Abstract
This work investigated the delivery of marrow mesenchymal stem cells (MSCs), with or without the growth factor transforming growth factor-β1 (TGF-β1), from biodegradable hydrogel composites on the repair of osteochondral defects in a rabbit model. Three formulations of oligo(poly(ethylene glycol) fumarate) (OPF) hydrogel composites containing gelatin microparticles (GMPs) and MSCs were implanted in osteochondral defects, including (1) OPF/GMP hydrogel composites; (2) OPF/GMP hydrogel composites encapsulating MSCs; and (3) OPF hydrogel composites containing TGF-β1 loaded GMPs and MSCs. At 12 weeks, the quality of new tissue formed in chondral and subchondral regions of defects was evaluated based on subjective and quantitative histological analysis. OPF hydrogel composites were partially degraded and the defects were filled with newly formed tissue at 12 weeks with no sign of persistent inflammation. With the implantation of scaffolds alone, newly formed chondral tissue had an appearance of hyaline cartilage with zonal organization and intense staining for glycosaminoglycans, while in the subchondral region hypertrophic cartilage with some extent of bone formation was often observed. The addition of MSCs, especially with TGF-β1 loaded GMPs, facilitated subchondral bone formation, as evidenced by more trabecular bone appearance. However, the delivery of MSCs with or without TGF-β1 at the dosage investigated did not improve cartilage morphology. While OPF-based hydrogel composites supported osteochondral tissue generation, further investigations are necessary to elucidate the effects of MSC seeding density and differentiation stage on new tissue formation and regeneration.
Keywords: cartilage tissue engineering, mesenchymal stem cells, hydrogel composites, osteochondral defects
Introduction
Although articular cartilage has a complex, highly organized structure responsible for its important function, it lacks an intrinsic capability to repair itself and is difficult to fully regenerate with current treatments 1. Recently, tissue engineering strategies combining cells, scaffolds and bioactive factors have been investigated for the replacement of structural and functional aspects of native cartilage 1, 2. Among the different cell populations investigated for cartilage tissue engineering applications, mesenchymal stem cells (MSCs) hold great promise for the generation of constructs as MSCs can be easily isolated from bone marrow and expanded in vitro 3. Numerous studies have demonstrated the successful transplantation of autologous MSCs for bone and cartilage tissue engineering 4-6.
Various types of scaffolds made of synthetic and natural polymers have been found to provide a favorable environment for small cartilage lesions 7. However, the repair of extended osteochondral lesions may require a suitable scaffold that can carry both growth factors and cells 8. A novel oligomer, oligo(poly(ethylene glycol) fumarate) (OPF), has been recently developed as a cell carrier and growth factor delivery system for cartilage tissue engineering 9, 10. OPF can be synthesized by esterification of fumaryl chloride and poly(ethylene glycol) (PEG). The double bonds in the main chain enable this material to crosslink into a hydrogel, and the hydrolysis of ester linkages along the chain leads to degradation of a crosslinked hydrogel 11. Previous work in our laboratory has shown that incorporation of gelatin microparticles (GMPs) into hydrogels is a promising strategy for controlled release of growth factors 12, 13. The GMPs encapsulated in the hydrogel composites function as both a digestable porogen and a drug delivery vehicle, which have been shown to improve the sustained release of loaded drug compared to hydrogels or GMPs alone, and to enhance the proliferation of cells co-encapsulated 9, 12, 14. In vivo studies also demonstrated the therapeutic effect of insulin-like growth factor-1 (IGF-1) released from hydrogel composites implanted into osteochondral defects in rabbits 15, 16. However, fibrous tissue was observed in the neo-surface with the delivery of transforming growth factor-β1 (TGF-β1) from OPF/GMP hydrogel composites 15, 16.
A previous study in our laboratory also investigated the encapsulation of rabbit marrow MSCs in OPF-based hydrogel composites containing growth factor-loaded GMPs and measured in vitro chondrogenesis 17. Rabbit MSCs encapsulated in hydrogels combined with TGF-β1-loaded GMPs showed an increase in gene expression of type II collagen and aggrecan, which are characteristic of chondrocytes, indicating that MSCs undergo chondrogenic differentiation in this approach 17. Therefore, in this study, we hypothesized that the combined delivery of MSCs and TGF-β1 would enhance the quality of new cartilage formation in a rabbit osteochondral defect model by influencing not only host cells but also implanted cells. More specifically, this study investigated (1) the effect of MSC delivery on the quality of new cartilage and bone tissue formation; and (2) the effect of the combined delivery of MSCs and TGF-β1 on the quality of new cartilage and bone tissue formation, both involving MSCs in a rabbit osteochondral defect model.
Materials and Methods
Three different formulations were designed for the present study and implanted in rabbits, including (1) OPF/GMP hydrogel composites without cells or growth factors (OPF group); (2) OPF hydrogel composites encapsulating blank GMPs and MSCs (10 million cells/ml hydrogel) (OPF/MSC group); and (3) OPF hydrogel composites containing TGF-β1 loaded GMPs (600 ng/ml hydrogel) and MSCs (10 million cells/ml hydrogel) (OPF/MSC/TGF group).
OPF Synthesis
OPF macromer was synthesized from PEG (Sigma, St. Louis, MO) with nominal molecular weight of 10,000 according to a method developed in our laboratory 11. The resulting OPF was sterilized by exposure to ethylene oxide gas for 14 h.
Gelatin Microparticle Fabrication and Loading
Gelatin microparticles were fabricated from acidic gelatin (Nitta Gelatin Inc., Osaka, Japan) and crosslinked in 10 mM glutaraldehyde (Sigma, St. Louis, MO) according to previously established methods 18. After lyophilization, the GMPs were sieved to obtain particles 50-100 μm in size and then sterilized with ethylene oxide.
Sterile GMPs were then loaded with recombinant human TGF-β1 (R&D Systems, Minneapolis, MN) by swelling in an aqueous solution of the growth factor at pH 7.4 for 15 hrs before composite fabrication according to established methods 19. In particular, 110 μl of TGF-β1 loading solution (volume needed for equilibrium swelling of the GMPs) was combined with 22 mg of GMPs. The loading solution had a concentration of 3.6 μg TGF-β1/ml phosphate buffered saline (PBS) to achieve a final loading of 600 ng TGF-β1/ml crosslinked scaffolds. This growth factor concentration has been shown to promote the chondrogenic differentiation of rabbit MSCs encapsulated in hydrogel composites in vitro 17. Blank GMPs were loaded with PBS in a similar fashion and served as control.
Rabbit Marrow MSC Isolation and Pre-culture
Rabbit marrow MSCs were isolated from the tibias of 6-month-old New Zealand White rabbits (specified-pathogen-free, different rabbits from those used for implantation) as previously described 4, 17. Two isolations were performed in this study, respectively, for the two groups treated with cells (OPF/MSC and OPF/MSC/TGF groups). Each isolation involved 3 rabbits in an effort to minimize any interanimal variation. Specifically, harvested bone marrow from each rabbit was cultured in Dulbecco's modified Eagle's medium-low glucose (DMEM-LG; Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Gemini, Calabasas, CA), 250 μg/l fungizone, 100 mg/l ampicillin, 50 mg/l gentamicin. After 2 weeks, the cells were lifted with a trypsin-EDTA solution (passage one cells) and mixed with cells from the other two rabbits of an isolation for composite fabrication. Previously, rabbit MSCs isolated and cultured using this method have been tested for their multi-potentiality to differentiate into osteoblast-like cells and chondrocyte-like in vitro 17.
Hydrogel Composite Fabrication
Before implantation, hydrogel composites were fabricated according to the formulations shown in Table 1 following established methods 9. Briefly, 0.1 g of sterile OPF and 0.05 g of sterile poly(ethylene glycol) diacrylate (PEG-DA; nominal MW 3400, Nektar Therapeutics, Huntsville, AL) in 300 μl of PBS were transferred to a vial containing the swelling microparticle solution (either blank or TGF-β1-loaded). For crosslinking, equal parts (46.8 μl) of initiator solutions, 0.3 M ammonium persulfate (APS) and 0.3 M N,N,N’,N’,-tetramethylethylenediamine (TEMED), were first mixed with the polymer solution, followed by the addition of 168 μl of a cell suspension containing 6.7 million rabbit MSCs to reach a final concentration of 10 million cells/ml solution. The solution was then quickly injected into Teflon molds (2.2 mm in diameter × 2.2 mm in depth) and incubated at 37°C for 8 minutes. Cell-free constructs were fabricated in a similar fashion using PBS instead of the cell suspension. After crosslinking, hydrogel constructs with or without MSCs were transferred in PBS before they were implanted in the osteochondral defects in rabbits within 2 h. The final dimensions of the scaffolds after swelling were 3 mm in diameter and 3 mm in height, which match the dimensions of the osteochondral defect.
Table 1.
Experimental Groups Tested in This Study
| Groups | Scaffolds | Cell seeding density | Growth factor dose | Repetitions |
|---|---|---|---|---|
| OPF | OPF+GMPs | ---- | ---- | 12 |
| OPF/MSC | OPF+GMPs | MSCs 10 million cells/ml | ---- | 12 |
| OPF/MSC/TGF | OPF+GMPs | MSCs 10 million cells/ml | TGF-β1 600 ng/ml | 12 |
Animal Surgery
A total of 18 specified-pathogen-free New Zealand white rabbits (6-month-old) were used for the implantation study based on a well-established rabbit osteochondral (full-thickness) defect model 4, 16. Rabbits 6 months in age were selected for this study since they are skeletally mature; a previous study has shown histologically and radiographically that their growth plates in the distal femur closed at 19-24 weeks 20. All in vivo work was conducted in accordance with ISO standards, and protocols of the Radboud University Nijmegen Medical Center, Nijmegen, the Netherlands. National guidelines for the care and use of laboratory animals were observed, and approval of the Experimental Animal Ethical Committee was obtained (RUDEC 2007-019).
Animal surgeries were performed as described previously 16. Briefly, bilateral osteochondral defects (3 mm in diameter × 3 mm in depth) were first created on the weight-bearing surface of medial femoral condyles. Then a prefabricated hydrogel composite, which had similar dimensions of the defect, was press-fit into the defect. Subsequently, the muscle and skin were closed. Each rabbit received 2 hydrogel composites with the same formulation, one per knee, and the procedure was repeated for 6 rabbits per formulation, resulting in n=12 implants per group. To minimize post-operative discomfort, Fynadyne® was administered for two days postoperatively. The animals were returned to their cages after surgery and allowed unrestricted weight-bearing activity. Signs of pain, infection and proper activity were monitored and carefully recorded.
Tissue Processing
Rabbits were euthanized at twelve weeks after surgery by intravenous administration of Nembutal (pentobarbital). The tissue surrounding the medial femoral condyle was retrieved en bloc. Specimens were fixed in 10% buffered formalin (pH 7.4) for 1 week, decalcified in Formical2000 (Decal Corporation, Congers, NY, USA) for 2 weeks, dehydrated through a graded series of ethanol, and then embedded in paraffin. Longitudinal sections of 6 μ were taken from the center (within the central 1 mm), lateral edge (within the lateral 1 mm) and medial edge (within the medial 1 mm) of each defect. Two sections from each region were then stained with hematoxylin and eosin (H&E), Safranin O/Fast green as well as Masson's trichrome separately, and subsequently scored 15.
Histological Scoring
Histological sections were blindly and independently scored by three evaluators (FKK, SY, JDK) using an established scoring system consisting of 11 parameters for osteochondral repair, as shown in Table 2 4, 15, 16, 21-24. Analysis was done over the whole defect for both chondral (within the upper 1 mm of the defect) and subchondral regions (within the bottom 2 mm of the defect). In particular, the chondral region was scored for its morphology, thickness, regularity, and chondrocyte clustering, as well as cell and GAG content. The cell and GAG content of the cartilage tissue adjacent to the defect was also examined to assess possible tissue degeneration near the implant site. Additionally, bone filling, integration, and morphology in the subchondral region of defects were assessed during this evaluation.
Table 2.
Histological Scoring System for Evaluation of Overall Tissue Filling (a), Subchondral Bone Repair (b), and Cartilage Repair (c) in Rabbit Osteochondral Defects
|
(a) |
Overall defect evaluation (throughout the entire defect depth) |
Score |
| 1. | Percent filling with neo-formed tissue | |
| 100% | 3 | |
| >50% | 2 | |
| <50% | 1 | |
| 0% | 0 | |
| 2. | Percent degradation of the implant | |
| 100% | 3 | |
| >50% | 2 | |
| <50% | 1 | |
| 0% | 0 | |
|
(b) |
Subchondral bone evaluation (within the bottom 2 mm of defect) |
|
| 3. | Percent filling with neo-formed tissue | |
| 100% | 3 | |
| >50% | 2 | |
| <50% | 1 | |
| 0% | 0 | |
| 4. | Subchondral bone morphology | |
| Normal, trabecular bone | 4 | |
| Trabecular bone, with some compact bone | 3 | |
| Compact bone | 2 | |
| Compact bone and fibrous tissue | 1 | |
| Only fibrous tissue or no tissue | 0 | |
| 5. | Extent of neo-tissue bonding with adjacent bone | |
| Complete on both edges | 3 | |
| Complete on one edge | 2 | |
| Partial on both edges | 1 | |
| Without continuity on either edge | 0 | |
|
(c) |
Cartilage evaluation (within the upper 1 mm of defect) |
|
| 6. | Morphology of neo-formed surface tissue | |
| Exclusively articular cartilage | 4 | |
| Mainly hyaline cartilage | 3 | |
| Fibrocartilage (spherical morphology observed with ≥75% of cells) | 2 | |
| Only fibrous tissue (spherical morphology observed with <75% of cells) | 1 | |
| No tissue | 0 | |
| 7. | Thickness of neo-formed cartilage | |
| Similar to the surrounding cartilage | 3 | |
| Greater than the surrounding cartilage | 2 | |
| Less than the surrounding cartilage | 1 | |
| No cartilage | 0 | |
| 8. | Joint surface regularity | |
| Smooth, intact surface | 3 | |
| Surface fissures (<25% neo-surface thickness) | 2 | |
| Deep fissures (≥25% neo-surface thickness) | 1 | |
| Complete disruption of the neo-surface | 0 | |
| 9. | Chondrocyte clustering | |
| None at all | 3 | |
| <25% chondrocytes | 2 | |
| 25-100% chondrocytes | 1 | |
| No chondrocytes present (no cartilage) | 0 | |
| 10. | Chondrocyte and GAG content of neo-cartilage | |
| Normal cellularity with normal Safranin O staining | 3 | |
| Normal cellularity with moderate Safranin O staining | 2 | |
| Clearly less cells with poor Safranin O staining | 1 | |
| Few cells with no or little Safranin O staining or no cartilage | 0 | |
| 11 | Chondrocyte and GAG content of adjacent cartilage | |
| Normal cellularity with normal Safranin O staining | 3 | |
| Normal cellularity with moderate Safranin O staining | 2 | |
| Clearly less cells with poor Safranin O staining | 1 | |
| Few cells with no or little Safranin O staining or no cartilage | 0 |
Statistical Analysis
Prior to the study, the number of defects needed in each group (n=12) was determined by power analysis and consideration of previous studies 15, 16. For histological data analysis, ordered logistic regression was performed on each of the parameters in the scoring system to determine the potential effects of implant formulation, location within the defect (lateral, medial edges and center) and knee joint (left and right) on tissue regeneration following previous methods 15, 16. A significance level of 0.05 was used for the statistical analysis.
Results
Macroscopic Observation
All animals regained full movement within one week, and they continued to exhibit normal behavior and movement during the 12-week period. No gross signs of inflammation, infection or swelling were observed upon visual inspection of the joint surface at the time of tissue retrieval. Migration of the hydrogel composites from the defect was not found.
Histological Observation
For all three treatments, complete degradation of the implanted hydrogel composites was seen in approximately half of the specimens. More specifically, the frequency for complete implant degradation was 5/12, 6/12 and 7/12 for the OPF, OPF/MSC and OPF/MSC/TGF groups, respectively. However, in the cases where the hydrogels were not completely degraded, less degradation was seen in the defects when TGF-β1 was not present. Four out of twelve specimens had less than 50% degradation in the OPF and OPF/MSC groups, whereas only one was found in the OPF/MSC/TGF group. A similar extent of implant degradation and tissue filling was seen in the left and right knees of each rabbit.
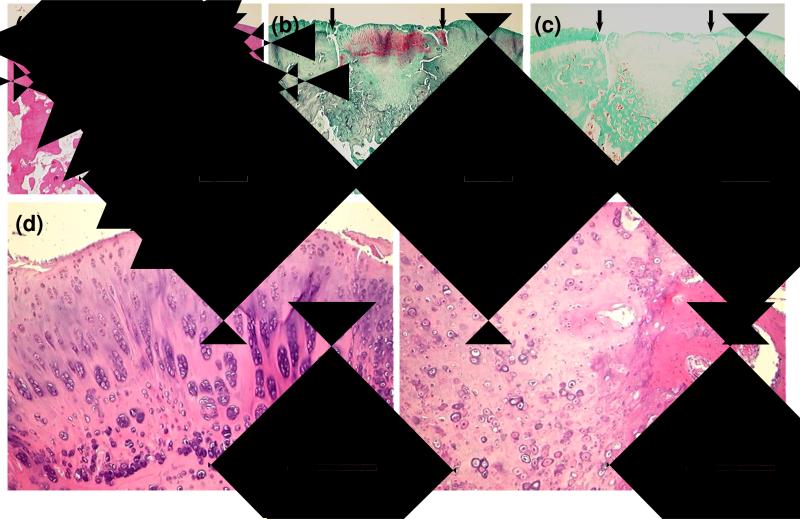
In the OPF group, the defects were filled with newly formed cartilage tissue in the superficial part and bone tissue in the deep part. In seven out of twelve specimens, cartilage developed in the subchondral bone area and occupied more than 2/3 of the whole defect. Neo-formed cartilage tissue on the surface had an appearance of hyaline cartilage, evidenced by an intense Safranin O staining as shown in Figure 1 (b). At higher magnification, the cells were round, clustered, and surrounded by extracellular matrix, resembling well differentiated chondrocytes [Figure 1 (d)]. In some sections, chondrocytes were even arranged with a zonal organization, indicative of true articular cartilage. As also shown in Figure 1 (d), slightly elongated cells were located near the articular surface. Below these cells, more rounded cells were clustered in columns, as expected for the cells in middle and deep zones of articular cartilage.
Figure 1.
Histological sections showing representative 12-week tissue repair at defect sites treated with OPF hydrogel composites alone. Sections stained with H&E (a), Safranin O (b), and Masson's trichrome (c) at 2× display cartilaginous tissue growth into the subchondral region. Small arrows define the edges of the defects. Boxed regions are shown at 10× magnification to illustrate clustered, spherical chondrocyte-like cells in the neo-surface (d) and hypertrophic cartilage with calcified matrix in the subchondral region (e). Bar is 1 mm for (a-c) and 250 μm for (d, e).
Although trabecular bone was present in some areas of the subchondral region, the bone regeneration seemed incomplete because of incomplete degradation of the hydrogel composites and the presence of cartilage tissue. Hypertrophic cartilage was seen and appeared calcified in some sections, indicative of the process of cartilage resorption and bone formation. In most specimens, tissue in the subchondral region integrated well with the surrounding host tissue and cartilage tissue.
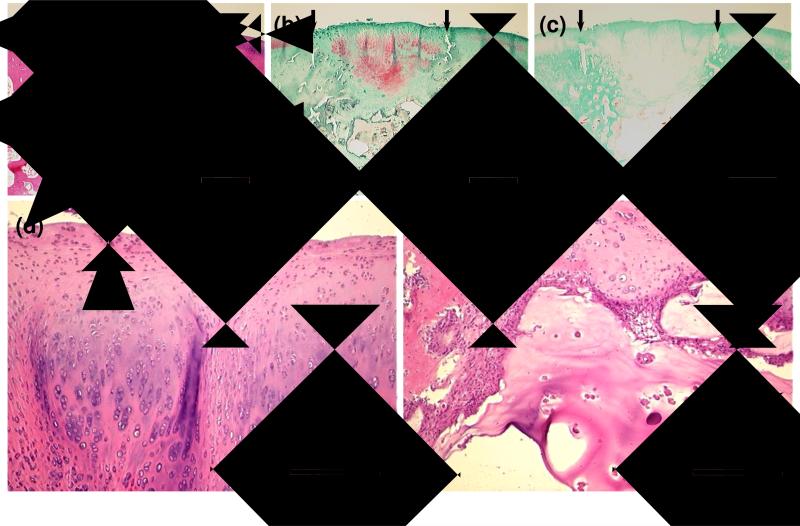
Similarly, in the OPF/MSC group, the subchondral region contained remaining hydrogel composite material, hypertrophic cartilage, and some trabecular bone. Cartilage had an appearance of hyaline cartilage and fibrocartilage with varying thickness, as seen in Figure 2.
Figure 2.
Histological sections showing representative 12-week tissue repair at defect sites treated with OPF hydrogel composites containing MSCs. Sections stained with H&E (a), Safranin O (b), and Masson's trichrome (c) are demonstrated at 2× magnification. Small arrows define the edges of the defects. Boxed regions are shown at 10× magnification to illustrate a mixture of hyaline cartilage and fibrocartilage in the chondral region (d) and partially degraded composites remaining in the subchondral region (e). The big arrow indicates microparticles in a hydrogel composite remained in the defect. Bar is 1 mm for (a-c) and 250 μm for (d, e).
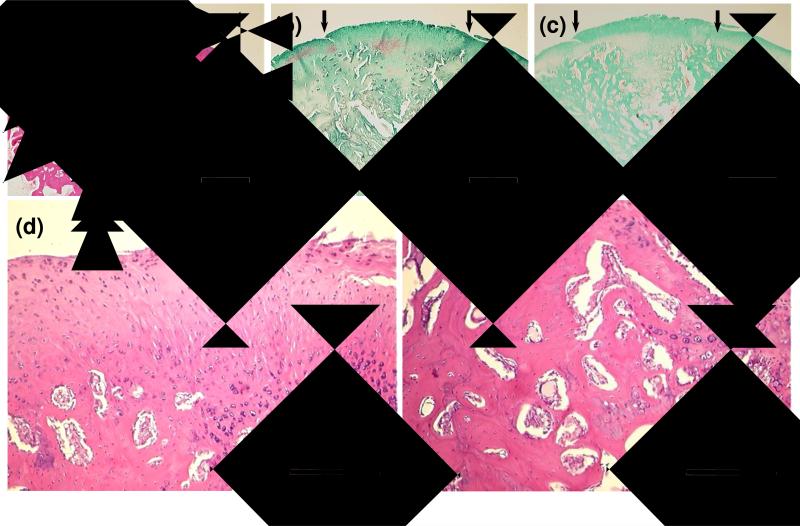
In the group with TGF-β1, neo-cartilage tissue exhibited a fibrocartilage-like appearance. In particular, a thicker fibrous layer with faint staining for GAG and intense staining for collagen was seen near the articular surface as compared to the other two groups [Figure 3 (a-c)]. At higher magnification, the cells in this layer appeared as small dots and were surrounded by fibrillar matrix [Figure 3 (d)]. In the subchondral region, although remaining hydrogel and hypertrophic cartilage were still observed in some sections, more trabecular bone formation could be seen as compared to the groups without TGF-β1.
Figure 3.
Histological sections showing representative 12-week tissue repair at defect sites treated with OPF hydrogel composites containing MSCs and TGF-β1. Sections stained with H&E (a), Safranin O (b), and Masson's trichrome (c) at 2× display a thick fibrous layer at the articular surface and significant restoration in the subchondral region. Small arrows define the edges of the defects. Boxed regions are shown at 10× magnification to illustrate the excessive fibrous tissue growth into the joint surface (d) and remodeling tissue in the subchondral region (e). Bar is 1 mm for (a-c) and 250 μm for (d, e).
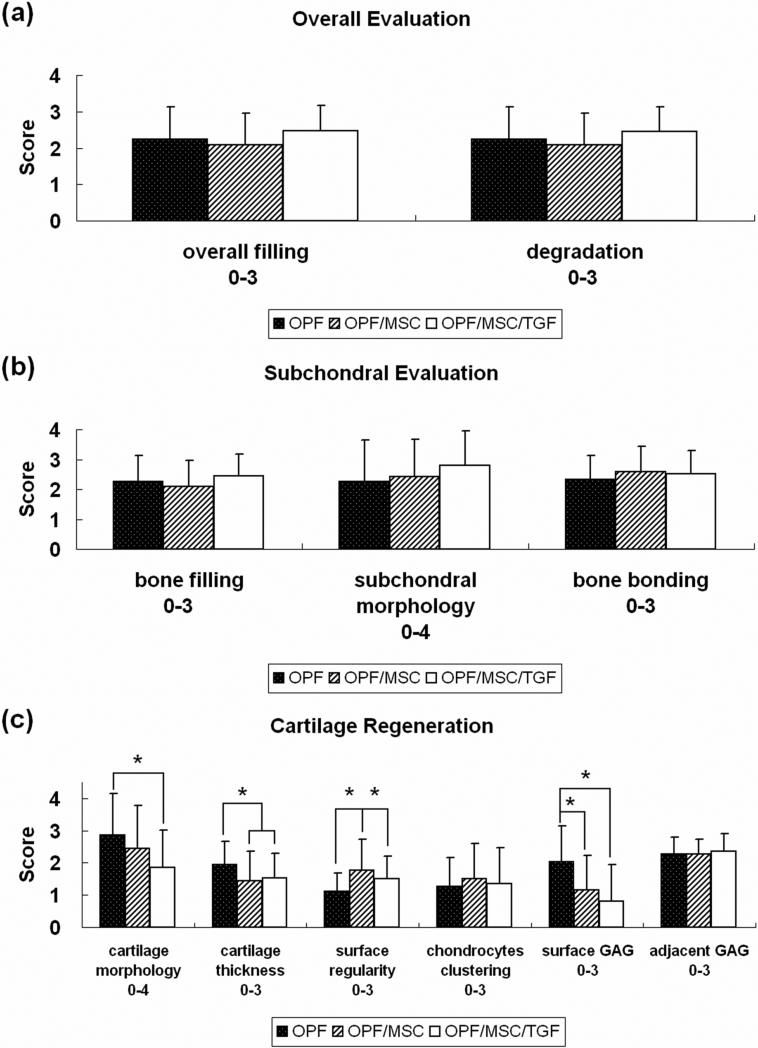
Histological Scoring
Figure 4 displays the quantitative scoring of 11 parameters of osteochondral restoration. Statistical analysis was performed to determine the effects of implant formulation, position in the defect site (lateral, center or medial), and knees (left and right) on each of the 11 parameters. The analysis revealed that implant formulation affected significantly the surface morphology, cartilage thickness, surface regularity, and cell and GAG content of the neo-surface. Position within the defect was determined to be a significant factor affecting only cell and GAG content of the adjacent surface, but not for any of the other parameters. No significant difference was found between left and right knees for all the parameters except bone bonding.
Figure 4.
Histological scoring for overall defect (a), subchondral region (b) and cartilage region (c). Data are shown as average scores with error bars representing standard derivation for n=12. The symbol (*) indicates a statistical difference between groups (P<0.05).
Mean scores for overall tissue filling and scaffold degradation were around two [Figure 4 (a)], indicating that more than 50% of the implant was degraded and filled with tissue [according to the scoring system, Table 2 (a)] over the 12-week period. However, no significant difference existed between the various implant formulations.
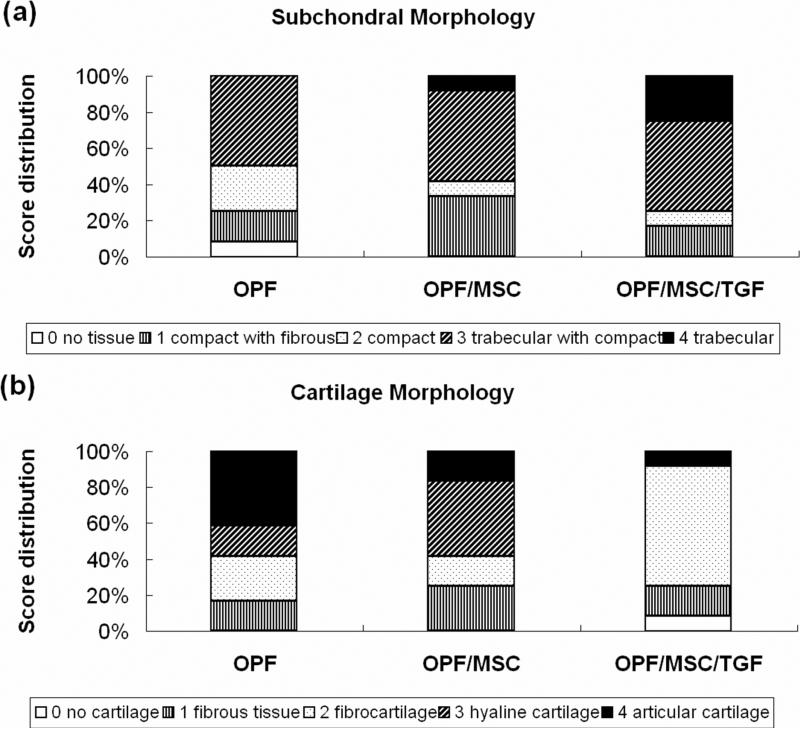
Similarly, no significant difference was observed for bone filling, subchondral morphology or bone bonding among the implant formulations [Figure 4 (b)]. Bone filling scored slightly above two, indicating that more than 50% of the subchondral region was filled with tissue at 12 weeks. Bone bonding had an average score between two and three, which was consistant with the histological apprearence that the neo-formed tissue in the subchodral region was well integrated with the surrounding host tissue. The detailed differences in subchondral bone morphology are shown in Figure 5 (a). In the OPF/MSC/TGF group, more sections scored ‘normal, trabecular bone’ (score 4), indicative of the formation of normal trabecular bone, than the other two groups. These findings corroborate the subjective histological observation that more remodeling of bone tissue was seen in the subchondral region of the defects treated with TGF-β1.
Figure 5.
Histological score distribution for subchondral morphology (a) and cartilage morphology (b).
Further, the OPF group had a higher score in cartilage morphology as well as cell and GAG content of neo-cartilage than the other two groups [Figure 4 (c)]. Specifically, the score for the cartilage morphology of the OPF group was 2.9 ± 1.3, reflecting the majority of hyaline cartilage near the joint surface. In the OPF/MSC group, the mean score was 2.4 ± 1.3, which was consistent with the histological appearance of a mixture of hyaline cartilage and fibrocartilage. When TGF-β1 was incorporated, the neo-formed tissue had a score of 1.9 ± 1.2 in cartilage morphology, indicating the fibrous nature of the neo-surface. A detailed score distribution of cartilage morphology for each formulation is displayed in Figure 5 (b). Scoring for cell and GAG content demonstrated the same trend and additional statistical evaluation revealed that for both of the two markers, significant differences were observed between the OPF and OPF/MSC/TGF groups.
For neo-cartilage thickness, the OPF group had a mean value around two [Figure 4 (c)], suggesting thicker neo-cartilage formed than host cartilage accroding to the scoring system. Although the scores of this group were significantly higher than those of both the OPF/MSC and OPF/MSC/TGF groups, the results may be due to the fact that more hypertrophic cartilage developed in the subchondral area and less remodeling of bone tissue was observed in this group. Additionally, when compared to the group implanted with OPF scaffolds alone, the delivery of cells (OPF/MSC group) significantly improved scores for surface regularity [Figure 4 (c)].
No significant difference was seen for chondrocyte clustering or cell and GAG content of adjacent cartilage among the formulations [Figure 4 (c)]. The values indicated that no severe degenerative changes occurred in the adjacent cartilage. However, the position within the defect was determined statistically to have an influence on cell and GAG content of adjacent cartilage. Specifically, significant differences in cell and GAG content of adjacent cartilage were seen between the lateral edge and the center of the defect.
Discussion
In this study, we investigated the delivery of MSCs, with or without TGF-β1, from biodegradable hydrogel composites on the repair of osteochondral defects in a rabbit model. Specifically, we investigated (1) whether the implantation of MSCs would influence the quality of new cartilage and bone tissue formation; and (2) whether the combined delivery of MSCs and TGF-β1 would affect the quality of new cartilage and bone tissue formation in a rabbit osteochondral defect model using OPF-based hydrogel composites as cell and growth factor carriers.
At 12 weeks post-surgery, implants were not found to migrate from the defect, as reported previously using OPF constructs in the same model 16. Hydrogel composites were found to be partially degraded and neo-tissue filling was observed within defects for all three formulations. Although scaffold degradation rate slightly varied among the animals, no signs of prolonged inflammatory response, osteoarthritis, or cartilage destruction were observed, which demonstrated the biocompatibility of OPF-based hydrogel composites and their degradation products.
Tissue formation and integration were seen in the group with the implantation of OPF/GMP scaffolds alone. As shown in Figure 1, hyaline cartilage with well-organized chondrocytes and intense GAG staining was seen to fill the chondral portion of the defect at 12 weeks, while hypertrophic cartilage with remodeling matrix was often observed filling the subchondral region. Many previous studies suggested that the post-injury response involves a rapid influx of donor MSCs and growth factors into the defect area and the fabrication of embryonic-like cartilage tissue throughout the defect 6, 25, 26. This was confirmed by the results of the present study. Although this study did not include an experimental group of untreated defects (empty control), comparison of the current results with the results of a previous study using the same defect model suggests that the quality of neo-tissue is superior to that of untreated defects 16. The normal cellularity and GAG deposition of neo-cartilage indicated that the OPF/GMP scaffold allowed for the recruitment, infiltration and differentiation of MSCs from the defect site or synovium. It has been reported that gelatin GMPs can bind with growth factors through polyionic interactions 12, 13, 27, therefore we speculate that GMPs incorporated in the hydrogel composites can adsorb and localize the growth factors from the defect site and synovial fluid. The subsequent release of these bioactive factors may contribute to the chondrogenic differentiation of the stem cells.
Many investigators have reported that implantation of MSCs within scaffolds improves bone formation and to a lesser degree cartilage formation 28-31. However, in the present study, differences in the three markers for subchondral bone formation were not significant among groups. The lack of difference could be due to a comparatively lower cell density in the scaffolds in the present study (10 million cells/ml) as compared to some other studies using a seeding density of 50 million cells/ml 5, 32.
For cartilage regeneration, implantation of MSCs significantly reduced cartilage thickness, which also provided evidence for faster erosion of hypertrophic cartilage and remodeling of subchondral bone in the presence of MSCs. It should also be noted that surface regularity was significantly improved with transplanted MSCs [see Figure 4 (c)]. Previous reports suggested that cartilage fibrillation would proceed when immature surface cartilage lost sufficient mechanical support during the degradation of the scaffold, especially when experiencing vigorous repetitive loading at the weight bearing regions of articular cartilage 30, 33, 34. Therefore, we hypothesize that the smoother articular surface observed in the OPF/MSC group was a result of faster subchondral bone formation in this group (as compared to the OPF group), which provided sufficient mechanical support for the articular surface. In addition to biomechanical considerations, implanted cells may also contribute to cartilaginous matrix secretion and remodeling, which result in better surface regularity and integration as other investigators reported 6, 31.
Histological scores of other neo-cartilage parameters did not reveal statistical differences for the implantation of MSCs. This is not an uncommon finding for the selected 12 week implantation time and the rabbit model used. For example, Solchaga et al. reported that the implantation of bone marrow in hyaluronan-based sponges accelerated the first stages of the osteochondral repair progress but did not significantly affect medium- (12 week) and long-term (24 week) outcomes of the repair process 4. Several other studies, which involved cell implantation and cell tracking in osteochondral defects also demonstrated that implanted cells can survive and participate in the initial repair process, but are finally replaced by host cells 30, 32, 35. We hypothesize that a similar phenomenon occurred in our study, as we know from previous studies that the cells survive the crosslinking process 9, 17. Additionally, cells from surrounding host tissues can migrate into the defect, thus explaining the dependence of cell and GAG content on position within the defect. Further research is necessary to elucidate the fate of encapsulated MSCs during implantation and the migration of host cells as well as their involvement in osteochondral tissue repair.
TGF-β1 has been reported to promote not only MSC chondrogenic differentiation, but also to induce osteogenic differentiation in vivo 36, 37. Therefore, not surprisingly, the highest scores in bone filling and bone morphology occurred for the group including both TGF-β1 and MSCs [see Figure 4(b)]. According to previous studies, TGF-β1 probably participates indirectly in vascularization, which controls the rate of bone formation 25. Additionally, previous investigations suggested that TGF-β1 also affects chondro/osteoclasts and facilitates cartilage matrix resorption and bone remodeling during endochondral ossification 37. This may explain the occurrence of more remodeled trabecular bone as seen in the group with TGF-β1 compared to the other two groups. Although TGF-β1 delivery to the bony tissue of osteochondral defects may elicit side effects, such as bone upgrowth into the cartilaginous region of the defects and inflammation 25, 38, such adverse effects were not observed in our study.
Surprisingly, in the current study cell morphology and GAG production of neo-cartilage were not improved by the addition of MSCs or TGF-β1. In fact, a thick fibrous layer was frequently noticed at the articular surface in the presence of TGF-β1, as shown in Figure 3. Since many studies indicated that a high dosage of TGF-β1 is related to the occurrence of fibrosis and osteophyte formation in articular cartilage defects 39, 40, we hypothesize that the fibrous layer as seen in the MSC/OPF/TGF group may be linked to the high local concentration of TGF-β1. A previous in vitro study investigating the release of TGF-β1 from similar OPF/GMP composites has shown that TGF-β1 had a burst release within the first 3 days (65.5 ± 3.3% in PBS and 60.8 ± 3.4% in PBS containing collagenase), and a sustained release up to 28 days (84.6 ± 1.1% in PBS and 83.6 ± 1.5% in PBS containing collagenase) 16. The TGF-β1 concentration was selected based on an in vitro study, where 600 ng TGF-β1/ml hydrogel achieved the best chondrogenic differentiation of MSCs in similar hydrogel composites 17. However, the formation of fibrous tissue in vivo emphasizes the significant difference between the in vitro and in vivo environments. In addition to TGF-β1 loaded on GMPs, some other bioactive agents from both the synovial joint and the defect site of the wound may also be involved in the repair process, as evidenced by the cartilage repair for the OPF and MSC/OPF groups.
Comparing the results of this study to those from a previous study in our laboratory, where TGF-β1 alone (200 ng/g gel) was delivered from a similar OPF/GMP composite to an osteochondral defect 16, we found that the combined delivery of both MSCs and TGF-β1 did not exhibit significant improvement in neo-cartilage formation for the MSC seeding density and differentiation stage tested. Although MSCs are reported to be immunoprivileged and immunosuppressive 41-43, histoincompatibility may be a factor that could affect neo-cartilage formation in the presence and absence of TGF-β1 in this case.
However, controversial results were found in some other studies, demonstrating that delivery of cells and growth factors enhanced cartilage repair as compared to the individual delivery of cells or growth factors. Sharma et al. injected PEG-DA and hyaluronic acid solution containing MSCs and 150 ng of TGF-β3 subcutaneously in nude mice, and they found that scaffolds with MSCs and TGF-β3 produced the highest quality cartilage 44. Similarly, another study investigating the transplantation of periosteal cells in a fibrin gel to a full-thickness defect in rabbit knees showed that the addition of TGF-β1 (5 ng/ml gel) resulted in better osteochondral integration and improved zonal architecture 31.
The different results for cartilage repair from similar strategies may be due to the differences in cellular microenvironment of defects, scaffold properties as well as growth factor release patterns. The complexity of the in vivo healing environment requires further understanding of the roles of cells and growth factors and their interactions during tissue repair. While OPF/GMP composites supported osteochondral tissue growth, further in vivo assessment (with and without cells) is necessary to optimize scaffold properties, stem cell seeding density and differentiation stage, growth factor dosage and release kinetics for the best osteochondral tissue regeneration. Design of an osteochondral construct of a layered architecture, which allows the delivery of suitable growth factors and cells to both cartilage and bone regions, may also be necessary.
Conclusions
This study investigated the effect of MSC delivery with a biodegradable hydrogel composite in the presence or absence of TGF-β1 on osteochondral tissue repair in a rabbit model. The results revealed that the OPF-based hydrogel composites partially degraded at 12 weeks after implantation, which allowed for cartilaginous and bony tissue formation. The implantation of MSCs with the hydrogel composites did not elicit a persistent inflammation, and facilitated subchondral bone formation in the presence of TGF-β1. However, the delivery of MSCs either with or without TGF-β1 did not improve cartilage morphology for the MSC seeding density and differentiation stage tested.
Acknowledgements
This work has been supported by the National Institutes of Health (R01 AR48756). X. Guo acknowledges the technical support by Ms. Natasja van Dijk.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Temenoff JS, Mikos AG. Review: tissue engineering for regeneration of articular cartilage. Biomaterials. 2000;21:431–440. doi: 10.1016/s0142-9612(99)00213-6. [DOI] [PubMed] [Google Scholar]
- 2.Jackson DWST. Tissue engineering principles in orthopaedic surgery. Clin Orthop. 1999;367(Suppl):31–45. doi: 10.1097/00003086-199910001-00005. [DOI] [PubMed] [Google Scholar]
- 3.Caplan AI. Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11:1198–1211. doi: 10.1089/ten.2005.11.1198. [DOI] [PubMed] [Google Scholar]
- 4.Solchaga LA, Gao J, Dennis JE, Awadallah A, Lundberg M, Caplan AI, Goldberg VM. Treatment of osteochondral defects with autologous bone marrow in a hyaluronan-based delivery vehicle. Tissue Eng. 2002;8:333–347. doi: 10.1089/107632702753725085. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, Shu XZ, Prestwich GD. Osteochondral defect repair with autologous bone marrow-derived mesenchymal stem cells in an injectable, in situ, cross-linked synthetic extracellular matrix. Tissue Eng. 2006;12:3405–3416. doi: 10.1089/ten.2006.12.3405. [DOI] [PubMed] [Google Scholar]
- 6.Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, Goldberg VM. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76:579–592. doi: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Lu L, Zhu X, Valenzuela RG, Currier BL, Yaszemski MJ. Biodegradable polymer scaffolds for cartilage tissue engineering. Clin Orthop Relat Res. 2001:S251–270. doi: 10.1097/00003086-200110001-00024. [DOI] [PubMed] [Google Scholar]
- 8.Martin I, Miot S, Barbero A, Jakob M, Wendt D. Osteochondral tissue engineering. J Biomech. 2007;40:750–765. doi: 10.1016/j.jbiomech.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Park H, Temenoff JS, Holland TA, Tabata Y, Mikos AG. Delivery of TGF-beta1 and chondrocytes via injectable, biodegradable hydrogels for cartilage tissue engineering applications. Biomaterials. 2005;26:7095–7103. doi: 10.1016/j.biomaterials.2005.05.083. [DOI] [PubMed] [Google Scholar]
- 10.Temenoff JS, Park H, Jabbari E, Sheffield TL, LeBaron RG, Ambrose CG, Mikos AG. In vitro osteogenic differentiation of marrow stromal cells encapsulated in biodegradable hydrogels. J Biomed Mater Res A. 2004;70:235–244. doi: 10.1002/jbm.a.30064. [DOI] [PubMed] [Google Scholar]
- 11.Jo S, Shin H, Shung AK, Fisher JP, Mikos AG. Synthesis and characterization of oligo(poly(ethylene glycol) fumarate) macromer. Macromolecules. 2001;34:2839–2844. [Google Scholar]
- 12.Holland TATY, Mikos AG. In vitro release of transforming growth factor-beta 1 from gelatin microparticles encapsulated in biodegradable, injectable oligo(poly(ethylene glycol) fumarate) hydrogels. Journal of Controlled Release. 2003;91:299–313. doi: 10.1016/s0168-3659(03)00258-x. [DOI] [PubMed] [Google Scholar]
- 13.Holland TA, Tabata Y, Mikos AG. Dual growth factor delivery from degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds for cartilage tissue engineering. J Control Release. 2005;101:111–125. doi: 10.1016/j.jconrel.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Holland TA, Tessmar JK, Tabata Y, Mikos AG. Transforming growth factor-beta 1 release from oligo(poly(ethylene glycol) fumarate) hydrogels in conditions that model the cartilage wound healing environment. J Control Release. 2004;94:101–114. doi: 10.1016/j.jconrel.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Holland TA, Bodde EW, Baggett LS, Tabata Y, Mikos AG, Jansen JA. Osteochondral repair in the rabbit model utilizing bilayered, degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds. J Biomed Mater Res A. 2005;75:156–167. doi: 10.1002/jbm.a.30379. [DOI] [PubMed] [Google Scholar]
- 16.Holland TA, Bodde EW, Cuijpers VM, Baggett LS, Tabata Y, Mikos AG, Jansen JA. Degradable hydrogel scaffolds for in vivo delivery of single and dual growth factors in cartilage repair. Osteoarthritis Cartilage. 2007;15:187–197. doi: 10.1016/j.joca.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Park H, Temenoff JS, Tabata Y, Caplan AI, Mikos AG. Injectable biodegradable hydrogel composites for rabbit marrow mesenchymal stem cell and growth factor delivery for cartilage tissue engineering. Biomaterials. 2007;28:3217–3227. doi: 10.1016/j.biomaterials.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tabata Y, Hijikata S, Muniruzzaman M, Ikada Y. Neovascularization effect of biodegradable gelatin microspheres incorporating basic fibroblast growth factor. J Biomater Sci Polym Ed. 1999;10:79–94. doi: 10.1163/156856299x00298. [DOI] [PubMed] [Google Scholar]
- 19.Holland TATJ, Tabata Y, Mikos AG. Transforming growth factor-1 release from oligo(poly(ethylene glycol) fumarate) hydrogels in conditions that model the cartilage wound healing environment. Journal of Controlled Release. 2003;94:101–114. doi: 10.1016/j.jconrel.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Kaweblum M, Aguilar MC, Blancas E, Kaweblum J, Lehman WB, Grant AD, Strongwater AM. Histological and radiographic determination of the age of physeal closure of the distal femur, proximal tibia, and proximal fibula of the New Zealand white rabbit. J Orthop Res. 1994;12:747–749. doi: 10.1002/jor.1100120519. [DOI] [PubMed] [Google Scholar]
- 21.O'Driscoll SW, Keeley FW, Salter RB. The chondrogenic potential of free autogenous periosteal grafts for biological resurfacing of major full-thickness defects in joint surfaces under the influence of continuous passive motion. J Bone Joint Surg Am. 1986;68:1017–1035. [PubMed] [Google Scholar]
- 22.O'Driscoll SW, Keeley FW, Salter RB. Durability of regenerated articular cartilage produced by free autogenous periosteal grafts in major full-thickness defects in joint surfaces under the influence of continuous passive motion. A follow-up report at one year. J Bone Joint Surg Am. 1988;70:595–606. [PubMed] [Google Scholar]
- 23.Buma P, et al. Cross-linked type I and type II collagenous matrices for the repair of full-thickness articular cartilage defects--a study in rabbits. Biomaterials. 2003;24:3255–3263. doi: 10.1016/s0142-9612(03)00143-1. [DOI] [PubMed] [Google Scholar]
- 24.Carranza-Bencano A, Garcia-Paino L, Armas Padron JR, Cayuela Dominguez A. Neochondrogenesis in repair of full-thickness articular cartilage defects using free autogenous periosteal grafts in the rabbit. A follow-up in six months. Osteoarthritis Cartilage. 2000;8:351–358. doi: 10.1053/joca.1999.0309. [DOI] [PubMed] [Google Scholar]
- 25.Hunziker EB, Driesang IM. Functional barrier principle for growth-factor-based articular cartilage repair. Osteoarthritis Cartilage. 2003;11:320–327. doi: 10.1016/s1063-4584(03)00031-1. [DOI] [PubMed] [Google Scholar]
- 26.Shapiro F, Koide S, Glimcher MJ. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1993;75:532–553. doi: 10.2106/00004623-199304000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Hong L, et al. Promoted bone healing at a rabbit skull gap between autologous bone fragment and the surrounding intact bone with biodegradable microspheres containing transforming growth factor-beta1. Tissue Eng. 2000;6:331–340. doi: 10.1089/107632700418056. [DOI] [PubMed] [Google Scholar]
- 28.Arinzeh TL, et al. Allogeneic mesenchymal stem cells regenerate bone in a critical-sized canine segmental defect. J Bone Joint Surg Am. 2003;85-A:1927–1935. doi: 10.2106/00004623-200310000-00010. [DOI] [PubMed] [Google Scholar]
- 29.De Kok IJ, Peter SJ, Archambault M, van den Bos C, Kadiyala S, Aukhil I, Cooper LF. Investigation of allogeneic mesenchymal stem cell-based alveolar bone formation: preliminary findings. Clin Oral Implants Res. 2003;14:481–489. doi: 10.1034/j.1600-0501.2003.110770.x. [DOI] [PubMed] [Google Scholar]
- 30.Shao XX, Hutmacher DW, Ho ST, Goh JC, Lee EH. Evaluation of a hybrid scaffold/cell construct in repair of high-load-bearing osteochondral defects in rabbits. Biomaterials. 2006;27:1071–1080. doi: 10.1016/j.biomaterials.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 31.Perka C, Schultz O, Spitzer RS, Lindenhayn K. The influence of transforming growth factor beta1 on mesenchymal cell repair of full-thickness cartilage defects. J Biomed Mater Res. 2000;52:543–552. doi: 10.1002/1097-4636(20001205)52:3<543::aid-jbm13>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 32.Koga H, et al. Synovial stem cells are regionally specified according to local microenvironments after implantation for cartilage regeneration. Stem Cells. 2007;25:689–696. doi: 10.1634/stemcells.2006-0281. [DOI] [PubMed] [Google Scholar]
- 33.Kerin AJ, Coleman A, Wisnom MR, Adams MA. Propagation of surface fissures in articular cartilage in response to cyclic loading in vitro. Clin Biomech (Bristol, Avon) 2003;18:960–968. doi: 10.1016/j.clinbiomech.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Solchaga LA, Temenoff JS, Gao J, Mikos AG, Caplan AI, Goldberg VM. Repair of osteochondral defects with hyaluronan- and polyester-based scaffolds. Osteoarthritis Cartilage. 2005;13:297–309. doi: 10.1016/j.joca.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 35.Ostrander RV, Goomer RS, Tontz WL, Khatod M, Harwood FL, Maris TM, Amiel D. Donor cell fate in tissue engineering for articular cartilage repair. Clin Orthop Relat Res. 2001:228–237. doi: 10.1097/00003086-200108000-00032. [DOI] [PubMed] [Google Scholar]
- 36.Linkhart TA, Mohan S, Baylink DJ. Growth factors for bone growth and repair: IGF, TGF beta and BMP. Bone. 1996;19:1S–12S. doi: 10.1016/s8756-3282(96)00138-x. [DOI] [PubMed] [Google Scholar]
- 37.Thorp BH, Anderson I, Jakowlew SB. Transforming growth factor-beta 1, -beta 2 and -beta 3 in cartilage and bone cells during endochondral ossification in the chick. Development. 1992;114:907–911. doi: 10.1242/dev.114.4.907. [DOI] [PubMed] [Google Scholar]
- 38.Hunziker EB. Growth-factor-induced healing of partial-thickness defects in adult articular cartilage. Osteoarthritis Cartilage. 2001;9:22–32. doi: 10.1053/joca.2000.0346. [DOI] [PubMed] [Google Scholar]
- 39.Mierisch CM, Cohen SB, Jordan LC, Robertson PG, Balian G, Diduch DR. Transforming growth factor-beta in calcium alginate beads for the treatment of articular cartilage defects in the rabbit. Arthroscopy. 2002;18:892–900. doi: 10.1053/jars.2002.36117. [DOI] [PubMed] [Google Scholar]
- 40.Holland TA, Mikos AG. Advances in drug delivery for articular cartilage. J Control Release. 2003;86:1–14. doi: 10.1016/s0168-3659(02)00373-5. [DOI] [PubMed] [Google Scholar]
- 41.Le Blanc K. Mesenchymal stromal cells: Tissue repair and immune modulation. Cytotherapy. 2006;8:559–561. doi: 10.1080/14653240601045399. [DOI] [PubMed] [Google Scholar]
- 42.Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–896. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 43.Liu H, Kemeny DM, Heng BC, Ouyang HW, Melendez AJ, Cao T. The immunogenicity and immunomodulatory function of osteogenic cells differentiated from mesenchymal stem cells. J Immunol. 2006;176:2864–2871. doi: 10.4049/jimmunol.176.5.2864. [DOI] [PubMed] [Google Scholar]
- 44.Sharma B, Williams CG, Khan M, Manson P, Elisseeff JH. In vivo chondrogenesis of mesenchymal stem cells in a photopolymerized hydrogel. Plast Reconstr Surg. 2007;119:112–120. doi: 10.1097/01.prs.0000236896.22479.52. [DOI] [PubMed] [Google Scholar]