Abstract
Although substantial evidence indicates that the progression of pathological changes of the neuronal cytoskeleton is crucial in determining the severity of dementia in Alzheimer's disease (AD), the exact causes and evolution of these changes, the initial site at which they begin, and the neuronal susceptibility levels for their development are poorly understood. The current clinical criteria for diagnosis of AD are focused mostly on cognitive deficits produced by dysfunction of hippocampal and high-order neocortical areas, whereas non-cognitive, behavioural, and psychological symptoms of dementia such as disturbances in mood, emotion, appetite, and wake-sleep cycle, confusion, agitation, and depression, have been less considered. The early occurrence of these symptoms suggests brainstem involvement, and more specifically of the serotonergic nuclei. In spite of the fact that the Braak staging system and NIA-RI criteria do not include their evaluation, several recent reports drew attention to the possibility of selective and early involvement of raphe nuclei, particularly the dorsal raphe nucleus (DRN), in the pathogenesis of AD. Based on these findings of differential susceptibility and anatomical connectivity, a novel pathogenetic scheme of AD progression was proposed. Although the precise mechanisms of neurofibrillary degeneration still await elucidation, we speculated that cumulative oxidative damage may be the main cause of DRN alterations, as the age is the main risk factor for sporadic AD. Within such a framework, β–amyloid production is considered only as one of the factors (although a significant one in familial cases) that promotes molecular series of events underlying AD-related neuropathological changes.
Keywords: aging, Alzheimer's disease, behavioural and psychological symptoms, cerebrospinal fluid, dorsal raphe nucleus, early diagnosis, fetal brain development, neurofibrillary degeneration, serotonin, tau protein
Introduction
Many neurological conditions, and particularly neurodegenerative diseases, involve the selective loss of specific neuronal populations. One notable example is Alzheimer's disease (AD) in which an identifiable subpopulation of large, cortically projecting, pyramidal neurons in high-order regions of the neocortex, are preferentially affected in the course of the disease [1, 2]. These neurons share a particular morphologic and neurochemical phenotype characterized by their size, distribution, and connectivity and their high expression of dephosphorylated epitopes of neurofilament proteins. They are affected by neurofibrillary tangles (NFTs) formation at very early stages of AD, degenerate at a faster rate than other pyramidal neurons, and shrink significantly in the process [2, 3]. However, even more than 100 years after its initial clinicopathological description, no satisfactory explanation for the neuronal specificity of the degenerative process in AD is available.
The amyloid cascade hypothesis began to take shape in late 1980s when a gene on chromosome 21 that produces amyloid precursor protein (APP) was discovered [4]. In short, the APP gene contains the sequence for the β–amyloid peptide, which is concentrated in the senile plaques (SPs) used to diagnose AD (Table 1). Some people who inherit a form of early-onset familial AD have a mutation in the APP gene that results in the overproduction of the β–amyloid. Similarly, people with Down syndrome, who invariably develop AD in middle age, have an extra copy of chromosome 21 containing the APP gene, causing them to produce excess amount of the β–amyloid protein [5]. A variety of enzymes in the brain normally process APP into soluble, seemingly harmless fragments. However, a specific β–secretase cleaves APP in its extracellular domain and leaves a fragment (known as C99) that is secondarily cleaved by the transmembrane γ-secretase complex (that includes presenilin) to cleave APP to yield the β–amyloid peptide. It is now well established that β–amyloid exists as monomers, oligomers, and fibrils. It seems that β–amyloid peptide has a spontaneous tendency to form oligomers that eventually accumulate into diffuse deposits. Diffuse deposits of β–amyloid have been found in large numbers even in subjects whose intellectual status had been evaluated as normal both neuropathologically [6] and by in vivo visualization using Pittsburgh compound B (PIB PET, see below) [7]. It is not known how long diffuse deposits may remain asymptomatic in the brains of cognitively intact subjects and why only certain regions of the brain are susceptible to their “maturation” into focal deposits and neuritic (“mature”) plaques (characterized by a corona of degenerating neurites containing tau protein paired helical filaments around an amyloid core). One hypothetical view for maturation of diffuse α–helical deposits of β–amyloid into focal deposits made of firm β–pleated sheet fibrils involves the presence of copper and zinc ions [8]. The presence of unsoluble β–amyloid fibrils in the neuropil is thought to be one of the major mechanisms that compromises axoplasmic flow and induces neurofibrillary changes (see below).
Table 1.
Chronological list of major neuropathological criteria and recommendations for neuropathological diagnosis of AD.
| Author(s) and year | Criterion name and its main feature | Major advances | Major drawbacks | Conclusion |
|---|---|---|---|---|
| Khachaturian et al., 1985 | NIA criteria: quantification of SPs cortical densities | First widely accepted criterion | Clinical history and NFTs were not considered | Unsatisfying because SPs were shown to be partly a benign age-related phenomenon (Arriagada et al., 1992) and since clinical history, NPs and NFTs were not taken into account |
| Mirra et al., 1991 | CERAD criteria: semiquantitative assessment of the highest density of NPs in superior temporal gyrus, frontal cortex and parietal lobule from 0 (none) to C (abundant) | Combinationa of clinical history and NPs score; four levels of diagnostic certainty: normal brain, possible AD, probable AD and definite AD | NFTs and hippocampal formation are not considered | Criteria were abandoned because NFTs were shown to correlate best with dementia severity (Arriagada et al., 1992; Bierer et al., 1995; Grossi et al., 2007) |
| NIA-RI, 1997 | NIA-RI criteria: integrate CERAD and Braak criteria (Braak and Braak, 1991) thus evaluating „likelihood“ of AD-changes to cause dementia | Three levels of probability were proposed: high (CERAD NP C – abundant / BBV or VI), intermediate (CERAD NP B – moderate / BB III or IV) and low (CERAD NP A – sparse / BB I or II); include semiquantitative assessment of AD lesions in hippocampus, nucleus niger and LC | There is a considerable number of demented patients with AD who have low numbers of neocortical NFTs | The combination of BB NFTs stage equal to or above IV and CERAD NP stage C together with CDR equal to or above 1, provides the highest sensitivity and specificity; NIA-RI criteria are more specific than CERAD, but less sensitive |
Abbreviations: AD, Alzheimer's disease; BB, Braak and Braak stage; CDR, Clinical Dementia Rating; CERAD, Consortium to Establish a Registry for Alzheimer's Disease; LC, locus coeruleus; NIA, National Institutes of Aging; NIA-RI, National Institutes of Aging – Reagan Institute; NFTs, neurofibrillary tangles; NPs, neuritic plaques; SPs, senile plaques
On the other hand, it has been shown that even naturally secreted oligomers of β–amyloid may inhibit hippocampal long-term potentiation in vivo [9]. Moreover, it has recently been demonstrated that amyloid oligomers isolated from the brains of AD patients and injected into rodents are capable of disrupting NMDA receptors and impairing memory [10]. Thus, free-floating oligomers may affect synaptic function years if not decades before symptoms of AD appear. On these premises it can be further speculated that soluble amyloid oligomers interfere with neurotrophic factors released into the synaptic cleft, producing anterograde neurodegeneration during the evolution of AD. Obviously, if this concept is correct, the anti-amyloid therapies would be far more effective if started earlier, before the “toxic” oligomers have had time to alter synaptic transmission (at the same time this would confirm that formation of β–amyloid deposits is actually a defense mechanism). However, although most current research still follows the path charted by the concept of amyloid cascade hypothesis, it seems that efforts toward an effective treatment and prevention will require some new perspectives, particularly for sporadic AD. Namely, data collected so far strongly indicate that mutations in PRESENILIN-1 (PS-1), PRESENILIN-2 (PS-2) and AMYLOID PRECURSOR PROTEIN (APP) genes have more widespread effects on broader range of cellular functions compared to sporadic cases [11]. One obvious example is that the enhanced γ-secretase production of β–amyloid1–42 is not a feature of sporadic AD.
Current state of knowledge
In recent years it became clearer that cerebrospinal fluid (CSF) biomarkers, particularly phosphorylated tau proteins, demonstrate a higher predictive value over algorithms comprised of clinical, neuropsychological and imaging modalities (MRI, SPECT, PET) [12]. Since the use of some of those biomarkers has been improved to a level that relatively early and reliable diagnosis of AD became possible, CSF examination is becoming a part of the routine diagnostic workup for suspected AD in both clinical and neuropathological evaluation of a patient.
Considering the therapy of AD besides the currently registered cholinomimetic agents for early (donepezil, galantamin, rivastigmine, tacrine) and non-competitive antagonist of glutamatergic NMDA receptors (which is also an antagonist of serotonin 5-HT3 receptors) for moderate/late AD (memantin), new drugs, mainly β– and γ–secretase inhibitors, were recently being developed and are currently being tested through various phases of clinical trials in order to prevent the formation and accumulation of the β–amyloid peptide, at least in those subjects with familial early-onset AD (FAD) due to mutations in PS-1, PS-2 and APP genes [13]. However, for the vast majority of sporadic, late-onset AD subjects, the neuropathological evidence collected so far does not support the amyloid cascade hypothesis, which is considered too narrow to explain all of the molecular mechanisms that lead to the characteristic accumulation of the neuropathological hallmarks of the disease [14]. It is therefore not suprising that all therapeutic efforts aimed at lowering β–amyloid production and aggregation (including passive and active immunizations) failed to show clinical improvement in AD patients [14].
Unanswered questions
The original amyloid hypothesis contains a conspicious inconsistency that the number of SPs found in elderly brains does not correlate with dementia. On the contrary, substantial evidence has been gathered that the progression of pathological changes of the neuronal cytoskeleton is crucial in determining the severity of dementia in AD [12, 15–17]. This fact is strengthened by the numerous reports showing that mutations of TAU gene cause hereditary tauopathies with dementia, most notably frontotemporal lobar degeneration (FTLD), in absence of β–amyloid accumulation [18–21]. Although there has been some experimental support for a possible direct causal relationship between the fibrillar β–amyloid [22] or more recently the intraneuronal prefibrillar β–amyloid1–40, β—amyloid1–42 and β–amyloid oligomers [23–25] and the acute hyperphosphorylation of tau proteins (that is presumed to be continued into full-blown neurofibrillary degeneration) in several cell cultures and transgenic animal models, the exact causes and evolution of human AD-related cytoskeletal changes, their starting point, mechanisms of spreading and the precise causal relationships between plaques and tangles all await elucidation. These problems are also related to the question of AD as a single disease. From the genetic, clinical, and neuropathological point of view, AD is a multifactorial and very heterogeneous disorder [14, 26]. Nevertheless, the existence of a common pathophysiological mechanism together with spreading of neurofibrillary degeneration in a more or less orderly fashion in most patients, would justify keeping AD as a single disease as originally believed by Alzheimer himself.
Why only certain types of neurons are preferentially affected in the brain, principally the cerebral cortex, in a layer- and region-specific manner remains unclear. There is ample evidence that in the neocortex, large pyramidal neurons in the deep part of layer III (layer IIIc) and the superficial part of layer V (layer Va), characterized by high somatodendritic levels of nonphosphorylated neurofilament protein are selectively vulnerable in the course of AD [2]. Stereologic analyses showed that their numbers decrease dramatically in cases with definite dementia compared to other neurons not characterized by this phenotype, to a nearly complete loss (> 90%) in endstage AD [3]. These neurons shrink considerably (by about 50% of their initial perikaryal volume) during NFT formation, the ones larger than 6,000 μm3 being preferentially affected. Stereologic data show that these neurons are lost at a faster rate in the course of AD than other and smaller pyramidal neurons, which being less prone to NFT formation, may die through other mechanisms in AD, such as oxidative stress, abortive apoptosis (abortosis), and excitotoxic mechanisms [27–30]. Considering that these neurofilament enriched neurons represent only about 30% of the total number of pyramidal neurons in dorsolateral prefrontal cortex [3], their preferential involvement suggests that highly specific cortical pathways are at higher risk for degeneration in AD and that the neurons providing such projections represent important cortical targets for early neuroprotective interventions in AD. In addition to the known alterations of myelin sheath integrity observed during normal brain aging in primates [32], the progressive loss of white matter that is observed over the course of AD [31] ultimately leads to a syndrome of neocortical disconnection. The periventricular white matter changes (leukoaraiosis) that are commonly seen in AD and are associated with low cognitive performance, seem to result from a combination of ischaemia and a failure of CSF to be drained along perivascular pathways that are blocked by the deposition of β–amyloid (cerebral amyloid angiopathy, CAA) and fibrosis in the walls of arteriolosclerotic arteries in the white matter [33]. As a consequence, CSF infuses into periventricular white matter thus increasing signal in T2 weighted MRI images [34, 35].
Neuropathological diagnostic criteria for AD
NIA (Khachaturian) criteria
The first neuropathological diagnostic criteria for AD were based on quantification of minimal SPs cortical densities as a function of age [36] (Table 1). They were not broadly accepted because SP formation was later shown to be partly a benign age-related phenomenon (making criteria less sensitive) [15]. Moreover, the cortical regions for quantification as well as the role of the clinical history were not well defined (making criteria less specific and non-comparable) and neurofibrillary changes (NFTs, neuritic plaques, and neuropil threads) were not considered. Therefore, another set of standardized criteria known as CERAD (Consortium to Establish a Registry for Alzheimer's disease) was proposed [37].
CERAD criteria
CERAD semiquantitative criteria were determined as a function of neocortical neuritic plaques (NPs) development in the superior temporal gyrus, prefrontal cortex and lower parietal lobule using modified Bielschowsky or thioflavin S staining in three age groups (less than 50, 50 to 75, and over 75 years) [37] (Table 1). Based on the combination of clinical information and NPs score, three levels of diagnostic certainty were assessed (definite, probable, or possible AD). Besides the fact that the hippocampal formation was again absent from criteria (despite its well known characteristic and crucial involvement in the initial stages of typical AD), the major weakness of CERAD criteria was that they relied chiefly upon the overproduction and accumulation of β–amyloid, and did not consider neocortical NFTs. Unlike SPs, except in low quantities in the entorhinal cortex [38, 39], neocortical NFTs are not present in normal aging and were later confirmed (in contrast to SPs) to correlate strongly with dementia severity [12, 15]. However, although clinico-pathological correlations showed the best correlation of dementia with the quantity of NFTs, the pattern of synapse and neuron loss in AD does not necessarily match the pattern of NFTs formation [29, 38, 40].
Braak and Braak staging system
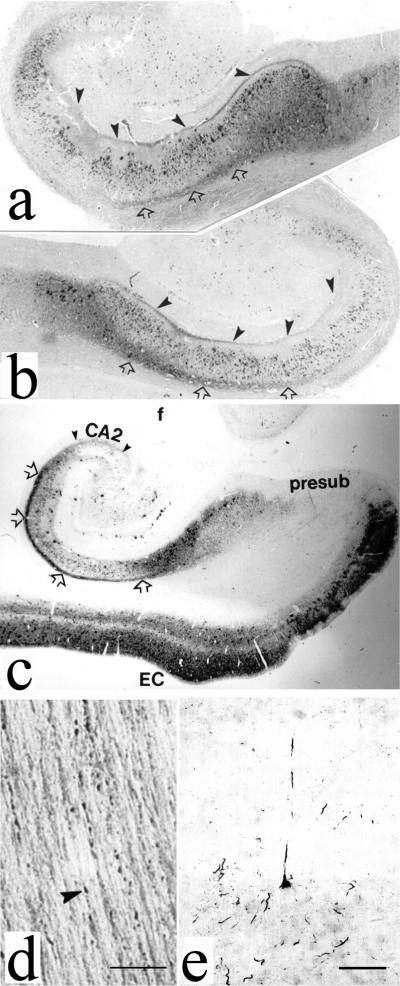
In the meantime, the Braak and Braak Staging System (BBSS) became widely accepted for scoring the neurofibrillary changes in AD [41]. The BBSS classifies the progression of AD neurofibrillary degeneration in six stages, spreading from the transentorhinal region (stage I) to the hippocampal formation (stage II), the temporal, frontal, and parietal neocortex (stages III and IV), and finally to unimodal and primary sensory and motor areas of the neocortex (stages V and VI). Illustration of early neurofibrillary changes in entorhinal and temporal cortex and hippocampal formation in two patients who fulfilled clinical criteria for AD is given in Figure 1. In short, our observations indicate that changes in limbic tau pathology spread in a highly ordered manner from layer II entorhinal neurons to cells located in deeper entorhinal layers, hippocampal formation and temporal isocortex. For example, the epitope Ser199/Ser202/Thr205 (as revealed by monoclonal antibody AT8) has been always found phosphorylated before the Ser396/Ser404 epitope (monoclonal antibody AD2) [42, 43], whereas cleaved tau (detected by antibody MN423) could not be found in layers free of phosphorylated tau at these sites [43].
Fig. 1.
Illustration of neurofibrillary changes, which in AD spread in an anterograde fashion through identified neuronal circuits due to yet unknown cause(s). All figures were obtained using the mouse monoclonal antibody AT8 (Innogenetics, Temse, Belgium) used at a 1:200 dilution in indirect immunocytochemistry. It should be stressed that AT8 reacts with tau only when multiple sites around Ser202, including Ser199, Ser202, and Thr205, are phosphorylated. Single phosphorylation of any of these residues is not enough for AT8 reactivity. Thus, AT8 immunoreactivity is useful in detecting phosphorylation of Ser202/Ser202/Thr205 for proline-directed kinases. a. Left hippocampus of 73-year old AD man who fulfilled NINCDS-ADRDA and DSM-IV-TR clinical criteria for AD and had a 7-year history of slowly progressive cognitive deterioration and various behavioral symptoms (middle level of hippocampal body, cause of death was bronchopneumonia), black arrowheads show perforant pathway fibers (originating from layer II islands of entorhinal cortex) that contact the apical dendrites of CA1 pyramidal neurons directly, open arrows denote subicular axons, magnification 18.75x; b. Right hippocampus of 83-year old woman with AD (middle level of hippocampal body), who had behavioral and memory problems for 5.5 years before death from myocardial infarction and was also fulfilling NINCDS-ADRDA and DSM-IV-TR clinical criteria for AD; c. Left hippocampus of the same case as in a; black arrowheads delineate CA2 region (most tangle-bearing neurons are not AT8-immunoreactive). Legend: prosub, prosubiculum; EC, entorhinal cortex; magnification 10x. d. Beaded-like appearance of AT8-positive axons, same subject as in a and c, scale bar = 10 μm, e. Early neurofibrillary changes of a temporal neocortex layer V pyramidal neuron, scale bar = 100 μm.
NIA-RI criteria
In an attempt to reconciliate the amyloid hypothesis with the major role of NFT in clinicopathological correlations, in 1997, the more rigorous National Institute on Aging - Reagan Institute (NIA-RI) consensus recommendations for the postmortem diagnosis of AD were issued [44] (Table 1). NIA-RI criteria use CERAD protocols for tissue processing, as well as a semi-quantitative assessment of AD lesions that must be made in several neocortical areas, hippocampus, substantia nigra and locus coeruleus, while also taking into account the BBSS for neurofibrillary changes. In contrast to CERAD criteria, which incorporate clinical data to provide a neuropathological diagnosis, NIA-RI criteria primarily aim to define the probability that clinical dementia was really due to AD-related lesions. Because NIA-RI criteria take into account the apparent number of neocortical NFT, in comparison to CERAD they are more specific but less sensitive, as many AD patients have low numbers of neocortical NFT [45]. As such, definitive neuropathological criteria for AD are still lacking.
The role of reactive processes in the pathogenesis of AD
Generally, the AD pathological changes can be broadly divided into three categories: lesions related to accumulation (“positive lesions”), lesions due to losses (“negative lesions”) and those related to the reactive processes such as inflammation and plasticity (“reactive lesions”) [46]. The losses of synapses and neurons are probably most directly related to the neurological deficits observed in AD patients. However, since they are difficult to estimate and evaluate neuropathologically, so far only the “positive lesions” that are easy to detect constituted the basis for the postmortal diagnotic criteria. On the other hand, the reactive processes are often thought to appear late during the course of the disease and were therefore not considered adeqate to be included in the neuropathological diagnostic criteria for AD.
Neuroinflammation as a possible link between amyloid deposition and neurofibrillary degeneration
It has been recently suggested that microglial activation may occur very early during the course of AD and that neuroinflammation may represent the possible link between amyloid deposition and neurofibrillary degeneration. A recent study investigating plasma biomarkers that could predict conversion from MCI to clinical AD identified raised levels of several cytokines and interleukins associated with inflammation (TNFα, IL-3, IL-1α and IL-11), which are secreted by microglial cells [47]. In turn these pro-inflammatory factors may change the substrate specificity of kinases/phosphatases leading to tau phosphorylation at pathological sites [48]. Albeit indirect, further evidence to support apossible link between inflammation and AD comes from increased risk of dementia in people who suffered a serious head injury [49, 50], patients with cerebral ischaemia [51] and subjects with Type 2 diabetes mellitus and insulin resistance [52, 53]. All of these three conditions are associated with increased production of pro-inflammatory molecules and a higher propensity to develop AD or to exacerbate AD symptoms.
The key role of tau protein in development and progression of cytoskeletal neurofibrillary changes and reactivation of fetal plasticity in AD
The key role of microtubule-associated protein tau in AD and related neurodegenerative disorders stems from its roles in neural development and cytoskeleton maintenance. Under normal circumstances, by binding to microtubules, tau regulates their stability and dynamics [54]. The human protein tau is encoded by a single gene located on the long arm of chromosome 17. Tau promoter activity increases significantly during neurite initiation and outgrowth. The human tau primary transcript contains 13 exons of which exons 2, 3 and 10 alternatively spliced and give rise a family of six isoforms [55]. As in aged and cognitively impaired animals the neurofibrillary degeneration due to abnormal phosphorylation of tau protein is scarcely found, the unique expression pattern of tau isoforms in the human central nervous system has been suggested as a possible trade-off between increased cognitive capabilities and decreased resistance to neurodegenerative disorders with tauopathy, especially AD and FTLD [56]. Except for AD and adult cases of Down syndrome that are characterized by both neurofibrillary pathology due to abnormally phosphorylated tau and β–amyloid plaques, all other tauopathies, such as FTLD due to tau mutations, argyrophilic grain disease, corticobasal degeneration and progressive supranuclear palsy (PSP), boxer's dementia, Guam parkinsonism-dementia complex, pallido-ponto-nigral degeneration, and others [57], are characterized by neurofibrillary pathology in the absence of β–amyloid plaques [21, 58]. In all of these tauopathies, the tau mutation is sufficiently potent to induce tau aggregation and NFTs in absence of β–amyloid, and is associated with cognitive deterioration and dementia (later appearance of β–amyloid may accelerate this process significantly) [59]. Furthermore, in many sporadic cases of AD, particularly in subjects over 80-year of age, the so-called „tangle only“ AD may be the predominant form [60] comprising about 4% of all AD cases [61].
The molecular species and biological activity of tau is developmentally controlled mainly by phosphorylation of its six isoforms [55, 62, 63]. The majority of the phosphorylation sites are clustered in the flanking regions of the microtubule-binding domain [55]. In turn, tau proteins change physical characteristics of MTs such as rigidity, length, stability and integrative capacity with other organelles. While normal tau promotes and stabilizes MTs, the non-fibrillized, abnormally hyperphosphorylated tau disrupts MTs, sequesters normal tau, decreases its turnover (due to increased resistance to degradation by calcium-activated neutral proteases, calpains, and ubiquitin-proteosome pathway), misfolds and finally promotes self-assembly into straight or paired helical filaments (PHF), thus impairing axoplasmic flow and leading to slow degeneration [14, 64]. In the fetal human brain, there is only one isoform referred to as fetal tau [65], which has three domains (3R) for binding to microtubules (MT). It is thought that the absence of expression of the fourth MT-binding domain during fetal development prevents MTs bundling [14] and allows for the cytoskeletal plasticity required for immature growing neurons [66, 67]. Adult tau isoforms have four MT-binding domains (4R tau) and are about 40-fold more efficient at promoting MT assembly [68–70]. The 4R tau protein and particularly the mutated tau protein studied in the most common missense mutations causing frontotemporal dementia with parkinsonism (G272V, P301L, V337M, and R406W) are more easily abnormally phosphorylated and more readily self-aggregate into filaments [67], which may explain an earlier onset and greater severity of the disease in the inherited FTDP-17 cases. As in situ hybridization analysis showed no significant change in the expression of tau mRNA in AD brain [71], the increase in tau level observed in AD brains is probably due to a decrease in its turnover caused by phosphorylation. Out of 80 putative Ser or Thr phosphorylation sites on the longest brain tau isoform, so far 45 of them have been characterized by monoclonal antibodies, mass spectrometry, and sequencing [14, 55]. Most of the phosphorylation sites are on Ser-Pro and Thr-Pro motives, but a number of sites on other residues have also been reported [72]. A different states of tau phosphorylation result from the activity of many different specific kinases, of which the most important are probably proline-directed (i.e., acting on serine/threonine followed by proline) kinases glycogen synthase kinase 3β (GSK-3β), mitogen-activated protein kinases ERK 1, ERK2, p70S6 and stress-activated kinases JNK and p38, cyclin-dependent protein kinase-5 (cdk5), and Dyrk1A [14, 73] because they have the ability to phosphorylate tau at a large number of sites. Although non-proline-directed protein kinases (such as microtubule affinity-regulating kinase, calcium/calmodulin kinase II, protein kinase A, and others) have been shown to phosphorylate tau at only a very limited number of sites, their importance may be underestimated because phoshorylation of tau by these enzymes significantly increases the phosphorylation of tau by GSK-3β and cdk5 [14]. The decreased activity of at least four different protein phosphatases (PP-1, PP-2A, PP-2B and PP-2C) can also lead to tau hyperphosphorylation in AD [55, 74]. Inhibitors of the main phosphatase PP-2A, such as I1PP-2A and I2PP-2A, thus also represent potential therapeutic targets for halting the progression of neurofibrillary degeneration in both AD and normal aging [14].
Hypothesis of reactivation of fetal plasticity in AD
Phosphorylation of tau proteins is high in the fetal period during axonal elongation (Figure 2), and decreases with advancing age due to activation of phosphatases [62, 63]. However, after about 30 years of age, yet unknown single or more probably multiple factors such as genetic dysfunction that may include both mutational (APP, PS1 and PS2, TAU genes) and diverse susceptibility loci (e.g., APOE, α2M, αACT, LRP1, IL1α, TNFα, ACE, BACE, BCHE, CST3, MTHFR, GSK3β, NOS3, mitochondrial CO1 and CO2), as well as environmental influences (e.g., head trauma), seem to converge to a single common pathogenic pathway, which cause the phosphorylation of tau to increase again (perhaps together with increased cleavage of tau, which may make tau an even more favorable substrate for abnormal hyperphosphorylation), causing tau polymerization and formation of straight and paired helical filaments that form NFTs, dystrophic neurites and neuropil threads [14]. Impaired brain glucose metabolism has also been shown to facilitate abnormal tau hyperphosphorylation in cultured cells and in mouse models of sporadic AD by at least two different mechanisms, abnormal glycosylation of tau [75] and impaired insulin receptor signalling cascade [76].
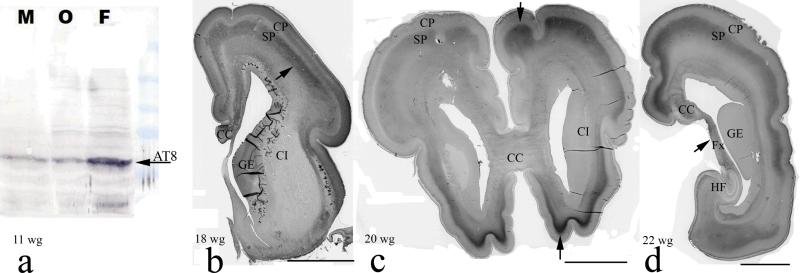
Fig. 2.
Tau protein phosphorylation during fetal brain development. a. Western blot of tau protein at 11weeks of gestation (w.g.) using monoclonal antibody AT8. Arrow shows positive bands from all three regions examined, particularly the frontal lobe, b. Arrow points to prominent AT8-immunoreactivity in the lower subplate zone of the frontal regions of the telencephalon at 18 w.g., c. At 20 w.g. AT8-immunoreactivity moves from lower to the upper subplate zone, d. During mid-gestation, the fornix as well as subset of callosal commissural fibers are unambiguously AT8-immunoreactive, whereas the hippocampal formation and internal capsule remained unstained; AT8-immunoreactivity then gradually appears in the cortical plate, diminishing and disappearing completely from the subplate zone at the end of 32 w.g. Fetal brains were part of the Zagreb Neuroembryological collection. Legend: M, mesencephalon, O, occipital lobe, F, frontal lobe, CP, cortical plate, SP, subplate, CI, internal capsule, GE, ganglionic eminence, CC, corpus callosum, Fx, fornix, HF, hippocampal formation, scale bars in b, c and d = 1 cm.
The genetic influences on lifespan were shown to be minimal prior to age 60 but increase thereafter [77]. Clearly, the aging process itself contributes to AD. However, AD should not be regarded as “accelerated aging” considering that many people live to extreme age with only minor loss of mental abilities. The autopsy performed on the world's oldest woman, who lived to be 115 years old, reported that her brain was virtually free of neuropathological signs of AD [78]. Besides the cumulative oxidative damage by reactive oxygen species (ROS) [79], which can be provoked by β–amyloid or its fragments [80], some of the hypotheses on putative initial triggers that may activate and speed-up AD-related pathological processes include proposal of neuronal failure due to plasticity burden [81], the potentially detrimental effect of Herpes simplex virus either through the activation of Toll-like receptor pathways in astrocytes and microglia or a change in the phosphorylation state of tau [82–84], brain ischaemia and ischaemic blood-brain barrier [85], and genetically programmed adaptive metabolic reduction [86]. One of the most plausible concepts that links plaques and tangles in a causative way stems from the idea that the key pathogenetic event and the main mechanism that induces physical damage to axons thus compromising axoplasmic flow is the fibrillogenesis of diffuse β–amyloid deposits (see above) [87]. Neuronal response to this kind of injury results in axonal sprouting [88], upregulation of tau mobilization and its excessive phosphorylation. In order to find the proper path to its targets and form synapses, in these regenerative processes of sprouting and pruning, axonal growth cones and their core MTs need to be less stable [88]. In other words, to induce this level of MTs plasticity, tau proteins need to be in a more phoshorylated state. As we found that the same epitope revealed by the antibody AT8 (Ser202/Ser202/Thr205) is phosphorylated both in growing fetal axons and in degenerating axons of AD brains (compare Fig. 1 and Fig. 2), and taking into account the fact that AT8-immunoreactivity permits evaluation of neuronal changes well before the actual formation of neurofibrillary tangles and neuropil threads [30, 89], we speculate neurofibrillary degeneration at least in its earliest stages may include pathological activation of some fetal proline-directed protein kinases, such as one of 100 kDa weight that phosphorylates Ser262 of tau protein [90]. In turn, such kinases may reactivate fetal plasticity mechanisms through phoshorylation of tau protein, this time however in degenerating axons of AD brains, further contributing to the development and progression of AD-related pathological changes.
Clinical criteria for AD and problems of antemortem diagnosis
Clinical criteria for AD and the concept of amnestic mild cognitive impairment (MCI)
The introduction of potential new treatments and drug-modifying therapies such as γ- and β-secretase inhibitors, blockers/inhibitors of β–amyloid aggregation and fibrillization, microtubule stabilizers, muscarinic agonists and others that are supposed to have the best effect if introduced early, as well as potentially better symptom-management therapies (5-HT1a agonists and antagonists, 5-HT4 agonists, mitochondrial permeability modulators, calcium channel blockers), increased awareness about AD in the population and have facilitated patients and their families to seek medical advice earlier during the course of the disease [91]. Therefore, in recent years, the preclinical phase of AD with mild memory impairment, but without overt dementia, has increasingly attracted attention.
According to all clinical criteria recommended so far, AD cannot be diagnosed until dementia is present. Although the NINCDS-ADRDA criteria [92] have a relatively high accuracy rate (around 80–90%) [93, 94], these percentages come from specialized expert research academic centers and are based on patients in later stages of the disease who were followed longitudinally for several years before autopsy. In clinical settings, there is no clinical method to determine which patients with mild cognitive impairment (MCI) will progress to dementia, except for a very long clinical follow-up.
Mild cognitive impairment is an aetiologically heterogeneous potential precursor stage of AD, defined by cognitive impairment, that can be shown by objective neuropsychological measures adjusted for age and education. About 40–60% of amnestic MCI patients develop AD during the first 5 years [95], whereas the remainder of MCI patients have a less progressive form of memory impairment [95, 96]. It shoud be kept in mind that other types of dementia, such as dementia with Lewy bodies, vascular dementia, and argyrophilic grain disease (Braak's dementia), may also be preceded by MCI [96, 97]. The amnestic subtype of MCI (i.e., memory complaint with objective memory impairment, but with preservation of general cognitive functioning without or with minimum impairment of activities of daily living) mostly represents prodromal AD [98], which is confirmed neuropathologically [99].
The major pitfall when studying CSF biomarkers to predict AD in MCI cohorts is the fact that conversion from MCI to AD is only about 12–15% per year (in contrast, only 1–2% of healthy older populations convert to AD per year) [95]. Therefore, only an extensive follow-up time (>5 years) of patients with stable MCI might further increase the specificity of CSF biomarkers [12]. On the other hand, neither the clinical nor the pathological aspects of AD evolve in a linear manner, but the predictable sequence of AD pathology allows for stage-based correlations with cognitive and behavioral symptomatology [100]. Besides CSF biomarkers, future neuropathological criteria and recommendations should therefore include more sensitive and specific immunohistochemical and immunocytochemical subgrouping [101] and neuropathological grading of AD.
Another crucial problem of antemortem diagnosis is that the reliability of both clinical and neuroimaging methods is a function of disease severity, and therefore, there is a risk of increasing overlap with non-AD pathology, psychiatric illnesses, and healthy aging (ICD-10, DSM-IV-TR, NINCDS-ADRDA) [92, 102, 103], particularly in presence of comorbidities and cases of atypical AD syndromes, such as anterior (frontotemporal) and posterior (parietotemporo-occipital) variants of AD, focal, lobar or gyral progressive degenerative syndromes [104]. Moreover, the clinical spectrum of symptoms of these conditions more closely corresponds to the functional anatomy of the affected brain areas than to the underlying pathology.
The current clinical criteria for diagnosis of dementia in AD are focused mostly on cognitive deficits produced by dysfunction of hippocampal and high-order neocortical areas [92, 102, 103]. In other words, during the neuropsychological part of the diagnostic procedure, clinicians are looking for impairment of memory for recent events (Braak stages I and II), impaired recall, delayed word recall and word finding difficulties, disorientation in time and space, and impaired concentration and comprehension (Braak stages III and IV), and disturbances in perceptual and motor skills (Braak stages V and VI) [41]. These neuropsychological findings are scored as number of points that, in the most commonly used Mini-Mental State Examination (MMSE), ranges from 0–30 points, where 30 reflects normal mental status across many cognitive domains [105]. Although many other tests for neuropsychological screening for AD are available, they are not used as much as MMSE, which has been criticized along several lines, including sensitivity to educational level, poor sensitivity and specificity, and poor negative predictive value (NPV) and positive predictive value (PPV), especially in early-stage disease [106] (see below).
Imaging modalities to diagnose MCI or early dementia in AD are tightly bound to the measurement of the structural and functional changes in the cerebral cortex, but not other, subcortical structures (see below why authors think this should be changed). Annual rates of cortical atrophy were found to be significantly different in normal aging (0.2–0.5%) and AD (2–3%) [107], particularly in the entorhinal cortex and hippocampus, as revealed by structural MRI volumetric measurement [108–112]. An even earlier characteristic sign of incipient AD is temporoparietal hypometabolism, as assessed by [18F]fluorodeoxyglucose (FDG) PET [113–115]. Recent neuroradiological advances now also allow the measurement of alterations in the white matter integrity in patients with MCI and AD using diffusion tensor imaging (DTI) [116], diffusion tensor tractography (DTT) [117] and the in vivo investigation of amyloid pathology, visualized by Pittsburgh Compound B PET (PIB PET) [118, 119], while neurofibrillary tangles and neuritic plaques may be visualized using 2–(1–(6–[(2–[18F]fluoroethyl)(methyl)amino]-2-naphthyl)ethylidene)malononitrile ([18F]FDDNP PET) [120–123]. Comparisons of PIB PET and FDG PET in AD have shown a reciprocal relationship most clearly in parietal regions, while studies in MCI subjects have shown a bimodal distribution of increased PIB binding, with one group demonstrating PIB retention at the level of AD subjects and the other only non-specific binding [124, 125]. Although differences in binding patterns between patients with AD, MCI and controls were reported to be more pronounced for 11C-PIB than for 18F-FDG PET [126] and for 18F-FDDNP [125], a positive relationship between the severity of depression and anxiety symptoms and 18F-FDDNP binding values that was found in nondemented middle age and older individuals, suggested a potentially important relationship between relatively mild mood symptoms and biomarkers of cerebral amyloid and tau deposition [123].
Clinicopathological correlations of behavioural symptoms and pathology of raphe nuclei
Behavioral and psychological symptoms in AD
Interestingly, in a study of 1,070 non-demented individuals, those who had depressed mood at baseline were found to have an increased relative risk of dementia of 2.94, even after adjustments made for age, gender, educational level and language [127], while in a longitudinal study of 235 patients with early probable AD only 8.5% were free of non-cognitive, so-called Behavioral and Psychological Symptoms of Dementia (BPSD), such as disturbances in mood, emotion, appetite and wake-sleep cycle, “sundowning”, confusion, agitation, depression and others, during the first 3 years of follow up [128]. A retrospective review of 100 autopsy-confirmed AD cases found that, on average, depression, mood change, social withdrawal and other BPSD were documented more than 2 years before the diagnosis of AD was made (the earliest non-cognitive symptom appeared, on average, 33 months before diagnosis) [129]. The early occurrence of BPSD is suggestive of early brainstem involvement and more specifically of serotonergic nuclei. However, although many published investigations initially reported early involvement of the brainstem nuclei by neurofibrillary degeneration during the course of AD (particularly the raphe nuclei - see Table 2), due to the fact that BBSS did not include raphe nuclei in postmortem investigation of AD-related pathology (BBSS criteria was later incorporated into NIA-RI criteria for neuropathological diagnosis of AD), their pathology in AD has not been systematically studied. Because age is the main risk factor for sporadic AD, for us it is logical to assume that a cumulative oxidative damage by ROS, particularly of mitochondrial DNA [130] and RNA (RNA seems to be more susceptible to oxidative insults than DNA since RNA is largely single-stranded, its bases are not protected by hydrogen bonding and specific proteins and it locates in the vicinity of mitochondria, the primary source of ROS) [131], together with other susceptibilities for “wear and tear” of somatic cells, may represent the main potential cause of neurofibrillary degeneration of neurons in DRN. Such a situation resembles the centrality and early involvement of substantia nigra in Parkinson's disease. Within such a framework, β–amyloid should be considered just as one of the factors (although obviously a very significant one in familial cases) that promote or speed-up the molecular series of events underlying AD-related neuropathological changes.
Table 2.
Chronological list of selected articles relevant for the transneuronal spreading of AD-related cytoskeletal changes
| Author(s) and year | Goal / Hypothesis | No. AD | No. CON | Major findings / Premises | Conclusion |
|---|---|---|---|---|---|
| Mann et al., 1983 | The goal of the study was to determine the role of serotonin-producing cells in AD by counting neuron numbers of the DRN | 20 | 22 | The major finding was 12% cell loss in DRN in AD | Biased methodology of estimation of nerve cell loss and frequency of NFTs from densities assessed, together with sampling done only at the level of trochlear nucleus prevents drawing firm conclusions |
| Curcio and Kemper, 1984 | The goal of the study was to determine neurofibrillary changes and neuronal packing density in DRN | 7 | 6 | Although cell density was similar between the groups studied, authors determined 8% loss of large neurons in the AD group | Biased methodology of estimation of nerve cell loss and frequency of NFTs from densities assessed, together with sampling done only at the level of trochlear nucleus prevents drawing firm conclusions |
| Yamamoto and Hirano, 1985 | The goal was to determine the number of NFTs and neurons in the DRN in AD | 5 | 7 | Authors reported 72% decrease in DRN cell density and 77% loss of large neurons in AD patients as compared with controls | Biased methodology of estimation of nerve cell loss and frequency of NFTs from densities assessed, together with sampling done only at the level of trochlear nucleus prevents drawing firm conclusions |
| Pearson et al., 1985 | A hypothesis was proposed that the distribution of neurofibrillary degeneration in AD may be accounted for on the basis of the connectivity of the involved structures | >14 | 0 | The largest number of NFTs in AD was found to occur typically in the CA1 subfield and subiculum in the hippocampal formation and in the regions of amygdala that have the most extensive interconnections with the entorhinal cortex layers II and IV in the parahippocampal gyrus | Since the entorhinal cortex is heavily interconnected with the layer II-III and V neurons of the temporal and posterior parietal higher order association areas that suffer the most profound degenerative change in AD, the authors concluded that the remarkable specificity of the distribution of NFTs reflects somehow the anatomical connectivity of the structures involved |
| German et al., 1987 | The goal was to analyze immunohystochemically the regional distribution of subcortical nuclei containing NFTs in brains from AD patients | 7 | 3 | The tangles were found not only in DRN and LC, but also in many other subcortical nuclei, such as nucleus paranigralis, peripeduncular nucleus, medial parabrachial nucleus and several midline thalamic nuclei | Authors concluded that AD is a disease of the cerebral cortex and the numerous subcortical nuclei which diffusely innervate it; they hypothesized that subcortical nuclei are secondarily affected due to retrograde transport of a cortical pathogen or failure of normal transport of a trophic agent |
| Hardy et al., 1987 | A hypothesis was proposed that the amyloid deposition in NPs and in cerebral blood vessels in AD is caused by neurofibrillary degeneration of central cerebrovascular regulatory neurons in the brainstem and basal forebrain | Two major premises were that: 1) NPs are similarly distributed in the forebrain and 2) NPs are seen in areas (such as striatum and thalamus) that receive axonal projections from the affected cortical neurons | The conclusion was that the brainstem and basal forebrain neurons that control the blood-brain barrier may die as a result of retrogradely transporting pathogenic agent from their axon collaterals involved in NPs formation in the cerebral cortex | ||
| Jellinger, 1987 | The goal of the study was to assess the number of cells in DRN of early and late AD patients and compare them with controls | 22 (13 early AD, 9 late AD) | 25 | The author reported DRN cell loss of 41% in early and 36.8%-55.8% in cases of late AD | Biased methodology of estimation of nerve cell loss and frequency of NFTs from densities assessed prevents drawing firm conclusions |
| Saper et al., 1987 | A hypothesis was given that a degenerative process in AD spreads transneuronally from specific subcortical cell groups (LC, DRN, A10 dopaminergic cell group, lateral hypothalamic area and n. basalis) to limbic and paralimbic cortex | Premise: several subcortical cell groups were found to contain large number of NFTs in AD and all have extensive cortical projections, particularly to the limbic and paralimbic cortex | Authors hypothesiued that AD is a result of transneuronal degeneration and speculated that AD may somehow involve either the transfer of toxic substance(s) across specific synapses or the lack of trophic substance(s) necessary for neuronal survival | ||
| Zweig et al., 1988 | The goal of the study was to detemine neuronal loss and NFTs presence within LC and DRN | 25 | 12 | Authors reported an average cell loss of DRN in AD of 64%; duration of AD correlated inversely with NFTs counts particularly within DRN; AD patients with major depression had fewer LC and DRN neurons in comparison with nondepressed patients | Biased methodology of estimation of nerve cell loss and frequency of NFTs from densities assessed, together with sampling done only at the level of trochlear nucleus prevents drawing firm conclusions; data obtained imply a correlation between NFTs counts in DRN and rate of AD progression |
| Wilcock et al., 1988 | The goal of the study was to determine DRN cell loss in AD | 18 | 10 | Authors reported an average loss of cell from DRN in AD patients of 33% | Biased methodology of estimation of nerve cell loss and frequency of NFTs from densities assessed, together with sampling done only at the level of trochlear nucleus prevents drawing firm conclusions |
| Hertz, 1989 | A hypothesis was given that AD is an anterograde degeneration originating in the brainstem | A degeneration of adrenergic neurons in LC and serotoninergic neurons in RN may lead to impairment in metabolic and functional interactions between neurons and astrocytes | Author gave a conceptual framework for AD pathogenesis: termination of many of ascending adrenergic and serotonergic fibers in varicosities, from which released transmitter molecules reach their targets by diffusion, suggests their potential impact on both neuronal and glial cells, with eventual influence on their membrane transport, energy metabolism and glutamate release | ||
| Van Domburg, 1990 | The goal of the study was to count the brainstem monoaminergic neurons in PD and AD | 5 | 5 | In comparison with controls, the author reported an average of 39.7% cell loss from DRN in AD patients | This is one of the first studies done with unbiased stereologic methods and throughout the whole rostrocaudal extent of DRN; the number of cases analysed was relatively low, but the amount of DRN cell loss was found to be substantial |
| Aletrino et al., 1992 | The study was devoted to the development of an efficient and reliable sampling scheme to determine the total cell number in DRN using the optical fractionator method | 8 | 10 | The study showed severe cell loss of AD patients in the DRN of 39.4% and a tendency for cell shrinkage of the remaining cells. | With slightly higher number of cases analyzed, the methodology and results obtained are the same as the study of Van Domburg (1990) |
| Halliday et al., 1992 | The goal of the study was to analyze the number of brain stem serotoninsynthesizing neurons AD patients and agematched controls using PH8 antibody | 11 | 5 | In comparison with controls, a large (70–80%) decrease in the number of PH8+ neurons in DRN and MRN of the AD+ subgroup (patients with significant AD-related pathology in RN) was found. In subgroup of AD-patients (those without pathology in RN), the reduction of PH8+ neurons was found only in DRN, but this change was not as dramatic as the „pure AD“ i.e. AD+ group | Authors concluded that RN are damaged only in certain cases of AD, indicating that, despite patient similarities in both clinical presentation and cortical neuropathology, the disease process is not homogeneous; the consistent and specific pathology of RN found in AD+ cases and the absence of such pathology in AD-cases suggested that pathology in RN is not essential and, therefore, causal for dementia: patients with earlier onset dementia had milder pathology in RN compared to later-onset patients |
| Brilliant et al., 1992 | The goal of the study was to determine distribution of β-amyloid in the brainstem | 19 (13 AD and 6 AD+P D) | 7 | In AD patients the density of β-amyloid was highest in the midbrain (nucleus ruber and nucleus niger were spared), moderate in pons and scarce in the medulla | β-amyloid deposition is a function of synaptic connectivity, rather than passive diffusion from vascular sources |
| Lippa et al., 1996 | The goal of the study was to determine does FAD and SAD cases differ neuropathologically | 25 FAD (19 due to PS-1 mut. + 6 due to APP mut.) and 11 SAD | none | There was no difference in the pattern of distribution of the neuronal loss, amyloid plaques, NFTs and NPs between FAD and SAD cases | Similarity of the neuropathological changes observed suggests a final common pathway for all forms of AD (however, FAD groups could be distinguished from SAD by the greater severity and the lack of influence of APOE genotype on pathology) |
| Mössner et al., 2000 | The goal of the study was to review allelic variations in the serotonin transporter in AD and PD | Association studies showed that the low-activity allele of the transporter is the risk factor for late-onset AD | Compromized serotoninergic system may play an important role in the pathophysiology of AD | ||
| Parvizi et al., 2000, 2001 | The goal of the study was to assess NFTs and SPs in PAG (Parvizi et al., 2000) and on serial sections from the entire brainstem (Parvizi et al., 2001) of AD and control subjects using thioflavin S-stained sections and immunocytochemis try using 10D5, ALZ-50 and AT8 antibodies | 32 | 26 (13 from normal subject s and 13 non-AD patients) | In 72% of AD cases there were only SPs, while both SPs and NFTs were found in 9%. These changes were absent in all 26 control brains (Parvizi et al., 2000); different monoaminergic, cholinergic and other nuclei of the brainstem were found affected to contain SPs and NFTs with varying degrees of severity, whereas no changes were seen in the brainstems of controls (Parvizi et al., 2001) | Authors concluded that PAG is selectively involved in AD; furthermore, the type and density of pathological changes correlated with the duration of dementia (Parvizi et al., 2001); authors concluded that the finding of severe pathological changes in some brainstem nuclei raises the possibility that the dysfunction of these nuclei may contribute to cognitive defects and increased rates of morbidity and mortality in AD patients |
| Rüb et al., 2000 | The goal of the study was to analyze AD-related cytoskeletal pathology in the raphe nuclei using modified silveriodide-Gallyas staining and the AT8 antibody | 27 | Examination of serial sections from the brainstems of 27 postmortem cases with BB I-VI of cortical cytoskeletal lesions showed that DRN manifests the cytoskeletal lesions early on (i.e. in cortical stages I-II), the central and linear RN do so in cortical stages III-IV while the caudal raphe nuclei only in cortical stages V-VI | Authors concluded that the developing damage within the nuclei of the raphe system correlates with the BB I-VI; as the source of the ascending serotoninergic system, the involvement of the oral raphe nuclei may be partially responsible for the early manifestations of the noncognitive and emotional deficiencies in AD | |
| Yang and Schmitt, 2001 | The goal of the study was to examine NCS, NRD and LC in FTLD, AD and non-demented controls | 30 AD, 12 FTLD | 35 | The FTLD cases showed a significant, 40%, decline in number of neurons in the NCS and NRD, while the LC was spared; the magnitude of neuronal loss matched that of AD where, by contrast, the LC was also severely changed; β-amyloid deposition and NFTs occurred in the aminergic nuclei almost exclusively in AD and, only to a minor extent, in some aged controls | Authors concluded that the serotoninergic raphe nuclei with ascending projections to the forebrain, but not the LC, become directly or indirectly involved in FTLD both with and without motor neuron disease (MND) |
| Kovacs et al., 2003 | One goal of the study was to compare the percentage of neurons synthesizing serotonin in NCS and NROP in AD and control brains | 10 | 11 | As compared with controls, the authors reported about 20% less neurons counted in NCS of AD subjects | Biased methodology of counting from densities observed and counting done only in 2 sections prevents drawing firm conclusions |
| Hendricksen et al., 2004 | The authors hypothesized that greater NFTs pathology and fewer serotonergic neurons would be found in the DRN in depressed compared to nondepressed elderly subjects and in AD patients with depression, compared to AD patients without depression | 8 AD with depression, 7 AD without depression | 14 elderly subject s with major depression, 10 nondepressed elderly subjects | No differences in neuritic pathology or neuronal density were found between the subjects with depression and the nondepressed controls; AD subjects taken altogether showed markedly fewer serotonergic neurons and associated higher levels of neuritic pathology, compared with subjects with primary depression and the nondepressed comparison subjects; however, AD subjects with with comorbid major depression did not differ from AD subjects without depression on these two measures | The authors concluded that if serotonergic dysfunction occurs in older depressed subjects, it is not due to neuronal loss in the brainstem; biased methodology of counting from densities observed and sampling done using only 3 sections prevents drawing firm conclusions |
| Kepe et al., 2006 | The goal of the study was quantification of 5-HT1A receptor densities in the living brains of AD, MCI and control subjects using PET | 8 AD, 6 MCI | 5 | AD patients had significantly decreased 5-HT1A receptor densities in both hippocampi and raphe nuclei; when volume losses were included, the 5-HT1A density losses were even more severe (average mean decrease of 24% in MCI and 49% in AD); these decreases correlated with decreased glucose utilization | The authors anticipated that the combined evaluation of 5-HT1a receptors densities together with [18F]FDNNP PET (targeting NFTs and NPs) and [18F]FDG PET (targeting neuronal function) offers a great opportunity for reliable, noninvasive detection of early neuropathological changes in AD |
| Bernedo et al., 2009 | The goal of the study was to estimate the number of DRN and MRN serotonergic neurons using optical fractionator method in young and age dogs with and without β-amyloid deposits | 8 young and 11 aged dogs | Aged dogs with β-amyloid cortical pathology had 33% fewer tryptophan-hydroxylase positive (TH+) serotonergic neurons in the DRN and MRN than aged dogs without β-amyloid cortical deposits; no significant variations were found between young and aged dogs without β-amyloid cortical deposits | Authors suggested that degeneration of the serotonergic neurons could be involved in the cognitive damage that accompanies β-amyloid cortical pathology in aged dogs | |
| Grinberg et al., 2009 | The goal of the study was to determine during which BB stage and how frequently the DRN is affected by AD-related neurofibrillary changes | 80 (with BB ≥1) | 38 (with BB = 0) | More than 20% of BB 0 and all of BB ≥ 1 cases were found to have substantial neurofibrillary changes in DRN | Authors concluded that their observations support the hypothesis of transneuronal spread of neurofibrillary changes from the DRN to its interconnected cortical brain areas; supratrochlear subnucleus of the DRN is affected by neurofibrillary changes prior to transentorhinal cortex during the disease process underlying AD |
Abbreviations: 5-HT, 5-hydroxytryptamine (serotonine); AD, Alzheimer's disease; APOE, APOLIPOPROTEIN E gene; APP, AMYLOID PRECURSOR PROTEIN gene; BB, Braak and Braak stage; CON, control cases; [18F]FDG PET, positron emission tomography with 2-deoxy-2-[18F]fluoro-D-glucose as a radioligand; [18F]FDNNP PET, positron emission tomography with 2-(1-{6-[(2-[18F]fluoroethyl)(methyl)amino]-2-naphthyl}ethylidene) malononitrile as a radioligand; FAD, familial AD; FTLD, frontotemporal lobar degeneration; DRN, dorsal raphe nucleus, LC, locus coeruleus; MCI, mild cognitive impairment; MND, motor neuron disease; MRN, median raphe nuclei; NCS, nucleus centralis superior; NFD, neurofibrillary degeneration; NFTs, neurofibrillary tangles; NROP, nucleus raphe obscurus and pallidus; PAG, periaqueductal gray matter; PH8, monoclonal antibody raised to phenylalanine hydroxylase, which cross-reacts with tryptophan hydroxylase, the serotonin synthesis enzyme; PD, Parkinson's disease; PET, positron emission tomography; PS-1, PRESENILIN-1 gene; RN, raphe nuclei; SAD, sporadic AD; TH+, tryptophan-hydroxlase positive neurons
The possible role and the significance of the early dorsal raphe nucleus involvement during AD
The human raphe nuclei consist of the rostral subdivision that gives rise to the ascending projections and caudal subdivision (nuclei raphe magnus, pallidus, and obscurus) that projects into the brainstem and spinal cord. The rostral subdivision comprises the centromedian part (including the annular, linear, and central raphe nuclei) and the dorsal part. The dorsal part, also known as the dorsal raphe nucleus (DRN) contains the largest aggregates of serotonergic neurons (about 80%) and is divided into the interfascicular, supratrochlear, and caudal subnuclei [132, 133].
Before the introduction of BBSS many investigators reported AD-related pathology and cell loss in the raphe brain stem nuclei (in addition to those in the basal nucleus of Meynert and entorhinal cortex) during the early course of AD [134–145] (see Table 2). However, most of them used biased methodology of estimation of nerve cell loss and frequency of tangles from densities assessed, while sampling was done usually only at the level of trochlear nucleus, thus preventing firm conclusions to be made (see Table 2). In spite of the fact that after the introduction of BBSS the raphe brainstem nuclei were not in the focus of investigation, several reports, including some of which used unbiased methodology for quantification, again drew attention to the possibility of their selective and early involvement, particularly the dorsal raphe nucleus, in the pathogenesis of AD [146–159] (see Table 2).
Braak and colleagues later confirmed very early AD-related cytoskeletal changes in the DRN in 27 AD cases [160] (see Table 2). They claimed that in accordance with the cross-sectional data available to them, the ascending system source, the rostral raphe group, is affected as early as in stage I – some 30 years prior to dysmnesia – by the AD-related cytoskeletal lesions [160]. Furthermore, it was recently reported that the DRN may actually display the earliest AD-related cytoskeletal pathology [159] (see Table 2). In this clinicopathological series of 118 cases, out of which 38 were categorized as Braak stage 0 (at least four sections at different levels of transentorhinal cortex were free of neurofibrillary changes, based on immunonegativity for monoclonal antibodies PHF-1 and AT8), and 80 as Braak stage ≥ 1 (rare neurofibrillary changes in the transentorhinal cortex are considered as Braak stage 1), more than 20% of Braak stage 0 and all of Braak stage ≥ 1 cases had substantial neurofibrillary changes in the DRN. An illustration of early neurofibrillary changes of the DRN is given in Fig. 3. Therefore, the putative staging scheme updated with the DRN (and considering the DRN as the first brain structure affected by neurofibrillary pathology and the “earliest source” of transneuronal degeneration), the crude scheme of differential susceptibility levels for neurofibrillary degeneration should be as follows: dorsal raphe nucleus --> entorhinal cortex --> hippocampal formation --> amygdala --> orbitofrontal and prefrontal cortex --> posterior association cortical regions --> primary sensory and motor cortical areas (from highest to lowest susceptibility). However, because the raphe nuclei dysfunction due to neurofibrillary changes is not included in the BBSS, its possible behavioural correlates are not yet considered as a potential early characteristic clinical feature of AD. As a matter of fact, studies of the brainstem in AD generally do not pay much attention to the connectivity of the affected nuclei and related functional consequences. In a similar fashion, the molecular characteristics of the dorsal raphe alterations are not yet determined (see below), and cannot be used for diagnostic purposes.
Fig. 3.
Gallyas silver iodide staining of the dorsal part of the supratrochlear subnucleus of the dorsal raphe nucleus of a mildly cognitively impaired 69-year old woman. This method specifically stains paired helical filaments (PHFs) in neurofibrillary tangles (NFTs) (arrows) and degenerated neurites (arrowheads). An initial degree of Gallyas-positive cytoskeletal changes in the dorsal part of ST DRN is seen. Legend: ST DRN, supratrochlear subnucleus of the dorsal raphe nucleus; aq, cerebral aqueduct (of Sylvius). Scale bar = 100 μm.
Summary and future prospects
In summary, research studies conducted so far indicate that AD in familial cases due to known mutations in APP, PS1 and PS2 genes is probably caused by a pathological cascade of events in which formation of β–amyloid oligomers lies upstream of neurofibrillary degeneration. Neuropathologically, some of the familiar AD forms are indeed of the “plaque only type”. Since the “toxic” β–amyloid oligomers are first being accumulated as diffuse deposits in the neuropil and later as SPs, this AD pathology is, however, an end-stage effect rather than cause or at least partially a response to initial insults, thus representing an adaptive, protective reaction [161, 162]. Despite intensive efforts to find the mechanistic missing link between plaques and tangles, the steps that connect β–amyloid and its potentially toxic oligomers to cytoskeletal tau pathology still remained largely undefined. On the other hand, in many or even most sporadic cases neurofibrillary degeneration can be caused in the absence of amyloid pathology. Thus a more coherent scheme in these cases can be based on the centrality of the cytoskeletal abnormalities [18, 163]. According to this view, the hyperphosphorylation of tau protein aids in both the development and progression of AD, while regional differences in compartmentalization and metabolism of tau protein possibly represent a molecular basis for individually and regionally different neuronal vulnerability in AD [164]. As it has been documented that hyperphosphorylated tau, but not PHFs, inhibits regeneration of MT network in cultured cells, the formation of PHF may be considered as a self-defense reaction (similarly to the formation of SPs) by the affected neurons [165]. The relationship between the quantity and distribution of NFTs, the loss of neurons and dementia, is not a linear one [29]. This could partially be attributed to the fact that due to the redundancy of nervous elements and compensatory structural, physiological and biochemical processes, a loss of neurons is to a certain limit tolerated without any functional consequences [18]. An important source of discrepancy among studies arises from the fact that most AD studies have focused on elderly people without considering the possibility of a gradual neuronal loss over adult life prior to the occurrence of AD-related changes. As long as elderly patients do not suffer from AD, they appear neuropathologically quite comparable as a group [38, 166]. It is therefore not surprising that significant neuron loss due solely to aging cannot be revealed without younger adult cases included in the regressions [167]. On the other hand, when neuronal loss attributable to aging is superimposed to an unbiased estimate of the number of NFTs in AD, regions like the entorhinal cortex and hippocampal formation may display neuronal losses larger than that accounted for by NFT counts alone [18, 29, 167–169]. It can be assumed that the pattern of neuron loss does not necessarily match the pattern of NFT formation due to mechanisms other than neurofibrillary degeneration [18, 29, 38, 39].
Based on CSF levels of proteins associated with SPs and NFTs, i.e. β–amyloid, total tau and ubiquitin, five different subgroups of AD have already been proposed [170]. As there is a tendency for phosphorylated tau proteins to behave differently in the different primary dementing disorders, the phospho-tau proteins in CSF come closest to fulfilling criteria of biological markers of AD [12]. It is therefore likely that additional subgroups of AD may be identified from phosphorylation patterns of CSF tau. This would help preclinical identification of specific signaling pathways involved selectively in different subgroups of AD patients and also facilitate the development of more specific therapeutic drugs.
In the context of early involvement of the raphe nuclei in AD degenerative process it would for instance be relevant to assess whether 5-HIAA (5-hydroxyindoleacetic acid), the main metabolite of serotonin in CSF, is decreased in patients with BPSD typical for very early AD and whether therefore it could be used as an early biological marker. Perhaps of equal importance would be to test for the functional correlation of genes related to serotonergic transmission that might be altered during the early course of AD by genome and transcriptome microarray analysis of blood and postmortem brain samples. A compensatory mechanism illustrated by an up-regulation of serotonergic metabolism has been shown at the stage of amnestic MCI in contrast with a dramatic decrease at later stages of AD [171]. This difference of hippocampal serotonergic receptor labeling allows distinguishing of patients with amnestic MCI from those with mild AD. Exploring 5-HT1a receptors with 2'-methoxyphenyl-(N-2'-pyridinyl)-p-(18)F-fluoro-benzamidoethylpiperazine PET may therefore be useful for better understanding of pathophysiologic changes at early stages of AD [171].
The serotonergic neurons in the DRN can also be easily visualized and quantified by immunohistochemistry using monoclonal antibody PH8 raised against phenylalanine hydroxylase, which cross-reacts with tryptophan hydroxylase, the serotonin synthesis enzyme [148]. Expanding our understanding of the raphe nuclei, particularly the DRN involvement in the early stages of AD to functional concepts beyond neuropathological descriptions will likely have a strong impact on our understanding, detection and tracking of AD progression, and on the development of new therapeutic strategies.
Acknowledgement
This work was supported by NIH grants AG02219 and AG05138 (to P.R.H.) and grant no. 108-1081870-1942 from Croatian Ministry of Science, Education and Sports (to G.S.).
References
- 1.Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–9. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- 2.Hof PR, Morrison JH. The aging brain: morphomolecular senescence of cortical circuits. Trends Neurosci. 2004;27:607–13. doi: 10.1016/j.tins.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 3.Bussiere T, Giannakopoulos P, Bouras C, Perl DP, Morrison JH, Hof PR. Progressive degeneration of nonphosphorylated neurofilament protein-enriched pyramidal neurons predicts cognitive impairment in Alzheimer's disease: stereologic analysis of prefrontal cortex area 9. J Comp Neurol. 2003;463:281–302. doi: 10.1002/cne.10760. [DOI] [PubMed] [Google Scholar]
- 4.Tanzi RE, Bertram L. Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–55. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 5.George-Hyslop P, Tanzi RE, Polinsky RJ, Haines JL, Nee L, Watkins PC, Myers PC, Feldman RG, Pollen D, Drachman D, Growdon J, Bruni A, Foncin JF, Salmon D, Hobbs WJ, Conneally PM, Gusella JF. The genetic defect causing familial Alzheimer's disease maps on chromosome 21. Science. 1987;235:885–90. doi: 10.1126/science.2880399. [DOI] [PubMed] [Google Scholar]
- 6.Dickson DW, Crystal HA, Mattiace LA, Masur DM, Blau AD, Davies P, Yen SH, Aronson MK. Identification of normal and pathological aging in prospectively studied nondemented elderly humans. Neurobiol Aging. 1992;13:179–89. doi: 10.1016/0197-4580(92)90027-u. [DOI] [PubMed] [Google Scholar]
- 7.Lockhart A, Lamb JR, Osredkar T, Sue LI, Joyce JN, Ye L, Libri V, Leppert D, Beach TG. PIB is a non-specific imaging marker of amyloid-beta peptide-related cerebral amyloidosis. Brain. 2007;130:2607–15. doi: 10.1093/brain/awm191. [DOI] [PubMed] [Google Scholar]
- 8.Dong J, Atwood CS, Anderson VE, Siedlak SL, Smith MA, Perry G, Carey PR. Metal binding and oxidation of amyloid-beta within isolated senile plaque cores: Raman microscopic evidence. Biochemistry. 2003;42:2768–73. doi: 10.1021/bi0272151. [DOI] [PubMed] [Google Scholar]
- 9.Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein inhibit hippocampal long-term potentiation in vivo. Nature. 2002;426:535–9. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 10.Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–42. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheperd C, McCann H, Halliday GM. Variations in the neuropathology of familial Alzheimer's disease. Acta Neuropathol. 2009;118:37–52. doi: 10.1007/s00401-009-0521-4. [DOI] [PubMed] [Google Scholar]
- 12.Simic G, Boban M, Hof PR. Cerebrospinal fluid phosphorylated tau proteins as predictors of Alzheimer's disease in subjects with mild cognitive impairment. Period Biol. 2008;110:27–30. [Google Scholar]
- 13.Tomita T. Secretase inhibitors and modulators for Alzheimer's disease treatment. Expert Rev Neurother. 2009;9:661–679. doi: 10.1586/ern.09.24. [DOI] [PubMed] [Google Scholar]
- 14.Iqbal K, Liu F, Gong CX, Alonso AD, Grundke-Iqbal I. Mechanisms of tau-induced neurodegeneration. Acta Neuropathol. 2009;118:53–69. doi: 10.1007/s00401-009-0486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 16.Bierer L, Hof PR, Purohit DP, Carlin L, Schmeidler J, Davis KL, Perl DP. Neocortical neurofibrillary tangles correlate with dementia severity in Alzheimer's disease. Arch Neurol. 1995;52:81–88. doi: 10.1001/archneur.1995.00540250089017. [DOI] [PubMed] [Google Scholar]
- 17.Grossi E, Buscema MP, Snowdon D, Antuono P. Neuropathologic findings processed by artificial neural networks (ANNs) can perfectly distinguish Alzheimer's patients from controls in the Nun study. BMC Neurol. 2007;7:15. doi: 10.1186/1471-2377-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simic G, Gnjidic M, Kostovic I. Cytoskeletal changes as an alternative view on pathogenesis of Alzheimer's disease. Period Biol. 1998;100:165–73. [Google Scholar]
- 19.Jellinger KA, Bancher C. Senile dementia with tangles (tangle predominant form of senile dementia) Brain Pathol. 1998;8:367–76. doi: 10.1111/j.1750-3639.1998.tb00160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noda K, Sasaki K, Fujimi K, Wakisaka Y, Tanizaki Y, Wakugawa Y, Kiyohara Y, Iida M, Aizawa H, Iwaki T. Quantitative analysis of neurofibrillary pathology in a general population to reappraise neuropathological criteria for senile dementia of the neurofibrillary tangle type (tangle-only dementia): the Hisayama Study. Neuropathology. 2006;26:508–18. doi: 10.1111/j.1440-1789.2006.00722.x. [DOI] [PubMed] [Google Scholar]
- 21.Spillantini MG, Goedert M. Tau protein pathology in neurodegenerative diseases. Trends Neurosci. 1998;21:428–33. doi: 10.1016/s0166-2236(98)01337-x. [DOI] [PubMed] [Google Scholar]
- 22.Götz J, Chen F, van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P301L tau transgenic mice induced by A-beta 42 fibrils. Science. 2001;293:1491–5. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- 23.King ME, Kan HM, Baas PW, Erisir A, Glabe CG, Bloom GS. Tau-dependent microtubule disassembly initiated by prefibrillar beta-amyloid. J Cell Biol. 2006;175:541–6. doi: 10.1083/jcb.200605187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oddo S, Caccamo A, Tran L, Lambert MP, Glabe CG, Klein WL, LaFerla FM. Temporal profile of amyloid-beta oligomerization in an in vivo model of Alzheimer disease. A link between Abeta and tau pathology. J Biol Chem. 2006;281:1599–604. doi: 10.1074/jbc.M507892200. [DOI] [PubMed] [Google Scholar]
- 25.De Felice FG, Wu D, Lambert MP, Fernandez SJ, Velasco PT, Lacor PN, Bigio EH, Jerecic J, Acton PJ, Shughrue PJ, Chen-Dodson E, Kinney GG, Klein WL. Alzheimer's disease-type neuronal tau hyperphosphorylation induced by A beta oligomers. Neurobiol Aging. 2008;29:1334–47. doi: 10.1016/j.neurobiolaging.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vogt BA, Vogt LJ, Hof PR. Functional Neurobiology of Aging. Academic Press; New York: 2001. Patterns of cortical neurodegeneration in Alzheimer's disease: subgroups, subtypes, and implications for staging strategies; pp. 111–29. [Google Scholar]
- 27.Jellinger K. Challenges in neuronal apoptosis. Curr Alzheimer Res. 2006;3:377–91. doi: 10.2174/156720506778249434. [DOI] [PubMed] [Google Scholar]
- 28.Raina AK, Hochman A, Zhu X, Rottkamp CA, Nunomura A, Siedlak SL, Boux H, Castellani RJ, Perry G, Smith MA. Abortive apoptosis in Alzheimer's disease. Acta Neuropathol. 2001;101:305–310. doi: 10.1007/s004010100378. [DOI] [PubMed] [Google Scholar]
- 29.Simic G, Winblad B, Bogdanovic N. Relationship between hippocampal neurofibrillary degeneration and neuronal loss in aging and Alzheimer's disease. Neurobiol Aging. 1998;19(Suppl 4):239. [Google Scholar]
- 30.Simic G, Lucassen PJ, Krsnik Z, Kruslin B, Kostovic I, Winblad B, Bogdanovic N. nNOS expression in reactive astrocytes correlates with increased cell death in the hippocampus and entorhinal cortex in Alzheimer's disease. Exp Neurol. 2000;165:12–26. doi: 10.1006/exnr.2000.7448. [DOI] [PubMed] [Google Scholar]
- 31.Piguet O, Double KL, Kril JJ, Harasty J, Macdonald V, McRitchie DA, Halliday GM. White matter loss in healthy ageing: a postmortem analysis. Neurobiol Aging. 2009;30:1288–95. doi: 10.1016/j.neurobiolaging.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 32.Peters A. The effects of normal aging on myelin and nerve fibers: a review. J Neurocytol. 2002;31:581–93. doi: 10.1023/a:1025731309829. [DOI] [PubMed] [Google Scholar]
- 33.Roher AE, Kuo Y-M, Esh C, Knebel C, Weiss N, Kalback W, Luehrs DC, Childress JL, Beach TG, Weller RO, Kokjohn TA. Cortical and leptomeningeal cerebrovascular amyloid and white matter changes in Alzheimer's disease. Mol Med. 2003;9:112–22. [PMC free article] [PubMed] [Google Scholar]
- 34.Weller RO, Boche D, Nicoll JAR. Microvasculature changes and cerebral amyloid angiopathy in Alzheimer's disease and their potential impact on therapy. Acta Neuropathol. 2009;118:87–102. doi: 10.1007/s00401-009-0498-z. [DOI] [PubMed] [Google Scholar]
- 35.Bulat M, Lupret V, Oreskovic D, Klarica M. Transventricular and transpial absorption of cerebrospinal fluid into cerebral microvessels. Coll Antropol. 2008;32(Suppl 1):43–50. [PubMed] [Google Scholar]
- 36.Khachaturian ZS. Diagnosis of Alzheimer's disease. Arch Neurol. 1985;42:1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- 37.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer's disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 38.Hof PR, Bussiere T, Gold G, Kövari E, Giannakopoulos P, Bouras C, Perl DP, Morrison JH. Stereologic evidence for persistence of viable neurons in layer II of the entorhinal cortex and the CA1 subfield in Alzheimer's disease. J Neuropathol Exp Neurol. 2003;62:55–67. doi: 10.1093/jnen/62.1.55. [DOI] [PubMed] [Google Scholar]
- 39.Simic G, Bexheti S, Kelovic Z, Kos M, Grbic K, Hof PR, Kostovic I. Hemispheric asymmetry, modular variability and age-related changes in the human entorhinal cortex. Neuroscience. 2005;130:911–925. doi: 10.1016/j.neuroscience.2004.09.040. [DOI] [PubMed] [Google Scholar]
- 40.Simic G, Kostovic I, Winblad B, Bogdanovic N. Volume and number of neurons of the human hippocampal formation in normal aging and Alzheimer's disease. J Comp Neurol. 1997;379:482–494. doi: 10.1002/(sici)1096-9861(19970324)379:4<482::aid-cne2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 41.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 42.Guillozet AL, Weintraub S, Mash DC, Mesulam MM. Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Arch Neurol. 2003;60:729–736. doi: 10.1001/archneur.60.5.729. [DOI] [PubMed] [Google Scholar]
- 43.Simic G, Grbic K, Hof PR. Tau phosphorylation and selective neuronal vulnerability in Alzheimer's disease. Neurol Croat. 2003;52(Suppl 4):87. [Google Scholar]
- 44.The National Institute on Aging and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. Neurobiol Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- 45.Geddes JW, Tekirian TL, Soultanian NS, Ashford JW, Davis DG, Markesbery WR. Comparison of neuropathological criteria for the diagnosis of Alzheimer's disease. Neurobiol Aging. 1997;18(Suppl 4):S99–105. doi: 10.1016/s0197-4580(97)00063-8. [DOI] [PubMed] [Google Scholar]
- 46.Duyckaerts C, Delatour B, Potier M-C. Clasification and basic pathology of Alzheimer disease. Acta Neuropathol. 2009;118:5–36. doi: 10.1007/s00401-009-0532-1. [DOI] [PubMed] [Google Scholar]
- 47.Motta M, Imbesi R, Di Rosa M, Stivala F, Malaguarnera L. Altered plasma cytokine levels in Alzheimer's disease: correlation with the disease progression. Immunol Lett. 2007;114:46–51. doi: 10.1016/j.imlet.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 48.Arnaud L, Robakis NK, Fiqueiredo-Pereira ME. It may take inflammation, phosphorylation and ubiquitination to “tangle” in Alzheimer's disease. Neurodegener Dis. 2006;3:313–19. doi: 10.1159/000095638. [DOI] [PubMed] [Google Scholar]
- 49.Guo Z, Cupples LA, Kurz A, Auerbach SH, Volicer L, Chui H, Green RC, Sadovnick AD, Duara R, DeCarli C, Johnson K, Go RC, Growdon JH, Haines JL, Kukull WA, Farrer LA. Head injury and the risk of AD in the MIRAGE study. Neurology. 2000;54:1316–23. doi: 10.1212/wnl.54.6.1316. [DOI] [PubMed] [Google Scholar]
- 50.Roberts GW, Gentleman SM, Lynch A, Murray L, Landon M, Graham DI. Beta amyloid protein deposition in the brain after severe head injury: implications for the pathogenesis of Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1994;57:419–25. doi: 10.1136/jnnp.57.4.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kalaria RN, Cohen DL, Premkumar DR, Naq S, LaManna JC, Lust WD. Vascular endotelial growth factor in Alzheimer's disease and experimental cerebral ischemia. Mol Brain Res. 1998;62:101–5. doi: 10.1016/s0169-328x(98)00190-9. [DOI] [PubMed] [Google Scholar]
- 52.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O'Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 54.Feinstein SC, Wilson L. Inability of tau to properly regulate neuronal microtubule dynamics: a loss-of-function mechanism by which tau might mediate neuronal cell death. Biochim Biophys Acta. 2005;1739:268–279. doi: 10.1016/j.bbadis.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 55.Buée L, Bussière T, Buée-Scherrer V, Delacourte A, Hof PR. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res Rev. 2000;33:95–130. doi: 10.1016/s0165-0173(00)00019-9. [DOI] [PubMed] [Google Scholar]
- 56.Janke C, Beck M, Stahl T, Holzer M, Brauer K, Bigl V, Arendt T. Phylogenetic diversity of the expression of the microtubule-associated protein tau: implications for neurodegenerative disorders. Brain Res Mol Brain Res. 1999;68:119–128. doi: 10.1016/s0169-328x(99)00079-0. [DOI] [PubMed] [Google Scholar]
- 57.Simic G, Gnjidic M, Kostovic I. Cytoskeletal changes as an alternative view on pathogenesis of Alzheimer's disease. Period Biol. 1998;100:165–173. [Google Scholar]
- 58.Spires-Jones TL, Stoothoff WH, de Calignon A, Jones PB, Hyman BT. Tau pathophysiology in neurodegeneration: a tangled issue. Trends Neurosci. 2009;32:150–9. doi: 10.1016/j.tins.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 59.Zilka N, Korenova M, Novak M. Misfolded tau proein and disease modifying pathways in transgenic rodent models of human tauopathies. Acta Neuropathol. 2009;118:71–86. doi: 10.1007/s00401-009-0499-y. [DOI] [PubMed] [Google Scholar]
- 60.Jellinger KA, Bancher C. Senile dementia with tangles (tangle predominant form of senile dementia) Brain Pathol. 1998;8:367–376. doi: 10.1111/j.1750-3639.1998.tb00160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Noda K, Sasaki K, Fujimi K, Wakisaka Y, Tanizaki Y, Wakugawa Y, Kiyohara Y, Iida M, Aizawa H, Iwaki T. Quantitative analysis of neurofibrillary pathology in a general population to reappraise neuropathological criteria for senile dementia of the neurofibrillary tangle type (tangle-only dementia): the Hisayama Study. Neuropathology. 2006;26:508–18. doi: 10.1111/j.1440-1789.2006.00722.x. [DOI] [PubMed] [Google Scholar]
- 62.Riederer BM, Mourton-Gilles C, Frey P, Delacourte A, Probst A. Differential phosphorylation of tau proteins during kitten brain development and Alzheimer's disease. J Neurocytol. 2001;30:145–158. doi: 10.1023/a:1011991207942. [DOI] [PubMed] [Google Scholar]
- 63.Rösner H, Rebhan M, Vacun G, Vanmechelen E. Developmental expression of tau proteins in the chicken and rat brain: Rapid down-regulation of a paired helical filament epitope in the rat cerebral cortex coincides with the transition from immature to adult tau isoforms. Int J Dev Neurosci. 1995;13:607–617. doi: 10.1016/0736-5748(95)00042-f. [DOI] [PubMed] [Google Scholar]
- 64.Lindwall G, Cole RD. Phosphorylation affects the ability of tau protein to promote microtubule assembly. J Biol Chem. 1984;259:5301–5305. [PubMed] [Google Scholar]
- 65.Andreadis A, Brown WM, Kosik KS. Structure and novel exons of the human tau gene. Biochemistry. 1992;31:10626–33. doi: 10.1021/bi00158a027. [DOI] [PubMed] [Google Scholar]
- 66.Goode BL, Feinstein SC. Identification of a novel microtubule binding and assembly domain in the developmentally regulated inter-repeat region of tau. J Cell Biol. 1994;124:769–82. doi: 10.1083/jcb.124.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alonso AD, Mederlyova A, Novak M, Grundke-Iqbal I, Iqbal K. Promotion of hyperphosphorylation by frontotemporal dementia tau mutations. J Biol Chem. 2004;279:34873–81. doi: 10.1074/jbc.M405131200. [DOI] [PubMed] [Google Scholar]
- 68.Goedert M, Jakes R. Expression of separate isoforms of human tau protein: correlation with the tau pattern in brain and effects on tubulin polymerization. EMBO J. 1990;9:4225–30. doi: 10.1002/j.1460-2075.1990.tb07870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Butner KA, Kirschner MW. Tau protein binds to microtubules through a flexible array of distributed weak sites. J Cell Biol. 1991;115:717–30. doi: 10.1083/jcb.115.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Panda D, Goode BL, Feinstein SC, Wilson L. Kinetic stabilization of microtubule dynamics at steady state by tau and microtubule-binding domains of tau. Biochemistry. 1995;34:11117–27. doi: 10.1021/bi00035a017. [DOI] [PubMed] [Google Scholar]
- 71.Poppek D, Keck S, Ermak G, Jung T, Stolzing A, Ullrich O, Davies KJ, Grune T. Phosphorylation inhibits turnover of the tau protein by the proteasome: influence of RCAN1 and oxidative stress. Biochem J. 2006;400:511–20. doi: 10.1042/BJ20060463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morishima-Kawashima M, Hasegawa M, Takio K, Suzuki M, Titani K, Ihara Y. Proline-directed and non-proline directed phosphorylation of PHF-tau. J Biol Chem. 1995;270:823–829. doi: 10.1074/jbc.270.2.823. [DOI] [PubMed] [Google Scholar]
- 73.Kimura R, Kamino K, Yamamoto M, Nuripa A, Kida T, Kazui H, Hashimoto R, Tanaka T, Kudo T, Yamagata H, TAbara Y, Miki T, Akatsu H, Kosaka K, Funakoshi E, Nishitomi K, Sakaguchi G, Kato A, Hattori H, Uema T, Takeda M. The DYRK1A gene, encoded in chromosome 21 Down syndrome critical region, bridges between beta-amyloid production and tau phosphorylation in Alzheimer disease. Hum Mol Genet. 2007;16:15–23. doi: 10.1093/hmg/ddl437. [DOI] [PubMed] [Google Scholar]
- 74.Gong CX, Singh TJ, Grundke-Iqbal I, Iqbal K. Phosphoprotein phosphatase activities in Alzheimer disease brain. J Neurochem. 1993;61:921–7. doi: 10.1111/j.1471-4159.1993.tb03603.x. [DOI] [PubMed] [Google Scholar]
- 75.Liu F, Zaidi T, Iqbal K, Grundke-Iqbal I, Merkle RK, Gong CX. Role of glycosylation in hyperphosphorylation of tau in Alzheimer's disease. FEBS Lett. 2002;512:101–6. doi: 10.1016/s0014-5793(02)02228-7. [DOI] [PubMed] [Google Scholar]
- 76.Salkovic-Petrisic M, Tribl F, Schmidt M, Hoyer S, Riederer P. Alzheimer-like changes in protein kinase B and glycogen synthase kinase-3 in rat frontal cortex and hippocampus after damage to the insulin signalling pathway. J Neurochem. 2006;96:1005–15. doi: 10.1111/j.1471-4159.2005.03637.x. [DOI] [PubMed] [Google Scholar]
- 77.vB Hjelmborg J, Iachine I, Skytthe A, Vaupel JW, McGue M, Koskenvuo M, Kaprio J, Pedersen NL, Christensen K. Genetic influence on human lifespan and longevity. Hum Genet. 2006;119:312–21. doi: 10.1007/s00439-006-0144-y. [DOI] [PubMed] [Google Scholar]
- 78.den Dunnen WFA, Brouwer WH, Bijlard E, Kamphuis J, van Linschoten K, Eggens-Meijer E, Holstege G. No disease in the brain of a 115-year-old woman. Neurobiol Aging. 2008;29:1127–32. doi: 10.1016/j.neurobiolaging.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 79.Marlatt MW, Lucassen PJ, Perry G, Smith MA, Zhu X. Alzheimer's disease: cerebrovascular dysfunction, oxidative stress, and advanced clinical therapies. J Alzheimers Dis. 2008;15:199–210. doi: 10.3233/jad-2008-15206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mark RJ, Blanc EM, Mattson MP. Amyloid beta-peptide and oxidative cellular injury in Alzheimer's disease. Mol Neurobiol. 1996;12:211–24. doi: 10.1007/BF02755589. [DOI] [PubMed] [Google Scholar]
- 81.Mesulam MM. Neuroplasticity failure in Alzheimer's disease: bridging the gap between plaques and tangles. Neuron. 1999;24:521–529. doi: 10.1016/s0896-6273(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 82.Otth C, Zambrano A, Concha M. The possible link between Herpes simplex virus type 1 infection and neurodegeneration. In: Maccioni RB, Perry G, editors. Current hypotheses and research milestones in Alzheimer's disease. Springer; New York: 2009. pp. 181–188. [Google Scholar]
- 83.Ball MJ. Limbic predilection in Alzheimer's dementia: is reactivated herpesvirus involved? Can J Neurol Sci. 1982;9:303–306. doi: 10.1017/s0317167100044115. [DOI] [PubMed] [Google Scholar]
- 84.Letenneur L, Peres K, Fleury H, Garrigue I, Barberger-Gateau P, Helmer C, Orgogozo JM, Gauthier S, Dartigues JF. Seropositivity to herpes simplex virus antibodies and risk of Alzheimer's disease: a population-based cohort study. PLoS ONE. 2008;3:e3637. doi: 10.1371/journal.pone.0003637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pluta R, Ułamek M, Januszewski S. Micro-blood-brain barrier openings and cytotoxic fragments of amyloid precursor protein accumulation in white matter after ischemic brain injury in long-lived rats. Acta Neurochir Suppl. 2006;96:267–71. doi: 10.1007/3-211-30714-1_57. [DOI] [PubMed] [Google Scholar]
- 86.Reser JE. Alzheimer's disease and natural cognitive aging may represent adaptive metabolism reduction programs. Behav Brain Funct. 2009;5:13. doi: 10.1186/1744-9081-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vickers JC, Dickson TC, Adlard PA, Saunders HL, King CE, McCormack G. The cause of neuronal degeneration in Alzheimer's disease. Prog Neurobiol. 2000;60:139–65. doi: 10.1016/s0301-0082(99)00023-4. [DOI] [PubMed] [Google Scholar]
- 88.Teter B, Ashford JW. Neuroplasticity in Alzheimer's disease. J Neurosci Res. 2002;70:402–37. doi: 10.1002/jnr.10441. [DOI] [PubMed] [Google Scholar]
- 89.Braak E, Braak H, Mandelkow EM. A sequence of cytoskeleton changes related to the formation of neurofibrillary tangles and neuropil threads. Acta Neuropathol. 1994;87:554–67. doi: 10.1007/BF00293315. [DOI] [PubMed] [Google Scholar]
- 90.Jenkins SM, Johnson GV. Phosphorylation of microtubule-associated protein tau on Ser 262 by an embryonic 100 kDa protein kinase. Brain Res. 1997;767:305–13. doi: 10.1016/s0006-8993(97)00615-x. [DOI] [PubMed] [Google Scholar]
- 91.Melnikova I. Therapies for Alzheimer's disease. Nat Rev Drug Discov. 2007;6:341–2. doi: 10.1038/nrd2314. [DOI] [PubMed] [Google Scholar]
- 92.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 93.Galasko D, Hansen LA, Katzman R, Wiederholt W, Masliah E, Terry R, Hill LR, Lessin P, Thal LJ. Clinical-neuropathological correlations in Alzheimer's disease and related dementias. Arch Neurol. 1994;51:888–895. doi: 10.1001/archneur.1994.00540210060013. [DOI] [PubMed] [Google Scholar]
- 94.Jellinger KA. Structural basis of dementia in neurodegenerative disorders. J Neural Transm Suppl. 1996;47:1–29. doi: 10.1007/978-3-7091-6892-9_1. [DOI] [PubMed] [Google Scholar]
- 95.Decarli C. Mild cognitive impairment: prevalence, prognosis, aetiology, and treatment. Lancet Neurol. 2003;2:15–21. doi: 10.1016/s1474-4422(03)00262-x. [DOI] [PubMed] [Google Scholar]
- 96.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 97.Simic G. Pathological tau proteins in argyrophilic grain disease. Lancet. 2002;1:276. doi: 10.1016/s1474-4422(02)00130-8. [DOI] [PubMed] [Google Scholar]
- 98.Dubois B, Albert ML. Amnestic MCI or prodromal AD? Lancet Neurol. 2004;3:246–248. doi: 10.1016/S1474-4422(04)00710-0. [DOI] [PubMed] [Google Scholar]
- 99.Markesbery WR, Schmitt FA, Kryscio RJ, Davis DG, Smith CD, Wekstein DR. Neuropathologic substrate of mild cognitive impairment. Arch Neurol. 2006;63:38–46. doi: 10.1001/archneur.63.1.38. [DOI] [PubMed] [Google Scholar]
- 100.Nelson PT, Braak H, Markesbery WR. Neuropathology and cognitive impairment in Alzheimer's disease: a complex but coherent relationship. J Neuropathol Exp Neurol. 2009;68:1–14. doi: 10.1097/NEN.0b013e3181919a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Murayama S, Saito Y. Neuropathological diagnostic criteria for Alzheimer's disease. Neuropathology. 2004;24:254–260. doi: 10.1111/j.1440-1789.2004.00571.x. [DOI] [PubMed] [Google Scholar]
- 102.American Psychiatric Association . Diagnostic and statistical manual of mental disorders (IV-TR) American Psychiatric Publishing Inc; Washington DC: 2000. [Google Scholar]
- 103.World Health Organization . ICD-10: international statistical classification of diseases and related health problems. 10th ed. World Health Organization; Geneva: 2004. [Google Scholar]
- 104.van Gunten A, Bouras C, Kövari E, Giannakopoulos P, Hof PR. Neural substrates of cognitive and behavioral deficits in atypical Alzheimer's disease. Brain Res Rev. 2006;51:176–211. doi: 10.1016/j.brainresrev.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 105.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 106.Pasquier F. Early diagnosis of dementia: neuropsychology. J Neurol. 1999;246:6–15. doi: 10.1007/s004150050299. [DOI] [PubMed] [Google Scholar]
- 107.Fox NC, Schott JM. Imaging cerebral atrophy: normal ageing to Alzheimer's disease. Lancet. 2004;363:392–4. doi: 10.1016/S0140-6736(04)15441-X. [DOI] [PubMed] [Google Scholar]
- 108.deToledo-Morrell L, Stoub TR, Bulgakova M, Wilson RS, Bennett DA, Leurgans S, Wuu J, Turner DA. MRI-derived entorhinal volume is a good predictor of conversion from MCI to AD. Neurobiol Aging. 2004;25:1197–203. doi: 10.1016/j.neurobiolaging.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 109.Teipel SJ, Pruessner JC, Faltraco F, Born C, Rocha-Unold M, Evans A, Möller HJ, Hampel H. Comprehensive dissection of the medial temporal lobe in AD: measurement of hippocampus, amygdala, entorhinal, perirhinal and parahippocampal cortices using MRI. J Neurol. 2006;253:794–800. doi: 10.1007/s00415-006-0120-4. [DOI] [PubMed] [Google Scholar]
- 110.Devanand DP, Pradhaban G, Liu X, Khandji A, De Santi S, Segal S, Rusinek H, Pelton GH, Honig LS, Mayeux R, Stern Y, Tabert MH, de Leon MJ. Hippocampal and entorhinal atrophy in mild cognitive impairment: prediction of Alzheimer's disease. Neurology. 2007;68:828–36. doi: 10.1212/01.wnl.0000256697.20968.d7. [DOI] [PubMed] [Google Scholar]
- 111.McEvoy LK, Fennema-Notestine C, Roddey JC, Hagler DJ, Jr, Holland D, Karow DS, Pung CJ, Brewer JB, Dale AM. Alzheimer disease: quantitative structural neuroimaging for detection and prediction of clinical and structural changes in mild cognitive impairment. Radiology. 2009;251:195–205. doi: 10.1148/radiol.2511080924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Henneman WJ, Sluimer JD, Barnes J, van der Flier WM, Sluimer IC, Fox NC, Scheltens P, Vrenken H, Barkhof F. Hippocampal atrophy rates in Alzheimer disease: added value over whole brain volume measures. Neurology. 2009;72:999–1007. doi: 10.1212/01.wnl.0000344568.09360.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.O'Brien JT. Role of imaging techniques in the diagnosis of dementia. Br J Radiol. 2007;80:71–77. doi: 10.1259/bjr/33117326. [DOI] [PubMed] [Google Scholar]
- 114.Mequro K, LeMestric C, Landeau B, Desgranges B, Eustache F, Baron JC. Relations between hypometabolism in the posterior association neocortex and hippocampal atrophy in Alzheimer's disease: a PET/MRI correlative study. J Neurol Neurosurg Psychiatry. 2001;71:315–321. doi: 10.1136/jnnp.71.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chételat G, Desgranges B, de la Sayette V, Vaider F, Eustache F, Baron JC. Mild cognitive impairment: Can FDG-PET predict who is to rapidly convert to Alzheimer's disease? Neurology. 2003;60:1374–7. doi: 10.1212/01.wnl.0000055847.17752.e6. [DOI] [PubMed] [Google Scholar]
- 116.Wang L, Goldstein FC, Veledar E, Levey AI, Lah JJ, Meltzer CC, Holder CA, Mao H. Alterations in cortical thickness and white matter integrity in mild cognitive impairment measured by whole brain cortical thickness mapping and diffusion tensor imaging. Am J Neuroradiol. 2009;30:893–9. doi: 10.3174/ajnr.A1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yasmin H, Nakata Y, Aoki S, Abe O, Sato N, Nemoto K, Arima K, Furuta N, Uno M, Hirai S, Masutani Y, Ohtomo K. Diffusion abnormalities of the uncinate fasciculus in Alzheimer's disease: diffusion tensor tract-specific analysis using a new method to measure the core of the tract. Neuroradiology. 2008;50:293–9. doi: 10.1007/s00234-007-0353-7. [DOI] [PubMed] [Google Scholar]
- 118.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergström M, Savitcheva I, Huang GF, Estrada S, Ausén B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Långström B. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–19. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 119.Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–17. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shoghi-Jadid K, Small GW, Agdeppa ED, Kepe V, Ercoli LM, Siddarth P, Read S, Satyamurthy N, Petric A, Huang SC, Barrio JR. Localization of neurofibrillary tangles and beta-amyloid plaques in the brains of living patients with Alzheimer disease. Am J Geriatr Psychiatry. 2002;10:24–35. [PubMed] [Google Scholar]
- 121.Smid LM, Vovko TD, Popovic M, Petric A, Kepe V, Barrio JR, Vidmar G, Bresjanac M. The 2,6-disubstituted naphtalene derivative FDDNP labeling reliably predicts Congo red birefringence of protein deposits in brain sections of selected human neurodegenerative diseases. Brain Pathol. 2006;16:124–130. doi: 10.1111/j.1750-3639.2006.00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Braskie MN, Klunder AD, Hayashi KM, Protas H, Kepe V, Miller KJ, Huang SC, Barrio JR, Ercoli LM, Siddarth P, Satyamurthy N, Liu J, Toga AW, Bookheimer SY, Small GW, Thompson PM. Plaque and tangle imaging and cognition in normal aging and Alzheimer's disease. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.09.012. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lavretsky H, Siddarth P, Kepe V, Ercoli LM, Miller KJ, Burggren AC, Bookheimer SY, Huang SC, Barrio JR, Small GW. Depression and anxiety symptoms are associated with cerebral FDDNP-PET binding in middle-aged and older nondemented adults. Am J Geriatr Psychiatry. 2009;17:493–502. doi: 10.1097/jgp.0b013e3181953b82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Edison P, Archer HA, Hinz R, Hammers A, Pavese N, Tai YF, Hotton G, Cutler D, Fox N, Kennedy A, Rossor M, Brooks DJ. Amyloid, hypometabolism, and cognition in Alzheimer's disease: an [11C]PIB and [18F]FDG PET study. Neurology. 2007;68:501–8. doi: 10.1212/01.wnl.0000244749.20056.d4. [DOI] [PubMed] [Google Scholar]
- 125.Talboom N, Yaqub M, van der Flier WM, Boellaard R, Luurtsema G, Windhorst AD, Barkhof F, Scheltens P, Lammertsma AA, van Berckel BN. Detection of Alzheimer pathology in vivo using both 11C-PIB and 18F-FDDNP PET. J Nucl Med. 2009;50:191–7. doi: 10.2967/jnumed.108.056499. [DOI] [PubMed] [Google Scholar]
- 126.Lowe VJ, Kemp BJ, Jack CR, Jr, Senjem M, Weigand S, Shiung M, Smith G, Knopman D, Boeve B, Mullan B, Petersen RC. Comparison of 18F-FDG and PiB PET in cognitive impairment. J Nucl Med. 2009;50:878–86. doi: 10.2967/jnumed.108.058529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Devanand DP, Sano M, Tang MX, Taylor S, Taylor BJ, Wilder D, Stern Y, Mayeux R. Depressed mood and the incidence of Alzheimer's disease in the elderly living in the community. Arch Gen Psychiatr. 1996;53:175–182. doi: 10.1001/archpsyc.1996.01830020093011. [DOI] [PubMed] [Google Scholar]
- 128.Devanand DP, Jacobs DM, Tang MX, Del Castillo-Castaneda C, Sano M, Marder K, Bell K, Bylsma FW, Brandt J, Albert M, Stern Y. The course of psychopathologic features in mild to moderate Alzheimer disease. Arch Gen Psychiatr. 1997;54:257–263. doi: 10.1001/archpsyc.1997.01830150083012. [DOI] [PubMed] [Google Scholar]
- 129.Jost BC, Grossberg GT. The evolution of psychiatric symptoms in Alzheimer's disease: A natural history study. J Am Geriatr Soc. 1996;44:1078–1081. doi: 10.1111/j.1532-5415.1996.tb02942.x. [DOI] [PubMed] [Google Scholar]
- 130.Diana A, Simic G, Sinforiani E, Orrù N, Pichiri G, Bono G. Mitochondria morphology and DNA content upon sublethal exposure to β–amyloid1–42 peptide. Coll Antropol. 2008;32(Suppl 1):51–8. [PubMed] [Google Scholar]
- 131.Nanomura A, Hofer T, Moreira PI, Castellani RJ, Smith MA, Perry G. RNA oxidation in Alzheimer disease and related neurodegenerative disorders. Acta Neuropathol. 2009;118:151–66. doi: 10.1007/s00401-009-0508-1. [DOI] [PubMed] [Google Scholar]
- 132.Braak H. On the nuclei of the human brain stem. II. The raphe nuclei. Z Zellforsch Mikrosk Anat. 1970;107:123–141. [PubMed] [Google Scholar]
- 133.Michelsen KA, Prickaerts J, Steinbusch HW. The dorsal raphe nucleus and serotonin: implications for neuroplasticity linked to major depression and Alzheimer's disease. Prog Brain Res. 2008;172:233–264. doi: 10.1016/S0079-6123(08)00912-6. [DOI] [PubMed] [Google Scholar]
- 134.Mann DM, Yates PO. Serotonin nerve cells in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 1983;46:96. doi: 10.1136/jnnp.46.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Curcio CA, Kemper T. Nucleus raphe dorsalis in dementia of the Alzheimer type: neurofibrillary changes and neuronal packing density. J Neuropathol Exp Neurol. 1984;43:359–368. doi: 10.1097/00005072-198407000-00001. [DOI] [PubMed] [Google Scholar]
- 136.Yamamoto T, Hirano A. Nucleus raphe dorsalis in Alzheimer's disease: neurofibrillary tangles and loss of large neurons. Ann Neurol. 1985;17:3–7. doi: 10.1002/ana.410170608. [DOI] [PubMed] [Google Scholar]
- 137.Pearson RC, Esiri MM, Hiorns RW, Wilcock GK, Powell TP. Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer disease. Proc Natl Acad Sci USA. 1985;82:4531–4534. doi: 10.1073/pnas.82.13.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.German DC, White CL, 3rd, Sparkman DR. Alzheimer's disease: neurofibrillary tangles in nuclei that project to the cerebral cortex. Neuroscience. 1987;21:305–312. doi: 10.1016/0306-4522(87)90123-0. [DOI] [PubMed] [Google Scholar]
- 139.Hardy JA, Mann DM, Wester P, Winblad B. An integrative hypothesis concerning the pathogenesis and progression of Alzheimer's disease. Neurobiol Aging. 1986;7:489–502. doi: 10.1016/0197-4580(86)90086-2. [DOI] [PubMed] [Google Scholar]
- 140.Jellinger K. Neuropathological substrates of Alzheimer's disease and Parkinson's disease. J Neural Transm Suppl. 1987;24:109–129. [PubMed] [Google Scholar]
- 141.Saper CB, Wainer BH, German DC. Axonal and transneuronal transport in the transmission of neurological disease: potential role in system degenerations, including Alzheimer's disease. Neuroscience. 1987;23:389–398. doi: 10.1016/0306-4522(87)90063-7. [DOI] [PubMed] [Google Scholar]
- 142.Zweig RM, Ross CA, Hedreen JC, Steele C, Cardillo JE, Whitehouse PJ, Folstein MF, Price DL. The neuropathology of aminergic nuclei in Alzheimer's disease. Ann Neurol. 1988;24:233–242. doi: 10.1002/ana.410240210. [DOI] [PubMed] [Google Scholar]
- 143.Wilcock GK, Esiri MM, Bowen DM, Hughes AO. The differential involvement of subcortical nuclei in senile dementia of Alzheimer's type. J Neurol Neurosurg Psychiatry. 1988;51:842–849. doi: 10.1136/jnnp.51.6.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hertz L. Is Alzheimer's disease an anterograde degeneration, originating in the brainstem, and disrupting metabolic and functional interactions between neurons and glial cells? Brain Res Rev. 1989;14:335–353. doi: 10.1016/0165-0173(89)90017-9. [DOI] [PubMed] [Google Scholar]
- 145.Whitehouse PJ, Price DL, Clark AW, Coyle JT, DeLong MR. Alzheimer's disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann Neurol. 1981;10:122–6. doi: 10.1002/ana.410100203. [DOI] [PubMed] [Google Scholar]
- 146.Van Domburg P. Thesis. University of Nijmegen; 1990. Human brainstem monoaminergic structures in Parkinson's disease and Alzheimer's disease. [Google Scholar]
- 147.Aletrino MA, Vogels OJ, Van Domburg PH, Ten Donkelaar HJ. Cell loss in the nucleus raphes dorsalis in Alzheimer's disease. Neurobiol Aging. 1992;13:461–468. doi: 10.1016/0197-4580(92)90073-7. [DOI] [PubMed] [Google Scholar]
- 148.Halliday GM, McCann HL, Pamphlett R, Brooks WS, Creasey H, McCusker E, Cotton RG, Broe GA, Harper CG. Brain stem serotonin-synthesizing neurons in Alzheimer's disease: a clinicopathological correlation. Acta Neuropathol. 1992;84:638–650. doi: 10.1007/BF00227741. [DOI] [PubMed] [Google Scholar]
- 149.Brilliant M, Elble RJ, Ghobrial M, Struble RG. Distribution of amyloid in the brainstem of patients with Alzheimer's disease. Neurosci Lett. 1992;148:23–26. doi: 10.1016/0304-3940(92)90795-9. [DOI] [PubMed] [Google Scholar]
- 150.Lippa CF, Saunders AM, Smith TW, Swearer JM, Drachman DA, Ghetti B, Nee L, Pulaski-Salo D, Dickson D, Robitaille Y, Bergeron C, Crain B, Benson MD, Farlow M, Hyman BT, George-Hyslop SP, Roses AD, Pollen DA. Familial and sporadic Alzheimer's disease: neuropathology cannot exclude a final common pathway. Neurology. 1996;46:406–12. doi: 10.1212/wnl.46.2.406. [DOI] [PubMed] [Google Scholar]
- 151.Mössner R, Schmitt A, Syagailo YV, Gerlach P, Riederer P, Lesch KP. The serotonin transporter in Alzheimer's and Parkinson's disease. J Neural Transm Suppl. 2000;60:345–350. [PubMed] [Google Scholar]
- 152.Parvizi J, Van Hoesen GW, Damasio A. Selective pathological changes of the periaqueductal gray matter in Alzheimer's disease. Ann Neurol. 2000;48:344–353. [PubMed] [Google Scholar]
- 153.Parvizi J, Van Hoesen GW, Damasio A. The selective vulnerability of brainstem nuclei to Alzheimer's disease. Ann Neurol. 2001;49:53–66. doi: 10.1002/1531-8249(200101)49:1<53::aid-ana30>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 154.Yang Y, Schmitt HP. Frontotemporal dementia: evidence for impairment of ascending serotoninergic but not noradrenergic innervation. Immunocytochemical and quantitative study using a graphy method. Acta Neuropathol. 2001;101:256–270. doi: 10.1007/s004010000293. [DOI] [PubMed] [Google Scholar]
- 155.Kovacs GG, Klöppel S, Fischer I, Dorner S, Lindeck-Pozza E, Birner P, Bötefür IC, Pilz P, Volk B, Budka H. Nucleus-specific alteration of raphe neurons in human neurodegenerative disorders. Neuroreport. 2003;14:73–76. doi: 10.1097/00001756-200301200-00014. [DOI] [PubMed] [Google Scholar]
- 156.Hendricksen M, Thomas AJ, Ferrier IN, Ince P, O'Brien JT. Neuropathological study of the dorsal raphe nuclei in late-life depression and Alzheimer's disease with and without depression. Am J Psychiatry. 2004;161:1096–1102. doi: 10.1176/appi.ajp.161.6.1096. [DOI] [PubMed] [Google Scholar]
- 157.Kepe V, Barrio JR, Huang SC, Ercoli L, Siddarth P, Shoghi-Jadid K, Cole GM, Satyamurthy N, Cummings JL, Small GW, Phelps ME. Serotonin 1A receptors in the living brain of Alzheimer's disease patients. Proc Natl Acad Sci USA. 2006;103:702–707. doi: 10.1073/pnas.0510237103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bernedo V, Insua D, Suarez ML, Santamarina G, Sarasa M, Pesini P. Beta-amyloid cortical deposits are accompanied by the loss of serotonergic neurons in the dog. J Comp Neurol. 2009;513:417–429. doi: 10.1002/cne.21985. [DOI] [PubMed] [Google Scholar]
- 159.Grinberg LT, Rüb U, Ferreti REL, Nitrini R, Farfel JM, Polichiso L, Gierga K, Jacob-Filho W, Heinsen H. The dorsal raphe nucleus shows phospho-tau neurofibrillary changes before the transentorhinal region in AD. A precocious onset? Neuropathol Appl Neurobiol. 2009;35:406–16. doi: 10.1111/j.1365-2990.2009.00997.x. [DOI] [PubMed] [Google Scholar]
- 160.Rüb U, Del Tredici K, Schultz C, Thal DR, Braak E, Braak H. The evolution of Alzheimer's disease-related cytoskeletal pathology in the human raphe nuclei. Neuropathol Appl Neurobiol. 2000;26:553–67. doi: 10.1046/j.0305-1846.2000.00291.x. [DOI] [PubMed] [Google Scholar]
- 161.Castellani RJ, Lee HG, Zhu X, Perry G, Smith MA. Alzheimer disease pathology as a host response. J Neuropathol Exp Neurol. 2008;67:523–531. doi: 10.1097/NEN.0b013e318177eaf4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Castellani RJ, Zhu X, Lee HG, Smith MA, Perry G. Molecular pathogenesis of Alzheimer's disease: reductionist versus expansionist approaches. Int J Mol Sci. 2009;10:1386–406. doi: 10.3390/ijms10031386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Terry RD. The pathogenesis of Alzheimer disease: an alternative to the amyloid hypothesis. J Neuropathol Exp Neurol. 1996;55:1023–5. [PubMed] [Google Scholar]
- 164.Holzer M, Holzapfel HP, Zedlick D, Brückner MK, Arendt T. Abnormally phoshorylated tau protein in Alzheimer's disease: heterogeneity of individual regional distribution and relationship to clinical severity. Neuroscience. 1994;63:499–516. doi: 10.1016/0306-4522(94)90546-0. [DOI] [PubMed] [Google Scholar]
- 165.Alonso AD, Li B, Grundke-Iqbal I, Iqbal K. Polymerization of hyperphosphorylated tau into filaments eliminates its inhibitory activity. Proc Natl Acad Sci USA. 2006;23:8864–69. doi: 10.1073/pnas.0603214103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kordower JH, Chu Y, Stebbins GT, DeKosky ST, Cochran EJ, Bennett D, Mufson EJ. Loss and atrophy of layer II entorhinal cortex neurons in elderly people with mild cognitive impairment. Ann Neurol. 2001;49:202–13. [PubMed] [Google Scholar]
- 167.Gómez-Isla T, Price JL, McKeel DW, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer's disease. J Neurosci. 1996;16:4491–500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gómez-Isla T, Hollister R, West H, Mui S, Growdon JH, Petersen JC, Parisi JE, Hyman BT. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer's disease. Ann Neurol. 1997;41:17–24. doi: 10.1002/ana.410410106. [DOI] [PubMed] [Google Scholar]
- 169.Krill JJ, Patel S, Harding AJ, Halliday GM. Neuron loss from the hippocampus of Alzheimer's disease exceeds extracellular neurofibrillary tangle formation. Acta Neuropathol. 2002;103:370–376. doi: 10.1007/s00401-001-0477-5. [DOI] [PubMed] [Google Scholar]
- 170.Iqbal K, Flory M, Khatoon S, Soininen H, Pirttila T, Lehtovirta M, Alafuzoff I, Blennow K, Andreasen N, Vanmechelen E, Grundke-Iqbal I. Subgroups of Alzheimer's disease based on cerebrospinal fluid molecular markers. Ann Neurol. 2005;58:748–57. doi: 10.1002/ana.20639. [DOI] [PubMed] [Google Scholar]
- 171.Truchot L, Costes SN, Zimmer L, Laurent B, Le Bars D, Thomas-Antérion C, Croisile B, Mercier B, Hermier M, Vighetto A, Krolak-Salmon P. Up-regulation of hippocampal serotonin metabolism in mild cognitive impairment. Neurology. 2007;69:1012–1017. doi: 10.1212/01.wnl.0000271377.52421.4a. [DOI] [PubMed] [Google Scholar]