Abstract
Group I p21-activated kinases are a highly conserved three-member family of serine/threonine kinases that act as key effectors for the small GTPases Cdc42 and Rac. In man, these enzymes have been implicated in a wide range of biological processes and are beginning to draw the attention of the pharmaceutical industry as potential therapeutic targets in cancer and in inflammatory processes. In this review, we summarize basic properties of group I Paks and discuss recently uncovered roles for these kinases in immune function and in viral infection.
Keywords: p21 activated kinases, cell signalling, Rho GTPases, viral pathogenesis, immune function
1. Introduction
p21-activated kinase 1 (Pak1) was first identified as a protein kinase activated by the small GTPases Cdc42 and Rac. Two other closely related protein kinases, Pak2 and Pak3, were subsequently identified, and together are termed group I Paks to distinguish them from the more distantly group II Paks. Group I Paks are highly conserved in evolution and have dozens of known substrates whose phosphorylation affects numerous cellular processes, including cytoskeletal organization, cell cycle progression, and cell survival (Bokoch, 2003, Dummler et al., 2009), as well as significant non-kinase related scaffold effects (Arias-Romero and Chernoff, 2008, Dummler et al., 2009). While the three group I Paks share many features, recent biochemical and genetic evidence is beginning to point to important functional differences among these enzymes. In this review, we focus on emerging roles for these kinases in immune function and in viral pathogenesis.
2. Structure
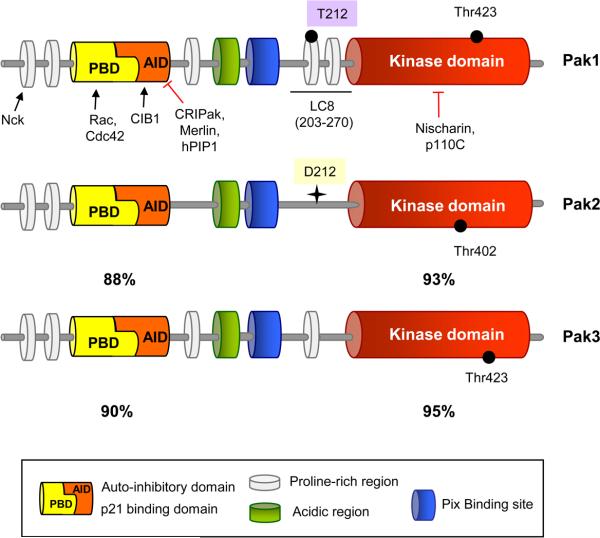
Group I Paks are characterized by an N-terminal regulatory domain and a C-terminal kinase domain (Fig. 1). The regulatory domain contains a conserved p21 GTPase binding domain (PBD), partially overlapping with an autoinhibitory domain (AID), and several proline-rich regions which serve as binding sites for SH3 domain containing proteins (Bokoch, 2003). The remainder of the N-terminus is quite variable, especially between Pak1 and Pak2; this distinction is likely to explain some of the functional differences between these two kinases.
Fig. 1. Structure of the group I Paks.
The p21-GTPase binding domain (PBD), autoinhibitory domain (AID), proline-rich regions, and acidic regions are shown. Between these conserved domains, the three group I Paks differ substantially. Pak1 has a unique T212 site that is phosphorylated by certain cyclin-dependent kinases and also by extracellular regulated kinase (ERK), as well as a nearby binding site for dynein light chain (LC8). Pak1 also contains a nuclear localization sequence, which, together with the LC8 binding site, is required for nuclear entry (Lightcap et al., 2009). Pak2 lacks these sites (D212), but contains a caspase cleavage site that, when cleaved during apoptosis, liberates the highly active protein kinase domain. Proteins described in the text that positively (arrow) or negatively (red line) regulate Pak1 function are indicated, as are percent sequence identities of Pak2 and Pak3 relative to Pak1.
In man, Pak1 has two mRNA splice variants, Pak1 and Pak1B, with different C-terminal regions (Kreis and Barnier, 2009). Pak3 also has multiple alternate splicing forms that are highly expressed in neurons. These alternate splices result in insertions into the AID, reduced binding to GTPases, and constitutive activation of the kinase.
3. Activation, Regulation, and Turnover
Under basal conditions, group I Paks exist in a trans-autoinhibited, inactive, dimeric conformation, with the AID region of one Pak molecule packed against the C-terminal catalytic domain of the other. Upon activation, the AID is displaced, leading to sequential autophosphorylation of a number of sites on Pak, including the critical T423 residue in the activation loop of the kinase domain, with subsequent monomerization of the activated enzyme (Bokoch, 2003). Additional activating inputs derive from PDK1, which has been shown to transphosphorylate T423, and also interaction with certain lipids, including sphingosine and phosphatidic acid. Other protein kinases also phosphorylate Pak1, but these phosphorylations have not been reported to alter the catalytic activity of Pak1. Additional factors that contribute to the full activation of Pak include its SH3-domain containing binding partners such as Nck and PIX, which recruit Pak1 to the plasma membrane and to focal adhesions and centrosomes, respectively, and the calcium- and integrin-binding protein, Cib1, which plays an important role in integrin-mediated Pak activation (Arias-Romero and Chernoff, 2008) (Fig. 1). Regarding negative regulation, the protein phosphatases POPX1 and POPX2 bind to the PIX/Pak complex and contribute to the deactivation of Pak by dephosphorylating its activation loop threonine residue as well as other autophosphorylation sites. In addition to dephosphorylation, Paks are also subject to inhibition by interaction with various proteins, including hPIP1, CRIPak, Nischarin, p110C, and Merlin (Arias-Romero and Chernoff, 2008), as well as down regulation by ubiquitin-mediated proteosomal degradation following binding to the small GTPase Chp (Cdc42 homologous protein) (Kreis and Barnier, 2009).
Pak1 expression is also regulated at the level of gene copy number and transcription. Pak1 mRNA levels are frequently elevated in human breast, colon, bladder, brain, and ovarian cancers as a result of gene amplification of the 11q13.5 locus. Interestingly, breast cancer patients with this genetic profile have been shown to be less sensitive to tamoxifen treatment, suggesting an oncogenic role for Pak1 in these cancers (Dummler et al., 2009). Although the identity of Pak1's transcriptional regulators are not known, Pak1 expression is negatively regulated by a microRNA, mIR-7 (Dummler et al., 2009).
4. Biological Functions
Since the three group I Paks are so similar in sequence, it is sometime assumed that they are interchangeable. However, this view is almost certainly incorrect. While Pak1 and Pak2 have identical peptide substrate preferences, as assessed by peptide arrays (Rennefahrt et al., 2007), emerging genetic and biochemical evidence suggest that Pak1 and Pak2 play different roles even when expressed in the same cells. For example, while all three group I Paks are highly expressed in the brain, and Pak1 and Pak2 are both highly expressed in most cells of hematopoietic origin, deletion of the Pak1 gene in mice leads to subtle defects in neuronal function and obvious defects in mast cell degranulation (see below), whereas deletion of the Pak2 gene results in embryonic lethality at day E8 (Arias-Romero and Chernoff, 2008). Furthermore, loss of Pak3 function in man is linked to mental retardation (Kreis and Barnier, 2009), despite continued normal expression of Pak1 and Pak2. At the cellular level, siRNA mediated Pak1 knockdown in breast epithelial cells leads to decreased myosin light chain phosphorylation, smaller focal adhesions, and unchanged RhoA activity, whereas knockdown of Pak2 has the opposite effects (Coniglio et al., 2008). Similarly, in prostate cancer cells, knockdown of Pak1 inhibits hepatocyte growth factor-stimulated migration and loss of cell-cell junctions, whereas knockdown of Pak2 increases lamellipodium extension but does not affect migration speed and enhances loss of cell-cell junctions (Bright et al., 2009). These data point to clear functional distinctions between these two Pak isoforms.
Biological processes known to be regulated by group I Paks are too numerous to review in detail, but include cytoskeletal remodelling, cell proliferation, and cell survival, mediated chiefly by effects on actin dynamics, gene transcription and translation, and apoptotic signalling pathways, respectively. Given this broad scope of activity, it is not surprising that Paks have also been implicated in a number of pathological conditions in man, most prominently mental retardation and cancer. Because these areas have been extensively reviewed in recent publications, we will here focus on recently discovered roles for group I Paks in immune function and viral infection.
4.1 Pak function in leukocytes
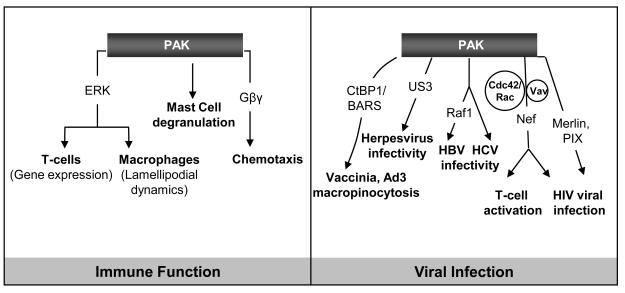
Pak1 and Pak2, but not Pak3, are expressed at high levels in most leukocytes. Pak1 and Pak2 have important functions in a variety of white blood cells, including T cells, neutrophils, macrophages, and mast cells (Fig. 2). While the function(s) of Pak in these cells is undoubtedly distinct, in each case, Pak plays an important role in cytoskeletal structure and activation of the Erk pathway, and hence affects the functional capabilities of these cells in immune response. T-cell receptor-mediated activation of Erk and NFAT depend on Pak1, which acts downstream of a Nck/Vav/SLP-76 signaling complex (Wu and Koretzky, 2004). Macrophages derived from Pak1−/− mice also display reduced Erk activation in response to growth factors, as well as defects in the stability of lamellipodia. These two phenomena are likely to be causally linked, since the effect on lamellipodial dynamics can be mimicked by Erk inhibitors (Smith et al., 2008).
Fig. 2. Group I Pak signaling networks in immune function and viral infection.
In T cells and macrophages, Paks, acting at least in part through ERK, are involved in the regulation of gene expression and lamellipodia formation. Paks, in particular Pak1, have a major role in mast cell degranulation, and are also important in directed migration of neutrophils via interactions with G proteins such as Gβγ. Group I Paks also play an important role in the life cycles of several viruses, as discussed in detail in the text. In the case of HBV, Raf1-mediated translocation of HBx depends on phosphorylation of Raf-1 by Pak1. PAK1 suppression of HCV replication is regulated by mTOR, through p70 S6 kinase activation of Pak1.
Activation of Pak1 is required for chemotaxis of leukocytes in response to the chemokine CXCR2 (Wang et al., 2002). In this case, however, the Pak requirement is apparently not related to effects on Erk activation. In myeloid cells, the role of Pak in chemotaxic signalling involves a self-reinforcing amplification circuit, involving Gβγ binding to Pak1, which, in association with bound PIX, activates Cdc42, leading to further Pak activation (Li et al., 2003). These findings provide another illustration of the importance of Pak's scaffolding effects in signal transduction.
Pak1 has recently been shown to play a key role in mast cells. Mast cells are important participants in allergic diseases via activation of high-affinity IgE receptors, resulting in release of proinflammatory mediators. Using Pak1−/− bone marrow derived mast cells, Allen et al. demonstrated that Pak1 is required for IgE-mediated calcium influx, and degranulation (Allen et al., 2009). These effects were also apparent in vivo, as Pak1−/− mice showed marked decreases in allergen-induced vascular permeability compared to controls. These data implicate Pak1 as an essential molecular target for modulating acute mast cell responses that contribute to allergic diseases. These findings may also be relevant to other mast-cell dependent pathologic processes, such as neurofibroma formation in NF1.
4.2 Paks and viral infection
In mammalian cells, group I Paks have been implicated in several aspects of virus/host interactions, in particular HIV pathogenesis. The role of Pak in HIV infectivity is likely to be mediated by several distinct Pak functions, but one aspect that has received particular attention is the association of Pak with the HIV-encoded protein Nef. The particular Pak isoform that interacts with Nef has been the subject of dispute, but there is reasonably strong evidence that the interaction of one or more of these kinases is important for HIV replication. Structural studies have shown that Pak2 associates with a hydrophobic binding surface on Nef (Agopian et al., 2006), and that the interaction of these proteins activates Pak2 (Linnemann et al., 2002). Nef-Pak complexes also contain the GTPases Rac and Cdc42, which might further activate Pak, as well as GTPase activators such as Vav (Rauch et al., 2008). A Nef-associated phosphatidylinositol-3 kinase/Pak complex has been reported to phosphorylate the proapoptotic Bad protein, independent of Akt. Since Nef, but not a Nef mutant incapable of binding or activating Pak, blocks apoptosis in T cells induced by HIV replication, it is possible that such Pak-mediated anti-apoptotic effects are important for HIV replication (Wolf et al., 2001).
In addition to its role in HIV, Pak function is also germane to additional viruses, such as HBV, HCV, and herpesvirus. Alphaherpesvirus encodes a protein kinase, US3, which induces alterations of the actin cytoskeleton that are associated with virus spread. Interestingly, US3-mediated stress fiber disassembly is drastically impaired in Pak2−/− mouse embryo fibroblasts (MEFs), whereas US3-mediated cell projections are impaired in Pak1−/− MEFs, implying distinct roles for these two forms of Pak in cell/virus interactions. The molecular mechanism underlying these effects are not fully understood, but US3 directly binds and activates Pak, suggesting a straightforward link between this viral protein and it cytoskeletal effects. In support of this view, loss of group I Pak activity in MEFs correlates with inefficient herpesvirus spread (Van den Broeke et al., 2009).
Group I Paks are also involved in other aspects of the viral life cycle. Pak1 activation is essential for vaccinia virus cellular entry, a process mediated by macropinocytosis and membrane blebbing (Mercer and Helenius, 2008). These effects might be mediated by Pak-catalyzed phosphorylation of CtBP/BARS, an event necessary for fission of the macropinocytic cup (Liberali et al., 2008). Similar endocytic pathways are also used by human adenovirus serotype 3 (Ad3). Infectious Ad3 macropinocytosis requires viral activation of Pak1 and a phosphorylation-defective mutant form of CtBP1 blocks Ad3, but not Ad5, infection (Amstutz et al., 2008). These results imply a selective role for Pak for viruses that use dynamin-independent endocytosis to enter cells.
5. Possible Medical Applications
The pleiotropic roles of group I Paks make them potential pharmacological targets. To date a handful of synthetic small-molecules, most notably the allosteric inhibitor IPA-3, have been used to target Pak (Arias-Romero and Chernoff, 2008). As Pak plays a fundamental role in ERK activation in many cell types, anti-Pak agents might be useful in diseases characterized by elevated ERK activity, such as NF1 or malignancies associated with activating RAS or BRAF mutations, as well as in mast-cell dependent allergic states and certain viral infections. We anticipate that these developments, along with increased understanding of specific Paks functions, will lead to improved compounds of clinical benefit.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Agopian K, Wei BL, Garcia JV, Gabuzda D. A hydrophobic binding surface on the human immunodeficiency virus type 1 Nef core is critical for association with p21-activated kinase 2. J Virol. 2006;80:3050–3061. doi: 10.1128/JVI.80.6.3050-3061.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JD, Jaffer ZM, Park SJ, Burgin S, Hofmann C, Sells MA, Chen S, Derr-Yellin E, Michels EG, McDaniel A, Bessler WK, Ingram DA, Atkinson SJ, Travers JB, Chernoff J, Clapp DW. p21-activated kinase regulates mast cell degranulation via effects on calcium mobilization and cytoskeletal dynamics. Blood. 2009;113:2695–2705. doi: 10.1182/blood-2008-06-160861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amstutz B, Gastaldelli M, Kälin S, Imelli N, Boucke K, Wandeler E, Mercer J, Hemmi S, Greber UF. Subversion of CtBP1-controlled macropinocytosis by human adenovirus serotype 3. EMBO J. 2008;27:956–969. doi: 10.1038/emboj.2008.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias-Romero LE, Chernoff J. A tale of two Paks. Biol Cell. 2008;100:97–108. doi: 10.1042/BC20070109. [DOI] [PubMed] [Google Scholar]
- Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- Bright MD, Garner AP, Ridley AJ. PAK1 and PAK2 have different roles in HGF-induced morphological responses. Cell Signal. 2009 doi: 10.1016/j.cellsig.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Coniglio SJ, Zavarella S, Symons MH. Pak1 and Pak2 mediate tumor cell invasion through distinct signaling mechanisms. Mol Cell Biol. 2008;28:4162–4172. doi: 10.1128/MCB.01532-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dummler B, Ohshiro K, Kumar R, Field J. Pak protein kinases and their role in cancer. Cancer Metastasis Rev. 2009;28:51–63. doi: 10.1007/s10555-008-9168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis P, Barnier JV. PAK signalling in neuronal physiology. Cell Signal. 2009;21:384–393. doi: 10.1016/j.cellsig.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Li Z, Hannigan M, Mo Z, Liu B, Lu W, Wu Y, Smrcka AV, Wu G, Li L, Liu M, Huang CK, Wu D. Directional sensing requires G beta gamma-mediated PAK1 and PIX alpha-dependent activation of Cdc42. Cell. 2003;114:215–227. doi: 10.1016/s0092-8674(03)00559-2. [DOI] [PubMed] [Google Scholar]
- Liberali P, Kakkonen E, Turacchio G, Valente C, Spaar A, Perinetti G, Bockmann RA, Corda D, Colanzi A, Marjomaki V, Luini A. The closure of Pak1-dependent macropinosomes requires the phosphorylation of CtBP1/BARS. EMBO J. 2008;27:970–981. doi: 10.1038/emboj.2008.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightcap CM, Kari G, Arias-Romero LE, Chernoff J, Rodeck U, Williams JC. Interaction with LC8 is required for Pak1 nuclear import and is indispensable for zebrafish development. PLoS One. 2009;4:e6025. doi: 10.1371/journal.pone.0006025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnemann T, Zheng YH, Mandic R, Peterlin BM. Interaction between Nef and phosphatidylinositol-3-kinase leads to activation of p21-activated kinase and increased production of HIV. Virology. 2002;294:246–255. doi: 10.1006/viro.2002.1365. [DOI] [PubMed] [Google Scholar]
- Mercer J, Helenius A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science. 2008;320:531–535. doi: 10.1126/science.1155164. [DOI] [PubMed] [Google Scholar]
- Rauch S, Pulkkinen K, Saksela K, Fackler OT. Human immunodeficiency virus type 1 Nef recruits the guanine exchange factor Vav1 via an unexpected interface into plasma membrane microdomains for association with p21-activated kinase 2 activity. J Virol. 2008;82:2918–2929. doi: 10.1128/JVI.02185-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennefahrt UE, Deacon SW, Parker SA, Devarajan K, Beeser A, Chernoff J, Knapp S, Turk BE, Peterson JR. Specificity profiling of Pak kinases allows identification of novel phosphorylation sites. J Biol Chem. 2007;282:15667–15678. doi: 10.1074/jbc.M700253200. [DOI] [PubMed] [Google Scholar]
- Smith SD, Jaffer ZM, Chernoff J, Ridley AJ. PAK1-mediated activation of ERK1/2 regulates lamellipodial dynamics. J Cell Sci. 2008;121:3729–3736. doi: 10.1242/jcs.027680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Broeke C, Radu M, Deruelle M, Nauwynck H, Hofmann C, Jaffer ZM, Chernoff J, Favoreel HW. Alphaherpesvirus US3-mediated reorganization of the actin cytoskeleton is mediated by group A p21-activated kinases. Proc Natl Acad Sci U S A. 2009;106:8707–8712. doi: 10.1073/pnas.0900436106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Sai J, Carter G, Sachpatzidis A, Lolis E, Richmond A. PAK1 kinase is required for CXCL1-induced chemotaxis. Biochemistry. 2002;41:7100–7107. doi: 10.1021/bi025902m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D, Witte V, Laffert B, Blume K, Stromer E, Trapp S, d'Aloja P, Schurmann A, Baur AS. HIV-1 Nef associated PAK and PI3-kinases stimulate Akt-independent Bad-phosphorylation to induce anti-apoptotic signals. Nat Med. 2001;7:1217–1224. doi: 10.1038/nm1101-1217. [DOI] [PubMed] [Google Scholar]
- Wu JN, Koretzky GA. The SLP-76 family of adapter proteins. Semin Immunol. 2004;16:379–393. doi: 10.1016/j.smim.2004.08.018. [DOI] [PubMed] [Google Scholar]