Abstract
Mammalian genomes encode only a small number of cuproenzymes. The many genes involved in coordinating copper uptake, distribution, storage and efflux make gene/nutrient interactions especially important for these cuproenzymes. Copper deficiency and copper excess both disrupt neural function. Using mice heterozygous for peptidylglycine α-amidating monooxygenase (PAM), a cuproenzyme essential for the synthesis of many neuropeptides, we identified alterations in anxiety-like behavior, thermoregulation and seizure sensitivity. Dietary copper supplementation reversed a subset of these deficits. Wildtype mice maintained on a marginally copper deficient diet exhibited some of the same deficits observed in PAM+/− mice and displayed alterations in PAM metabolism. Altered copper homeostasis in PAM+/− mice suggested a role for PAM in the cell type specific regulation of copper metabolism. Physiological functions sensitive to genetic limitations of PAM that are reversed by supplemental copper and mimicked by copper deficiency may serve as indicators of marginal copper deficiency.
Keywords: nutrition, thermogenesis, anxiety, seizure, neuropeptides, haploinsufficiency, PAM
INTRODUCTION
In both Menkes Disease, with low levels of copper, and Wilson's disease, with elevated levels of copper, neural function is impaired (Madsen and Gitlin, 2007; Schlief and Gitlin, 2006; Cunliffe et al., 2001; Tumer and Horn, 1997). These diseases are rare, caused by mutations in ATP7A and ATP7B, respectively. In addition to these two copper transporting P type ATPases, the uptake and tissue-specific distribution of dietary copper involves additional transporters and copper-binding chaperones (Madsen and Gitlin, 2007; Schlief and Gitlin, 2006). Menkes patients and mottled/brindled (ATP7aMo-br) mice, an animal model for Menkes Disease, exhibit increased susceptibility to seizures and neurodegeneration which can be partially reversed with copper supplementation (Sheela et al., 2005). Similar symptoms result from dietary copper deficiency; livestock consuming a copper-deficient diet develop progressive ataxic myelopathy (Madsen and Gitlin, 2007; Kumar, 2006). In humans, acquired copper deficiency can accompany long-term parenteral nutrition or malabsorption; in addition to hematological symptoms, sensory ataxia and spastic gait may occur (Madsen and Gitlin, 2007). Biomarkers that accurately reflect mild changes in copper status have not yet been established (Harvey and McArdle, 2008).
Copper-dependent enzymes, which evolved with the appearance of atmospheric molecular oxygen, are rare, with about a dozen in mammals (Critchton and Pierre, 2001; Ridge et al., 2008). Peptidylglycine α-hydroxylating monooxygenase (PHM) and dopamine β-monooxygenase (DBM) are homologous copper-dependent monooxygenases, essential for the synthesis of α-amidated peptides and norepinephrine/epinephrine, respectively. The biosynthesis of amidated peptides, conserved from Planaria to humans, is compromised in mottled/brindled mice (Asada et al., 2005; Prigge et al., 2000; Steveson et al., 2003; Niciu et al., 2007). ATP7A must interact with Atox1, a cytoplasmic copper chaperone, to transport Cu(I) from the cytoplasm into the secretory pathway (Madsen and Gitlin, 2007; Prohaska and Gybina, 2004; Puig and Thiele, 2002).
Mutations of either PAM or ATP7A affect the vascular system and thermoregulation (Niciu et al., 2006; Niciu et al., 2007; Bousquet-Moore et al., 2009; Czyzyk et al., 2005; Al-Bitar et al., 2005; Ozawa et al., 2002; Maury et al., 2007). Mice lacking PAM do not survive past E13.5; thin arterial walls and edema are apparent at E12.5 (Czyzyk et al., 2005). Mice with a single copy of the PAM gene are viable (Czyzyk et al., 2005; Bousquet-Moore et al., 2009). Along with mild glucose intolerance, PAM+/− mice showed deficits in peripheral vasoconstriction; supplemental copper reversed this deficit (Bousquet-Moore et al., 2009). By coupling our analysis of functional impairments in PAM heterozygous mice (PAM+/−) to dietary manipulations of copper, we sought to identify indicators of peptidergic dysfunction and mild copper deficiency.
We exposed PAM+/− mice and their wildtype (WT) littermates to either a copper deficient or copper supplemented diet. Dietary copper deficiency in WT mice increased anxiety-like behavior and seizure sensitivity, deficits similar to those observed in PAM+/− mice. Supplementary copper ameliorated some, but not all, of the deficits observed in PAM+/− mice. In addition, we found that copper status differed in PAM+/− and WT mice on the control diet. This unexpected finding led to the hypothesis that PAM is linked to the ability of an organism to provide copper to tissues. Comparisons of gene expression profiles in PAM+/− vs. WT mice confirmed this hypothesis.
MATERIALS AND METHODS
Animal Care and Use
Male and female PAM+/− and C57BL/6J WT littermates (2 to 5 months old) were used in these experiments; male PAM+/− mice were bred with female C57BL/6J WT mice from Jackson Labs (Bar Harbor ME) backcrossed more than 12 generations. Tail clips and/or ear tags taken at the time of weaning were used to determine the genotype (Bousquet-Moore et al., 2009). All protocols were approved by the Animal Care and Use Committee at the University of Connecticut Health Center.
Manipulation of dietary copper
WT and PAM+/− mice received one of three dietary treatments: (1) Control - Harlan Teklad #2018 diet (15 ppm copper per manufacturer) and deionized, reverse osmosis treated water (MilliQ, or Sarstedt) or reverse osmosis treated water; (2) Copper Deficient - Harlan Teklad copper deficient diet (TD80388; 0.6 ppm copper per manufacturer) and reverse osmosis treated water (0.007 ppm), for 9 to 10 weeks; (3) Copper Supplemented - Harlan Teklad control diet and deionized reverse osmosis treated water supplemented with 300 ppm CuSO4·5H2O (measured as 70 ppm Cu) for 14 to 16 days unless noted otherwise. Over the course of each treatment, body weight and appearance were monitored. At the time of euthanasia, trunk blood was collected without anesthesia; serum was prepared and stored frozen for quantification of ceruloplasmin and PAM enzymatic activities. Fragments of forebrain, cerebellum, liver, and atria (0.1 to 0.2 gm for each tissue) were flash frozen (on dry ice) or immediately homogenized in buffer appropriate for the assays to be performed.
Assessment of total tissue copper
Total copper and iron levels were measured in flash-frozen fragments of forebrain and liver by atomic absorption spectroscopy (Prohaska and Bailey, 1994).
TRH and TRH-Gly Levels
For measurement of TRH and TRH-Gly, hypothalamic fragments were extracted into 2N acetic acid/2 mM EGTA/protease inhibitors (Bousquet-Moore et al., 2009); lyophilized samples were reconstituted in phosphate-buffered saline for radioimmunoassay (Nillni et al., 2002; Bousquet-Moore et al., 2009). Hypothalamic TRH and TRH-Gly levels were determined by radioimmunoassay as described (Bousquet-Moore et al., 2009; Nillni et al., 2002).
Immunoblots
Liver, cerebellum, and atria were homogenized in 10 vol SDS lysis buffer (50 mM TrisHCl, pH 8.0, 0.5% SDS, 2 mM EDTA, 50 mM NaF, with proteinase inhibitors), heated at 95°C for 5 min, sonicated and centrifuged at 13,0000 × g before analysis of supernatants. Protein concentrations were determined using the bicinchoninic acid assay (Pierce, Rockford IL) with bovine serum albumin as the standard. Samples (18-40 µg protein) were subjected to Western blot analysis using antibodies to PHM or PAM-1 (JH 1761 or JH 629; 1:1000) (Czyzyk et al., 2005), copper chaperone for superoxide dismutase (CCS, 1:1000, (Prohaska et al., 2003), or γ-adaptin (BD Tranduction Laboratories 610385; 1:1000). Antibody JH1761 was raised to the catalytic monooxygenase domain of PAM region of PAM-1; JH629 was raised against the Exon A region of PAM-1 and recognizes intact PAM-1, soluble PHM and membrane PAL (Czyzyk et al., 2005). Second antibodies were conjugated to horse-radish peroxidase and chemiluminescent signals were captured and analyzed using GeneGnome software (Syngene, Frederick MD).
Ceruloplasmin and PAM Assays
Serum was isolated from trunk blood. Fragments of atria and hypothalami were homogenized directly in 20 mM NaTES, 10 mM mannitol, pH 7.4, 1% TX-100 (TMT) with protease inhibitors (Czyzyk et al., 2005; Bousquet-Moore et al., 2009); samples were clarified by centrifugation at 3000 × g for 30 min before assay. PAM activity in serum and extracts of atria was determined using [125I]-Ac-Tyr-Val-Gly plus 0.5 μM Ac-Try-Val-Gly, 0.5 mM ascorbate and optimal levels of exogenous copper in 150 mM NaTES buffer (pH 5.5). Following incubation at 37°C for 1 h, base was used to convert Ac-Tyr-Val-αhydroxyglycine into amidated product (Bousquet-Moore et al., 2009). Copper dose-response curves using pooled samples of WT and PAM+/− serum revealed no activity in the absence of exogenous CuSO4 and no shift in the concentration of exogenous CuSO4 needed for maximal activity. Assays of serum included 10 μM CuSO4 and assays of hypothalamus/atrium included 7.5 μM CuSO4. Atrial and hypothalamic extracts were analyzed for PAM activity. The PHM assay used in these studies differed in substrate, pH, and key buffer components (absence of N-ethylmaleimide and KI) from the PHM assay used in some prior studies (Prohaska et al., 2005; Prohaska and Broderius, 2006). The addition of 25 mM N-ethylmaleimide plus 25 mM KI to purified PHM assayed as described here reduced activity to 30% of control.
Serum ceruloplasmin activity was measured using O-dianisidine dihydrochloride (Prohaska and Broderius, 2006; Lehmann et al., 1974).
Response to cold
Mice were handled daily for 5 days before core body temperature was monitored. Core temperature was measured using a rectal probe (Harvard Apparatus, Holliston, MA); body temperature was measured before and every 40 min following placement in a 4°C environment (Bousquet-Moore et al., 2009). Changes in velocity of blood flow in the tail in response to decreased body temperature in anesthetized mice (1.5% isoflurane in air-oxygen mixture) were determined using laser velocimetry and are expressed as a percent increase over baseline.
Sensitivity to pentylenetetrazole-induced seizures
Mice were monitored for 45 min following a single intraperitoneal injection of pentylenetetrazole (PTZ, 30 mg/kg, dissolved in saline), a GABAA receptor antagonist. This dose was selected based on a pilot dose-response study (0, 15, 30 and 45 mg/kg PTZ); in PAM+/− mice, administration of 30 mg/kg PTZ typically produced a stage 2 seizure (dose response data not shown). Seizure severity was scored according to the scale described by Ferraro and colleagues (Ferraro et al., 1999); animals with a stage 2 seizure exhibited multiple bouts of twitching.
Assessment of anxiety-like behavior
Mice were handled daily for 5 days prior to placement in the elevated zero maze (San Diego Instruments, San Diego CA) or open field apparatus (San Diego Instruments). On the day of testing, mice were given at least 3 h to acclimate to the Scoville Neurobehavioral Suite before testing was initiated. Each mouse was placed into the closed area of the elevated zero maze and monitored for 5 min. The percent of the total time spent in the open area of the maze, the number of stretch-attend postures (extending the forelimbs from the closed into the open compartment), and the number of head-dips (over the edge of the open area) were quantified.
Q-PCR
RNA was prepared using Trizol reagent (Invitrogen, Carlsbad CA) from the anterior pituitaries and atria of 5 WT and 6 PAM+/− mice maintained on the control diet. Each pituitary and atrium yielded 2-4 μg total RNA. RNA integrity was verified by electrophoresis on denaturing gels. cDNA was reverse transcribed using 0.5 μg RNA and Superscript II reverse transcriptase (Invitrogen). Primer sequences are identified in Supplementary Table 1; product size was verified by agarose gel electrophoresis. Quantitative-PCR was conducted with Sybr-Green (Bio Rad) using the Eppendorf AG 22331 thermal cycler to compare expression of actin, GAPDH, copper transporter 1 (Ctr1), the copper transporter ATP7A, and copper chaperones Atox1, CCS and Cox17 (primer binding at 53°C or 57°C with 55 sec for extension). Samples were analyzed in duplicate and expression levels relative to actin or GAPDH were normalized to the average WT level.
Statistical analysis
Homogeneity of variance was evaluated using Hartley's F-max test. For serum PAM with copper deficiency and supplementation and serum ceruloplasmin with copper deficiency, independent t-tests were used to compare the effect of genotype or copper availability without assuming equal variance. For each experiment of 2×2 factorial design with either copper deficiency or supplementation, two-way ANOVAs were used to determine the overall effect of either copper deficiency or supplementation or genotype on levels of copper, iron, CCS, ceruloplasmin (copper supplemented), TRH, tissue PAM, and percent time spent in the open arm of the elevated zero maze with either copper deficiency or supplementation compared to the control diet. Independent t-tests (assuming equal variance and adjusted criterion via. Bonferroni correction) were used to compare PAM+/− and WT mice at baseline or to compare the effect of either dietary copper deficiency or supplementation within either genotype on levels of copper, CCS, ceruloplasmin (copper supplemented), TRH, tissue PAM, ATP7A, and percent time spent in the open arms of the elevated zero maze. ANOVAs of mixed design were used to determine the effect of dietary copper on the repeated measures of core temperature and blood flow velocity over time during cold exposure and the between subjects factors of genotype and diet. Chi square analysis was used to analyze the non-parametric, frequency distribution of seizure severity. In every figure, one star (*) indicates significance with p< 0.02, two stars (**) indicate where p<0.005, three stars (***) indicate where p<0.0005, and an additional symbol (#) indicates where significance is between 0.02 and 0.07.
RESULTS
Copper status in WT mice on copper deficient and supplemented diets
Because our previous work demonstrated that copper supplementation restored the ability of PAM+/− mice to maintain body temperature in the cold (Bousquet-Moore et al., 2009), we wanted to assess the effects of marginal copper deficiency on WT mice. Copper deficiency was created by feeding adult male mice a diet containing 0.6 ppm Cu/kg instead of 15 ppm Cu/kg for 9 weeks. Copper status was assessed by measuring total liver copper (Fig.1A), serum ceruloplasmin (Fig. 1B) and liver levels of copper chaperone of superoxide dismutase (CCS) (Fig. 1C). As expected, total hepatic copper levels were 21% lower, serum ceruloplasmin levels were 68% lower, and CCS levels were 52% higher during copper deficiency compared to the control diet in WT mice (Prohaska et al., 2003; Prohaska and Broderius, 2006). Copper deficient WT mice did not differ from WT mice on the control diet in body weight or heart weight (Supplementary Table 2), and showed normal locomotor activity (Fig. S1) and grooming behavior.
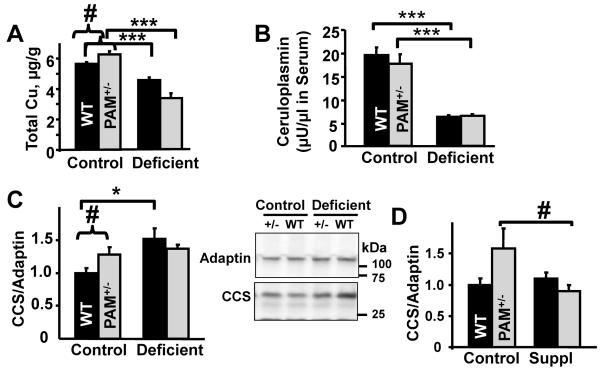
Fig.1. Copper Status Differs in PAM+/− and WT mice.
A. Total liver copper was measured in PAM+/− (Total N=13; N per group = 6,7) and WT mice (Total N=14; N per group=8,6) on the control or copper deficient diet. Copper deficiency reduced total copper (two-way deficiency main effect, p<0.0005; t-tests, p<0.0005). Liver copper was higher in PAM+/− than WT mice on the control diet (t-test p=0.023) and the sensitivity of PAM+/− mice to copper deficiency was increased (two-way interaction, p<0.0005). B. Serum ceruloplasmin activity was determined in PAM+/− (Total N=22; N per group= 9,13) and WT mice (Total N=24; N per group = 11,13) on the two diets; levels were lower in copper deficient WT and PAM+/− mice (t-tests, p<0.0005). CCS levels were quantified in separate liver samples by immunoblot (40 μg protein) in PAM+/− (Total N=28; N per group =7) and WT mice (Total N=23; N per group = 5-6) with deficiency (C) or supplementation (D). CCS levels were normalized to γ-adaptin. CCS levels in WT mice were higher with the copper deficient vs. the control diet, where the PAM+/− mice did not respond (two-way deficiency main effect, p=0.011; two-way interaction p=0.05; t-test, p=0.03). Hepatic CCS levels in PAM+/− mice were slightly lower with copper supplementation (two-way interaction p=0.06; independent t-test p=0.06).
In a separate series of experiments, mice were fed the control diet with copper supplemented or control drinking water for two weeks. The copper supplemented water contained 67 ppm Cu while control water contained 0.007 ppm Cu. The 70 ppm level of copper was selected to avoid toxicity (Herbert et al., 1993) and the copper supplemented mice maintained body weight (Supplementary Table 2) and exhibited normal grooming behavior. Liver copper levels, serum ceruloplasmin activity and liver CCS levels did not vary with copper supplementation (Fig. 1D and Supplementary Table 2).
Copper status was also assessed in the central nervous system of WT mice kept on the copper deficient or copper supplemented diet. As expected, dietary copper had a smaller effect on central nervous system copper levels than it did on hepatic copper levels; total forebrain copper levels were slightly lower in WT mice kept on the copper deficient diet, but were not higher following copper supplementation (Supplementary Table 2). Cerebellar CCS levels did not change with variations in copper availability (Supplementary Table 2).
Copper status and responsiveness differ in PAM+/− and WT mice
To our surprise, several measures of copper status differed between genotypes maintained on the control diet (Fig. 1A, C). Total liver copper was slightly, but significantly, higher (up 11%; p=0.023) in PAM+/− mice compared to their WT littermate controls. Hepatic CCS levels were also significantly higher in PAM+/− mice. Although the changes in liver CCS observed in PAM+/− mice were in the direction associated with copper deficiency, total liver Cu levels were higher in PAM+/− mice than in WT mice, suggesting potential differences in copper localization, trafficking or regulation. Serum ceruloplasmin was not significantly different in WT and PAM+/− mice.
In addition, changes in dietary copper availability affected PAM+/− and WT mice differently. The copper deficient diet had a larger effect on total liver copper in PAM+/− mice than it did in WT mice (Fig. 1A). In contrast, the copper deficient diet increased liver CCS levels in WT mice but not in PAM+/− mice (Fig. 1C). Copper supplementation reduced liver CCS levels in PAM+/− mice without affecting liver CCS levels in WT mice (Fig. 1D). The PAM+/− mice exhibit differences in baseline copper status and in their sensitivity to copper deficiency and supplementation.
As in WT mice, the copper deficient diet produced slightly lower total forebrain copper levels in PAM+/− mice and the copper supplemented diet was without effect (Supplementary Table 2). Cerebellar CCS levels in PAM+/− mice did not vary with copper availability (Supplementary Table 2). In sum, it is clear we have created models of marginal copper deficiency and excess in WT and PAM+/− mice.
Copper deficiency impairs thermoregulation and peripheral vasoconstriction in WT mice
In our earlier study of PAM+/− mice, we explored thermoregulation because several amidated neuropeptides (e.g. neuropeptide Y, vasopressin, TRH) play critical roles in this response (Nillni et al., 2002; Woods and Stock, 1996; Hwa et al., 1999; Pelz and Dark, 2007; Dark and Pelz, 2008; Ye et al., 1995). We previously identified a deficit in the ability of PAM+/− mice to vasoconstrict and maintain core body temperature in response to cold exposure (Bousquet-Moore et al., 2009). The fact that 14 days of supplemental dietary copper reversed both the vascular and thermoregulatory deficits observed in PAM+/− mice motivated the current study.
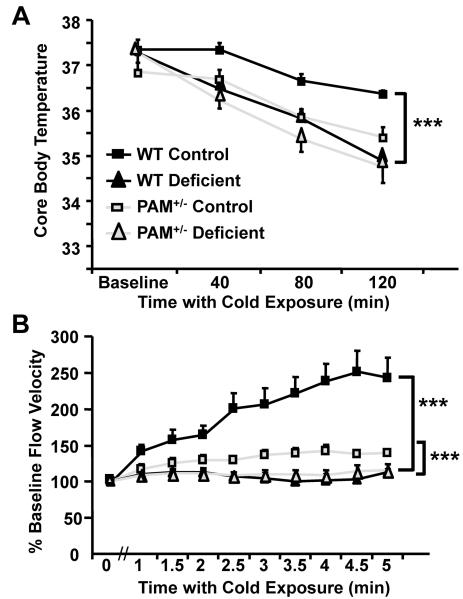
We reasoned that a dietary deficiency in copper in WT mice might, among its many other effects, result in decreased metallation of PAM and a decrease in its ability to produce multiple amidated peptides, thus mimicking phenotypes observed in PAM+/− mice. As predicted, WT mice maintained on the copper deficient diet were unable to maintain body temperature when kept at 4°C (Fig. 2A). In WT mice on the control diet, core body temperature decreased only 1°C after 120 min at 4°C; in copper deficient WT mice, core temperature decreased 2.5°C. Copper deficiency did not further impair thermoregulation in PAM+/− mice.
FIG. 2. Copper Deficiency Impairs Thermoregulation in WT Mice.
(A) PAM+/− (Total N=15; N per group =7,8) and WT mice (Total N=10; N per group=4,6) on the control or copper-deficient diet were exposed to a 4°C environment and core body temperature was assessed every 40 min. As observed previously, core temperature fell more in PAM+/− mice than in WT mice on the control diet (multivariate ANOVA, p=0.014). Core temperature dropped more quickly and to a lower level when WT mice were kept on a copper deficient diet (multivariate ANOVA, p<0.0005). Core temperature was not altered with copper deficiency in PAM+/− mice (two-way interaction, p<0.0005). (B) Laser Doppler velocimetry was used to measure tail blood flow velocity with cold exposure in WT (Total N=12; N per group=6) and PAM+/− mice (Total N=13; N per group= 7,6) on the two diets. The robust cold-induced increase in blood flow velocity observed in WT mice on the control diet was eliminated by copper deficiency (multivariate ANOVA p<0.0005). The limited ability of PAM+/− mice to increase flow velocity in response to cold exposure was further reduced by the copper deficient diet (multivariate ANOVA, p=0.004). The impairment of vasoconstriction was more pronounced in WT than PAM+/− mice (two-way interaction, p=0.008). Error bars, SEM.
We next assessed the effect of copper deficiency on cold-induced vasoconstriction. WT mice maintained on the control diet showed the expected vasoconstriction-induced increase in velocity of blood flow following cold exposure (Fig. 2B). Copper deficient WT mice exposed to the cold failed to exhibit an increase in the velocity of blood flow, suggesting that their ability to constrict their peripheral vasculature was compromised. As reported previously, PAM+/− mice showed deficits in their ability to vasoconstrict in response to cold (Fig. 2B) (Bousquet-Moore et al., 2009); this deficit was exacerbated when PAM+/− mice were fed a copper deficient diet.
Anxiety-like behavior is increased in WT mice by copper deficiency and by PAM haploinsufficiency
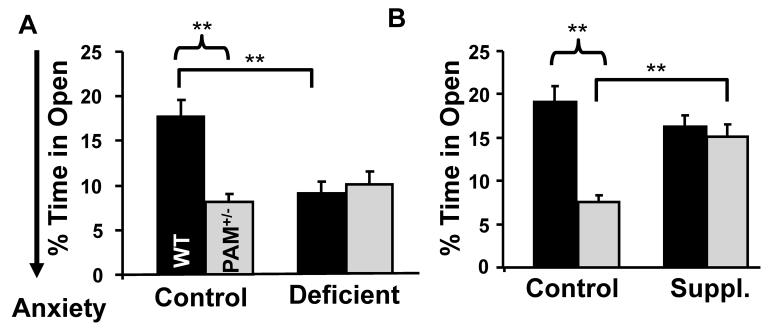
Amidated peptides are expressed in most neurons, with especially high levels in GABAergic interneurons (Cope et al., 2002; Pawelzik et al., 2002). Central levels of amidated peptides such as cholecystokinin, TRH and CRH have been implicated in a variety of behaviors including anxiety-like behavior (Tschenett et al., 2003; Gutierrez-Mariscal et al., 2008; Abramov et al., 2008). Anxiety-like behavior was assessed by quantifying time spent in the open areas of an elevated zero maze. On the control diet, PAM+/− mice spent substantially less time in the open arms of the elevated zero maze than did WT mice (Fig. 3). When fed a copper deficient diet for nine weeks, WT mice exhibited an increase in anxiety-like behavior, spending substantially less time in the open arms of the elevated zero maze when compared to the control diet (Fig. 3A). Compared to the control diet, no changes in anxiety-like behavior were observed when PAM+/− mice were kept on the copper deficient diet. Importantly, PAM+/− mice kept on the copper supplement for 14 days spent more time in the open areas of the zero maze compared to those on the control diet, mimicking the behavior of WT mice (Fig. 3B). Copper supplementation did not affect the behavior of WT mice.
FIG. 3. Anxiety-like Behavior in WT Mice Is Sensitive to Copper Status.
PAM+/− (Total N=29; N per group=6-8) and WT mice (Total N =22; N per group=5-6) on the indicated diet were placed in the elevated zero maze for 5 min; the amount of time spent in the open areas was assessed by a blinded observer and is expressed as a percentage of the total time in the maze. Copper deficient WT mice spent less time in the open areas than WT mice on the control diet (two-way deficiency main effect p=0.025; independent t-test, p=0.004). On the control diet, PAM+/− mice spent less time in the open arms than WT mice (two-way genotype main effect p=0.008; independent t-test, p=0.001), suggesting an anxiety-like phenotype. While PAM+/− mice demonstrated a lower behavioral sensitivity to copper deficiency compared to WT mice (two-way interaction, p=0.001), PAM+/− mice fed the copper supplemented diet spent more time in the open areas than PAM+/− mice on the control diet (independent t-test, p=0.002). The behavior of WT mice did not change significantly with copper supplementation (two-way interaction p=0.005). Error bars, SEM.
The time course with which supplementary dietary copper affected the anxiety-like behavior of PAM+/− mice was evaluated. A single day of copper supplementation failed to increase the amount of time PAM+/− mice spent in the open arms of the elevated zero maze (Fig. S2A). Similarly, two days of copper supplementation did not alter thermoregulation (Fig. S2B). However, eight days of copper supplementation reversed the thermoregulatory deficit in PAM+/−mice (Fig. S2C). Copper-dependent reversal of both the thermoregulatory deficit and increased anxiety-like behavior observed in PAM+/− mice required several days.
Sensitivity to pentylenetetrazole-induced seizures is increased by copper deficiency and by PAM haploinsufficiency
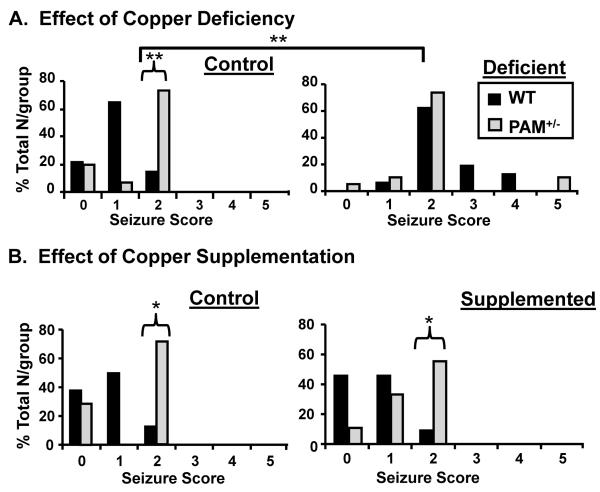
Expression of a number of amidated neuropeptides and PAM is increased following seizure induction by electroconvulsive shock, kainate, or pentylenetetrazole (PTZ, a GABAA receptor antagonist) (Ma et al., 2002; Bhat et al., 1993; Baraban et al., 1997). Neuropeptide Y (NPY), named for its α-amidated Tyr, plays a protective role and mice lacking NPY are seizure prone (Erikson et al., 1996). Cholecystokinin, with its essential –Phe-NH2, is a potent anticonvulsant, inhibiting kindling and PTZ-induced seizures (Tirassa et al., 2005; Tirassa et al., 2002). Based on dose-response studies (data not shown), we selected a dose of pentylenetetrazole (30 mg/kg) that caused only mild seizures in WT mice on a normal diet (Fig. 4A, left). When PAM+/− mice on the control diet received the same dose of PTZ, they had significantly more severe seizures.
FIG. 4. Copper Deficiency Increases Seizure Sensitivity in WT Mice.
PAM+/− (N=15 control; N=18 copper deficient) and WT (N= 14 control; N=16 copper deficient) mice were given an injection of PTZ (30 mg/kg, i.p.) and behavior was scored as described (83). The percentages of each group exhibiting seizures of each rating (0-5) are shown. Chi-square analyses revealed increased seizure severity in PAM+/− mice compared to WT mice on either the copper supplemented diet (p=0.05) or the control diet (p =0.03). Compared to the control diet, copper deficiency (A) increased seizure severity in WT (chi square p = 0.001), but not in PAM+/− mice. Data shown are for male mice; the same experiment was repeated with female mice [WT, N=8 control; N=10 copper deficient; PAM+/−, N=8 control; N=11 copper deficient) with the same answer. Seizure severity did not differ with copper supplementation in either PAM+/− (N=8 control; N=9 supplemented) or WT (N=8 control; N=11 supplemented) male mice compared to the control diet (B).
Dietary copper deficiency increased the sensitivity of WT mice to PTZ-induced seizures (Fig. 4A, right). On the copper deficient diet, five times as many WT mice showed twitching and brief seizures (Behavioral Score 2) compared to the control diet. PAM+/− mice were not further sensitized to PTZ by copper deficiency. Copper supplementation did not alter seizure severity in either genotype compared to the control diet (Fig. 4B).
Dietary copper availability alters the extent of peptide amidation
Given the mild nature of the copper deficiency and supplementation paradigms utilized, we were surprised at the ease with which we found an effect of dietary copper on the physiology and behavior of WT and PAM+/− mice. In several tests, copper deficient WT mice exhibited a phenotype resembling that of PAM+/− mice on the control diet, and copper supplementation ameliorated the effects of PAM heterozygosity. To elucidate the underlying mechanism, we first asked whether production of amidated peptides was affected by dietary copper.
The active site of PAM recognizes the carboxylate of the glycine residue at the C-terminus of its peptidylglycine substrates and accommodates any penultimate amino acid side chain (Prigge et al., 2000). Test substrates with Pro or Gly at the penultimate position preceding the final Gly are amidated at a significantly slower rate than those with either Tyr or Val at the penultimate position (Merkler, 1994; Murthy et al., 1987), suggesting that the effects of limited PAM activity will be peptide specific. The conversion of inactive TRH-Gly into bioactive TRH requires amidation of a Pro residue and we previously demonstrated that PAM+/− mice had twice as much TRH-Gly as WT mice on the control diet (Bousquet-Moore et al., 2009).
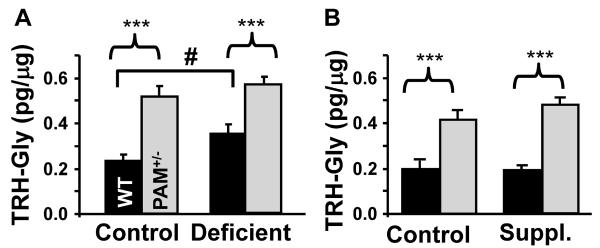
We therefore explored the possibility that dietary copper deficiency might result in diminished availability of copper in the lumen of the secretory pathway, leading to accumulation of the TRH-Gly intermediate in the hypothalamic neurons that produce preproTRH (Fig. 5). When compared to WT mice, levels of hypothalamic TRH-Gly were increased in PAM+/− mice on the control, deficient, and copper supplemented treatments (Fig. 5). In WT mice, levels of hypothalamic TRH-Gly were increased by copper deficiency (Fig. 5A). Copper supplementation was without effect on TRH-Gly levels in WT or PAM+/− mice (Fig. 5B). Although levels of TRH exceed levels of TRH-Gly even in copper-deficient PAM+/− mice, the fact that copper deficiency produces an observable accumulation of TRH-Gly supports the suggestion that deficits in peptide amidation contribute to the phenotypes observed. Data for TRH-Gly and amidated TRH are presented in Supplementary Table 3.
FIG. 5. Copper Deficiency Leads to Accumulation of TRH-Gly in WT Mice.
Hypothalami from PAM+/− and WT mice were extracted and the inactive precursor to amidated TRH, TRH-Gly, was quantified by radioimmunoassay with copper deficiency (A) and supplementation (B). For the copper deficiency experiment, 26 WT mice (11 control;15 deficient) and 37 PAM+/− mice (17 control; 20 deficient) were used. For the copper supplementation experiment, 20 WT mice (9 control; 11 supplemented) and 14 PAM+/− mice (7 for each group) were used. A significant accumulation of TRH-Gly was noted in PAM+/− mice (two-way genotype main effect, p<0.0005; independent t-test p<0.0005) as previously described (Bousquet-Moore et al., 2009). Significantly higher levels of TRH-Gly were seen in copper deficient WT mice compared to WT mice on the control diet (two-way deficiency main effect, p = 0.036; independent t-test, p=0.03). Error bars, SEM.
Dietary copper alters serum PAM activity
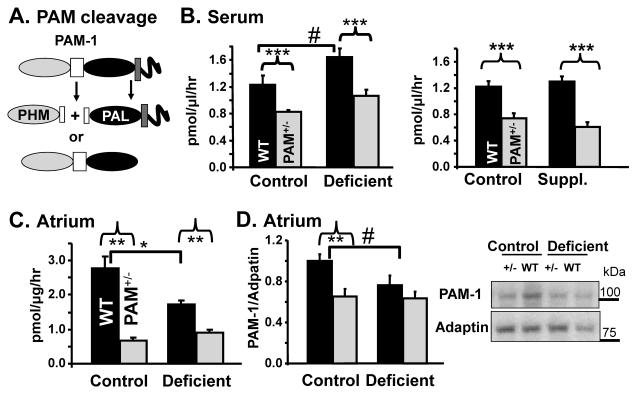
Since the effects of PAM heterozygosity and copper deficiency on TRH amidation were modest, we explored other mechanisms that might contribute to the phenotypes observed. Earlier cell culture experiments revealed effects of copper on the endocytic trafficking of PAM-1 (De et al., 2007) and non-catalytic effects of the PAM-1 protein on cytoskeletal organization and gene expression (Ciccotosto et al., 1999; Rajagopal et al., 2009). Although the major PAM isoforms are synthesized as integral membrane proteins, endoproteolytic cleavage generates soluble PHM and bifunctional PAM that are secreted (Fig. 6A) (De et al., 2007).
FIG. 6. Copper Deficiency Affects PAM Processing.
(A) The major cleavage products of PAM-1 are shown. (B) Serum levels of copper-optimized PAM activity were determined in WT and PAM+/− mice on the indicated diet. For the copper deficiency experiment, 41 WT mice (19 control; 22 deficient) and 52 PAM+/− (24 control; 28 deficient) were used. For the copper supplementation experiment, 19 WT mice (8 control;11 supplemented) and 16 PAM+/− mice (8 per group) were used. An increase in serum activity with copper deficiency was noted in WT mice (independent t-test, p=0.048). Copper supplementation did not alter serum activity. Copper optimized PAM activity was also assessed in atria from PAM+/− (Total N=14; N per group=7) and WT (Total N=11; N=5,6) mice on the control or copper deficient diet (C). Separate samples were subjected to Western blot analysis using antibody to PHM (9 WT and 10 PAM+/−mice on either the control or deficient diets). Data for PAM-1 were quantified and normalized to γ-adaptin (D). Atrial PAM activity was lower in PAM+/− vs. WT mice on both diets (two-way genotype main effect, p<0.0005; independent t-test, p=0.002) and declined with copper deficiency in WT mice (two-way interaction p=0.022; independent t-test p=0.01). Levels of intact PAM-1 protein were lower in PAM+/− mice than in WT mice on the control diet (two-way genotype main effect, p=0.006; independent t-test, p=0.01) and only slightly decreased with copper deficiency in WT mice (independent t-test, p=0.07). Error bars, SEM.
We examined serum as well as lysates from tissues that express high levels of PAM to determine whether the cleavage and secretion of PAM were altered by copper status. For many cuproenzymes, protein stability is diminished when copper is less available. Apoceruloplasmin, for example, has a much shorter plasma half-life than holoceruloplasmin (Holtzman and Gaumnitz, 1970a; Holtzman and Gaumnitz, 1970b) and apohephaestin is degraded by the proteasome (Nittis and Gitlin, 2004). In contrast, cell culture studies have demonstrated that decreased copper availability resulted in increased secretion and decreased endocytic degradation of PAM-1, leading to the speculation that PAM may be involved in the regulation of copper metabolism (De et al., 2007).
Serum PAM levels were assessed by measuring the conversion of Ac-Tyr-Val-Gly into Ac-Tyr-Val-NH2 following the addition of exogenous copper. Without exogenous copper, serum PAM activity was undetectable. Dietary treatment did not alter the amount of exogenous copper needed to optimize serum PAM activity (Fig. S3). In WT mice, serum PAM activity was higher in copper deficient animals than in the corresponding control animals (Fig. 6B). Activity levels for PAM+/− mice were lower than those of WT controls and were less sensitive to copper deficiency. Copper supplementation did not alter serum PAM activity in WT or PAM+/− mice. The stimulatory effects of dietary copper deficiency on serum PAM levels in WT mice are consistent with the effects of copper on PAM-1 cleavage and secretion by corticotrope tumor cells (De et al., 2007).
Dietary copper alters atrial PAM metabolism
Since assays of serum PAM activity suggested that dietary copper availability affected PAM metabolism, we used Western blots to examine the proteolytic processing of PAM-1 in atrium and hypothalamus. Atrium was selected because it is the site of highest PAM expression, develops abnormally in PAM−/− embryos (Czyzyk et al., 2005) and is known to be sensitive to dietary copper levels (Jiang et al., 2007). Hypothalamus was examined because it contains the cell bodies of neurons specializing in the synthesis of amidated peptides such as vasopressin, oxytocin, TRH and CRH.
Because it is more homogeneous, we first assessed copper-optimized PAM activity in atrial homogenates; PAM activity was reduced more than two-fold in PAM+/− verses WT atria (Fig. 6C). Following dietary copper deficiency, PAM activity was lower in WT atria, but not in atria from PAM+/− mice. Alternative splicing yields several major isoforms of PAM; each encodes active enzyme and each contributes to the activity measurement reported. For studies of PAM metabolism, we focused on a single isoform, PAM-1, because it is cleaved more extensively than other isoforms. Western blot analysis using an antibody specific for PAM-1 revealed a lower level of intact PAM-1 in atria from WT mice on the copper deficient diet compared to the control diet (Fig. 6D). The lower levels of PAM-1 protein in the atrium paralleled the decline in PAM activity. PAM activity was lower in hypothalamic homogenates from PAM+/− vs. WT mice, but was unaltered by copper deficiency (Fig. S4).
Alterations in PAM affect expression of genes involved in copper metabolism
Our data suggest a role for PAM itself in regulating copper metabolism. Studies carried out in a corticotrope tumor cell line in which expression of membrane PAM can be induced with doxycycline (Ciccotosto et al., 1999), revealed a cell autonomous role for PAM in controlling the expression of specific genes (Francone et al., submitted). Amongst the genes whose expression was affected by levels of PAM were several copper chaperones (Atox1, CCS and Cox17) and copper transporters (Ctr1 and ATP7A) (Supplementary Table 4). Expression of the cuproenzymes present in corticotrope cells (Aoc2, Aoc3, PAM, SOD1, and SOD3) was not altered in response to changes in levels of PAM.
If PAM has similar effects on gene expression in vivo, we reasoned that we should see differences in gene expression in PAM+/− vs. WT mice. We used qPCR to compare levels of a subset of these transcripts in atria and anterior pituitaries from PAM+/− and WT mice. RNA prepared from individual pituitaries and atria was used to prepare cDNA; β-actin and GAPDH transcripts analyzed at the same time served as internal controls. The data normalized to either internal control yielded the same result. Pituitary levels of transcripts encoding the copper chaperones Atox1 and Cox17 were lower in PAM+/− mice compared to WT mice (Table 1). Both decreases were predicted by the changes observed with doxycycline-induced overexpression of PAM in corticotrope cells. For the genes examined, the PAM-induced increases in gene expression observed in corticotrope tumor cells predict the decreases in gene expression observed in PAM+/− mice. We next used qPCR to compare gene expression in PAM+/− vs. WT atria (Table 1). In contrast to pituitary, atrial expression of Atox1 was unaltered. Atrial levels of transcripts encoding ATP7A and Ctr1 were lower in PAM+/− mice compared WT mice. The effects of PAM on gene expression are tissue specific.
Table 1.
QPCR Analysis of Copper-Related Gene Expression in PAM+/− vs. WT Mouse Atrium and Pituitary.
| Atrium | Pituitary | |||||
|---|---|---|---|---|---|---|
| Gene | WT | PAM+/− | Two-tailed P | WT | PAM+/− | Two-tailed P |
| Atox1 | 1.0 ± 0.10 | 0.90 ± 0.08 | NS | 1.0 ± 0.09 | 0.67 ± 0.03 | 0.005 |
| CCS | 1.0 ± 0.13 | 0.87 ± 0.11 | NS | 1.0 ± 0.09 | 0.81 ± 0.13 | NS |
| Cox17 | 1.0 ± 0.17 | 0.93 ± 0.08 | NS | 1.0 ± 0.02 | 0.58 ± 0.16 | 0.001 |
| Ctr1 | 1.0 ± 0.14 | 0.62 ± 0.10 | 0.040 | 1.0 ± 0.23 | 0.85 ± 0.14 | NS |
| ATP7A | 1.0 ± 0.17 | 0.60 ± 0.07 | 0.038 | 1.0 ± 0.24 | 1.44 ± 0.31 | NS |
RNA prepared from the atria and pituitaries of 5 WT and 6 PAM+/− mice was analyzed in duplicate by QPCR. Data were normalized to β-actin. Similar differences were observed when data were normalized to GAPDH.
DISCUSSION
Effects of PAM heterozygosity are widespread and diverse
In organisms as simple as Planaria and Hydra, amidated peptides play multiple roles in normal physiology (Asada et al., 2005). Since PAM is the only enzyme known to synthesize amidated peptides, we used mice with a single functional copy of PAM to identify functions that were most sensitive to a decrease in PAM and dietary copper. While haploinsufficiency of many genes is without effect, a single functional copy of the vesicular monoamine transporter 2 gene (Fukui et al., 2007) or various ion channel genes (Moseley et al., 2007) results in impaired function. Based on sites rich in amidated peptides, deficits in neuronal function were anticipated. Based on the phenotype of PAM−/− embryos, which die in utero, deficits in the vascular system were also expected.
Deficient vasoconstriction in PAM+/− mice contributes to their inability to maintain body temperature in the cold. Changes in peripheral levels of neuropeptide Y or vasopressin may contribute to this phenotype. Studies in human subjects demonstrate a key role for peptidergic transmission in cold-induced peripheral vasoconstriction, with deficits often apparent in elderly individuals (Stephens et al., 2004). Behavioral tests revealed increased anxiety-like behavior in PAM+/− mice. Central levels of amidated neuropeptides such as NPY (Tschenett et al., 2003) and TRH (Gutierrez-Mariscal et al., 2008) play a role in controlling anxiety-like behavior, but further studies are required to determine the mechanisms through which PAM heterozygosity results in this phenotype. Patch clamp recordings from amygdalar slices of PAM+/− and WT mice reveal significant alterations in synaptic transmission in the mutant mice (Gaier et al., 2008).
Similarities between PAM heterozygosity and copper deficiency
The increased sensitivity of PAM+/− mice to drug-induced seizures is of particular interest. Central expression of several neuropeptides increases substantially following seizures induced by PTZ (Jaworska Feil et al., 1999), electroconvulsive shock (Ma et al., 2002) or kainate (Mahata et al., 1993; Woldbye et al., 1997). There is evidence that central administration of several amidated neuropeptides reduces seizure severity (Woldbye et al., 1997; Woldbye et al., 1996; Woldbye et al., 2005). The effects of PAM heterozygosity on seizure sensitivity were not reversed by supplementary copper. However, dietary copper deficiency increased the sensitivity of wildtype mice to PTZ-induced seizures. Menkes disease is associated with increased seizure frequency (Tumer and Horn, 1997; Sheela et al., 2005; Schlief et al., 2006), and alterations in amidated peptides and ATP7A-dependent release of copper may contribute to this phenotype (Schlief et al., 2006; Schlief et al., 2005). PAM binds copper weakly compared to most cuproenzymes (0.06 μM affinity) (Bell et al., 2003) and may be especially sensitive to copper deficiency. While PAM retains copper in secretory granules, where the copper concentration is 30 μM (Thorn et al., 1986), it loses copper when tissue is homogenized (Bousquet-Moore et al., 2009). Haploinsufficient levels of PAM and copper deficiency produce similar physiological and behavioral phenotypes: seizure sensitivity, cold sensitivity, and anxiety-like behavior.
Several single nucleotide polymorphisms (SNPs) have been identified in the human PAM gene (http://www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?locusId=5066), but their consequences have not been explored. Based on linkage studies, PAM is associated with nicotine addiction (Gelernter et al., 2007) and alcoholism (Ehlers and Wilhelmson, 2008), but the underlying mechanisms have not been examined. Exposure of PAM+/− mice to altered diets and different environments may reveal additional physiological functions that are sensitive to limitations in PAM function in human patients.
PAM: a Target for Nutrient Deficiency
While bacteria, archaea and eukaryotes share cuproenzymes like cytochrome c oxidase, a number of new copper domains evolved in eukaryotes and ancient domains adopted new roles (Andreini et al., 2008). Amongst the cuproenzymes unique to multicellular organisms is the monooxygenase family that includes PAM, DBM and monooxygenase X (Prigge et al., 2000). In addition to an absolute requirement for copper, PAM and DBM require ascorbate. While most vertebrates can synthesize ascorbate, mutations in the gene encoding L-gulono-γ-lactone oxidase make humans dependent upon dietary ascorbate (Nishikimi et al., 1994; Linster and Schaftingen, 2007). Hence, dietary ascorbate deficiency could result in similar physiological phenotypes and decreases in peptide amidation.
Marginal deficiency of trace metals, which is thought to be common even in developed countries, affects distinct cell types differently (Merchant and Sagasti, 2006). Acquired copper deficiency can be caused by long-term parenteral nutrition, malabsorption or increased zinc intake, and is being recognized more frequently in some human populations. In addition to anemia, symptoms often include sensory ataxia (Kumar, 2006; Madsen and Gitlin, 2007). By analyzing the effects of copper on early development in zebrafish, Mendelsohn and colleagues (Mendelsohn et al., 2006) identified a hierarchy of copper-sensitive events. Peripheral neurons may be more sensitive to marginal copper deficiency than central neurons, which benefit from the ability of the central nervous system to accumulate the limited amount of copper available. There is substantial disagreement on the recommended daily intake of copper in people and on biomarkers that can detect marginal copper deficiency.
Our studies suggest that a similar spectrum of sensitivity to copper availability exists in adult mice and that the physiological functions impaired by PAM heterozygosity and reversed by copper supplementation may be useful indicators of copper status. The fact that marginally copper deficit mice display deficits similar to some of those observed in PAM+/− mice supports this possibility. Measures of both anxiety-like behavior, which presumably reflect central levels of copper, and the ability to thermoregulate, more a reflection of peripheral copper availability, should be explored to see whether they could be used as indicators of marginal copper deficiency.
Variations in severity of copper deficiency
More extreme copper deficiency during embryonic and early post-natal development produces effects distinct from those observed with marginal copper deficiency in adults. Severe developmental copper deficiency was sufficient to cause diminished weight gain and cardiac hypertrophy, which were not observed in the present study (Prohaska and Broderius, 2006; Prohaska and Gybina, 2004; Penland and Prohaska, 2004). Levels of Ctr1 were higher in many tissues during severe copper deficiency, perhaps compensating for diminished copper availability (Kuo et al., 2006). Again, the response was tissue specific, with choroid plexus showing an increase in Ctr1 with no change in CCS, and cerebellum demonstrating higher CCS levels (Gybina and Prohaska, 2006). ATP7a Mo-br mice do not survive to weaning. Genetic deletion of Ctr1 in mice is also lethal. The differences between the physiological effects of copper deficiency in adult animals and during development reflect the critical biological importance of copper during development. In rodents, brain copper levels increase substantially between P7 and P11 (Prohaska and Wells, 1974). Expression of ATP7A in mice similarly peaks at P4 (Niciu et al., 2006). ATP7a Mo-br mice respond to supplemental copper (50 μg, subcutaneous injection) when it is provided at P7 (Mann et al., 1979).
Copper metabolism is altered by PAM
We had not expected to see differences between WT and PAM+/− mice in baseline copper status. However, PAM+/− liver copper levels are slightly, but significantly, higher than those in their WT littermate controls, despite lower levels of liver CCS. While some of these differences may reflect altered feedback from systems whose metabolism is affected by lack of the signaling molecules made by this peptide processing enzyme, others may be a direct result of the decreased levels of PAM protein in PAM+/− mice.
Expression of exogenous PAM in corticotrope tumor cells alters the expression of approximately 1000 of the 25,000 genes expressed at readily detectable levels in corticotrope tumor cells (Francone et al, submitted). Many of the copper transporter and copper binding protein genes expressed in AtT-20 cells are sensitive to the level of PAM expression. With the exception of SOD1, genes encoding cuproenzymes were not affected by changes in PAM expression. For the limited number of transcripts examined, the response of corticotrope tumor cells to increases in PAM expression predicted the response of the PAM+/− pituitary to decreases in PAM expression. Expression of Atox1 and Cox17, cytosolic chaperones that deliver Cu(I) to ATP7A and Sco1 (Prohaska and Gybina, 2004) respectively, rose when PAM expression increased in a corticotrope tumor cell line with doxycycline-inducible expression of PAM (Ciccotosto et al., 1999) and fell when PAM expression decreased in PAM+/− mice.
The changes in gene expression associated with PAM heterozygosity are tissue-specific. If PAM is part of the systems that allow an organism to adjust to variations in copper availability, tissue specificity makes sense. The interaction of PAM with key players in copper metabolism may reflect a role for PAM and amidated peptides as the vascular system evolved. PAM, a cuproenzyme that requires molecular oxygen to produce its amidated peptide products, would be well suited to a role producing signaling molecules involved in organizing the vascular system. Consistent with this suggestion, a recent study identified a role for protease-mediated activation of PAM and two of its amidated peptide products, NPY and substance P, in the response to intermittent hypoxia (Sharma et al., 2009).
Underlying mechanism
A variety of pathways may link alterations in PAM expression to physiological function. Although conversion of some peptidylglycine precursors into amidated products is compromised in PAM+/− mice, our data for total hypothalamic TRH suggest that production of most amidated peptides is not greatly altered by PAM heterozygosity. Developmental deficits due to PAM heterozygosity may contribute to the differences observed in adult behavior, but both the ability of supplementary copper to reverse the differences and mimicry by adult copper deficiency demonstrate that this is not the entire explanation. The effects of PAM on gene expression may explain many of the differences observed. The soluble, cytoplasmic fragment generated from PAM and targeted to the nucleus may play an essential role in this response. PAM-mediated alterations in Atox1, the cytosolic chaperone that delivers copper to ATP7A, may control a subset of the alterations observed. Atox1 is also a transcription factor, binding to a defined sequence in the promoter region of its target genes (Muller and Klomp, 2009). Any changes in copper levels and localization as a result of PAM heterozygosity are likely to be of great significance. Copper itself can act as a neuromodulator and copper is secreted upon glutamatergic stimulation of NMDA receptors (Schlief et al., 2005). While trafficking of ATP7A is known to respond to copper levels, it also responds to NMDA receptor activation (Schlief et al., 2005; Schlief et al., 2006).
Neuropeptides, many of which are amidated, are expressed in almost every neuron. Production of every amidated neuropeptide requires PAM. With their dependence on the PAM gene, dietary copper and dietary ascorbate, pathways that rely on peptidergic transmission may be sensitive indicators of this essential gene/environment interaction.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramov U, Raud S, Innos J, Lasner H, Kurrikoff K, Turna T, Puussaar T, Okva K, Matsui T, Vasar E. Different housing conditions alter the behavioral phenotypes of CCS(2) receptor-deficient mice. Behav. Brain Res. 2008;193:108–116. doi: 10.1016/j.bbr.2008.04.027. [DOI] [PubMed] [Google Scholar]
- Al-Bitar Y, Azam JS, Azam T. Menke's kinky hair syndrome- a rare medical condition. J. Pak. Med. Assoc. 2005;55:40–42. [PubMed] [Google Scholar]
- Andreini C, Banci L, Bertini I, Rosato A. Occurence of copper proteins through the three domains of life: a bioinformatic approach. J. Proteome Res. 2008;7:209–216. doi: 10.1021/pr070480u. [DOI] [PubMed] [Google Scholar]
- Asada A, Orii H, Watanabe K, Tsubaki M. Planarian peptidylglycine-hydroxylating monooxygenase, a neuropeptide processing enzyme, colocalizes with cytochrome b561 along the central nervous system. FEBS J. 2005;272:942–955. doi: 10.1111/j.1742-4658.2004.04528.x. [DOI] [PubMed] [Google Scholar]
- Baraban SC, Hollopeter G, Erickson JC, Schwartzkroin PA, Palmiter RD. Knockout mice reveal a critical antiepileptic role for neuropeptide Y. J. Neurosci. 1997;17:8927–8936. doi: 10.1523/JNEUROSCI.17-23-08927.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J, El Meskini R, D'Amato D, Mains RE, Eipper BA. Mechanistic investigation of peptidylglycine alpha-hydroxylating monooxygenase via intrinsic tryptophan fluorescence and mutagenesis. Biochemistry. 2003;42:7133–7142. doi: 10.1021/bi034247v. [DOI] [PubMed] [Google Scholar]
- Bhat RV, Tausk FA, Baraban JM, Mains RE, Eipper BA. Rapid increases in peptide processing enzyme expression in hippocamal neurons. J. Neurochem. 1993;61:1315–1322. doi: 10.1111/j.1471-4159.1993.tb13624.x. [DOI] [PubMed] [Google Scholar]
- Bousquet-Moore D, Ma XM, Nillni EA, Czyzyk TA, Pintar JE, Eipper BA, Mains RE. Reversal of physiological deficits caused by diminished levels of peptidylglycine alpha-amidating monooxygenase by dietary copper. Endocrinology. 2009;150:1739–1737. doi: 10.1210/en.2008-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccotosto GD, Schiller MR, Eipper BA, Mains RE. Induction of integral membrane PAM expression in AtT-20 cells alters the storage and trafficking of POMC and PC1. J. Cell Biol. 1999;144:459–471. doi: 10.1083/jcb.144.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope DW, Maccaferri G, Marton LF, Roberts JD, Cobden PM, Somogyi P. Cholecystokinin-immunopositive basket and Schaffer collateral-associated interneurons target different domains of pyramidal cells in the CA1 area of the hippocampus. Neuroscience. 2002;109:63–80. doi: 10.1016/s0306-4522(01)00440-7. [DOI] [PubMed] [Google Scholar]
- Critchton RR, Pierre JL. Old iron, young copper: from Mars to Venus. Biometals. 2001;14:99–112. doi: 10.1023/a:1016710810701. [DOI] [PubMed] [Google Scholar]
- Cunliffe P, Reed V, Boyd Y. Intragenetic deletions at ATP7a in mouse models for Menkes disease. Genomics. 2001;74:155–162. doi: 10.1006/geno.2001.6529. [DOI] [PubMed] [Google Scholar]
- Czyzyk TA, Ning Y, Hsu MS, Peng B, Mains RE, Eipper BA, Pintar JE. Deletion of peptide amidation enzymatic activity leads to edema and embryonic lethality in the mouse. Dev. Biol. 2005;287:301–313. doi: 10.1016/j.ydbio.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Dark J, Pelz KM. NPY Y1 receptor antagonist prevents NPY-induced torporlike hypothermia in cold-acclimated Siberian hamsters. Am. J Physiol. Reg. Integr. Comp. Physiol. 2008;294:R236–R245. doi: 10.1152/ajpregu.00587.2007. [DOI] [PubMed] [Google Scholar]
- De M, Ciccotosto GD, Mains RE, Eipper BA. Trafficking of a secretory granule membrane protein is sensitive to copper. J. Biol. Chem. 2007;282:23362–23371. doi: 10.1074/jbc.M702891200. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Wilhelmson KC. Association between single nucleotide polymorphisms in the mu opioid receptor gene (OPRM1) and self reported responses to alcohol in American Indians. BMC Med. Genet. 2008;9:35. doi: 10.1186/1471-2350-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson JC, Clegg KE, Palmiter RD. Sensitivity to leptin and susceptibility to seizures of mice lacking Neuropeptide Y. Nature. 1996;381:415–421. doi: 10.1038/381415a0. [DOI] [PubMed] [Google Scholar]
- Ferraro TN, Golden GT, Smith GM, John P, Schork NJ, Mulholland N, Ballas C, Schill J, Buono RJ, Berrettini WH. Mapping loci for pentylenetetrazol-induced seizure susceptibility in mice. J. Neurosci. 1999;19:6733–6739. doi: 10.1523/JNEUROSCI.19-16-06733.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui M, Rodriguiz R, Zhou J, Jiang S, Phillips L, Caron MG, Wetsel WC. Vmat2 heterozygous mutant mice display a depressive-like phenotype. J. Neurosci. 2007;27:10520–10529. doi: 10.1523/JNEUROSCI.4388-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaier ED, Sivaramakrishnan S, Rodriguiz RM, Czyzyk TA, Pintar JE, Wetsel WC, Eipper BA, Mains RE. The role of PAM in amygdala physiology and behavior. Soc. Neurosci. Proc. 2008 634.15/E28. [Google Scholar]
- Gelernter J, Panhuysen C, Weiss R, Brady K, Poling J, Krauthammer M, Farrer L, Kranzler HR. Genomewide linkage scan for nicotine-dependence: identification of a chromosome 5 risk locus. Biol. Psychiatry. 2007;61:119–126. doi: 10.1016/j.biopsych.2006.08.023. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Mariscal M, de Gortari P, Lopez-Rubaicava C, Joseph-Bravo P. Analysis of the anxiolytic-like effect of TRH and the response of amygdalar TRHergic neurons in anxiety. Psychoneuroendocrinology. 2008;33:198–213. doi: 10.1016/j.psyneuen.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Gybina AA, Prohaska JR. Variable response of selected cuproproteins in rat choroid plexus and cerebellum following perinatal copper deficiency. Genes Nutr. 2006;1:51–59. doi: 10.1007/BF02829936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey LJ, McArdle HJ. Biomarkers of copper status: a brief update. Br. J Nutr. 2008;99:S10–S13. doi: 10.1017/S0007114508006806. [DOI] [PubMed] [Google Scholar]
- Herbert CD, Elwell MR, Travlos GS, Fitz CJ, Bucher JR. Subchronic toxicity of cupric sulfate administered in drinking water and feed of mice and rats. Fundam. Appl. Toxicol. 1993;21:461–475. doi: 10.1006/faat.1993.1122. [DOI] [PubMed] [Google Scholar]
- Holtzman NA, Gaumnitz BM. Identification of an apoceruloplasmin-like substance in the plasma of copper deficient rats. J Biol. Chem. 1970a;245:2350–2353. [PubMed] [Google Scholar]
- Holtzman NA, Gaumnitz BM. Studies of the rate of release and turnover of ceruloplasmin and apoceruloplasmin in rat plasma. J Biol. Chem. 1970b;245:2354–2358. [PubMed] [Google Scholar]
- Hwa J, Witten M, Williams P, Ghibaudi L, Gao J, Salisbury B, Mullins D, Hamud F, Strader C, Parker EM. Activation of the NPY Y5 receptor regulates both feeding and energy expenditure. Am. J Physiol. Reg. Integr. Comp. Physiol. 1999;277:R1428–R1434. doi: 10.1152/ajpregu.1999.277.5.R1428. [DOI] [PubMed] [Google Scholar]
- Jaworska Feil L, Turchan J, Przewlocka B, Budziszewska B, Leskiewicz M, Lason W. Effects of pentylenetetrazole-induced kindling on thyrotropin-releasing hormone biosynthesis and receptors in rat brain. Neuroscience. 1999;90:695–704. doi: 10.1016/s0306-4522(98)00446-1. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Reynolds C, Xiao C, Feng W, Zhou Z, Rodriguez W, Tyagi S, Eaton J, Saari J, Kang Y. Dietary copper supplementation reverses hypertrophic cardiomyopathy induced by chronic pressure overload in mice. J Exp. Med. 2007;204:657–666. doi: 10.1084/jem.20061943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N. Copper deficiency myelopathy (human swayback) Mayo Clin. Proc. 2006;81:1371–1384. doi: 10.4065/81.10.1371. [DOI] [PubMed] [Google Scholar]
- Kuo YM, Gybina AA, Pyatskowit JW, Gitschier J, Prohaska JR. Copper transport protein 1 (Ctr1) levels in mice are tissue specific and dependent on copper status. J Nutr. 2006;136:21–26. doi: 10.1093/jn/136.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann HP, Schosinsky KH, Beeler MF. Standardization of serum ceruloplasmin concentrations in international enzyme units with o-dianisidine dihydrochloride as substrate. Clin. Chem. 1974;20:1564–1567. [PubMed] [Google Scholar]
- Linster CL, Schaftingen EV. Vitamin C: biosynthesis, recycling, and degradation in mammals. FEBS J. 2007;274:1–22. doi: 10.1111/j.1742-4658.2006.05607.x. [DOI] [PubMed] [Google Scholar]
- Ma XM, Mains RE, Eipper BA. Plasticity in hippocampal peptidergic systems induced by repeated electroconvulsive shock. Neuropsychopharmacology. 2002;27:55–71. doi: 10.1016/S0893-133X(02)00284-1. [DOI] [PubMed] [Google Scholar]
- Madsen E, Gitlin JD. Copper and iron disorders of the brain. Annu. Rev. Neurosci. 2007;30:317–337. doi: 10.1146/annurev.neuro.30.051606.094232. [DOI] [PubMed] [Google Scholar]
- Mahata SK, Gruber B, Mahata M, Roder C, Fischer-Colbrie R, Sperk G. Kainic acid seizures in rat: differential expression of chromogranin A, carboxypeptidase H, and peptidylglycine alpha-amidated monooxygenase in subfields of the hippocampal formation. Acta Neuropathol. 1993;86:590–595. doi: 10.1007/BF00294297. [DOI] [PubMed] [Google Scholar]
- Mann JR, Camakaris J, Danks DM, Walliczek EG. Copper metabolism in mottled mouse mutants: copper therapy of brindled (Mobr) mice. Biochem. J. 1979;180:605–612. doi: 10.1042/bj1800605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maury A, Payen V, Toutain A, Guiraud P, Saliba E, Labarthe F. Neonatal onset of Menkes disease: diagnosis interest of cupremia and microscopic examination of the hairs. Arch. Pediatr. 2007;14:1216–1218. doi: 10.1016/j.arcped.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Mendelsohn BA, Yin C, Johnson SL, Wilm TP, Solnica-Krezel L, Gitlin JD. ATP7a determines a hierarchy of copper metabolism essential for notochord development. Cell Metab. 2006;4:155–162. doi: 10.1016/j.cmet.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Merchant SS, Sagasti A. Precious metal economy. Cell Metab. 2006;4:99–101. doi: 10.1016/j.cmet.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Merkler DJ. C-terminal amidated peptides: Production by the in vitro enzymatic amdiation of glycine-extended peptides and the importance of the amide to biological activity. Ezyme Microb. Technol. 1994;16:450–456. doi: 10.1016/0141-0229(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Moseley AE, Williams MT, Schaefer TL, Bohanan CS, Neumann JC, Behbehani MM, Vorhees CV, Lingrel JB. Deficiency in Na, K-ATPase alpha-isoform genes alters spatial learning, motor activity, and anxiety in mice. J. Neurosci. 2007;27:616–626. doi: 10.1523/JNEUROSCI.4464-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller PA, Klomp LW. Atox1: a novel copper-responsive transcription factor in mammals? Int. J Biochem. Cell Biol. 2009;41:1233–1236. doi: 10.1016/j.biocel.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Murthy AS, Keutmann HT, Eipper BA. Further characterization of peptidylglycine α-amidating monooxygenase from bovine intermediate pituitary. Mol. Endocrinol. 1987;1:290–299. doi: 10.1210/mend-1-4-290. [DOI] [PubMed] [Google Scholar]
- Niciu MJ, Ma XM, El Meskini R, Pachter JS, Mains RE, Eipper BA. Altered ATP7a expression and other compensatory responses in a murine model of Menkes disease. Neurobiol. Dis. 2007;27:278–291. doi: 10.1016/j.nbd.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niciu MJ, Ma XM, El Meskini R, Ronnett GV, Mains RE, Eipper BA. Developmental changes in the expression of ATP7a during a critical period in postnatal neurodevelopment. Neuroscience. 2006;139:947–964. doi: 10.1016/j.neuroscience.2006.01.044. [DOI] [PubMed] [Google Scholar]
- Nillni EA, Xie W, Mulcahy L, Sanchez VC, Wetsel WC. Deficiencies in prothyroptropin-releasing hormone processing and abnormalities in thermoregulation in CPEfat/fat mice. J Biol. Chem. 2002;277:48587–48595. doi: 10.1074/jbc.M206702200. [DOI] [PubMed] [Google Scholar]
- Nishikimi M, Fukuyama R, Minoshima S, Shimizu N, Yagi K. Cloning and chromosomal mapping of the human nonfunctional gene for L-gulono-gamma-lactone oxidase, the enzyme for L-ascorbic acid biosynthesis missing in man. J. Biol. Chem. 1994;269:13685–13688. [PubMed] [Google Scholar]
- Nittis T, Gitlin JD. Role of copper in the proteosome-mediated degradation of the multicopper oxidase hephaestin. J Biol. Chem. 2004;279:25696–25702. doi: 10.1074/jbc.M401151200. [DOI] [PubMed] [Google Scholar]
- Ozawa H, Nakamoto N, Kodama H. Clinical manifestations for early diagnosis of the patient with classical Menkes disease. No To Hattatsu. 2002;34:387–390. [PubMed] [Google Scholar]
- Pawelzik H, Hughs DI, Thomson AM. Physiological and morphological diversity of immunocytochemically defined parvalbumin- and cholecystokinin-positive interneurons in the CA1 of the adult rat hippocampus. J. Comp. Neurol. 2002;443:346–367. doi: 10.1002/cne.10118. [DOI] [PubMed] [Google Scholar]
- Pelz K, Dark J. ICV NPY Y1 agonist but not Y5 agonist induces torpor-like hypothermia in cold-acclimated Siberian hamsters. Am. J Physiol. Reg. Integr. Comp. Physiol. 2007;292:R2299–R2311. doi: 10.1152/ajpregu.00790.2006. [DOI] [PubMed] [Google Scholar]
- Penland JG, Prohaska JR. Abnormal motor function persists following recovery from perinatal copper deficiency in rats. J. Nutr. 2004;134:1984–1988. doi: 10.1093/jn/134.8.1984. [DOI] [PubMed] [Google Scholar]
- Prigge ST, Mains RE, Eipper BA, Amzel LM. New insights into copper monooxygenases and peptide amidation: structure, mechanism and function. Cell Mol. Life Sci. 2000;57:1236–1259. doi: 10.1007/PL00000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prohaska JR, Broderius M. Plasma peptidylglycine alpha-amidating monooxygenase (PAM) and ceruloplasmin are affected by age and copper status in rats and mice. Comp. Biochem. Physiol B Biochem. Mol. Biol. 2006;143:360–366. doi: 10.1016/j.cbpb.2005.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prohaska JR, Broderius M, Brokate B. Metallochaperone for Cu,Zn-superoxide dismutase (CCS) protein, but not mRNA is higher in organs from copper deficient mice and rats. Arch. Biochem. Biophys. 2003;417:227–234. doi: 10.1016/s0003-9861(03)00364-3. [DOI] [PubMed] [Google Scholar]
- Prohaska JR, Bailey WR. Regional specificity in alterations of rat brain copper and catecholamines following perinatal copper deficiency. J Neurochem. 1994;63:1551–1557. doi: 10.1046/j.1471-4159.1994.63041551.x. [DOI] [PubMed] [Google Scholar]
- Prohaska JR, Gybina AA. Intracellular copper transport in mammals. J. Nutr. 2004;134:1003–1006. doi: 10.1093/jn/134.5.1003. [DOI] [PubMed] [Google Scholar]
- Prohaska JR, Gybina AA, Broderius M, Brokate B. Peptidylglycine-alpha-amidating monooxygenase activity and protein are lower in copper-deficient rats and suckling copper-deficient mice. Arch. Biochem. Biophys. 2005;434:212–220. doi: 10.1016/j.abb.2004.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prohaska JR, Wells WW. Copper deficiency in the developing rat brain: a possible model for Menkes' steely hair disease. J Neurochem. 1974;23:91–98. doi: 10.1111/j.1471-4159.1974.tb06920.x. [DOI] [PubMed] [Google Scholar]
- Puig S, Thiele DJ. Molecular mechanisms of copper uptake and distribution. Curr. Opin. Chem. Biol. 2002;6:171–180. doi: 10.1016/s1367-5931(02)00298-3. [DOI] [PubMed] [Google Scholar]
- Rajagopal C, Francone V, Mains RE, Eipper BA. Multiple phosphorylation, cleavage, and nuclear translocation of the unstructured PAM cytosolic domain. J. Biol. Chem. 2009 in press. [Google Scholar]
- Ridge PG, Zhang Y, Gladeyshev VN. Comparative genomic analyses of copper transporters and cuproproteomes reveal evolutionary dynamics of copper utilization and its link to oxygen. PLoS ONE. 2008;3:e1378. doi: 10.1371/journal.pone.0001378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlief ML, Craig AM, Gitlin JD. NMDA receptor activation mediates copper homeostasis in hippocampal neurons. J. Neurosci. 2005;251:239–246. doi: 10.1523/JNEUROSCI.3699-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlief ML, Gitlin JD. Copper homeostasis in the CNS. Mol. Neurobiol. 2006;33:81–90. doi: 10.1385/MN:33:2:81. [DOI] [PubMed] [Google Scholar]
- Schlief ML, West T, Craig AM, Holtzman DM, Gitlin JD. Role of Menkes copper-transporting ATPase in NMDA receptor-mediated neuronal toxicity. Proc. Natl. Acad. Sci. U. S. A. 2006;103:14919–14924. doi: 10.1073/pnas.0605390103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SD, Raghuraman G, Lee MS, Prabhakar NR, Kumar GK. Intermittent hypoxia activates peptidylglycine alpha-amidating monooxygenase in rat brain stem via reactive oxygen species-mediated proteolytic processing. J Appl. Physiol. 2009;106:12–19. doi: 10.1152/japplphysiol.90702.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheela SR, Latha M, Lui P, Lem K, Kaler SG. Copper-replacement treatment for symptomatic Menkes disease: ethical considerations. Clin. Genet. 2005;68:278–283. doi: 10.1111/j.1399-0004.2005.00496.x. [DOI] [PubMed] [Google Scholar]
- Stephens DP, Saad AR, Bennett LA, Kosiba WA, Johnson JM. Neuropeptide Y antagonism reduces reflex cutaneous vasoconstriction in humans. Am. J Physiol. Heart Circ. Physiol. 2004;287:H1404–H1409. doi: 10.1152/ajpheart.00061.2004. [DOI] [PubMed] [Google Scholar]
- Steveson TC, Ciccotosto GD, Ma XM, Mueller GP, Mains RE, Eipper BA. Menkes protein contributes to the function of peptidylglycine alpha-hydroxylating monooxygenase. Endocrinology. 2003;144:188–200. doi: 10.1210/en.2002-220716. [DOI] [PubMed] [Google Scholar]
- Thorn NA, Nielson FS, Jeppsen CK, Christensen BL, Farver O. Uptake of dehydroascorbic acid and ascorbic acid to isolated nerve terminals and secretory granules from ox neurohypophyses. Acta. Physiol. Scand. 1986;128:629–638. doi: 10.1111/j.1748-1716.1986.tb08021.x. [DOI] [PubMed] [Google Scholar]
- Tirassa P, Costa N, Aloe L. CCK-8 prevents the development of kindling and regulates the GABA and NPY expression in the hippocampus. Neuropharmacology. 2005;48:732–742. doi: 10.1016/j.neuropharm.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Tirassa P, Manni L, Aloe L, Lundeberg T. Cholecystokinin-8 and nerve growth factor: two endogenous molecules working for the upkeep and repair of the nervous system. Curr. Drug Targets CNS Neurol. Disord. 2002;1:495–510. doi: 10.2174/1568007023338978. [DOI] [PubMed] [Google Scholar]
- Tschenett A, Siongewald N, Carli M, Balducci C, Salchner P, Vezzani A, Herzog H, Sperk G. Reduced anxiety and improved stress coping ability in mice lacking NPY-Y2 receptors. Eur. J Neurosci. 2003;18:143–148. doi: 10.1046/j.1460-9568.2003.02725.x. [DOI] [PubMed] [Google Scholar]
- Tumer Z, Horn N. Menkes disease: recent advances and new aspects. J Med. Genet. 1997;34:265–274. doi: 10.1136/jmg.34.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldbye DP, Larsen PK, Mikkelsen J, Klemp K, Madsen TM, Bolwig TG. Powerful inhibition of kainic acid seizures by Neuropeptide Y via Y5-like receptors. Nat. Med. 1997;3:761–764. doi: 10.1038/nm0797-761. [DOI] [PubMed] [Google Scholar]
- Woldbye DP, Madsen TM, Larsen PK, Mikkelsen J, Bolwig TG. Neuropeptide Y inhibits hippocampal seizures and wet dog shakes. Brain Res. 1996;737:162–168. doi: 10.1016/0006-8993(96)00730-5. [DOI] [PubMed] [Google Scholar]
- Woldbye DP, Nanobashvili A, Sorensen AT, Ernfors P, Kokaia M. Differential suppression of seizures via Y2 and Y5 receptors. Neurobiol. Dis. 2005;20:760–772. doi: 10.1016/j.nbd.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Woods A, Stock M. Inhibition of brown fat activity during hypothalamic stimulation in the rat. Am. J Physiol. 1996;270:R605–R613. doi: 10.1152/ajpregu.1996.270.3.R605. [DOI] [PubMed] [Google Scholar]
- Ye JM, Clark MG, Colquhoun EQ. Constant-pressure perfusion of rat hindlimb shows alpha- and beta-adrenergic stimulation of oxygen consumption. Am. J Physiol. 1995;269:E960–E968. doi: 10.1152/ajpendo.1995.269.5.E960. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.