Abstract
Gastrin-17-Gly (G17-Gly) has been shown to bind to non-CCK nanomolar and micromolar affinity sites on DLD-1 and HT-29 human colonic carcinoma cells and to stimulate cellular proliferation. However, in previous studies, we showed that C-terminal truncation of the gastrin-17 (G17) to the G17 analog G17(1–12) and then to G17(1–6)-NH2 did not remove the ability to bind to DLD-1 cells or to activate proliferation. This implies that residues and/or structural motifs required for bioactivity at these receptors rest in the N-terminal region of G17. In this work, radioligand binding studies conducted with further C-terminally truncated analogs revealed that sequences as short as G17(1–4) still bind to a single receptor with micromolar affinity. Additionally, cell proliferation assays showed that G17(1–12) stimulates proliferation of DLD-1 cells, as of HT-29 cells, but the sequences shorter than G17(1–6)-NH2, including nonamidated G17(1–6), were incapable of stimulating proliferation. These observations indicate that the tetrapeptide pGlu-Gly-Pro-Trp is the minimum N-terminal sequence for binding to the probable growth-promoting site on DLD-1 cells. Since analogs shorter than G17(1–6) are able to bind the receptor, these peptides may be of use for developing selective antagonists.
1. Introduction
While it is accepted that gastrin-17 (G17) (pGlu-Gly-Pro-Trp-Leu-Glu-Glu-Glu-Glu-Glu-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2) has a role in the development and growth of gastric cancers, consensus has not been reached regarding its effect on colon cancers. Recent research has suggested that elevated gastrin levels in the circulation do not play a role in cancer promotion in the colon [8, 15–16, 28, 30, 35], and that CCK2 receptors, responsible for the mediation of gastric acid secretion and epithelial growth promoting effects of G17, are largely absent from the colon and thus do mediate such effects [5, 12–14, 31–32]. Additionally, it has been found that processing intermediates in the formation of G17 including progastrin and G17-Gly are secreted by colon cancer cells and are more frequently present in the colon than is G17 [9, 20, 27]. These intermediates have been shown to stimulate the growth of the colonic mucosa in vitro and in rats [4, 10, 21, 29, 34–35], and to stimulate the proliferation of normal and neoplastic colon cells not expressing CCK2 receptors [6, 18, 22, 33, 36].
The growth-promoting effects of G17-Gly have been shown to be mediated by a putative, non-CCK receptor by several groups, through a mechanism not involving the C-terminal tetrapeptide sequence Trp-Met-Asp-Phe-NH2 of G17, which is essential for binding and activation of the CCK2 receptor [3, 7,23–24,26, 37, 39–40]. Nanomolar and micromolar affinity receptors for G17-Gly on primary tissues and on cultured cell lines have been detected [17, 19, 25, 33, 36, 38, 41]. In our previous study we demonstrated the simultaneous presence of G17-Gly and G17 nanomolar and micromolar binding sites on the DLD-1 and HT-29 human colon cancer lines by performing radioligand binding assays employing a wide concentration range of unlabeled G17-Gly [1]. Subsequently it was shown that these two sites are responsible for a biphasic growth effect when DLD-1 cells are treated with G17-Gly. Further studies revealed that both C-terminal analogs [Leu15]G17(6–17)-Gly and [Leu15]G17(11–17)-Gly bind to a single site on DLD-1 cells with close to micromolar affinity, while the N-terminal analog G17(1–12) stimulates nonbiphasic proliferation of HT-29 cells [2]. These results suggest that the N-terminal region of G17 is essential for binding and activation of a nanomolar affinity receptor that mediates the growth-promoting effects of the peptide.
In our previous study, we showed that G17(1–12) binds to two sites on DLD-1 cells with similar affinities to that of G17-Gly[11]. We further truncated G17 to produce G17(1–6)-NH2 to find that it binds to DLD-1 cells at a single site with micromolar affinity and it also can stimulate cell proliferation. Thus, neither the C-terminal tetrapeptide of G17 which is essential for binding the CCK2 receptor nor even the full pentaglutamyl sequence of the central portion of the peptide is necessary for binding or activation of the putative receptor on the cancer cells. In this work, we examined the growth effect of G17(1–12) on DLD-1 cells, as well as further truncated G17 (Table 1.) to determine the minimal N-terminal sequence required for binding and activation of the putative growth-promoting receptor. We also examined the effect of C-terminal capping with an amide group on the ability of these analogs to bind and activate the receptor.
Table 1.
Synthetic N-terminal analogs of G17.
| Peptide | Sequence |
|---|---|
| G17(1–12) | pGlu-Gly-Pro-Trp-Leu-(Glu)5-Ala-Tyr |
| G17(1–6) | pGlu-Gly-Pro-Trp-Leu-Glu |
| G17(1–5) | pGlu-Gly-Pro-Trp-Leu |
| G17(1–5)-NH2 | pGlu-Gly-Pro-Trp-Leu-NH2 |
| G17(1–4) | pGlu-Gly-Pro-Trp |
| G17(1–4)-NH2 | pGlu-Gly-Pro-Trp-NH2 |
| G17(1–3)-NH2 | pGlu-Gly-Pro-NH2 |
2. Materials and methods
2.1. Solid phase peptide synthesis resins and amino acids
Rink Amide AM, Fmoc-Trp(Boc)-Wang, and Fmoc-Tyr(OtBu)-Wang resins were from NovaBioChem (San Diego, CA, USA). Fmoc-Glu(OtBu)-Wang and Fmoc-Leu-Wang resins were from Advanced ChemTech (Louisville, KY, USA). H-Tyr(OtBu)-HMPB-ChemMatrix resin was from Matrix Innovations (Montreal, Quebec, Canada). Fmoc-Gly-OH, Fmoc-Leu-OH, Fmoc-Trp(Boc)-OH, Fmoc-Pro-OH, Fmoc-Ala-OH, and pGlu-OH were from Advanced ChemTech. Fmoc-Glu(OtBu)-OH and Fmoc-Tyr(OtBu)-OH were from NovaBioChem.
2.2. Peptide synthesis, cleavage, and purification reagents
DMF, DMSO, NMP, DCM, acetone, methanol, ammonium bicarbonate, acetonitrile, sodium hydroxide, and sodium chloride were from Fisher (Fair Lawn, NJ, USA). DIEA and HBTU were from Chem-Impex (Wood Dale, IL). TIS, thioanisole, EDT, piperidine, and diethyl ether were from Sigma-Aldrich (Milwaukee, WI, USA). TFA was from Sigma-Aldrich (peptide synthesis grade), Chem-Impex (peptide synthesis grade), and Pierce (Rockford, IL) (HPLC grade).
2.3. Cell culture and radioligand binding materials and reagents
DLD-1 human colon carcinoma cells were from American Type Tissue Collection (ATCC) (Manassas, VA, USA). RPMI-1640 (with L-glutamine) growth media, Cellstripper non-enzymatic cell dissocation solution and antibiotic/antimycotic solution (10,000 I.U. penicillin, 10,000 μg/mL streptomycin, and 25 mg/mL amphotericin B) were from Mediatech Inc. (Herndon, VA, USA). Trypsin-EDTA (0.25% trypsin and 1.0 M EDTA) and fetal bovine serum were from Atlanta Biologicals (Lawrenceville, GA, USA). HEPES, Tris-HCl, potassium chloride, potassium phosphate (monobasic), and sodium phosphate (dibasic) were from Fisher. Tris, magnesium chloride, sodium chloride, potassium phosphate (dibasic), calcium chloride, D-(+)-glucose, and trypan blue were from Sigma Aldrich. [Leu15]G17-Gly was prepared as previously reported [1].
2.4. Radiolabeling of G17(1–12)
Custom iodination of G17(1–12) and subsequent HPLC purification was carried out by Peninsula Laboratories using the chloramine T method (Peninsula Laboratories, San Carlos, CA, USA). The specific activity of [125I-Tyr12]G17(1–12) was 782–1832 Ci/mmol.
2.5. Peptide synthesis and cleavage
Peptides (Table 1.) were synthesized, as previously described [11], on an Advanced ChemTech “Apogee” synthesizer in 0.25 mmol scale using N-α-Fmoc protected amino acids and the appropriate polystyrene resins: a Rink Amide AM resin for all amidated, and the appropriate Wang linker resins for all non-amidated peptides. G17(1–12) was additionally synthesized using a H-Tyr(OtBu)-HMPB-ChemMatrix resin. The side chains of Tyr and Glu were t-Bu protected, while the side chain of Trp was t-Boc protected.
Peptides were cleaved from their respective resins using thioanisole / EDT / water / phenol / TIS / TFA (5:2.5:5:5:1:81.5, v/v/v/v/v) cleavage cocktail. This mixture was prepared and chilled to −10 °C before use. The resin was agitated in the mixture with a magnetic stirrer for 15 minutes at 0 °C, and then for 105 minutes at room temperature. After cleavage, the peptide was precipitated by adding ice cold diethyl ether. The peptide and resin were collected by filtration using a medium porosity sintered glass filter, washed several times with ice-cold diethyl ether, and dried under vacuum. The peptide was then dissolved in neat TFA. The solution was filtered and the volume was reducted to 1–2 mL using a Buchi rotary evaporator (Buchi, Flawil, Switzerland). The peptide was again precipitated by adding 100-fold excess (by volume) of ice-cold diethyl ether followed by incubation at 4 °C for 30 minutes. The peptide was collected by filtration using a fine porosity sintered glass filter.
2.6. Peptide purification and characterization
Peptides were analyzed and purified on a dual pump HPLC apparatus (Gilson, Middleton, WI, USA) as previously described [2, 11]. For analytical HPLC either a Vydac 218TP54 column (C18, 5 μm particle size, 4.6 mm × 250 mm) (Grace Vydac, Hesperia, CA, USA) or a Phenomenex 00G-4252-Y0 “Luna” column (C18, 5 μm particle size, 3 mm × 250 mm) (Phenomenex, Torrance, CA, USA) were used at flow rates of 1 mL/min and 0.5 mL/min, respectively, with an initial injection concentration of 1 mg/mL. For purification, a semi-preparative Phenomenex 00G-4252-N0 “Luna” column (C18, 5 μm particle size, 10 mm × 250 mm) was used at a flow rate of 5 mL/min, with an initial injection concentration of 4 mg/mL. The molecular weights of peptides were confirmed by ES-MS using a SCIEX API150EX mass spectrometer (Perkin-Elmer Life Sciences, Boston, MA, USA). Peptides were obtained at greater than 95% purity.
2.7. Cell culture
DLD-1 human colon carcinoma cells were grown in monolayer culture in RPMI 1640 media containing 10% fetal bovine serum and 1% (v/v) of antibiotic/mycotic solution (Gibco, Invitrogen Corporation, Carlsbad, CA, USA). This medium was changed every 2–3 days. Cells were incubated at 37 °C in a 5% CO2 atmosphere and maintained at subconfluent levels. Cultures were passaged by detachment of cells with trypsin-EDTA and reseeding cells 1:4 to 1:10. Cells after passage 25–30 were harvested at 70–90% confluency.
2.8. Radioligand binding assays with intact cells
The binding of peptides in competition with [125I-Tyr12]G17(1–12) to receptors on DLD-1 cells was determined. Cells were detached from culture flasks using a non-enzymatic cell dissociation solution (Cellstripper). Cells (3–4 million cells/well) were incubated with shaking for 5 h at 28 °C in a buffer solution (pH 7.4; 300 μL/) containing 20 mM HEPES, 1 mM calcium chloride, 5 mM potassium chloride, 2.2 mM magnesium chloride, 120 mM sodium chloride and 6 mg/mL glucose with 50 pM [125I-Tyr12]G17(1–12) and 10−12 M to 10−4 M test peptide. Suspensions were rapidly filtered through glass fiber filters (pore size 3.1 mm; Pall Life Sciences, East Hills, NY, USA) that had been presoaked at 4 °C in 50 mM Tris-HCl buffer (pH 7.4) containing 0.1% bovine serum albumin using a Brandel cell harvester (Biomedical R&D Laboratories, Gaithersburg, MD, USA). Filters were washed twice with ice-cold 50 mM Tris-HCl buffer, air-dried and were transferred to scintillation tubes. Bound radioactivity was counted using a Wallac model 1277 Gammamaster automated gamma counter (EG&G, Inc., Wellesley, MA, USA) with a counting efficiency of 50%. Non-specific binding was determined in the presence of 10−4 M G17(1–12).
2.9. Cell proliferation assays
Cells (7–10 × 103) were seeded in culture medium (400 μL) containing 10% fetal calf serum in 48-well plates and allowed to attach and reach 40–50% confluence. The medium was then replaced with serum-free growth medium and cells were starved for 24 h. 10−12 M to 10−5 M test peptide was added to the inner 24 wells of the 48-well plates, and cells were allowed to grow for 72 h. Medium was removed and the cells were detached using trypsin-EDTA and counted visually using a hemocytometer under a brightfield microscope.
2.10. Statistical analysis
All data were analyzed using GraphPad Prism software (GraphPad Software Inc., San Diego, CA, USA). Comparison of fit for one and two site binding was determined using the F-test. The statistical significance of cell proliferation data was determined using one-way ANOVA with the Dunnett post-test.
3. Results
3.1. Competition binding to intact DLD-1 cells
[125I-Tyr12]G17(1–12) bound to DLD-1 cells in a saturable and displaceable manner. Total binding represented less than 1% of the total added radioligand in all assays.
3.1.1. G17(1–6)
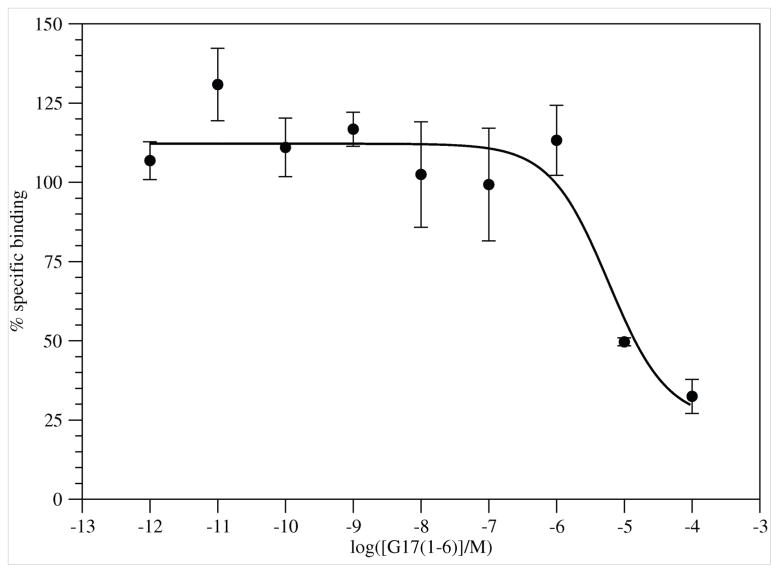
G17(1–6) displaced [125I-Tyr12]G17(1–12) in a dose dependent manner (Figure 1). Specific binding was 44–52% of total binding. The peptide failed to displace all radioligand. Nonlinear regression of the binding results fit the data to a single-site model. The IC50 value for binding was 5.9×10−6 M.
Figure 1.
Binding of G17(1–6) (●) to DLD-1 cells. Results are from 3 experiments with 6 replicates for each concentration. Error bars are ± SEM.
3.1.2 G17(1–5)-NH2 and G17(1–5)
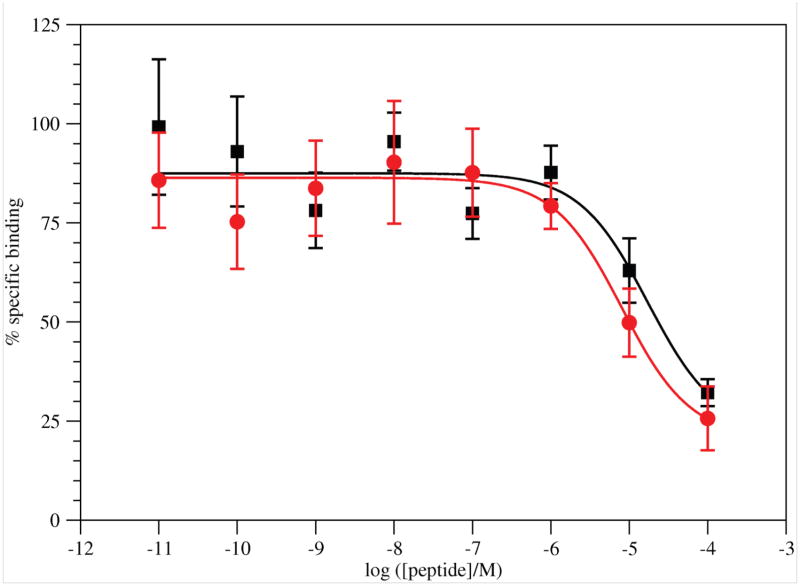
G17(1–5)-NH2 displaced [125I-Tyr12]G17(1–12) in a dose dependent manner (Figure 2). Specific binding was 47–67% of total binding. The peptide failed to displace all radioligand. Nonlinear regression of the binding results fit the data to a single-site model. The IC50 value for binding was 1.7×10−5 M.
Figure 2.
Binding of G17(1–5)-NH2 (■; Results are from 7 experiments with 18 replicates for each concentration.) and G17(1–5) ( Results are from 5 experiments with 16 replicates for each concentration.) to DLD-1 cells. Error bars are ± SEM.
Results are from 5 experiments with 16 replicates for each concentration.) to DLD-1 cells. Error bars are ± SEM.
The nonamidated peptide again displaced [125I-Tyr12]G17(1–12) in a dose dependent manner, but once again, not all radioligand was displaced. Specific binding was 39–64% of total binding. Nonlinear regression of the binding results fit the data to a single-site model. The IC50 value for binding was 8.1×10−6 M.
3.1.3 G17(1–4)-NH2 and G17(1–4)
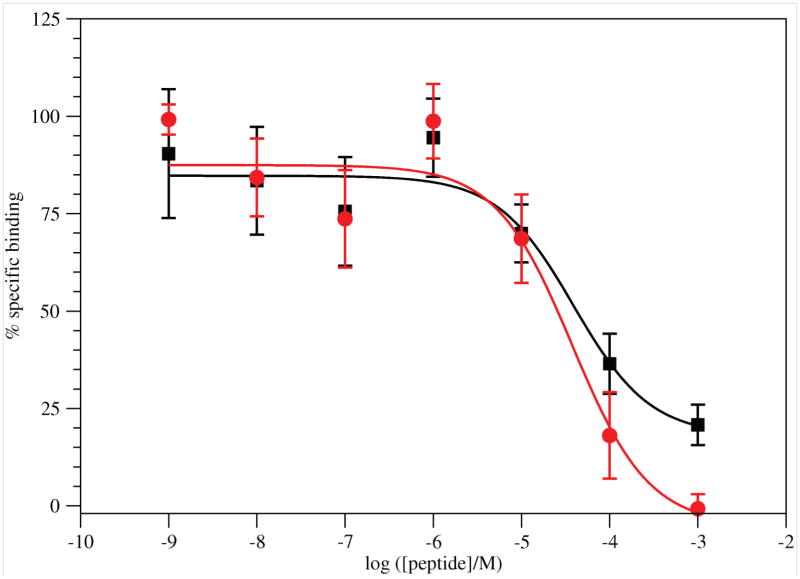
G17(1–4)-NH2 displaced [125I-Tyr12]G17(1–12) in a dose dependent manner (Figure 3). Not all radioligand was displaced. Nonlinear regression of the binding results fit the data to a single-site model. The IC50 value for binding was 3.9×10−5 M.
Figure 3.
Binding of G17(1–4)-NH2 (■; Results are from 7 experiments with 15 replicates for each concentration.) and G17(1–4) ( ; Results are from 4 experiments with 10 replicates for each concentration.) to DLD-1 cells. Error bars are ± SEM.
; Results are from 4 experiments with 10 replicates for each concentration.) to DLD-1 cells. Error bars are ± SEM.
The nonamidated peptide again displaced [125I-Tyr12]G17(1–12) in a dose dependent manner, but all radioligand was displaced. Specific binding was 59–71% of total binding. Nonlinear regression of the binding results fit the data to a single-site model. The IC50 value for binding was 3.9×10−5 M.
3.1.4 G17(1–3)-NH2
G17(1–3)-NH2 did not displace [125I-Tyr12]G17(1–12) at concentrations as high as 10−3 M.
3.2 Cell proliferation assays
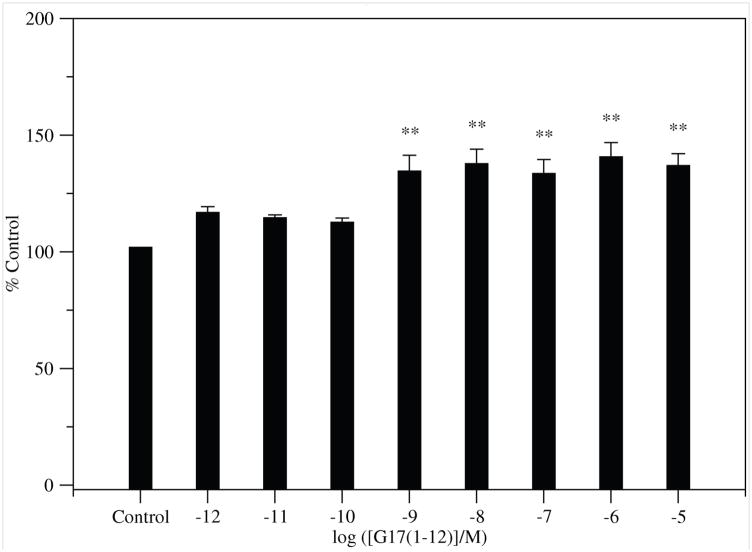
G17(1–12) stimulated significant proliferation of DLD-1 cells in a non-biphasic, dose-dependent manner. (Figure 4). N-terminal G17 analogs shorter than G17(1–6) did not stimulate proliferation of DLD-1 cells at concentrations as high as 10−5 M in 8 experiments with 4 replicates for each concentration of peptides.
Figure 4.
Proliferation of DLD-1 cells treated with G17(1–12). Error bars are ± SEM. (ANOVA: Dunnett post-test: * p < 0.05; ** p < 0.01; *** p < 0.001).
4. Discussion
4.1 Radioligand binding assays with while DLD-1 cells
G17(1–6) bound to and displaced [125I-Tyr12]G17(1–12) from a single site on DLD-1 cells. The analog bound a receptor with low (micromolar) affinity, and failed to displace all radioligand, resembling G17(1–6)-NH2 but with slightly lower affinity [11]. The low binding might be either to the G17-Gly low affinity site, or to the G17-Gly high affinity site. [Leu15]G17(6–17)-Gly and [Leu15]G17(11–17)-Gly also bound one site with similarly low affinity and displacement of receptors [2].
Sequential C-terminal truncation of G17(1–6) resulted in analogs with decreasing affinities for the receptor. G17(1–5) and G17(1–5)-NH2 have slightly lower binding affinity than the G17(1–6) analogs, and similarly fail to displace all radioligand.
G17(1–4) and G17(1–4)-NH2 had identical IC50 values for the receptor, at 3.9×10−5 M. Another drop in affinity was seen, for a total of about an order of magnitude drop from the G17(1–6) set of analogs. The concentration range was extended to 10−3 M because of the extremely low affinity exhibited by the analogs. Strangely, here G17(1–4)-NH2 shows similar behavior to the longer analogs in displacing about ¾th of the specifically bound radioligand, but G17(1–4) displaces all specifically bound radioligand. It is not clear what this means, given the incomplete displacement of radioligand by all of the other unlabeled analogs, especially G17(1–4)-NH2 at concentrations as high as 10−3 M. It is presumably possible that the analogs still bind two sites similar to G17(1–12), though this seems highly unlikely given the binding of [Leu15]G17(6–17) and [Leu15]G17(11–17). Additionally, the short length of the analogs seems to belie the possibility that they would be able to bind to two separate sites, except perhaps in the case that high and low affinity sites are simply conformational states of the same receptor, such as with GPCRs bound and unbound to their accompanying G-proteins.
The failure of G17(1–3)-NH2 to displace specifically bound radioligand at concentrations as high as 10−3 M indicates that the Trp residue is essential for binding to the receptor. Thus, the N-terminal region contains a four residue sequence which is essential for binding to the putative G17-Gly receptor, much like the C-terminal tetragastrin sequence is essential for binding CCK2-R. This finding is in agreement with our previous results [1], which showed that [Leu15]G17-Gly and [Leu15]G17 displace tritiated [Leu15]G17-Gly with similar affinities at both binding sites on DLD-1 membranes. Clearly, unlike CCK2-R, the putative G17-Gly receptor or receptors do not rely on tetragastrin and the amidated C-terminus for binding, which explains the much greater ability of G17-Gly to bind and activate the receptor(s), as opposed to CCK2-R, upon which G17-Gly has virtually no effect.
4.2 Proliferation effect of analogs on DLD-1 cells
The stimulation of proliferation of DLD-1 cells by G17(1–12) is less than the maximum stimulation effected by [Leu15]G17-Gly or [Leu15]G17 on DLD-1 cells seen previously [1]. Therefore, it is possible that the C-terminal region of both G17 and G17-Gly acts to stabilize any structures important for binding that exist in the N-terminal region of G17(1–12).
The non-biphasic stimulation of proliferation by G17(1–12) is resembles that that of the effect of the peptide on HT-29 cells [2]. The revelation that G17(1–12) binds both high and low affinity sites on DLD-1 cells indicates that this sequence is an antagonist to the low affinity (proliferation retarding) receptor site. It appears that he two receptor sites work in tandem to promote and retard proliferation, and that the residues responsible for binding the low affinity site rest in the C-terminal portion of the peptide.
Contrary to the results seen with its amidated form [11], G17(1–6) did not stimulate proliferation of DLD-1 cells at concentrations as high as 10−5 M. This seems at variance with the close binding affinities. However, as C-terminal amidation has been shown to be essential to binding of CCK2-R, C-terminal capping by an amide group may also be necessary to stabilize this short analog to permit activation of the putative receptor, in lieu of the longer sequence of G17(1–12) and the full G17.
All analogs shorter than G17(1–6) were unable to stimulate proliferation of DLD-1 cells. These results show that a peptide length of at least six residues from the N-terminus, along with C-terminal amidation, is required to form structures important to activation of the receptor. Since analogs shorter than G17(1–6) are able to bind to the receptor, these peptides may be of use for developing selective antagonists.
Acknowledgments
This work was supported by NIH-INBRE grant (1 P20 RR16469) and the Carpenter Endowed Chair in Biochemistry, Creighton University.
Abbreviations
- Boc
butyloxycarbonyl
- CCK
cholecystokinin
- DCM
dichloromethane
- DIEA
N,N-diisopropylethylamine
- DMF
N,N-dimethylformamide
- DMSO
dimethyl sulfoxide
- EDT
1,2-ethanedithiol
- EDTA
ethylenediaminetetraacetic acid
- ESI-MS
electrospray ionization mass spectrometry
- Fmoc
9-fluorenylmetoxycarbonyl
- G17
gastrin-17
- G17-Gly
gastrin-17-Gly
- HBTU
O-benzotriazolyl-N,N,N′,N′-tetramethyluronium hexafluorophosphate
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- HPLC
high performance liquid chromatography
- NMP
N-methylpyrrolidinone
- OtBu
O-t-butyl
- pGlu
pyroglutamic acid
- TFA
trifluoroacetic acid
- TIS
triisopropylsilane
- Tris
tris(hydroxymethyl)aminomethane
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahmed S, Budai B, Heredi-Szabo K, Farkas J, Toth G, Murphy RF, Lovas S. High and low affinity receptors mediate growth effects of gastrin and gastrin-Gly on DLD-1 human colonic carcinoma cells. FEBS Lett. 2004;556:199–203. doi: 10.1016/s0014-5793(03)01408-x. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed S, Murphy RF, Lovas S. Importance of N- and C-terminal regions of gastrin-Gly for preferential binding to high and low affinity gastrin-Gly receptors. Peptides. 2005;26:1207–1212. doi: 10.1016/j.peptides.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed S, Wibowo F, Gembitsky DS, Bozso Zs, Lovas S, Murphy RF. Importance of the C-terminal phenylalanine of gastrin for binding to the human CCK2 receptor. J Pept Res. 2001;58:332–337. doi: 10.1034/j.1399-3011.2001.00942.x. [DOI] [PubMed] [Google Scholar]
- 4.Aly A, Shulkes A, Baldwin GS. Short term infusion of glycine-extended (17) stimulates both proliferation and formation of aberrant crypt foci in rat colonic mucosa. Int J Cancer. 2001;94:307–313. doi: 10.1002/ijc.1483. [DOI] [PubMed] [Google Scholar]
- 5.Aly A, Shulkes A, Baldwin GS. Gastrins, cholecystokinins and gastronintestinal cancer. Biochimica et Biophysica Acta. 2004;1704:1–10. doi: 10.1016/j.bbcan.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Baldwin GS. The role of gastrin and cholecystokinin in normal and neoplastic gastrointestinal growth. J Gastroenterol Hepatol. 1995;10:215–232. doi: 10.1111/j.1440-1746.1995.tb01083.x. [DOI] [PubMed] [Google Scholar]
- 7.Black JW, Kalindjian SB. Gastrin agonists and antagonists. Pharmacol Toxicol. 2002;91:275–281. doi: 10.1034/j.1600-0773.2002.910602.x. [DOI] [PubMed] [Google Scholar]
- 8.Chen D, Destree M, Hakanson R, Willems G. Endogenous hypergastrinemia does not promote growth of colonic mucosa or of transplanted colon adenocarcinoma in rats. Eur J Gastroenterol Hepatol. 1998;10:293–299. doi: 10.1097/00042737-199804000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Ciccotosto G, McLeish A, Hardy K, Shulkes A. Expression processing and secretion of gastrin in patients with colorectal carcinoma. Gastroenterology. 1995;109:1142–1153. doi: 10.1016/0016-5085(95)90572-3. [DOI] [PubMed] [Google Scholar]
- 10.Cobb S, Wood T, Ceci J, Varro A, Velasco M, Singh P. Intestinal expression of mutant and wild-type progastrin significantly increases colon carcinogenesis in response to azooxymethane in transgenic mice. Cancer. 2004;100:1311–1323. doi: 10.1002/cncr.20094. [DOI] [PubMed] [Google Scholar]
- 11.Copps J, Ahmed S, Murphy RF, Lovas S. Gastrin 1–6 promotes growth of colon cancer cells through non-CCK receptors. Peptides. 2007;28:632–635. doi: 10.1016/j.peptides.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Dufresne M, Seva C, Fourmy D. Cholecystokinin and gastrin receptors. Physiol Rev. 2006;86:805–847. doi: 10.1152/physrev.00014.2005. [DOI] [PubMed] [Google Scholar]
- 13.Fontana MG, Donato F, Villanacci V, Ghirardi M, Moneghini D, Di Betta E, Salerni B. Inhibitory effect of a gastrin receptor antagonist, CR 2495, on 1,2-dimethylhydrazine-induced colorectal cancer in mice. Eur Surg Res. 1999;31:406–411. doi: 10.1159/000008719. [DOI] [PubMed] [Google Scholar]
- 14.Fontana MG, Moneghini D, Villanacci V, Donato F, Rindi G. Effect of cholecystokinin-B gastrin receptor blockade on chemically induced colon carcinogenesis in mice: follow up at 52 weeks. Digestion. 2002;65:35–40. doi: 10.1159/000051929. [DOI] [PubMed] [Google Scholar]
- 15.Hakanson R, Axelson J, Ekman R, Sundler F. Hypergastrinemia evoked by omprazole stimulates growth of gastric mucosa but not of pancreas or intestines in hamster, guinea pig and chicken. Regul Pept. 1988;23:105–115. doi: 10.1016/0167-0115(88)90426-0. [DOI] [PubMed] [Google Scholar]
- 16.Hakanson R, Blom H, Carlsson E, Larsson H, Ryberg B, Sundler F. Hypergastrinemia produces trophic effects in stomach but not in pancreas and intestine. Regul Pept. 1986;13:225–233. doi: 10.1016/0167-0115(86)90041-8. [DOI] [PubMed] [Google Scholar]
- 17.Higashide S, Gomez G, Greeley GH, Jr, Townsend CM, Jr, Thompson JC. Glycine-extended gastrin potentiates gastrin-stimulated gastric acid secretion in rats. Am J Physiol Gastrointest Liver Physiol. 1996;270:G220–224. doi: 10.1152/ajpgi.1996.270.1.G220. [DOI] [PubMed] [Google Scholar]
- 18.Hollande F, Imdahl A, Mantamadiotis T, Ciccotosto GD, Shulkes A, Baldwin GS. Glycine-extended gastrin acts as an autocrine growth factor in a nontransformed colon cell line. Gastroenterology. 1997;113:1576–1588. doi: 10.1053/gast.1997.v113.pm9352860. [DOI] [PubMed] [Google Scholar]
- 19.Imdahl A, Mantamadiotis T, Eggstein S, Farthmann EH, Baldwin GS. Expression of gastrin, gastrin/CCK-B and gastrin/CCK-C receptors in human colorectal carcinomas. J Cancer Res Clin Oncol. 1995;121:661–666. doi: 10.1007/BF01218524. [DOI] [PubMed] [Google Scholar]
- 20.Kochman ML, Del Valle J, Dickinson CJ, Borland CR. Post-translational processing of gastrin in neoplastic human colonic tissues. Biochem Biophys Res Commun. 1992;189:1165–1169. doi: 10.1016/0006-291x(92)92326-s. [DOI] [PubMed] [Google Scholar]
- 21.Koh TJ, Dockray GJ, Varro A, Cahill RJ, Dangler CA, Fox JG, Wang TC. Overexpression of glycine-extended gastrin in transgenic mice results in increased colonic proliferation. J Clin Invest. 1999;103:1119–1126. doi: 10.1172/JCI4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Litvak DA, Hellmich MR, Iwase K, Evers BM, Martinez J, Amblard M, Townsend CM., Jr JMV1155: a novel inhibitor of glycine-extended progastrin-mediated growth of a human colon cancer in vivo. Anticancer Res. 1999;19:45–49. [PubMed] [Google Scholar]
- 23.Low CMR, Black JW, Broughton HB, Buck IM, Davies JMR, Dunstone DJ, Hull RAD, Kalindjian SB, McDonald IM, Pether MJ, Shankley NP, Steel KIM. Development of peptide 3D structure mimetics: rational design of novel peptoid cholecystokinin receptor antagonists. J Med Chem. 2000;43:3505–3517. doi: 10.1021/jm000937a. [DOI] [PubMed] [Google Scholar]
- 24.Magous R, Bali J, Moroder L, Previero A. Effect of Nin-formylation of the tryptophan residue on gastrin (HG-13) binding and on gastric acid secretion. Eur J Pharmacol. 1982;77:11–16. doi: 10.1016/0014-2999(82)90528-3. [DOI] [PubMed] [Google Scholar]
- 25.Mauss S, Niederau C, Hengels KJ. Effects of gastrin, proglumide, loxiglumide and L-365,260 on growth of human colon carcinoma cells. Anticancer Res. 1994;14:215–220. [PubMed] [Google Scholar]
- 26.Morley JS. Structure-function relationships in gastrin-like peptides. Proc R Soc Lond B Biol Sci. 1968;170:97–111. doi: 10.1098/rspb.1968.0028. [DOI] [PubMed] [Google Scholar]
- 27.Nemeth J, Taylor B, Pauwels S, Varro A, Dockray GJ. Identification of progastrin derived peptides in colorectal carcinoma extracts. Gut. 1993;34:90–95. doi: 10.1136/gut.34.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oscarson J, Veen H, Ross J, Malt R. Dimethylhydrazine induced colonic neoplasia: dissociation from endogenous gastrin levels. Surgery. 1982;91:525–530. [PubMed] [Google Scholar]
- 29.Ottewell PD, Varro A, Dockray GJ, Kirton CM, Watson AJ, Wang TC, Dimaline R, Pritchard DM. COOH-terminal 26-amino acid residues of progastrin are sufficient for stimulation of mitosis in murine colonic epithelium in vivo. Am J Physiol Gastrointest Liver Physiol. 2005;288:G541–G549. doi: 10.1152/ajpgi.00268.2004. [DOI] [PubMed] [Google Scholar]
- 30.Pinson DM, Havu N, Sztern MI, Mattsson H, Looney GA, Kimler BF, Hurwitz A. Drug-induced hypergastrinemia: absence of trophic effects on colonic carcinoma in rats. Gastroenterology. 1995;108:1068–1074. doi: 10.1016/0016-5085(95)90204-x. [DOI] [PubMed] [Google Scholar]
- 31.Reubi JC, Waser B, Schmassmann A, Laissue JA. Receptor autoradiographic evaluation of cholecystokinin, neurotensin, somatostatin and vasoactive intestinal peptide receptors in gastro-intestinal adenocarcinoma samples: where are they really located? Int J Cancer. 1999;81:376–386. doi: 10.1002/(sici)1097-0215(19990505)81:3<376::aid-ijc11>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 32.Schmitz F, Otte JM, Stechele HU, Reimann B, Banasiewicz T, Folsch UR, Schmidt WE, Herzig KH. CCK-2/gastrin receptors in human colorectal cancer. Eur J Clin Investig. 2001;31:812–820. doi: 10.1046/j.1365-2362.2001.00870.x. [DOI] [PubMed] [Google Scholar]
- 33.Singh P, Owlia A, Espeijo R, Dai B. Novel gastrin receptors mediate mitogenic effects of gastrin and processing intermediates of gastrin on Swiss 3T3 fibroblasts. Absence of detectable cholecystokinin CCK-A and CCK-B receptors. J Biol Chem. 1995;270:8429–8438. doi: 10.1074/jbc.270.15.8429. [DOI] [PubMed] [Google Scholar]
- 34.Singh P, Velasco M, Given R, Varro A, Wang TC. Progastrin expression predisposes mice to colon carcinomas and adenomas in response to a chemical carcinogen. Gastroenterology. 2000;119:162–171. doi: 10.1053/gast.2000.8527. [DOI] [PubMed] [Google Scholar]
- 35.Singh P, Velasco M, Given R, Wargovich M, Varro A, Wang TC. Mice overexpressing progastrin are predisposed for developing aberrant colonic crypt foci in response to AOM. Am J Physiol Gastroenterol Liver Physiol. 2000;278:G390–G399. doi: 10.1152/ajpgi.2000.278.3.G390. [DOI] [PubMed] [Google Scholar]
- 36.Stepan VM, Sawada M, Todisco A, Dickinson CJ. Glycine-extended gastrin exerts growth-promoting effects on human colon cancer cells. Mol Med. 1999;5:147–159. [PMC free article] [PubMed] [Google Scholar]
- 37.Tracy HJ, Gregory RA. Physiological properties of a series of synthetic peptides structurally related to gastrin I. Nature (London) 1964;204:935–938. doi: 10.1038/204935a0. [DOI] [PubMed] [Google Scholar]
- 38.Upp JR, Singh P, Townsend CM, Jr, Thompson JC. Clinical significance of gastrin receptors in human colon cancers. Cancer Res. 1989;49:488–492. [PubMed] [Google Scholar]
- 39.Weng JH, Blommaert AGS, Moizo L, Bado A, Ducos B, Bohme A, Garbay C, Roques BP. Role of N- and C-terminal substituents on the CCK-B agonist-antagonist pharmacological profile of Boc-Trp-Phg-Asp-Nal-NH2 derivatives. Bioorg Med Chem. 1996;4:563–573. doi: 10.1016/0968-0896(96)00050-8. [DOI] [PubMed] [Google Scholar]
- 40.Yabe Y, Morita A, Miura C, Kobayashi S, Baba Y. Synthesis and biological activity of tetragastrin analogues modifying the tryptophan residue. Chem Pharm Bull. 1977;10:2731–2734. doi: 10.1248/cpb.25.2731. [DOI] [PubMed] [Google Scholar]
- 41.Yang C-H, Ford J, Karelina Y, Shulkes A, Xiao S-D, Baldwin GS. Identification of a 70-kDa gastrin-binding protein on DLD-1 human colorectal carcinoma cells. Int J Biochem Cell Biol. 2001;33:1071–1079. doi: 10.1016/s1357-2725(01)00077-2. [DOI] [PubMed] [Google Scholar]