Abstract
Oxidative damage contributes to cone cell death in retinitis pigmentosa and death of rods, cones, and retinal pigmented epithelial (RPE) cells in age-related macular degeneration. In this study, we explored the strategy of overexpressing components of the endogenous antioxidant defense system to combat oxidative damage in RPE cells and retina. In transfected cultured RPE cells with increased expression of superoxide dismutase1 (SOD1) or SOD2, there was increased constitutive and stress-induced oxidative damage measured by the level of carbonyl adducts on proteins. In contrast, RPE cells with increased expression of glutathione peroxidase 1 (Gpx1) or Gpx4 did not show an increase in constitutive oxidative damage. An increase in Gpx4, and to a lesser extent Gpx1, reduced oxidative stress-induced RPE cell damage. Co-expression of Gpx4 with SOD1 or 2 partially reversed the deleterious effects of the SODs. Transgenic mice with inducible expression of Gpx4 in photoreceptors were generated, and in three models of oxidative damage-induced retinal degeneration, increased expression of Gpx4 provided strong protection of retinal structure and function. These data suggest that gene therapy approaches to augment the activity of Gpx4 in the retina and RPE should be considered in patients with retinitis pigmentosa or age-related macular degeneration. Antioxid. Redox Signal. 11, 715–724.
Introduction
Retinal photoreceptors are packed with mitochondria and have extremely high metabolic activity and oxygen consumption. Since run-off from the electron transport chain is a major source of oxidative stress, photoreceptors are challenged under normal circumstances. In patients with retinitis pigmentosa (RP), one of a number of different mutations causes death of rods which drastically reduces oxygen consumption and elevates oxygen levels in the outer retina (16). Prolonged exposure to high levels of oxygen causes progressive oxidative damage to cones (14), and their gradual death results in progressive constriction of visual fields and eventual blindness. Antioxidants significantly slow cone cell death in several models of RP (8, 9); therefore, clinical trials investigating the effects of antioxidants in patients with RP are being planned.
Oxidative damage has also been implicated in another highly prevalent eye disease, age-related macular degeneration (AMD). One of the first hints came from epidemiologic studies that showed a negative correlation between the presence of AMD and consumption of a diet rich in antioxidants. This led to the Age-Related Eye Disease Study (AREDS) in which it was shown that antioxidant vitamins and/or zinc reduced the risk of progression to advanced AMD and severe loss of vision (1). The protective effects of AREDS formulation is clinically meaningful and it is now part of standard care in AMD patients with phenotypic characteristics associated with a high risk of progression; however, despite its use there are still large number of patients that develop advanced AMD. Reactive oxygen species are continuously generated in different cellular compartments and rapidly interact with critical host macromolecules unless they are intercepted. Oral administration of antioxidants is a relatively inefficient way to counter the constant bombardment by reactive oxygen species (ROS). A complementary strategy is to increase expression of components of the endogenous antioxidant defense system. But there are several components of the antioxidant defense system, and it is difficult to know which component might be best for a particular application without systematic testing. We have previously demonstrated that superoxide dismutase 1 (SOD1) is an important component of the antioxidant defense system in the retina, because compared to the retinas of wild-type mice, those from mice deficient in SOD1 show high basal levels of oxidative damage and more extensive retinal degeneration when challenged by exposure to oxidants (6). Transgenic mice with increased expression of SOD1 driven by the β-actin promoter showed partial protection of the retina from severe oxidative stress compared to wild-type mice, but also showed increased basal oxidative stress. This study provided proof-of-concept for the overall approach of bolstering the endogenous antioxidant defense system for treatment of oxidative damage-induced retinal degeneration, but left doubt as to whether SOD1 is the best transgene candidate.
The SODs convert superoxide radicals to hydrogen peroxide which is then metabolized by glutathione peroxidases (Gpx) and catalase. In this study, we compared the effects of overexpressing SOD1, SOD2, Gpx1, and Gpx4 in RPE cells exposed to various types of oxidative stress. Cells expressing Gpx4 were particularly well-protected against oxidative stress, and therefore the effect of increased expression of Gpx4 in photoreceptors of the retina was also examined.
Materials and Methods
Construction of expression plasmids
The pIRES2-EGFP vector (BD Biosciences Clontech, Mountain View, CA) was used as the expression vector in RPE cells. The primers for construction were mouse Gpx1: forward: 5′ GCCTCGAGATGTGTGCTGCTCGGCTCTC 3′, reverse: 5′ GCGGATCCTTAGGAGTTGCCAGACTGCT 3′, mouse Gpx4: forward: 5′ GCCTCGAGATGTGTGCATCCCGCGATGA 3′, reverse: 5′ GCGGATCCCTAGAGATAGCACGGCAGGT 3′, mouse Sod1: forward, ATGGCGATGAAAGCGGTGTGC, reverse: 5′ TTACTGCGCAATCCCAATCAC 3′, mouse Sod2, forward: 5′ ATGTTGTGTCGGGCGGCGTGC 3′, reverse; 5′ TCACTTCTTGCAAGCTGTGTA 3′. Fragments of DNA containing full-length murine Gpx1, Gpx4, Sod1, or Sod2 were subcloned into pGEM-T vector (Promega, Madison, WI). Each construct was sequenced to confirm the correct sequence and then excised from pGEM-T and ligated into pIRES2-EGFP expression vector. The expression vectors were used in transient transfections in ARPE19 cells (American Type Culture Collection, Manassas, VA) using Lipofectamin (Invitrogen Corp., Carlsbad, CA). Control cells were prepared by transfection with pIRES2-EGFP vector that did not contain an insert.
Cell culture
Transfected and control cells were grown in Dulbecco's Modified Eagles's Medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin (all from Invitrogen Corp, Carlsbad, CA) at 37°C and 5% CO2. Confluent cells were washed and placed in growth medium supplemented with or without 7 mM paraquat (Aldrich, Wilwaukee, WI), or 0.5 mM H2O2 (Sigma, St. Louis, MO) for 1 day. To expose cells to hyperoxia, cells were grown to confluence in a 25 cm2 flask, which was filled with 100% oxygen for 1 min, then the cap was loosened and the flask was returned to the 5% CO2 incubator. This was repeated twice a day until the cells were scraped into lysis buffer and collected as described previously (11).
Cell viability
Cells were plated (50,000 cells per well) in 96-well plates and after attachment they were transiently transfected with one of the experimental or control expression vector. The following day, the transfected cells were incubated with 7 mM paraquat or 0.5 mM H2O2 for 24 h. The medium was then replaced with normal growth medium. The number of viable cells was determined with the methylthiazoletetrazolium (MTT) cell viability assay kit (American Type Culture Collection, Manassas, VA), which determines the number of viable cells by bioreduction of MTT into a colored formazan product which is detected by absorbance at 590 nm with a 96-well plate reader.
ELISA for protein carbonyl content
Cells were scraped into lysis buffer (10 mM Tris-HCl, pH 7.2, 50 mM NaCl, 1 mM EDTA 0.5% Triton X-100). One proteinase inhibitor cocktail tablet (Roche, Indianapolis, IN) was added to each 10 ml of lysis buffer. Mouse retina was dissected and placed into lysis buffer. Cells or retinas were vortexed and freeze-thawed three times, centrifuged at 16,000 g for 10 min at 4°C, and supernatants were collected and protein concentrations were determined using the BCA protein assay kit (BioRad, Hercules, CA). Protein concentrations were adjusted to 4 mg/ml by dilution with TBS, and protein carbonyl content was measured by ELISA as previously described (5, 11). Briefly, cell or retinal lysates (15 μl of 4 mg/ml) were incubated with 45 μl of 10 mM 2, 4-dinitrophenylhydrazine (DNPH; Sigma, St. Louis, MO) in 6 M guanidine-HCl, 0.5 M potassium phosphate, pH 2.5 for 45 min at room temperature, mixing every 15 min. Five μl of each sample was then added to 995 μl of PBS and 200 μl aliquots were added to triplicate wells of a 96-well plate with a MaxiShorp surface (Nalgene Nunc International, Rochester, NY), and incubated overnight at 4°C. Dilutions of oxidized bovine serum albumin (BSA) were also added to triplicate wells to generate a standard curve. Oxidized BSA was prepared and determined as described (5, 10). Unbound protein was washed away with PBS (3 × 400 μl) and nonspecific sites were blocked for 2 h at 37°C with 250 μl per well of 0.1% reduced BSA in PBS. After five washes with 400 μl of phosphate-buffered saline (PBS), the wells were incubated with 200 μl of anti-DNPH mouse monoclonal IgE (1:1,000 dilution in PBS with 0.1% reduced BSA and 0.1% Tween 20; Sigma, St. Louis, MO) at room temperature for 1 h with shaking. After three washes with PBS, 200 μl of rat anti-mouse monoclonal IgE conjugated to alkaline phosphatase (1:2,000 dilution in PBS with 0.1% reduced BSA and 0.1% Tween 20; Southern Biotechnology Associates. Inc, Birmingham, AL) was added to each well and incubated at room temperature for 1 h. After three washes with PBS and three washes with alkaline phosphatase buffer (100 mM NaCl, 5 mM MgCl2, 100 mM Tris-HCl, pH 9.5), 200 μl of paranitrophenyl phosphate (pNPP, Sigma, St. Louis, MO, 2 mg/ml in alkaline phosphatase buffer) was added to each well and incubated at 37°C for 30 min. The absorbance was measured at 405 nm using a 96-well plate reader. The carbonyl content (nmol/mg protein) of cell lysates was calculated using the oxidized BSA standard curve.
Construction of double transgenic mice with inducible expression of Gpx4
A 529 bp BamH I and Hind III fragment containing full-length murine Gpx4 cDNA was subcloned into pGEM-T vector (Promega, Madison, WI) and then excised and ligated into pTRE2 (Clontech. Mountain View, CA) containing the tetracycline response element (TRE). After transformation, a clone with correct orientation of the Gpx4 fragment was identified by DNA sequencing. Purified DNA was linearized with Aat II and SpaI, yielding a 2437 bp TRE2/Gpx4/β-globin poly A fusion gene. The fusion gene was purified and transgenic mice were generated by Johns Hopkins Transgenic Mouse Core Laboratory. Mice were screened by polymerase chain reaction (PCR) of tail DNA using an upstream primer in the TRE domain (5′ CACGCTGT TTTGACCTCC 3′) and a downstream primer in the Gpx4 domain (5′ GTCTGGCAACTCCTAA 3′). Tail DNA was obtained by digestion of a 1-cm tail segment in 0.4 ml of 50 mM Tris-HCl, pH 7.5. 400 mM NaCl, 20 mM EDTA, and 0.1% sodium dodecyl sulfate with 5 μl of 20 mg/ml proteinase K, at 55°C. Founders of transgenic TRE2/Gpx4 mice were crossed with C57BL/6 mice to obtain independent lines of TRE2/Gpx4 transgenic mice and crossed with homozygous opsin promoter/reverse tetracycline transactivator (opsin/rtTA) transgenic mice that have been previously described (3, 12) to yield opsin/rtTA-TRE/Gpx4 (Tet/opsin/Gpx4) double transgenic mice. The expression level of Gpx4 was assessed by Western blot analysis after treatment with 2 mg/ml of doxycycline in drinking water for 2 weeks.
Western blot analysis
Retinal or RPE cell lysates containing 50 μg of protein were subjected to SDS-PAGE using 12% polyacrylamide resolving gel (BioRad). After electrophoresis, the slab gel was transferred onto a nitrocellulose membrane (Amersham, Piscataway, NJ). The membrane was incubated with rabbit anti-Gpx4 polyclonal antibody (1:1,000, Cayman, Ann Arbor, MI), followed by incubation with horseradish peroxidase conjugated to goat anti-rabbit IgG (1:2,000, Sigma). For SOD2, the membrane was incubated with goat anti-SOD2 polyclonal antibody (1:1,000, Santa Cruz Biotechnology, Santa Cruz, CA), and followed by incubation with horseradish peroxidase conjugated to rabbit anti-goat IgG (1:2,000, Sigma). Chemiluminescence reaction product was detected using the ECL kit (Amersham). To assess loading levels of protein, blots were incubated with rabbit anti-actin polyclonal antibody (1:1,000, Sigma), followed by incubation with horseradish peroxidase conjugated to goat anti-rabbit IgG (1:2,000, Sigma).
Paraquat model of oxidative damage-induced retinal degeneration
Tet/opsin/Gpx4 mice were tested in the paraquat model of oxidative damage-induced retinal degeneration (4) using techniques similar to those previously described (6). Briefly, double hemizygous transgenic mice were given unsupplemented drinking water (controls) or water containing 2 mg/ml of doxycycline, and after 2 weeks a 1 μl intraocular injection of 0.75 mM paraquat (Sigma) was done in the left eye and 1 μl of PBS was injected in the right eye. Electroretinograms (ERGs) were done 1 and 8 days after injection. After 2 weeks, the mice were euthanized and protein carbonyl content was measured in the retinas of some mice, while outer nuclear layer thickness was measured in others.
Hyperoxia-induced oxidative damage
Tet/opsin/Gpx4 mice were tested in a model of hyperoxia-induced retinal degeneration (6, 13, 15). Double hemizygous Tet/opsin/Gpx4 mice from the same litters received unsupplemented water or water containing 2 mg/ml of doxycycline. As an additional control, wild-type C57BL/6 mice. All were exposed to 75% oxygen for 2 weeks and then had ERGs and were euthanized for measurement of carbonyl protein content and measurement of outer nuclear layer (ONL) thickness.
Recording of ERGs
Scotopic ERGs were recorded (Espion ERG; Diagnosys LLL, Littleton, MA), as previously described (13). Briefly, mice were dark adapted overnight and anesthetized with an intraperitoneal injection of ketamine and xylazine. Pupils were dilated with Midrin P consisting of 0.5% tropicamide and 0.5%. phenylephrine hydrochloride (Santen Pharmaceutical Co., Osaka, Japan). The mice were placed on a pad heated to 39°C and platinum loop electrodes were placed on each cornea after application of gonioscopic prism solution (Alcon Laboratories, Fort Worth, TX). A reference electrode was placed subcutaneously in the anterior scalp between the eyes, and a ground electrode was inserted into the tail. The head of the mouse was held in a standardized position in a Ganzfeld bowl illuminator that ensured equal illumination of the eyes. Recordings for both eyes were made simultaneously with electrical impedance balanced. The a-wave was measured from the baseline to the negative peak and the b-wave was measured from peak to peak. An average was calculated from six measurements at eleven intensity levels of white light ranging from −3.00 to +1.40 log cd-s/m2.
Measurement of outer nuclear layer thickness
The ONL consists of the cell bodies of photoreceptors and its thickness provides an assessment of photoreceptor survival. Thickness of the ONL was done as previously described (13). Briefly, mice were killed and the eyes were removed and frozen in embedded compound. Ten μm frozen sections were cut parallel to 12:00 meridian through the optic nerve and fixed in 4% paraformaldehyde. The sections were stained with hematoxylin and eosin and examined with an Axioskop microscope (Zeiss, Thornwood, NY). Images were digitalized using a three-charge coupled device color video camera (IK-TU40A; Toshiba, Tokyo, Japan) and a frame grabber. Image-Pro Plus software (Media Cybernetics, Silver Spring, MD) was used to calculate the area of the ONL. The Images for display were captured with a Nikon microscope equipped with Nikon Digital Still Camera DXM1200.
Statistical analysis
Statistical comparisons were done using analysis of variance (ANOVA) with Dunnett's test for multiple comparisons. Differences were judged statistically significant at p < 0.05.
Results
Increased expression of SOD1 or 2 causes oxidative damage in RPE cells, while increased expression of Gpx1 or 4 provides protection against oxidative stress
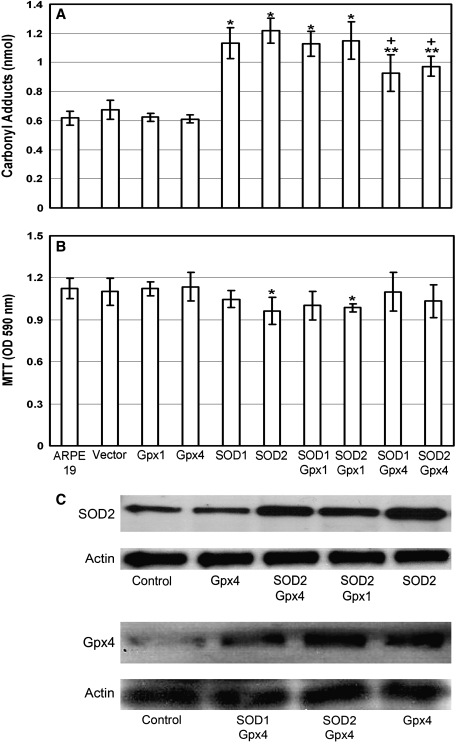
Measurement of the carbonyl content of proteins by ELISA provides a good quantitative assessment of oxidative damage (2, 4). Compared to control RPE cells, those overexpressing Gpx1 or 4 showed similar protein carbonyl content, whereas those overexpressing SOD1 or 2 showed significant increases in carbonyl content (Fig. 1A). Cells with increased levels of SOD2 also showed reduced viability (Fig. 1B). Co-transfections showed that increased levels of Gpx4, but not Gpx1, partially reversed the deleterious effects of increased SOD1 or 2. Figure 1C demonstrates that cells co-transfected with expression constructs for Gpx4 and SOD1 or 2 showed increased levels of both enzymes. This suggests that increased levels of SOD1 or 2 enhance constitutive oxidative damage in RPE cells secondary at least in part to elevated levels of H2O2 that is partially detoxified by a simultaneous increase in Gpx4.
FIG. 1.
Increased oxidative damage and reduced viability in retinal pigmented epithelial (RPE) cells overexpressing superoxide dismutase 1 (SOD1) or SOD2. Untransfected ARPE 19 cells (control) or those transfected with empty plasmid or plasmid containing an expression construct for glutathione peroxidase 1 (Gpx1), Gpx4, SOD1, or SOD2 were scraped into lysis buffer 48 h after transfection. The bars represent the mean (±SEM) calculated from four experimental values. (A) Protein carbonyl content was measured by ELISA and was not significantly different in cells overexpressing Gpx1 or Gpx4 compared to control cells, but it was significantly elevated in cells overexpressing SOD1 or SOD2 (*p <0.01; **p < 0.05 for difference from untransfected cells and +p < 0.05 for difference from cells transfected with SOD1 or 2 alone by ANOVA with Dunnett's correction for multiple comparisons). (B) Cell viability was measured by MTT and was not different in cells overexpressing Gpx1 or Gpx4 compared to untransfected cells, but it was significantly reduced in cells overexpressing SOD2 or co-expressing SOD2 and Gpx1 (*p < 0.05 by ANOVA with Dunnett's correction). (C) Forty-eight hours after transfections, cell lysates (50 μg total protein) were run in immunoblots for SOD2 or Gpx4, and then stripped and reblotted for actin to control for loading differences. There were increased levels of SOD2 in cells transfected with the Sod2 expression construct or cells co-transfected with Sod2 and Gpx expression constructs (top row) with no difference in actin levels (second row). Immunoblots for Gpx4 showed increased levels of Gpx4 in cells transfected with the Gpx4 expression construct or co-transfected with Gpx4 and Sod2 expression constructs (third row) with no difference in actin levels ( fourth row).
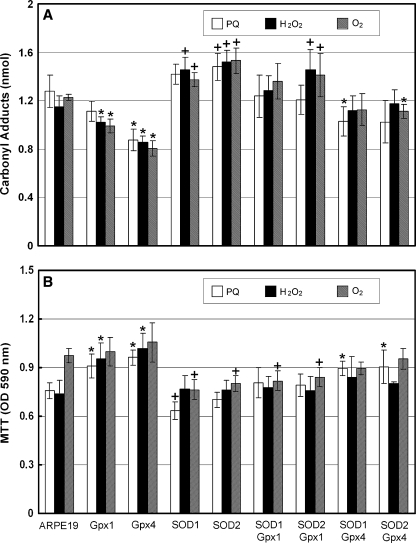
Control RPE cells that were challenged with paraquat, hydrogen peroxide, or hyperoxia had carbonyl levels in the range of 1.2 nM, compared to 0.6 nM in unchallenged cells. In the presence of each of the three types of oxidative stress, RPE cells overexpressing Gpx4 had significantly less carbonyl content than control RPE cells (Fig. 2A). Cells overexpressing Gpx1 had less carbonyl adducts on proteins than that seen in control cells when exposed to H2O2 or hyperoxia. Viability of untransfected RPE cells was not reduced by hyperoxia, but was significantly reduced by exposure to paraquat or H2O2 and the paraquat- and H2O2-induced cell death was significantly blocked by over-expression of Gpx4 or Gpx1 (Fig. 2B). In contrast, cells overexpressing SOD1 or SOD2 showed significantly increased carbonyl levels compared to control RPE cells when challenged with oxidative stress and increased hyperoxia-induced cell death. Increased levels of Gpx4, but not Gpx1, partially reversed the deleterious effects of overexpression of SODs in RPE cells challenged with oxidative stress. Oxidative damage and viability were not worse in cells co-expressing Gpx4 and an SOD than that seen in control RPE cells and was significantly better for some types of stress, but in general, protection from oxidative stress was less in cells overexpressing both Gpx4 and an SOD compared to cells overexpressing Gpx4 alone. These data suggest that increased levels of Gpx4 or Gpx1 improve the antioxidant defense system of RPE cells, whereas increased levels of SOD1 or SOD2 have a negative effect. While in some instances overexpression of Gpx1 provides comparable protection to overexpression of Gpx4, the latter provided protection for the greatest number of stress conditions, particularly the oxidative damage associated with overexpression of the SODs.
FIG. 2.
Glutathione peroxidase 1 (Gpx1) and Gpx4 protect RPE cells from oxidative stress. Twenty-four hours after transfection with an expression construct for glutathione peroxidase 1 (Gpx1), Gpx4, SOD1, or SOD2, RPE cells were treated with 7 mM paraquat, 0.5 mM H2O2, or hyperoxia for 24 h. Untransfected RPE cells were treated in the same way to serve as controls. Cell lysates were used to measure protein carbonyl content by ELISA (A) and cell viability by MTT (B). The bars represent the mean (±SEM) calculated from four experimental values. (A) When exposed to oxidative stress, compared to control cells, those overexpressing Gpx1 or Gpx4 showed significantly less carbonyl content for two of three, or three of three types of stress, respectively. In contrast, cells overexpressing SOD1 or SOD2 showed significantly greater carbonyl content for two of three, or three of three types of stress, respectively. Cells co-expressing SOD1 and Gpx1 had carbonyl contents no different from control cells and those co-expressing SOD2 and Gpx1 had a significant increase in carbonyl content compared to controls for two of three types of stress. In contrast, cells co-expressing Gpx4 and SOD1 or SOD2 had significantly less carbonyl content than control cells for one of three types of stress. *p < 0.05 for decrease from control cells; +p < 0.05 for increase from control cells by ANOVA with Dunnett's correction for multiple comparisons. (B) Compared to control cells, those overexpressing Gpx1 or Gpx4 showed a significant increase in viability for two of three types of oxidative stress, whereas those overexpressing SOD1 or SOD2 had a significant reduction in viability for two of three, or one of three types of oxidative stress, respectively. Cells co-expressing Gpx1 with SOD1 or 2 showed reduced viability for one type of oxidative stress, while cells co-expressing Gpx4 with SOD1 or 2 showed increased viability for one type of oxidative stress. *p < 0.05 for increase from control cells; +p < 0.05 for decrease from control cells by ANOVA with Dunnett's correction for multiple comparisons.
Increased expression of Gpx4 in photoreceptors reduces paraquat- and hyperoxia-induced oxidative damage
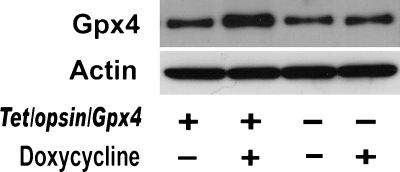
The encouraging protective effects seen with increased expression of Gpx4 in RPE cells prompted investigation of the effects of increasing Gpx4 in photoreceptors in vivo. To accomplish this goal, TRE/murine Gpx4 transgenic mice were generated. Six founders were obtained and crossed with opsin/rtTA mice to generate opsin/rtTA-TRE/Gpx4 (Tet/opsin/Gpx4) double transgenic mice. Mice from each of the six double transgenic lines were given drinking water containing 2 mg/ml of doxycycline for 2 weeks, and immunoblots of retinal homogenates were performed. Only one line showed increased levels of Gpx4 in the retina after treatment with doxycycline (Fig. 3); this line was used for all subsequent experiments.
FIG. 3.
Transgenic mice with doxycycline-inducible expression of glutathione peroxidase 4 (Gpx4). Tetracycline response element (TRE)/Gpx4 mice were generated as described in Methods and crossed with opsin/rtTA transgenic mice to generate Tet/opsin/Gpx4 double transgenic mice. Adult Tet/opsin/Gpx4 mice or littermates lacking one of the transgenes were given drinking water containing (+) or lacking (-) 2 mg/ml doxycycline. After 2 weeks, mice were euthanized, and retinal homogenates were assayed for protein concentration; samples containing 50 μg were run in immunoblots for Gpx4. The blots were stripped and reprobed for actin. There was an increase in Gpx4 in the retinas of Tet/opsin/Gpx4 mice treated with doxycycline.
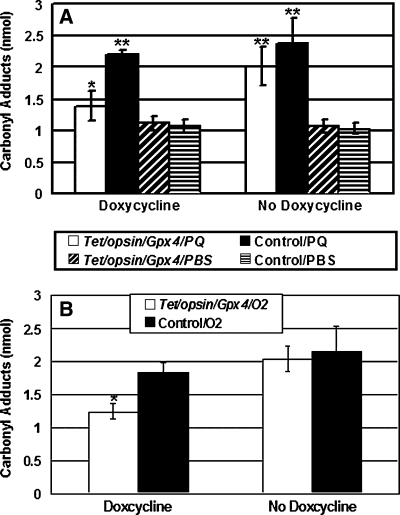
When 1 μl of 0.75 mM paraquat was injected into the vitreous cavity of littermate control mice or doxycycline-treated Tet/opsin/Gpx4 mice, the protein carbonyl content in the retina was increased compared to mice injected with PBS, but the latter had significantly lower levels than the former (Fig. 4A). In contrast, Tet/opsin/Gpx4 mice that were not treated with doxycycline had similar paraquat-induced elevation of protein carbonyl levels in the retina compared to littermate control mice. When placed in 75% hyperoxia for 2 weeks, Tet/opsin/Gpx4 mice that were treated with doxycycline had significantly lower protein carbonyl content in the retina than doxycycline-treated littermate control mice. Tet/opsin/Gpx4 mice that were not treated with doxycycline had similar hyperoxia-induced elevation of protein carbonyl levels in the retina compared to littermate control mice (Fig. 4B).
FIG. 4.
Doxycycline-induced expression of Gpx4 in Tet/opsin/Gpx4 double transgenics reduces oxidative damage in the retina. Tet/opsin/Gpx4 double transgenic mice or littermates lacking one of the transgenes (controls) were given drinking water containing or lacking 2 mg/ml of doxycycline for 2 weeks and then assessed for effects of paraquat (A) or hyperoxia (B) on carbonyl content in the retina. (A) Mice were given an intravitreous injection of 1 μl of PBS containing 0.75 mM paraquat in one eye and 1 μl of PBS in the other eye, and after 24 h the protein carbonyl content in the retina was measured by ELISA. The bars represent the mean (±SEM) calculated from 6 mice in each group. For paraquat-injected eyes, the carbonyl content was significantly less (*p < 0.05 by ANOVA with Dunnett's correction) in the retinas of Tet/opsin/Gpx4 mice that received doxycycline compared to retinas of Tet/opsin/Gpx4 mice that did not receive doxycycline or retinas of control mice either treated with doxycycline or not (**p < 0.005). Paraquat-injected eyes had greater carbonyl content in the retina than fellow eyes-injected with PBS. (B) Mice were placed in 75% oxygen for 2 weeks and then carbonyl content was measured in the retina. The bars represent the mean (±SEM) calculated from 5 mice in each group. Retinal carbonyl content was significantly less in Tet/opsin/Gpx4 mice treated with doxycycline (*p < 0.05) compared to Tet/opsin/Gpx4 mice that did not receive doxycycline or control mice whether or not they received doxycycline.
Increased expression of Gpx4 in photoreceptors reduces paraquat- and hyperoxia-induced thinning of the outer nuclear layer (ONL)
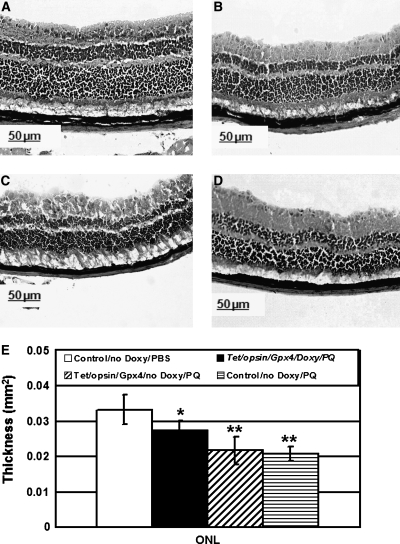
The ONL of the retina contains the cell bodies of the photoreceptors and death of photoreceptors results in thinning of the ONL. Two weeks after intraocular injection of 1 μl of 0.75 mM paraquat, Tet/opsin/Gpx4 mice that were treated with doxycycline had significantly thicker ONLs than Tet/opsin/Gpx4 mice that were not treated with doxycycline or doxycycline-treated littermate control mice (Fig. 5). The protection of photoreceptors by induced expression of Gpx4 was partial, because ONL thickness was significantly less in paraquat-injected Tet/opsin/Gpx4 mice that were treated with doxycycline than in PBS-injected littermate control mice.
FIG. 5.
Induced expression of Gpx4 reduces paraquat-induced thinning of the outer nuclear layer (ONL) of the retina. Tet/opsin/Gpx4 double transgenic mice received drinking water containing or lacking 2 mg/ml of doxycycline, and littermate control mice were given normal drinking water. After 1 week, the mice were given an intraocular injection of 1 μl of 0.75 mM paraquat in the left eye and 1 μl of PBS in right eye. After another 2 weeks of water containing or lacking doxycycline, the mice were euthanized and outer nuclear layer (ONL) thickness was measured as described in Methods. The bars represent the mean (±SEM) calculated from 5 mice in each group. Compared to identical regions of the retina in eyes of control mice injected with PBS (A), those from paraquat-injected eyes of doxycycline-treated Tet/opsin/Gpx4 mice appeared to have a slightly thinner ONL, and this was confirmed by image analysis (E, *p < 0.05, by ANOVA with Dunnett's correction for multiple comparisons), but significantly thicker than the ONL from paraquat-injected eyes of Tet/opsin/Gpx4 mice that were not treated with doxycycline (C, **p < 0.001) or paraquat-injected eyes of control mice (D, **p < 0.001).
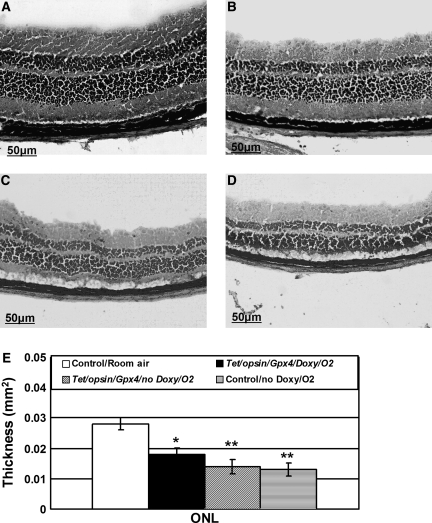
After 2 weeks in 75% oxygen, Tet/opsin/Gpx4 mice that were treated with doxycycline had significantly thicker ONLs than Tet/opsin/Gpx4 mice that were not treated with doxycycline or doxycycline-treated littermate control mice (Fig. 6). The protection of photoreceptors by induced expression of Gpx4 was partial, because ONL thickness was significantly less in hyperoxia-exposed Tet/opsin/Gpx4 mice that were treated with doxycycline than in littermate controls that were not exposed to hyperoxia.
FIG. 6.
Induced expression of Gpx4 reduces hyperoxia-induced thinning of the outer nuclear layer (ONL) of the retina. Tet/opsin/Gpx4 double transgenic mice were placed in 75% O2 and given drinking water containing or lacking 2 mg/ml of doxycycline. Littermate controls were also placed in 75% oxygen or left in room air. After 2 weeks, the mice were euthanized, 10 μm ocular frozen sections were stained with hematoxylin and eosin, and the ONL thickness was measured as described in Methods. The bars represent the mean (±SEM) calculated from 5 mice in each group. Compared to control mice that remained in room air (A, n = 5), the ONL of the same region of the retina from eyes of hyperoxia-treated Tet/opsin/Gpx4 mice (B, n = 5) appeared somewhat thinner which was confirmed by image analysis (E, *p < 0.05 by ANOVA with Dunnett's correction), but was significantly thicker than the ONL of hyperoxia-exposed Tet/opsin/Gpx4 mice that did not receive doxycycline (C, n = 5, **p <0.002) or hyperoxia-treated control mice (D, n = 5, **p < 0.002).
Increased expression of Gpx4 in photoreceptors reduces loss of retinal function after injection of paraquat or exposure to hyperoxia
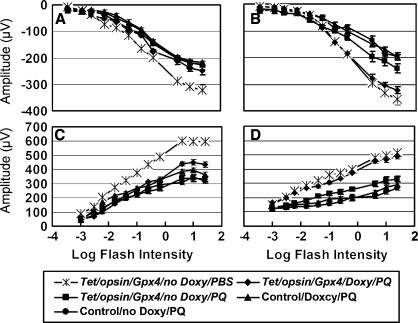
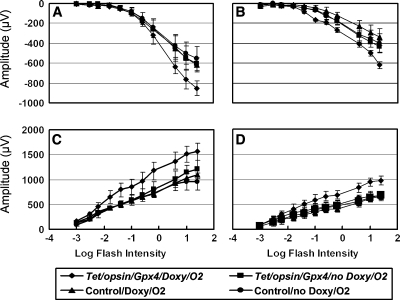
ERGs provide a global assessment of retinal functioning. One day after injection of 1 μl of 0.75 mM paraquat, all mice injected with paraquat showed significantly reduced ERG a- and b-wave amplitudes compared to mice injected with PBS (Fig. 7A and C). However, 8 days after paraquat injection, Tet/opsin/Gpx4 mice that were treated with doxycycline had a- and b-wave amplitudes that were significantly greater than those seen in littermate controls or Tet/opsin/Gpx4 mice that were not treated with doxycycline, and were no different from those seen in mice that had been injected with PBS (Fig. 7B and D). After 2 weeks in 75% oxygen, Tet/opsin/Gpx4 mice that were treated with doxycycline had a- and b-wave amplitudes that were significantly greater than those seen in littermate controls or Tet/opsin/Gpx4 mice that were not treated with doxycycline (Fig. 8).
FIG. 7.
Induced expression of Gpx4 prevents loss of retinal function assessed by electroretinograms (ERGs) after intraocular injection of paraquat. Tet/opsin/Gpx4 double transgenic or littermate control mice were given water containing or lacking 2 mg/ml of doxycycline, and after 1 week received an intraocular injection of 1 μl of 0.75 mM paraquat in one eye and PBS in the fellow eye. Scotopic ERGs were performed at 2 and 8 days after injection. The points represent the mean (±SEM) calculated from 6 mice in each group. At 2 days after injection, all eyes injected with paraquat showed a significant reduction in a-wave (A) and b-wave (C) amplitude compared to eyes injected with PBS. However, at 8 days after injection, paraquat-injected eyes of Tet/opsin/Gpx4 mice that received doxycycline showed a-wave (B) and b-wave (D) amplitudes that were essentially identical to those of PBS-injected eyes, and significantly greater than all other paraquat-injected eyes.
FIG. 8.
Induced expression of Gpx4 prevents hyperoxia-induced loss of retinal function assessed by electroretinograms (ERGs). Tet/opsin/Gpx4 double transgenic or littermate control mice were given water containing or lacking 2 mg/ml of doxycycline, and after 1 week were placed in 75% oxygen. After another 1 (A and C) and 2 (B and D) weeks, scotopic ERGs (the points represent the mean ± SEM calculated from 6 mice in each group) showed that eyes of Tet/opsin/Gpx4 mice exposed to hyperoxia had significantly greater a-wave (A, B) and b-wave (C, D) amplitudes than Tet/opsin/Gpx4 that did not receive doxycycline or control mice that received water containing or lacking doxycycline.
Discussion
Increased expression of components of the antioxidant defense system is an appealing strategy for treatment of retinal degenerations in which oxidative damage plays an important role. However, there are several components of the antioxidant defense system, and it appears that the effects of increased expression of various components may vary depending upon the cell type and the nature of the oxidative stress. We had previously found that transgenic mice with increased expression of SOD1 had reduced damage to photoreceptors when challenged with severe oxidative stress, but in unchallenged mice there was higher than normal constitutive oxidative stress, resulting in mild reduction in retinal function (6). In this study, we compared the effects of increased expression of SOD1, SOD2, Gpx1, and Gpx4 in cultured RPE cells. Similar to the situation in vivo, increased expression of SOD1 or 2 in RPE cells enhanced oxidative damage in unchallenged cells, but surprisingly exposure to oxidative stress resulted in greater increases in oxidative damage in cells overexpressing SOD1 or 2 than in control cells. In contrast, RPE cells overexpressing Gpx1 or 4 showed no increase in constitutive oxidative damage and less oxidative damage than control cells when challenged. Cells in which expression of Gpx1 was increased in combination with SOD1 or 2, showed high carbonyl content no different from that in cells with increased expression of SOD1 or 2 alone. Cells in which expression of Gpx4 and SOD1 or 2 was increased showed a significant reduction in carbonyl content compared to cells with increased expression of SOD1 or 2 alone, but still higher than that in untransfected cells. Also, in co-transfected cells challenged with oxidative stress, increased levels of Gpx4, but not Gpx1, partially reversed the deleterious effects of overexpression of SODs. These data suggest that the more efficient dismutation of superoxide radicals by high levels of SOD yielding a higher steady state concentration of H2O2 is likely to contribute to toxicity from overexpression of SOD1 or 2 in RPE cells and the excess H2O2 is better detoxified by Gpx4 than Gpx1.
There are some theoretical advantages of Gpx4 that may help to explain its ability to provide greater protection than Gpx1 against oxidative stress in RPE cells. In addition to reducing hydrogen peroxide, alkyl peroxide, and fatty acid peroxide, it also reduces hydroperoxides in lipoproteins, complex lipids, and phospholipids (7). Therefore, overexpression of Gpx4 might be particularly advantageous in tissues with high content of polyunsaturated fatty acids, such as the photoreceptors and RPE cells.
It is clear that SOD1 is an important component of the endogenous antioxidant defense system in the retina because mice that lack SOD1 are much more susceptible to oxidative stress (6), but that is a different issue than whether its overexpression can provide therapeutic benefits. Our data demonstrate that despite their role in the endogenous antioxidant defense system, increased levels of SOD1 or 2 in RPE cells have deleterious effects. Furthermore, one might expect that co-expression of Gpx4 with SOD1 or 2 would provide superior protection against oxidative stress than increased expression of Gpx4 alone, but this was not the case. There are a number of possible reasons for this observation. It may be that a precise balance between SOD and Gpx activity is required and was not achieved. Mismatches in terms of activity levels within particular cellular compartments might also lead to problems. For instance, overexpression of SOD2 would be expected to increase levels of H2O2 most within mitochondria, and combining this with increased levels of Gpx1 or Gpx4 in cytoplasm, as was the case in this study, might cause a problem that might have been circumvented if the mitochondrial form of Gpx4 had been used. Also, catalase is another component of the defense system that helps to detoxify H2O2 and we do not know if its overexpression would provide advantages. Finally, the lack of complete reversal of SOD-induced damage by Gpx1 or 4 leaves open the possibility that there is another mechanism in addition to excess production of H2O2 that contributes to oxidative damage in cells overexpressing SOD1 or 2 such as misfolding of the overexpressed SODs. All of these potential pitfalls underscore the need to carefully investigate effects of overexpressing components of the endogenous antioxidant defense system to make sure theoretical benefits are truly realized.
All in vitro model systems are imperfect. For example, cultured cells are normally exposed to relative hyperoxia compared to cells in vivo which could cause adaptations that alter their response to more severe hyperoxia or other oxidative stresses. Thus, while in vitro experiments can help to select promising candidates, the effects of overexpressing antioxidant enzymes within cell types of interest in relevant animal models is likely to be more predictive for gene-based treatment strategies. Increased expression of Gpx4 in photoreceptors of double transgenic mice provided strong protection against paraquat- and hyperoxia-induced damage indicated by reduced protein carbonyl content, preservation of retinal function assessed by ERGs, and reduced photoreceptor cell death. Considering these results in transgenic mice, the in vitro results in RPE cells, and the results of a previous study in which effects of overexpressing SOD1 were investigated in these models (6), it appears that Gpx4 may be a better choice for a therapeutic transgene than Sod1 for treatment of RP and AMD. While additional studies are needed to evaluate other transgene candidates, it will also be important to assess the effects of viral vector-mediated overexpression of Gpx4 in animal models of retinal degeneration to further evaluate the potential of that therapeutic strategy.
Acknowledgments
This research is supported by EY05951 and core grant P30EY1765 from the NEI and Dr. and Mrs. William Lake. PAC is the George S. and Dolores Dore Eccles Professor of Ophthalmology.
Abbreviations
AREDS, Age-Related Eye Disease Study; AMD, age-related macular degeneration; ANOVA, analysis of variance; BSA, bovine serum albumin; DNPH, dinitrophenylhydrazine; ERGs, electroretinograms; Gpx, glutathione peroxidase; MTT, methylthiazoletetrazolium; ONL, outer nuclear layer; PBS, phosphate-buffered saline; ROS, reactive oxygen species; RPE, retinal pigmented epithelium; RP, retinitis pigmentosa; rtTA, reverse tetracycline transactivator; SOD, superoxide dismutase; Tet/opsin/Gpx4 mice, rhodopsin promoter/rtTA-TRE/Gpx4 double transgenic mice; TRE, tetracycline response element.
Disclosure Statement
No competing financial interests exist.
References
- 1.The Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss. Arch Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buss H. Chan TP. Sluis KB. Domigan NM. Winterbourn CC. Protein carbonyl measurement by a sensitive ELISA method. Free Radical Biol Med. 1997;23:361–366. doi: 10.1016/s0891-5849(97)00104-4. [DOI] [PubMed] [Google Scholar]
- 3.Chang MA. Horner JW. Conklin BR. DePinho RA. Bok D. Zack DJ. Tetracycline-inducible system for photoreceptor-specific gene expression. Invest Ophthalmol Vis Sci. 2000;41:4281–4287. [PubMed] [Google Scholar]
- 4.Cingolani C. Rogers B. Lu L. Kachi S. Shen J. Campochiaro PA. Retinal degeneration from oxidative damage. Free Radic Biol Med. 2006;40:660–669. doi: 10.1016/j.freeradbiomed.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 5.Davies SMK. Poljak A. Duncan MW. Smythe GA. Murphy MP. Measurement of protein carbonyls, ortho- and meta-tyrosine and oxidative phosphorylation complex activity in mitochondria from young and old rats. Free Radical Biol Med. 2001;31:181–190. doi: 10.1016/s0891-5849(01)00576-7. [DOI] [PubMed] [Google Scholar]
- 6.Dong A. Shen J. Krause M. Akiyama H. Hackett SF. Lai H. Campochiaro PA. Superoxide dismutase 1 protects retinal cells from oxidative damage. J Cell Physiol. 2006;208:516–526. doi: 10.1002/jcp.20683. [DOI] [PubMed] [Google Scholar]
- 7.Girotti AW. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J Lipid Res. 1998;39:1529–1542. [PubMed] [Google Scholar]
- 8.Komeima K. Rogers BS. Campochiaro PA. Antioxidants slow photoreceptor cell death in mouse models of retinitis pigmentosa. J Cell Physiol. 2007;213:809–815. doi: 10.1002/jcp.21152. [DOI] [PubMed] [Google Scholar]
- 9.Komeima K. Rogers BS. Lu L. Campochiaro PA. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc Natil Acad Sci USA. 2006;103:11300–11305. doi: 10.1073/pnas.0604056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levine RL. Garland D. Oliver CN. Amici A. Climent I. Lenz A–G. Bong-Whan A. Shaltiel S. Stadtman ER. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 11.Lu L. Hackett SF. Mincey A. Lai H. Campochiaro PA. Effects of different types of oxidative stress in RPE cells. J Cell Physiol. 2006;206:119–125. doi: 10.1002/jcp.20439. [DOI] [PubMed] [Google Scholar]
- 12.Ohno–Matsui K. Hirose A. Yamamoto S. Saikia J. Okamoto N. Gehlbach P. Duh EJ. Hackett SF. Chang M. Bok D. Zack DJ. Campochiaro PA. Inducible expression of vascular endothelial growth factor in photoreceptors of adult mice causes severe proliferative retinopathy and retinal detachment. Am J Pathol. 2002;160:711–719. doi: 10.1016/S0002-9440(10)64891-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okoye G. Zimmer J. Sung J P. G, Deering T, Nambu N, Hackett SF, Melia M, Esumi N, Zack DJ, and Campochiaro PA. Increased expression of BDNF preserves retinal function and slows cell death from rhodopsin mutation or oxidative damage. J Neurosci. 2003;23:4164–4172. doi: 10.1523/JNEUROSCI.23-10-04164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen J. Yan X. Dong A. Petters RM. Peng Y–W. Wong F. Campochiaro PA. Oxidative damage is a potential cause of cone cell death in retinitis pigmentosa. J Cell Physiol. 2005;203:457–464. doi: 10.1002/jcp.20346. [DOI] [PubMed] [Google Scholar]
- 15.Yamada H. Yamada E. Ando A. Esumi N. Bora N. Saikia J. Sung C-H. Zack DJ. Campochiaro PA. FGF2 decreases hyperoxia-induced cell death in mice. J Am Pathol. 2001;159:1113–1120. doi: 10.1016/S0002-9440(10)61787-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu DY. Cringle SJ. Su EN. Yu PK. Intraretinal oxygen levels before and after photoreceptor loss in the RCS rat. Invest Ophthalmol Vis Sci. 2000;41:3999–4006. [PubMed] [Google Scholar]