Abstract
Hyperinsulinemia associated with type II diabetes mellitus is a risk factor for non-alcoholic steatohepatitis (NASH) and hepatic fibrosis. Hepatic stellate cells (HSCs) are the major effectors in collagen production during hepatic fibrogenesis. Elevated levels of insulin stimulate HSC activation. In addition to its anti-diabetic effects, the antioxidant curcumin, the yellow pigment in curry from turmeric, suppresses HSC activation and protects the liver from fibrogenesis in vitro and in vivo. This study aims at evaluating the effect of curcumin on insulin-induced HSC activation and further elucidating the underlying mechanisms. We report that curcumin dose-dependently eliminates insulin-induced HSC activation demonstrated by suppressing expression of type I collagen gene and other key genes relevant to HSC activation. Additional experiments indicate that curcumin interrupts insulin signaling in HSCs by reducing the phosphorylation level of insulin receptor (InsR) and suppressing gene expression of InsR. Furthermore, curcumin attenuates insulin-induced oxidative stress in HSCs by inducing gene expression of glutamate-cysteine ligase, leading to de novo synthesis of glutathione and the suppression of gene expression of InsR. These results support our initial hypothesis that curcumin inhibits the effects of insulin on stimulating HSC activation by interrupting insulin signaling and attenuating oxidative stress Our results provide novel insights into the mechanisms by which curcumin inhibits the insulin-induced HSC activation.
Keywords: Hepatic fibrosis, hepatic stellate cell, hyperinsulinemia, insulin, non-alcoholic fatty liver disease, phytochemical
INTRODUCTION
Hyperinsulinemia, i.e. abnormally elevated levels of plasma insulin, is a feature of type II diabetes mellitus (T2DM). T2DM is recognized as the etiology of over 80 percent of all diabetes and is dramatically increasing in incidence as a result of changes in human behavior and increased body mass index 1. Hyperinsulinemia is a condition in which normal amounts of insulin could not produce a normal insulin response from fat, muscle and liver cells 1, 2. As a compensatory mechanism, pancreatic beta cells excessively secret insulin to maintain euglycemia, resulting in abnormally elevated levels of blood insulin 3. Insulin in normal state carries out its functions through binding to insulin receptor (InsR), leading to the auto-phosphorylation of InsR and the activation of downstream signaling cascades, including the mitogen-activated protein kinase (MAPK) pathway and the PI3K-AKT pathway 4.
The liver is an insulin sensitive organ that plays a critical role in regulating the whole body energy homeostasis 2. It has been noted that T2DM is often associated with non-alcoholic fatty liver diseases (NAFLD) and is a risk factor for non-alcoholic steatohepatitis (NASH), an advanced form of NAFLD 5. NAFLD are found in a quarter of the general population in U. S. and approximately 15–40% of NASH patients develop hepatic fibrosis 6. Hepatic stellate cells (HSCs) are the key players in the development of hepatic fibrosis, regardless of etiology 7, 8. HSCs normally reside in the space of Disse in a quiescent, non-proliferative state. They are characterized by abundant lipid droplets, composed of retinyl esters, triglycerides, cholesteryl esters, cholesterol, phospholipids, and free fatty acids 9, 10. During hepatic injury, quiescent HSCs undergo profound phenotypic changes, including enhanced cell proliferation, loss of lipid droplets, de novo expression of α-smooth muscle actin (α-SMA), and excessive production of ECM. This process is called HSC activation. Freshly-isolated HSCs in culture gradually and spontaneously become fully activated in seven days 11, mimicking the process seen in vivo, which provides a good model for elucidating underlying mechanisms of HSC activation and studying potential therapeutic intervention of the process 7, 8. Studies have demonstrated that insulin stimulates HSC activation in vitro by inducing mitogenesis and collagen synthesis 12.
Despite considerable accomplishments in research on NASH-associated hepatic fibrogenesis, the underlying mechanisms remain largely undefined. It is widely accepted that oxidative stress plays critical roles in hepatic fibrosis, regardless of etiology 13. For instance, during the pathogenesis of NASH, fat accumulation in the liver is considered as “the first hit” 1, which makes the liver vulnerable to endotoxins and impairs liver regeneration. Oxidative stress is recognized as “the second hit” 1, which causes peroxidation of lipids in cell membranes, pro-inflammatory cytokine induction, and the activation of HSCs. NASH patients have increased levels of oxidative stress and lipid peroxidation products 1, 2, which, in turn, promotes the development of hepatic fibrogenesis 1, 2. Activities of antioxidant enzymes in NASH patients are dramatically reduced 14. Oxidative stress stimulates collagen production in HSCs and hepatic fibrogenesis 14. Prior reports have shown protective effects of antioxidants, including vitamin E, in the suppression of HSC activation 13 and the inhibition of hepatic fibrogenesis 13. However, the efficiency of currently well-known antioxidants in protecting the liver from fibrogenesis is still not very impressive 13, 15.
Few effective therapies are currently available for treatment of hepatic fibrosis 16. Research identifying anti-fibrotic agents that are innocuous is, therefore, of high priority and urgently needed. Curcumin, the yellow pigment in curry from turmeric, is a potent antioxidant, whose antioxidant capacity is 100-fold stronger than that of vitamin E/C 17. Curcumin has received attention as a promising dietary component for the protection against fibrogenic insults 18. We recently showed that curcumin inhibited HSC activation, including inducing gene expression of endogenous peroxisome proliferator-activated receptor-gamma (PPARγ), and suppressing gene expression of αI(I) collagen, α-SMA, PDGF-beta receptor (PDGF-βR), EGF receptor (EGFR), type I and II transforming growth factor-beta receptors (Tβ-RI & Tβ-RII) and connective tissue growth factor (CTGF) and protected the liver from CCl4-caused fibrogenesis in vitro and in vivo 18–22. The current study aims at evaluating the effect of curcumin on eliminating the role of insulin in the stimulation of HSC activation and further elucidating the underlying mechanisms. Results in this report provide evidence to support our initial hypothesis that curcumin inhibits the effects of insulin on stimulating HSC activation by interrupting insulin signaling and attenuating oxidative stress.
MATERIALS AND METHODS
Materials
Insulin and curcumin (purity > 94%) were purchased from Sigma-Aldrich (St. Louis, MO). Insulin was dissolved in 0.01N HCl and a stocking concentration at 1 mM was prepared. Curcumin was dissolved in 100% ethanol and a stocking concentration at 100 mM was made.
HSC isolation and culture
Hepatic stellate cells (HSCs) were isolated from the liver of normal male Sprague-Dawley rats (200–250 g) through portal vein perfusion with collagenase-pronase and subsequent density gradient centrifugation as previously described 11, 20. After isolation, HSCs were identified by their typical morphology, the vitamin A-droplet-dependent auto-fluorescence and their inability to phagocytose latex beads. Freshly-isolated HSCs were plated on plastic plates and cultured in Dulbecco's modified Eagle medium (DMEM) supplemented with 20% of fetal bovine serum (FBS) at 37°C in a 5% CO2 atmosphere for the first 48 hr. The purity of HSCs was greater than 95% as assessed 48 hr after seeding. HSCs were subsequently cultured in regular DMEM with FBS (10%). Semi-confluent HSCs with 4–8 passages were used in experiments. In some experiments, cells were cultured in serum-depleted media for 24 hr before treatment, which rendered HSCs more sensitive to stimuli, including exogenous insulin. Cells were subsequently treated and cultured in serum-depleted media for additional 24 hr, which excluded the interference from other factors in FBS.
Oil Red O staining
After fixation with 4% paraformaldehyde for 15 minutes (min), HSCs were stained with Oil Red O in isopropanol (60%) for 10 min, followed by differentiation with isopropanol (60%) for 2 min. Cells were counterstained with hematoxylin for 15 seconds and washed with distilled water thoroughly. Lipid droplets in HSCs were evaluated using a light microscope with 40x amplification. Lipid droplets in quiescent HSCs were colored dark red by Oil Red O.
mRNA extraction and real-time PCR
Total RNA was extracted using TRI-reagent according to the manufacturer's instruction (Sigma). Total RNA was treated with DNase I prior to the synthesis of the first strand of cDNA. Real-time PCR were performed as we previously described using SYBR Green Supermix 23. mRNA levels were expressed as fold changes after normalization with glyceraldehyde-3-phosphate dehydrogenase (GAPDH), as described by Schmittgen et al 24. The following primers were used for real-time PCR. InsR: (F) 5'-GCC TGG GCA ACT GTT CAG A-3', (R) 5'-GTT TCG ACA GGC CAC ACA CTT-3'. Gclc: (F) 5'-TGT GTG ATG AGC CCA AGG AC-3'; (R) 5'-AGT TGG CTC GCA TCA TAG TTG-3'; Gclm: (F) 5'-CTG CTA AACTGT TCA TTG TAG G-3'; (R) 5'-CTA TGG GTT TTA CCT GTG-3'. Other primers were recently described 25.
Western blotting analyses
Whole cell extracts were prepared as we previously described 20. Protein concentrations were determined using the BCA Protein Assay Kit according to the manufacturer's instruction (Pierce, Rockford, IL). SDS-PAGE, transblotting and subsequent immuno-reactions were conducted as we previously described 20. Antibodies used in this study were described in Table 1.
Table 1.
Antibodies use for Western blotting analyses
| Company | Description | Catalog number |
|---|---|---|
| Santa Cruz Biotech. Inc. | Rabbit α-PDGF-βR antibody | sc-432 |
| Rabbit α-EGFR antibody | sc-03 | |
| Rabbit α-Tβ-RII antibody | sc-400 | |
| Rabbit αTβ-RI antibody | sc-399 | |
| Rabbit α-p27 antibody | sc-528 | |
| Rabbit α-p21 antibody | sc-397 | |
| Rabbit α-Bax antibody | sc-493 | |
| Rabbit α-Bcl-2 antibody | sc-492, | |
| Rabbit α-InsR antibody | sc-20739 | |
| Rabbit α-p-InsR antibody | sc-25103-R | |
| Rabbit α-p-ERK1/ antibody | sc-16982-R | |
| Rabbit α- total ERK1 antibody | sc-94 | |
| Rabbit α-total ERK2 antibody | sc-153 | |
| Rabbit α-p-AKTser 473 antibody | sc-7985-R | |
| Rabbit α-total AKT antibody | sc-8312 | |
| Rabbit α-p-JNK antibody | sc-12882-R | |
| Rabbit α-total JNK antibody | sc-474 | |
| Rabbit α-p-PI3K antibody | sc-12929-R | |
| Rabbit α-total PI3K antibody | sc-423 | |
| Goat α-CTGF antibody | sc-14939 | |
| Goat α-pro-αl(l)col antibody | sc-25974 | |
| Bovine α-goat-IgG-HRP | sc-2350 | |
| Goat α-mouse-IgG-HRP | sc-2005 | |
| Goat α-rabbit-IgG-HRP | Sc-2004 | |
| Sigma Company | Mouse α-alpha-SMA monoclonal antibody | A2547 |
| Rabbit α-β-actin antibody: | A2066 |
Determination of the level of intracellular ROS
The level of intracellular ROS in HSCs was determined by analyzing dichlorofluorescein (DCF) fluorescence, as described previously 26.
LPO assays
LPO assays were performed by using the Lipid Hydroperoxide Assay kit purchased from Cayman Chemical (Ann Arbor, MI).
GSH assays
Levels of GSH and GSSG were determined by the Glutathione Assay Kit from Cayman Chemical, following the protocol provided by the manufacturer. The concentration of total GSH was calculated according to the equation in the protocol.
Analyses of GCL activities
GCL activities were spectrophotometrically determined as previously described with slight modifications 27. Briefly, a sample of cell extracts (20 μl) was mixed with the reaction solution (0.21 ml) containing Tris-HCl (100 mM), pH 8.0, KCl (150 mM), MgCl2 (20 mM), Na2EDTA (2 mM), Na2ATP (5 mM), phosphoenolpyruvate (2 mM), L-glutamate (10 mM), L-α-aminobutyrate (10 mM), NADH (0.27 mM), type II rabbit muscle pyruvate (2 μg) and lactate dehydrogenase (2 μg). The reaction was initiated by the addition of ATP to a final concentration at 5 mM. The decrease in the absorbance of NADH at 340 nm was monitored for 30 min with interval of 2 min by using a SpectraMax 190 plate reader (Molecular Device, Sunnyvale, CA) and expressed as micro-moles of NADH oxidized/min. Protein concentration was quantitated by BCA assay (Pierce). The final GCL activity was calculated and expressed as micro-moles (mmol) of NADH oxidized/min/mg protein.
Statistical analyses
Differences between means were evaluated by an unpaired two-sided Student's t-test (p<0.05 considered as significant). Where appropriate, comparisons of multiple treatment conditions with controls were analyzed by ANOVA with the Dunnett's test for post hoc analysis.
RESULTS
Curcumin eliminates the effects of insulin on regulating expression of genes closely relevant to HSC activation in culture-activated HSCs
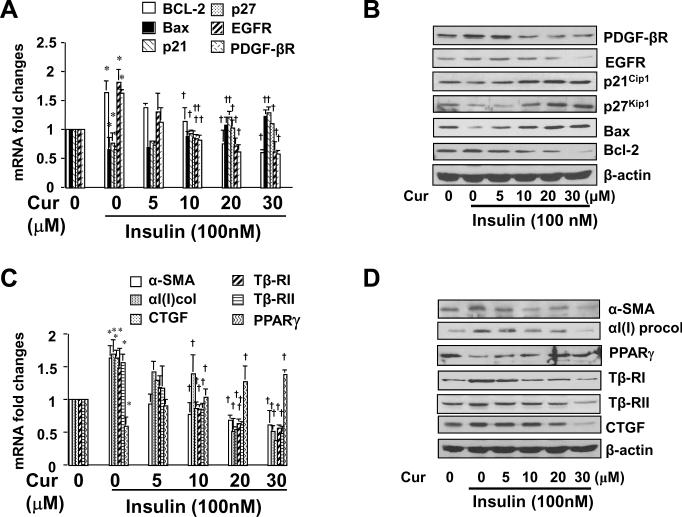
Prior studies have demonstrated that insulin stimulates HSC activation in vitro by inducing mitogenesis and collagen synthesis 12. To evaluate the effect of curcumin on insulin-induced HSC activation, after cultured in serum-depleted media for 24 hr, semi-confluent HSCs were stimulated with insulin (100 nM) in the presence of curcumin at 0–30 μM in serum-depleted DMEM for additional 24 hr. Results from our pilot experiments indicated that compared with serum-starved HSCs, HSCs cultured in regular DMEM with FBS (10%) required higher concentrations of insulin to achieve the same level of changes in regulating expression of genes, including αI(I) collagen and α-SMA, the two established markers for activated HSCs (data not shown). These observations suggested that serum-starvation rendered HSCs in vitro more sensitive to exogenous stimuli. The subsequent culture in serum-depleted media excluded the interference from other factors in FBS 21, 28. Total RNA and whole cell extracts were prepared from the cells. To evaluate the effects of curcumin on insulin-induced cell growth, genes relevant to cell proliferation and to apoptosis were selectively studied. As shown by real-time PCR assays (Fig. 1A), compared to the untreated control (the corresponding 1st columns), insulin significantly increased, as expected, the mRNA levels of pro-mitogenic PDGF-βR and EGFR (the corresponding 2nd columns), and reduced the mRNA levels of the potent cyclin-dependent kinase inhibitors p21Cip1 and p27Kip1 (the corresponding 2nd columns). In addition, insulin increased the mRNA level of anti-apoptotic protein Bcl-2 and reduced the mRNA level of pro-apoptotic protein Bax in the cells (the corresponding 2nd columns). Further experiments indicated that curcumin dose-dependently eliminated the insulin effects (the corresponding 3rd –6th columns). These observations were verified by Western blotting analyses (Fig. 1B).
Figure 1. Curcumin attenuates the stimulatory effects of insulin on the activation of HSCs.
Serum-starved HSCs were stimulated with or without insulin (100 nM) plus curcumin at various concentrations in serum-depleted DMEM for 24 hr. Total RNA or whole cell extracts were prepared for real-time PCR assays (A & C), or for Western blotting analyses (B & D). Values in A & C were presented as mRNA fold changes (mean ± S. D., n=3), *p<0.05, versus the untreated control (the corresponding 1st columns); †p<0.05, versus cells treated with insulin only (the corresponding 2nd columns). Blots were representatives from three independent experiments. β-actin was used as an internal control for equal loading for Western. A & B: Analyses of expression of genes relevant to cell proliferation or apoptosis; C & D: Analyses of expression of genes relevant to fibrogenesis.
To assess the effect of curcumin on insulin-induced fibrogenesis, genes relevant to fibrogenesis were selectively studied. Real-time PCR (Fig. 1C) and Western blotting analyses (Fig. 1D) demonstrated that compared to the untreated control (the corresponding 1st columns or wells), insulin stimulated, as expected, gene expression of α-SMA and αI(I) collagen, the markers of activated HSCs, and pro-fibrogenic Tβ-RI & Tβ-RII and CTGF (the corresponding 2nd columns or well). The stimulatory effects of insulin were dose-dependently attenuated by curcumin (the corresponding 3rd –6th columns). It is noteworthy that insulin suppressed gene expression of PPARγ in cultured HSCs (Fig. 1C & D). Curcumin dose-dependently abrogated the inhibitory role of insulin and induced gene expression of endogenous PPARγ, which had been shown to play a critical role in the curcumin-caused inhibition of HSC activation 20, 21. Taken together, our results suggested that curcumin eliminated the effects of insulin on stimulating HSC activation in vitro.
Insulin stimulates the activation of quiescent HSCs in vitro, which is attenuated by curcumin
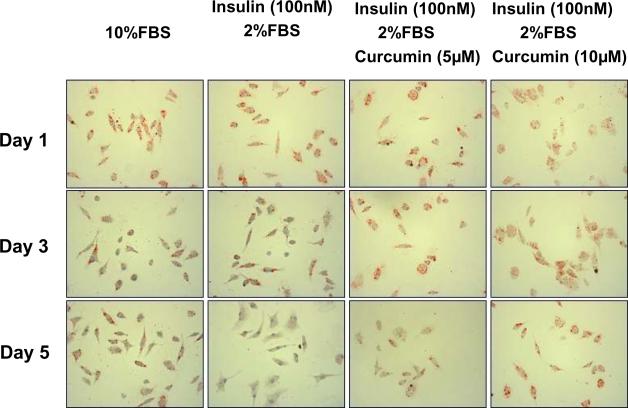
To evaluate the effect of insulin on the activation of quiescent HSCs and the potential inhibitory role of curcumin in the process, additional experiments were conducted. After culturing for 24 hr in serum-rich media (20% of FBS) for cell attachment, freshly-isolated quiescent HSCs were cultured in DMEM with 10% of FBS, or 2% of FBS plus insulin (100 nM) with or without curcumin at 5 or 10 μM for additional 1, 3, or 5 days. These HSCs were stained with Oil Red O for detecting intracellular lipid droplets. As shown in Fig. 2, after one-day culture, no matter what supplements in the media with or without curcumin, HSCs showed high levels of intracellular lipid droplets, which were stained dark red by Oil Red O, demonstrating that these HSCs were still in quiescent state. Insulin (100 nM) plus 2% of FBS, like FBS (10%), day-dependently and apparently reduced the contents of lipid droplets in HSCs, suggesting that insulin, like FBS, stimulated the activation of HSCs in culture. Most of freshly-isolated quiescent HSCs were detached within 3 days in the media with only 2% of FBS (data not shown). In great contrast, the presence of curcumin in the media with insulin plus 2% of FBS dose-dependently maintained higher levels of intracellular lipid droplets after 5 days of culture, suggesting that curcumin attenuated the stimulatory role of insulin, which might delay the process of HSC activation in culture. Lower concentrations of curcumin at 5 and 10 μM were used because of higher sensitivity of freshly-isolated HSCs to the phytochemical. Most of the cultured cells were detached within 7 days in the media with insulin (100 nM) plus 2% of FBS in the presence of curcumin (data not shown). Quiescent HSCs were reported to become fully activated after 7 days of culture in DMEM with 10% of FBS 11. However, the technical barrier prevented from conducting the experiments for more than 5 days. Taken together, our results demonstrated that insulin stimulated the activation of quiescent HSCs in vitro, which was attenuated by curcumin.
Figure 2. Insulin stimulates the activation of quiescent HSCs in vitro, which is attenuated by curcumin.
After culturing for 24 hr in serum-rich media (20% of FBS) for cell attachment, freshly-isolated HSCs were cultured in DMEM with 10% of FBS, or 2% of FBS plus insulin (100 nM) with or without curcumin at indicated concentrations for additional 1, 3, or 5 days. Each treatment had triplicates. After fixation, the cells were stained with Oil Red O for detecting intracellular lipid droplets, which were colored dark red by Oil Red O. Representative views were presented.
Curcumin reduces the phosphorylation of InsR and its downstream inter-mediators in HSCs
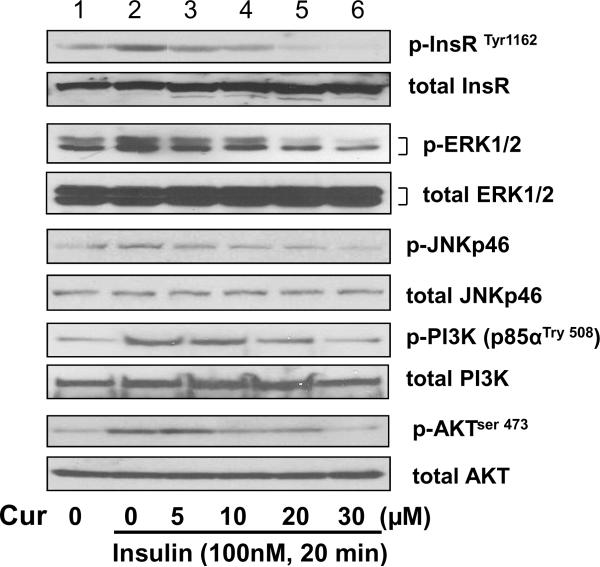
To explore the underlying mechanisms by which curcumin eliminated the insulin-induced HSC activation, we hypothesized that curcumin might interrupt insulin signaling pathways, which was initiated by binding of insulin to its receptor InsR, leading to the auto-phosphorylation of the receptor and the subsequent activation of downstream signaling inter-mediators, including ERK, JNK, PI3K and AKT 4. Pilot experiments indicated that insulin rapidly initiated its signaling by stimulating the phosphorylation of InsR in HSCs, which reached its peak within 20–30 min and gradually faded thereafter (data not shown here). To evaluate the impact of curcumin on insulin signaling pathways, serum-starved HSCs were pretreated with curcumin at various concentrations for 1 hr prior to the stimulation with insulin (100 nM) in serum-depleted DMEM for additional 20 min. The pretreatment with curcumin inhibited activating signal pathways and excluded their interference. Whole cell extracts were prepared. As in Fig. 3 by Western blotting analyses, compared to the untreated control (the corresponding 1st wells), insulin significantly increased the abundance of phosphorylated InsRTyr 1162, ERK1/2, JNKp46, PI3K (p85α Tyr 508) and AKTser 473 (the corresponding 2nd wells) in cultured HSCs. The insulin stimulatory effects were dose-dependently attenuated by curcumin (the corresponding 2nd–6th wells), indicating that curcumin interrupted the insulin signaling pathways. It is noteworthy that the phosphorylation of InsR and its downstream inter-mediators was an instant and short-term action following the insulin stimulation. The impact of curcumin on it was a short-term event, which lasted only few hours.
Figure 3. Curcumin reduces phosphorylation levels of InsR and its downstream signaling intermediators in HSCs.
Serum-starved HSCs were pretreated with curcumin for 1 hr at various concentrations prior to the stimulation with insulin (100 nM) for additional 20 min. Whole cell extracts were prepared for Western blotting analyses of phosphorylated proteins and their corresponding total proteins. The total protein for each corresponding phospho-protein was used as the internal control for equal loading. Representatives were shown from three independent experiments.
Curcumin dose-dependently suppresses gene expression of InsR in cultured HSCs
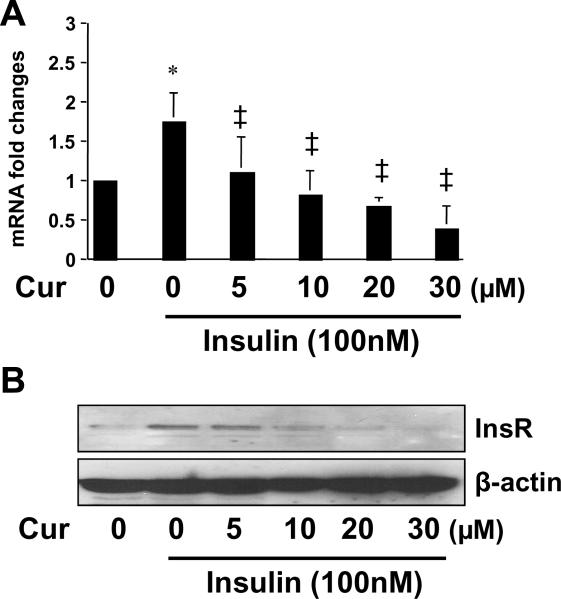
To further explore the mechanisms by which curcumin eliminated the stimulatory effects of insulin on the activation of HSCs, we assumed that in addition to the instant and short-term action, curcumin should exert a slow but long-lasting action. We postulated that curcumin suppressed gene expression of InsR, leading to a reduction in the bioavailability of InsR to insulin. To test this postulation, serum-starved HSCs were stimulated with insulin at 100 nM in the presence of curcumin at 0–30 μM in serum-depleted media for 24 hr. Total RNA and whole cell extracts were prepared for analyses of gene expression of InsR. As shown in Fig. 4 by real-time PCR and Western blotting analyses, compared to the untreated control (the 1st column, or well), insulin significantly increased the steady state level of transcript and the abundance of protein of InsR (the 2nd column, or well). The stimulatory effects were dose-dependently abolished by curcumin (the 3rd –6th columns, or wells). Taken together, these results supported our postulation and demonstrated that curcumin suppressed gene expression of InsR in activated HSCs in vitro.
Figure 4. Curcumin dose-dependently suppresses gene expression of InsR in cultured HSCs.
Serum-starved HSCs were stimulated with or without insulin (100 nM) in the presence or absence of curcumin at indicated concentrations in serum-free media for 24 hr. Total RNA or whole cell extracts were prepared. (A). Real-time PCR assays of the steady state level of InsR mRNA. β-actin was used as an invariant internal control for calculating mRNA fold changes (n=3). *p<0.05 versus cells with no treatment (the 1st column on the left); ‡p<0.05 versus cells treated with insulin only (the 2nd column on the left); (B). Western blotting analyses of the abundance of InsR. β-actin was used as an internal control for equal loading. A representative was shown from three independent experiments.
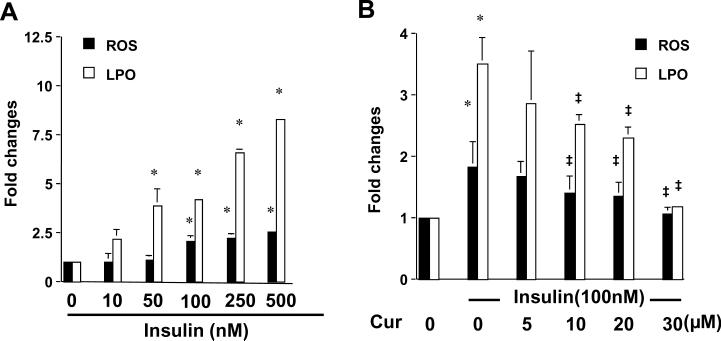
Insulin increases the levels of intracellular ROS and LPO in HSCs, which is dose-dependently attenuated by curcumin
Oxidative stress has been known to play a critical role in HSC activation and in hepatic fibrogenesis, regardless of etiology 13, 29. We have previously reported the role of curcumin alone in attenuating oxidative stress in cultured HSCs 23. To explore the mechanisms by which insulin stimulated the activation of HSCs, experiments were conducted to evaluate the impact of insulin on oxidative stress. Serum-starved HSCs were stimulated with different concentrations of insulin in serum-depleted media for 24 hr. Levels of lipid peroxides (LPO) and reactive oxygen species (ROS) in the cells were determined. As shown in Fig. 5A, insulin elevated the levels of cellular ROS and LPO in a dose-dependent manner. To assess the role of curcumin in attenuating insulin-induced oxidative stress, serum-starved HSCs were stimulated with insulin (100 nM) in the presence or absence of curcumin at indicated concentrations in serum-depleted media for 24 hr. As shown in Fig. 5B, ROS and LPO levels elevated by insulin in the cells were dose-dependently reduced by curcumin. These results collectively indicated that insulin induced oxidative stress in cultured HSCs, which was attenuated by curcumin.
Figure 5. Insulin stimulates oxidative stress in cultured HSCs by increasing the levels of ROS and LPO, which is attenuated by curcumin.
Serum-starved HSCs were stimulated with insulin at indicated concentrations in the absence (A) or presence (B) of curcumin at 0–30μM in serum-free media for 24 hr. The levels of intracellular ROS and LPO were assessed and expressed as fold changes compared to that in cells with no treatment (the 1st column on the left). Values were presented as means ± S.D. (n≥3). *p<0.05, versus cells with no treatment; ‡p<0.05, versus cells treated with insulin only (the 2nd column on the left in B).
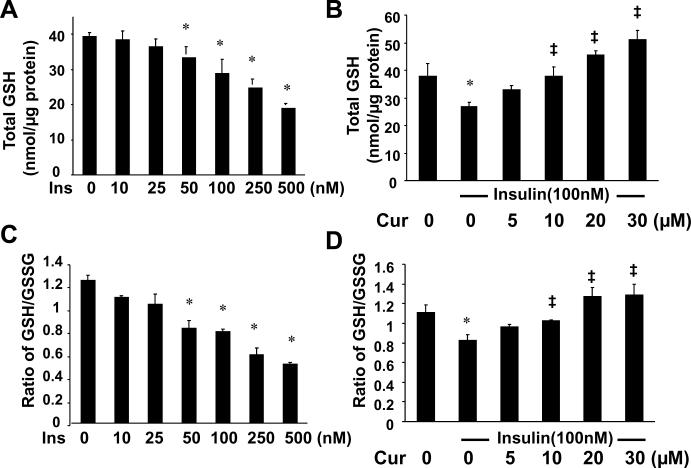
Curcumin attenuates insulin-induced oxidative stress in activated HSCs, at least partially, by increasing the level of intracellular GSH and improving the ratio of GSH/GSSG
Glutathione (GSH) is the most abundant and effective thiol antioxidant in eukaryotic cells 30. GSH is converted to its oxidized form (GSSG), when attenuating oxidative stress 30. To elucidate the mechanisms by which insulin induced oxidative stress and curcumin attenuated the insulin-induced oxidative stress, serum-starved HSCs were stimulated with insulin at indicated concentrations in the presence or absence of curcumin at various concentrations in serum-depleted media for 24 hr. As shown in Fig. 6A & C, insulin reduced, in a dose-dependent manner, the levels of intracellular GSH and the ratio of GSH/GSSG, a sensitive indicator of oxidant stress 31, 32. On the other hand, curcumin dose-dependently eliminated the inhibitory effects of insulin on the level of cellular GSH (Fig. 6B) and improved the ratio of GSH/GSSG (Fig. 6D). These results indicated that curcumin attenuated insulin-induced oxidative stress in activated HSCs, at least partially, by increasing the level of intracellular GSH and improving the ratio of GSH/GSSG.
Figure 6. Insulin reduces the level of intracellular GSH and the ratio of GSH/GSSG in cultured HSCs, which are dose-dependently eliminated by curcumin.
Serum-starved HSCs were stimulated with insulin at indicated concentrations in the absence (A & C) or presence (B & D) of curcumin at 0–30μM in serum-free media for 24 hr. The levels of total cellular GSH (A & B) and the ratio of GSH/GSSG (C & D) were determined. The levels of total GSH were expressed as nmol/μg protein. Values were presented as means ± S.D. (n≥3). *p<0.05, versus cells with no treatment (the 1st column on the left). ‡p<0.05, versus cells treated with insulin only (the 2nd column on the left in B & D)
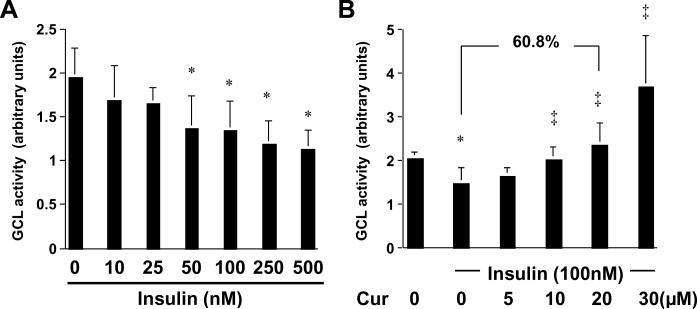
Curcumin increases the level of cellular GSH and attenuates insulin-induced oxidative stress in activated HSCs in vitro by stimulating the activity of GCL
The level of cellular GSH is mainly determined by GSH synthesis (GSH supply) and GSH-consuming (GSH demand). Glutamate-cysteine ligase (GCL) is the key rate-limiting enzyme in de novo synthesis of GSH 27. To understand the mechanisms by which insulin reduced the levels of cellular GSH and curcumin eliminated the inhibitory effects, we assumed that insulin might reduce the GCL activity in HSCs, which was eliminated by curcumin. To test the assumption, serum-starved HSCs were stimulated with insulin at indicated concentrations in the presence or absence of curcumin at various doses in serum-free media for 24 hr. Whole cell extracts were prepared for analyzing the GCL activity. Results in Fig. 7A demonstrated that insulin dose-dependently reduced the activity of GCL in these cells. The inhibitory effect of insulin was eliminated by curcumin (Fig. 7B). For example, compared to the control cells treated with insulin only (the 2nd column in Fig. 7B), curcumin at 20 μM abrogated the inhibitory effect of insulin and caused a significant increase in the activity of GCL by 60.8%. Taken together, these results supported our assumption and demonstrated that curcumin increased the level of cellular GSH and attenuated insulin-induced oxidative stress in activated HSCs in vitro by stimulating the activity of GCL.
Figure 7. Curcumin eliminates the role of insulin in the reduction of the activity of GCL in activated HSCs in vitro.
Serum-starved HSCs were stimulated with insulin at indicated concentrations in the absence (A) or presence (B) of curcumin at 0–30μM in serum-free media for 24 hr. The activities of GCL in the cells were determined. Values were expressed as means ± S. D. (n≥6). The number of percentage indicated the increase in the GCL activity in cells treated with insulin (100 nM) plus curcumin (20 μM) compared to that in cells treated with insulin only. *p<0.05, versus cells with no treatment (the 1st column on the left). ‡p<0.05, versus cells treated with insulin only (the 2nd column on the left in B)
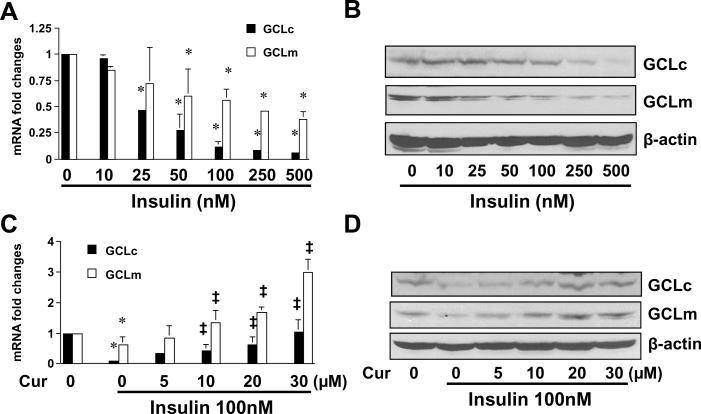
Curcumin increases the activity of GCL and the level of cellular GSH in insulin-activated HSCs by inducing gene expression of GCLc and GCLm
The enzyme of GCL is composed of a large catalytic subunit (GCLc, ~73kDa) and a small modulatory subunit (GCLm, ~30kDa). To answer the question how curcumin could increase the activity of GCL and elevate the level of cellular GSH in insulin-activated HSCs, we postulated that curcumin might induce gene expression of the GCL subunits GCLc and GCLm, leading to the increase in the GCL activity and the elevation of the level of cellular GSH. To test the postulation, serum-starved HSCs were stimulated with insulin at various concentrations in the presence or absence of curcumin at indicated doses in serum-depleted media for 24 hr. Total RNA and whole cell extracts were prepared from the cells. Gene expression of GCLc and GCLm were analyzed by real-time PCR and Western blotting analyses. It was observed that insulin reduced the levels of GCLc and GCLm transcripts (Fig. 8A) and proteins (Fig. 8B). The inhibitory effect of insulin was dose-dependently abrogated by curcumin (Fig. 8C and D). These results supported our postulation and demonstrated that curcumin increased the activity of GCL and elevated the level of cellular GSH in insulin-activated HSCs by inducing gene expression of GCLc and GCLm.
Figure 8. Curcumin eliminates the effect of insulin and induces gene expression of GCLc and GCLm in activated HSCs in vitro.
Serum-starved HSCs were stimulated with insulin at indicated concentrations in the absence (A & B) or presence (C & D) of curcumin at 0–30μM in serum-free media for 24 hr. Total RNA or whole cell extracts were prepared for real-time PCR assays (A & C), or Western blotting analyses (B & D). β-actin was used as an invariant internal control for calculating mRNA fold changes (n=3). *p<0.05, versus cells with no treatment (the corresponding 1st column on the left in A & C); ‡p<0.05, versus cells treated with insulin only (the corresponding 2nd column on the left in A & C). β-actin was used as an internal control for equal loading (B & D). A representative was shown from three independent experiments.
de novosynthesis of GSH is required for curcumin to inhibit gene expression of InsR and other key proteins relevant to HSC activation induced by insulin
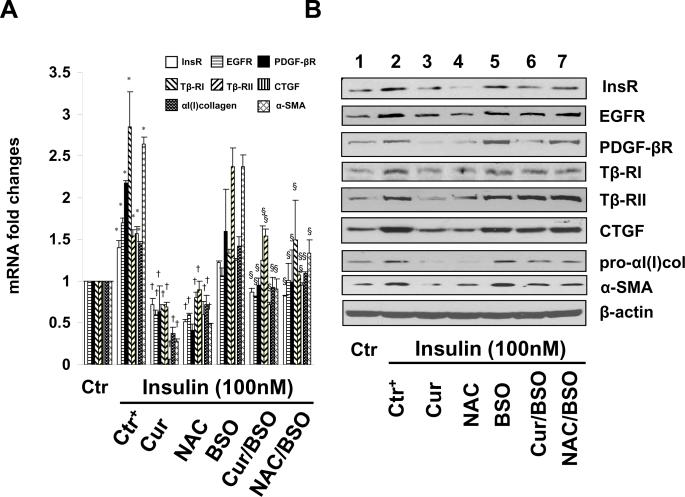
To elucidate the mechanisms by which curcumin inhibited InsR gene expression in HSCs, we presumed that the elevation of the level of cellular GSH played a critical role in the curcumin-dependent suppression of expression of InsR gene and other genes relevant to HSC activation observed in Fig. 1 & 4. To test this presumption, levels of cellular GSH in cultured HSCs were altered by the following well-known manipulators of GSH synthesis. N-acetyl-cysteine (NAC) is a precursor of GSH and increases GSH contents by supplying cysteine 33. L-Buthionine sulfoximine (BSO) is a specific inhibitor of GCL, which depletes cellular GSH 34. We previously demonstrated that BSO alone caused a time- and dose-dependent reduction in the level of GSH in cultured HSCs 23. Serum-starved HSCs were divided into two groups. In one group, cells were stimulated with or without insulin (100 nM) plus curcumin (20 μM) or NAC (5 mM) in serum-free media for 24 hr. In another group, cells were pretreated with BSO (0.25 mM) for 1 hr prior to the addition of insulin (100 nM) plus curcumin (20 μM) or NAC (5 mM) in serum-free media for additional 24 hr. Total RNA and whole cell extracts were prepared. As demonstrated by real-time PCR (Fig. 9A) and Western blotting analyses (Fig. 9B), compared to the untreated controls (the corresponding 1st columns and wells), insulin increased, as expected, the levels of mRNA and proteins of InsR and other key genes relevant to HSC activation (the corresponding 2nd columns and wells), including αI(I) collagen, α-SMA, and pro-mitogenic PDGF-βR and EGFR, as well as pro-fibrogenic Tβ-RI, & Tβ-RII and CTGF. NAC (the 4th columns and wells) mimicked the role of curcumin (the 3rd columns and wells) and dramatically eliminated the inhibitory effect of insulin on the expression of InsR and the other key genes relevant to HSC activation. The depletion of intracellular GSH by the pretreatment with BSO apparently eliminated the impacts of curcumin (the 6th columns and wells), as well as NAC (the 7th columns and wells) on the suppression of expression of the genes, including InsR, in activated HSCs in vitro. Taken together, these results demonstrated that de novo synthesis of GSH was required for curcumin to inhibit gene expression of InsR and other key proteins relevant to HSC activation induced by insulin.
Figure 9. de novo synthesis of GSH is required for curcumin to suppress gene expression of InsR and other proteins relevant to HSC activation induced by insulin.
Serum-starved HSCs were divided into two groups. One group of cells was treated with or without insulin (100 nM) plus curcumin (Cur) (20 μM) or NAC (5 mM) in serum-free media for 24 hr. Another group of cells was pretreated with BSO (0.25 mM) for 1 hr prior to the addition of insulin (100 nM) plus curcumin (20 μM) or NAC (5 mM) in serum-free media for additional 24 hr. Total RNA or whole cell extracts were respectively prepared for real-time PCR assays (A) or Western blotting analyses (B). β-actin was used as an invariant internal control for calculating mRNA fold changes (n=3). *p<0.05 versus cells with no treatment (the 1st column); †p<0.05 versus cells treated with insulin only (the 2nd column); §p<0.05 versus cells treated with insulin plus Cur (the 3rd columns), or NAC (the 4th columns). β-actin was used as an internal control for equal loading (B). Blots were representatives from three independent Western experiments.
DISCUSSION
Development of steatosis, steatohepatitis and fibrosis is a common outcome of type II diabetic patients with hyperinsulinemia. A large number of studies have focused on the impact of the elevated levels of insulin on hepatocytes 2. However, very few studies have addressed effects of the elevated levels of insulin on activation of HSCs, which are targets and responding cells to pathological insults in the state of hyperinsulinemia 2. Prior studies suggested that insulin stimulated HSC activation in vitro by inducing mitogenesis and collagen synthesis 12. The underlying mechanisms remain largely to be defined. Curcumin has received attention as a promising dietary component for the protection against fibrogenic insults 18. The current study was designed to evaluate the effect of curcumin on eliminating the role of insulin in the stimulation of HSC activation and to further elucidate the underlying mechanisms. Our results suggested that curcumin eliminated the role of insulin in stimulating HSC activation. Curcumin interrupted insulin signaling in HSCs by reducing the phosphorylation levels of InsR and downstream inter-mediators and by dramatically suppressing gene expression of InsR. Additional experiments demonstrated that the phytochemical attenuated insulin-induced oxidative stress in HSCs by increasing the activity of GCL and stimulating de novo synthesis of GSH. The latter was required for curcumin to suppress expression of InsR and other genes relevant to HSC activation.
Insulin signaling is initiated by binding of insulin to InsR and subsequently activating downstream signaling cascades. We postulated that InsR might be the first target for curcumin to interrupt insulin signaling. Our results supported the postulation and demonstrated that curcumin exerted its action by two steps. Curcumin eliminated the instant action of insulin by reducing the phosphorylation levels of InsR and its downstream inter-mediators, including ERK, JNK, PI3K and AKT (Fig. 3). This action was short-term. On the other hand, curcumin suppressed gene expression of InsR, leading to the reduction in the bioavailability of InsR to the ligand insulin (Fig. 4). This action was slower, but lasted longer, no less than 24 hr. Prior studies have shown similar functions of curcumin in interrupting signal transduction pathways for PDGF, EGF and TGF-β 20, 28, 35. Curcumin reduced the phosphorylation levels of PDGF-βR and TGF-β receptors, as well as suppressed gene expression of the receptors in activated HSCs in vitro. These results suggest that curcumin might be a broad spectrum inhibitor and non-specifically interrupts signaling pathways involved in the activation of HSCs. The underlying mechanisms remain elusive. Other studies have indicated the role of GSH in the physiological modulation of protein tyrosine phosphatase 1B, a key enzyme in the down-regulation of insulin signaling 36. We previously observed that it took several hours for curcumin to increase the level of cellular GSH in HSCs 26, because the process required the transcription and translation of GCL genes. Since curcumin rapidly reduced the phosphorylation levels of InsR and the downstream inter-mediators, it is unlikely that the inhibitory effect of curcumin results from its capability to elevate the level of cellular GSH. We recently observed that curcumin instantly and dramatically reduced the level of cellular Ca++ in cultured HSCs (unpublished observations). Accumulating evidence has indicated the role of calcium in regulation of activities of protein tyrosine kinases and phosphatases 37–39. Additional experiments are necessary to explore the mechanisms by which curcumin acts as a protein tyrosine kinase inhibitor and/or a phosphatase activator to reduce the phosphorylation levels of InsR and downstream signaling inter-mediators.
A critical question was raised during doing our pilot experiments whether curcumin would interrupt the insulin signaling in other insulin responding cells, including hepatocytes and muscle cells. If so, curcumin would deteriorate hyperglycemia and diabetes. It is noteworthy that curcumin has shown distinct effects on regulating gene expression depending on cell types 40. Curcumin induces gene expression of low-density lipoprotein receptor (LDLR) in hepatoma cell line HepG2 41, which might result in an increase in the endocytosis of plasma LDL by hepatocytes and a reduction in the level of plasma LDL. However, curcumin suppresses gene expression of LDLR in cultured HSCs 42, which attenuates the stimulatory role of LDL in the activation of HSCs. Curcumin and its major metabolite increase the number of total cellular insulin binding sites in circulating erythrocytes in a streptozotocin-induced diabetic rat model 43. In addition, curcumin significantly reduces blood glucose levels, improves insulin resistance, glucose tolerance, as well as lipid profiles in db/db mice 44. Current available results, together with our pilot observations, have suggested that curcumin has anti-hyperglycemic and anti-diabetic effects in diabetic animal models 44–47. Although beyond the scope of this report, it is of interest to address the underlying mechanisms by which curcumin shows distinct effects on different cell types, including HSCs and hepatocytes.
Curcumin is a potent antioxidant, whose antioxidant capacity is 100-fold stronger than that of vitamin E/C 17. The underlying mechanisms are largely undefined. NAC, as an antioxidant, has been studied to inhibit hepatic fibrosis in animal models 48–50. However, results are not always promising and consistent. It is worth notice that compared to the effects of curcumin at 20 μM, NAC at a much higher concentration (5 mM) showed similar, if not weaker, impacts on the attenuation of the insulin-stimulatory effects (Fig. 9). This observation suggested that curcumin, compared to NAC, might act in a unique and more efficient mechanism to exert its antioxidant properties. In general, one molecule of a “regular” antioxidant, such as vitamin E, directly scavenges one or two free radicals, leading to the attenuation of oxidative stress. In this report, we observed that curcumin indirectly attenuated oxidative stress by inducing gene expression of GCL, a key rate-limiting enzyme in de novo synthesis of GSH. The enhanced GCL activity catalyzes de novo synthesis of GSH, resulting in the removal of many molecules of ROS and the reduction of LPO production induced by insulin in HSCs. This unique mechanism explains, at least partially, its potent antioxidant capacity, which might allow curcumin to succeed where other “regular” antioxidants have failed to inhibit hepatic fibrogenesis. The level of cellular GSH is mainly determined by GSH synthesis (GSH supply) and GSH-consuming (GSH demand). It bears emphasis that our results do not exclude the role of curcumin in the suppression of GSH-consuming to elevate the level of cellular GSH in activated HSCs.
The toxicity of curcumin to cultured HSCs was previously evaluated 20. Based on results from LDH release assays, trypan blue exclusion assays and a rapid recovery of cell proliferation after withdrawal of curcumin, it was concluded that curcumin up to 100 μM was not toxic to cultured HSCs. Curcumin at 20 μM was used in most of our experiments in this report. This working concentration of curcumin is in the range used by many other researchers 51–53. The systemic bioavailability of curcumin is relatively low 54. Curcumin concentrations in human plasma can reach up to 2 μM following oral intake of very high amounts of curcumin 51. Although higher concentrations of curcumin in the blood might be possible, depending on the actual composition of the food 54, most pharmacokinetic studies in humans point out that only low micromole levels of curcumin can be found in the blood. It is noteworthy that because the in vivo system is multi-factorial, directly extrapolating in vitro conditions and results, e.g. effective concentrations, to the in vivo system might be misleading.
In summary, our results demonstrated that curcumin attenuated the effects of insulin on stimulating HSC activation, at least partially, by interrupting insulin signaling and attenuating oxidative stress. It bears emphasis that our results do not exclude any other mechanisms by which curcumin eliminates the effects of insulin on stimulating HSC activation. Our results in this report suggested that curcumin could be an anti-fibrotic candidate in therapeutic treatment of T2DM & NASH-associated hepatic fibrogenesis.
ACKNOWLEDGEMENT
The work was supported by the grant RO1 DK 047995 from NIH/NIDDK to A. Chen
ABBREVIATIONS
- α-SMA
alpha-smooth muscle actin
- BSO
L-buthionine sulfoximine
- CTGF
connective tissue growth factor
- DCF
dichlorofluorescein
- DMEM
Dulbecco's modified Eagle's medium
- EGFR
epidermal growth factor receptor
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GCL
glutamate-cysteine ligase
- GCLc
GCL catalytic subunit
- GCLm
GCL modulatory subunit
- GSH
glutathione
- HSCs
hepatic stellate cells
- LDLR
low-density lipoprotein receptor
- LPO
lipid peroxides
- InsR
insulin receptor
- MAPK
mitogen-activated protein kinase
- NAC
N-acetyl-cysteine
- NAFLD
non-alcoholic fatty liver diseases
- NASH
non-alcoholic steatohepatitis
- PDGF-ßR
platelet-derived growth factor-beta receptor
- PPARγ
peroxisome proliferator-activated receptor-gamma
- ROS
reactive oxygen species
- TGF-ß
transforming growth factor-beta
- Tβ-RI & Tβ-RII
type I and II TGF-ß receptor
- T2DM
type II diabetes mellitus
Footnotes
AUTHOR DISCLOSURE STATEMENT No competing financial interests exist.
REFERENCE
- 1.Maiese K, Morhan SD, Chong ZZ. Oxidative stress biology and cell injury during type 1 and type 2 diabetes mellitus. Curr Neurovasc Res. 2007;4:63–71. doi: 10.2174/156720207779940653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leclercq IA, Da Silva Morais A, Schroyen B, Van Hul N, Geerts A. Insulin resistance in hepatocytes and sinusoidal liver cells: mechanisms and consequences. J Hepatol. 2007;47:142–156. doi: 10.1016/j.jhep.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Dandona P, Aljada A, Chaudhuri A, Mohanty P, Rajesh G. A novel view of metabolic syndrome. Metab Syndr Relat Disord. 2004;2:2–8. doi: 10.1089/met.2004.2.2. [DOI] [PubMed] [Google Scholar]
- 4.Khamzina L, Gruppuso PA, Wands JR. Insulin signaling through insulin receptor substrate 1 and 2 in normal liver development. Gastroenterology. 2003;125:572–585. doi: 10.1016/s0016-5085(03)00893-x. [DOI] [PubMed] [Google Scholar]
- 5.Tsochatzis E, Papatheodoridis GV, Manesis EK, Kafiri G, Tiniakos DG, Archimandritis AJ. Metabolic syndrome is associated with severe fibrosis in chronic viral hepatitis and non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2008;27:80–89. doi: 10.1111/j.1365-2036.2007.03538.x. [DOI] [PubMed] [Google Scholar]
- 6.Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol. 2006;40(Suppl 1):S5–10. doi: 10.1097/01.mcg.0000168638.84840.ff. [DOI] [PubMed] [Google Scholar]
- 7.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kisseleva T, Brenner DA. Hepatic stellate cells and the reversal of fibrosis. J Gastroenterol Hepatol. 2006;21(Suppl 3):S84–87. doi: 10.1111/j.1440-1746.2006.04584.x. [DOI] [PubMed] [Google Scholar]
- 9.Hendriks HF, Brekelmans PJ, Buytenhek R, Brouwer A, de Leeuw AM, Knook DL. Liver parenchymal cells differ from the fat-storing cells in their lipid composition. Lipids. 1987;22:266–273. doi: 10.1007/BF02533990. [DOI] [PubMed] [Google Scholar]
- 10.Yamada M, Blaner WS, Soprano DR, Dixon JL, Kjeldbye HM, Goodman DS. Biochemical characteristics of isolated rat liver stellate cells. Hepatology. 1987;7:1224–1229. doi: 10.1002/hep.1840070609. [DOI] [PubMed] [Google Scholar]
- 11.Friedman SL, Rockey DC, McGuire RF, Maher JJ, Boyles JK, Yamasaki G. Isolated hepatic lipocytes and Kupffer cells from normal human liver: morphological and functional characteristics in primary culture. Hepatology. 1992;15:234–243. doi: 10.1002/hep.1840150211. [DOI] [PubMed] [Google Scholar]
- 12.Svegliati-Baroni G, Ridolfi F, Di Sario A, Casini A, Marucci L, Gaggiotti G, Orlandoni P, Macarri G, Perego L, Benedetti A, Folli F. Insulin and insulin-like growth factor-1 stimulate proliferation and type I collagen accumulation by human hepatic stellate cells: differential effects on signal transduction pathways. Hepatology. 1999;29:1743–1751. doi: 10.1002/hep.510290632. [DOI] [PubMed] [Google Scholar]
- 13.Di Sario A, Candelaresi C, Omenetti A, Benedetti A. Vitamin E in chronic liver diseases and liver fibrosis. Vitam Horm. 2007;76:551–573. doi: 10.1016/S0083-6729(07)76021-1. [DOI] [PubMed] [Google Scholar]
- 14.Das KS, Balakrishnan V, Mukherjee S, Vasudevan DM. Evaluation of blood oxidative stress-related parameters in alcoholic liver disease and non-alcoholic fatty liver disease. Scand J Clin Lab Invest. 2008;68:323–334. doi: 10.1080/00365510701673383. [DOI] [PubMed] [Google Scholar]
- 15.Halliwell B. Drug antioxidant effects. A basis for drug selection? Drugs. 1991;42:569–605. doi: 10.2165/00003495-199142040-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Svegliati-Baroni G, De Minicis S, Marzioni M. Hepatic fibrogenesis in response to chronic liver injury: novel insights on the role of cell-to-cell interaction and transition. Liver Int. 2008;28:1052–1064. doi: 10.1111/j.1478-3231.2008.01825.x. [DOI] [PubMed] [Google Scholar]
- 17.Sreejayan, Rao MN. Curcuminoids as potent inhibitors of lipid peroxidation. J Pharm Pharmacol. 1994;46:1013–1016. doi: 10.1111/j.2042-7158.1994.tb03258.x. [DOI] [PubMed] [Google Scholar]
- 18.O'Connell MA, Rushworth SA. Curcumin: potential for hepatic fibrosis therapy? Br J Pharmacol. 2008;153:403–405. doi: 10.1038/sj.bjp.0707580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu Y, Zheng S, Lin J, Ryerse J, Chen A. Curcumin protects the rat liver from CCl4-caused injury and fibrogenesis by attenuating oxidative stress and suppressing inflammation. Mol Pharmacol. 2008;73:399–409. doi: 10.1124/mol.107.039818. [DOI] [PubMed] [Google Scholar]
- 20.Xu J, Fu Y, Chen A. Activation of peroxisome proliferator-activated receptor-gamma contributes to the inhibitory effects of curcumin on rat hepatic stellate cell growth. Am J Physiol Gastrointest Liver Physiol. 2003;285:G20–30. doi: 10.1152/ajpgi.00474.2002. [DOI] [PubMed] [Google Scholar]
- 21.Zheng S, Chen A. Activation of PPARgamma is required for curcumin to induce apoptosis and to inhibit the expression of extracellular matrix genes in hepatic stellate cells in vitro. Biochem J. 2004;384:149–157. doi: 10.1042/BJ20040928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng S, Chen A. Curcumin suppresses the expression of extracellular matrix genes in activated hepatic stellate cells by inhibiting gene expression of connective tissue growth factor. Am J Physiol Gastrointest Liver Physiol. 2006;290:G883–893. doi: 10.1152/ajpgi.00450.2005. [DOI] [PubMed] [Google Scholar]
- 23.Chen A, Zhang L. The antioxidant (−)-epigallocatechin-3-gallate inhibits rat hepatic stellate cell proliferation in vitro by blocking the tyrosine phosphorylation and reducing the gene expression of platelet-derived growth factor-beta receptor. J Biol Chem. 2003;278:23381–23389. doi: 10.1074/jbc.M212042200. [DOI] [PubMed] [Google Scholar]
- 24.Schmittgen TD, Zakrajsek BA, Mills AG, Gorn V, Singer MJ, Reed MW. Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Anal Biochem. 2000;285:194–204. doi: 10.1006/abio.2000.4753. [DOI] [PubMed] [Google Scholar]
- 25.Lin J, Chen A. Activation of peroxisome proliferator-activated receptor-gamma by curcumin blocks the signaling pathways for PDGF and EGF in hepatic stellate cells. Lab Invest. 2008;88:529–540. doi: 10.1038/labinvest.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng S, Yumei F, Chen A. De novo synthesis of glutathione is a prerequisite for curcumin to inhibit hepatic stellate cell (HSC) activation. Free Radic Biol Med. 2007;43:444–453. doi: 10.1016/j.freeradbiomed.2007.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fraser JA, Kansagra P, Kotecki C, Saunders RD, McLellan LI. The modifier subunit of Drosophila glutamate-cysteine ligase regulates catalytic activity by covalent and noncovalent interactions and influences glutathione homeostasis in vivo. J Biol Chem. 2003;278:46369–46377. doi: 10.1074/jbc.M308035200. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Y, Zheng S, Lin J, Zhang QJ, Chen A. The interruption of the PDGF and EGF signaling pathways by curcumin stimulates gene expression of PPARgamma in rat activated hepatic stellate cell in vitro. Lab Invest. 2007;87:488–498. doi: 10.1038/labinvest.3700532. [DOI] [PubMed] [Google Scholar]
- 29.De Minicis S, Brenner DA. Oxidative stress in alcoholic liver disease: role of NADPH oxidase complex. J Gastroenterol Hepatol. 2008;23(Suppl 1):S98–103. doi: 10.1111/j.1440-1746.2007.05277.x. [DOI] [PubMed] [Google Scholar]
- 30.Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 31.Fridovich I. The biology of oxygen radicals. Science. 1978;201:875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- 32.Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol. 2002;348:93–112. doi: 10.1016/s0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- 33.Cotgreave IA. N-acetylcysteine: pharmacological considerations and experimental and clinical applications. Adv Pharmacol. 1997;38:205–227. [PubMed] [Google Scholar]
- 34.Anderson ME, Luo JL. Glutathione therapy: from prodrugs to genes. Semin Liver Dis. 1998;18:415–424. doi: 10.1055/s-2007-1007174. [DOI] [PubMed] [Google Scholar]
- 35.Zheng S, Chen A. Disruption of transforming growth factor-beta signaling by curcumin induces gene expression of peroxisome proliferator-activated receptor-gamma in rat hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G113–123. doi: 10.1152/ajpgi.00200.2006. [DOI] [PubMed] [Google Scholar]
- 36.Mueller AS, Bosse AC, Most E, Klomann SD, Schneider S, Pallauf J. Regulation of the insulin antagonistic protein tyrosine phosphatase 1B by dietary Se studied in growing rats. J Nutr Biochem. 2008;20:235–247. doi: 10.1016/j.jnutbio.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Gerthoffer WT. Signal-transduction pathways that regulate visceral smooth muscle function. III. Coupling of muscarinic receptors to signaling kinases and effector proteins in gastrointestinal smooth muscles. Am J Physiol Gastrointest Liver Physiol. 2005;288:G849–853. doi: 10.1152/ajpgi.00530.2004. [DOI] [PubMed] [Google Scholar]
- 38.Nilsson BO, Gomez MF, Sward K, Hellstrand P. Regulation of Ca2+ channel and phosphatase activities by polyamines in intestinal and vascular smooth muscle--implications for cellular growth and contractility. Acta Physiol Scand. 2002;176:33–41. doi: 10.1046/j.1365-201X.2002.01013.x. [DOI] [PubMed] [Google Scholar]
- 39.Tani E, Matsumoto T. Continuous elevation of intracellular Ca2+ is essential for the development of cerebral vasospasm. Curr Vasc Pharmacol. 2004;2:13–21. doi: 10.2174/1570161043476492. [DOI] [PubMed] [Google Scholar]
- 40.Syng-Ai C, Kumari AL, Khar A. Effect of curcumin on normal and tumor cells: role of glutathione and bcl-2. Mol Cancer Ther. 2004;3:1101–1108. [PubMed] [Google Scholar]
- 41.Peschel D, Koerting R, Nass N. Curcumin induces changes in expression of genes involved in cholesterol homeostasis. J Nutr Biochem. 2007;18:113–119. doi: 10.1016/j.jnutbio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 42.Kang Q, Chen A. Curcumin suppresses gene expression of low-density lipoprotein receptor, leading to the inhibition of LDL-induced activation of hepatic stellate cell. Brit. J. Pharmacol. 2009;158 doi: 10.1111/j.1476-5381.2009.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murugan P, Pari L, Rao CA. Effect of tetrahydrocurcumin on insulin receptor status in type 2 diabetic rats: studies on insulin binding to erythrocytes. J Biosci. 2008;33:63–72. doi: 10.1007/s12038-008-0022-y. [DOI] [PubMed] [Google Scholar]
- 44.Seo KI, Choi MS, Jung UJ, Kim HJ, Yeo J, Jeon SM, Lee MK. Effect of curcumin supplementation on blood glucose, plasma insulin, and glucose homeostasis related enzyme activities in diabetic db/db mice. Mol Nutr Food Res. 2008;52:995–1004. doi: 10.1002/mnfr.200700184. [DOI] [PubMed] [Google Scholar]
- 45.Suryanarayana P, Saraswat M, Mrudula T, Krishna TP, Krishnaswamy K, Reddy GB. Curcumin and turmeric delay streptozotocin-induced diabetic cataract in rats. Invest Ophthalmol Vis Sci. 2005;46:2092–2099. doi: 10.1167/iovs.04-1304. [DOI] [PubMed] [Google Scholar]
- 46.Pari L, Murugan P. Effect of tetrahydrocurcumin on blood glucose, plasma insulin and hepatic key enzymes in streptozotocin induced diabetic rats. J Basic Clin Physiol Pharmacol. 2005;16:257–274. doi: 10.1515/jbcpp.2005.16.4.257. [DOI] [PubMed] [Google Scholar]
- 47.Sharma S, Kulkarni SK, Chopra K. Curcumin, the active principle of turmeric (Curcuma longa), ameliorates diabetic nephropathy in rats. Clin Exp Pharmacol Physiol. 2006;33:940–945. doi: 10.1111/j.1440-1681.2006.04468.x. [DOI] [PubMed] [Google Scholar]
- 48.Galicia-Moreno M, Rodriguez-Rivera A, Reyes-Gordillo K, Segovia J, Shibayama M, Tsutsumi V, Vergara P, Moreno MG, Muriel P. N-acetylcysteine prevents carbon tetrachloride-induced liver cirrhosis: role of liver transforming growth factor-beta and oxidative stress. Eur J Gastroenterol Hepatol. 2009;21:908–914. doi: 10.1097/MEG.0b013e32831f1f3a. [DOI] [PubMed] [Google Scholar]
- 49.Pereira-Filho G, Ferreira C, Schwengber A, Marroni C, Zettler C, Marroni N. Role of N-acetylcysteine on fibrosis and oxidative stress in cirrhotic rats. Arq Gastroenterol. 2008;45:156–162. doi: 10.1590/s0004-28032008000200013. [DOI] [PubMed] [Google Scholar]
- 50.Tahan G, Tarcin O, Tahan V, Eren F, Gedik N, Sahan E, Biberoglu N, Guzel S, Bozbas A, Tozun N, Yucel O. The effects of N-acetylcysteine on bile duct ligation-induced liver fibrosis in rats. Dig Dis Sci. 2007;52:3348–3354. doi: 10.1007/s10620-006-9717-9. [DOI] [PubMed] [Google Scholar]
- 51.Garcea G, Jones DJ, Singh R, Dennison AR, Farmer PB, Sharma RA, Steward WP, Gescher AJ, Berry DP. Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br J Cancer. 2004;90:1011–1015. doi: 10.1038/sj.bjc.6601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Motterlini R, Foresti R, Bassi R, Green CJ. Curcumin, an antioxidant and anti-inflammatory agent, induces heme oxygenase-1 and protects endothelial cells against oxidative stress. Free Radic Biol Med. 2000;28:1303–1312. doi: 10.1016/s0891-5849(00)00294-x. [DOI] [PubMed] [Google Scholar]
- 53.Shakibaei M, John T, Schulze-Tanzil G, Lehmann I, Mobasheri A. Suppression of NF-kappaB activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: Implications for the treatment of osteoarthritis. Biochem Pharmacol. 2007;73:1434–1445. doi: 10.1016/j.bcp.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 54.Sharma RA, McLelland HR, Hill KA, Ireson CR, Euden SA, Manson MM, Pirmohamed M, Marnett LJ, Gescher AJ, Steward WP. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res. 2001;7:1894–1900. [PubMed] [Google Scholar]