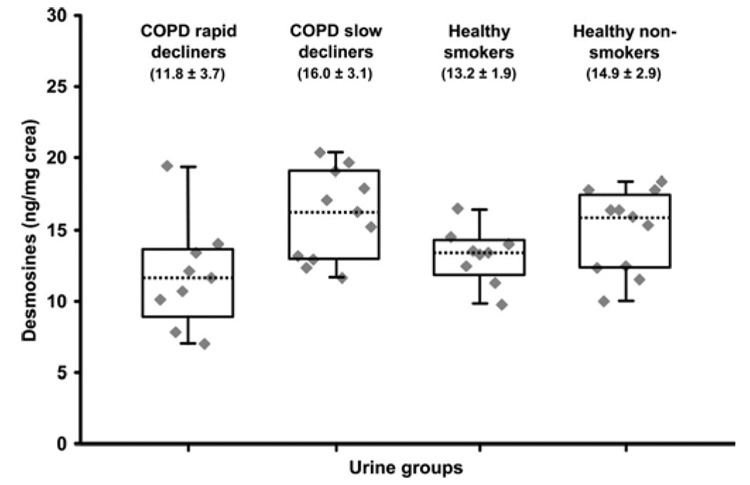
The DES levels measured for each of the four groups of urine samples are presented in Figure 5. All of these DES levels were corrected, as mentioned in Nano-LC—MS Analysis of the DES Propionyl Derivatives, for the D0-DES contained in the IS. The RSDs measured for the four groups of samples ranged from 14 to 31%. The method variability of 0.9% (NanoLC—MS/MS Analyses of DESs in Urine Samples) was thus negligible compared to the population variability of each of these groups. The mean DESs both concentrations for the healthy nonsmokers group (14.9 ± 2.9 ng/mg crea) was similar to those previously reported by Viglio et al.8 (16.65 ± 4.70 ng/mg of crea) and Ma et al.1 (12.78 ± 4.78 ng/mg of crea). We performed one-way analysis of variance (ANOVA) to test for differences among the four independent groups examined. Pair-wise comparison of DESs levels from the four groups identified statistically significant differences (p-values <0.05) between the rapid decliners (group 1) and the slow decliners (group 2) at p < 0.002 and between the rapid decliners (group 1) and the nonsmoker controls (group 4) at p < 0.013. Even if the mean level of DESs was higher in the COPD slow decliners group (16.0 ± 3.1 ng/mg of crea) than for the nonsmokers control (14.9 ± 2.9 ng/mg of crea), the difference between these two groups was not statistically significant (one way ANOVA test). A similar observation was made by Ma et al.1 where a DESs level of 13.42 ± 4.16 ng/mg of crea was obtained for the COPD group compared to 12.78 ± 4.78 ng/mg of crea for the healthy nonsmokers group.
Figure 5.
Box-whisker plot of the DESs concentrations measured for the four groups of urine samples. Box lines indicate lower and higher quartile values with the median as dotted bar. The whiskers show the smallest and largest nonoutlier DESs concentrations. Mean and relative square deviations are presented in brackets for each group.
We observed statistically significant lower levels of DES concentrations in the urine from the COPD rapid decliners group (11.8 ± 3.7 ng/mg of crea) compared to the slow decliners group (16.0 ± 3.1 ng/mg of crea) and the healthy nonsmokers (14.9 ± 2.9 ng/mg of crea) and a trend toward a decrease compared to the healthy smokers group (13.2 ± 1.9 ng/mg of crea). The decreasing levels of DES concentration from COPD rapid and slow decliner groups can be partly explained by a progressive decrease in elastin catabolism due to reduced lung elastin pool during COPD progression. It is noteworthy that an inverse correlation between the urinary DESs excretion and the extent of COPD was also observed during a study conducted by Cocci et al.7 where they observed significantly higher levels of DESs for COPD patients with no evidence or only mild emphysema compared to patients with moderate to severe emphysema. However, these results differ from those of Gottlieb et al.25 who observed a greater excretion of DES in urine for the COPD rapid decliners group compared to the COPD slow decliners group. Results obtained from this study and others1,15,16 suggest that clear distinction in DESs levels across the different groups remains difficult to establish. This variability could possibly be due to factors such as an elastin-rich diet36 and potential contributions from other elastin-containing organs13,27 that can vary significantly across individuals.
CONCLUSION
This study presents a sensitive and reproducible analytical approach using a nanoLC—MS/MS method for the analysis of urinary DESs. This method provided a detection limit of 0.1 ng/mL for DESs with RSD values of 0.9%. The derivatization of DESs with propionic anhydride was successfully used to decrease the polarity of DESs thereby facilitating the analysis of DES by nanoLC—MS/MS using a C18 stationary phase. Reduction of side reaction products such as esterification and amidation was achieved using the solvent isopropanol instead of methanol typically used with anhydride reagents. We also developed a convenient straightforward protocol to synthesize the D4-DES internal standard from inexpensive chemicals. The application of this method was demonstrated for the analysis of urine samples from four groups of individuals (n = 9–11): (a) COPD rapid decliners, (b) COPD slow decliners, (c) healthy smokers, and (d) healthy nonsmokers. We applied one-way analysis of variance to test for statistical differences among these four independent groups. These analyses revealed that statistically meaningful differences (p-values <0.05) were noted only between the rapid decliners and the slow decliners (p < 0.002) and between the rapid decliners and the nonsmoker controls (p < 0.013). We also observed an inverse correlation of DESs levels and COPD suggesting that lower DESs urinary concentration of rapid decliners could possibly be due to a reduction in the lung elastin pool during the progression of the disease. It is important to note that these subjects were studied when they were not acutely ill. Active elastin degradation may occur primarily in times of disease activity, such as COPD exacerbations. Thus, this turnover may not be reflected in our patient groups who were studied during a steady-state phase of their disease. These analyses indicated that clear distinction among groups remains challenging due to overlap and biological variability that could compromise the use of desmosine/isodesmosine as a COPD disease biomarker. However, its full analytical potential might be best realized in studies where biological variability can be better controlled such as those involving the monitoring of impaired lung functions over time for the same COPD patients or for studies using animal models.
ACKNOWLEDGMENT
This work was financially supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) and Merck Frosst Canada via a collaborative research and development grant to P.T. and a NSERC fellowship to M.B. IRIC is supported in part by a multiresource grant from the Canadian Institute for Health Research (CIHR), the Canadian Center of Excellence in Commercialization and Research (CECR), the Canada Foundation for Innovation (CFI), and by the Fonds de Recherche en Santé du Québec (FRSQ). This work was also funded by a grant from the National Institutes of Health, Grant NIDA/NHLBI P01-HL72903. Informed consent from subjects was obtained under the University of Utah IRB Protocol No. 8969.
References
- 1.Ma S, Lin YY, Turino GM. Chest. 2007;131:1363–1371. doi: 10.1378/chest.06-2251. [DOI] [PubMed] [Google Scholar]
- 2.Mahadeva R, Shapiro SD. Thorax. 2002;57:908–914. doi: 10.1136/thorax.57.10.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshida T, Tuder RM. Physiol. Rev. 2007;87:1047–1082. doi: 10.1152/physrev.00048.2006. [DOI] [PubMed] [Google Scholar]
- 4.Mithieux SM, Wise SG, Raftery MJ, Starcher B, Weiss AS. J. Struct. Biol. 2005;149:282–289. doi: 10.1016/j.jsb.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Moroy G, Alix AJ, Hery-Huynh S. Biopolymers. 2005;78:206–220. doi: 10.1002/bip.20276. [DOI] [PubMed] [Google Scholar]
- 6.Senior RM, Griffin GL, Mecham RP. J. Clin. Invest. 1980;66:859–862. doi: 10.1172/JCI109926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cocci F, Miniati M, Monti S, Cavarra E, Gambelli F, Battolla L, Lucattelli M, Lungarella G. Int. J. Biochem. Cell Biol. 2002;34:594–604. doi: 10.1016/s1357-2725(02)00015-8. [DOI] [PubMed] [Google Scholar]
- 8.Viglio S, Iadarola P, Lupi A, Trisolini R, Tinelli C, Balbi B, Grassi V, Worlitzsch D, Doring G, Meloni F, Meyer KC, Dowson L, Hill SL, Stockley RA, Luisetti M. Eur. Respir. J. 2000;15:1039–1045. doi: 10.1034/j.1399-3003.2000.01511.x. [DOI] [PubMed] [Google Scholar]
- 9.Brown-Augsburger P, Tisdale C, Broekelmann T, Sloan C, Mecham RP. J. Biol. Chem. 1995;270:17778–17783. doi: 10.1074/jbc.270.30.17778. [DOI] [PubMed] [Google Scholar]
- 10.Foster JA, Rubin L, Kagan HM, Franzblau C, Bruenger E, Sandberg LB. J. Biol. Chem. 1974;249:6191–6196. [PubMed] [Google Scholar]
- 11.Rucker RB, Kosonen T, Clegg MS, Mitchell AE, Rucker BR, Uriu-Hare JY, Keen CL. Am. J. Clin. Nutr. 1998;67:996S–1002S. doi: 10.1093/ajcn/67.5.996S. [DOI] [PubMed] [Google Scholar]
- 12.Ryvkin F, Greenaway FT. J. Inorg. Biochem. 2004;98:1427–1435. doi: 10.1016/j.jinorgbio.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Annovazzi L, Viglio S, Gheduzzi D, Pasquali-Ronchetti I, Zanone C, Cetta G, Iadarola P. Eur. J. Clin. Invest. 2004;34:156–164. doi: 10.1111/j.1365-2362.2004.01306.x. [DOI] [PubMed] [Google Scholar]
- 14.Annovazzi L, Viglio S, Perani E, Luisetti M, Baraniuk J, Casado B, Cetta G, Iadarola P. Electrophoresis. 2004;25:683–691. doi: 10.1002/elps.200305607. [DOI] [PubMed] [Google Scholar]
- 15.Bode DC, Pagani ED, Cumiskey WR, von Roemeling R, Hamel L, Silver PJ. Pulm. Pharmacol. Ther. 2000;13:175–180. doi: 10.1006/pupt.2000.0245. [DOI] [PubMed] [Google Scholar]
- 16.Boschetto P, Quintavalle S, Zeni E, Leprotti S, Potena A, Ballerin L, Papi A, Palladini G, Luisetti M, Annovazzi L, Iadarola P, De Rosa E, Fabbri LM, Mapp CE. Thorax. 2006;61:1037–1042. doi: 10.1136/thx.2006.058321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cumiskey WR, Pagani ED, Bode DC. J. Chromatogr., B: Biomed. Appl. 1995;668:199–207. doi: 10.1016/0378-4347(95)00092-w. [DOI] [PubMed] [Google Scholar]
- 18.Fill JA, Brandt JT, Wiedemann HP, Rinehart BL, Lindemann CF, Komara JJ, Bowsher RR, Spence MC, Zeiher BG. Biomarkers. 2006;11:85–96. doi: 10.1080/13547500500343225. [DOI] [PubMed] [Google Scholar]
- 19.Kaga N, Soma S, Fujimura T, Seyama K, Fukuchi Y, Murayama K. Anal. Biochem. 2003;318:25–29. doi: 10.1016/s0003-2697(03)00134-9. [DOI] [PubMed] [Google Scholar]
- 20.Lucattelli M, Bartalesi B, Cavarra E, Fineschi S, Lunghi B, Martorana PA, Lungarella G. Respir. Res. 2005;6:83. doi: 10.1186/1465-9921-6-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma S, Lieberman S, Turino GM, Lin YY. Proc. Natl. Acad. Sci. U.S.A. 2003;100:12941–12943. doi: 10.1073/pnas.2235344100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stolk J, Veldhuisen B, Annovazzi L, Zanone C, Versteeg EM, van Kuppevelt TH, Nieuwenhuizen W, Iadarola P, Berden JH, Luisetti M. Respir. Res. 2006;7:20. doi: 10.1186/1465-9921-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiorenza D, Viglio S, Lupi A, Baccheschi J, Tinelli C, Trisolini R, Iadarola R, Luisetti M, Snider GL. Respir. Med. 2002;96:110–114. doi: 10.1053/rmed.2001.1224. [DOI] [PubMed] [Google Scholar]
- 24.Viglio S, Zanaboni G, Luisetti M, Trisolini R, Grimm R, Cetta G, Iadarola P. J. Chromatogr., B: Biomed. Sci. Appl. 1998;714:87–98. doi: 10.1016/s0378-4347(98)00046-2. [DOI] [PubMed] [Google Scholar]
- 25.Gottlieb DJ, Stone PJ, Sparrow D, Gale ME, Weiss ST, Snider GL, O’Connor GT. Am. J. Respir. Crit. Care Med. 1996;154:1290–1295. doi: 10.1164/ajrccm.154.5.8912738. [DOI] [PubMed] [Google Scholar]
- 26.McClintock DE, Starcher B, Eisner MD, Thompson BT, Hayden DL, Church GD, Matthay MA. Am. J. Physiol.: Lung Cell. Mol. Physiol. 2006;291:L566–L571. doi: 10.1152/ajplung.00457.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osakabe T, Usami E, Sato A, Sasaki S, Watanabe T, Seyama Y. Biol. Pharm. Bull. 1999;22:854–857. doi: 10.1248/bpb.22.854. [DOI] [PubMed] [Google Scholar]
- 28.Viglio S, Annovazzi L, Luisetti M, Stolk J, Casado B, Iadarola P. J. Sep. Sci. 2007;30:202–213. doi: 10.1002/jssc.200600260. [DOI] [PubMed] [Google Scholar]
- 29.Getie M, Raith K, Neubert RH. Biochim. Biophys. Acta. 2003;1624:81–87. doi: 10.1016/j.bbagen.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 30.Scheigetz J, Berthelette C, Li C, Zamboni RJ. J. Label Compd. Radiopharm. 2004;47:881–889. [Google Scholar]
- 31.Durkin DA, Kjelsberg MO, Buist AS, Connett JE, Owens GR. Control Clin. Trials. 1993;14:20S–37S. doi: 10.1016/0197-2456(93)90022-6. [DOI] [PubMed] [Google Scholar]
- 32.Garcia BA, Busby SA, Barber CM, Shabanowitz J, Allis CD, Hunt DF. J. Proteome Res. 2004;3:1219–1227. doi: 10.1021/pr0498887. [DOI] [PubMed] [Google Scholar]
- 33.Nordstrom A, Tarkowski P, Tarkowska D, Dolezal K, Astot C, Sandberg G, Moritz T. Anal. Chem. 2004;76:2869–2877. doi: 10.1021/ac0499017. [DOI] [PubMed] [Google Scholar]
- 34.Syka JE, Marto JA, Bai DL, Horning S, Senko MW, Schwartz JC, Ueberheide B, Garcia B, Busby S, Muratore T, Shabanowitz J, Hunt DF. J. Proteome Res. 2004;3:621–626. doi: 10.1021/pr0499794. [DOI] [PubMed] [Google Scholar]
- 35.Boutin M, Bateman K, Thibault P. Proceedings of the 55th ASMS Conference on Mass Spectrometry and Allied Topics; June 3–7, 2007; Indianapolis, IN. [Google Scholar]
- 36.Pai V, Guz A, Phillips GJ, Cooke NT, Hutchison DC, Tetley TD. Metabolism. 1991;40:139–145. doi: 10.1016/0026-0495(91)90164-r. [DOI] [PubMed] [Google Scholar]