Abstract
Central neuropathic pain (CNP) developing after spinal cord injury (SCI) is described by the region affected: above-level, at-level and below-level pain occurs in dermatomes rostral, at/near, or below the SCI level, respectively. People with SCI and rodent models of SCI develop above-level pain characterized by mechanical allodynia and thermal hyperalgesia. Mechanisms underlying this pain are unknown and the goals of this study were to elucidate components contributing to the generation of above-level CNP. Following a thoracic (T10) contusion, forelimb nociceptors had enhanced spontaneous activity and were sensitized to mechanical and thermal stimulation of the forepaws 35 days post-injury. Cervical dorsal horn neurons showed enhanced responses to non-noxious and noxious mechanical stimulation as well as thermal stimulation of receptive fields. Immunostaining dorsal root ganglion (DRG) cells and cord segments with activating transcription factor 3 (ATF3, a marker for neuronal injury) ruled out neuronal damage as a cause for above-level sensitization since few C8 DRG cells expressed AFT3 and cervical cord segments had few to no ATF3-labeled cells. Finally, activated microglia and astrocytes were present in thoracic and cervical cord at 35 days post-SCI, indicating a rostral spread of glial activation from the injury site. Based on these data, we conclude that peripheral and central sensitization as well as reactive glia in the uninjured cervical cord contribute to CNP. We hypothesize that reactive glia in the cervical cord release pro-inflammatory substances which drive chronic CNP. Thus a complex cascade of events spanning many cord segments underlies above-level CNP.
Keywords: neuroplasticity, primary afferents, nociception, microglia activation, astrocyte activation
1. Introduction
Following both complete and partial spinal lesions, chronic central pain syndromes develop in the majority of spinal cord injured (SCI) patients [14, 99], usually within a month following injury [73]. The pain so greatly affects the quality of life that depression and suicide frequently result [7, 80]. This painful condition is permanent and because the lesion is often contained within the central nervous system (CNS), the condition is referred to as central neuropathic pain (CNP). Three regions exhibiting CNP after SCI have been defined [84]: 1) above-level pain occurs in dermatomes cranial to the injury site, 2) at-level or “girdle” pain occurs in dermatomes at or near the spinal injury and 3) below-level pain is localized to dermatomes caudal to the injury site [85, 90]. Mechanisms underlying at- and below-level pain have been an intense focus of research with the ultimate goal of improving treatment for CNP in SCI patients [42, 43, 100]. Almost nothing is known about the mechanisms underlying above-level CNP. The pain abnormalities include two components: spontaneous and evoked pain. Spontaneous pain occurs in the absence of any peripheral stimulus. Evoked pain occurs in the form of allodynia, in which a non-noxious stimulus is perceived as noxious; and hyperalgesia, when a noxious stimulus produces an exaggerated pain response, being perceived as even more painful. Rats with a moderate T10 SCI develop above-level pain, evidenced by mechanical allodynia and heat hyperalgesia in the forelimbs [45]. In the present study we provide evidence that both peripheral and central mechanisms contribute to the sensory abnormalities occurring in these remote, non-injured segments.
2. Methods
2.1 Animals
Adult male, Sprague-Dawley rats (230–250 g) were used in all experiments. They were housed on soft bedding in groups of two under a 12 hr reverse light/dark cycle. All procedures were reviewed and approved by the University of Texas Medical Branch Animal Care and Use Committee and followed the guidelines of the International Association for the Study of Pain (IASP) for the ethical treatment of animals [103]. Every effort was made to reduce the number of animals needed and their discomfort.
2.2 Thoracic contusion surgery to create a spinal cord injury (SCI)
Rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p.). Sterile procedures were used throughout the surgery. The back of each animal was shaved and cleaned with betadine. A 5 cm incision was made along the vertebral column from ~T3 to T13 vertebra. The muscle surrounding the T9–11 vertebrae was retracted and a laminectomy was performed at the T9–10 level to expose the spinal cord. The vertebral column was fixed beneath an Infinite Horizons (IH) impactor with forceps and a contusion injury at T10 was induced by applying a force of 150 kdynes at a velocity of 1mm/s and a dwell time of 1s. Sham animals underwent the same surgery but received no contusion. After closing the incision in layers with 3–0 silk suture and wound clips, the animals were placed on a heating pad to recover from anesthesia before being returned to their home cages. Postoperative care of SCI animals required the manual expression of bladders and subcutaneous injections of Baytril® (0.3 ml, sc) twice daily until bladder function was regained (3 consecutive days with no or only a few drops of urine present at each voiding attempt). Animals were given Ensure or Boost (nutritional supplements) twice daily (1ml) until bladder function was regained. Following surgery, all animals were monitored for locomotor recovery, as well as for mechanical and tactile sensitivity as described below.
2.3 Behavioral studies
Habituation: Animals were habituated to the mechanical testing apparatus by being placed in transparent acrylic restrainers (8 × 8 × 10 cm) over a metal mesh platform for 30 min per day for 3 days. They were acclimated to thermal testing by being placed in acrylic restrainers on a ¼” thick glass plate maintained at 23 °C. Each forepaw of each rat was habituated by exposure to the laser stimulus (see below) alternating every 5 min for 4 trials on each of 5 consecutive days.
Open-Field Locomotion: Recovery of hindlimb locomotor function was assessed while animals moved freely in a rectangular open field enclosure. Animals were observed for 2 min while hindlimb locomotion was scored using the Basso, Beattie, and Bresnahan scale (BBB) [1]. Briefly, the BBB Scale is a 21 point scale that can show the recovery of hindlimb function by a change in score from “0” (no hindlimb movement) to a score of “21” (normal hindlimb locomotion). Hindlimbs were scored once prior to contusion surgery to establish that all animals had intact locomotion. Following contusion surgery, hindlimb function was scored daily for 2 weeks (days 1–14), and then weekly on days 21, 28, and 35.
Mechanical Sensitivity: For some animals (n = 11), paw withdrawal thresholds (PWT) were assessed on the ventral surface of the forepaw using von Frey filaments (Stoelting, Wood Dale, IL, USA) and the up-down method [12, 22]. The PWT during the week pre-surgery and during the 5th week post-surgery was assessed once each day for 3 days and the values were averaged to determine changes in mechanical sensitivity.
Thermal sensitivity: Paw withdrawal latency (PWL) to a thermal stimulus was tested using a solid state laser and a modified Hargreaves method [40]. On the testing day, animals were placed in acrylic restrainers on the glass plate and acclimated for 15–30 min or until exploratory behavior ceased. The laser system (made in-house) was comprised of a microprocessor-controlled 980 nm (near infrared) continuous wave, solid-state laser (4 watt) with a spot diameter of 2 mm. The parameters of the laser were set so that the temperature of the glass ramped from 23 °C to 52 °C over 12 s with a cutoff at 15 s. PWLs were determined for both the left and right forepaws with a 5 min interval between tests. For baselines, PWL was measured each day over 3 days and averaged. Following surgery, PWL was assessed on day 35 by testing for 3 trials with a 5-min inter-trial interval; the trials were averaged to determine the mean PWL.
Once baseline mechanical and thermal reactivity was assessed, animals were assigned to either sham or contusion groups. The best attempts were made to perform behavioral tests in a blinded fashion following SCI/sham surgery.
2.4 In vitro peripheral nerve recordings from the forelimb median nerve
Naïve, sham or 35 day SCI rats (male, Sprague Dawley) were sacrificed with an overdose of CO2. The glabrous skin from the wrist to the fingertips was dissected from the forepaw. The median nerve was dissected free and kept intact with the glabrous skin. All muscle and tendon tissue were removed from the preparation. The preparation was placed corium side up in an organ bath and superfused (15 ml/min, 34 °C) with an oxygen-saturated, modified synthetic interstitial fluid solution (SIF, in mM: NaCl, 123; KCl, 3.5; MgSO4, 0.7; CaCl2, 2.0; Na gluconate, 9.5; NaH2PO4, 1.7; Glucose, 5.5; Sucrose, 7.5; and HEPES, 10; pH 7.45 ± 0.05). The median nerves were moved into a separate chamber containing a superficial layer of mineral oil and a bottom layer of SIF. The nerves were desheathed and axon bundles teased apart on a mirror stage. Small filaments were repeatedly split with sharpened forceps until single unit activity was obtained in recordings made with gold wire electrodes. This preparation was adapted from a hindpaw glabrous skin preparation that has been used successfully to record from peripheral primary afferents in our laboratory [10, 11, 24–26] and in those of others [52, 56, 71, 76].
Neural activity was recorded using a DAM80 Differential Amplifier (World Precision Instruments, New Haven, CT). Action potentials were acquired and later analyzed with the CED 1401 (Cambridge, UK) using Spike 2 (v5.08) software. The conduction velocity of each unit was determined by monopolar electrical stimulation (1 ms duration, 1 Hz) at the most mechanosensitive site in the receptive field of each unit using a Teflon-coated steel electrode (5 Ω impedance, 250 μm shaft diameter with an uninsulated tip) that was gently lowered into the receptive field. The conduction velocity of each unit was determined from the latency of the action potential and the distance from the stimulating electrode to the recording site. Based on measurements using our in vitro chamber with a 34°C bath temperature and isolated plantar nerves (n=3), the average conduction velocity for C fibers was ≤ 1.2 ± 0.1 m/s. This agrees with previous in vitro nerve recordings in rats [52, 55].
Only units responding to mechanical probing of the glabrous skin with a blunt glass rod and with a clearly defined receptive field were studied in detail. For thermal stimulation, radiant heat was applied to the receptive field by a feedback-controlled lamp placed beneath the organ bath. The beam was focused through the bottom of the bath onto the epidermal surface of the skin. A thermocouple was placed into the corium above the light beam to measure intracutaneous temperature. A standard heat ramp starting from an adapting temperature of 34 °C and rising to 51 °C in 10 s was applied to the receptive field of each unit from the epidermal side (51 °C on the epidermal side was equal to 47 °C on the corium side). The threshold of the heat response was defined as the temperature evoking the second spike following the initiation of the ramp [24, 55]. A Dual Mode Lever System (Aurora Scientific Inc. Ontario, Canada) was used to determine mechanical threshold. The system had a motor-driven stylus that delivered force in a continuous ascending ramp (from 0–250 mN in 20 s). The stylus was placed on the corium side of the skin in the most sensitive region of the receptive field of the unit. The first spike occurring once the ramping force was activated was considered threshold for that unit. This same instrument was used to determine magnitude of response of units to a suprathreshold mechanical stimulus. The stylus was placed on the corium side in the most sensitive region of the receptive field and a square wave pulse was used to deliver a 250 mN constant stimulus for 20 s.
As previously described [71] the majority of units from normal skin had no spontaneous activity but the mechanical search stimulus (probing with a glass rod) sometimes resulted in ongoing activity at a low frequency in some units. Therefore, the spontaneous activity (imp/s) in each unit was measured for 2 min prior to stimulation and this was subtracted from the evoked responses.
2.5 In vivo dorsal horn neuron recordings in the cervical enlargement
Extracellular single-unit recordings were made from spinal cord dorsal horn neurons in sham and 35 day SCI rats. Rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) during the initial surgery. Anesthesia was maintained by continuous intravenous infusion of pentobarbital (5–8 mg/kg/hr, i.v.) through an indwelling catheter in the external jugular vein. Adequacy of anesthesia was monitored by the lack of withdrawal reflexes to noxious stimuli and the absence of corneal blink reflexes. After a tracheotomy the rats were artificially ventilated to maintain end-tidal CO2 at 3.5–4.5%. After deep, stable anesthesia was established, the animals were paralyzed with pancuronium (0.3–0.4 mg/kg/hr, i.v.). Core body temperature was regulated at 37 °C using a thermostatically controlled heating blanket. A laminectomy was performed over the cervical enlargement (C7–T1), an oil pool formed with skin flaps, and dorsal horn neurons isolated. Extracellular single-unit recordings were made with a low impedance (0.4–0.8Ω at 1KHz; Kation Scientific) glass carbon fiber microelectrode. All cells recorded from the cervical enlargement responded to stimulation of the glabrous skin of the fore paw. An average of three to four neurons per animal was collected.
Once a neuron was isolated, background activity was measured followed by cutaneous receptive field mapping with von Frey filaments and brief pinches. Peripheral stimuli included the following: brush (with a makeup brush), pressure (with large arterial clip at 144 g/mm2), pinch (with small arterial clip at 583 g/mm2), graded von Frey filament application (0.6 g, 2.0 g, 6.0 g, 15.0 g, and 60.0 g). A 45 °C stimulus (10 s) was applied to the receptive field with a Physitemp NTE-2A temperature stimulus probe. Unit activity was acquired with the set-up as described above. A stimulus-evoked response was calculated by subtracting the baseline activity from evoked activity. Responsiveness to thermal stimuli was calculated by subtracting the response to 25 °C (ambient temperature) from that at 45 °C (this factored out the unit response due to pressure produced by the temperature probe on the skin). Cumulative time histograms were constructed to compare group responses between sham and SCI animals.
Cells were classified as wide dynamic range (WDR) if they responded maximally to pinch and had a brush response greater than 10% of the maximal response [15]. Cell depth and distance from midline were recorded and mapped as described previously [36].
2.6 Immunostaining of ATF3 in the DRG and spinal cord dorsal horn
Some SCI and sham rats were perfused at 2 days or 35 days (n = 2 – 4) post-SCI for analysis of possible injury or death of DRG or dorsal horn neurons. Rats were transcardially perfused with 4% paraformaldehyde with 0.1% picric acid in 0.1 M phosphate buffer (PB) at 4 °C. The ipsilateral and contralateral C8 and T10 DRG and spinal cord segments C5–C6 and T7–9 were removed and cryoprotected overnight in 30% sucrose-PB before processing. The DRG were frozen and sectioned at 8 μm through their short axis on a cryostat, and spinal cord segments were sectioned at 30 μm in the transverse plane on a sliding microtome. The sections were immunostained on slides overnight at room temperature with antibody to activating transcription factor 3 (rabbit anti-ATF3, 1:500, Santa Cruz Biotechnology, Santa Cruz, CA), a marker of cellular stress and injury. Following rinses in 0.1 M phosphate buffered saline (PBS), the sections were incubated for 1 h in goat anti-rabbit IgG cyanine 3 (Cy3) conjugate (1:400, Jackson ImmunoResearch, West Grove, PA). After rinsing again in PBS the sections were coverslipped with Vectashield (Vector Labs, Burlingame, CA). As a control for antibody specificity, sections were incubated in antiserum in which the corresponding ATF3 peptide (100 μg peptide/1 ml antiserum) was preincubated overnight at room temperature. None of the sections stained with this solution showed positive immunofluorescence.
Some T9 DRG and spinal cord sections from 2 day SCI animals were immunostained with mouse anti-NeuN (1:5000, Chemicon, a neuron-specific label) and rabbit anti-ATF3 (1:500, Santa Cruz Biotechnology), visualized with Cy3- and Cy2-conjugated secondary antibodies, respectively. This double label allowed us to determine whether ATF3-expressing profiles were neurons or glia.
2.7 Stereological estimates of ATF3 and total cell numbers in DRGs
In a blinded fashion, estimates of ATF3-labeled and total DRG cell numbers in SCI or sham animals were analyzed using stereological protocols that have been previously described [8, 9]. After ATF3 staining, color digitized images of sections to be analyzed (24 bits per pixel) were captured using the 20x objective on an Olympus BX51 microscope with Cy3 filter cube and an attached Spot RT digital camera (Diagnostic Instruments, Sterling Heights, MI). Camera exposures were adjusted automatically by Spot software (version 3.1) and brightness and gamma corrections were made manually to achieve the best signal-to-noise ratios. The physical disector (pairs of sections) was used to achieve unbiased estimates of ATF3-labeled and total (stained with cresyl violet) DRG cells [16]. Equally spaced sections were analyzed throughout each DRG (k is the section separation). The size of most DRG sections and the magnification needed to identify labeled cells necessitated analysis of a randomly chosen subregion or fraction (f) in each section in a disector pair. Once identified, images in the subregion in each pair were outlined from the computer screen onto clear mylar sheets including all labeled cell profiles and an appropriate number of fiduciary landmarks. The mylar sheets from a pair were matched with one another using the fiduciary landmarks, and all labeled profiles that appeared in both sections were disallowed. Labeled profiles remaining were “tops” or “counts” (identified as Q- in the stereological literature). Using “fractionator sampling” [28, 93], total numbers of cresyl violet stained neurons and numbers of ATF3-labeled neurons were estimated by multiplying “Q-” by the reciprocal of the fraction (1/f) of each sampled section pair. These numbers (N) for all pairs in a DRG were summed (ΣN). The total number of neurons in the entire DRG was then estimated by (ΣN × k)/2 (divided by two because each section in a pair was used both as reference and look-up sections) [16]. Percentages of AFT3-labeled neurons were calculated by dividing the numbers of labeled neurons by the total DRG cell number × 100.
2.8 Analysis of ATF3 staining in the Spinal Cord
Using a 40X objective, spinal cord sections were analyzed using the optical disector method. Eight to ten equally spaced sections throughout the length of the thoracic (T7–9) and cervical segments (C5–6) were viewed under a Cy3 filter cube for ATF3 immunofluorescence in laminae I–VI. For each section the reference space analyzed was demarcated between the focal planes at the top and bottom of the section. Only those nuclei that came into focus as one passed from the first to the second plane were counted. Total numbers of ATF3-labeled nuclei in laminae I–VI were estimated by summing the counts from each section and multiplying by section separation (ΣN × k). In contused animals, if there was damage preventing accurate counting on one side of the dorsal horn, counts from the one undamaged side were doubled. Again the investigator was blinded as to which group of animals was being analyzed.
2.9 Protein Extraction/Western blotting from thoracic and cervical cord segments
Sham and SCI animals with a 35 day survival were perfused with 120–150 ml of saline containing heparin (1000 units/L) to eliminate blood from the spinal cord tissue. Spinal cord segments were removed and immediately placed on dry ice to freeze fully. For protein extraction, 200 μl of homogenizing buffer (10 mM Tris Base, 300 mM sucrose, 1 mM EDTA, 1mM DTT, 0.5 mM PMSF, 2 μg/ml antipain, 2 μg/ml chymostatin, 2 μg/ml pepstatin, 2 μg/ml leupeptin, pH 7.5) was added to each individual spinal cord segment for homogenizing on ice with a clear grinder (05-559-27; Fisher) that tightly fit inside the 1.5 ml tubes. After homogenization samples were vortexed for 5 s, and then centrifuged at 7,500 rpm for 5 min at 4°C. Protein concentrations were determined using the BioRad Protein Assay (500–0006; BioRad).
Samples containing 40 μg of protein were boiled for 10 min at 100 °C with an appropriate volume of 6X sample buffer (350 mM Tris–HCl, pH 6.8, 1 M urea, 1% 2-mercaptoethanol, 9.3% DTT, 13% SDS, 0.06% Bromophenol Blue, 30% glycerol). Samples were then placed on ice to cool before being loaded onto a 12% sodium dodecyl sulfate–polyacrylamide gel and separated at 150V for 4 h. Gels were then transferred overnight onto a PVDF membrane at 4 °C and 25V. Membranes were reversibly stained with Ponceau S to confirm the transfer of proteins, and destained in water. Membranes were then incubated for 1 h at room temperature (23 °C) in blocking solution (5.0% nonfat dry milk in Tris-buffered saline with 0.2% Tween-20). Primary and secondary antibodies were also diluted in blocking solution, and washes were done with Tris-buffered saline containing 0.2% Tween-20. Peroxidase activity was detected using the Amersham enhanced chemiluminescence lighting system (ECL) (RPN2209; Amersham Biosciences, Piscataway, NJ, USA). Antibodies used were directed against GFAP (Mouse anti-GFAP, Chemicon International Temecula, CA; 1: 71,000 dilution), Iba-1 (Rabbit anti-Iba-1, Wako Pure Chemicals Ind. Richmond, VA; 0.5 μg/ml), and β-Actin (anti-β-actin, Sigma; 1:10,000) which was used as a loading control in all Western blots.
2.10 Statistical Analysis
All data are presented as mean ± SEM unless otherwise noted. SigmaStat 3.1 software was used for statistical analysis. Parametric or non-parametric analyses were used, whichever was appropriate. Behavioral data were analyzed using repeated measures ANOVA. For unit recordings in the in vitro preparation, differences between groups were analyzed using a Kruskal-Wallis test followed by a Dunn's post hoc analysis. For cells in the in vivo recordings, differences in activity between groups were analyzed using a one way ANOVA followed by Tukey's test. For anatomical data and western blot data, differences between groups were determined using a Student's t-test. P<0.05 was considered significant.
3. Results
3.1 General condition of SCI rats
Using 150 kdynes and a 1 s dwell time resulted in a moderate contusion such that at 35 days, the cords showed necrosis, cavitation of the grey matter and a decreased density of axons in the white matter [42]. All animals were monitored for recovery of spontaneous bladder function and maintenance of body weight. SCI animals lost the ability to void spontaneously, while sham-operated animals did not lose bladder control. On average, contused animals recovered bladder control 14 ± 1.6 days following injury. Prior to surgery, no differences in weight existed between groups (p > 0.05). Following surgery, SCI animals lost an average of 5–10% of their body weight and by day 35, SCI rats weighed significantly less than sham animals (327.80 ± 13.74 vs. 388.58 ± 13.63 g, respectively; p < 0.05).
Recovery of hindlimb locomotor function was monitored using the BBB open-field rating scale. One day following SCI, rats exhibited nearly complete hindlimb paralysis (BBB = 0.59 ± 0.27), compared to sham rats whose hindlimb motor function remained completely intact and stable (BBB = 21.0 ± 0.00). The spinal cord injury parameters used (150 kdyne, 1 s dwell time) produced a pattern of recovery consistent with a moderate contusion injury, with rats showing a slow progressive increase in BBB score over the 35 day survival period (p < 0.01). On Day 35, contused rats had significantly impaired hindlimb function (BBB = 8.09 ± 0.44) compared to sham rats (all animals BBB = 21.0, p < 0.01). A BBB score of 8 indicates extensive movement of all hindlimb joints (ankle, knee, and hip) with plantar placement or sweeping motions in the absence of weight support or stepping. The forelimbs were never compromised by the surgery or the contusion injury.
3.2 T10 SCI resulted in forelimb hypersensitivity: behavioral studies
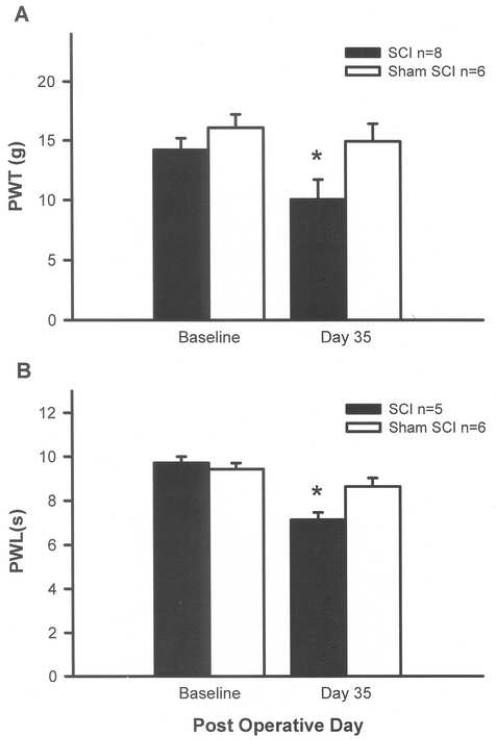
Mechanical sensitivity: Rats did not differ in their baseline PWTs following mechanical stimulation of the forepaws prior to injury. By 35 days after injury, SCI rats had a decrease in mean PWT compared to baseline (10.1 ± 1.6 g vs. 14.2 ± 1.0 g, p < 0.01; Fig. 1A) but sham rats showed no change from baseline (16.1 ± 1.1 g vs. 15.0 ± 1.5 g). Thus, the data indicate that a mechanical hypersensitivity developed in the forepaws of T10 SCI rats tested 35 days post-injury.
Fig. 1.
SCI effects forelimb sensitivity. At 35 days post-contusion, SCI animals have mechanical allodynia evidenced by a decrease in paw withdrawal threshold (PWT) to von Frey stimulation (A) and have thermal hyperalgesia evidenced by a decrease in paw withdrawal latency (PWL) to thermal stimulation (B). Sham rats show no change from baseline. *p < 0.05, repeated measures ANOVA.
Thermal Sensitivity: Rats did not differ in their baseline PWLs following thermal stimulation of the forepaws prior to injury (Fig. 1B). By 35 days post-injury, SCI animals demonstrated significant forelimb hypersensitivity with PWLs decreased as much as 2.91 s, a 31% decrease from baseline (9.7 ± 0.3 s vs 7.1 ± 0.3 s, p < 0.05); however, sham rats showed no change from baseline in thermal sensitivity (9.4 ± 0.3 s vs. 8.6 ± 0.4 s). Thus, the data indicate that a thermal hypersensitivity developed in the forepaws of T10 SCI rats.
3.3 T10 SCI resulted in peripheral hyperexcitability
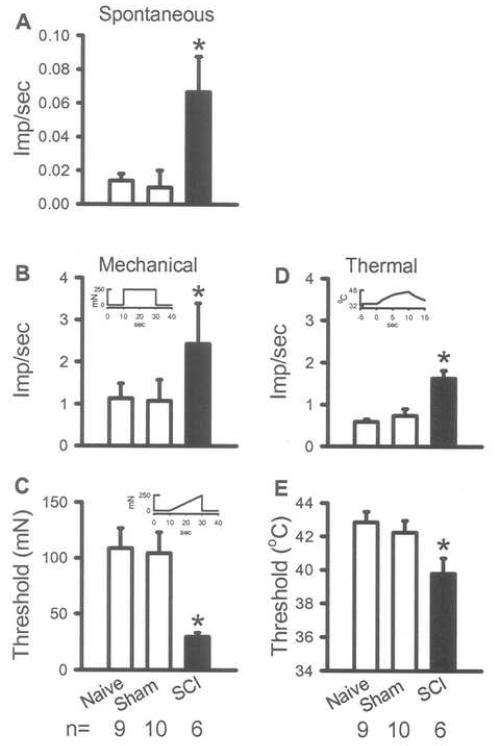
Using in vitro recording from nociceptors innervating the forelimb, it was shown that the hypersensitivity to mechanical and thermal stimulation observed in forelimb behavioral testing was mirrored by hyperexcitability of nociceptors in the median nerve 35 days post-injury. In the absence of stimulation, the spontaneous activity of nociceptors in the median nerve of SCI rats was significantly increased compared to sham and naïve rats (Table 1, Fig. 2A and 3). Hypersensitivity to mechanical stimulation developed in nociceptors in SCI rats, evidenced by an increased discharge rate in response to a suprathreshold stimulus and a decreased threshold to activation compared to sham and naïve rats (Table 1, Fig. 2B and C, Fig. 3). Nociceptors in SCI rats also developed hypersensitivity to thermal stimulation evidenced by a higher discharge rate and a lower threshold to activation to a heat stimulus compared to sham and naïve rats (Table 1, Fig. 2D and E, Fig. 3). The data indicate that nociceptors innervating the forelimb have increased spontaneous activity and are sensitized to mechanical and heat stimulation at 35 days following a T10 SCI.
Table 1.
In vitro nociceptor responses
| Groups | Spontaneous (Imp/s) | Mechanical (Imp/s) | Mechanical Threshold (mN) | Thermal (Imp/s) | Thermal Threshold (°C) |
|---|---|---|---|---|---|
| Naïve | 0.014 ± 0.01 | 1.13 ± 0.35 | 108.75 ± 17.97 | 0.59 ± 0.06 | 42.85 ± 0.63 |
| Sham | 0.010 ± 0.01 | 1.07 ± 0.51 | 104.28 ± 18.88 | 0.74 ± 0.16 | 42.24 ± 0.71 |
| SCI | 0.067 ± 0.02* | 2.43 ± 0.96* | 29.58 ± 3.66* | 1.63 ± 0.18* | 39.80 ± 0.91* |
p<0.05
Fig. 2.
Peripheral sensitization of forelimb primary afferents 35 days following SCI. In vitro recordings from nociceptors demonstrate an increased spontaneous activity compared to naïve and sham animals (A). These units also demonstrate mechanical sensitization evidenced by an increased response to a suprathreshold mechanical stimulation (B) and a decreased threshold to activation (C). Units demonstrate thermal hyperalgesia evidenced by an increased response to thermal stimulation and a decreased threshold to activation (E). Insets in B, C and D show stimulus parameters. *p<0.05, Kruskal-Wallis test followed by a Dunn's post hoc analysis.
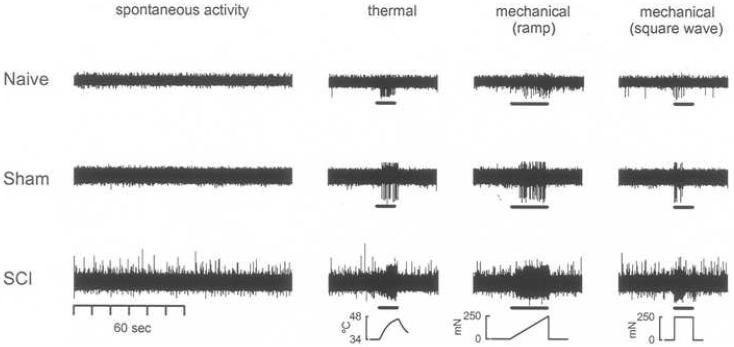
Fig. 3.
Recordings from nociceptors in SCI rats show sensitization. At 35 days post-injury, spontaneous activity in nociceptors is clearly enhanced in SCI compared to naïve and sham animals. Evoked responses following thermal and mechanical stimulation are also greater in magnitude in SCI compared to naïve and sham animals. (Data is unfiltered; stimulus is shown at the bottom of each column.)
3.4 T10 SCI resulted in central hyperexcitability
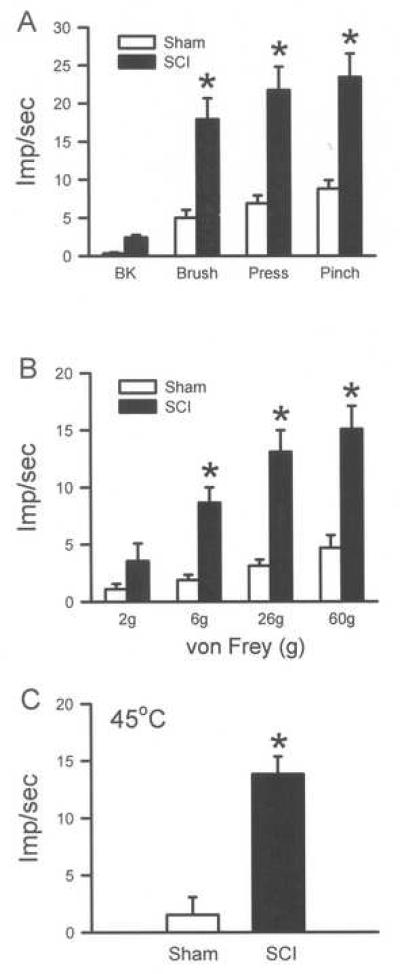
Using in vivo recordings of dorsal horn neurons in the cervical cord, it was shown that the hypersensitivity to mechanical and thermal stimulation observed in forelimb behavioral testing was mirrored by hyperexcitability of wide dynamic range (WDR) neurons at 35 days post-injury. The WDR neurons (n = 13, depth from cord surface was 545 ± 57 μm), in SCI rats showed enhanced background activity compared to sham (n = 15, depth from cord surface was 622 ± 43 μm), however, this did not reach significance (Fig. 4A, Table 2). In contrast, responses of WDR neurons to brush, press and pinch in the forepaw receptive fields in SCI rats were significantly increased compared to sham (p<0.05, Fig. 4A and 5, Table 2). Mechanical stimulation on the forepaw with ascending von Frey filaments also demonstrated enhanced discharge rates in SCI compared to sham rats, particularly following the 6, 26 and 60g forces (p<0.05, Fig. 4B, Table 2). Responses to a 45°C peripheral stimulus were more robust in SCI compared to sham rats (p<0.05, Fig. 4C, Table 2). The data indicate that cervical dorsal horn neurons were sensitized to mechanical and thermal stimulation in the forepaw receptive fields following a T10 SCI.
Fig. 4.
Sensitization of responses of dorsal horn neurons 35 days following SCI. Recordings from unidentified dorsal horn neurons demonstrate significantly enhanced responses to brush, press and pinch in the receptive fields of the neurons. Responses to the von Frey filaments and thermal stimulation are also significantly enhanced. *p<0.05, one way ANOVA followed by a Tukey's test.
Table 2.
In vivo WDR dorsal horn responses
| SCI (Imp/sec) | Sham (Imp/sec) | |
|---|---|---|
| Background (BK) | 2.42 ± 0.34 | 0.34 ± 0.15 |
| Stimulus | ||
| Brush | 17.94 ± 2.76* | 5.0 ± 1.03 |
| Press | 21.74 ± 3.1* | 6.9 ± 1.03 |
| Pinch | 23.46 ± 3.11* | 8.8 ± 1.11 |
| 2 g VF | 3.55 ± 1.54 | 1.11 ± 0.44 |
| 6 g VF | 8.66 ± 1.33* | 1.89 ± 0.44 |
| 26 g VF | 13.1 ± 1.89* | 3.11 ± 0.55 |
| 60 g VF | 15.1 ± 2.0* | 4.66 ± 1.11 |
| 45 °C | 13.84 ± 1.54* | 1.54 ± 1.54 |
p<0.05
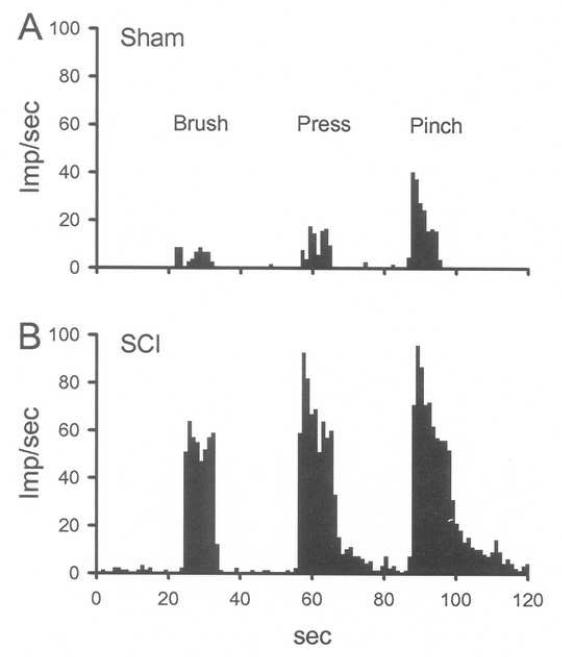
Fig. 5.
Recordings from dorsal horn neurons in SCI rats show sensitization. Peristimulus histograms show that compared to sham (A), cervical WDR neurons in SCI rats (B) are sensitized evidenced by the enhanced responses to brush, press and pinch in their receptive fields.
3.5 Sensitization of forelimb nociceptors is not due to injury of DRG neurons
To determine whether sensitization was the result of injury to forelimb peripheral afferents, cervical and thoracic DRG from naïve (n = 3), sham (n = 4) and contused (n = 4) rats were immunostained for ATF3 at 2 days and 35 days post-injury. As a marker of cellular injury [41, 89], ATF3-positive cells contained brightly fluorescing nuclei but the cytoplasm was unstained (Fig. 6). No ATF3-labeled DRG neurons were present in naïve rats (data not shown). At 2 days post-injury the T10 DRG showed an abundance of ATF3-labeled cells (39 ± 5%); these estimates were significantly higher compared to T10 sham rats (4 ± 3%, Fig. 6A and B, p < 0.05, t-test). At 35 days post-injury, however, there was no difference in T10 DRG labeling between SCI (2 ± 1%) and sham (3 ± 2%). In the cervical region, the percentage of ATF3-labeled cells in C8 DRG was extremely low regardless of the time point analyzed (< 2 ± 1%) and there was no difference between SCI and shams (Fig. 6E and F). Furthermore, there was no difference in total cell number at either time point when comparing SCI and sham DRGs from T10 or comparing SCI and sham DRGs from C8. This indicates no cell death occurred in the SCI DRG as a result of the injury. Double labeling with NeuN and ATF3 demonstrated that those cells expressing ATF3 were neurons, not glia (Fig. 7A and B). The data demonstrate that little or no damage or cell death occurs in the cervical DRG following a T10 SCI. Injury to the central processes of cervical primary afferents is not the cause of the hyperexcitability of forelimb primary afferents.
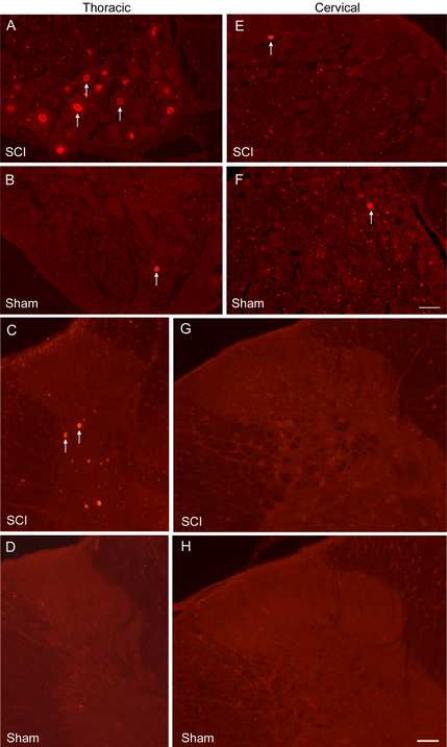
Fig. 6.
ATF3 immunostaining in DRG and spinal cord. At 2 days post-contusion, the T10 DRG has numerous ATF3-stained nuclei (A); however, the sham T10 DRG has few to none (B). The spinal cord near T10 in SCI rats has many ATF3-labeled neurons (C) but the thoracic cord in sham rats has none (D). In the cervical region, a very small number of DRG cells from SCI (E) or sham (F) rats are labeled for ATF3. Similarly, in the cervical cord in SCI (G) or sham (H) rats, almost no ATF3 cells are observed. White arrows indicate ATF3-stained nuclei. Bar = 50μm
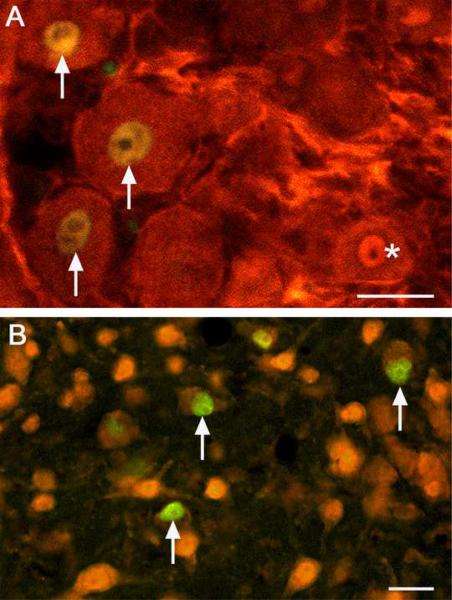
Fig. 7.
ATF3 labels neurons. Sections from the T9 DRG (A) and spinal cord (B) 2 days post-injury were double labeled for ATF3 (green) and NeuN (red, a neuronal marker) to confirm neuronal, and not glial, expression of ATF3. All AFT3-expressing cells also stained for NeuN (arrows). The asterisk identifies a single labeled NeuN cell. Bar = 25μm
3.6 Sensitization of dorsal horn neurons is not due to injury in the cervical cord
The possibility existed that cervical dorsal horn neurons became sensitized because their cell bodies or processes were disrupted or damaged by the T10 contusion. Analysis of ATF3-stained cervical sections ruled out this possibility. Sections analyzed near the contusion site (n = 2) contained an estimated 2868 ± 73 AFT3-labeled neurons at 2 days post-injury (Fig. 6C). T10 sections from sham rats (n = 2) contained only a small number of labeled cells (108 ± 17, Fig. 6D) and naïve rats had none (data not shown). In the C8 cord segment from SCI rats (n = 2), the estimated number of AFT3-labeled cells in the dorsal horn was only 39 ± 54 and sham rats (n = 2) had 18 ± 25 labeled cells (Fig. 6G and H). Considering that these counts represent the mean number of cells labeled for left and right sides of the entire C8 cord segment, this small population becomes insignificant. The data demonstrate that little or no cell damage occurs in the cervical cord following a T10 contusion. Injury to cervical dorsal horn cells is not the cause of the central sensitization.
3.7 Spreading of glial activation in chronically injured spinal cords
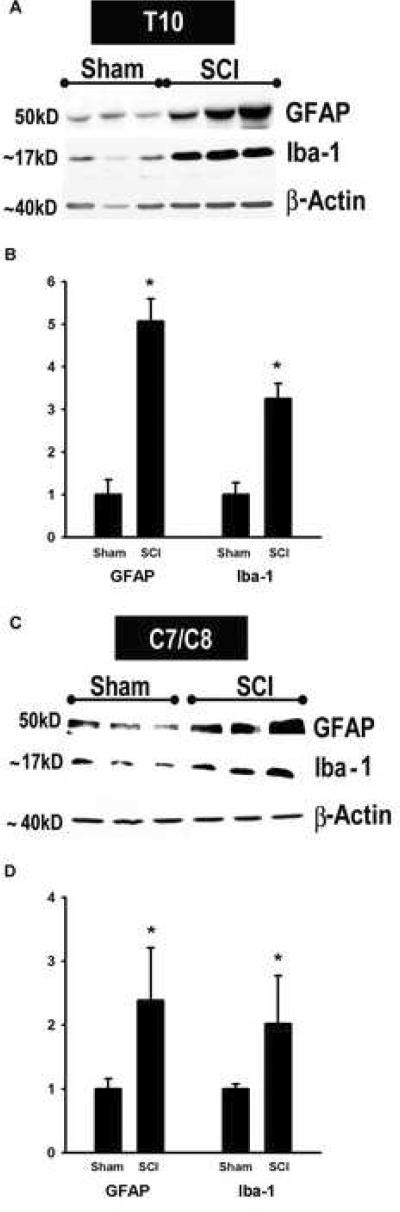
At T10, western blot analysis and quantitative assessment demonstrated a significant 5-fold elevation in GFAP protein 35 days post-injury (Fig. 8A and B). Similarly, the microglial marker Iba-1 showed greater than a 3-fold increase at T10. At C7/C8, more than a 2-fold increase in GFAP levels was present 35d after SCI (Fig. 8C and D). We also found a significant 2-fold increase in Iba-1 in the same cervical SCI samples. Thus, in the chronically injured spinal cord, there was spread of astrocytic and microglial activation rostrally from thoracic into the cervical segments.
Fig. 8.
Spread of glial activation in chronically injured spinal cords. Using Western blots, GFAP (marker of astrocytic activation, ~ 50kD) and Iba-1 (marker of microglial activation, ~17kD) levels were measured at the site of injury (T10) and in cervical segments (C7/C9), 35 days post-injury (A and C). n = 3 sham and 3 SCI. Quantitative analyses of immunoblotted GFAP and Iba-1 expression in T10 (B, n = 6 sham, 9 SCI) and C7/C8 (D, n = 3 sham, 3 SCI). The Y-axis represents the relative intensity of the GFAP and Iba-1 bands normalized to β-actin and then to sham values (set to 1). GFAP and Iba-1 protein levels show significant increases 35d after SCI at both the site of injury and in the cervical cord. *p<0.05, Student's t-test.
4. Discussion
The present study demonstrates that 35 days following CNS injury in the thoracic cord, rats develop a variety of pathophysiological changes in the cervical region including: 1) behavioral signs of mechanical allodynia and thermal hyperalgesia in the forepaws, 2) mechanical and thermal sensitization of nociceptors in the median nerve, 3) mechanical and thermal sensitization of cervical WDR dorsal horn neurons, 4) astrocytic and microglial activation. These changes occur in segments that are uninjured, rostral and far removed from the injury site.
4.1 Peripheral sensitization of cervical primary afferents contributes to CNP
Analysis of changes in the cervical region following SCI has been limited to behavioral changes, such as development of mechanical and heat sensitivity in the forelimbs [2, 13, 14, 33, 37, 45], and anatomical changes, such as sprouting of CGRP-containing fibers in cervical segments [54, 66]. This study confirms the presence of forelimb mechanical allodynia and heat hyperalgesia. An unexpected finding was the presence of sensitized nociceptors in the forelimbs. This aberrant condition would certainly contribute to the forelimb behavioral changes. The increased background activity could give rise to the spontaneous pain reported in human SCI patients [73, 74]. The lowered thresholds and enhanced responses of nociceptors could contribute to enhanced evoked pain sensations upon mechanical and thermal stimulation. That peripheral primary afferent activity can contribute to CNP was reported in a human case study [91]. In this patient, topical lidocaine significantly ameliorated the at-level pain, indicting peripheral nerve activity was involved in its generation. The authors concluded that nociceptors were not sensitized because the patient showed no heat hyperalgesia. But, he did have mechanical allodynia in the area of ongoing neuropathic pain. Based on animals studies, nociceptors can contribute to mechanical allodynia with lowered thresholds and increased discharge rates to mechanical stimulation [46, 47, 83]. Thus, this patient could have had sensitized nociceptors.
Concerning possible mechanisms that might lead to this sensitization, our DRG cell counts demonstrate no cell loss in the cervical ganglia thus, we can rule out peripheral sensitization arising from products of Wallerian degeneration [77, 78, 96, 97]. The fact that the sensitization is still present after the fibers are disconnected from the DRG and cord suggests there is either a permanent change in the fibers and/or in the chemical environment in the skin. At this time, nothing is known about what form these above-level changes might take.
4.2 Central Sensitization of cervical WDR neurons contributes to CNP
Another unexpected finding was the presence of sensitized WDR neurons in the cervical cord. This phenomenon would also influence behavior expressed in the forepaws, contributing to the mechanical allodynia and heat hyperalgesia. Hyperexcitability in dorsal horn neurons has been previously reported near the level of SCI [23, 39, 98], as well as considerably below level (lumbar enlargement) [34, 36]. Exaggerated responses of WDR neurons in the cervical region indicates that transmission of noxious input to supraspinal sites is enhanced, ultimately intensifying perceived pain.
There are several possible mechanisms for chronic sensitization of cervical dorsal horn cells. First and foremost, the peripheral sensitization most likely drives the central changes. The WDR activity reflects the increased mechanical and thermal sensitivity observed in the nociceptors. However, other contributing factors could include a glutamate surge that extends at least 5 mm rostral to the impact site following contusion [58, 60]. While this distance does not explain the changes we observe in the cervical region, the excess glutamate is neurotoxic to the surrounding tissue [58] and contributes to dorsal horn neuron hyperexcitability [94], as well as glial cell reactivity near the injury site [64]. This reactivity spreads away from the injury site evidenced by upregulation of astrocytes and/or microglia at-level [17, 63, 69, 70, 87] and well below-level in the lumbar enlargement [19, 30, 35, 63, 68, 101]. We demonstrate glial activation in the cervical enlargement, confirming and extending observations from transcriptional profiling studies where it was shown that glia markers are upregulated 5 segments rostral to the injury site for up to 9 months post-injury, consistent with the chronic nature of CNP [6, 63]. Chronic activation of astrocytes and microglia in the cervical cord could result in a chemical environment conducive to sensitization of dorsal horn neurons [44, 62, 79]. Once activated, glia can release a variety of neurotransmitters and proinflammatory cytokines and chemokines [61, 75] which could promote sensitization of the cervical dorsal horn neurons [27, 29, 49]. We cannot rule out other contributions to dorsal horn hyperexcitability since many pathological events can lead to a similar outcome, most notably loss of descending inhibitory controls [88] or disinhibition related to intrinsic cord circuitry [20, 32, 59, 102].
4.3 Implications of ATF3 expression
Large, medium and small diameter DRG neurons [38, 41, 51, 81, 82, 89], and neurons in the dorsal [57] and ventral horns [89] express ATF3 when they are injured or stressed. ATF3 has also been associated with regeneration and plasticity [81, 82]. ATF3 is clearly not a marker for pain. This is based on the finding that 35 days post-injury, ATF3 expression is greatly reduced or absent in cervical DRG and cord but pain behaviors and neuronal hyperexcitability/sensitization are well established.
At the level of the injury, our results are similar to those of Huang et al. (2006) who reported ATF3 label at 2 days post-injury in T10–T12 DRG after a T12 compression. Importantly, they reported that injury to the central processes of DRG cells (rhizotomy) also resulted in ATF3 expression in the DRG. In the current study, the large percentage of AFT3-expressing cells in the thoracic DRG 2 days post–injury most likely results from surgery, where peripheral axons in the dorsal primary rami would be cut. The contusion injury itself would also sever and/or damage the central process of T10 primary afferents (similar to a rhizotomy). Transsynaptic induction of ATF3 does not occur [89], and hence the ATF3-labeled neurons in the thoracic dorsal horn most likely result from direct injury to cells during the contusion. Double labeling for ATF3 and NeuN confirmed ATF3 was expressed in neurons and not glia [4, 48].
At 2 and 35 days post-injury, there were a few labeled neurons in the cervical DRG and few to none in cervical cord. These findings support the conclusion there was little or no injury/stress to these cell populations. Those neurons that express ATF3 in the C7/C8 DRG probably reflect axons that were cut during surgery by the skin incision. From these data, we conclude that injury to cervical DRG cells, to the central processes of cervical DRGs or to cervical dorsal horn neurons is not the mechanism underlying sensitization of forelimb nociceptors or cervical dorsal horn neurons.
4.4 Spreading of glial activation
We documented glial activation more than 10 segments rostral to the injury site. Activation of astrocytes in cervical cord can be attributed to the fact that these glial cells create an extensive functional syncytium [67], interconnected via gap junctions [21]. This would allow the initial glial activation at the injury site to spread throughout this astrocytic network to distant (i.e. cervical) segments. Microglial activation in segments far from the injury site is most likely due to Wallerian degeneration occurring in ascending and descending tracts that pass through the injury level [53, 92], although microglia also extend a network of processes that infiltrate and monitor all extracellular space in the CNS and spinal cord [65].
Several lines of evidence indicate that activated astrocytes and microglia contribute to the maintenance of chronic SCI pain at level and below level, strengthening the line of reason that the same is happening above level. Treatment with glial inhibitors such as minocycline or propentofylline return glia to a quiescent morphological state, reduce the hyperexcitability of lumbar dorsal horn neurons and block mechanical allodynia and heat hyperalgesia that develops below level [30, 35]. Furthermore, the reported increase in phosphorylated p-38 MAPK in microglia [17, 35] is correlated with development of SCI aberrant pain behaviors [18]; inhibiting its enzymatic activity reverses allodynia and decreases the hyperexcitability of thoracic dorsal horn cells [17]. Concerning astrocytes, GFAP expression in the contused cord corresponds with the presence of mechanical allodynia [31]. Evidence suggests that GFAP serves as a marker for astrocyte activation and plays a critical role in the maintenance of neuropathic pain [50].
5. Conclusion
These data highlight forelimb primary afferent, cervical dorsal horn neuron, and cervical glia contributions to the CNP pain that develops following T10 SCI. The data clearly challenge the concept that CNP is a strictly central phenomenon, demonstrating that changes in primary afferents contribute to CNP after SCI. Importantly, the data also provide evidence that a CNS lesion can have profound effects on the function of uninjured primary afferent fibers that are far removed from the injury site. There are other reports of the CNS modulating peripheral nerve activity and function. The degree of peripheral inflammation [5, 86] and peripheral neutrophil accumulation [3] can be controlled by CNS activity. Possible efferent signaling pathways underlying this control include dorsal root reflexes [5, 72, 95]. We are currently investigating the presence of dorsal root reflex activity in cervical cord of SCI rats. The data demonstrate that the “central injury cascade” initiated by spinal cord insult [99] can extend not only caudal but far rostral to the injury. Our data enhance our understanding of mechanisms underlying CNP and identify additional targets for CNP therapy.
Acknowledgements
This work was supported by NIH NS54765 and NS027910 to SMC; NS11255, NS39161 and the Dunn and the West Foundations to CEH; Mission Connect and the Moody Foundations to SMC, CEH and ON.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest The authors declare no conflict of interest.
References
- [1].Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;121:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- [2].Bennett AD, Everhart AW, Hulsebosch CE. Intrathecal administration of an NMDA or a non-NMDA receptor antagonist reduces mechanical but not thermal allodynia in a rodent model of chronic central pain after spinal cord injury. Brain Res. 2000;8591:72–82. doi: 10.1016/s0006-8993(99)02483-x. [DOI] [PubMed] [Google Scholar]
- [3].Bong GW, Rosengren S, Firestein GS. Spinal cord adenosine receptor stimulation in rats inhibits peripheral neutrophil accumulation. The role of N-methyl-D-aspartate receptors. J Clin Invest. 1996;9812:2779–2785. doi: 10.1172/JCI119104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bontioti E, Dahlin LB, Kataoka K, Kanje M. End-to-side nerve repair induces nuclear translocation of activating transcription factor 3. Scand J Plast Reconstr Surg Hand Surg. 2006;406:321–328. doi: 10.1080/02844310600999956. [DOI] [PubMed] [Google Scholar]
- [5].Boyle DL, Moore J, Yang L, Sorkin LS, Firestein GS. Spinal adenosine receptor activation inhibits inflammation and joint destruction in rat adjuvant-induced arthritis. Arthritis Rheum. 2002;4611:3076–3082. doi: 10.1002/art.10595. [DOI] [PubMed] [Google Scholar]
- [6].Byrnes KR, Garay J, Di Giovanni S, De Biase A, Knoblach SM, Hoffman EP, Movsesyan V, Faden AI. Expression of two temporally distinct microglia-related gene clusters after spinal cord injury. Glia. 2006;534:420–433. doi: 10.1002/glia.20295. [DOI] [PubMed] [Google Scholar]
- [7].Cairns D, Adkins RH, Scott MD. Pain and depression in acute traumatic spinal cord injury: origins of chronic problematic pain. Arch Phys Med Rehab. 1996;77:329–335. doi: 10.1016/s0003-9993(96)90079-9. [DOI] [PubMed] [Google Scholar]
- [8].Carlton SM, Hargett GL. Stereological analysis of Ca(2+)/calmodulin-dependent protein kinase II alpha -containing dorsal root ganglion neurons in the rat: colocalization with isolectin Griffonia simplicifolia, calcitonin gene-related peptide, or vanilloid receptor 1. J Comp Neurol. 2002;4481:102–110. doi: 10.1002/cne.10250. [DOI] [PubMed] [Google Scholar]
- [9].Carlton SM, Hargett GL. Colocalization of metabotropic glutamate receptors in rat dorsal root ganglion cells. J Comp Neurol. 2007;5015:780–789. doi: 10.1002/cne.21285. [DOI] [PubMed] [Google Scholar]
- [10].Carlton SM, Hargett GL, Coggeshall RE. Localization and activation of glutamate receptors in unmyelinated axons of rat glabrous skin. Neurosci Lett. 1995;1971:25–28. doi: 10.1016/0304-3940(95)11889-5. [DOI] [PubMed] [Google Scholar]
- [11].Carlton SM, Zhou S, Du J, Hargett GL, Ji G, Coggeshall RE. Somatostatin modulates the transient receptor potential vanilloid 1 (TRPV1) ion channel. Pain. 2004;1103:616–627. doi: 10.1016/j.pain.2004.04.042. [DOI] [PubMed] [Google Scholar]
- [12].Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;531:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- [13].Christensen MD, Everhart AW, Pickelman JT, Hulsebosch CE. Mechanical and thermal allodynia in chronic central pain following spinal cord injury. Pain. 1996;681:97–107. doi: 10.1016/S0304-3959(96)03224-1. [DOI] [PubMed] [Google Scholar]
- [14].Christensen MD, Hulsebosch CE. Chronic central pain after spinal cord injury. J Neurotrauma. 1997;148:517–537. doi: 10.1089/neu.1997.14.517. [DOI] [PubMed] [Google Scholar]
- [15].Chung JM, Surmeier DJ, Lee KH, Sorkin LS, Honda CN, Tsong Y, Willis WD. Classification of primate spinothalamic and somatosensory thalamic neurons based on cluster analysis. J Neurophysiol. 1986;562:308–327. doi: 10.1152/jn.1986.56.2.308. [DOI] [PubMed] [Google Scholar]
- [16].Coggeshall RE. A consideration of neural counting methods. Trends Neurosci. 1992;151:9–13. doi: 10.1016/0166-2236(92)90339-a. [DOI] [PubMed] [Google Scholar]
- [17].Crown ED, Gwak YS, Ye Z, Johnson KM, Hulsebosch CE. Activation of p38 MAP kinase is involved in central neuropathic pain following spinal cord injury. Exp Neurol. 2008;2132:257–267. doi: 10.1016/j.expneurol.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Crown ED, Ye Z, Johnson KM, Xu GY, McAdoo DJ, Hulsebosch CE. Increases in the activated forms of ERK 1/2, p38 MAPK, and CREB are correlated with the expression of at-level mechanical allodynia following spinal cord injury. Exp Neurol. 2006;1992:397–407. doi: 10.1016/j.expneurol.2006.01.003. [DOI] [PubMed] [Google Scholar]
- [19].Detloff MR, Fisher LC, McGaughy V, Longbrake EE, Popovich PG, Basso DM. Remote activation of microglia and pro-inflammatory cytokines predict the onset and severity of below-level neuropathic pain after spinal cord injury in rats. Exp Neurol. 2008;2122:337–347. doi: 10.1016/j.expneurol.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Devor M, Wall PD. Plasticity in the spinal cord sensory map following peripheral nerve injury in rats. J Neurosci. 1981;17:679–684. doi: 10.1523/JNEUROSCI.01-07-00679.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dienel GA, Cruz NF. Neighborly interactions of metabolically-activated astrocytes in vivo. Neurochem Int. 2003;434–5:339–354. doi: 10.1016/s0197-0186(03)00021-4. [DOI] [PubMed] [Google Scholar]
- [22].Dixon WJ. Staircase bioassay: the up-and-down method. Neurosci Biobehav Rev. 1991;151:47–50. doi: 10.1016/s0149-7634(05)80090-9. [DOI] [PubMed] [Google Scholar]
- [23].Drew GM, Siddall PJ, Duggan AW. Responses of spinal neurones to cutaneous and dorsal root stimuli in rats with mechanical allodynia after contusive spinal cord injury. Brain Res. 2001;8931–2:59–69. doi: 10.1016/s0006-8993(00)03288-1. [DOI] [PubMed] [Google Scholar]
- [24].Du J, Koltzenburg M, Carlton SM. Glutamate-induced excitation and sensitization of nociceptors in rat glabrous skin. Pain. 2001;892–3:187–198. doi: 10.1016/s0304-3959(00)00362-6. [DOI] [PubMed] [Google Scholar]
- [25].Du J, Zhou S, Carlton SM. Kainate-induced excitation and sensitization of nociceptors in normal and inflamed rat glabrous skin. Neuroscience. 2006;1373:999–1013. doi: 10.1016/j.neuroscience.2005.10.008. [DOI] [PubMed] [Google Scholar]
- [26].Du J, Zhou S, Carlton SM. Group II metabotropic glutamate receptor activation attenuates peripheral sensitization in inflammatory states. Neuroscience. 2008;1542:754–766. doi: 10.1016/j.neuroscience.2008.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ, Park JY, Lind AL, Ma Q, Ji RR. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci. 2009;2913:4096–4108. doi: 10.1523/JNEUROSCI.3623-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. Apmis. 1988;9610:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- [29].Gustafson-Vickers SL, Lu VB, Lai AY, Todd KG, Ballanyi K, Smith PA. Long-term actions of interleukin-1beta on delay and tonic firing neurons in rat superficial dorsal horn and their relevance to central sensitization. Mol Pain. 2008;4:63. doi: 10.1186/1744-8069-4-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gwak YS, Crown ED, Unabia GC, Hulsebosch CE. Propentofylline attenuates allodynia, glial activation and modulates GABAergic tone after spinal cord injury in the rat. Pain. 2008;1382:410–422. doi: 10.1016/j.pain.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gwak YS, Hulsebosch CE. Remote astrocytic and microglial activation modulates neuronal hyperexcitability and below-level neuropathic pain after spinal injury in rat. Neuroscience. 2009;1613:895–903. doi: 10.1016/j.neuroscience.2009.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gwak YS, Tan HY, Nam TS, Paik KS, Hulsebosch CE, Leem JW. Activation of spinal GABA receptors attenuates chronic central neuropathic pain after spinal cord injury. J Neurotrauma. 2006;237:1111–1124. doi: 10.1089/neu.2006.23.1111. [DOI] [PubMed] [Google Scholar]
- [33].Hains BC, Chastain KM, Everhart AW, McAdoo DJ, Hulsebosch CE. Transplants of adrenal medullary chromaffin cells reduce forelimb and hindlimb allodynia in a rodent model of chronic central pain after spinal cord hemisection injury. Exp Neurol. 2000;1642:426–437. doi: 10.1006/exnr.2000.7439. [DOI] [PubMed] [Google Scholar]
- [34].Hains BC, Johnson KM, Eaton MJ, Willis WD, Hulsebosch CE. Serotonergic neural precursor cell grafts attenuate bilateral hyperexcitability of dorsal horn neurons after spinal hemisection in rat. Neuroscience. 2003;1164:1097–1110. doi: 10.1016/s0306-4522(02)00729-7. [DOI] [PubMed] [Google Scholar]
- [35].Hains BC, Waxman SG. Activated microglia contribute to the maintenance of chronic pain after spinal cord injury. J Neurosci. 2006;2616:4308–4317. doi: 10.1523/JNEUROSCI.0003-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hains BC, Willis WD, Hulsebosch CE. Temporal plasticity of dorsal horn somatosensory neurons after acute and chronic spinal cord hemisection in rat. Brain Res. 2003;9701–2:238–241. doi: 10.1016/s0006-8993(03)02347-3. [DOI] [PubMed] [Google Scholar]
- [37].Hains BC, Yucra JA, Hulsebosch CE. Reduction of pathological and behavioral deficits following spinal cord contusion injury with the selective cyclooxygenase-2 inhibitor NS-398. J Neurotrauma. 2001;184:409–423. doi: 10.1089/089771501750170994. [DOI] [PubMed] [Google Scholar]
- [38].Hamann W, Abou-Sherif S, Thompson S, Hall S. Pulsed radiofrequency applied to dorsal root ganglia causes a selective increase in ATF3 in small neurons. Eur J Pain. 2006;102:171–176. doi: 10.1016/j.ejpain.2005.03.001. [DOI] [PubMed] [Google Scholar]
- [39].Hao JX, Xu XJ, Yu YX, Seiger A, Wiesenfeld-Hallin Z. Transient spinal cord ischemia induces temporary hypersensitivity of dorsal horn wide dynamic range neurons to myelinated, but not unmyelinated, fiber input. J Neurophysiol. 1992;682:384–391. doi: 10.1152/jn.1992.68.2.384. [DOI] [PubMed] [Google Scholar]
- [40].Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;321:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- [41].Huang WL, Robson D, Liu MC, King VR, Averill S, Shortland PJ, Priestley JV. Spinal cord compression and dorsal root injury cause up-regulation of activating transcription factor-3 in large-diameter dorsal root ganglion neurons. Eur J Neurosci. 2006;231:273–278. doi: 10.1111/j.1460-9568.2005.04530.x. [DOI] [PubMed] [Google Scholar]
- [42].Hulsebosch CE. Recent advances in pathophysiology and treatment of spinal cord injury. Adv Physiol Educ. 2002;261–4:238–255. doi: 10.1152/advan.00039.2002. [DOI] [PubMed] [Google Scholar]
- [43].Hulsebosch CE. Mechanisims and treatment strategies for chronic central neuropathic pain after spinal cord injury. Top Spinal Cord Inj Rehabil. 2003;8:76–91. [Google Scholar]
- [44].Hulsebosch CE. Gliopathy ensures persistent inflammation and chronic pain after spinal cord injury. Exp Neurol. 2008 doi: 10.1016/j.expneurol.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hulsebosch CE, Xu GY, Perez-Polo JR, Westlund KN, Taylor CP, McAdoo DJ. Rodent model of chronic central pain after spinal cord contusion injury and effects of gabapentin. J Neurotrauma. 2000;1712:1205–1217. doi: 10.1089/neu.2000.17.1205. [DOI] [PubMed] [Google Scholar]
- [46].Ji G, Zhou S, Carlton SM. Intact Adelta-fibers up-regulate transient receptor potential A1 and contribute to cold hypersensitivity in neuropathic rats. Neuroscience. 2008;1543:1054–1066. doi: 10.1016/j.neuroscience.2008.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ji G, Zhou S, Kochukov MY, Westlund KN, Carlton SM. Plasticity in intact A delta- and C-fibers contributes to cold hypersensitivity in neuropathic rats. Neuroscience. 2007;1501:182–193. doi: 10.1016/j.neuroscience.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kataoka K, Kanje M, Dahlin LB. Induction of activating transcription factor 3 after different sciatic nerve injuries in adult rats. Scand J Plast Reconstr Surg Hand Surg. 2007;414:158–166. doi: 10.1080/02844310701318288. [DOI] [PubMed] [Google Scholar]
- [49].Kiguchi N, Maeda T, Kobayashi Y, Kishioka S. Up-regulation of tumor necrosis factor-alpha in spinal cord contributes to vincristine-induced mechanical allodynia in mice. Neurosci Lett. 2008;4452:140–143. doi: 10.1016/j.neulet.2008.09.009. [DOI] [PubMed] [Google Scholar]
- [50].Kim DS, Figueroa KW, Li KW, Boroujerdi A, Yolo T, David Luo Z. Profiling of dynamically changed gene expression in dorsal root ganglia post peripheral nerve injury and a critical role of injury-induced glial fibrillary acetic protein in maintenance of pain behaviors. Pain. 2009;1431–2:114–122. doi: 10.1016/j.pain.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kim HY, Park CK, Cho IH, Jung SJ, Kim JS, Oh SB. Differential Changes in TRPV1 expression after trigeminal sensory nerve injury. J Pain. 2008;93:280–288. doi: 10.1016/j.jpain.2007.11.013. [DOI] [PubMed] [Google Scholar]
- [52].Koltzenburg M, Kress M, Reeh PW. The nociceptor sensitization by bradykinin does not depend on sympathetic neurons. Neuroscience. 1992;462:465–473. doi: 10.1016/0306-4522(92)90066-b. [DOI] [PubMed] [Google Scholar]
- [53].Koshinaga M, Whittemore SR. The temporal and spatial activation of microglia in fiber tracts undergoing anterograde and retrograde degeneration following spinal cord lesion. J Neurotrauma. 1995;122:209–222. doi: 10.1089/neu.1995.12.209. [DOI] [PubMed] [Google Scholar]
- [54].Krenz NR, Weaver LC. Sprouting of primary afferent fibers after spinal cord transection in the rat. Neuroscience. 1998;852:443–458. doi: 10.1016/s0306-4522(97)00622-2. [DOI] [PubMed] [Google Scholar]
- [55].Kress M, Koltzenburg M, Reeh PW, Handwerker HO. Responsiveness and functional attributes of electrically localized terminals of cutaneous C-fibers in vivo and in vitro. J Neurophysiol. 1992;682:581–595. doi: 10.1152/jn.1992.68.2.581. [DOI] [PubMed] [Google Scholar]
- [56].Lang E, Novak A, Reeh PW, Handwerker HO. Chemosensitivity of fine afferents from rat skin in vitro. J Neurophysiol. 1990;634:887–901. doi: 10.1152/jn.1990.63.4.887. [DOI] [PubMed] [Google Scholar]
- [57].Latremoliere A, Mauborgne A, Masson J, Bourgoin S, Kayser V, Hamon M, Pohl M. Differential implication of proinflammatory cytokine interleukin-6 in the development of cephalic versus extracephalic neuropathic pain in rats. J Neurosci. 2008;2834:8489–8501. doi: 10.1523/JNEUROSCI.2552-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Liu D, Xu GY, Pan E, McAdoo DJ. Neurotoxicity of glutamate at the concentration released upon spinal cord injury. Neuroscience. 1999;934:1383–1389. doi: 10.1016/s0306-4522(99)00278-x. [DOI] [PubMed] [Google Scholar]
- [59].Liu J, Wolfe D, Hao S, Huang S, Glorioso JC, Mata M, Fink DJ. Peripherally delivered glutamic acid decarboxylase gene therapy for spinal cord injury pain. Mol Ther. 2004;101:57–66. doi: 10.1016/j.ymthe.2004.04.017. [DOI] [PubMed] [Google Scholar]
- [60].McAdoo DJ, Xu GY, Robak G, Hughes MG. Changes in amino acid concentrations over time and space around an impact injury and their diffusion through the rat spinal cord. Exp Neurol. 1999;1592:538–544. doi: 10.1006/exnr.1999.7166. [DOI] [PubMed] [Google Scholar]
- [61].McMahon SB, Cafferty WB, Marchand F. Immune and glial cell factors as pain mediators and modulators. Exp Neurol. 2005;1922:444–462. doi: 10.1016/j.expneurol.2004.11.001. [DOI] [PubMed] [Google Scholar]
- [62].Milligan ED, Sloane EM, Watkins LR. Glia in pathological pain: a role for fractalkine. J Neuroimmunol. 2008;1981–2:113–120. doi: 10.1016/j.jneuroim.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Nesic O, Lee J, Johnson KM, Ye Z, Xu GY, Unabia GC, Wood TG, McAdoo DJ, Westlund KN, Hulsebosch CE, Regino Perez-Polo J. Transcriptional profiling of spinal cord injury-induced central neuropathic pain. J Neurochem. 2005;954:998–1014. doi: 10.1111/j.1471-4159.2005.03462.x. [DOI] [PubMed] [Google Scholar]
- [64].Nesic O, Svrakic NM, Xu GY, McAdoo D, Westlund KN, Hulsebosch CE, Ye Z, Galante A, Soteropoulos P, Tolias P, Young W, Hart RP, Perez-Polo JR. DNA microarray analysis of the contused spinal cord: effect of NMDA receptor inhibition. J Neurosci Res. 2002;684:406–423. doi: 10.1002/jnr.10171. [DOI] [PubMed] [Google Scholar]
- [65].Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;3085726:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- [66].Ondarza AB, Ye Z, Hulsebosch CE. Direct evidence of primary afferent sprouting in distant segments following spinal cord injury in the rat: colocalization of GAP-43 and CGRP. Exp Neurol. 2003;1841:373–380. doi: 10.1016/j.expneurol.2003.07.002. [DOI] [PubMed] [Google Scholar]
- [67].Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;504:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- [68].Peng XM, Zhou ZG, Glorioso JC, Fink DJ, Mata M. Tumor necrosis factor-alpha contributes to below-level neuropathic pain after spinal cord injury. Ann Neurol. 2006;595:843–851. doi: 10.1002/ana.20855. [DOI] [PubMed] [Google Scholar]
- [69].Popovich PG, van Rooijen N, Hickey WF, Preidis G, McGaughy V. Hematogenous macrophages express CD8 and distribute to regions of lesion cavitation after spinal cord injury. Exp Neurol. 2003;1822:275–287. doi: 10.1016/s0014-4886(03)00120-1. [DOI] [PubMed] [Google Scholar]
- [70].Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol. 1997;3773:443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- [71].Reeh PW. Sensory receptors in mammalian skin in an in vitro preparation. Neurosci Lett. 1986;662:141–146. doi: 10.1016/0304-3940(86)90180-1. [DOI] [PubMed] [Google Scholar]
- [72].Rees H, Sluka KA, Westlund KN, Willis WD. Do dorsal root reflexes augment peripheral inflammation? Neuroreport. 1994;57:821–824. doi: 10.1097/00001756-199403000-00021. [DOI] [PubMed] [Google Scholar]
- [73].Richards JS, Meredith RL, Nepomuceno C, Fine PR, Bennett G. Psycho-social aspects of chronic pain in spinal cord injury. Pain. 1980;83:355–366. doi: 10.1016/0304-3959(80)90079-2. [DOI] [PubMed] [Google Scholar]
- [74].Rinaldi PC, Young RF, Albe-Fessard D, Chodakiewitz J. Spontaneous neuronal hyperactivity in the medial and intralaminar thalamic nuclei of patients with deafferentation pain. J Neurosurg. 1991;743:415–421. doi: 10.3171/jns.1991.74.3.0415. [DOI] [PubMed] [Google Scholar]
- [75].Rostene W, Kitabgi P, Parsadaniantz SM. Chemokines: a new class of neuromodulator? Nat Rev Neurosci. 2007;811:895–903. doi: 10.1038/nrn2255. [DOI] [PubMed] [Google Scholar]
- [76].Rueff A, Mendell LM. Nerve growth factor NT-5 induce increased thermal sensitivity of cutaneous nociceptors in vitro. J Neurophysiol. 1996;765:3593–3596. doi: 10.1152/jn.1996.76.5.3593. [DOI] [PubMed] [Google Scholar]
- [77].Schafers M, Lee DH, Brors D, Yaksh TL, Sorkin LS. Increased sensitivity of injured and adjacent uninjured rat primary sensory neurons to exogenous tumor necrosis factor-alpha after spinal nerve ligation. J Neurosci. 2003;237:3028–3038. doi: 10.1523/JNEUROSCI.23-07-03028.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Schafers M, Sorkin LS, Geis C, Shubayev VI. Spinal nerve ligation induces transient upregulation of tumor necrosis factor receptors 1 and 2 in injured and adjacent uninjured dorsal root ganglia in the rat. Neurosci Lett. 2003;3473:179–182. doi: 10.1016/s0304-3940(03)00695-5. [DOI] [PubMed] [Google Scholar]
- [79].Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;1011:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- [80].Segatore M. Understanding chronic pain after spinal cord injury. J Neurosci Nurs. 1994;264:230–236. doi: 10.1097/01376517-199408000-00007. [DOI] [PubMed] [Google Scholar]
- [81].Seijffers R, Allchorne AJ, Woolf CJ. The transcription factor ATF-3 promotes neurite outgrowth. Mol Cell Neurosci. 2006;321–2:143–154. doi: 10.1016/j.mcn.2006.03.005. [DOI] [PubMed] [Google Scholar]
- [82].Seijffers R, Mills CD, Woolf CJ. ATF3 increases the intrinsic growth state of DRG neurons to enhance peripheral nerve regeneration. J Neurosci. 2007;2730:7911–7920. doi: 10.1523/JNEUROSCI.5313-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Shim B, Kim DW, Kim BH, Nam TS, Leem JW, Chung JM. Mechanical and heat sensitization of cutaneous nociceptors in rats with experimental peripheral neuropathy. Neuroscience. 2005;1321:193–201. doi: 10.1016/j.neuroscience.2004.12.036. [DOI] [PubMed] [Google Scholar]
- [84].Siddall P, Yezierski R, Loeser J. Taxomony and epidemiology of spinal cord injury pain. In: Yezierski R, Burchiel K, editors. Spinal cord Injury Pain: Assessment, Mechanisms, Management. Vol 23. IASP Press; Seattle: 2002. pp. 9–24. [Google Scholar]
- [85].Sjolund BH. Pain and rehabilitation after spinal cord injury: the case of sensory spasticity? Brain Res Brain Res Rev. 2002;401–3:250–256. doi: 10.1016/s0165-0173(02)00207-2. [DOI] [PubMed] [Google Scholar]
- [86].Sluka KA, Westlund KN. Centrally administered non-NMDA but not NMDA receptor antagonists block peripheral knee joint inflammation. Pain. 1993;552:217–225. doi: 10.1016/0304-3959(93)90150-N. [DOI] [PubMed] [Google Scholar]
- [87].Sroga JM, Jones TB, Kigerl KA, McGaughy VM, Popovich PG. Rats and mice exhibit distinct inflammatory reactions after spinal cord injury. J Comp Neurol. 2003;4622:223–240. doi: 10.1002/cne.10736. [DOI] [PubMed] [Google Scholar]
- [88].Sweet WH. Deafferentation syndromes in humans: A general discussion. In: Nashold BS, Ovelman-Levitt J, editors. Deafferentation pain syndromes: Pathophysiology and treatment. Raven Press; New York: 1991. pp. 259–283. [Google Scholar]
- [89].Tsujino H, Kondo E, Fukuoka T, Dai Y, Tokunaga A, Miki K, Yonenobu K, Ochi T, Noguchi K. Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: A novel neuronal marker of nerve injury. Mol Cell Neurosci. 2000;152:170–182. doi: 10.1006/mcne.1999.0814. [DOI] [PubMed] [Google Scholar]
- [90].Vierck CJ, Jr., Light AR. Allodynia and hyperalgesia within dermatomes caudal to a spinal cord injury in primates and rodents. Prog Brain Res. 2000;129:411–428. doi: 10.1016/S0079-6123(00)29032-8. [DOI] [PubMed] [Google Scholar]
- [91].Wasner G, Naleschinski D, Baron R. A role for peripheral afferents in the pathophysiology and treatment of at-level neuropathic pain in spinal cord injury? A case report. Pain. 2007;1311–2:219–225. doi: 10.1016/j.pain.2007.03.005. [DOI] [PubMed] [Google Scholar]
- [92].Watanabe T, Yamamoto T, Abe Y, Saito N, Kumagai T, Kayama H. Differential activation of microglia after experimental spinal cord injury. J Neurotrauma. 1999;163:255–265. doi: 10.1089/neu.1999.16.255. [DOI] [PubMed] [Google Scholar]
- [93].West MJ, Gundersen HJ. Unbiased stereological estimation of the number of neurons in the human hippocampus. J Comp Neurol. 1990;2961:1–22. doi: 10.1002/cne.902960102. [DOI] [PubMed] [Google Scholar]
- [94].Willcockson WS, Chung JM, Hori Y, Lee KH, Willis WD. Effects of iontophoretically released amino acids and amines on primate spinothalamic tract cells. J Neurosci. 1984;43:732–740. doi: 10.1523/JNEUROSCI.04-03-00732.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Willis WD, Sluka KA, Rees H, Westlund KN. A contribution of dorsal root reflexes to peripheral inflammation. In: Rudomin P, Romo R, Mendell LM, editors. Presynaptic inhibition and neural control. Oxford University Press; New York: 1998. pp. 407–423. [Google Scholar]
- [96].Wu G, Ringkamp M, Hartke TV, Murinson BB, Campbell JN, Griffin JW, Meyer RA. Early onset of spontaneous activity in uninjured C-fiber nociceptors after injury to neighboring nerve fibers. J Neurosci. 2001;218:RC140. doi: 10.1523/JNEUROSCI.21-08-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Wu G, Ringkamp M, Murinson BB, Pogatzki EM, Hartke TV, Weerahandi HM, Campbell JN, Griffin JW, Meyer RA. Degeneration of myelinated efferent fibers induces spontaneous activity in uninjured C-fiber afferents. J Neurosci. 2002;2217:7746–7753. doi: 10.1523/JNEUROSCI.22-17-07746.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Xu XJ, Hao JX, Wiesenfeld-Hallin Z. Physiological and pharmacological characterization of a rat model of spinal cord injury pain after spinal ischemia. [Google Scholar]
- [99].Yezierski RP. Pain following spinal cord injury: the clinical problem and experimental studies. Pain. 1996;682–3:185–194. doi: 10.1016/s0304-3959(96)03178-8. [DOI] [PubMed] [Google Scholar]
- [100].Yezierski RP. Pathophysiology and Animal Models of Spinal Cord Injury Pain. In: Yezierski R, Burchiel K, editors. Spinal cord Injury Pain: Assessment, Mechanisms, Management. Vol 23. IASP Press; Seattle: 2002. pp. 117–154. [Google Scholar]
- [101].Zai LJ, Wrathall JR. Cell proliferation and replacement following contusive spinal cord injury. Glia. 2005;503:247–257. doi: 10.1002/glia.20176. [DOI] [PubMed] [Google Scholar]
- [102].Zeilhofer HU. Loss of glycinergic and GABAergic inhibition in chronic pain--contributions of inflammation and microglia. Int Immunopharmacol. 2008;82:182–187. doi: 10.1016/j.intimp.2007.07.009. [DOI] [PubMed] [Google Scholar]
- [103].Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;162:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]