Abstract
Objective
To test whether alternate day low-dose aspirin affects incidence of age-related macular degeneration (AMD) in a large-scale randomized trial of women.
Design
Randomized, double-masked, placebo-controlled trial
Participants
Thirty-nine thousand eight hundred seventy six healthy female health professionals aged 45 years or older.
Intervention
Participants were randomly assigned to receive either 100 mg of aspirin on alternate days or placebo and were followed for the presence of AMD for an average of 10 years.
Main Outcome Measure
Incident AMD responsible for a reduction in best-corrected visual acuity to 20/30 or worse based on self-report confirmed by medical record review.
Results
After 10 years of treatment and follow-up, there were 111 cases of AMD in the aspirin group and 134 cases in the placebo group (hazard ratio [HR], 0.82; 95 percent confidence interval [CI], 0.64 to 1.06).
Conclusions
In a large-scale randomized trial of female health professionals with 10 years of treatment and follow-up, low-dose aspirin had no large beneficial or harmful effect on risk of AMD.
Introduction
Age-related macular degeneration (AMD) is a chronic eye disease that involves the central retina, retinal pigment epithelium, and the subjacent choriocapillaris. An estimated 7.3 million persons in the United States (U.S) (1) have early AMD which is usually associated with little or no vision loss (2, 3). However, these persons are at increased risk of developing advanced AMD (ie. geographic atrophy or neovascular AMD) (4, 5) which is the leading cause of severe irreversible visual impairment in the US affecting an estimated 1.75 million individuals (1). At present, treatment options are limited to a minority of persons with late-stage, neovascular AMD (6-9) or intermediate AMD (10). For the majority of persons who have the earlier, atrophic form of AMD, there is no treatment and, except for avoidance of cigarette smoking (11), no recognized method of disease prevention. For these reasons, the National Eye Institute has included the development of new treatments for AMD as an important program goal for vision research (12).
The observation in some epidemiologic studies that risk factors for cardiovascular disease may also be associated with increased risks of AMD has led to a renewed interest in the vascular hypothesis of AMD (13), first proposed more than 70 years ago (14). A vascular mechanism in AMD is likely to involve a complex interplay of inflammatory (15), atherosclerotic (16), and oxidative processes (17), and underlie the characteristic decrease in choroidal blood flow detected early in the disease process (18, 19). If vascular factors do indeed play an important causal role in the pathogenesis of AMD, then agents that maintain or increase choroidal blood flow might be expected to decrease risks of AMD.
Low-dose aspirin has been shown to lead to a substantial reduction in the risk of serious occlusive vascular events (20), and could have a beneficial effect on the vasculature in AMD. Evidence from observational studies is limited and inconsistent. Two studies of patients with existing AMD showed that patients who chose to use aspirin or other nonsteroidal antiinflammatory agents (NSAIDS) had lower risks of disease progression (21, 22). Other observational studies found no significant association between aspirin use and risks of early or late AMD (23-28). In the only randomized trial reported to date, conducted among 22,071 U.S. male physicians, men assigned to alternate-day low-dose aspirin (325 mg) had a statistically non-significant 22% reduced risk of visually-significant AMD during a five year treatment period (29).
In this report we present the results for AMD from the aspirin component of the recently-completed Women's Health Study (WHS) (30, 31). The WHS was a large randomized, double-blind, placebo-controlled trial of low-dose aspirin and vitamin E in the primary prevention of cardiovascular disease and cancer among 39,876 apparently healthy women followed for a mean of 10 years.
Methods
Study Design
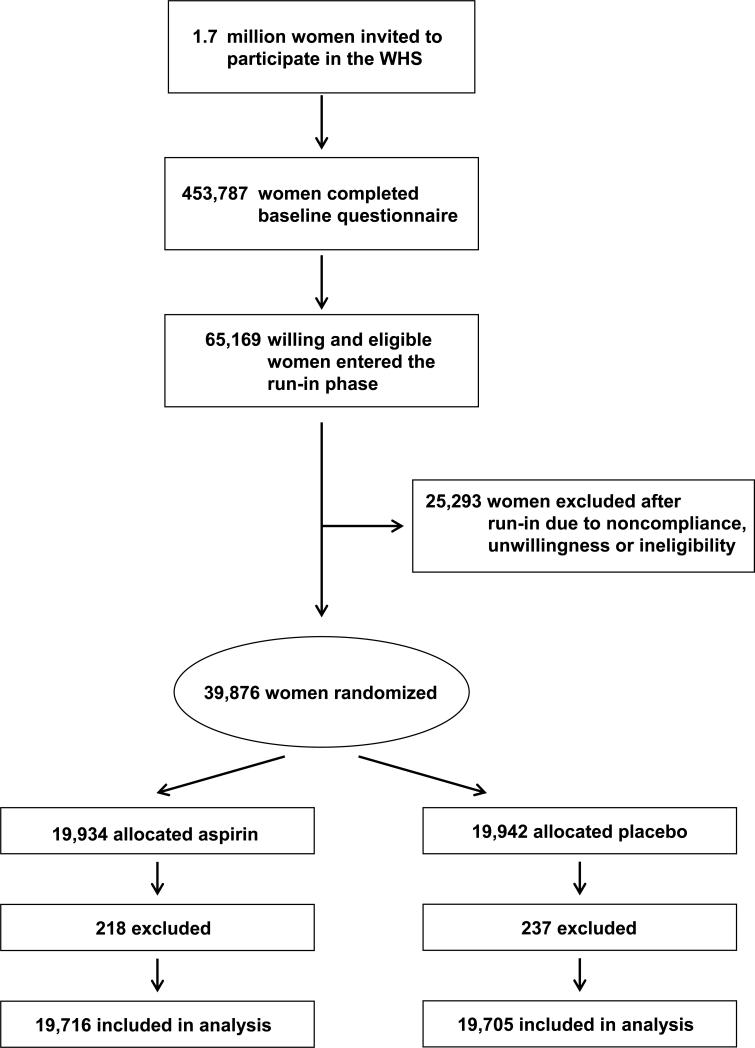
The WHS was a randomized, double-blind, placebo-controlled, 2 × 2 factorial trial testing low-dose aspirin (100 mg every other day) and vitamin E in the primary prevention of cardiovascular disease and cancer among 39,876 female health professionals aged 45 years or older (30, 31). A third component, beta carotene, was terminated early in January 1996 after a median treatment duration of 2.1 years (32, 33). Baseline information included height, weight, history of cigarette smoking, history of alcohol use, blood pressure level, cholesterol level, history of diabetes mellitus, history of multivitamin use, parental history of myocardial infarction, postmenopausal hormone use, and history of an eye exam in the last 2 years. Information on a personal history of AMD was also obtained at baseline. A total of 39,876 women were randomized in blocks of 16 within 5-year age strata to aspirin (n=19,934) or placebo (n=19,942) using computer-generated random numbers. A total of 39,421 women were without a diagnosis of AMD at baseline and are included in these analyses:19,716 were in the aspirin group and 19,705 were in the placebo group (Figure 1). Informed consent was obtained from all participants, and the research protocol was reviewed and approved by the institutional review board at Brigham and Women's Hospital in Boston. This trial is registered at clinicaltrials.gov (NCT00000161).
Annual questionnaires were sent to all participants to monitor their compliance with pill-taking and the occurrence of any relevant events including AMD. Pill-taking and end point ascertainment were continued in blinded fashion through the scheduled end of the trial on March 31, 2004. Morbidity and mortality follow-up were 97.2% and 99.4% complete, respectively. Compliance (defined as taking at least two thirds of the study aspirin or aspirin placebo) was 76% at 5 years, 67% at 10 years, and averaged 73% throughout the trial.
Ascertainment and definition of endpoints
At baseline, participants were asked “Have you ever had macular degeneration diagnosed?” Those who responded affirmatively were excluded from this analysis. Information on new diagnoses of AMD was requested on annual questionnaires. Participants were asked “In the past year, have you had any of the following?” with response options including “macular degeneration right eye” and “macular degeneration left eye”. If yes, participants were requested to provide the month and year of the diagnosis and to complete a consent form granting permission to examine medical records pertaining to the diagnosis. Ophthalmologists and optometrists were contacted by mail and requested to complete an AMD questionnaire supplying information about the date of initial diagnosis, the best-corrected visual acuity at the time of diagnosis, and the date when best-corrected visual acuity reached 20/30 or worse (if different from the date of initial diagnosis). Information was also requested about signs of AMD observed (drusen, retinal pigment epithelium [RPE] hypo/hyperpigmentation, geographic atrophy, RPE detachment, subretinal neovascular membrane, or disciform scar) when visual acuity was first noted to be 20/30 or worse, and the date when exudative neovascular disease, if present, was first noted (defined by presence of RPE detachment, subretinal neovascular membrane, or disciform scar). We also asked whether there were other ocular abnormalities that would explain or contribute to vision loss and if so, whether the AMD, by itself, was significant enough to cause the best-corrected visual acuity to be reduced to 20/30 or worse. Alternatively, ophthalmologist(s) and optometrist(s) could provide the requested information by supplying photocopies of the relevant medical records. Medical records were obtained for 85.2% of participants reporting AMD.
Medical records were reviewed without knowledge of treatment assignment. The primary endpoint was visually-significant AMD defined as a self-report confirmed by medical record evidence of an initial diagnosis after randomization but before March 31, 2004, with best-corrected vision loss to 20/30 or worse attributable to AMD. We also examined two secondary endpoints: advanced AMD, comprised of those cases of exudative neovascular AMD (defined by presence of RPE detachment, subretinal neovascular membrane, or disciform scar) plus cases of geographic atrophy; and AMD with or without vision loss, comprised of all incident cases confirmed by medical records.
Data analysis
Cox proportional hazards regression was used to estimate the hazard ratio (HR) of AMD among those assigned to receive aspirin compared with those assigned to receive placebo after adjustment for age (years) at baseline and randomized vitamin E and beta carotene assignments (34). Models were also fit separately within three age groups; 45−54, 55−64, 65+ years. The proportionality assumption was tested by including an interaction term of aspirin with the logarithm of time in the Cox models. The proportionality assumption was not violated for visually-significant AMD (P = 0.60), or for either of the secondary endpoints (advanced AMD, P=0.76); AMD with or without vision loss, P = 0.75). For each HR, we also calculated the 95% confidence interval (CI) and two-sided P value.
We also analyzed subgroup data by categories of baseline variables that are possible risk factors for AMD. We explored possible modification of any effect of aspirin by using interaction terms between subgroup indicators and aspirin assignment, testing for trend when subgroup categories were ordinal.
Individuals, rather than eyes, were the unit of analysis because eyes were not examined independently, and participants were classified according to the status of the worse eye as defined by disease severity. When the worse eye was excluded because of visual acuity loss attributed to other ocular abnormalities, the fellow eye was considered for classification.
Results
The baseline characteristics of participants in the aspirin and placebo groups are shown in Table 1. As expected in this large, randomized trial, baseline characteristics were equally distributed between the two treatment groups.
Table 1.
Baseline Characteristics in Randomized Aspirin and Placebo Treatment Groups in the Women's Health Study.*
|
Characteristics |
Aspirin (n=19,716) |
|
Placebo (n=19,705) |
|
|---|---|---|---|---|
| Mean age, y |
54.5 |
|
54.5 |
|
| 45−54 |
60.7 |
|
60.6 |
|
| 55−64 |
29.4 |
|
29.4 |
|
| 65+ |
9.9 |
|
9.9 |
|
| Cigarette smoking | ||||
| Current | 13.0 | 13.4 | ||
| Past/Never |
87.0 |
|
86.6 |
|
| Alcohol use | ||||
| <1/wk | 58.3 | 58.2 | ||
| ∃1/wk |
41.7 |
|
41.8 |
|
| Body-mass index† | ||||
| Mean | 26.1 | 26.0 | ||
| <25.0 | 50.8 | 50.9 | ||
| 25.0−29.9 | 30.9 | 31.0 | ||
| ∃30.0 |
18.3 |
|
18.1 |
|
| Hypertension‡ | ||||
| Yes | 25.8 | 25.5 | ||
| No |
74.2 |
|
74.5 |
|
| Hyperlipidemia§ | ||||
| Yes | 29.7 | 28.9 | ||
| No |
70.3 |
|
71.1 |
|
| Diabetes mellitus | ||||
| Yes | 2.6 | 2.4 | ||
| No |
97.4 |
|
97.6 |
|
| Menopausal status and use of HT | ||||
| Premenopausal | 27.7 | 27.8 | ||
| Uncertain | 17.8 | 18.3 | ||
| Postmenopausal and current HT | 30.3 | 29.7 | ||
| Postmenopausal and no HT |
24.2 |
|
24.2 |
|
| Parental history of MI before 60 yrs of age | ||||
| Yes | 13.0 | 12.9 | ||
| No |
87.0 |
|
87.1 |
|
| Multivitamin use | ||||
| Current | 36.8 | 36.6 | ||
| Past/Never |
63.2 |
|
63.4 |
|
| Eye exam in past 2 yrs | ||||
| Yes | 82.7 | 82.6 | ||
| No | 17.3 | 17.4 | ||
Abbreviations: HT, hormone therapy; MI, myocardial infarction.
Data are given as the percentage of participants, unless otherwise indicated. Percentages may not total 100 because of rounding.
Body-mass index is the weight in kilograms divided by the square of the height in meters.
Hypertension was defined as a systolic blood pressure of at least 140 mm Hg, a diastolic blood pressure of at least 90 mm Hg, or self-reported physician-diagnosed hypertension.
Hyperlipidemia was defined as a total cholesterol of at least 240 mg per deciliter or self-reported physician-diagnosed high cholesterol levels.
During an average of 10 years of treatment and follow-up, a total of 245 cases of visually-significant AMD were confirmed. More than eighty percent of these cases were characterized by some combination of drusen and RPE changes at the time vision was first noted to be 20/30 or worse. Fifty-five visually-significant cases developed signs of advanced AMD during follow-up.
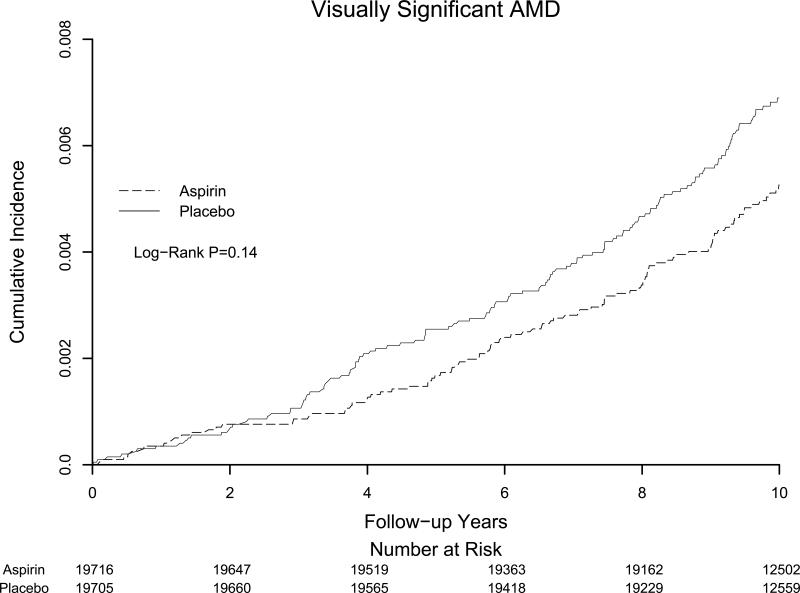
Women in the aspirin group had a statistically non-significant 18% reduced risk of the primary study endpoint of visually-significant AMD (111 cases in the aspirin group vs 134 cases in the placebo group; hazard ratio [HR], 0.82; 95% confidence interval [CI], 0.64−1.06) (Table 2). For advanced AMD, there was a statistically non-significant 10% reduced risk in the aspirin group (26 cases in the aspirin group vs 29 cases in the placebo group; HR, 0.90; CI, 0.53−1.52). When we considered all cases of AMD with or without vision loss, 302 cases were documented in the aspirin group and 291 in the placebo group (HR, 1.03, CI, 0.88 to 1.21). Cumulative incidence rates of visually significant AMD according to the year of follow-up are shown in Figure 2. The curves appeared to separate at around 3 years of follow-up, but never attained statistical significance.
Table 2.
Confirmed Cases of AMD
| Endpoint | Aspirin (N=19,716) | Placebo (N=19,705) | RR* | (95% CI) |
|---|---|---|---|---|
| Visually significant AMD | 111 | 134 | 0.82 | 0.64−1.06 |
| Advanced AMD | 26 | 29 | 0.90 | 0.53−1.52 |
| AMD with or without vision loss | 302 | 291 | 1.03 | 0.88−1.21 |
Abbreviations: AMD, age-related macular degeneration; RR, relative risk; CI, confidence interval
Adjusted for age and vitamin E and beta carotene treatment assignment
The effect of aspirin on the primary study endpoint of visually-significant AMD appeared to be modified by reported multivitamin use at baseline. Among current nonusers of multivitamins, those in the aspirin group had a statistically-significant 32% reduced risk of AMD (HR, 0.68; 95% CI, 0.49−0.95) whereas among current users of multivitamins, there was a nonsignificant 14% increased risk of AMD in the aspirin group (HR, 1.14; 95% CI, 0.76−1.70) (p interaction = 0.053) (Table 3).
Table 3.
Effect of Randomized Aspirin Assignment on Visually Significant AMD by Subgroups.
| |
No. of AMD/Total |
|
|
|
|
|---|---|---|---|---|---|
| |
Aspirin (N=19,716) |
Placebo (N=19,705) |
RR* |
95% CI |
P-value |
| Age, y | |||||
| 45−54 | 9/11,959 | 20/11,948 | 0.46 | 0.21−0.99 | 0.05 |
| 55−64 | 39/5,806 | 39/5,801 | 1.00 | 0.64−1.55 | 0.98 |
| ∃65 |
63/1,951 |
75/1,956 |
0.84 |
0.60−1.17 |
0.30 |
| Smoke Cigarettes | |||||
| Current | 16/2,558 | 25/2,633 | 0.73 | 0.39−1.38 | 0.34 |
| Past/Never |
95/17,143 |
108/17,052 |
0.86 |
0.65−1.14 |
0.29 |
| Alcohol Use | |||||
| <1/wk | 67/11,493 | 88/11,455 | 0.75 | 0.55−1.03 | 0.08 |
| ∃1/wk |
44/8,218 |
46/8,245 |
0.97 |
0.64−1.47 |
0.88 |
| Body-mass index | |||||
| <25.0 | 48/9,803 | 72/9,826 | 0.67 | 0.46−0.96 | 0.03 |
| 25.0−29.9 | 32/5,967 | 36/5,985 | 0.86 | 0.54−1.39 | 0.54 |
| ∃30.0 |
28/3,535 |
25/3,504 |
1.16 |
0.68−2.00 |
0.58 |
| Hypertension | |||||
| Yes | 51/5,093 | 50/5,032 | 0.99 | 0.67−1.46 | 0.94 |
| No |
60/14,617 |
84/14,670 |
0.73 |
0.52−1.01 |
0.06 |
| Hyperlipidemia | |||||
| Yes | 40/5,858 | 53/5,697 | 0.77 | 0.51−1.16 | 0.20 |
| No |
71/13,848 |
81/14,002 |
0.86 |
0.63−1.19 |
0.37 |
| Diabetes | |||||
| Yes | 4/514 | 5/477 | 0.77 | 0.21−2.87 | 0.69 |
| No |
107/19,190 |
129/19,216 |
0.83 |
0.64−1.07 |
0.15 |
| Menopausal status and use of HT | |||||
| Premenopausal | 3/5,456 | 7/5,465 | 0.43 | 0.11−1.65 | 0.22 |
| Uncertain | 7/3,493 | 8/3,595 | 0.89 | 0.32−2.45 | 0.82 |
| Postmenopausal and current HT | 33/5,967 | 44/5,832 | 0.74 | 0.47−1.16 | 0.18 |
| Postmenopausal and no HT |
68/4,752 |
75/4,761 |
0.91 |
0.65−1.26 |
0.56 |
| Parental history of MI before 60 yrs of age | |||||
| Yes | 9/2,299 | 13/2,286 | 0.82 | 0.35−1.92 | 0.64 |
| No |
93/15,381 |
101/15,458 |
0.90 |
0.68−1.19 |
0.46 |
| Multivitamin Use | |||||
| Current | 50/7,155 | 46/7,110 | 1.14 | 0.76−1.70 | 0.53 |
| Past/Never |
61/12,289 |
87/12,313 |
0.68 |
0.49−0.95 |
0.02 |
| Eye exam in past 2 yrs | |||||
| Yes | 97/16,307 | 112/16,276 | 0.86 | 0.66−1.13 | 0.29 |
| No | 14/3,408 | 22/3,423 | 0.64 | 0.33−1.25 | 0.19 |
Abbreviations: AMD, age-related macular degeneration; CI, confidence interval; HT, hormone therapy; MI, myocardial infarction.
Adjusted for age and vitamin E and beta carotene treatment assignment.
Discussion
In this large randomized trial, women assigned to alternate-day treatment with low-dose aspirin and followed for an average of 10 years had a non-significant 18% reduced risk of visually-significant AMD compared to women assigned to placebo. The 95% CI around this estimate could not rule out a possible beneficial effect as large as 36%, or a small harmful effect of 6% or less. Aspirin treatment appeared to have little effect on the endpoints of advanced AMD or all AMD cases with or without vision loss.
Evidence from observational studies on aspirin use and AMD is limited. A retrospective analysis of clinic patients with prevalent AMD showed that patients with choroidal neovascularization, compared to patients with dry AMD, were significantly less likely to use aspirin based on review of medication records (21). Prospective observational data from the Age-Related Eye Disease Study (AREDS), comprised of patients at high risk of developing advanced AMD, showed no association between self-selected aspirin use at baseline and risk of neovascular AMD or geographic atrophy during 6.3 years of follow-up. However, the risk of geographic atrophy was inversely related to use of NSAIDS in that study (22). In the Macular Photocoagulation Study, there was no significant difference between users and nonusers of aspirin in recurrence rate of AMD during 4 years of follow-up after initially successful laser treatment (23, 24). Three other prospective studies with mean follow-up of 4.8 to 6.5 years also reported no relation between use of aspirin or other NSAIDS and risk of early or late AMD (26-28). Taken together, these observational studies provide little support for a benefit of aspirin treatment in AMD. Of note, however, these studies lacked detailed information on frequency, dosage, and duration of aspirin use, and thus had limited ability to assess the potential benefits of long-term aspirin treatment on AMD. Finally, several anecdotal reports of an increased risk of retinal hemorrhage in persons with AMD who used aspirin or other antiplatelet agents were limited by the absence of a comparison group and thus were impossible to interpret (35-37).
Only one previous randomized trial has examined aspirin treatment in AMD. In Physicians’ Health Study I, men assigned to alternate-day low-dose aspirin (325 mg) had a non-significant 22% reduced risk of visually-significant AMD (HR, 0.78; CI, 0.46−1.32) during 5 years of follow-up (29). However, the power of that trial to detect a benefit of aspirin treatment was limited by the early termination of the randomized aspirin component of PHS I (due primarily to a statistically extreme benefit of aspirin on first myocardial infarction [38]) which resulted in a far lower number of incident cases of visually-significant AMD (n=57) than would have accrued without early termination. The present finding of a non-significant 18% reduced risk of visually-significant AMD in WHS is similar in magnitude to that reported for men in PHS I. When we combined data from the WHS and PHS I in a stratified proportional hazards model (with study as the strata), overall results indicate a possible, but statistically non-significant, 18% reduced risk of visually-significant AMD for those assigned to aspirin (HR, 0.82; 95% CI, 0.65−1.03). These combined data from women and men without a prior diagnosis of AMD tend to rule out a large beneficial effect for low-dose aspirin treatment on AMD occurrence, but are consistent with a modest benefit which, if confirmed in other trials of sufficient size and duration, would be of potential public health importance. Although most of the documented cases in the WHS and PHS I were characterized by a combination of drusen and RPE changes reflecting an early stage of AMD development, persons with early AMD are at increased risk of developing advanced AMD (4,5), the leading cause of severe irreversible visual impairment in the US. Finally, the finding that the effect of aspirin on visually-significant AMD appeared to be modified by reported multivitamin use at baseline is intriguing and deserves further investigation. On the other hand, this could simply be a chance observation in view of the multiple comparisons.
There are several possible pathways through which aspirin could potentially exert a beneficial effect in AMD. At the dose tested in WHS (100 mg every other day), aspirin irreversibly inhibits platelet cyclooxygenase, resulting in a rapid and marked inhibition of platelet function and an immediate decrease in platelet aggregability and risk of thrombosis (39-41). This mechanism of action is believed to underlie the protective effects of low-dose aspirin in CVD (42, 43), but seems unlikely to be an important mechanism in AMD. A more likely mechanism may involve the long-term effects of platelet inhibition on initiation and progression of atherosclerosis. Platelet inhibition may limit platelet adherence or aggregation on vascular endothelium and existing plaque, and may alter the chemotactic and adhesive properties of endothelial cells, which may be an important early pathophysiological event in atherogenesis (44). At present, however, it remains unclear whether atherosclerosis is an important pathogenic mechanism in AMD (45, 46). Aspirin may also influence AMD development through pathways not dependent on platelet inhibition. For example, in the vascular endothelium aspirin has been shown to initiate production of 15-epi-lipoxin A4 which functions as a local endogenous antiinflammatory mediator (47, 48). Aspirin may also exert an antioxidant effect (49) by protecting endothelial cells from the deleterious effects of hydrogen peroxide and other oxidative agents (50-52), and perhaps by suppressing lipid peroxidation (53).
Several possible limitations of the study need to be considered. Ascertainment of AMD cases was based on participant reports and thus some degree of underascertainment of AMD is plausible. Such underascertainment would likely reduce study power and also limit the comparison of incidence rates between this and other populations. However, underascertainment of disease is not associated with bias in randomized comparisons. Random misclassification of reported AMD, which would tend to shift the relative risk estimate toward the null, was reduced by the use of medical records to confirm the participant reports. Non-random or differential misclassification was unlikely since medical records were reviewed without knowledge of aspirin treatment assignment, and study participants and treating ophthalmologists and optometrists were unaware of aspirin treatment assignment. Confounding is unlikely in this large randomized trial since, as expected, baseline characteristics were equally distributed between the aspirin and placebo groups. This provides reassurance that other potential confounders, which were either unmeasured or unknown, were also likely to be evenly distributed between the two treatment groups.
In summary, these randomized trial data from a large population of healthy women indicate that 10 years of treatment with low-dose aspirin has no large beneficial effect in reducing risk of visually-significant AMD. However, a modest, but potentially important, beneficial effect on visually-significant AMD could not be ruled out and warrants continued examination in other populations of men and women.
Supplementary Material
Acknowledgments
Supported by research grants CA 47988, HL 43851, and EY 06633 from the National Institutes of Health, Bethesda, Md. Pills and packaging were provided by Bayer Healthcare and the Natural Source Vitamin E Association.
Footnotes
The authors report no conflicts of interest. Dr. Christen had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis
References
- 1.Eye Diseases Prevalence Research Group Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–72. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 2.Klein R, Wang Q, Klein BE, et al. The relationship of age-related maculopathy, cataract, and glaucoma to visual acuity. Invest Ophthalmol Vis Sci. 1995;36:182–91. [PubMed] [Google Scholar]
- 3.Hogg RE, Chakravarthy U. Visual function and dysfunction in early and late age-related maculopathy. Prog Retin Eye Res. 2006;25:249–76. doi: 10.1016/j.preteyeres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Klein R, Klein BE, Tomany SC, et al. Ten-year incidence and progression of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 2002;109:1767–79. doi: 10.1016/s0161-6420(02)01146-6. [DOI] [PubMed] [Google Scholar]
- 5.Age-Related Eye Disease Study Research Group A simplified severity scale for age-related macular degeneration: AREDS report no. 18. Arch Ophthalmol. 2005;123:1570–4. doi: 10.1001/archopht.123.11.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macular Photocoagulation Study Group Laser photocoagulation of subfoveal neovascular lesions of age-related macular degeneration: updated findings from two clinical trials. Arch Ophthalmol. 1993;111:1200–9. doi: 10.1001/archopht.1993.01090090052019. [DOI] [PubMed] [Google Scholar]
- 7.Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials-TAP report 2. Arch Ophthalmol. 2001;119:198–207. [PubMed] [Google Scholar]
- 8.Gragoudas ES, Adamis AP, Cunningham ET, Jr, et al. VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351:2805–16. doi: 10.1056/NEJMoa042760. [DOI] [PubMed] [Google Scholar]
- 9.Rosenfeld PJ, Brown DM, Heier JS, et al. MARINA Study Group Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 10.Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–36. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christen WG, Glynn RJ, Manson JE, et al. A prospective study of cigarette smoking and risk of age-related macular degeneration in men. JAMA. 1996;276:1147–51. [PubMed] [Google Scholar]
- 12.National Eye Institute [May 21, 2009];National Plan for Eye and Vision Research: Retinal Diseases Program. (updated October 2008). Available at: http://www.nei.nih.gov/strategicplanning/np_retinal.asp.
- 13.Snow KK, Seddon JM. Do age-related macular degeneration and cardiovascular disease share common antecedents? Ophthalmic Epidemiol. 1999;6:125–43. doi: 10.1076/opep.6.2.125.1558. [DOI] [PubMed] [Google Scholar]
- 14.Verhoeff FH, Grossman HP. The pathogenesis of isciform degeneration of the macula. Trans Am Ophthalmol Soc. 1937;35:262–94. [PMC free article] [PubMed] [Google Scholar]
- 15.Hageman GS, Luthert PJ, Victor Chong NH, et al. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch's membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res. 2001;20:705–32. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- 16.Friedman E. The role of the atherosclerotic process in the pathogenesis of age-related macular degeneration. Am J Ophthalmol. 2000;130:658–63. doi: 10.1016/s0002-9394(00)00643-7. [DOI] [PubMed] [Google Scholar]
- 17.Beatty S, Koh H, Phil M, et al. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45:115–34. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 18.Pauleikhoff D, Chen JC, Chisholm IH, Bird AC. Choroidal perfusion abnormality with age-related Bruch's membrane change. Am J Ophthalmol. 1990;109:211–7. doi: 10.1016/s0002-9394(14)75989-6. [DOI] [PubMed] [Google Scholar]
- 19.Grunwald JE, Metelitsina TI, Dupont JC, et al. Reduced foveolar choroidal blood flow in eyes with increasing AMD severity. Invest Ophthalmol Vis Sci. 2005;46:1033–8. doi: 10.1167/iovs.04-1050. [DOI] [PubMed] [Google Scholar]
- 20.Antithrombotic Trialists’ Collaboration Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson HL, Schwartz DM, Bhatt HR, et al. Statin and aspirin therapy are associated with decreased rates of choroidal neovascularization among patients with age-related macular degeneration. Am J Ophthalmol. 2004;137:615–24. doi: 10.1016/j.ajo.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 22.Age-Related Eye Disease Study Research Group Risk factors for the incidence of advanced age-related macular degeneration in the Age-Related Eye Disease Study (AREDS): AREDS report no. 19. Ophthalmology. 2005;112:533–9. doi: 10.1016/j.ophtha.2004.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macular Photocoagulation Study Group Recurrent choroidal neovascularization after argon laser photocoagulation for neovascular maculopathy. Arch Ophthalmol. 1986;104:503–12. doi: 10.1001/archopht.1986.01050160059012. [DOI] [PubMed] [Google Scholar]
- 24.Macular Photocoagulation Study Group Persistent and recurrent neovascularization after krypton laser photocoagulation for neovascular lesions of age-related macular degeneration. Arch Ophthalmol. 1990;108:825–31. doi: 10.1001/archopht.1990.01070080067037. [DOI] [PubMed] [Google Scholar]
- 25.Klein ML. Macular degeneration: is aspirin a risk for progressive disease? JAMA. 1991;266:2279. doi: 10.1001/jama.266.16.2279b. [DOI] [PubMed] [Google Scholar]
- 26.Klein R, Klein E, Jensen SC, et al. Medication use and the 5-year incidence of early age-related maculopathy: the Beaver Dam Eye Study. Arch Ophthalmol. 2001;119:1354–9. doi: 10.1001/archopht.119.9.1354. [DOI] [PubMed] [Google Scholar]
- 27.Wang JJ, Mitchell P, Smith W, et al. Systemic use of anti-inflammatory medications and age-related maculopathy: the Blue Mountains Eye Study. Ophthalmic Epidemiol. 2003;10:37–48. doi: 10.1076/opep.10.1.37.13776. [DOI] [PubMed] [Google Scholar]
- 28.van Leeuwen R, Tomany SC, Wang JJ, et al. Is medication use associated with the incidence of early age-related maculopathy? Pooled findings from 3 continents. Ophthalmology. 2004;111:1169–75. doi: 10.1016/j.ophtha.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 29.Christen WG, Glynn RJ, Ajani UA, et al. Age-related maculopathy in a randomized trial of low-dose aspirin among US physicians. Arch Ophthalmol. 2001;119:1143–9. doi: 10.1001/archopht.119.8.1143. [DOI] [PubMed] [Google Scholar]
- 30.Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 31.Cook NR, Lee IM, Gaziano JM, et al. Low-dose aspirin in the primary prevention of cancer: The Women's Health Study: a randomized controlled trial. JAMA. 2005;294:47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 32.Lee IM, Cook NR, Manson JE, et al. Beta-carotene supplementation and incidence of cancer and cardiovascular disease: the Women's Health Study. J Natl Cancer Inst. 1999;91:2102–6. doi: 10.1093/jnci/91.24.2102. [DOI] [PubMed] [Google Scholar]
- 33.Christen W, Glynn R, Sperduto R, et al. Age-related cataract in a randomized trial of beta-carotene in women. Ophthalmic Epidemiol. 2004;11:401–12. doi: 10.1080/09286580490515152. [DOI] [PubMed] [Google Scholar]
- 34.Cox DR. Regression models and life tables. J R Stat Soc Series B Stat Methodol. 1972;34:187–220. [Google Scholar]
- 35.el Baba F, Jarrett WH, II, Harbin TS, Jr, et al. Massive hemorrhage complicating age-related macular degeneration: clinicopathologic correlation and role of anticoagulants. Ophthalmology. 1986;93:1581–92. doi: 10.1016/s0161-6420(86)33540-1. [DOI] [PubMed] [Google Scholar]
- 36.Kingham JD, Chen MC, Levy MH. Macular hemorrhage in the aging eye: the effects of anticoagulants [letter]. N Engl J Med. 1988;318:1126–7. doi: 10.1056/NEJM198804283181710. [DOI] [PubMed] [Google Scholar]
- 37.Kinshuck D, Stevenson L. Complications of NSAID therapy in patients with macular disease [letter]. Surv Ophthalmol. 1992;3:149. doi: 10.1016/0039-6257(92)90085-8. [DOI] [PubMed] [Google Scholar]
- 38.Steering Committee of the Physicians’ Health Study Research Group Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med. 1989;321:129–35. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 39.Roth GJ, Stanford N, Majerus PW. Acetylation of prostaglandin synthase by aspirin. Proc Natl Acad Sci U S A. 1975;72:3073–6. doi: 10.1073/pnas.72.8.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burch JW, Stanford N, Majerus PW. Inhibition of platelet prostaglandin synthetase by oral aspirin. J Clin Invest. 1978;61:314–9. doi: 10.1172/JCI108941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ridker PM, Hennekens CH, Tofler GH, et al. Anti-platelet effects of 100 mg alternate day oral aspirin: a randomized, double-blind, placebo-controlled trial of regular and enteric coated formulations in men and women. J Cardiovasc Risk. 1996;3:209–12. [PubMed] [Google Scholar]
- 42.Ridker PM, Manson JE, Buring JE, et al. The effect of chronic platelet inhibition with low-dose aspirin on atherosclerotic progression and acute thrombosis: clinical evidence from the Physicians’ Health Study. Am Heart J. 1991;122:1588–92. doi: 10.1016/0002-8703(91)90275-m. [DOI] [PubMed] [Google Scholar]
- 43.Eidelman RS, Hebert PR, Weisman SM, Hennekens CH. An update on aspirin in the primary prevention of cardiovascular disease. Arch Intern Med. 2003;163:2006–10. doi: 10.1001/archinte.163.17.2006. [DOI] [PubMed] [Google Scholar]
- 44.Gawaz M, Neumann FJ, Dickfeld T, et al. Activated platelets induce monocyte chemotactic protein-1 secretion and surface expression of intercellular adhesion molecule-1 on endothelial cells. Circulation. 1998;98:1164–71. doi: 10.1161/01.cir.98.12.1164. [DOI] [PubMed] [Google Scholar]
- 45.Cheung N, Liao D, Amirul Islam FM, et al. Is early age-related macular degeneration related to carotid artery stiffness? The Atherosclerosis and Risk in Communities Study. Br J Ophthalmol. 2007;91:430–3. doi: 10.1136/bjo.2006.106054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong TY, Klein R, Sun C, et al. Atherosclerosis Risk in Communities Study Age-related macular degeneration and risk for stroke. Ann Intern Med. 2006;145:98–106. doi: 10.7326/0003-4819-145-2-200607180-00007. [DOI] [PubMed] [Google Scholar]
- 47.Serhan CN. Lipoxins and aspirin-triggered 15-epi-lipoxin biosynthesis: an update and role in anti-inflammation and pro-resolution. Prostaglandins Other Lipid Mediat. 2002;68−69:433–55. doi: 10.1016/s0090-6980(02)00047-3. [DOI] [PubMed] [Google Scholar]
- 48.Chiang N, Bermudez EA, Ridker PM, et al. Aspirin triggers antiinflammatory 15-epilipoxin A4 and inhibits thromboxane in a randomized human trial. Proc Natl Acad Sci U S A. 2004;101:15178–83. doi: 10.1073/pnas.0405445101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Awtry EH, Loscalzo J. Aspirin. Circulation. 2000;101:1206–18. doi: 10.1161/01.cir.101.10.1206. [DOI] [PubMed] [Google Scholar]
- 50.Aruoma OI, Halliwell B. The iron-binding and hydroxyl radical scavenging action of anti-inflammatory drugs. Xenobiotica. 1988;18:459–70. doi: 10.3109/00498258809041682. [DOI] [PubMed] [Google Scholar]
- 51.Grosser N, Schroder H. Aspirin protects endothelial cells from oxidant damage via the nitric oxide-cGMP pathway. Arterioscler Thromb Vasc Biol. 2003;23:1345–51. doi: 10.1161/01.ATV.0000083296.57581.AE. [DOI] [PubMed] [Google Scholar]
- 52.Podhaisky HP, Abate A, Polte T, et al. Aspirin protects endothelial cells from oxidative stress--possible synergism with vitamin E. FEBS Lett. 1997;417:349–51. doi: 10.1016/s0014-5793(97)01307-0. [DOI] [PubMed] [Google Scholar]
- 53.Steer KA, Wallace TM, Bolton CH, Hartog M. Aspirin protects low density lipoprotein from oxidative modification. Heart. 1997;77:333–7. doi: 10.1136/hrt.77.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.