Abstract
Objective
To evaluate the efficacy of vitamin B-6 in the treatment of premenstrual syndrome.
Design
Systematic review of published and unpublished randomised placebo controlled trials of the effectiveness of vitamin B-6 in the management of premenstrual syndrome.
Subjects
Nine published trials representing 940 patients with premenstrual syndrome.
Main outcome measures
Proportion of women whose overall premenstrual symptoms showed an improvement over placebo. A secondary analysis was performed on the proportion of women whose premenstrual depressive symptoms showed an improvement over placebo.
Results
Odds ratio relative to placebo for an improvement in overall premenstrual symptoms was 2.32 (95% confidence interval 1.95 to 2.54). Odds ratio relative to placebo for an improvement in depressive symptoms was 1.69 (1.39 to 2.06) from four trials representing 541 patients.
Conclusion
Conclusions are limited by the low quality of most of the trials included. Results suggest that doses of vitamin B-6 up to 100 mg/day are likely to be of benefit in treating premenstrual symptoms and premenstrual depression.
Key messages
Randomised placebo controlled studies of vitamin B-6 treatment for premenstrual symptoms were of insufficient quality to draw definitive conclusions
Limited evidence exists to suggest that 100 mg of vitamin B-6 daily (and possibly 50 mg) are likely to be beneficial in the management of premenstrual syndrome
Vitamin B-6 was significantly better than placebo in relieving overall premenstrual symptoms and in relieving depression associated with premenstrual syndrome, but the response was not dose dependent
No conclusive evidence was found of neurological side effects with these doses
A randomised controlled trial of sufficient power and quality is needed to compare vitamin B-6 with placebo to establish definitive recommendations for treatment
Introduction
The UK Department of Health and the Medical Control Agency have recently published recommendations to restrict the dose of vitamin B-6 available generally to 10 mg and to limit the dose sold by a pharmacist to less than 50 mg.1 Vitamin B-6 is often used to treat premenstrual syndrome without clear evidence of its efficacy, hence it is timely to re-evaluate vitamin B-6 in the treatment of premenstrual syndrome.
Premenstrual syndrome exists when women complain of regularly recurring psychological or somatic symptoms, or both, which occur specifically during the luteal phase of the menstrual cycle and which are relieved by the onset of, or during, menstruation. Symptoms can be severe enough to disrupt every day life.2 Mild physiological symptoms occur in approximately 95% of all women of reproductive age. Approximately 5% of symptomatic women complain of such severe symptoms that their lives are completely disrupted.3
Somatic symptoms of premenstrual syndrome include bloating, weight gain, mastalgia, abdominal discomfort and pain, lack of energy, headache, and exacerbations of chronic illnesses such as asthma, allergies, epilepsy, or migraine. Commonly reported affective changes are dysphoria, irritability, anxiety, tension, aggression, feelings of being unable to cope, and a sense of loss of control.4
Since the original description of the syndrome in 19315 numerous hypotheses have been advanced to explain premenstrual syndrome, but to date the pathogenesis remains unclear and speculative.6 This uncertainty reflects the many treatments available7; one reviewer suggested that there were as many as 327 different treatments for premenstrual syndrome.6 Most interventions, however, have been on the basis of informal observations, retrospective data collection, or inadequately controlled trials.
The recommended dietary allowance for vitamin B-6 is around 2.0 mg/day, depending on age and protein intake,8 and deficiency of vitamin B-6 is rare.9 Excessive ingestion (2000-6000 mg) of vitamin B-6 causes peripheral neuropathy,10–17 and doses of 200 mg/day may cause similar, although probably reversible, effects.18
Because the efficacy of vitamin B-6 has not yet been proved, and in light of recent government recommendations, we undertook a systematic review of published and unpublished randomised controlled trials where efficacy of vitamin B-6 was compared with placebo in women with premenstrual syndrome.
Methods
Trials
We found reports of published and unpublished clinical trials by searching medical databases for trials of vitamin B-6 (pyridoxine) in the management of premenstrual syndrome (MeSH terms used were premenstrual syndrome and pyridoxine, together with title and abstract searches for keywords vitamin and pyridoxine, premenstrual syndrome, PMT, LLPDD, and PMDD). We also contacted relevant pharmaceutical companies manufacturing vitamin B-6 preparations. The trials were identified by searching Embase (1988 to 1996), Medline (1966 to 1998), Psychlit (1974 to 1997), Cinahl (1982 to 1997), and the database of the Cochrane Controlled Trials Register. We searched references cited in all included and excluded trials to identify any missing studies. All languages were included. Trials investigating the effect of vitamin B-6 on premenstrual symptoms were included if they were randomised, placebo controlled, double blind, parallel, or crossover studies (where the first treatment period could be treated as a parallel trial) for which data could be acquired. We also included studies investigating the effect of multivitamin supplements on premenstrual symptoms, when the vitamin preparation contained 50 mg or more of vitamin B-6.
Data extraction and outcome measures
We extracted the data from each trial that met the inclusion criteria. When there were insufficient data presented for inclusion, we contacted authors for further details. Data on the dosage and preparation of vitamin B-6 were collected. The main outcome measure was an improvement in overall premenstrual symptoms. Combined or overall symptoms was chosen in an attempt to overcome the clinical heterogeneity concerned with the measurement and scoring of premenstrual symptoms. As a secondary outcome we recorded the improvement in depressive premenstrual symptoms when suitable information was presented. Where possible we also recorded the number of women withdrawing from treatment and those complaining of side effects.
Quality assessment
We assessed the quality of each trial with two different methods. Firstly, we assessed the methodology of each trial with a scale developed by Jadad and colleagues.19 This scale assesses the randomisation and double blinding and reports of dropouts and withdrawals. Secondly, we developed a second quality scale to assess the trials for study design, reproducibility, and statistical analysis. The eight point scale measured: preliminary diagnosis of premenstrual syndrome for all participants in the trial; confirmation that no other vitamin supplements or oral contraceptives were being concurrently taken; the randomisation procedure described in detail; a power calculation to justify participant numbers, or >65 participants in each arm of the study; a single clearly stated dose of vitamin B-6 only; reproducible measurement of premenstrual symptoms such as use of visual analogue scales or Moos' menstrual distress questionnaire; clear presentation of results; and a description of the number and reason for trial withdrawals. One point was awarded for each category. Each trial was independently scored by KMW and PWD, and any areas of disagreement arbitrated by PMSO. A predetermined criterion for the recognition of the highest quality trials was established. A score of 3 or more was required in the Jadad score (as recommended by Jadad and colleagues19) and a score of 6 or more was required in our eight item quality assessment.
Statistical analysis
Where dichotomous data were presented, odds ratios with 95% confidence intervals were generated for the primary and, where possible, secondary outcome in each trial.20 We calculated odds ratios by categorising patients with a subjective improvement as “better,” and those with no change or worse symptoms as “not better.” Odds ratios of >1 indicated that an event was more likely to occur in the group receiving vitamin B-6 than in the group receiving placebo. We calculated an overall odds ratio with fixed and random effects models.20 Homogeneity was tested for with a χ2 test, with P<0.05 indicating significant heterogeneity. For trials using continuous measures of premenstrual symptomology, we calculated a standardised mean difference (or effect size), and converted to an odds ratio using a relation given by Hasselblad and Hedges.21
To detect bias (such as publication and location) within the analysed trials, we constructed a funnel plot.22 To assess quantitatively the asymmetry of the funnel plot we used the method of Egger and colleagues.22 Briefly, a linear regression of the standard normal deviate defined as the odds ratio is divided by its SE against precision (inverse of the standard error). A regression line which passes through the origin of the plot (within error limits) indicates symmetry and hence the absence of bias.
Results
We identified 25 published trials.2,18,23–45 Of these, four were excluded because they did not include a placebo group,34,38,39,43 two were retrospective studies,40,41 and nine presented quantitative data that could not be analysed,2,18,23,27,35–37,42,45 leaving 10 placebo controlled trials.24–26,28–44 Two of the trials had two separate dosing regimens and were effectively treated as four studies. Only one trial contained details of the method of randomisation.25
Quality assessment of the trials
Three of the included trials met the Jadad scale cut off point of a score of ⩾3.25,26,33 However, only one of the trials included in the meta-analysis scored 6 on our eight point criteria for classification as a high quality trial.31 Unfortunately this trial had too few subjects to achieve sufficient power and had a low Jadad score. The overall methodology of the trials was poor, with none of the included trials justifying patient numbers with a power calculation. Three of the 10 studies reported the number of withdrawals from a trial, together with reasons, and side effects when taking either vitamin B-6 or placebo.26,30,33
Of the nine trials included in the meta-analysis (one trial was excluded owing to statistical heterogeneity), a statement regarding whether women taking oral contraceptives were excluded from the trial was reported in three trials,25,28,31 three other trials do not mention this as an exclusion criterion,24,29,30 and one trial excluded women “on medication.”33 Two trials26,32 included women who were taking the contraceptive pill, but they both flagged those women who did and analysed them separately and found no statistically significant difference between those taking oral contraceptives and those not taking oral contraceptives.
As none of the trials met both our and the Jadad quality criteria, we did not take the quality score into account when considering trials for inclusion. Tables 1 and 2 present the quality scores for included and excluded trials.
Table 1.
Characteristics of studies included in meta-analysis of vitamin B-6 in premenstrual syndrome
| Study | Participants | Intervention | Outcome measures | Reported results | Withdrawals and side effects* | Jadad (authors’) quality score | Comments |
|---|---|---|---|---|---|---|---|
| Colin 198224 | 32 with diagnosed mastalgia; 17 given vitamin B-6, 15 given placebo | 500 mg/day for 4 months | Mastalgia symptoms worse or better | 58% better taking vitamin B-6, 59% better taking placebo | None reported | 1(3) | Only studied mastalgia Exclusion of women on oral contraceptives not mentioned |
| Barr 198425 | 48; crossover study design | 100 mg/day day 10 to day 3 after cycle for 2 months | Depression, irritability, tiredness, headache, stomach ache, and swollen breasts, abdomen, fingers, or ankles | Vitamin B-6 significantly better than placebo | None reported | 3 (4) | “Most” women were not on oral contraceptives |
| Williams et al 198526 | 434 with diagnosed premenstrual syndrome; 204 given vitamin B-6, 230 given placebo | 100 mg/day (55%) 200 mg/day (29%) 50 mg/day (3%) | Depression, irritability, tension, violence, coordination, breast tenderness, bloating, headache, acne rated on a 4 point scale | Significant improvement of global symptoms only for vitamin B-6 over placebo | Owing to side effects 11 women withdrew on vitamin B-6 and 8 withdrew on placebo | 3 (4) | Patients included were allowed drugs: 74 psychotropics; 60 analgesics; 33 diuretics; 100 oral contraceptives Only analgesia significantly affected analysis |
| Chakmakjian et al 198528 | 31 with diagnosed premenstrual syndrome from hospital and general population; crossover study design | Multivitamin containing 300 mg vitamin B-6/day for 2 × 3 cycles | 19 symptoms in 4 subgroups: PMT-A, PMT-C, PMT-D, and PMT-H | Significant effect for symptom groups PMT-A and PMT-C but not for PMT-H and PMT-D | None reported | 2 (3) | Women on oral contraceptives excluded |
| Smallwood et al 198629 | 42 hospital patients “with severe cyclical mastalgia"; crossover study design | 200 mg/day for 2 × 2 cycles | Breast pain and tenderness rated on chart and visual analogue scale | No significant effect | None reported | 2 (4) | Only studied mastalgia Exclusion of women on oral contraceptives not mentioned |
| Stewart 198730* | 222 ” with premenstrual syndrome symptomology"; 49 given 200 mg vitamin B-6, 70 given placebo 46 given 100 mg vitamin B-6, 58 given placebo | Multivitamin containing 100 mg vitamin B-6 day 1 to day 14 then 200 mg for 4 cycles Multivitamin containing 50 mg vitamin B-6 day 1 to day 14 then 100 mg | 19 symptoms in 4 subgroups: PMT-A, PMT-C, PMT-D, and PMT-H rated worse, no better, slightly better, substantially better, or cured | 100 mg and 200 mg significantly better than placebo, 50 mg and 100 mg not significantly better than placebo | 30 withdrew on placebo, 26 withdrew on vitamin B-6, 5 had side effects on vitamin B-6 and 1 on placebo, 17 withdrew on placebo, 22 withdrew on vitamin B-6, 5 had side effects on vitamin B-6 and 2 on placebo | 1 (4) | Exclusion of women on oral contraceptives not mentioned Study blinded to patient only |
| Kendall and Schnurr 198731 | 55 (financially reimbursed) with moderate to severe premenstrual mood changes recruited form general population; 29 given vitamin B-6, 26 given placebo | 150 mg/day for 2 cycles | Pain, water retention, negative effect, positive effect, control, impaired concentration, behaviour change, autonomic reactions | No significant effect | None reported | 1 (6) | Women on oral contraceptives excluded |
| Doll et al 198932 | 32 with moderate to severe premenstrual symptoms in previous year recruited from general practitioner; crossover study design | 50 mg/day for 3 cycles with one washout cycle between | 3 symptom groups: emotional, somatic, and menstrual | Significant effect on emotional symptoms | None reported | 2 (5) | Included women on oral contraceptives but data flagged |
| London et al 199133 | 44 with self perceived premenstrual syndrome recruited form the general population; 28 given vitamin B-6, 16 given placebo | 2 parallel arms Multivitamin containing 300 mg vitamin B-6 or 600 mg vitamin B-6/day for 3 cycles | 19 symptoms in 4 subgroups: PMT-A, PMT-C, PMT-D, and PMT-H with London variation | Significant effect observed | Withdrawals: 1 taking placebo, 1 taking 300 mg vitamin B-6, 2 taking 600 mg vitamin B-6, 1 noted “tingling in fingers” at the end of the 600 mg study | 3 (5) | Exclusion of women on oral contraceptives not mentioned Women on “medication” excluded |
Neurological side effects reported in 1 of 934 patients while taking 600 mg/day vitamin B-6 as part of multivitamin preparation.33
Table 2.
Characteristics of studies not included in meta-analysis of vitamin B-6 in premenstrual syndrome
| Study | No of participants | Intervention | Reason for exclusion | Reported results | Side effects* | Jadad (authors’) quality score | Comments |
|---|---|---|---|---|---|---|---|
| Kerr 197734 | 70 | 40-100 mg | Not placebo controlled | Considerable benefit | None observed | 0 (1) | |
| Day 197935 | 57 | 100 mg | No detailed data | Improvement in 63% of patients on pyroxidine | No significant side effects observed | 0 (3) | |
| O’Brien 19872 | 53; crossover study design | 100 mg | Data not suitable for meta-analysis | No benefit of drug over placebo | None reported | 1 (4) | 24 patients preferred pyridoxine v 14 taking placebo, but results not significant |
| Abraham and Hargrove 198036 | 25 | 500 mg | Data not suitable for meta-analysis | 21 patients showed significant improvement over placebo | None reported | 2 (5) | |
| Mattes and Martin 198237 | 3 | 50 mg | Anecdotal study, no assessment of symptoms | Some beneficial effects | None reported | 2 (1) | |
| Goei and Abraham 198338 | 31 | Multivitamin containing 150-600 mg vitamin B-6 | Not placebo controlled | Significant decrease in symptoms in all patients | 5 patients reported gastrointestinal side effects | 1 (5) | Multivitamin |
| Harrison et al 198445 | 30 | 50-150 mg | Sequential design; data not suitable for analysis | No benefit | >100 mg/3 g tryptophan caused drowsiness, nausea, headache, overstimulation | 1 (5) | Cointervention each tablet: 50 mg vitamin B-6 + 1.5 g of tryptophan; up to 3 tablets per day |
| Hagen et al 198527 | 34 | 100 mg | Data not suitable for meta-analysis | No significant effect | 6 reported nausea: 1 taking vitamin B-6 and 1 taking placebo | 3 (5) | Large phase effect: first treatment, whether placebo or vitamin B-6, gave same result Women on oral contraceptives excluded |
| Fuchs et al 198539 | 16 | Multivitamin containing 300-600 mg vitamin B-6 | Not placebo controlled | Beneficial effect | None reported | 0 (2) | Multivitamin study centred on analysis of liver enzymes |
| Malmgren et al 198718 | 19 | 300 mg | No data | No significant effect over placebo | None reported | 1 (4) | Study centred on uptake of platelet serotonin |
| Brush et al 198840 | 630 | 40-200 mg | Retrospective study, not placebo controlled | 40% “good” response 65%-88% partial response depending on dose | 7 patients reported indigestion, 5 reported nausea, 3 reported breast soreness No neuropathy reported | 0 (1) | Other side effects were termed “coincidental” and included worse premenstrual symptoms and depression |
| Brush 198841 | 336 | ⩽200 mg | Retrospective study, not placebo controlled | 70% reported good or partial response | 8 patients reported nausea, 5 dizziness, 6 mild tingling or numbness | 0 (1) | 5 of the patients reporting tingling or numbness were on the highest dose (200 mg) |
| Van den Berg et al 198942 | 19 | 120 mg | Data not suitable for meta-analysis | No beneficial effect | None reported | 1 (4) | |
| Berman et al 199043 | 28 | 250 mg | Not placebo controlled | Some favourable trends in improvement of symptoms | None reported | 0 (5) | |
| De Souza et al 199644 | 44; crossover study design | 50 mg every day for 2 × 1 cycle | Statistically heterogeneous | No significant effect | None reported | 2 (4) | 4 crossovers for magnesium, vitaminB-6, magnesium + vitamin B-6, placebo |
Of 1395 patients taking vitamin B-6, six reported neurological side effects: five patients taking 200 mg/day and one patient taking 100-200 mg/day.
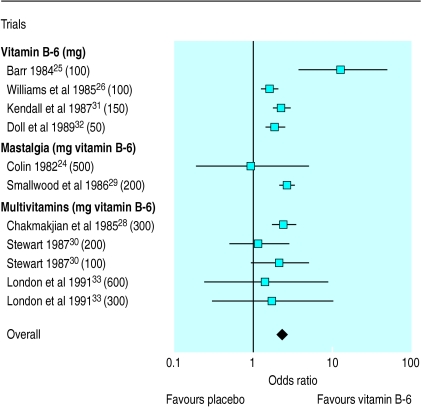
We calculated the odds ratios with both fixed and random effects models. Minimal difference was found between the two methods, and so the results from the more conservative random effects model were used. The overall odds ratio in favour of vitamin B-6 was 1.57 (95% confidence interval 1.40 to 1.77). One trial caused significant heterogeneity in the overall odds ratio (homogeneity test, P=<0.001),44 and removal of this trial resulted in homogeneity (homogeneity test, P=0.187). The recalculated odds ratio in favour of vitamin B-6 was 2.32 (1.95 to 2.54). This heterogeneity may be due to an unexpectedly high placebo effect, which resulted in an odds ratio in favour of placebo. It should be noted, however, that the overall odds ratio remained significantly in favour of vitamin B-6 even with the inclusion of this trial. Table 1 lists the nine included trials, and table 2 lists the other 16 trials that were identified. Figure 1 shows the odds ratio and dosage for each of the nine included trials.
Figure 1.
Odds ratios (95% confidence intervals) for proportion of patients showing improvement in overall premenstrual symptoms with vitamin B-6
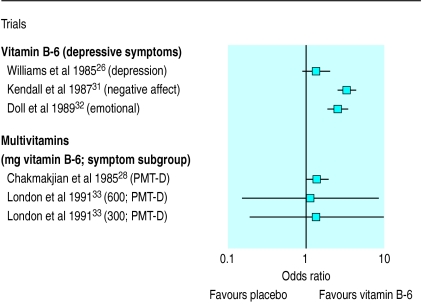
We extracted data on the efficacy of vitamin B-6 in treating depressive premenstrual symptoms from five trials.26,28,31–33 Figure 2 shows the odds ratio for each trial and the term used by those trials to describe depression. The overall odds ratio in favour of vitamin B-6 was 2.12 (1.80 to 2.48; homogeneity test P<0.001). This combined result showed significant heterogeneity with both fixed and random effects models. Exclusion of one trial31 gave an overall odds ratio of 1.69 (1.39 to 2.06), which was homogeneous (homogeneity test, P=0.079).
Figure 2.
Odds ratios (95% confidence intervals) for proportion of patients showing improvement in depressive premenstrual symptoms with vitamin B-6
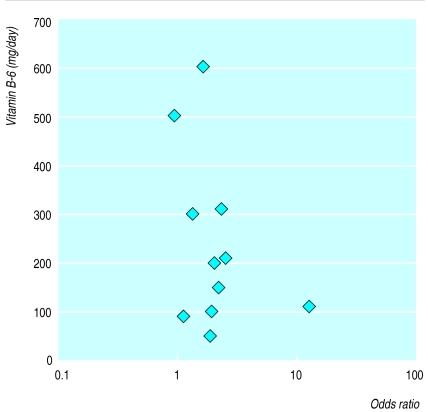
Figure 3 shows a dose response plot of vitamin B-6 dosage against the odds ratio for each of the nine trials. There was no correlation between the amount of vitamin B-6 given and its efficacy.
Figure 3.
Dose response of vitamin B-6 against individual odds ratios for patients who showed improvement in premenstrual symptoms with vitamin B-6 (when two trials used same dose, data points offset for clarity)
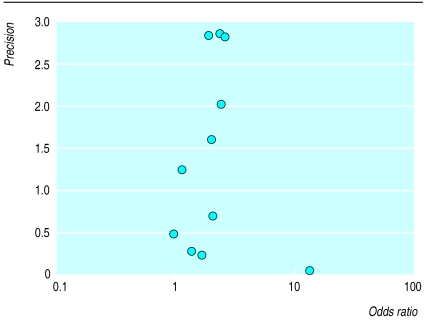
Figure 4 details a funnel plot of the included trials. Regression analysis of this plot indicated no significant asymmetry (intercept –0.25 (90% confidence interval –0.88 to 0.38) P=0.49),22 and thus no evidence of bias.
Figure 4.
Funnel plot of included studies: precision of study (inverse of SE) against odds ratios
Discussion
The conclusions that can be drawn from our systematic review are limited. Although the results from the available data suggest that vitamin B-6 is more effective than placebo, there is insufficient evidence of high enough quality to give a confident recommendation for using vitamin B-6 in the treatment of premenstrual syndrome. For example, the evidence from the overall analysis would be more compelling if there were large scale rigorous trials with sufficient power to detect a clinically significant effect. To detect a medium effect size (0.5) at a significance level of 0.05 and 80% power, approximately 65 subjects would be required in each arm, using a two tailed test. Only one of the nine studies included a sufficient number of patients,26 and all the other trials had low statistical power (<70%). The methodologies of each trial varied considerably using differing dosage regimens and different outcome measures, which makes intercomparisons difficult. In addition, the possible inclusion of women taking oral contraceptives complicates the overall analysis of the effect of vitamin B-6 on premenstrual syndrome as the vitamin may be treating pill induced premenstrual symptoms or depression.
An overall odds ratio was calculated from the nine included trials. Of these trials, two24,29 only studied the effect of vitamin B-6 on mastalgia and three28,30,33 used the multivitamin preparation Optivite (Optimox, Torrance, CA, USA). We believed it was appropriate to include the trials of mastalgia, as breast pain and breast swelling are frequent premenstrual symptoms. As many as 60% of women with premenstrual syndrome report cyclical breast pain as a primary component.46
The odds ratios in the four included trials that used only vitamin B-6 and studied premenstrual symptomatology25,26,31,32 were found to be heterogeneous. The combined odds ratio for the four trials was 2.15 (95% confidence interval 1.79 to 2.59; homogeneity test, P=0.035), and when one heterogeneous trial was excluded,25 the recalculated odds ratio was 2.07 (1.72 to 2.50; homogeneity test, P=0.61).
The use of funnel plots in meta-analysis gives a simple graphical method of assessing bias. A regression analysis to assess quantitatively potential asymmetry of the funnel plot shown in figure 4 indicated no significant asymmetry.22 The size and quality of the included trials, however, makes any definite statement concerning bias inappropriate.
Depression
One of the rationales behind vitamin B-6 being recommended as a treatment for premenstrual syndrome was the observation that it could ease induced depression in several conditions47–49 particularly that associated with contraceptive pills high in oestrogen and progesterone.50,51 The combined odds ratio of the four trials (representing 541 patients) that presented data for depressive symptoms was 1.69 (1.39 to 2.06), and this was homogeneous. This indicates that vitamin B-6 is better than placebo in treating premenstrual depression. It is unlikely, however, that this is the central mode of action of vitamin B-6 in treating premenstrual syndrome, as the odds ratio for overall symptomatology is more favourable than when considering premenstrual depression alone.
Dose response
The lack of a dose response (fig 3) indicates that the amount of vitamin B-6 given has no impact on the efficacy of its use as treatment for premenstrual syndrome, for either vitamin B-6 alone or when given in a multivitamin preparation. This result adds to the misgivings surrounding the use of vitamin B-6 as a treatment. The toxic effects of vitamin B-6 at doses higher than 200 mg/day are well characterised. As the recommended daily allowance of vitamin B-6 is around 2 mg,8 any effect of vitamin B-6 on premenstrual symptomatology would be pharmacological. However, there does remain some controversy as to the precise vitamin B-6 requirement in disease states, highlighted by the successful use of vitamin B-6 doses of around 100 mg/day in the management of carpal tunnel syndrome.52
Multivitamins
The rationale for including multivitamin treatments in a review on vitamin B-6 is the very large amount of vitamin B-6 in the tablets used, 50 mg per tablet (600 mg/day), which represents over 25 times the recommended daily allowance.8 Figure 1 shows the results of the published trials on use of multivitamins in the treatment of premenstrual syndrome, with an overall odds ratio of 2.08 (1.55 to 2.78; homogeneity test, P=0.61). The lack of a dose response makes the daily consumption of more than 100 mg of vitamin B-6 inadvisable, and doses in excess of 200 mg/day cannot be recommended in the light of the proved toxic side effects of vitamin B-6, even in a multivitamin preparation.
Side effects
Only one patient of the 940 participating in these trials indicated the presence of any side effects that could be attributed to the neuropathy associated with pyridoxine toxicity.33 This may be due to the comparatively low doses used and the short duration of the trials. The symptoms, however, may have been missed owing to the lack of assessment by a suitably qualified neurologist. Detailed animal studies on pyridoxine indicate that nerve damage can occur before manifestation of the gross symptoms such as ataxia and neuropathy.11,12
Conclusions
Conclusions from this meta-analysis are limited by the quality of the trials. No conclusive evidence of vitamin B-6 toxicity was reported, and there seems to be no dose related response to treatment. We conclude, therefore, that there is no rationale for giving daily doses of vitamin B-6 in excess of 100 mg. Such doses, and possibly doses of 50 mg/day, are likely to be of benefit in relieving premenstrual symptoms.
We believe that this systematic review does highlight the need for a randomised placebo controlled trial, which should be conducted with sufficient subjects and should have the power to detect any significant clinical difference between vitamin B-6 and placebo.
Acknowledgments
We thank Mrs Cornelia Dean for translating reference 42, and Mrs Olive Ford (National Association for Premenstrual Syndrome) and Ms Ruth Jepson (Cochrane Menstrual Disorders and Subfertility Group) for assistance with literature surveys.
Footnotes
Funding: None.
Competing interests: PMSO’B has been reimbursed for lectures and conferences by the following companies: Shire Pharmaceuticals, SmithKline Beecham, Eli Lilly, Searle, Sanofi Winthrop, Zeneca, and Solvay Pharmaceuticals. He has also received funds for research staff from Searle, SmithKline Beecham, Eli Lilly, and Sanofi Winthrop. He is married to a member of the research department of Zeneca Pharmaceuticals. KMW, PWD, and PWJ declare no competing interests.
References
- 1.Joint Food Safety and Standards Group. Survey of dietary supplements containing vitamin B6. London: Ministry of Agriculture Fisheries and Food; 1997. [Google Scholar]
- 2.O'Brien PMS. Premenstrual syndrome. London: Blackwell Scientific; 1987. [Google Scholar]
- 3.O'Brien PMS. Helping women with premenstrual syndrome. BMJ. 1993;307:1471–1475. doi: 10.1136/bmj.307.6917.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith R, Studd J. Premenstrual syndrome. Clin Rev Gynaecol 1994;939-47.
- 5.Frank RT. The hormonal causes of premenstrual tension. Arch Neurol Psychiatry. 1931;26:1053. [Google Scholar]
- 6.Chakmakjian ZH. A critical assessment of therapy for the premenstrual tension syndrome. J Reprod Med. 1983;28:532–538. [PubMed] [Google Scholar]
- 7.O'Brien PMS. The premenstrual syndrome: a review of the present status of therapy. Drugs. 1982;24:140–151. doi: 10.2165/00003495-198224020-00004. [DOI] [PubMed] [Google Scholar]
- 8.Driskell JA. Vitamin B6 requirements of humans. Nutr Res. 1994;14:293–324. [Google Scholar]
- 9.Abraham GE. Nutritional factors in the etiology of the premenstrual tension syndrome. J Reprod Med. 1983;28:446. [PubMed] [Google Scholar]
- 10.Antopol W, Thomas M. Experimental study of the effects produced by large doses of vitamin B6. J Neuropathol Exp Neurol. 1942;1:330–336. [Google Scholar]
- 11.Schaeppi U, Krinke G. Pyridoxine neuropathy: correlation of functional tests and neuropathology in beagle dogs treated with large doses of vitamin B6. Agents Actions. 1982;12:575–582. doi: 10.1007/BF01965944. [DOI] [PubMed] [Google Scholar]
- 12.Krinke G, Naylor DC, Skorpil V. Pyridoxine megavitaminosis: an analysis of the early changes induced with massive doses of vitamin B6 in rat primary sensory neurons. J Neuropathol Exp Neurol. 1985;44:117–129. [PubMed] [Google Scholar]
- 13.Windebank AJ. Neurotoxicity of pyridoxine analogs is related to coenzyme structure. Neurochem Pathol. 1985;3:159–167. doi: 10.1007/BF02834268. [DOI] [PubMed] [Google Scholar]
- 14.Parry GJ. Sensory neuropathy with low dose pyridoxine. Neurology. 1985;35:1466–1468. doi: 10.1212/wnl.35.10.1466. [DOI] [PubMed] [Google Scholar]
- 15.Windebank AJ, Low PA, Blexrud MD, Schmelzer JD, Schaumburg HH. Pyridoxine neuropathy in rats: specific degeneration of sensory axons. Neurology. 1985;35:1617–1622. doi: 10.1212/wnl.35.11.1617. [DOI] [PubMed] [Google Scholar]
- 16.Berger AR, Schaumburg HH, Schroeder C, Apfel S, Reynolds R. Dose response, coasting, and differential fiber vulnerability in human toxic neuropathy: a prospective study of pyridoxine neurotoxicity. Neurology. 1992;42:1367–1370. doi: 10.1212/wnl.42.7.1367. [DOI] [PubMed] [Google Scholar]
- 17.Schaumburg HH, Kaplan J, Windebank AJ, Vick N, Rasmus S, Pleasure D, et al. Sensory neuropathy from pyridoxine abuse: a new megavitamin syndrome. N Eng J Med. 1983;309:445–448. doi: 10.1056/NEJM198308253090801. [DOI] [PubMed] [Google Scholar]
- 18.Malmgren R, Collins A, Nilsson CG. Platelet serotonin uptake and effects of vitamin B6-treatment in premenstrual tension. Neuropsychobiology. 1987;18:83–86. doi: 10.1159/000118398. [DOI] [PubMed] [Google Scholar]
- 19.Jadad A, Moore M, Carrol D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomised clinical trials; is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 20.Computer programs for epidemiologic analysis: PEPI version 2. Gahlinger PM and Abramson JH. 2. Stone Mountain, GA: USD, 1995.
- 21.Hasselblad V, Hedges LV. Meta-analysis of screening and diagnostic tests. Psychol Bull. 1995;117:167–178. doi: 10.1037/0033-2909.117.1.167. [DOI] [PubMed] [Google Scholar]
- 22.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stokes J, Mendels J. Pyridoxine and premenstrual tension. Lancet. 1972;i:1177–1178. doi: 10.1016/s0140-6736(72)91399-2. [DOI] [PubMed] [Google Scholar]
- 24.Colin C. Studies on the treatment of mastalgia. Rev Med Brux. 1982;3:605–609. [PubMed] [Google Scholar]
- 25.Barr W. Pyridoxine supplements in the premenstrual syndrome. Practitioner. 1984;228:425–427. [PubMed] [Google Scholar]
- 26.Williams MJ, Harris RI, Dean BC. Controlled trial of pyridoxine in the premenstrual syndrome. J Int Med Res. 1985;13:174–179. doi: 10.1177/030006058501300305. [DOI] [PubMed] [Google Scholar]
- 27.Hagen I, Nesheim BI, Tuntland T. No effect of vitamin B-6 against premenstrual tension. A controlled clinical study. Acta Obstet Gynecol Scand. 1985;64:667–670. doi: 10.3109/00016348509158211. [DOI] [PubMed] [Google Scholar]
- 28.Chakmakjian ZH, Higgins CE, Abraham GE. The effect of a nutritional supplement, Optivite for women, on premenstrual tension syndromes. J Appl Nutr. 1985;37:12–17. [Google Scholar]
- 29.Smallwood J, Ah-Kye D, Taylor I. Vitamin B6 in the treatment of pre-menstrual mastalgia. Br J Clin Pract. 1986;40:532–533. [PubMed] [Google Scholar]
- 30.Stewart A. Clinical and biochemical effects of nutritional supplementation on the premenstrual syndrome. J Reprod Med. 1987;32:435–441. [PubMed] [Google Scholar]
- 31.Kendall KE, Schnurr PP. The effects of vitamin B6 supplementation on premenstrual symptoms. Obstet Gynecol. 1987;70:145–149. [PubMed] [Google Scholar]
- 32.Doll H, Brown S, Thurston A, Vessey M. Pyridoxine (vitamin B6) and the premenstrual syndrome: a randomized crossover trial. J R Coll Gen Pract. 1989;39:364–368. [PMC free article] [PubMed] [Google Scholar]
- 33.London RS, Bradley L, Chiamori NY. Effect of a nutritional supplement on premenstrual symptomatology in women with premenstrual syndrome: a double blind longitudinal study. J Am Coll Nutr. 1991;10:494–499. doi: 10.1080/07315724.1991.10718176. [DOI] [PubMed] [Google Scholar]
- 34.Kerr GD. The management of the premenstrual syndrome. Curr Med Res Opin. 1977;4:29–34. [Google Scholar]
- 35.Day JB. Clinical trials in the premenstrual syndrome. Curr Med Res Opin. 1979;6:40–45. [Google Scholar]
- 36.Abraham GE, Hargrove JT. Effect of vitamin B6 on premenstrual symptomology in women with premenstrual tension syndromes: a double blind crossover study. Infertility. 1980;3:155–165. [Google Scholar]
- 37.Mattes JA, Martin D. Pyridoxine in premenstrual depression. Hum Nutr Appl Nutr. 1982;36:131–133. [PubMed] [Google Scholar]
- 38.Goei GS, Abraham GE. Effect of a nutritional supplement, Optivite, on symptoms of premenstrual tension. J Reprod Med. 1983;28:527–531. [PubMed] [Google Scholar]
- 39.Fuchs N, Hakim M, Abraham GE. The effect of a nutritional supplement, Optivite for women, on premenstrual tension syndromes. J Appl Nutr. 1985;37:1–11. [Google Scholar]
- 40.Brush MG, Bennett T, Hansen K. Pyridoxine in the treatment of premenstrual syndrome: a retrospective survey in 630 patients. Br J Clin Pract. 1988;42:448–452. [PubMed] [Google Scholar]
- 41.Brush MG. Vitamin B6 treatment of premenstrual syndrome. In: Leklem JE, Reynolds RD, editors. Clinical and physiological applications of vitamin B6. New York: Liss; 1988. pp. 363–379. . (Current topics in nutrition and disease, vol 19). [Google Scholar]
- 42.Van den Berg H, Schrijver J, Bruinse HW, van der Ploeg HM. Vitamin B6 and premenstrual syndrome. Voeding. 1989;50:58–62. [Google Scholar]
- 43.Berman MK, Taylor ML, Freeman E. Vitamin B-6 in premenstrual syndrome. J Am Diet Assoc. 1990;90:859–861. [PubMed] [Google Scholar]
- 44.De Souza MC, Walker AF, Bolland KM, Robinson PA. A synergistic effect of magnesium and vitamin B6 supplementation for the relief of symptoms of the premenstrual syndrome (PMS) Proc Nutr Soc. 1996;56:75A. [Google Scholar]
- 45.Harrison W, Endicott J, Rabkin JG, Nee J. Treatment of dysphoric changes: clinical outcome and methodological implications. Psychol Bull 1984;118-22. [PubMed]
- 46.Blue J, Harman J. Mastalgia review: St Marks Breast Centre. NZ Med J. 1998. p. 111. :34-7. [Google Scholar]
- 47.Villegas-Salas E, Ponce dL, Juarez-Perez MA, Grubb GS. Effect of vitamin B6 on the side effects of a low-dose combined oral contraceptive. Contraception. 1997;55:245–248. doi: 10.1016/s0010-7824(97)00005-x. [DOI] [PubMed] [Google Scholar]
- 48.Bernstein AL. Vitamin B6 in clinical neurology. Ann NY Acad Sci. 1990;585:250–260. doi: 10.1111/j.1749-6632.1990.tb28058.x. [DOI] [PubMed] [Google Scholar]
- 49.Stewart JW, Harrison W, Quitkin F, Baker H. Low B6 levels in depressed outpatients. Biol Psychiatry. 1984;19:613–616. [PubMed] [Google Scholar]
- 50.Adams PW, Rose DP, Folkard J, Wynn V, Seed M, Strong R. Effects of pyridoxine hydrochloride upon depression associated with oral contraceptives. Lancet 1973;:897-904. [PubMed]
- 51.Winston F. Oral contraceptives, pyridoxine, and depression. Am J Psychiatry. 1973;130:1217–1221. doi: 10.1176/ajp.130.11.1217. [DOI] [PubMed] [Google Scholar]
- 52.Folkers K, Ellis J. Successful therapy with vitamin B6 and vitamin B2 of the carpal tunnel syndrome and need for determination of RDAs for vitamins B6 and B2 for disease states. Ann NY Acad Sci 1989;295-301. [DOI] [PubMed]