Abstract
To determine air-liquid interface (ALI) culture derived from cryopreserved mammalian tracheal ciliated cells is a viable ciliated cell model for the investigations of regulatory mechanisms of ciliary beat frequency (CBF), two studies were performed using ovine and porcine tracheae obtained from local slaughterhouses. The protease-digested tracheal ciliated cells were harvested and cultured at the ALI using collagen-coated, porous membrane inserts. In study 1, the ALI culturing protocols were established using non-cryopreserved ovine tracheal ciliated cells. Ciliogenesis was documented with immuno-histology and electron micrographs. Vigorous beating cilia were video-recorded. CBF was measured by laser light scattering. The functional integrity of the autonomic receptors of the ciliated cells was confirmed with the stimulatory responses of CBF using luminal methacholine and basolateral terbutaline. In study 2, porcine tracheal ciliated cells stored in liquid nitrogen for a minimum of four weeks were used. The cryopreserved cells were thawed and cultured using the ALI protocol established in study 1. After two months, cilia outgrowths were confirmed using video microscopy and scanning electron micrograph (SEM). The trans-epithelial resistances were 28.5 kΩ (n=4). Luminal applications of 1 µM and 10 µM methacholine stimulated CBF from a baseline of 7.4±0.2 Hz to 8.4±0.8 Hz and 7.7±0.4 Hz, respectively (n=5). Basolateral applications of 1 µM and 10 µM terbutaline stimulated CBF from a baseline of 7.5±0.3 Hz to 8.2±0.4Hz and 8.0±0.4 Hz, respectively (n=5). These data demonstrated that a ciliated cell bank can be established using cryo-preserved ciliated cells for pulmonary drug discovery and toxicological screening.
Keywords: ciliated cell bank, drug screening, retinoic acid, CBF, primary tracheal ciliated cells
Introduction
Airway ciliated epithelial cells are specialized to transport secretions in the airways. The asymmetrical location of ion pumps and transporters between the luminal and basolateral membranes polarizes these ciliated cells and is responsible for the transport of ions and water across the epithelia. Morphologically, cilia are located only at the luminal surface of the airway epithelial cells. They are immersed in an air-liquid interface (ALI) milieu with their basolateral membranes of the ciliated cell bodies being nourished by the capillary bed. Cultured epithelial cells with intact cilia should represent those in native epithelia and in vivo. All cryopreserved epithelial cell lines have lost most if not all of their cilia. A culturing technique that activates the mechanisms of ciliogenesis of cryopreserved tracheal and bronchial epithelial cells on demand could be utilized for the investigations of the luminal and basolateral regulatory mechanisms of ciliary activity and epithelial cell biology. At present, most of the investigations of the regulatory mechanisms of ciliary activity, in terms of ciliary beat frequency (CBF), are largely derived from submerged non-cryopreserved ciliated cell cultures [11,18,25] where the polarization properties of these ciliated cells are likely lost.
Herein we described a method for producing a culture of tracheal epithelial ciliated cells on semi-permeable transwell membrane inserts using cryopreserved porcine tracheal ciliated cells. The results were compared to those derived from non-cryopreserved ovine tracheal ciliated cells using the same culturing protocol. Under these conditions, the cells cultured at the ALI on the inserts achieved cilia reproduction after their native cilia were lost in both cryopreserved and non-cryopreserved samples. In both preparations, the cells form a tight epithelium that is morphologically and functionally similar to the airway epithelium found in native epithelia and in in vivo. By investigating the luminal and basolateral regulatory mechanisms of CBF using these polarized ALI cultures, we have shown that they are closely mimic the responses reported in native epithelia [22] and in vivo [21]. The ability to induce cilia regeneration in these cryopreserved airway epithelial cells and to maintain the active functional cilia of these cells for over 4 months provide an in vitro cell-based system for the evaluation of the long-term effects of respiratory drug candidates on mucociliary clearance.
Materials and Methods
Two studies were performed. In study 1, non-cryopreserved, ovine ciliated epithelial cell were first cultured to establish the ALI culturing conditions for the outgrowth of cilia. These include the types of culture media, the concentrations of the fetal calf serum (FCS) and the retinoic acid (RA). Observations were also made in the submerged culture conditions. In study 2, cryopreserved porcine ciliated epithelial cells were cultured using the culture conditions derived from study 1 to induce cilia outgrowth. Luminal and basolateral regulations of CBF were investigated in both studies.
Common Protocols for Study 1 and 2
Harvesting Airway Ciliated Epithelial Cell
Ovine and porcine tracheae were obtained from local slaughterhouses. The tracheae were submerged in chilled Hanks solution (Bio-Source International, Camarillo, CA) until the dissection of mucosa [11, 23]. Epithelial mucosa was removed from each trachea. A small piece of tissue was cut and examined under microscope to ensure the presence of vigorously beating cilia on the epithelial mucosa. The harvested mucosa was washed 5 times using M199 (Bio-source International, Camarillo, CA) with antibiotics prior to cutting them into small pieces. The resulting tissues were incubated at 4°C overnight in M199 supplemented with 1× of penicillin/streptomycin/fungizon mix (Cambrex, Wallersville, MD) and 0.6 mg/ml type IV protease (Sigma, St Louis, MO). Clusters of ciliated cells were harvested the next day by gently agitating each piece of the mucosa in a petri dish containing M199 with 10% FCS (ATCC, Manassas, VA). Cell clusters were settled in the plastic petri dish for an hour to minimize fibroblast contaminations. The resulting cell clusters were washed 3 times with M199 supplemented with 1× of penicillin/streptomycin/fungizone mix and were re-suspended in medium BEGM (Cambrex, Wallersville, MD) containing 5% FCS and 10−7M RA. The basic ingredients of the BEGM include: epidermal growth factor (0.5 ng/ml h_EGF), insulin (5 µg/ml), hydrocortisone (0.5 µg/ml), transferrin (10 µg/ml), epinephrine (0.5 µg/ml), triiodothyronine (6.5 ng/ml), bovine pituitary extract (60 µg/ml), gentamicin (50 µg/ml), cholera toxin (10 ng/ml), retinoic acid (0.1 ng/ml), amphotericin (50 ng/ml) and 0.8% penicillin-streptomycin and a minute quantities of RA (~0.1 nM).
Air-Liquid Interface Culture
Induction of cilia outgrowths by ALI method consisted of two steps. In step 1, cells were cultured to confluence in submerged condition such that culture media were present on both sides of the collagen membrane. In step 2, ALI was established by lowering the fluid on the luminal surface. Briefly, tracheal epithelial cells were grown on transparent, permeable polyester membrane (ϕ=6 mm) culture inserts (Transwell-COL, Corning Costar). The membrane of the insert was pre-coated with type I collagen by the manufacturer. Prior to seeding with the cells, these membrane inserts were placed in a 24-well plate. 200 µl growth medium was added to each insert. The plate containing the inserts was placed in a 37°C incubator for 2 hours. Cell suspension containing approximately 50,000 cells were added to the luminal compartment of each insert. The basolateral compartment was supplied with cell-free 0.5 ml medium. After 2 days incubation, the dead cells, cell debris and cells which failed to attach to the membrane were gently washed away using the growth medium. Based on the Study 1 results shown in Table 1, 2 and 3, BEGM with 5% FCS and 10−7M retinoic acid (RA) were used for all the cultures. The cells were incubated in a 37°C, 5% CO2 incubator with 100% humidity. Fresh culturing medium was changed every 2–3 days for both sides of the inserts. After five days, cells reached confluence and lost their cilia. An ALI culture was established by lowering the level of culture medium to the collagen substrate. Culture medium was exchanged to BEGM with 10−7M retinoic acid (RA). The cells at ALI were also maintained at 37°C, 5% CO2 incubator with 100% humidity. Culture medium was renewed every 4–5 days.
Table 1.
Comparisons of Culture Media to Promote Cell Growth
| BEGM with 5% FCS |
LHC-9 with 5% FCS |
F12/DMEM with 10% FCS |
|
|---|---|---|---|
| number of days for cell to reach confluence |
3 | 5 | 7 |
Table 2.
Effect of FCS concentration on cell growth using BEGM
| Concentrations of FCS | 0% | 1% | 5% | 10% |
|---|---|---|---|---|
| Days Cell Reach Confluence | 5 | 5 | 3 | 3 |
Table 3.
Effect of Retinoic Acid (RA) on the induction of ciliogenesis
| BEGM +5% FCS with RA |
LHC-9+5% FCS with RA |
BEGM +5% FCS without RA |
LHC-9+5% FCS without RA |
|
|---|---|---|---|---|
| Morphology of the cell | Cuboidal | Cuboidal | squamous | squamous |
| Cilia outgrowth | + | + | − | − |
Scanning Electron Microscopy (SEM)
Inserts were washed twice in PBS and fixed in 3.5% glutaraldehyde in 0.15 M phosphate buffer (pH 7.4) at room temperature for 1 hour. They were then dehydrated in graded ethanol series and treated with a series of different ratios of ethanol with hexamethyldisilazane (HMDS). After air-dried at room temperature, the cultured cells on the ALI membrane was detached from the plastic holder and mounted on an aluminum tube. Samples were sputter coated with platinum/palladium and analyzed with a scanning electron microscope.
Transmission Electron Microscopy (TEM)
Ciliated cells were fixed in 3.5% glutaraldehyde in 0.15M phosphate buffer for 30 minutes at room temperature and then post-fixed in 1% osmium tetroxide in 0.15 M phosphate buffer (pH 7.4) for 30 min at room temperature following two 5-minute washing with phosphate buffer. The samples were dehydrated in a series of graded ethanol. The dehydrated samples were infiltrated with a ratio of 100% ethanol per Pure Lx112 resin and embedded in Pure resin in BEEM® capsules with the sample facing toward the capsule bottom. They were cured at 60°C for 48 hours and sectioned on a Reichert ultramicrotome (100 nm). The samples were examined with a transmission electron microscope.
Immunohistochemistry
Cells (1×105) were plated on coverslips and maintained in 5% FBS in BEGM for 24 hours. Cells were washed in PBS and fixed with freshly prepared 3% para-formaldehyde for 30 minutes at room temperature. After washing with PBS, the cells were permeabilized and quenched with 0.05% Triton X-100 in PBS containing 0.3% H2O2 for 10 min. Following the washing with PBS, cells were treated with 5% BSA (Sigma-Aldrich Corp., St. Louis, MO) in PBS for 1 hour, then incubated for an hour with anti–Epithelial Keratin-antibodies (ICN Biomedicals, Aurora, OH) at a 1:100 dilution at room temperature. For the identification of the outgrowth of cilia on the insert, anti-β-tubulin-antibody (ICN Biomedicals, Aurora, OH) was used. After washing off the primary antibodies, the sample was incubated with biotinylated, linked-Abs for 20 min at room temperature and followed with the reactions of Streptavidin-HRP conjugates. Color development was achieved by adding 3-amino-9-ethylcarbazole (ACE) (Sigma-Aldrich Corp., St. Louis, MO) as a substrate. Cells were observed under light microscope. Substitution of the primary Abs with irrelevant normal serum was served as negative control.
Measurements of CBF
CBF was measured by a forward laser light scattering technique using an inverted microscope [11,12]. Briefly, cell culture insert was placed in a 2-well chambered coverglass and placed on an inverted microscope heated stage. 100 µl of Hank’s solution maintained at 30–32°C were placed on both sides of the insert. An attenuated He-Ne laser beam (λ=632.8 nm) was focused on the beating cilia from the top using a 100 mm focal length plano-lens. The dynamically Doppler-shifted light scattered by the beating cilia was observed by a 40× inverted microscope objective. Depending on the confluency of the cultured monolayer, the focal spot encompassed the cilia of one to five cells. The backscattered photons were collected following optical and spatial filtering and directed to a photon-counting photomultiplier tube. The heterodyne photon signals containing the embedded ciliary beat period were converted to a standard logic pulses using a pulse-amplifier-discriminator and routed to a photon counter housed in a personal computer. Photon counts were analyzed for the predominant frequency of each frame consisted of 1024-channel sequences sampled at 4 ms using frequency analysis. CBF, defined as the predominant frequency of each frame, was obtained in realtime every second.
Luminal and Basolateral Regulations of CBF by Autonomic Agonists
Five experiments were performed for each experimental condition using ALI cultures derived from non-cryopreserved ovine ciliated cells and cryopreserved porcine ciliated cells. In each experiment, following establishment of 5 minutes baseline measurements of CBF, 50 µl of Hank’s solution (control) was applied topically to the luminal compartment of the inserts and CBF was measured for the subsequent 5 minutes. Subsequently, 50 µl of 1 µM and 10 µM of methacholine (Sigma-Aldrich Corp., St. Louis, MO) or terbutaline (Sigma-Aldrich Corp., St. Louis, MO) were added cumulatively to the respective luminal or basolateral compartments of the inserts 10 minutes apart. CBF was measured continuously for each of the applications.
Protocols Specific to Study 1: Non-cryopreserved ovine tracheal ciliated cells
(i) Optimization of Culture Media
Three preliminary experiments were performed using ovine epithelial cells grown to determine the proper constituents of the growth medium. (a) Epithelial cell growth and expansion in submerged conditions were compared using BEGM+5% FCS, LHC-9+5% FCS and F12/DMEM+10% FCS. (b) the effect of FCS on cell growth in submerged condition were performed using BEGM containing 0%, 1–2%, 5% and 10% FCS. (c) Confluent monolayer cultured epithelial cells that lost all their cilia in experiment (a) and (b) were then transferred from the submerged culture to an ALI culturing condition described below. The cells were then cultured in either BEGM, or LHC-9 media with or without 10−7 M RA.
(ii) ALI Cultures
A minimum of 20 samples of ALI cultures were developed using freshly derived ovine tracheal ciliated epithelial cells. Ten samples were used for the experiments of luminal and basolateral stimulations of CBF. The remaining samples were used for the immuno-histology, SEM and TEM studies.
Protocols Specific to Study 2: Cryopreserved porcine tracheal ciliated cells
(i) Cryopreservation of Porcine Epithelial Cells
The ciliated epithelial cells were isolated from porcine trachea after overnight digestion with protease as described above. For long term storage of freshly harvested porcine tracheal ciliated epithelial cells, the ciliated cells were re-suspended at 2.5 × 107 cell/ml with BEGM containing 10% FCS and 1× penicillin/streptomycin/fungizone mix. 0.4 ml cell suspension was transferred to a 1.5-ml cryotube with 0.32 ml of FCS and 0.08 ml dimethyl sulfoxide (Me2SO) added to each tube. The final cryopreservation cell medium contains 45% FCS and 10 % Me2SO. After well-mixing of the content, cryotubes were placed in an insulated container, then placed in −80°C freezer overnight to allow the temperature to decrease at approximately 1°C/min. Cryotubes were transferred to liquid nitrogen (Nelgene Cryo Freezing Container) for long term storage. For thawing and culturing the cryopreserved cells, the tube was taken from liquid nitrogen and placed in dry ice. The tube was gently swirled in a 37°C water bath for 1–2 min until the content was completely thawed. The medium was exchanged to the completed culture medium as described.
(ii) ALI Cultures
A minimum of 20 samples of ALI cultures were developed using cryopreserved porcine tracheal ciliated epithelial cells. Ten samples were used for the experiments of luminal and basolateral stimulations of CBF. The remaining samples were used for the transmembrane resistance measurements and SEM studies. All samples were a minimum of 6 weeks old.
(iii) Transmembrane Resistance Measurements
Four samples of 2 months old ALI cultures were used. Transmemebrane resistance was measured using an epithelial Votohmmeter (EVOM™, WPI). The asymmetrical dual-probe STX2 electrode was used to measure the transmembrane resistance with one of the probes being placed at the luminal compartment and the remaining probe at the basolateral compartment of the ALI culture to complete the circuit.
Data Analysis
All mean (mean of means) data are expressed as arithmetic means ± SD. CBF responses of each experimental condition were summarized as the mean values of 90 sec of the peak CBF responses following the applications of each agent. ANOVA was applied to the data to determine the statistical significance of each experimental condition.
Results
Study 1: Non-cryopreserved Ovine Tracheal Epithelial Cells cultured at the ALI
(i) Culture media, FCS and RA
Culture media for ciliated cell growth
Cells multiplied much faster in BEGM+5%FCS and in LHC-9+5%FCS than in F12/DMEM+10%FCS. Cells cultured in BEGM+ 5% FCS became confluent in 3 days; cells cultured in LHC-9+5%FCS became confluent in 5 days, while cells cultured in F12/DMEM+10% serum did not reach confluence for more than one week. When observed under light microscopy, cultures in BEGM or LHC-9 also appeared to maintain a more stable pH (in terms of the color of the media), and healthier cellular morphology compared to cultures in F12/DMEM. These findings indicate that cells became confluent more easily in submerged culture in BEGM+5%FCS (Table 1).
Concentration of FCS on cell growth
Experiments were performed using BEGM containing 0%, 1–2%, 5% and 10% serum in submerged culture condition (Table 2). Cells cultured in media containing 5% serum became confluent in substantially less time than cells cultured in media with 1–2% serum or no serum at all. Cells cultured in 5% serum became confluent in 3 days, while cells cultured in 1–2% serum or without serum required more than 5 days to reach confluence. There was little observable difference between cells cultured in 5% or 10% serum, although cells cultured in 10% serum may have increased in cell number slightly faster.
RA on ciliated cell growth
As noted above, cells became confluent in submerged culture either in BEGM+5%FCS or LHC-9+5%FCS. Without RA in the medium, the epithelial cells became squamous (Table 3). RA (10−7 M) caused the cells to develop into a more cuboidal or columnar shape [27]. When these confluent monolayer cultured epithelial cells that lost all their cilia were transferred from submerged to ALI culture, cilia outgrowths occurred in 3 days after the cells were in ALI when 10−7 M RA was included in either the BEGM or LHC-9 media. In the absence of RA, cilia outgrowths did not occur after 2 weeks. Cells in ALI culture with RA continuously differentiated into cuboidal and columnar shapes.
(ii) Submerged and ALI Cultures
In submerged culture conditions, more than 80% of the seeded cells attached to the collagen layer in one day. Many of these cells were flattened, squamous-like, “primary” non-ciliated cells. Within one week, a confluent monolayer of newly divided cells without cilia were observed. At this time, the original primary cells with cilia were also lost most of their remaining cilia. Cell surfaces were predominantly covered with microvilli with no mature cilia. Short cilia (1–2 µm) were occasionally found in some areas (Fig 1). Cells in submerged culture (control) in these two weeks did not show any cilia outgrowths, indicated by the lack of β anti-tubulin staining (Fig 2a).
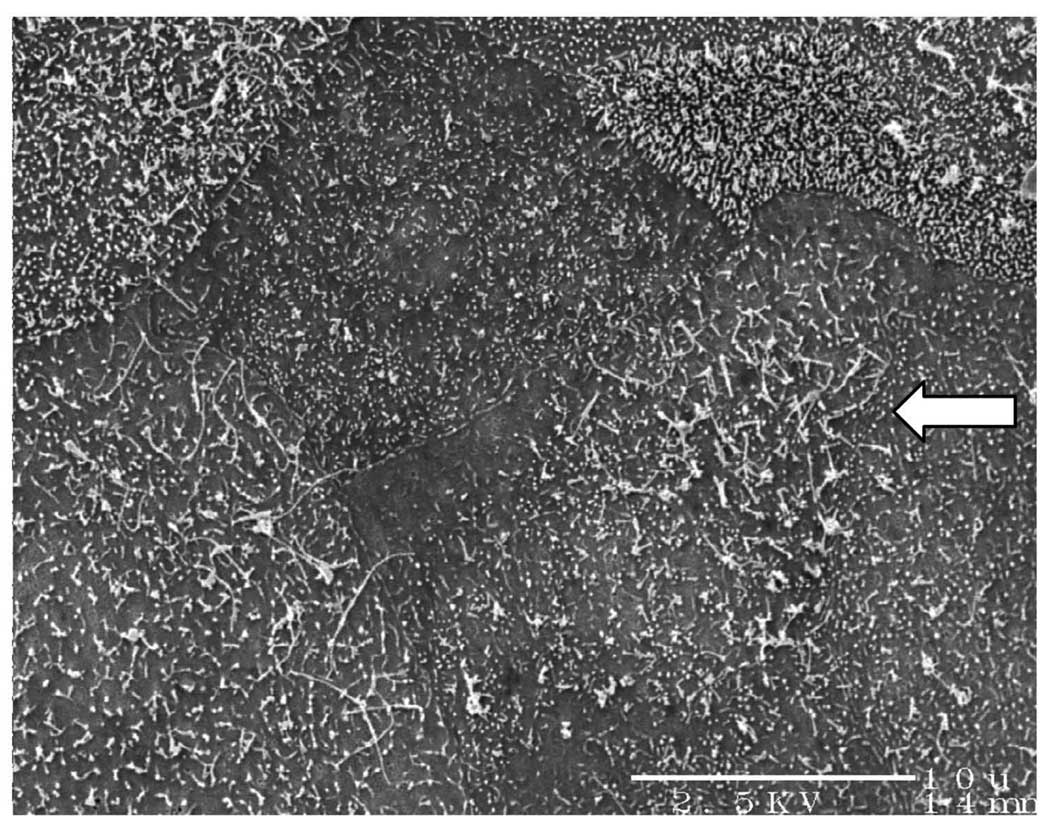
Fig. 1.
SEM of ovine tracheal epithelial cells cultured under submerged conditions for 3 weeks. The cell surfaces are covered with microvilli. Arrow indicates some short cilia which were occasionally found in some areas. However, no mature cilia were found (bar=10µm).
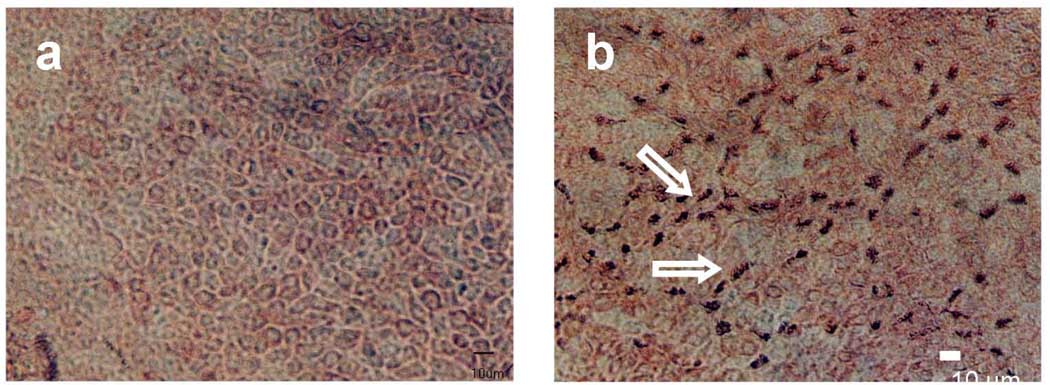
Fig. 2.
Anti-tubulin staining of airway epithelial cells in (a) submerged culture for two weeks (control), and (b) air-liquid interface culture after 3 days. Arrow shows the anti-tubulin stain of the cilia. Approximately 20–30% of the cell in the picture have cilia out-growths (bar=10 µm).
Cilia started to appear 3 days after the cells were in ALI culture. Cilia outgrowth occurred in ~25% of the epithelial cells as shown by positive anti-β tubulin staining of cilia [22] in ALI culture (Fig 2b). By the end of the first week, more than 25% of the cells were covered with cilia. The length of the cilia was also gradually increased. In the second week, cell differentiation continued with more cells became ciliated epithelial cells; the length of the cilia continued to increase. After 14 days of ALI culture, more than 50% of the cells were ciliated with the cilia length being about 6–7 µm. These newly grown cilia showed a rhythmic ciliary beating pattern similar to that observed under the light microscope in primary epithelial cell culture.
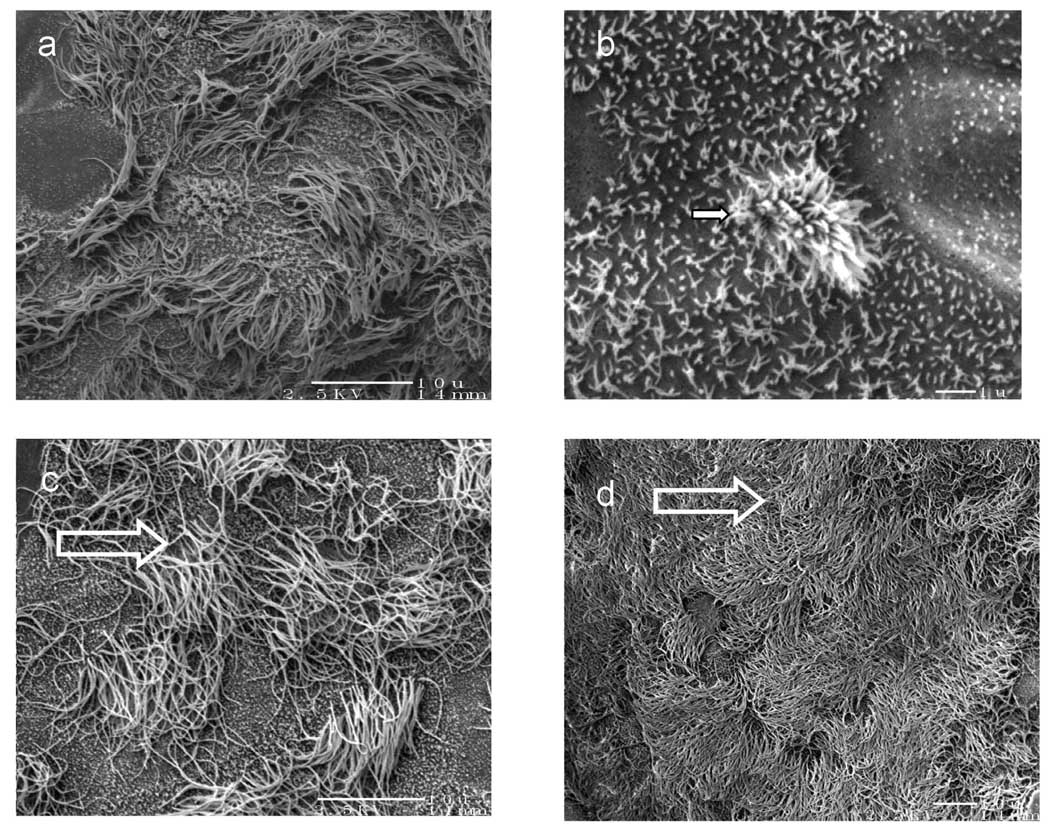
Examples of scanning electron micrographs (SEM) of ovine tracheal epithelial cells grown in ALI conditions for 2 weeks are shown in Figure 3. Stages of ciliogenesis were captured as illustrated in Fig 3a. Cilia in the active stage of ciliogenesis such as the budding cilia among microvilli are shown in Fig 3b, a 10 times enhanced magnification of Fig 3a. Most of the cilia reached a mature length of approximately 6–7 µm (Fig 3c). In some areas of the culture, over 50% of the cultured cell surface were covered with mature cilia (Fig 3d). These areas resemble native tracheal epithelia.
Fig. 3.
Scanning electron micrographs (SEM) of non-cryopreserved ovine tracheal epithelial cell cultured in air-liquid interface for 2 weeks. Samples were sputter-coated with Pt/Pd. (a) ciliogenesis was captured at several stages (bar=10 µm), (b) magnified SEM picture showing an active stage of ciliogenesis such as the budding cilia (arrow) among microvilli (star) (bar=1 µm), (c) mature cilia reached a length of approximately 6–7 µm (bar=10 µm), (d) over 50% of the cultured cell surface was covered with mature cilia (bar=10 µm).
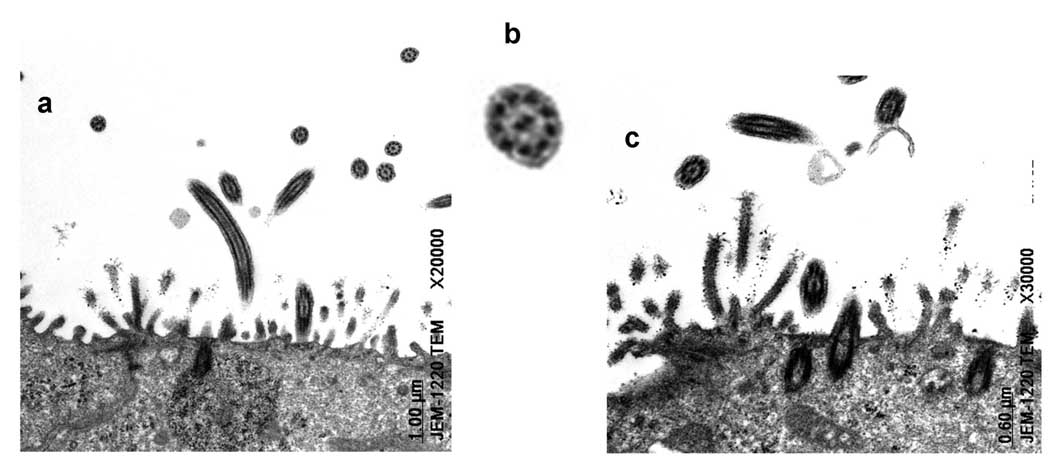
Transmission electron micrographs (TEM) show the transverse plane of a ciliated epithelial cell in ALI culture with mature cilia and microvilli (Fig 4). The TEM pictures (Fig 4a, 4b, 4c) revealed mature cilia with a normal ultra-structure of 9+2 axomeme: the nine doublets, the central pairs, the radial spokes, and the dynein arms in the ciliary shaft (Fig 4c).
Fig. 4.
Transmission electron micrographs (TEM) taken of the apical regions of ovine tracheal epithelial cells cultured in air-liquid interface for 3 weeks. The sample was cured at 60°C for 48 hours and sectioned using a Reichert ultramicrotome (100 nm). (a) transverse section of apical region showing transverse and oblique sections through cilia (bar=1 µm), (b) details of a transverse-sectioned mature cilium showing normal ultrastructure of axoneme, c) shows ciliary shafts which anchor the cilia in the apical region of the cell (arrow) (bar=0.6 µm).
An example of ovine tracheal epithelial cells grown in ALI for 2 weeks captured in video microscopy is shown in Figure 5. As visualized in the video, there were obvious identifiable patches of epithelial cells with cilia exhibited vigorous and synchronous beating almost everywhere in the ALI surface. The synchronous beating of cilia formed moving metachronal waves propagating on the ciliated surface, consistent with those observed in native epithelia.
Fig. 5.
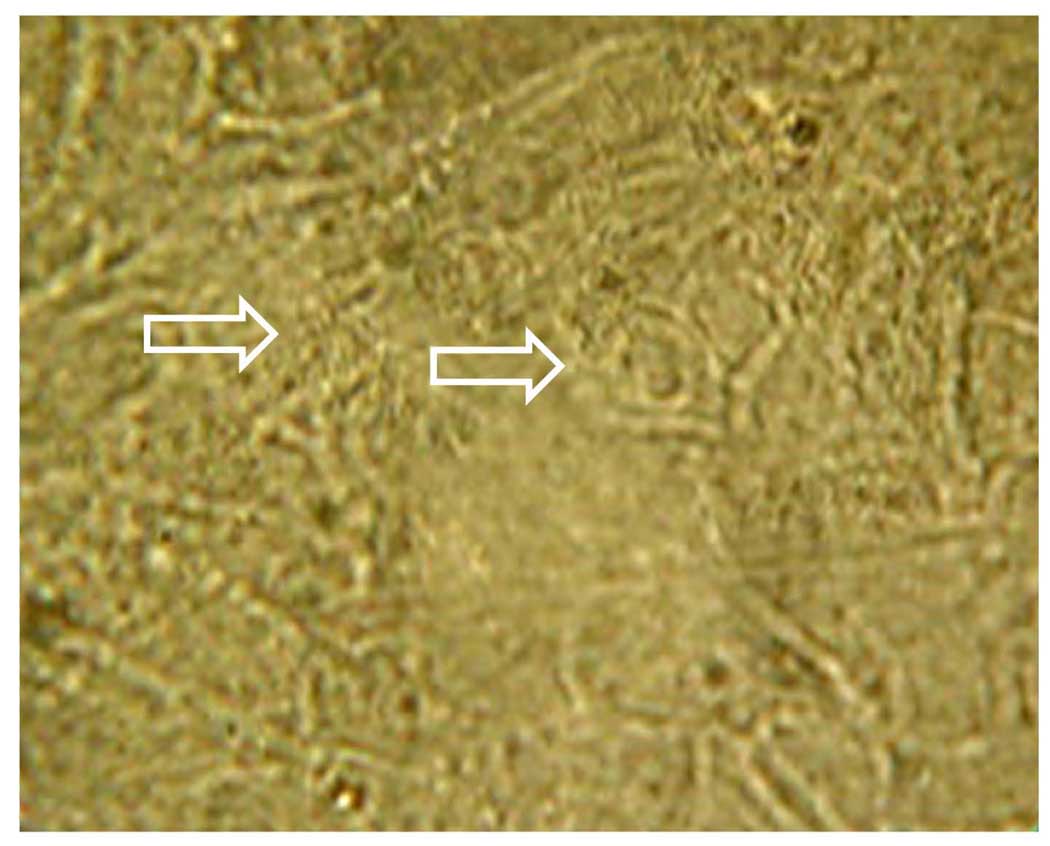
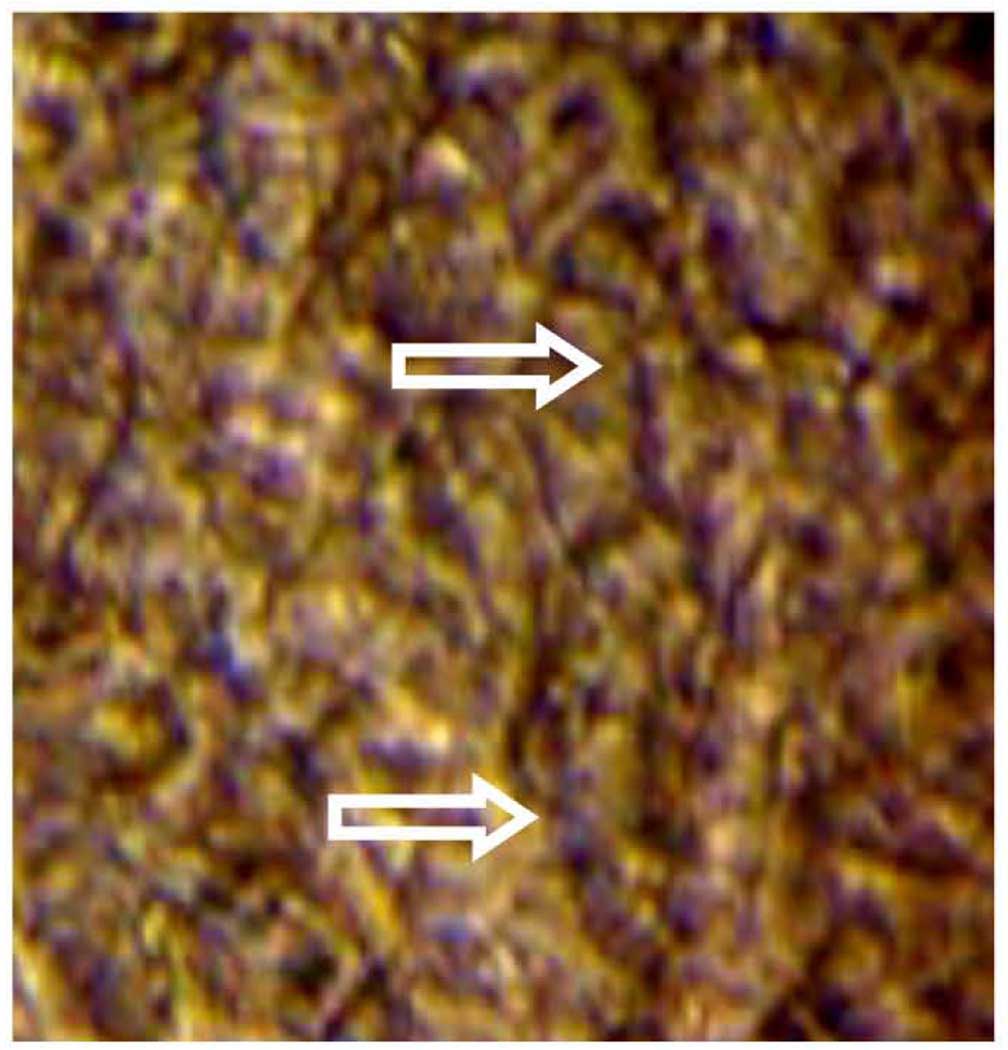
A sample picture from a video of beating cilia derived from non-cryopreserved ovine ciliated cells cultured at ALI (Video attached). The arrows indicated the positions of the beating cilia. Video were captured using a 40< objective coupled with a Sony video camera (DSC F707).
(iii) Luminal Muscarinic and Basolateral Adrenergic Regulation of CBF of Non-Cryopreseved Ovine Ciliated Cells
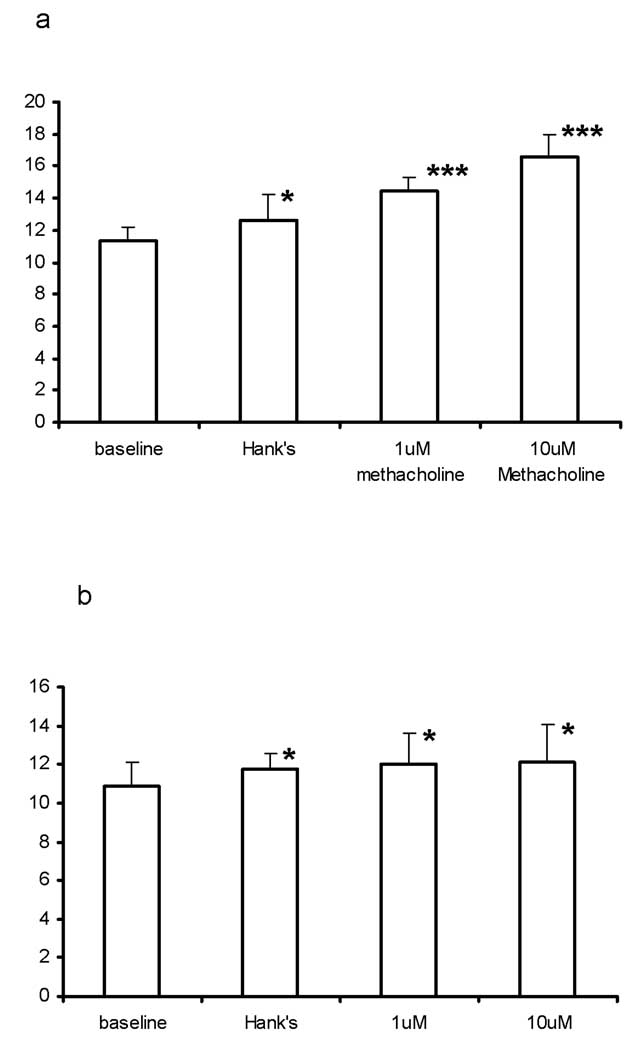
Figure 6 shows the muscarinic and adrenergic receptor-activated differential physiological responses of CBF using the non-cryopreserved ovine ALI culture. Cumulative luminal application of 1 µM and 10 µM methacholine stimulated CBF (n=5) from a baseline of 11.3±2.0 Hz to 14.4±1.9 Hz (p<0.05) and 16.5±3.2 Hz (p<0.05), respectively; while cumulative basolateral application of 1 µM and 10 µM terbutaline stimulated CBF (n=5) from a baseline of 10.8±2.7 Hz to 12.04±3.5 Hz (p<0.05) and 12.1±4.3 Hz (p<0.05), respectively. It should be noted that in both experiments, application of Hank’s solution caused a slight increase of CBF. This is consistent with our previous observations [22].
Fig. 6.
Regulations of CBF in non-cryopreserved ovine ciliated cells cultured at ALI. (a) Luminal stimulations of CBF by methacholine. (b) Basolateral stimulations of CBF by terbutaline. (* p <0.5 and *** p <0.005)
Study 2: Cryo-preserved Porcine Tracheal Ciliated Cell in ALI Cultures
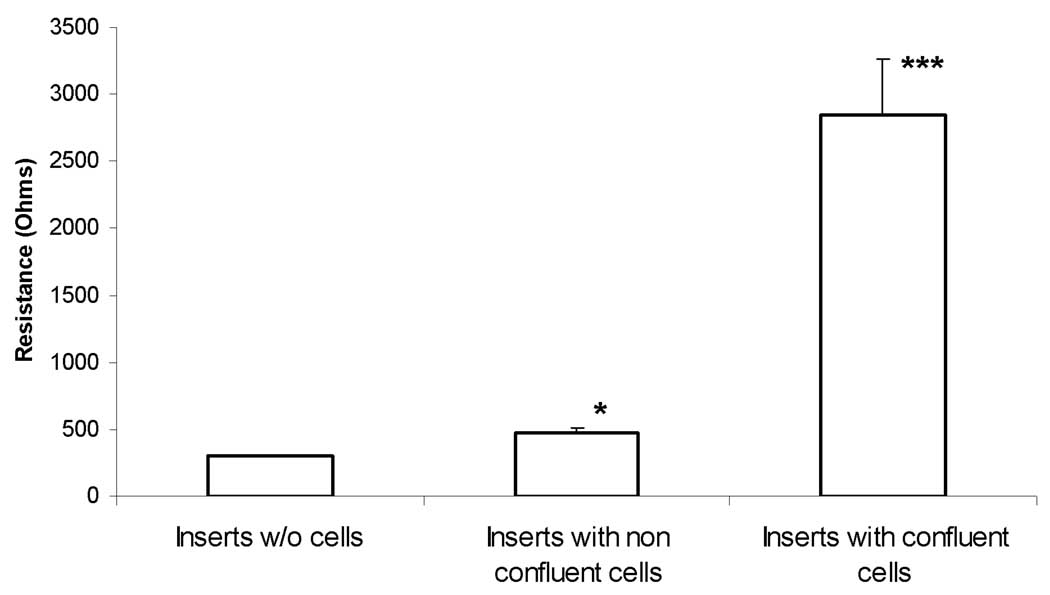
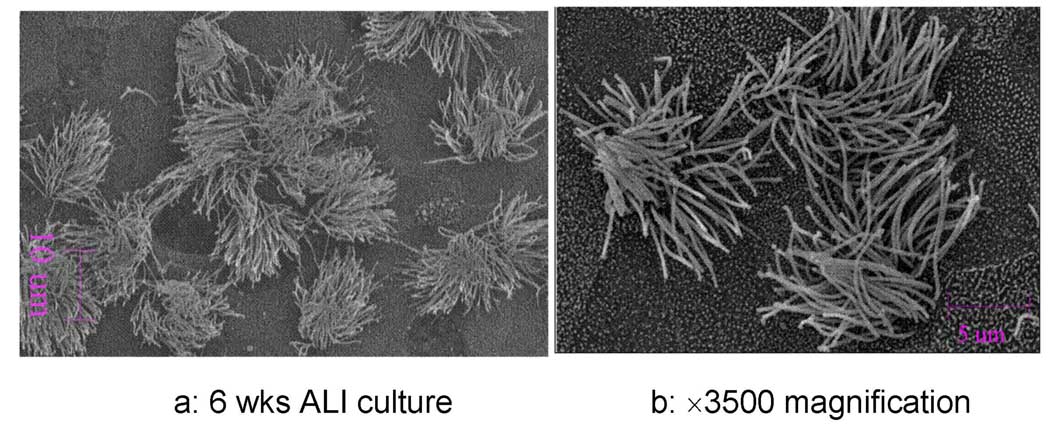
After a week of culturing the cryo-preserved porcine tracheal ciliated cell on transmembrane inserts in submerged conditions, cells reached confluence. Transmembrane resistance measurements indicate that tight junctions have been formed (Figure 7). 40% of the cells were observed to have new cilia outgrowth within two weeks after the creation of ALI in the new medium supplemented with RA. Examples of scanning electron micrographs (SEM) of cryopreserved porcine tracheal epithelial cells grown in ALI conditions for 6 weeks are shown in Figure 8. While there were no observable differences between cryopreserved and non-cryopreserved ciliated cell samples, cryo-preserved cells appeared to require longer time to induce cilia outgrowth.
Fig. 7.
Transmembrane resistance of cryopreserved porcine tracheal ciliated epithelial cells cultured in air-liquid interface. (*p <0.5 and ***p <0.005)
Fig. 8.
Scanning electron micrographs (SEM) of cryopreserved porcine tracheal epithelial cell cultured in air-liquid interface for 6 weeks. Samples were sputter-coated with Pt/Pd. (a) mature cilia (bar=10 µm), (b) magnified SEM picture (bar=5 µm).
An example of video microscopy of cryopreserved porcine tracheal epithelial cells grown in ALI conditions for 2 months is shown in Figure 9. Similarly, there were visible areas of ciliated cells with cilia exhibited vigorous and synchronous beating. There was no apparent observable differences regarding the ciliary beat pattern compared to the non-cryopreserved ovine ciliated cells cultured at the ALI.
Fig 9.
A sample picture from a video of beating cilia derived from cryopreserved porcine ciliated cells cultured at ALI (Video attached). The arrows indicated the positions of the beating cilia. Video were captured using a 40× objective coupled with a Sony video camera (DSC-F707).
Luminal Muscarinic and Basolateral Adrenergic Regulation of CBF of Cryo-preserved Porcine Ciliated Cells
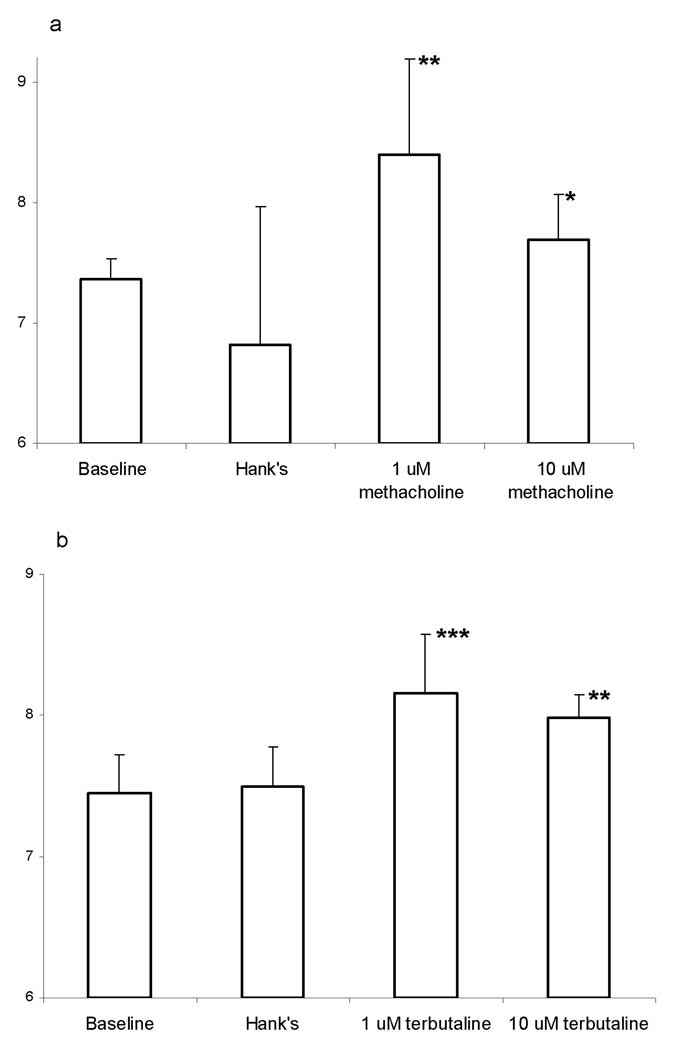
Figure 10 shows the muscarinic and adrenergic receptor-activated differential physiological responses of CBF using the cryopreserved porcine ALI culture. Cumulative luminal application of 1 µM and 10 µM methacholine stimulated CBF from a baseline of 7.4±0.2 Hz to 8.4±0.8 Hz and 7.7±0.4 Hz, respectively. Cumulative basolateral application of 1 µM and 10 µM terbutaline stimulated CBF from a baseline of 7.4±0.3 Hz to 8.1±0.4 Hz and 8.0±0.2 Hz, respectively. It appeared that high doses of either methacholine or terbutaline are toxic to the CBF [22].
Fig. 10.
Regulations of CBF in cryopreserved porcine ciliated cells cultured at ALI. (a) Luminal stimulations of CBF by methacholine. (b) Basolateral stimulations of CBF by terbutaline. (* p <0.5, ** p <0.05 and *** p <0.005)
Discussion
A method for producing a culture of airway ciliated epithelial cell system from cryopreserved and non-cryopreserved tracheal epithelial cells on semi-permeable transwell membrane inserts was developed. The cells cultured at the air-liquid interface achieved cilia regeneration after their native cilia were lost. The newly grown cilia of both cryopreserved and non-cryopreserved epithelial cells apparently preserved their cellular regulatory functions of ciliary activity as indicated by the luminal and basolateral stimulations of CBF by autonomic agonists. These data demonstrated that investigation of luminal and basolateral regulation of ciliary beat frequency by mediators can be investigated using ciliated cells derived from cryopreserved primary airway epithelial cells.
It has been previously reported in primary airway ciliated cells that cilia retained their intrinsic beat rate and the metachronal pattern following their revivals from cryopreservation [4,5,24]. The present report further these investigations by inducing ciliogenesis in cryopreserved primary airway epithelial cells with well-defined air-liquid interface culturing conditions. The morphological results derived from the cryopreserved and non-cryopreserved airway epithelial cell ALI cultures were consistent with those observed in native ciliated epithelia. This is the first report in the induction of ciliogenesis in cryopreserved mammalian ciliated cells using ALI technique. These data indicate that the mechanisms of ciliogenesis in cryopreserved airway epithelial cell are intact and they can be re-activated on demand.
Though grown on a semi-permeable membrane, the monolayer of cells is uniform and tightly packed as demonstrated by the high transmembrane resistance measurement. The ALI cultures described herein has an establishment of a luminal negative potential difference (PD) across them, similar to that found in native epithelia. Therefore the cell culture system on the insert provides both a luminal and a basolateral compartment at which different reagents could be administrated independently. Consequently their effects on the functions of beating cilia could be monitored accordingly. It has been shown that the luminal and basolateral regulations of CBF are mediated by separate receptor signal transduction mechanisms [22]. The luminal and basolateral stimulatory responses of CBF by autonomic agonists are consistent with the previous reports in non-cryopreserved airway epithelial ciliated cell cultures and native epithelia [11,18,22,25]. In addition, the ALI cultures could be maintained over 4 months with active cilia and tight transmembrane resistance. These data demonstrate that cryopreserved cells cultured in ALI conditions preserve the functional integrity of the luminal and basolateral regulatory mechanisms of CBF [22].
It has long been recognized that media have to be specifically designed for each cell line to support cell growth. Our data indicate that BEGM is an appropriate media to support the tracheal epithelial cell growth. The cell culturing strategy in using ALI with BGEM and retinoic acid is based on the following observations. Cilia outgrowths have never been reported in isolated airway epithelial cell. Whether inter-cellular communication or formation of tight-junctions among airway epithelial cells is prerequisite for promoting cilia outgrowth is uncertain. For the cells to grow to confluence, they have to anchor to a collagen or other substrate in submerged media conditions. Under submerged conditions [16], the newly divided cells do not have any cilia. To induce cilia outgrowths from these cells, it appears that luminal and basolateral orientations of the ion transporters and pumps of the epithelial cells must be re-established. Epithelial cell differentiation is a complex process that results from the integration of signals derived from growth factors, hormones and the extracellular matrix (ECM) [2,26]. One of the key elements for promoting cell differentiation is retinoic acid. The importance of retinoids for the maintenance of mucociliary differentiation of the tracheo-bronchial epithelium in vivo has been reported [20]. It has also been noted that airway epithelium of rats [9,13] and rabbits [8] cultured in “retinoid-depleted” serum free media were differentiated into a stratified squamous epithelium [6], while others have reported cellular differentiation without supplementing the media with retinoids [19,13,14]. The role of retinoic acid in ciliated cell differentiation is consistent with reported findings [1,2,4,13,26] and the recent identification of the retinoic acid responder (EST 36-5 cDNA) in cultures undergoing ciliogenesis. Retinoic acid responder may modulate and play key roles in a few dynein heavy-chain genes during ciliogenesis [10].
The utility of using ciliary function for screening drugs by the pharmaceutical industry has been proposed [17]. However, it has never been extensively adopted. One of the major technical factors is possibly the lack of suitable and abundance of ciliated cells for drug screening. A cryopreserved ciliated cell culture will resolve this issue. It is possible to harvest more than 100 million epithelial cells and to cryopreserve more than 90% of these primary cells from each ovine or porcine trachea. This is sufficient to generate more than 500 ALI inserts without cell expansion. The data described herein endows the potential of cryopreserved tracheal ciliated epithelial cells that can be used to develop into a ciliated cell bank for physiological, pharmacological and toxicological investigations as well as in pulmonary drug screening and discovery.
In conclusion, our results indicate that airway epithelial cells cultured at the ALI promote cilia outgrowths and preserve their ciliary function. Cryopreserved mammalian ciliated epithelial cell cultures exhibit similar morphological characteristics and physiological cell function as the cultures derived from non-cryopreserved ciliated cells. These data suggested that a mammalian ciliated cell bank can be established using cryopreserved primary airway ciliated epithelial cells derived from different species. The provision of a mammalian ciliated airway epithelial cells whose physiological cell function can be used to screen a wide range of receptor-mediated signal transduction mechanisms for a variety of agonists and antagonists will enhance pulmonary drug discovery processes.
Supplementary Material
Acknowledgements
This research was supported from the National Institutes of Health, National, Heart, Lung and Blood Institute under Grant No. R43 & R44 HL 067595 awarded to HM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Clark AB, Randell SH, Nettesheim P, Gray TE, Bagnell B, Ostrowski LE. Regulation of ciliated cell differentiation in cultures of rat tracheal epithelial cells. Am J Respir Cell Mol Biol. 1995;12:329–338. doi: 10.1165/ajrcmb.12.3.7873199. [DOI] [PubMed] [Google Scholar]
- 2.Davenport EA, Nettesheim P. Regulation of mucociliary differentiation of rat tracheal epithelial cells by type I collagen gel substratum. Am J Respir Cell Mol Biol. 1996;14:19–26. doi: 10.1165/ajrcmb.14.1.8534482. [DOI] [PubMed] [Google Scholar]
- 3.de Jong PM, van Sterkenburg MA, Hesseling SC, Kempenaar JA, Mulder AA, Mommaas AM, Dijkman JH, Ponec M. Ciliogenesis in human bronchial epithelial cells cultured at the air-liquid interface. Am J Respir Cell Mol Biol. 1994;10:271–277. doi: 10.1165/ajrcmb.10.3.8117445. [DOI] [PubMed] [Google Scholar]
- 4.Di Benedetto G, Gill J, Lopez-Vidriero MT, Clarke SW. The effect of cryopreservation on ciliary beat frequency of human respiratory epithelium. Cryobiology. 1989;26:328–332. doi: 10.1016/0011-2240(89)90056-4. [DOI] [PubMed] [Google Scholar]
- 5.Di Benedetto G, Gill J, Lopez-Vidriero MT, Clarke SW. Suitability of cryopreserved samples for pharmacological studies of ciliary activity. Cryobiology. 1990;27:591–595. doi: 10.1016/s0011-2240(05)80026-4. [DOI] [PubMed] [Google Scholar]
- 6.Harris CC, Kaufman DG, Sporn MB, Staffiotti U. Histogenesis of squamous metaplasia and squamous cell carcinoma of the respiratory epithelium in an animal model. Cancer Chemother Rep. 1973;3(4):43–47. [PubMed] [Google Scholar]
- 7.Huang TH, Ann DK, Zhang YJ, Chang AT, Crabb JW, Wu R. Control of keratin gene expression by vitamin A in tracheobronchial epithelial cells. Am J Respir Cell Mol Biol. 1994;10:192–201. doi: 10.1165/ajrcmb.10.2.7509163. [DOI] [PubMed] [Google Scholar]
- 8.Jetten AM, Brody AR, Deas MA, Hook GE, Rearick JI, Thacher SM. Retinoic acid and substratum regulate the differentiation of rabbit tracheal epithelial cells into squamous and secretory phenotype, Morphological and biochemical characterization. Lab Invest. 1987;56:654–664. [PubMed] [Google Scholar]
- 9.Kaartinen L, Nettesheim P, Adler KB, Randell SH. Rat tracheal epithelial cell differentiation in vitro. In Vitro Cell Dev Biol Anim. 1993;29A:481–492. [PubMed] [Google Scholar]
- 10.Maiti AK, Jorissen M, Bouvagnet P. Isolation, in silico characterization and chromosomal localization of a group of cDNAs from ciliated epithelial cells after in vitro ciliogenesis. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-7-research0026. RESEARCH0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mao H, Wong LB. Depolarization of cell membrane is associated with an increase in ciliary beat frequency (CBF) Biochem Biophys Res Commun. 1995;215:1014–1021. doi: 10.1006/bbrc.1995.2565. [DOI] [PubMed] [Google Scholar]
- 12.Mao H, Wong LB. Fluorescence and laser photon counting: measurements of epithelial [Ca2+]i or [Na+]i with ciliary beat frequency. Ann Biomed Eng. 1998;26:666–678. doi: 10.1114/1.124. [DOI] [PubMed] [Google Scholar]
- 13.Marchok AC, Cone V, Nettesheim P. Induction of squamous metaplasia (vitamin A deficiency) and hypersecretory activity in tracheal organ cultures. Lab Invest. 1975;33:451–460. [PubMed] [Google Scholar]
- 14.McDowell EM, Keenan KP, Huang M. Restoration of mucociliary tracheal epithelium following deprivation of vitamin A, A quantitative morphologic study. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;45:221–240. doi: 10.1007/BF02889866. [DOI] [PubMed] [Google Scholar]
- 15.Million K, Larcher J, Laoukili J, Bourguignon D, Marano F, Tournier F. Polyglutamylation and polyglycylation of alpha- and beta-tubulins during in vitro ciliated cell differentiation of human respiratory epithelial cells. J Cell Sci. 1999;112(Pt 23):4357–4366. doi: 10.1242/jcs.112.23.4357. [DOI] [PubMed] [Google Scholar]
- 16.Ostrowski LE, Nettesheim P. Inhibition of ciliated cell differentiation by fluid submersion. Exp Lung Res. 1995;21:957–970. doi: 10.3109/01902149509031773. [DOI] [PubMed] [Google Scholar]
- 17.Romet-Haddad S, Marano F, Blanquart C, Baeza-Squiban A. Tracheal epithelium in culture: a model for toxicity testing of inhaled molecules. Cell Biol Toxicol. 1992;8:141–150. doi: 10.1007/BF00130521. [DOI] [PubMed] [Google Scholar]
- 18.Salathe M, Bookman RJ. Mode of Ca2+ action on ciliary beat frequency in single ovine airway epithelial cells. J Physiol. 1999;520(Pt 3):851–865. doi: 10.1111/j.1469-7793.1999.00851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitcutt MJ, Adler KB, Wu R. A biphasic chamber system for maintaining polarity of differentiation of cultured respiratory tract epithelial cells. In Vitro Cell Dev Biol. 1988;24:420–428. doi: 10.1007/BF02628493. [DOI] [PubMed] [Google Scholar]
- 20.Wong YC, Buck RC. An electron microscopic study of metaplasia of the rat tracheal epithelium in vitamin A deficiency. Lab Invest. 1971;24:55–66. [PubMed] [Google Scholar]
- 21.Wong LB, Miller IF, Yeates DB. Stimulation of ciliary beat frequency by autonomic agonists: in vivo. J Appl Physiol. 1988;65:971–981. doi: 10.1152/jappl.1988.65.2.971. [DOI] [PubMed] [Google Scholar]
- 22.Wong LB, Miller IF, Yeates DB. Regulation of ciliary beat frequency by autonomic mechanisms: in vitro. J Appl Physiol. 1988;65:1895–1901. doi: 10.1152/jappl.1988.65.4.1895. [DOI] [PubMed] [Google Scholar]
- 23.Wong LB, Park CL, Yeates DB. Neuropeptide Y inhibits ciliary beat frequency in human ciliated cells via nPKC, independently of PKA. Am J Physiol. 1998;275:C440–C448. doi: 10.1152/ajpcell.1998.275.2.C440. [DOI] [PubMed] [Google Scholar]
- 24.Wulffraat NM, Veerman AJ, Stamhuis IH. Frequency and coordination of ciliary beat after cryopreservation of respiratory epithelium. Cryobiology. 1985;22:105–110. doi: 10.1016/0011-2240(85)90164-6. [DOI] [PubMed] [Google Scholar]
- 25.Wyatt TA, Spurzem JR, May K, Sisson JH. Regulation of ciliary beat frequency by both PKA and PKG in bovine airway epithelial cells. Am J Physiol. 1998;275:L827–L835. doi: 10.1152/ajplung.1998.275.4.L827. [DOI] [PubMed] [Google Scholar]
- 26.Yoon JH, Gray T, Guzman K, Koo JS, Nettesheim P. Regulation of the secretory phenotype of human airway epithelium by retinoic acid, triiodothyronine, and extracellular matrix. Am J Respir Cell Mol Biol. 1997;16:724–731. doi: 10.1165/ajrcmb.16.6.9191474. [DOI] [PubMed] [Google Scholar]
- 27.Yoshitsugu M, Hanamure Y, Furuta S, Deguchi K, Ueno K, Rautiainen M. Ciliary motility and surface morphology of cultured human respiratory epithelial cells during ciliogenesis. Biol Cell. 1994;82:211–216. doi: 10.1016/s0248-4900(94)80024-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.