Abstract
Fish in agricultural and remote areas may be exposed to endosulfan and its degradation products as a result of direct runoff, atmospheric transport and deposition. The following study used the zebrafish developmental model to investigate the responses to endosulfan I and endosulfan sulfate, the major degradation product of endosulfan I and II. Embryos were dechorionated and waterborne exposed to the endosulfan I or endosulfan sulfate from 6 to 120 hours post fertilization (hpf). Endosulfan I exposure concentrations ranged from 0.01 to 10 μg/L and endosulfan sulfate from 1 to 100 μg/L. Water solutions were renewed every 24 hours and fish were scored for overt developmental and behavioral abnormalities. Chemical analysis was performed on water, whole embryo, and larvae samples to determine waterborne exposure concentrations and tissue concentrations throughout the 5-day period. The most sensitive toxicity endpoint for both endosulfan I and endosulfan sulfate was an abnormal response of the embryo/larvae to touch, suggesting that endosulfan I and sulfate are developmentally neurotoxic. The waterborne exposure EC50s for inhibition of touch response for endosulfan I and endosulfan sulfate were 2.2 μg/L and 23 μg/L, respectively. The endosulfans were highly concentrated by the organisms, and the inhibition of touch response tissue EC50, determined from the measured tissue concentrations, was 367 ng/g for endosulfan I and 4552 ng/g for endosulfan sulfate.
Keywords: zebrafish, endosulfan, endosulfan sulfate, development
1. Introduction
Endosulfan (6,7,8,9,10,10-hexachloro-1,5,5a,6,9,9a-hexahydro-6,9-methano-2,3,4-benzodioxathiepin-3-oxide) is a cyclodiene insecticide currently used throughout the world. It is applied as a technical mixture of the I (α) and II (β) isomers in the ratios of 2:1 to 7:3 on a wide variety of crops (Wan et al., 2005a). It may enter the environment surrounding agricultural areas as a result of atmospheric transport and field runoff with concentrations ranging in the low part per billion measured in streams and rivers bordering these areas (Hose et al., 2003; Wan et al., 2005a; Wan et al., 2005b). Endosulfans have also been measured in fish from remote lakes throughout the U.S., Canada, and Europe where atmospheric transport and deposition accounts for its presence (Simonich and Hites, 1995; McConnell et al., 1998; LeNoir et al., 1999; Vilanova et al., 2001; Carrera et al., 2002; Stern et al., 2005; Usenko et al., 2007; Ackerman et al., 2008). Upon entering the environment endosulfan I and II are primarily converted to the diol form in water and the sulfate form in soil and sediment (Hose et al., 2003). The endosulfan sulfate and diol further break down to the ether, hydroxyl ether, lactone, and alcohol forms (Hose et al., 2003). Endosulfan sulfate is the only breakdown product considered to be toxic (Berntssen et al., 2008). The half-lives of endosulfan I and II in water are on the order of days to months, whereas endosulfan sulfate is on the order of weeks to years (Leonard et al., 2001; Wan et al., 2005a). In sediment, both forms are more persistent (Leonard et al., 2001; Wan et al., 2005a). Although fish tissue half-lives are on the order of days, potential exists for aquatic organisms to be impacted by endosulfan exposure, especially in agricultural areas where organisms are exposed to the highest environmental levels of endosulfan (Naqvi and Vaishnavi, 1993; Hose et al., 2003; Wan et al., 2005b).
Endosulfan I and II are highly toxic to aquatic organisms, including fish. In laboratory studies, the fish LC50s are in the range of 1 to 100 μg/L for endosulfan I, II, and the technical product (Jonsson and Toledo, 1993; Hose et al., 2003). In some fish species, endosulfan I is more toxic than endosulfan II and the technical product (Devi et al., 1981; Wan et al., 2005a). Based on acute studies, endosulfan sulfate appears to be as toxic as the parent isomers (Leonard et al., 2001). However, its toxicity is not well-characterized.
Endosulfan I and II are neurotoxic, thought to act through inhibition of the gamma-aminobutyric acid (GABA)-gated chloride channels (ATSDR, 2000; Jia and Misra, 2007). Symptoms of neurotoxicity, including hyperactivity, erratic swimming, and convulsions have been observed in adult fish (Jonsson and Toledo, 1993). Development is a particularly sensitive life stage, and fish are often more vulnerable to the adverse effects of chemicals during this period (Rosenthal, 1976; Lele and Krone, 1996). Few studies, conducted in rodents, have investigated the developmental neurotoxicity of endosulfan and reported possible neurotoxic effects as a result of alterations in physical brain or neurotransmitter and amino acid measurements (EPA, 2007; Cabaleiro et al., 2008). However, to our knowledge no studies have investigated the neurotoxicity of endosulfan in developing fish.
Zebrafish (Danio rerio) are a model organism (Spitsbergen and Kent, 2003) and are useful for studying developmental toxicity because organogenesis is largely complete by 48 hours post fertilization (hpf) (Kimmel C.B., 1995). Other benefits of using zebrafish in developmental studies include their transparency during early development, which allows for direct viewing of biological responses. Large clutch sizes and their small body size reduces the supplies required and costs to conduct experiments (Hill et al., 2005). The sequencing of the zebrafish genome is nearly complete and this promises to enhance it as a tool to study the mechanisms of developmental processes and how toxicants interfere with these processes (Spitsbergen and Kent, 2003; Hill et al., 2005).
Relatively few studies have investigated the toxicity of endosulfan sulfate despite its environmental persistence. In particular, information gaps exist regarding the potential developmental or developmental neurotoxicity of endosulfan. Endosulfan I was chosen in this study because it has been reported in some cases to be more toxic than endosulfan II. The objectives of this study were to determine if early zebrafish life stages were sensitive to endosulfan I or endosulfan sulfate, to determine the most sensitive endpoint of toxicity, and to define the relative developmental toxicity of endosulfan sulfate to endosulfan I.
2. Materials and methods
2.1 Fish care and husbandry
Adult zebrafish wild-type (Danio rerio) of the strains AB and 5D Tropical (5D) were reared at the Sinnhuber Aquatic Research Laboratory (SARL) at Oregon State University. Fish were housed in 2.0 L polycarbonate tanks filled with reverse osmosis (RO) water containing 0.6% instant ocean (Aquarium Systems, Inc., Mentor, Ohio) in a recirculating system. The water temperature was maintained at 28 °C (±1 °C), with a pH of 7.2 (±0.2), and the fish were kept on a 14 hour light/10 hour dark photoperiod. Fish were group spawned and embryos were collected at 2 to 3 hours post fertilization (hpf) and rinsed with water. They were dechorionated in a glass petri dish at 5 hpf using pronase (50 mg/ml), rinsed thoroughly with water, and allowed to recover 0.5 to 1 hour before they were placed into exposure solutions. Extensive comparative studies were completed between the AB and 5D and there were no significant differences in the dose response to endosulfan I and endosulfan sulfate (data not shown).
2.2 Waterborne exposures
Endosulfan I (99.5%) and sulfate (98.9%) were purchased from ChemService (West Chester, PA). A master stock solution of each chemical was prepared by dissolving the crystalline solids into ethyl acetate to obtain a concentration of 1 mg/ml. The solutions were stored at 4°C. A stock solution in DMSO was prepared for each experiment by reducing the ethyl acetate solution to dryness and immediately adding DMSO to the vial. Using the DMSO stocks a serial dilution was prepared to obtain endosulfan I and endosulfan sulfate DMSO solutions of varying concentrations. The final percentage of DMSO in the water solutions was 0.1 %. Experiments were conducted in 96-well styrene divinylbenzene plates, with 100 μL of solution and one organism per well. Zebrafish embryos were staged using the methods described in Kimmel et al. (1995) and exposed to the chemicals from 6 hpf to 120 hpf. Solutions were renewed with freshly prepared endosulfan or fish water solutions at 24, 48, 72, and 96 hpf.
2.3 Range finding experiments
Experiments were conducted to determine the waterborne concentration that produced mortality in 50 % of the embryos (LC50) and to determine the embryonic response most sensitive to endosulfan I and endosulfan sulfate using a large range of concentrations. Concentrations between 0.01 and 1000 μg/L were tested for endosulfan I and between 1 and 10,000 μg/L were tested for endosulfan sulfate. The results from these experiments were used to determine a concentration response curve for the most sensitive endpoint, an abnormal response to touch, of endosulfan I and endosulfan sulfate exposure in the embryos and larvae. Once an appropriate range for the concentration response curve was determined the experiment was repeated on three separate occasions. Final nominal concentrations were 0.01, 0.1, 0.5, 1, 5, and 10 μg/L for endosulfan I and 1, 10, 20, 40, 80, and 100 μg/L for endosulfan sulfate.
2.4 Abnormality scoring and behavioral scoring system
Embryos and larvae were scored for developmental abnormalities at 24, 48, 72, 96, and 120 hpf. Behavioral abnormalities were scored at 48, 72, 96, and 120 hpf by using a touch response scoring system, which was developed during the initial range finding experiments. The touch response was conducted by touching a thin needle head to the tip of the tail of the embryo or larvae and the resulting behavior of the zebrafish was recorded using a score of 1 through 5. The behaviors associated with each score are detailed in Table 1.
Table 1.
Associated behaviors for touch response scoring system.
| Number | Behavior |
|---|---|
| 1 | Normal: fish quickly swim away from the source of the touch, move across the length of the well |
| 2 | Fish quickly respond to touch, includes prolonged periods of swimming and fish acting disoriented |
| 3 | Fish are slower to respond to touch and swim a shorter distance |
| 4 | Fish flick the tail in response to touch, but do not swim |
| 5 | Paralysis: fish do not move |
2.5 Chemical analysis
All solvents used in analysis were of Optima grade (Fisher Scientific, Pittsburgh, PA). Water samples and tissue samples were analyzed using isotope dilution gas chromatography (GC)/mass spectrometry (MS). In order to determine the average concentrations of endosulfan I and endosulfan sulfate in the organisms over the course of the 5-day, static renewal (every 24 hours) exposure, a time course study was designed. Fish were exposed in groups of 12–24 in individual wells of a 24-well styrene divinylbenzene plate and exposure experiments were repeated in triplicate. The total amount of endosulfan I and sulfate exposure was kept consistent with dose response studies by adding 100 μl of exposure solution per embryo/larvae.
The water and tissue extraction procedures were based on the method developed by Isaacson et al. (2007). The samples were extracted and analyzed as follows. Water samples were collected (0.5 ml) and added to a 2.0 ml centrifuge tube. Embryos and larvae were anesthetized with tricaine amide methylsulfonate, rinsed thoroughly with water, and added to a pre-weighed 2.0 ml centrifuge tube (approximately 12 to 24 organisms pooled per sample). Water surrounding the embryos/larvae was removed with a 10 μl pipette and the tube was reweighed (Mettler B6 Analytical Balance, Hightstown, NJ). Embryos and larvae were ground using a Teflon pestle. Water and tissue samples were stored at −20 °C until analysis. Samples were thawed and spiked with 5 μl of isotopically labeled surrogate standards. In the case of water samples, 0.5 ml of hexane was added to each sample. In the case of embryo and larvae samples, 0.1 ml of glacial acetic acid was added to each sample, the tube was vortexed for 30 seconds, and 0.25 ml of ethyl acetate and 0.25ml of hexane was added to the sample. The water and tissue samples were vortexed for 1 minute followed by centrifugation for 10 min at 14,000 rpm. One-half of the top layer of solvent (0.25 ml) was removed and added to a 2.0 ml amber glass GC vial. The samples were solvent exchanged to ethyl acetate by reducing the solvent under a steady stream of nitrogen gas to approximately 0.1 ml and adding 10 times the amount of ethyl acetate (repeated 3 times). The samples were then reduced to 95 μl, spiked with 5 μl of internal standards and run on an Agilent 6890 gas chromatograph coupled to an Agilent 5973N mass selective detector in electron capture negative ionization (ECNI) mode using selected ion monitoring (SIM). A 12 point, solvent based, calibration curve was used for quantification. To monitor instrument performance, a standard was analyzed for every 3 to 4 samples. Endosulfans were identified using a standard based mass spectrometry library and matching retention times (±0.05 minutes). The GC/MS temperature method and SIM details are reported elsewhere (Usenko et al., 2005). In the case of the triplicate spike and recovery experiments the recovery from water and tissue were determined by adding a known concentration of endosulfan I and endosulfan sulfate (10 ng) to the samples (triplicate) prior to extraction. Both isotopically labeled surrogates and internal standards were added to the sample just prior to instrumental analysis to determine the loss of endosulfan I and endosulfan sulfate over the method.
2.6 Statistical analysis
A one-way ANOVA was performed in S-PLUS (version 8.0) to determine if there was a significant relationship between the dose and the response. Linear regression, using indicator variables for endosulfan I and endosulfan sulfate, was used to determine if there was a significant difference between the dose response curves of the two compounds (log-log transformed) (S-PLUS). Sigmoidal regression analysis was performed for endosulfan I and endosulfan sulfate dose response curves using Sigmastat (version 8.0) and the resulting equation for the curve was used to calculate the EC50. Fisher’s exact test was used to determine if there was a significant difference between the exposure groups and the control (S-PLUS) for abnormal touch response and a one-way ANOVA followed by Dunn’s multiple comparison procedure was used to determine if there was a significant difference between the exposure groups and the control for the touch score (Sigmastat version 11.0). For all analyses, a p-value ≤ 0.05 was considered significant. Results are presented as means ± standard deviation unless otherwise stated.
3. Results
3.1 Abnormal touch response is the most sensitive endpoint following endosulfan I and endosulfan sulfate exposure
Endosulfan I and endosulfan sulfate, even at relatively high concentrations did not produce visible or overt toxicity. No physical abnormalities were observed in larvae exposed to nominal concentrations of 5 μg/L or less of endosulfan I. Pericardial and yolk sac edema was observed in less than 20 % of larvae exposed to endosulfan I concentrations of 10 μg/L at 120 hpf. At the highest concentrations tested, 100 and 1000 μg/L, pericardial and yolk sac edema was visible in at least 80 % of larvae, a curved body axis in at least 40 % of larvae, and a wavy notochord in at least 66 % of larvae. Larvae exposed to the highest concentrations of endosulfan I also showed a less sensitive response to touch, reduced movement, and in some cases paralysis. No physical abnormalities were observed in larvae exposed to nominal concentrations of 40 μg/L or less of endosulfan sulfate at 120 hpf. Pericardial and yolk sac edema were observed in 20 to 50 % of larvae exposed to 80 and 100 μg/L and 80 to 100 % of larvae exposed to 1000 and 10000 μg/L of endosulfan sulfate (the highest concentrations tested). A curved body axis was visible in 33 to 80 % of larvae exposed to the highest concentrations of endosulfan sulfate and in 33 to 60 % of larvae wavy notochords were also apparent. Like larvae exposed to the highest concentrations of endosulfan I, those exposed to the highest concentrations of endosulfan sulfate showed a less sensitive response to touch, reduced movement, and in some cases paralysis. Mortality occurred in less than 20 % of the larvae exposed to the highest concentrations of endosulfan I and endosulfan sulfate.
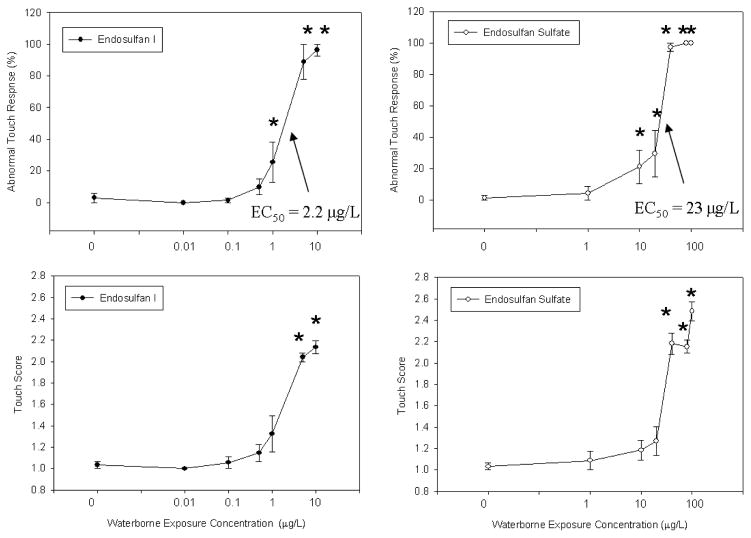
Preliminary range finding experiments indicated that endosulfan I and endosulfan sulfate induced abnormal behavior and reduced touch response in larval zebrafish. Endosulfan I and endosulfan sulfate showed the same progression of abnormal behaviors at the highest concentrations tested (100 and 1000 μg/L of endosulfan I and 1000 and 10,000 μg/L of endosulfan sulfate) during the range finding experiments, that is, fish first exhibited signs with a score of 2 followed by more severe symptoms with a score of 3, 4, and 5 as exposure time progressed (Table 1). The abnormal touch response was the most sensitive toxicity endpoint, that is, it is the response that was observed at the earliest developmental time point and at the lowest concentration, in zebrafish exposed to endosulfan I or endosulfan sulfate. Further concentration response studies more accurately determined the nominal exposure concentration and time point at which the abnormal touch response were produced (Fig. 1). An abnormal touch response was recorded in 100% of tested individuals exposed to a nominal concentration of 5 μg/L of endosulfan I and 40 μg/L of endosulfan sulfate at 72 hpf and there were no overt developmental abnormalities in the larvae from these exposure groups through 120 hpf. The concentration was significantly related to the abnormal touch response (one way ANOVA) and the endosulfan I and endosulfan sulfate curves were significantly different when tested using linear regression (average of the 3 replicate experiments was used (Fig. 1); endosulfan I and endosulfan II were indicator variables). The EC50 for each endosulfan I and endosulfan sulfate replicate experiment was calculated using the following equations: for endosulfan I, y=100(1+e^(−(x−1.1)/0.28)), y=89(1+ e^(−(x−4.1)/0.83)), y=100(1+ e^(−(x−1.2)/0.34)) and for endosulfan sulfate, y=100(1+ e^(−(x−20)/7.8)), y=100(1+ e^(−(x−30)/34)), y=102(1+ e^(−(x−20)/8.6)). The average abnormal touch response EC50 for endosulfan I and endosulfan sulfate were determined to be 2.2 ± 1.8 and 23 ± 5.8, respectively (Fig. 1). The no observable adverse effect concentration (NOAEC), determined from the highest concentration not significantly different from the control, was 0.5 μg/L for endosulfan I and 1 μg/L for endosulfan sulfate (Fig. 1). The lowest observable adverse effect concentration (LOAEC), determined from the lowest concentration significantly different from the control, was 1 μg/L for endosulfan I and 10 μg/L for endosulfan sulfate (Fig. 1). Observed abnormal behaviors included prolonged swimming and disorientation, spastic swimming behavior and in a few cases a slower response and shorter distance swam in response to touch (Fig. 1).
Fig. 1.
Concentration response curve for the nominal waterborne exposure concentration versus the % incidence of abnormal touch response at 72 hpf for endosulfan I and endosulfan sulfate and the nominal waterborne exposure concentration versus the touch score for abnormal touch response at 72 hpf for endosulfan I and endosulfan sulfate, averaged across 3 experiments. The error bars represent the standard error of the mean. * Indicates significantly different from control group (p ≤ 0.01).
3.2 Concentrations of endosulfan I and endosulfan sulfate in exposure water and zebrafish tissue
The analytical methods for measuring endosulfan I and endosulfan sulfate in water and tissue provided good accuracy and precision as determined by the average recovery and relative standard deviation (RSD) from the triplicate spike and recovery experiment. Spiked water samples had an average recovery of 87 % ± 2.3 (RSD) for endosulfan I and 105 % ± 3.6 for endosulfan sulfate, and fish samples had an average recovery of 91 % ± 7.8 for endosulfan I and 56 % ± 3.0 for endosulfan sulfate.
The endosulfan I and endosulfan sulfate water exposure solutions were measured 3 times over the course of the 5 day exposure by collecting water samples immediately following their preparation (0hpf, 48hpf, and 96hpf). Endosulfan I and endosulfan sulfate were not detected in fish or water samples of the control groups. The measured concentrations for the nominally prepared exposure concentrations of 0.1 μg/L, 2μg/L, and 10 μg/L endosulfan I were 0.12 ± 0.044 μg/L, 1.7 ± 0.75 μg/L, and 7.0 ± 4.0 μg/L, respectively. The measured concentrations for the nominally prepared exposure concentrations of 1 μg/L, 20μg/L, and 40 μg/L endosulfan sulfate were 1.6 ± 0.60 μg/L, 18 ± 7.3 μg/L, and 51 ± 19 μg/L, respectively.
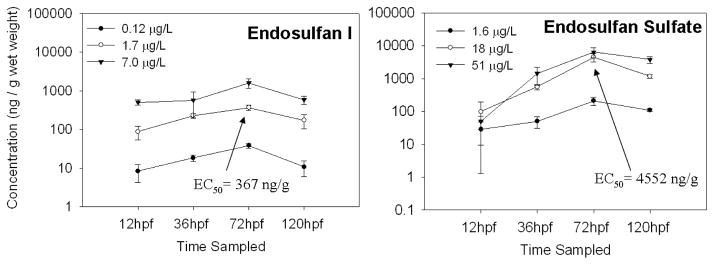
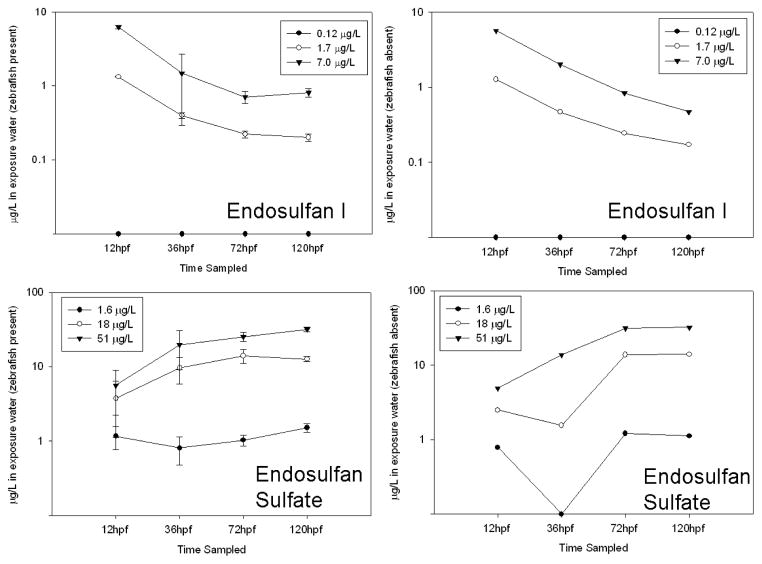
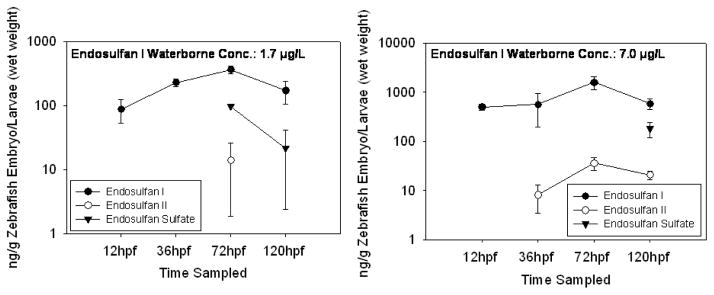
The embryos and larvae accumulated high concentrations of endosulfan I and endosulfan sulfate, 30 to 5300 and 7 to 160 times the exposure concentrations, respectively, over the course of the 5 day exposure (Fig. 2). The concentrations appeared to stabilize in water and fish by 120 hpf (Fig. 2 and Fig. 3), and thus the bioconcentration factor (BCF) for was calculated. To obtain BCF, the concentration in the fish was divided by the concentration in the exposure water at 120 hpf for each triplicate sample in each exposure group, triplicate samples were averaged, and then the results were averaged for all exposure groups. BCF was 94 ± 9.9 for endosulfan I and 69 ± 5.4 for endosulfan sulfate. The estimated EC50 for abnormal touch response in larvae (72 hpf), determined from the measured tissue concentrations, was 370 ± 54.6 for endosulfan I and 4600 ± 1300 ng/g wet weight (w.w.) for endosulfan sulfate. The estimated NOAEC, determined from the measured tissue concentrations in the low exposure groups, was 38 ± 5.2 for endosulfan I and 210 ± 60 ng/g w.w. for endosulfan sulfate. The embryos at 36 hpf and larvae at 72 hpf and 120 hpf appear to be capable of metabolizing endosulfan I. Endosulfan II was measured in fish exposed to 7.0 μg/L at 36 hpf, in fish exposed to 1.7 and 7.0 μg/L at 72 hpf and in fish exposed to 7.0 μg/L endosulfan I at 120 hpf (Fig. 6). Endosulfan sulfate was measured in fish exposed to 1.7 μg/L at 72 hpf and fish exposed to 1.7 and 7.0 μg/L endosulfan I at 120 hpf (Fig. 4). The fish also excreted some endosulfan I, at 120hpf a concentration of 0.12 ± 0.10 μg/L was measured in the 1.7 μg/L exposure group and 0.91 ± 0.30 μg/L was measured in the 7.0 μg/L exposure group. The measured endosulfan I and endosulfan sulfate water concentrations showed similar patterns when zebrafish were present and zebrafish were absent (Fig. 3). Endosulfan sulfate concentrations in the wells with and without the zebrafish present were lower than the exposure water concentrations during the first 72 hpf of the endosulfan sulfate experiment (Fig. 3). The concentrations in the water appeared to stabilize by 120 hpf and reached concentrations closer to exposure water concentrations (Fig. 3). In addition to uptake by the organisms, some of the loss of endosulfan I and sulfate in the water was attributed to loss by sorption to the polypropylene wells and/or hydrolysis (Guerin and I.R., 1992; Isaacson et al., 2007).
Fig. 2.
The average concentration (ng/g) in zebrafish embryos and larvae sampled over the course of a 5 day exposure to endosulfan I and endosulfan sulfate. Endosulfans were not detected in control fish or water. The bars represent the standard deviation; n = 3. The tissue EC50 for abnormal touch response is shown.
Fig. 3.
The average concentration (μg/L) in exposure water, with and without the embryos/larvae present, over the course of a 5 day exposure to endosulfan I and endosulfan sulfate. Endosulfans were not detected in control fish or water. Zebrafish present: the bars represent the standard deviation; n = 3, zebrafish absent: n = 1. Data points along the X-axis represent data below the detection limit.
Fig. 4.
The average concentration of metabolites (endosulfan II and endosulfan sulfate) of endosulfan I in zebrafish embryos and larvae (ng/g) in exposure water (μg/L) over the course of a 5 day exposure to endosulfan I. Endosulfans were not detected in control fish or water. The bars represent the standard deviation; n = 3, no bars present n = 1. Only detections are shown.
4. Discussion
These studies were aimed at determining if endosulfan I and II were developmentally toxic to zebrafish. The results indicated that abnormal touch response was the most sensitive endpoint, indicating potential neurotoxicity, following endosulfan I and endosulfan sulfate exposure. This was the first study, to our knowledge, that investigated developmental neurotoxicity of these compounds in fish. Behavioral abnormalities, associated with neurotoxicity, that resulted from endosulfan I and sulfate included extended periods of swimming and spastic behavior at the lower concentrations and reduced motility and paralysis at the highest concentrations tested.
An endosulfan LC50 for adult zebrafish was reported as 1.6 μg/L compared to 0.8 μg/L for rainbow trout (Oncorhychus mykiss) and 1.7 μg/L for the bluegill sunfish (Lepomis macrochirus) (Jonsson and Toledo, 1993; EPA, 2001). At the highest concentrations tested in this study, 1000 μg/L of endosulfan I and 10,000 μg/L of endosulfan sulfate, some larvae were paralyzed as a result of exposure. However, the occurrence of mortality was low. The lack of mortality in the developing zebrafish is likely due to the ability of the developing zebrafish to obtain oxygen through cutaneous respiration (Rombough, 2002) even after paralysis prevented gill ventilation.
Endosulfan, like other cyclodiene insecticides, has been proposed to cause neurotoxicity through GABA-gated chloride channel inhibition (Naqvi and Vaishnavi, 1993; ATSDR, 2000; Jia and Misra, 2007). Inhibition of these channels results in excitation because the neuron is unable to repolarize (Jia and Misra, 2007). Associated symptoms of neurotoxicity include convulsions and eventual paralysis. Studies in rats have shown that endosulfan I and II inhibit the influx of chloride and GABA-induced chloride influx across rat brain membranes, with endosulfan I being a more potent inhibitor than endosulfan II (Abalis et al., 1986; Gant et al., 1987). A mutation in an insect GABA receptor subunit gene has been shown to provide resistance to cyclodiene, including endosulfan, toxicity in some insects (Ffrench-Constant et al., 2000).
Although GABA-gated inhibition is widely suggested as the molecular endpoint underlying endosulfan neurotoxicity, the molecular mechanism has yet to be confirmed. This work provides a basis to begin investigations to elucidate the mechanism of endosulfan I and endosulfan sulfate neurotoxicity in developing zebrafish.
Endosulfan I is 10 times more toxic than endosulfan sulfate to zebrafish. For endosulfan I the BCF was calculated to be 94 ± 9.9 and for endosulfan sulfate it was calculated to be 69 ± 5.4. In comparison to the BCF for the technical mixture of endosulfan in adult zebrafish, 2650 (Toldeo and Jonsson, 1993), the measured BCF for endosulfan I and endosulfan sulfate is low. This may be the result of differences in skin, gill or gut uptake, and metabolism between adult and larvae; for example larvae may have less uptake from the gills than adults; little is known about the time of gill development in zebrafish (Rombough, 2002; ZFIN, 2008).
Developing zebrafish metabolized endosulfan I to endosulfan II at 36 hpf and to endosulfan sulfate at 72 hpf (Fig. 4). The sulfate is formed through oxidation by cytochrome P450s (CYP) (Lee et al., 2006). In humans metabolism was reported to occur via enzyme isoforms CYP3A4 and CYP2B6 (Lee et al., 2006). Developing organisms have large numbers of P450s (Juchau et al., 1992; Stoilov, 2001). CYP1A1 was detected in developing zebrafish as early as 36 hpf (Andreasen et al., 2002), supporting our findings that some metabolism of endosulfan I occurred at 36 hpf and cytochrome P450s may be involved.
Total endosulfan (I, II, and sulfate) concentrations in fish from agricultural areas were detected up to 310 ng/g w.w. and in remote areas concentrations were < 10 ng/g w.w. (Nowak, 1990; Ackerman et al., 2008). The tissue NOAEC and EC50 for developmental toxicity for endosulfan I was within the range measured in fish from agricultural areas and, at a minimum, 4 (NOAEC) and 37 (EC50) times higher than concentrations measured in remote ecosystems. The tissue NOAEC for endosulfan sulfate was within the range measured in fish from agricultural areas and 20 times higher than measured concentrations in fish from remote ecosystems. The EC50 for endosulfan sulfate was, at a minimum 15 times higher than fish tissue concentrations measured in agricultural areas and 450 times higher than measured concentrations in fish from remote ecosystems. The endosulfan I NOAEC and EC50 and the endosulfan sulfate NOAEC in this study were within the range of endosulfan concentrations measured in fish from agricultural areas. Thus, the current work demonstrated the potential for behavioral effects after exposure to endosulfan in the environment.
Acknowledgments
The authors thank Jane LaDu, and Eric Johnson and SARL for caring for the fish and for spawning and collection of embryos. Funding was provided in part by National Institute of Health (NIH)/National Institute of Environmental Health Sciences (NIEHS) grant ES00210. Funding was also provided by the John Hopkins Center for the Alternatives to Animal Testing.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abalis IM, Eldefrawi ME, Eldefrawi AT. Effects of insecticides on GABA-induced chloride influx into rat brain microsacs. J Toxicol Environ Health. 1986;18:13–23. doi: 10.1080/15287398609530844. [DOI] [PubMed] [Google Scholar]
- Ackerman LK, Schwindt AR, Massey Simonich SL, Koch DC, Blett TF, Schreck CB, Kent ML, Landers DH. Atmospherically deposited PBDEs, pesticides, PCBs, and PAHs in Western U.S. National Park fish: Concentrations and consumption guidelines. Environ Sci Technol. 2008;42:2334–2341. doi: 10.1021/es702348j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen EA, Spitsbergen JM, Tanguay RL, Stegeman JJ, Heideman W, Peterson RE. Tissue-Specific Expression of AHR2, ARNT2, and CYP1A in Zebrafish Embryos and Larvae: Effects of Developmental Stage and 2,3,7,8-Tetrachlorodibenzo-p-dioxin Exposure. Toxicol Sci. 2002;68:403–419. doi: 10.1093/toxsci/68.2.403. [DOI] [PubMed] [Google Scholar]
- ATSDR. Agency for Toxic Substances and Disease Registry (ATSDR), Toxicological Profile for Endosulfan. 2000 http://www.atsdr.cdc.gov/toxprofiles/tp41.html. [PubMed]
- Berntssen MHG, Glover CN, Robb DHF, Jakobsen JV, Petri D. Accumulation and elimination kinetics of dietary endosulfan in Atlantic salmon (Salmo salar) Aquat Toxicol. 2008;86:104–111. doi: 10.1016/j.aquatox.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Cabaleiro T, Caride A, Romero A, Lafuente A. Effects of in utero and lactational exposure to endosulfan in prefrontal cortex of male rats. Toxicol Letters. 2008;176:58–67. doi: 10.1016/j.toxlet.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Carrera G, Fernandez P, Grimalt JO, Ventura M, Camarero L, Catalan J, Nickus U, Thies H, Psenner R. Atmospheric deposition of organochlorine compounds to remote high mountain lakes of Europe. Environ Sci Technol. 2002;36:2581–2588. doi: 10.1021/es0102585. [DOI] [PubMed] [Google Scholar]
- Devi AP, Rato DMR, Tilak KS, Murty AS. Relative toxicity of the technical grade material, isomers, and formulations of endosulfan to the fish Channa punctata. Bull Environ Contam and Toxicol. 1981;27:239–243. doi: 10.1007/BF01611015. [DOI] [PubMed] [Google Scholar]
- EPA, 2007, U.S. Environmental Protection Agency (EPA), Summary of “A Developmental Neurotoxicity Study with Technical Grade Endosulfan in Wistar Rats” by Gilmore et al. 2006 Bayer CropScience. MRID#46968301. http://www.regulations.gov/fdmspublic/component/main?main=DocketDetail&d=EPA-HQ-OPP-2002-0262
- EPA. U.S. Environmental Protection Agency (EPA), Overview of Endosulfan Risk Assessment. 2001 http://www.epa.gov/oppsrrd1/reregistration/endosulfan/endosulfan_finaloverview.pdf.
- Ffrench-Constant RH, Anthony N, Aronstein K, Rocheleau T, Stilwell G. Cyclodiene insecticide resistance: from molecular to population genetics. Annu Rev Entomol. 2000;45:449–466. doi: 10.1146/annurev.ento.45.1.449. [DOI] [PubMed] [Google Scholar]
- Gant DB, Eldefrawi ME, Eldefrawi AT. Cyclodiene insecticides inhibit GABAA receptor-regulated chloride transport. Toxicol Appl Pharmacol. 1987;88:313–321. doi: 10.1016/0041-008x(87)90206-7. [DOI] [PubMed] [Google Scholar]
- Guerin TF, IRK Distribution and dissipation of endosulfan and related cyclodienes in sterile aqueous systems: Implications for studies on biodegredation. J Agric Food Chem. 1992;40:2315–2323. [Google Scholar]
- Hill AJ, Teraoka H, Heideman W, Peterson RE. Zebrafish as a Model Vertebrate for Investigating Chemical Toxicity. Toxicol Sci. 2005;86:6–19. doi: 10.1093/toxsci/kfi110. [DOI] [PubMed] [Google Scholar]
- Hose GC, Lim RP, Hyne RV. The transport, fate and effects of endosulfan in the Australian freshwater environment. Australas J Ecotoxicol. 2003;9:101–111. [Google Scholar]
- Isaacson CW, Usenko CY, Tanguay RL, Field JA. Quantification of Fullerenes by LC/ESI-MS and Its Application to in Vivo Toxicity Assays. Anal Chem. 2007;79:9091–9097. doi: 10.1021/ac0712289. [DOI] [PubMed] [Google Scholar]
- Jia Z, Misra HP. Developmental exposure to pesticides zineb and/or endosulfan renders the nigrostriatal dopamine system more susceptible to these environmental chemicals later in life. NeuroToxicol. 2007;28:727–735. doi: 10.1016/j.neuro.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Jonsson CM, Toledo MCF. Acute toxicity of endosulfan to the fish Hyphessobrycon bifasciatus and Brachydanio rerio. Arch Environ Contam Toxicol. 1993;24:151–155. [Google Scholar]
- Juchau MR, Lee QP, Fantel AG. Xenobiotic biotransformation/bioactivation in organogenesis-stage conceptual tissues: implications for embryotoxicity and teratogenesis. Drug Metab Rev. 1992;24:195–238. doi: 10.3109/03602539208996293. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, BWW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Lee HK, Moon JK, Chang CH, Choi H, Park HW, Park BS, Lee HS, Hwang EC, Lee YD, Liu KH, Kim JH. Stereoselective metabolism of endosulfan by human liver microsomes and human cytochrome P450 isoforms. Drug Metab Dispos. 2006;34:1090–1095. doi: 10.1124/dmd.105.009134. [DOI] [PubMed] [Google Scholar]
- Lele Z, Krone PH. The zebrafish as a model system in developmental, toxicological and transgenic research. Biotechnol Adv. 1996;14:57–72. doi: 10.1016/0734-9750(96)00004-3. [DOI] [PubMed] [Google Scholar]
- LeNoir JS, McConnell LL, Fellers GM, Cahill TM, Seiber JN. Summertime transport of current-use pesticides from California’s Central Valley to the Sierra Nevada Mountain Range, USA. Environ Toxicol Chem. 1999;18:2715–2722. [Google Scholar]
- Leonard AW, Hyne RV, Lim RP, Leigh KA, Le J, Beckett R. Fate and Toxicity of Endosulfan in Namoi River Water and Bottom Sediment. J Environ Qual. 2001;30:750–759. doi: 10.2134/jeq2001.303750x. [DOI] [PubMed] [Google Scholar]
- McConnell LL, LeNoir JS, Datta S, Seiber JN. Wet deposition of current-use pesticides in the Sierra Nevada Mountain Range, California, USA. Environ Toxicol Chem. 1998;17:1908–1916. [Google Scholar]
- Naqvi SM, Vaishnavi C. Bioaccumulative potential and toxicity of endosulfan insecticide to non-target animals. Comp Biochem Physiol. 1993;105C:347–361. doi: 10.1016/0742-8413(93)90071-r. [DOI] [PubMed] [Google Scholar]
- Nowak B. Residues of endosulfan in the livers of wild catfish from a cotton growing area. Environ Monit Assess. 1990;14:347–351. doi: 10.1007/BF00677926. [DOI] [PubMed] [Google Scholar]
- Rombough P. Gills are needed for ionoregulation before they are needed for O2 uptake in developing zebrafish, Danio rerio. J Exp Biol. 2002;205:1787–1794. doi: 10.1242/jeb.205.12.1787. [DOI] [PubMed] [Google Scholar]
- Rosenthal H. Sublethal effects of environmental stressors, natural and pollution, on marine fish eggs and larvae. J Fish Res Board Can. 1976;33:2047–2065. [Google Scholar]
- Simonich S, Hites R. Global distribution of persistent organochlorine compounds. Science. 1995;269:1851–1854. doi: 10.1126/science.7569923. [DOI] [PubMed] [Google Scholar]
- Spitsbergen JM, Kent ML. The State of the Art of the Zebrafish Model for Toxicology and Toxicologic Pathology Research--Advantages and Current Limitations. Toxicol Pathol. 2003;31:62–87. doi: 10.1080/01926230390174959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern GA, Braekevelt E, Helm PA, Bidleman TF, Outridge PM, Lockhart WL, McNeeley R, Rosenberg B, Ikonomou MG, Hamilton P, Tomy GT, Wilkinson P. Modern and historical fluxes of halogenated organic contaminants to a lake in the Canadian arctic, as determined from annually laminated sediment cores. Sci Total Environ. 2005;342:223–243. doi: 10.1016/j.scitotenv.2004.12.046. [DOI] [PubMed] [Google Scholar]
- Stoilov I. Cytochrome P450s: coupling development and environment. Trends Genet. 2001;17:629–632. doi: 10.1016/s0168-9525(01)02444-1. [DOI] [PubMed] [Google Scholar]
- Toldeo MCF, Jonsson CM. Bioaccumulation and elimination of endosulfan in Zebrafish (Brachydanio rerio) Pestic Sci. 1993;36:207–211. [Google Scholar]
- Usenko S, Hageman KJ, Schmedding DW, Wilson GR, Simonich SL. Trace analysis of semivolatile organic compounds in large volume samples of snow, lake water, and groundwater. Environ Sci Technol. 2005;39:6006–6015. doi: 10.1021/es0506511. [DOI] [PubMed] [Google Scholar]
- Usenko S, Landers DH, Appleby PG, Simonich SL. Current and historical deposition of PBDEs, pesticides, PCBs, and PAHs to Rocky Mountain National Park. Environ Sci Technol. 2007;41:7235–7241. doi: 10.1021/es0710003. [DOI] [PubMed] [Google Scholar]
- Vilanova R, Fernandez P, Martinez C, Grimalt JO. Organochlorine Pollutants in Remote Mountain Lake Waters. J Environ Qual. 2001;30:1286–1295. doi: 10.2134/jeq2001.3041286x. [DOI] [PubMed] [Google Scholar]
- Wan MT, Kuo J-n, Buday C, Schroeder G, Aggelen GV, Pasternak J. Toxicity of alpha-, beta-, and (alpha + beta)-endosulfan and their formulated and degredation products to Daphnia magna, Hyalella azteca, Oncorhynchus mykiss, Oncorhynchus kisutch, and biological implications in streams. Environ Toxicol Chem. 2005a;24:1146–1154. doi: 10.1897/04-300r1.1. [DOI] [PubMed] [Google Scholar]
- Wan MT, Kuo J-n, Pasternak J. Residues of Endosulfan and Other Selected Organochlorine Pesticides in Farm Areas of the Lower Fraser Valley, British Columbia, Canada. J Environ Qual. 2005b;34:1186–1193. doi: 10.2134/jeq2004.0361. [DOI] [PubMed] [Google Scholar]
- ZFIN. Anatomical Development: Gills. University of Oregon; Eugene, Oregon: 2008. Zebrafish Information Network (ZFIN) http://zfin.org/action/anatomy/term-detail?anatomyItem.zdbID=ZDB-ANAT-011113-279. [Google Scholar]