Abstract
Gadolinium-containing magnetic resonance imaging contrast agents such as Omniscan are associated with Nephrogenic Systemic Fibrosis (NSF). To determine if Omniscan can affect the differentiation of monocytes into fibroblast-like cells called fibrocytes that are found in the fibrotic lesions of NSF, peripheral blood mononuclear cells (PBMCs) from NSF patients, hemodialysis patients without NSF, and healthy, renally sufficient controls were exposed to Omniscan in a standardized in vitro fibrocyte differentiation protocol. When added to PBMCs, the gadolinium-containing magnetic resonance imaging contrast agent Omniscan generally had little effect on fibrocyte differentiation. However, 10-8 -10-3 mg/ml Omniscan reduced the ability of the fibrocyte differentiation inhibitor Serum Amyloid P (SAP) to decrease fibrocyte differentiation in PBMCs from 15 of 17 healthy controls and one of three NSF patients. Omniscan reduced the ability of SAP to decrease fibrocyte differentiation from purified monocytes, indicating that the Omniscan effect does not require the presence of other cells (such as T cells) in the PBMC. Omniscan also reduced the ability of a different fibrocyte differentiation inhibitor, interleukin-12, to decrease fibrocyte differentiation. These data suggest that Omniscan interferes with the regulatory action of signals that inhibit the differentiation of monocytes to fibrocytes.
Keywords: Nephrogenic Systemic Fibrosis, Peripheral blood mononuclear cells, Monocytes, Omniscan, Serum Amyloid P, IL-12
INTRODUCTION
Healing wounds and fibrotic lesions contain fibroblast-like cells called fibrocytes that originate from the circulation (1-3). Fibrocytes develop from CD14+ progenitors (4) and exhibit a distinctive spindle shape in vitro. Fibrocytes secrete collagen, contribute to scar tissue, and promote fibrosis by a variety of mechanisms (3,5). Besides releasing mediators that recruit additional cell types, fibrocytes may themselves differentiate into myofibroblasts. In addition, fibrocytes may secrete pro-angiogenic factors, thereby inducing capillary formation and nutrient entry into wound sites (5,6).
Nephrogenic systemic fibrosis (NSF) is a recently described fibrosing disorder that is associated with exposure to gadolinium-containing magnetic resonance imaging contrast agents in some renally insufficient subjects (7). The fibrotic lesions observed in NSF patients contain fibrocytes (8,9). Gadolinium-containing contrast agents (GCCA) contain a gadolinium atom bound to a proprietary gadolinium-binding linear or macrocyclic ligand (7). A commonly used GCCA is Omniscan, which contains the gadolinium-ligand complex gadodiamide and an excess of the gadolinium-binding ligand. In experimental animal studies, renally competent rats treated with doses of Omniscan or gadodiamide alone that result in gadodiamide levels similar to those seen in the peripheral tissues of renally incompetent humans developed skin lesions that resemble those observed in human NSF, including the appearance of fibrocytes in the skin (10).
When human peripheral blood mononuclear cells (PBMCs) are cultured in serum-free medium, fibrocytes appear in the culture within three days. Fibrocyte differentiation is generally suppressed in vivo; for instance, fibrocytes are present in very low numbers in circulation. The protein serum amyloid P (SAP) has been identified as a key inhibitor of fibrocyte differentiation (11,12). Signals such as IL-12 also decrease fibrocyte differentiation (13). As discussed below, in serum-free medium cultures, Omniscan appears to interfere with the regulatory action of signals that decrease fibrocyte differentiation.
MATERIALS AND METHODS
Patient Characteristics
Peripheral blood was obtained from volunteers recruited in accordance with protocols approved by Institutional Review Boards. Three patients on hemodialysis with biopsy-proven NSF were studied (age range 37-50, 2 males, 1 female). The diagnosis dates were 7/23/2002 for one of the male patients and 3/23/2006 for the female patient; the diagnosis date for the third NSF patient is unknown. Two hemodialysis patients (1 male: age 33, 1 female: age 57) and 9 normal, disease-free individuals (age range 20-50, 7 males, 2 females) were studied as controls. One of the control hemodialysis patients had received Omniscan in 2002 for a magnetic resonance imaging brain scan, while the other control hemodialysis patient had no record of receiving any gadolinium-containing contrast agent. None of the healthy controls had received a gadolinium-containing contrast agent.
Cell Preparation, Culture, and Imunocytochemistry
The isolation of PBMCs and their culture in serum-free medium (permitting fibrocyte differentiation) was performed following (11) with the exceptions described below. Monocytes were isolated and their purity checked following Shao et al. (13). Blood was collected in sodium heparin vacutainer tubes (BD Biosciences, Franklin Lakes, NJ). Human serum amyloid P (Calbiochem, La Jolla, CA) was resuspended in water to 1 mg/ml and buffer-exchanged 4 times with 20 mM sodium phosphate pH 7.4 using a Microcon 30 kDa cutoff centrifugal filter device (Millipore, Bedford, MA). Human interleukin-12 (IL-12) was from PeproTech, Rocky Hills, NJ, and was prepared as a 50 μg/ ml stock following the manufacturer's directions. A dilution series of Omniscan® injection 287 mg/ml (Novaplus, Irving, TX) was made in Eppendorf tubes by adding 10 μl of Omniscan to 90 μl of serum-free medium, mixing, and then adding 10 μl of this to 90 μl of serum-free medium. This dilution was repeated until a 1:1010 dilution was reached. Preliminary experiments were done with different dilutions of Omniscan (data not shown) to determine the dilution range needed for the assays. 100 μl of serum-free medium, or serum-free medium containing human SAP or IL-12 was added to each well of a Microtest 96 well tissue culture plate (BD Biosciences). 5 μl of serum-free medium, undiluted Omniscan, or an Omniscan dilution was added to each well, with each condition represented in duplicate wells. 100 μl of human PBMC or monocytes at a concentration of 5 × 105 cells/ml in serum-free medium was then added to each well. After 5 days in culture, cells were fixed and stained with hematoxylin/eosin, and fibrocytes were identified as spindle-shaped cells with an oval nucleus following (11). The average number of fibrocytes in the duplicate wells was calculated. For each independent experiment, fibrocyte numbers were normalized to the number present with no SAP (or IL-12) and no Omniscan. Immunocytochemistry to confirm that the spindle-shaped cells with oval nuclei were fibrocytes was done following (13).
SAP Assay and Statistics
An ELISA assay to measure serum SAP levels was performed following (11). The assay was performed three independent times for each sample, and the values were averaged. All statistical analysis was performed using GraphPad Prism software (GraphPad Software, San Diego, CA). Differences between two groups were assessed by Student's t-test or chi square as indicated. Significance was defined as p < 0.05.
RESULTS
Fibrocytes differentiate from circulating CD14+ monocyte precursors (4) by a process that is regulated by SAP (11,12). To determine if a GCCA affects the differentiation of monocytes into fibrocytes, we subjected human PBMCs (a population that includes CD14+ monocytes) to a standardized fibrocyte differentiation protocol (11) in the presence of Omniscan. We studied peripheral blood cells obtained from 17 healthy volunteers, 3 patients with biopsy-proven NSF, and 2 hemodialysis patients without NSF.
We observed that under baseline differentiation conditions (no Omniscan or SAP), the yield of fibrocytes from different donors, or from a single donor studied over a period of several months, could vary considerably. For instance, from 34 assays of PBMC from 17 healthy donors, the number of fibrocytes observed in the absence of SAP at 5 days varied from 9.3 × 101 to 2.0 × 103/ 2.5 × 105 PBMC. The number of fibrocytes from the NSF patients ranged from 1.0 × 103 to 2.1 × 103/ 2.5 × 105 PBMC, and the number for hemodialysis patients ranged from 7.8 × 102 to 1.3 × 103/ 2.5 × 105 PBMC. For quantification, we therefore normalized the fibrocyte number for each donor to the condition (presence or absence of SAP) in which Omniscan was added. SAP inhibits fibrocyte differentiation (11), and for the healthy controls, 1 μg/ml SAP decreased the number of fibrocytes by 71 ± 4% (mean ± SEM, n = 34 studies from 17 donors). In the NSF patients, SAP decreased the number of fibrocytes by 44 ± 7% (n=9 studies from 3 patients), and for the hemodialysis patients the decrease was 57 ± 10% (n=6 studies from 2 patients). The difference between controls and NSF patients was significant (p < 0.01, 1-way ANOVA, Tukey's test), while the difference between hemodialysis patients and either of the other groups was not significant. We also measured serum SAP levels by ELISA, but observed no significant differences among the studied groups (healthy controls 35 ± 2 μg/ ml (n=9), NSF 32 ± 3 μg/ ml (n=3), and the two hemodialysis controls 29 and 34 μg/ ml).
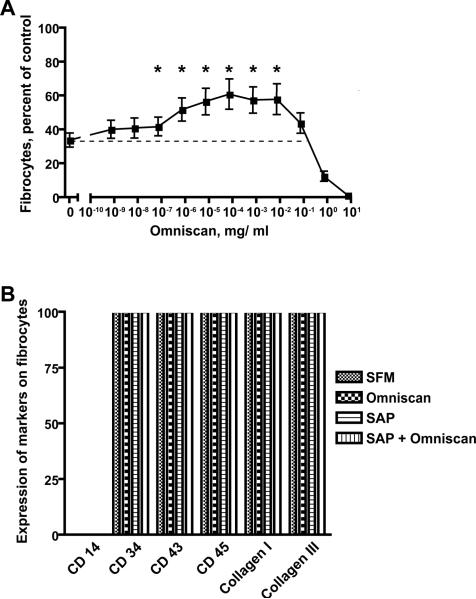
Very high concentrations of Omniscan (≥10-1 mg/ml) inhibited fibrocyte differentiation from PBMCs obtained from all donors under all conditions examined (Figures 1A, 2-4, and data not shown); this most likely reflects a direct toxic action of this agent on cells in our culture conditions. For the healthy volunteers, Omniscan in the absence of exogenous SAP sometimes appeared to increase the number of fibrocytes by ~20%, but this was not consistent even for different blood collections from a single donor. Since SAP is a key inhibitor of fibrocyte differentiation, we examined the effect of Omniscan on the ability of SAP to inhibit fibrocyte differentiation. In the presence of 1 μg/ ml SAP, in 15 of the 17 healthy donors, the addition of Omniscan (10-7 to 10-3 mg/ml) reduced the ability of SAP to inhibit the number of fibrocytes present in the culture (Figure 1A). To verify that the elongated cells we observed in the cultures were fibrocytes, we stained cells with a variety of markers. Using PBMCs from 3 different healthy donors, we found that the cells we were counting as fibrocytes showed no expression of CD14, a marker that is quickly lost by fibrocytes (1,11), but did stain for 5 different markers that are expressed by fibrocytes (Figure 1B). Together, the results indicate that for most healthy patients, Omniscan reduces the ability of SAP to inhibit fibrocyte differentiation.
Figure 1. Fibrocyte differentiation of PBMCs from healthy donors.
A) PBMC were cultured in the presence of 1 μg/ml SAP and the indicated concentrations of Omniscan, with the highest concentration (7 mg/ ml) representing 5 μl of a 1:9 dilution of the 287 mg/ ml Omniscan added to 200 μl of cells, SAP, and media. Values are mean ± SEM of the number of fibrocytes observed after 5 days relative to the no SAP/ no Omniscan control in each of 34 independent experiments from 17 donors. A * indicates that the value is significantly higher than the 1 μg/ ml SAP/ no-Omniscan (0) value with p < 0.05 (t-tests). B) PBMC were cultured in serum-free medium (SFM), SFM with 7 × 10-5 mg/ ml Omniscan, (Omniscan), SFM with 1 μg/ ml SAP (SAP), or SFM with 1 μg/ ml SAP and 7 × 10-5 mg/ ml Omniscan (SAP + Omniscan). After 5 days, cells were fixed and stained for the indicated markers. For each experiment for each marker, more than 100 spindle-shaped cells were examined. The values are the mean ± SEM (because of consistent results, no error bars are visible) of the percentage of spindle-shaped cells stained with the indicated marker from 3 independent experiments using PBMC from 3 different healthy donors.
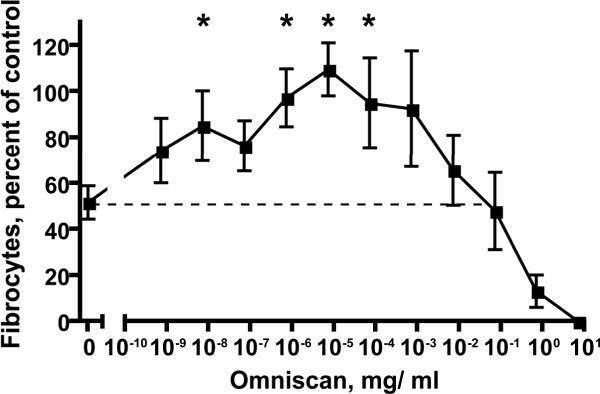
Figure 2. Fibrocyte differentiation of monocytes from healthy donors.
Monocytes from healthy donors were cultured in the presence of 1 μg/ml SAP and the indicated concentrations of Omniscan. Values are mean ± SEM of the number of fibrocytes observed after 5 days relative to the no SAP/ no Omniscan control from each of 4 different donors. A * indicates that the value is significantly higher than the 1 μg/ ml SAP/ no-Omniscan (0) value with p < 0.05 (t-tests).
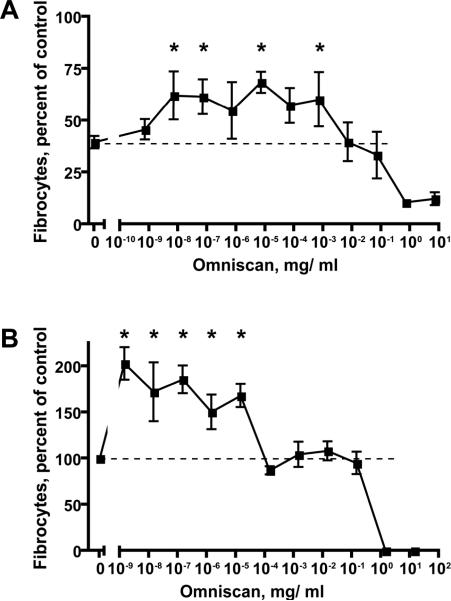
Figure 4. Fibrocyte differentiation of PBMCs from two NSF patients.
A) Fibrocyte appearance from PBMCs in NSF patient JS-5 after 5 days of culture in the presence of 1 μg/ ml SAP and the indicated concentrations of Omniscan. Values are mean ± SEM of the number of fibrocytes relative to the no SAP/ no Omniscan control from three independent experiments. A * indicates that the value is significantly higher than the 1 μg/ ml SAP/ no-Omniscan (0) value with p < 0.05 (t-tests). B) Fibrocyte appearance from PBMCs in NSF patient JS-2 after 5 days of culture in the absence of exogenous SAP with the indicated concentrations of Omniscan. Values are mean ± SEM of the number of fibrocytes relative to the no Omniscan control from three independent experiments. A * indicates that the values is significantly higher than the no Omniscan value with p < 0.05 (t-tests).
Fibrocytes differentiate from the monocytes in the PBMCs, and SAP acts directly on monocytes to inhibit fibrocyte differentiation (1,4,11,13). To determine if Omniscan requires the presence of an additional cell population in the PBMCs to decrease the effect of SAP on fibrocyte differentiation, we examined the effect of Omniscan and SAP on purified monocytes. As with the PBMC, Omniscan in the absence of SAP had little discernable effect on fibrocyte differentiation (data not shown). However, Omniscan reduced the ability of 1 μg/ ml SAP to decrease the differentiation of purified monocytes into fibrocytes (Figure 2).
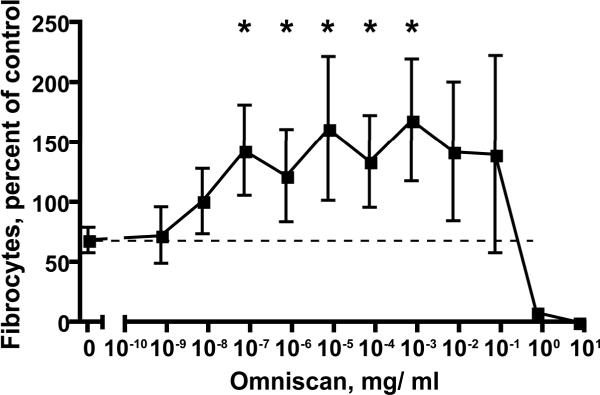
To determine if the effect of Omniscan on fibrocyte differentiation is limited to its ability to partially block the effect of SAP, we examined the effect of a different fibrocyte differentiation inhibitor, IL-12, in the presence or absence of Omniscan. The presence of ~10-7 to 10-3 mg/ ml Omniscan significantly decreased the ability of IL-12 to inhibit fibrocyte differentiation (Figure 3). These data indicate that Omniscan reduces the ability of at least two different signals to decrease fibrocyte differentiation.
Figure 3. Omniscan decreases the ability of IL-12 to decrease fibrocyte differentiation.
PBMC from healthy donors were cultured in the presence of 10 ng/ml IL-12 and the indicated concentrations of Omniscan. Values are mean ± SEM of the number of fibrocytes observed after 5 days relative to the no IL-12/ no Omniscan control from each of 7 different donors. A * indicates that the value is significantly higher than the 10 ng/ ml IL-12/ no Omniscan (0) value with p < 0.05 (t-tests).
In one of three NSF patients (JS-5), Omniscan reduced the ability of 1 μg/ ml SAP to decrease fibrocyte differentiation (Figure 4A). In PBMCs from this patient, Omniscan had no significant effect on fibrocyte differentiation in the absence of SAP. However, for the second NSF patient (JS-2), Omniscan in the absence of SAP consistently increased fibrocyte differentiation (Figure 4B), even though Omniscan did not affect the ability of SAP to decrease the number of fibrocytes. Interestingly, whereas for the healthy control PBMCs, Omniscan did not significantly increase fibrocyte numbers at 7 × 10-9 mg/ ml (Figure 1A), for both of these NSF patients, Omniscan at this concentration significantly increased fibrocyte numbers. In both the presence and absence of SAP, a promotional effect of Omniscan on fibrocyte differentiation in vitro was not observed in PBMCs obtained from the third NSF patient as well as the two hemodialysis patients without NSF.
DISCUSSION
We observed that in PBMCs from healthy donors, Omniscan in concentrations of 10-7 to 10-3 mg/ml reduced the ability of both SAP and IL-12 to decrease the number of fibrocytes. Even though the number of PBMCs that differentiated into fibrocytes varied by a factor of 21 (2.0 × 103/ 9.3 × 101), in the presence of either 1 μg/ ml SAP or 10 ng/ ml IL-12, the addition of Omniscan at 10-7 to 10-3 mg/ml caused a fairly consistent ~1.6-fold increase in the number of fibrocytes compared to no added Omniscan. Radiologic studies employ an Omniscan dose of ~0.1 mmol/ kg ((14) and Omniscan prescribing information), which corresponds to 0.23 to 0.26 mg/ ml of Omniscan in the blood immediately after the injection (15). Since Omniscan is excreted or dialyzed, at some point concentrations of Omniscan less than this level would be observed in a patient.
The NSF and hemodialysis patients all had impaired renal function. We observed that Omniscan reduced the ability of 1 μg/ ml SAP to decrease fibrocyte numbers in 15 of 17 healthy controls, but under the same conditions, Omniscan affected fibrocyte numbers in only 1 of 5 of the combined NSF and hemodialysis patients. While our conclusions must necessarily be qualified by the limited number of patients studied, a chi square analysis showed that the decreased response to Omniscan in the combined patient group was significant with p < 0.01. One possible explanation for this is that impaired renal function or some other factor may influence PBMC in some patients to render them less sensitive to Omniscan. We observed that at very low concentrations of Omniscan, there was a variability in the sensitivity of different donors' PBMCs or monocytes to Omniscan (Figures 1A, 2, 3, and 4). We hypothesize that this may explain why only a minority of patients with impaired renal function who receive Omniscan develop NSF.
Patients with normal renal function do not develop NSF, while we observed that for most healthy donors, Omniscan decreased the ability of both SAP and IL-12 to inhibit fibrocyte differentiation. One possible explanation for this is that PBMCs require a prolonged exposure to Omniscan for Omniscan to reduce the inhibitory action of SAP or IL-12 on fibrocyte differentiation. In patients with normal renal function, Omniscan is cleared with an elimination half-life of ~1.3 hours, while in patients with impaired renal function, Omniscan is cleared relatively slowly (15). In our experiments, PBMCs were exposed to Omniscan for 5 days, which is an exposure time more similar to that in a patient with a slow clearance of Omniscan than the exposure time in a patient with normal renal function and a rapid clearance of Omniscan.
In conclusion, our data suggest that Omniscan interferes with the regulatory action of signals that inhibit the differentiation of monocytes to fibrocytes. Fibrocytes are involved in a number of fibrosing diseases (1-3,16), and several mediators have been identified that control their differentiation, recruitment, and trafficking to tissue (13,16). Our observation that the number of fibrocytes that develop from PBMCs in culture varies from individual to individual or from the same individual over time also emphasizes the plasticity of the fibrocyte differentiation response (1,11). The identification of additional co-factors that influence fibrocyte differentiation may prove to be important in not only explicating the pathogenesis of NSF but also other fibrosing disorders that develop in response to injurious or immunologic stimuli.
Acknowledgments
Support: This work was supported by a gift from Promedior to Rice University, NIH R01 HL083029 (to RHG), and NIH/NCRR/CTSA Program Grant #1 UL1 RR024139-01.
REFERENCES
- 1.Bucala R, Spiegel L, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidt M, Sun G, Stacey M, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171:380–389. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- 3.Wang JF, Jiao H, Stewart TL, Shankowsky HA, Scott PG, Tredget EE. Fibrocytes from burn patients regulate the activities of fibroblasts. Wound Repair Regen. 2007;15:113–121. doi: 10.1111/j.1524-475X.2006.00192.x. [DOI] [PubMed] [Google Scholar]
- 4.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 5.Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol. 1998;160:419–425. [PubMed] [Google Scholar]
- 6.Hartlapp I, Abe R, Saeed RW, et al. Fibrocytes induce an angiogenic phenotype in cultured endothelial cells and promote angiogenesis in vivo. FASEB J. 2001;15:2215–2224. doi: 10.1096/fj.01-0049com. [DOI] [PubMed] [Google Scholar]
- 7.Cowper SE. Nephrogenic systemic fibrosis: a review and exploration of the role of gadolinium. Adv Dermatol. 2007;23:131–154. doi: 10.1016/j.yadr.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Cowper SE, Kuo PH, Bucala R. Nephrogenic systemic fibrosis and gadolinium exposure: association and lessons for idiopathic fibrosing disorders. Arthritis Rheum. 2007;56:3173–3175. doi: 10.1002/art.22926. [DOI] [PubMed] [Google Scholar]
- 9.Perazella MA. Tissue Deposition of Gadolinium and Development of NSF: A Convergence of Factors. Semin Dial. 2008;21:150–154. doi: 10.1111/j.1525-139X.2007.00403.x. [DOI] [PubMed] [Google Scholar]
- 10.Sieber MA, Pietsch H, Walter J, Haider W, Frenzel T, Weinmann HJ. A preclinical study to investigate the development of nephrogenic systemic fibrosis: a possible role for gadolinium-based contrast media. Invest Radiol. 2008;43:65–75. doi: 10.1097/RLI.0b013e31815e6277. [DOI] [PubMed] [Google Scholar]
- 11.Pilling D, Buckley CD, Salmon M, Gomer RH. Inhibition of fibrocyte differentiation by serum amyloid P. J Immunol. 2003;17:5537–5546. doi: 10.4049/jimmunol.171.10.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pilling D, Roife D, Wang M, et al. Reduction of bleomycin-induced pulmonary fibrosis by serum amyloid P. J Immunol. 2007;179:4035–4044. doi: 10.4049/jimmunol.179.6.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shao DD, Suresh R, Vakil V, Gomer RH, Pilling D. Pivotal Advance: Th-1 cytokines inhibit, and Th-2 cytokines promote fibrocyte differentiation. J Leukoc Biol. 2008;83:1323–1333. doi: 10.1189/jlb.1107782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thurnher SA, Capelastegui A, Del Olmo FH, et al. Safety and effectiveness of single-versus triple-dose gadodiamide injection- enhanced MR angiography of the abdomen: a phase III double-blind multicenter study. Radiology. 2001;219:137–146. doi: 10.1148/radiology.219.1.r01ap10137. [DOI] [PubMed] [Google Scholar]
- 15.Joffe P, Thomsen HS, Meusel M. Pharmacokinetics of gadodiamide injection in patients with severe renal insufficiency and patients undergoing hemodialysis or continuous ambulatory peritoneal dialysis. Acad Radiol. 1998;5:491–502. doi: 10.1016/s1076-6332(98)80191-8. [DOI] [PubMed] [Google Scholar]
- 16.Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest. 2007;87:858–870. doi: 10.1038/labinvest.3700654. [DOI] [PubMed] [Google Scholar]