Abstract
Although previous studies have examined the extent to which adrenocorticotropic hormone (ACTH) secretion depends on endogenous glucocorticoid levels, few have examined the parallel glucocorticoid dependency of gene expression within the corticotropin releasing hormone (CRH) neuron containing subregion of the hypothalamic paraventricular nucleus (PVN). This study examined resting and stress-induced expression of three immediate early genes (c-fos, zif268, and NGFI-B mRNAs) and two phenotypic restricted immediate early genes that code for ACTH secretagogues (CRH and arginine vasopressin [AVP] hnRNAs) in the PVN of adrenalectomized (ADX) rats given either 0.9% saline to drink for 5 days or saline with corticosterone (CORT; 25 µg/ml). CORT-containing saline was replaced with saline 18 h before testing to ensure clearance of CORT at the time of testing. Dependent measures were examined 0, 15, 30, 60, or 120 min after 30 min restraint. Compared to sham surgery, ADX produced a large upregulation of basal ACTH secretion but only a trend for an increase in basal PVN CRH and parvocellular (mp) PVN AVP hnRNA expression, and a marked augmentation of restraint-induced ACTH secretion and the expression of all five genes examined. CORT containing saline partially normalized basal and restraint-induced ACTH secretion and restraint-induced AVP hnRNA, c-fos mRNA, and zif268 mRNA in the PVN in ADX rats. In contrast, expression patterns of restraint-induced PVN CRH hnRNA and NGFI-B mRNA were not different between ADX rats with or without CORT replacement. Given that there was no circulating CORT present at the time of restraint challenge in either group of ADX rats, the differential impact of CORT replacement on restraint-induced PVN gene expression must reflect differential dependency of the expression of these genes in the PVN on the prior presence of CORT.
Keywords: AVP, CRH, glucocorticoid negative feedback, HPA axis, immediate early gene, PVN
Introduction
Circulating glucocorticoids modify hypothalamicpituitary adrenal (HPA) axis function by acting directly on cellular components of the HPA axis (i.e. corticotrophs within the pituitary and hypophysiotropic corticotropin releasing hormone [CRH] neurons of the paraventricular nucleus of the hypothalamus [PVN]). Glucocorticoids may also indirectly affect HPA axis activity by altering the activity of brain regions that provide afferents to the PVN (Bradbury et al. 1991; de Kloet et al. 1998), as well as by altering inflammatory signals and peripheral metabolic-related signals that impact on HPA axis activity (Turnbull and Rivier 1999; Laugero 2004). These different glucocorticoid actions ultimately limit output of the HPA axis (Dallman et al. 1987). This negative feedback influence has been most extensively studied by examining the effect of various glucocorticoid manipulations on basal and stress-induced plasma adrenocorticotropic hormone (ACTH) levels in rats (Keller-Wood and Dallman 1984; Akana et al. 1988; Jacobson et al. 1989; Jacobson and Sapolsky 1993; Andres et al. 1999; Tanimura and Watts 2000).
From a physiological standpoint, a useful distinction can be made between the permissive (or proactive) and stimulatory/inhibitory (or reactive) effects of glucocorticoids on physiology (de Kloet et al. 1998; Sapolsky et al. 2000). This distinction may also reflect different mechanistic actions of glucocorticoids. A permissive influence is generally attributed to the effects that the presence of basal levels of glucocorticoids in the system have on physiological measures, such as ACTH secretion (Sapolsky et al. 2000). Permissive effects may be due to both the prior as well as the more immediate presence of basal levels of glucocorticoids. Reactive glucocorticoid effects have been attributed to the effects that a recent phasic increase in glucocorticoids produce on physiological measures (de Kloet et al. 1998). An example of reactive glucocorticoid effects is the influence that a phasic rise in glucocorticoid level during acute stress may have on the magnitude and duration of that ongoing acute stress response, including effects on stress-induced ACTH secretion. Dallman’s group have examined in detail the nature of the inhibitory influence of glucocorticoids on ACTH secretion and they found evidence for both permissive and reactive glucocorticoid effects (Dallman et al. 1987; Jacobson et al. 1988; Bradbury et al. 1991; Jacobson and Sapolsky 1993). Normal basal ACTH secretion appears to depend solely on permissive glucocorticoid effects (Akana et al. 1986). Interestingly, much of the hyper-responsiveness of ACTH secretion with acute stress present in adrenalectomized (ADX) rats appears also to be the result of the absence of permissive glucocorticoid effects. Moreover, there appear to be multiple components to this permissive influence. Maintenance of low constant levels of corticosterone (CORT) in ADX rats (via implantation of slow release CORT pellets) is sufficient to normalize to a large extent stress-induced ACTH secretion. However, the normal quite rapid shut-off of the ACTH response to a temporally punctuated stressor appears compromised (Akana et al. 1988). In contrast, giving ADX rats a replacement regimen of CORT in the drinking water, which maintains a circadian varying pattern of CORT exposure (Jacobson et al. 1988; Jacobson et al. 1989; Sutton et al. 1994), is sufficient to normalize basal ACTH levels and the shut-off rate of stress-induced ACTH secretion; although the absolute stress-induced secretion levels remain elevated (Jacobson et al. 1988).
The studies described above, however, did not determine the extent to which permissive glucocorticoid effects on basal and stress-induced ACTH secretion are a result of a direct effect of glucocorticoids on corticotrophs, or reflect more central actions that impact on ACTH secretagogue patterns. Some support for the latter possibility is provided by studies finding that long-term ADX also produces increases in basal and/or stress-induced CRH and arginine vasopressin (AVP) gene expression in the PVN (Swanson and Simmons 1989; Imaki et al. 1995; Tanimura and Watts 1998; Ma and Aguilera 1999; Kovacs et al. 2000). Kovacs et al. (2000) examined the extent to which constant CORT levels normalize CRH and AVP hnRNA responses to acute stress. They found that constant exposure to a physiologically low basal CORT level constrains the basal and stress-induced levels of CRH hnRNA and AVP hnRNA, whereas higher tonic levels of CORT completely suppress the acute stress response. However, with constant CORT replacement it is not possible to determine the extent to which basal and stress-reactive gene expression is maintained by the presence of glucocorticoids during stress, or if this gene expression is dependent on some residual effect of the prior presence of glucocorticoids.
The present study was designed after considering the above. This study investigated the permissive effects of glucocorticoids on both ACTH levels and concurrent neuronal activity within the PVN, with the goal of eventually determining the extent to which the two are coupled. As a measure of neuronal activity, we examined the expression of five genes that are each rapidly induced within the PVN by acute stress (Imaki et al. 1996; Kovacs and Sawchenko 1996; Umemoto et al. 1997). Two of these genes (CRH and AVP genes) encode precursor proteins for the principal neurohormones that control ACTH secretion. The other three genes examined (c-fos, zif268, and NGFI-B genes) function as immediate early genes in a wide range of neuronal populations and encode proteins that serve as inducible transcription factors (Herdegen and Leah 1998). Since every gene has a unique configuration of associated enhancer and repressor elements, it is likely that the expression of each of these five genes somewhat specifically reports on the recent activity of intracellular signaling pathways within the PVN. Moreover, each gene may be differentially responsive to the intracellular presence of glucocorticoids. For example, functional negative glucocorticoid response elements have been described for the CRH and AVP genes, but not for the three immediate early genes (Herdegen and Leah 1998; Malkoski and Dorin 1999; Kim et al. 2001; Dostert and Heinzel 2004).
To investigate permissive glucocorticoid effects, we examined rats for 5 days after ADX with or without a CORT replacement regimen that approximates basal CORT circadian patterns. For the CORT replacement condition, ADX rats were given saline to drink that contained a low dose of CORT (Jacobson et al. 1988). We removed the CORT-containing saline approximately 18 h before acute restraint challenge in order to ensure that there was no circulating CORT, and thus no immediate glucocorticoid effects exerted at the time of stress challenge. This is a time-frame of “stress-less” glucocorticoid removal that begins to result in an upregulation of basal ACTH secretion (Jacobson et al. 1989).
Our working hypothesis was that permissive glucocorticoid effects on ACTH secretion reflect not only a direct glucocorticoid action at the level of the corticotroph, but also effects on PVN CRH neuron activity. We therefore expected to see some parallel changes in basal and stress-induced plasma ACTH levels and PVN gene expression with long-term ADX with or without CORT replacement. Further interest was determination of the extent to which the expression of the five genes examined exhibit parallel changes within the PVN under our experimental conditions. Differential gene expression responses may be indicative of selective glucocorticoid effects on stress-responsive neural afferents to the PVN, the coupling of gene expression to neural afferent activity, or direct modulation of gene expression.
Methods
Subjects
Adult male Sprague Dawley rats (Harlan Labs, Indianapolis, IA, USA) weighing between 280 and 330 g were pair housed in polycarbonate tubs (47 cm × 23 cm × 20 cm) with access to food and drinking fluid ad libitum. Rats were allowed a 2-week acclimation period after arrival and before surgery or testing. The colony room was maintained on a 12 h light/dark cycle (lights on at 0700 h), and the temperature was maintained at 20–24°C. All testing began within 2–3.5 h after lights on. Care and use of animals were in accordance with ethical procedures approved by the University of Colorado Institutional Animal Care and Use Committee, and followed the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Restraint stressor challenge
Rats were placed in clear Plexiglas containers (23.5 cm long, 7 cm diameter) for various durations before tissue and blood collection. Restrainers prevented subjects from turning around, but allowed for regular breathing.
Tissue and blood collection
Rats were killed without anesthesia because anesthetics can rapidly activate the HPA axis which would confound the brain gene expression measures of this study (Vahl et al. 2005). Trunk blood was collected into EDTA coated tubes, and plasma separated by centrifugation, and brains were rapidly excised and flash frozen in chilled (−30°C) isopentane. Plasma and brains were then stored at −80°C until radioimmunoassay, sectioning, or in situ hybridization. The thymus gland was excised and weighed immediately after decapitation.
Experimental procedure
Five days prior to testing, surgical procedures were performed that resulted in three different glucocorticoid status groups. One-third of all rats in the study underwent sham surgery (Sham) under general anesthesia with xylazine (10 mg/kg) and ketamine (50 mg/kg) administered by intraperitoneal injection, during which time the adrenal glands were visually identified but not excised. The remaining rats in the study underwent bilateral ADX via two dorsal lateral incisions with the same anesthetic. After surgery, ADX rats were treated in one of two ways: they either had free access to 0.9% saline to drink (ADX), or they had free access to 0.9% saline plus CORT (ADX + Bw). For the latter group, 25 µg/ml CORT (Steraloids Inc., Wilton, NH, USA) was first dissolved in absolute ethanol, and then diluted into saline to give a final ethanol concentration of 0.1%. Saline-containing CORT was then withdrawn approximately 18 h before testing and replaced with regular saline (bottle switch at 1600 h) in order to ensure complete clearance of CORT in these rats at the time of restraint challenge.
Five days after surgery, subjects underwent 1 of 5 possible test day restraint duration challenges. Three of the five groups of rats were exposed to restraint for 15, 30, or 60 min, after which time they were rapidly decapitated for collection of samples. The fourth group of rats was taken directly from their home cages and killed. The fifth group of rats (recovery group) was challenged with restraint for 1 h and then returned to the homecages. After 1 h in their homecages, rats in this fifth group were killed. The experiment was a fully factorial 3 × 5 design (CORT status × test day challenge; n = 5, N = 75).
Radioimmunoassays
Diluted plasma samples (20 µl plasma to 1 ml with 0.01 M phosphate buffered saline) were first heat inactivated (1 h at 70°C). Both diluted samples and standards (25–2000 pg/tube) were then incubated overnight with CORT antibody (Endocrine Sciences B3–163, Calabas Hills, CA, USA) and 3H-CORT (Amersham, Arlington Heights, IL, USA). Antibody-bound steroid was separated from free steroid by dextran-coated activated charcoal and centrifugation. Supernatant radioactivity was determined by a liquid scintillation analyzer (Packard Instruments 1600TR, Meriden, CT, USA). The detection limit was 0.5 µg/100ml, and the variability within the assay was 7%.
ACTH was measured with basic methods previously described (Nicholson et al. 1984). Briefly, all procedures of the ACTH radioimmunoassay were conducted at 4°C, unless otherwise noted. Plasma (125 µl) was first diluted 1:1 with 0.01 M phosphate buffered saline (pH 7.4). The primary ACTH antiserum Rb7 (courtesy of Dr Bill Engeland, Univ. of Minnesota) and 125I ACTH (Diasorin, Inc., Stillwater, MN, USA) were then added to the diluted plasma and hACTH standard curve tubes (7.5–100 pg/ml). Assay tubes were then incubated overnight. The next day, normal rabbit serum (Vector Labs, Burlingame, CA, USA) and goat anti-rabbit gamma globulin (Calbiochem, La Jolla, CA, USA) were added to the assay tubes, which were then incubated for 30 min at room temperature. Subsequently, 5% polyethylene glycol solution was added to the assay tubes immediately before centrifugation at 4000g at 4°C. Supernatant was then drawn off by aspiration and discarded. Radioactivity in the pellets in the assay tubes was measured with a gamma counter. The detection limit of the assay was 10 pg/ml, and the variability within the assay was 9%.
In situ hybridization and image analysis
Gene expression within the PVN was measured with in situ hybridization (Girotti et al. 2006). Rapid stress-induced increases in CRH and AVP gene expression are readily detected by monitoring the short-lived primary transcript (hnRNA) for these genes (Herman et al. 1991,1992). Coronal tissue sections (thickness 10 µM) were first cut on a cryostat and mounted onto poly-L-lysine coated slides, and were then post-fixed with 4% paraformaldehyde. Slides were then acetylated for 10 min with 0.1 M triethanolamine (pH 8) containing 0.25% acetic anhydride, and dehydrated with increasing graded ethyl alcohol concentrations and air-dried. 35S-UTP (c-fos, zif268, andNGFI-B) or 35S-UTP/35S-CTP (AVP and CRH) labeled cRNA probes were generated for CRH hnRNA, AVP hnRNA, c-fos mRNA, zif268 mRNA, and NGFI-B mRNAs from cDNA subclones in transcription vectors using standard in vitro transcription methods (Girotti et al. 2006). CRH hnRNA cDNA was kindly provided by Dr Robert Thompson (Univ. of Michigan), AVP hnRNA cDNA was kindly provided by Dr Thomas G. Sherman (Georgetown Univ. Medical Ctr), c-fos mRNA cDNA was kindly provided by Dr Tom Curran (St Jude’s Children’s Research Hospital, Memphis, TN, USA) and zif268 mRNA cDNA was kindly provided by Dr J. Milbrandt (Washington University School of Medicine, St Louis). The NGFI-B plasmid harboring a 839 base pair fragment spanning nucleotides 188–1027 of the mature mRNA (Genebank acc. #NM_024388) was generated in house. Briefly, total RNA from rat hippocampus was isolated using the SV. Total RNA extraction system (Promega, Fitchburg, WI, USA) and first strand cDNA was produced using SuperScript II Reverse Transcriptase reagents (Invitrogen, Carlsbad, CA, USA). Following PCR with NGFI-B specific oligonucleotides, the amplified product was cloned into pCRII-TOPO vector(Invitrogen) according to the manufacturer’s instructions. The identity of the cloned DNA was verified by DNA sequencing (University of Colorado Molecular, Cellular and Developmental Biology sequencing facility).
Probes were diluted into hybridization buffer (50% formamide, 10% dextran sulfate, 3 × SSC, 50 mM sodium phosphate buffer [pH 7.4], 1 × Denhardt’s solution, and 0.1 mg/ml yeast tRNA) to yield approximately 1.5 × 106 dpm/30 µl buffer, and applied to coronal tissue sections (65 µl/slide). Sections were then incubated overnight at 55°C in sealed chambers containing a 50% formamide solution in the base. The next day coverslips were floated off the slides, sections were treated with RNase at 37°C for 1 h, then subjected to a stringent high temperature (65°C) wash for 1 h before being dehydrated with increasing concentrations of ethyl alcohol followed by air drying. Sections were then exposed to X-ray film (Kodak XAR, X-ray film Eastman Kodak Company, Rochester, NY, USA) for a proper exposure time, as determined by quality control slides exposed to smaller test pieces of X-ray film. After exposure to X-ray film, slides treated with a probe for AVP hnRNA were dipped in photographic emulsion (Amersham LM-1) and allowed to expose for an appropriate period of time determined by test slides (20–40 days). Afterward, sections were developed (Kodak D-19 developer Eastman Kodak Company) and counterstained with cresyl violet for purposes of anatomical verification. Sections treated with one of the other probes were also counterstained with cresyl violet after exposure to X-ray film to assist with anatomical verification of the PVN location in the autoradiograms.
Brain sections chosen for analysis were located approximately 1.8 mm posterior to bregma and matched the general appearance of PVN topology illustrated in the rat brain atlas of Swanson (2004). Semiquantitative densiometric analysis of digitized X-ray film images was performed with NIH Image software. For analysis of autoradiograms, pixels within the region of interest defined as positive signal were 3.5 × standard deviation gray level units above representative background, which was set by an area adjacent to the region of interest that contained no signal. Integrated gray level for one PVN section was then computed from the product of the number of pixels comprising the positive signal and their average gray level within the region of interest. Overall integrated gray level for an individual rat was determined from the average integrated gray levels from four PVN sections (Campeau and Watson 1997). Quantitation of AVP hnRNA from emulsion dipped slides began with digitizing a given region of interest in both light field and dark field magnification, followed by separate quantitation of single clusters of grains (>5 pixels) within the parvocellular zone (defined as extending laterally from the third ventricle by 350 µm) and the magnocellular zone (lateral to the 350 µm point). The use of 350 µm as a dividing line between primarily parvocellular and magnocellular subdivisions of the PVN was determined empirically in a pilot study comparing the medial to lateral distribution of AVP hnRNA positive cells in the PVN rats restrained for 1 h or unstressed. This division corresponds well to the lateral extent of the medial parvocellular dorsal subdivision of the PVN at this rostral-caudal level of the rat brain (Swanson 2004). Density of positive cells for each rat was then computed as the average of three different serial PVN sections. Within each section, cell density was computed as the number of positive cell nuclei divided by the total square pixel area of the region of interest.
Statistics
Data were analyzed with a two-way ANOVA for independent measures (duration of restraint challenge × glucocorticoid status group), unless indicated otherwise. Post hoc comparisons were made with Fisher’s least significant difference test (FLSD). The level of statistical significance was set at p ≤ 0.05 for all tests, and a statistical analysis computer application was used (StatView v4.51 for Macintosh, Abacus Concepts, Berkeley, CA, USA). In order to determine for each dependent variable the approximate time of peak response to acute restraint we adopted the following criteria: the peak response was considered to be at the first time-point post-restraint onset where the value was significantly greater than the basal level (time 0) and not significantly less than the value at the subsequent time-point for that particular glucocorticoid status treatment group. Data presented in graphs are group means ± SEM.
Results
Plasma hormone concentrations
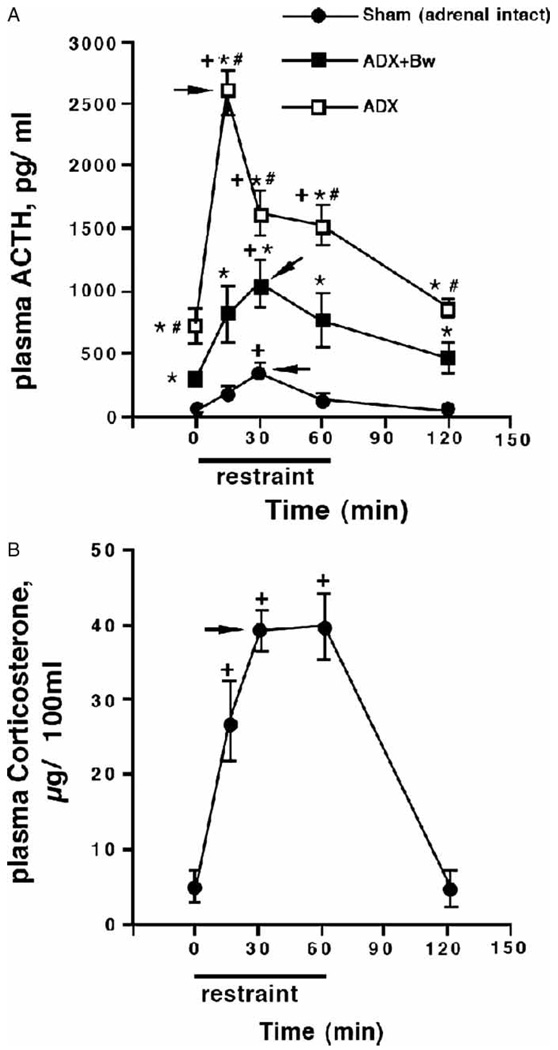
Across the three glucocorticoid status groups there was a significant increase in plasma ACTH concentration as a result of restraint challenge, F(4, 62) = 20.8, p < 0.001 (Figure 1(A)). ACTH concentrations depended upon glucocorticoid status before testing, regardless of the duration of restraint challenge, F(2, 62) = 119.01, p < 0.001. It is noteworthy that the timing of the peak plasma ACTH response to restraint challenge differed as a function of basal glucocorticoid status before testing, as reflected in a significant duration of restraint challenge by glucocorticoid status group interaction (F[8, 62] = 7.6, p < 0.001). Post hoc analysis indicated that basal (no stress) plasma ACTH concentrations differed among the three glucocorticoid status groups. ADX rats had the highest basal ACTH levels, which were partially normalized by treatment with CORT in the drinking water. The same pattern of relative differences for the three glucocorticoid status groups continued throughout the restraint time-course. However, ADX rats displayed a prominent peak of maximal plasma ACTH response after just 15 min of restraint, while ADX + Bw or sham rats reached peak levels of plasma ACTH after 30 min of restraint challenge. Adrenalectomized rats ± CORT replacement did not have detectable levels of circulating CORT at the time they were killed (Figure 1(B)).
Figure 1.
Effects of adrenalectomy ± CORT replacement on basal and restraint-induced plasma ACTH and CORT concentrations. Rats underwent sham adrenalectomy (ADX; Sham) or ADX 5 days before restraint challenge. After surgery, adrenalectomized rats received either 0.9% saline solution (ADX) or saline with 25µg/ml CORT (ADX + Bw) to drink. In the case of the latter, CORT saline was replaced with regular saline 18 h before testing. Trunk blood was obtained at the time from unstressed rats (0 min), from rats challenged with various durations of restraint (15, 30, or 60min), and from rats 1 h after 60 min of restraint (120 min); N = 75, n = 5. Both plasma ACTH (panel A) and plasma CORT (panel B) were measured in trunk blood samples; there were no detectable CORT levels in ADX or ADX + Bw rats. +p < 0.05, compared to no stress (0 min) timepoint in same glucocorticoid status group; *p < 0.05, compared to sham group at same time; #p < 0.05, compared to ADX + Bw rats (FLSD). Arrowheads indicate the peak response as determined by the criteria described in the Methods (statistics subheading).
Thymus gland
Mean (SEM) thymus gland weights by group were as follows: sham, 382.5 mg (13.0 mg); ADX + Bw, 411.8 mg (16.5 mg); and ADX, 485.6 mg (18.0 mg). Thymus gland weight depended upon glucocorticoid status group as determined by one-way ANOVA for independent measures, F(2, 74) = 11.20, p < 0.001. Post hoc analysis indicated that the only pairwise group difference that was statistically significant was between ADX and sham rats.
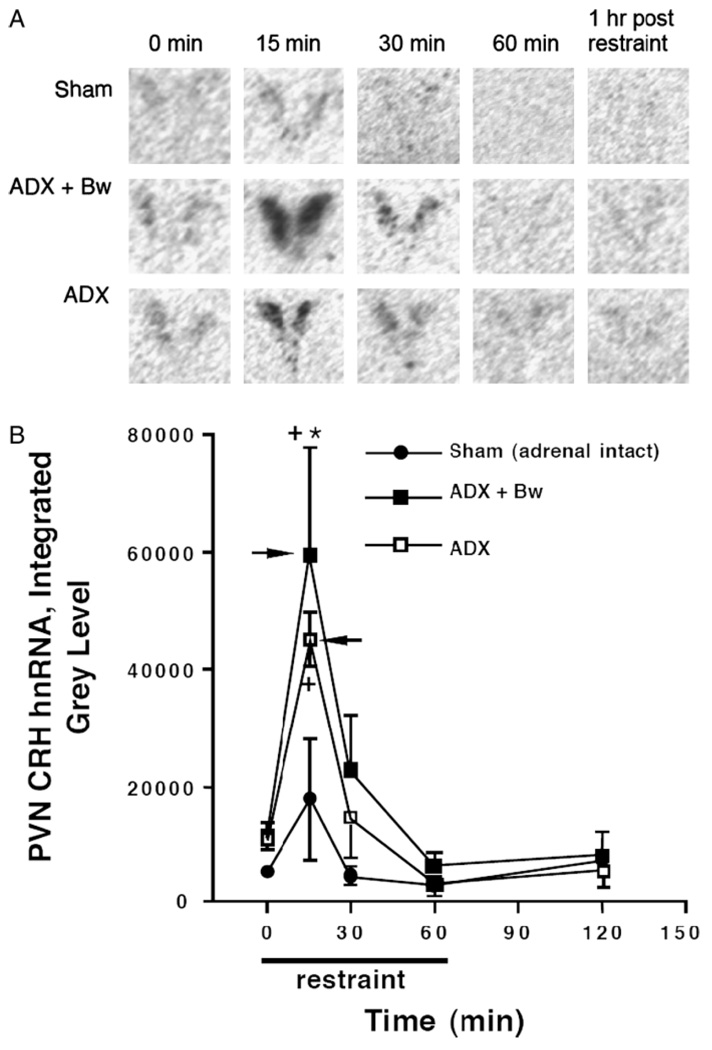
PVN CRH hnRNA
Representative autoradiograms of CRH hnRNA in the PVN across treatment groups are depicted in Figure 2(A). The overall amount of CRH hnRNA expression depended upon circulating glucocorticoid status before restraint challenge, F(2, 60) = 5.8, p < 0.001 (Figure 2(B)). Challenge with restraint also resulted in an overall increase in expression of CRH hnRNA regardless of glucocorticoid status group, F(4, 60) = 14.4, p < 0.001. Post hoc analysis indicated that there were strong trends for ADX rats (p = 0.07) and ADX + Bw rats (p = 0.06) to have higher basal (no stress) levels of CRH hnRNA expression than the sham group. Regardless of glucocorticoid status, maximal expression of CRH hnRNA occurred after 15 min of restraint and the expression levels returned to near basal levels within 30 min after restraint onset. In addition, ADX + Bw rats had significantly greater CRH hnRNA expression than the sham group after 15 min of restraint. A similar trend of augmented CRH hnRNA after 15 min of restraint for ADX rats compared to sham rats was evident.
Figure 2.
Effects of ADX ± CORT replacement on basal and restraint-induced CRH hnRNA in the PVN of the hypothalamus. Rats underwent sham ADX (Sham) or ADX 5 days before restraint challenge. After surgery, adrenalectomized rats received either saline solution (ADX) or saline with 25µg/ml CORT (ADX + Bw). In the case of the latter, CORT saline was substituted with regular saline 18 h before testing. Whole brains obtained by decapitation were rapidly frozen: from unstressed rats (0 min), from rats challenged with various durations of restraint (15, 30, or 60 min), and from rats 1 h after 60 min of restraint (120 min); N = 75, n = 5. Panel A: representative PVN CRH hnRNA autoradiograms for each group. Panel B: graph of group CRH hnRNA integrated gray levels. +p < 0.05, compared to no stress (0 min) timepoint in same glucocorticoid status group; *p < 0.05, compared to sham group at same time (FLSD). Arrowheads indicate the peak response as determined by the criteria described in the Methods (statistics subheading).
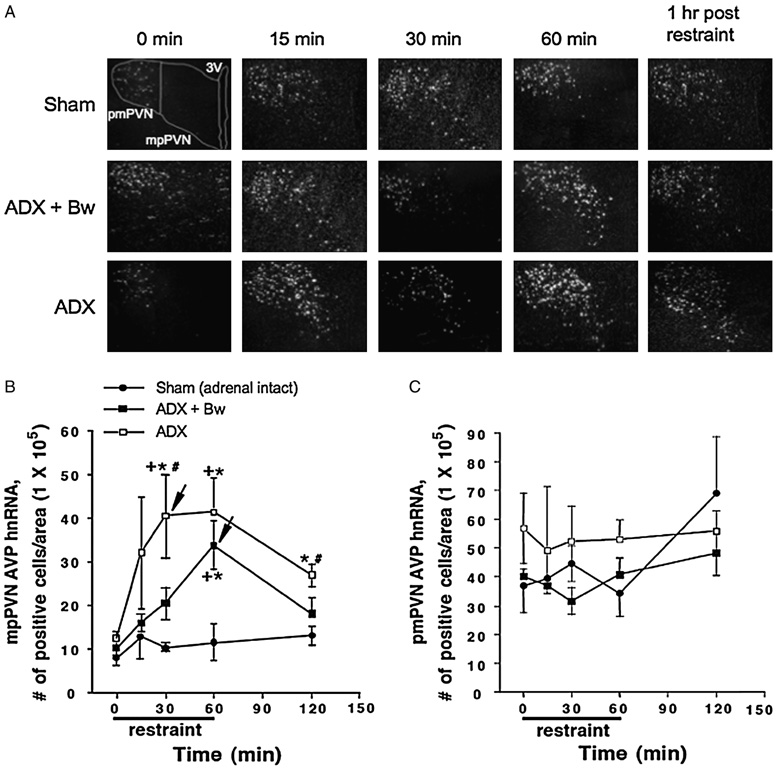
PVN AVP hnRNA
Representative darkfield photomicrographs of AVP hnRNA in the PVN across treatment groups are depicted in Figure 3(A). Overall, mpPVN (parvocellular subdivision) AVP hnRNA expression depended upon glucocorticoid status, F(2, 60) = 20.8, p < 0.001 (Figure 3(B)). Challenge with restraint also increased expression of AVP hnRNA in the mpPVN, irrespective of glucocorticoid status group, F(4, 60) = 6.4, p < 0.001. ADX rats exhibited a rapid stress-induced rise in AVP hnRNA expression that reached peak levels after 30 min of restraint. ADX + Bw rats also exhibited a large stress-induced increase in AVP hnRNA, however, they did not attain peak levels of expression until 60 min after stress onset. In sham-treated rats there was a small increase in AVP hnRNA levels at all post-stress time-points, but this increase was not statistically significant. Post hoc analysis indicated that ADX rats had significantly greater AVP hnRNA expression levels after 30 min of restraint compared to sham and ADX + Bw rats. In addition, ADX + Bw rats did not differ from sham rats 1 h after restraint had ended, while ADX rats had significantly higher levels than the other two groups at this time. In contrast to the mpPVN, there were no significant main effects of glucocorticoid status group or duration of restraint challenge on pmPVN (magnocellular subdivision) AVP hnRNA expression (Figure 3(C)).
Figure 3.
Effects of ADX ± CORT replacement on basal and restraint-induced AVP hnRNA in the medial parvocellular (mp) and posterior magnocellular (pm) PVN of the hypothalamus. See Figure 2 legend for description of treatment groups. Panel A: representative PVN AVP hnRNA dark field micrographs. Panel B: graph of AVP hnRNA positive cells/area in mpPVN. Panel C: graph of AVP hnRNA positive cells/area in pmPVN +p < 0.05, compared to no stress (0 min) timepoint in same glucocorticoid status group; *p < 0.05, compared to sham group at same time; #p < 0.05, compared to ADX + Bw rats (FLSD). Arrowheads indicate the peak response as determined by the criteria described in the Methods (statistics subheading).
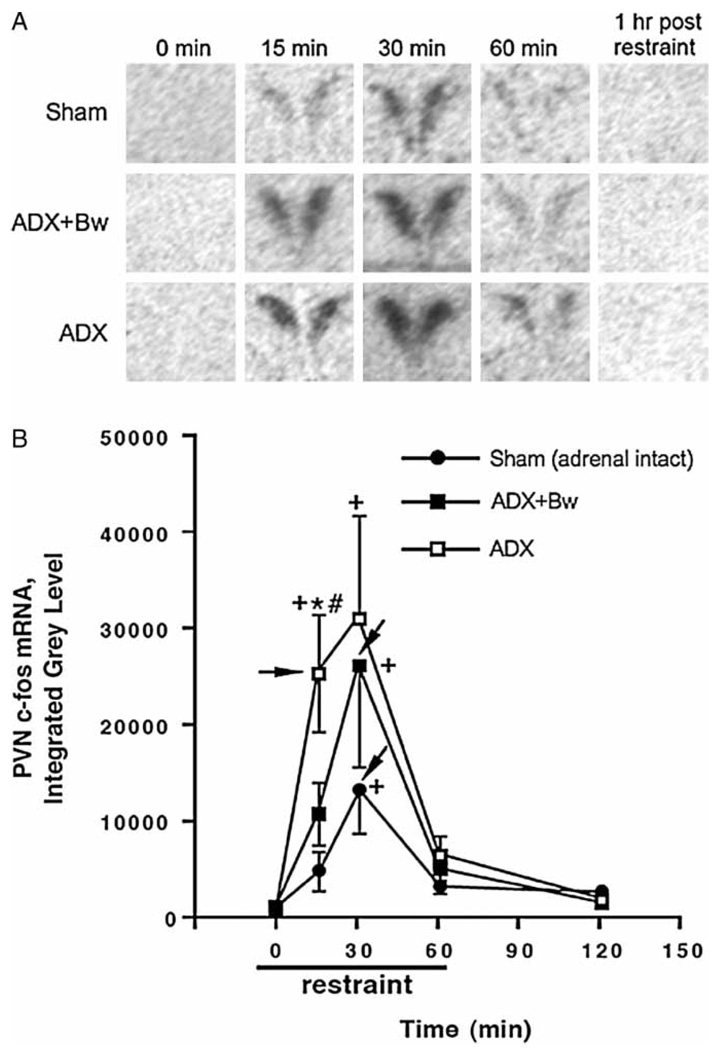
PVN c-fos mRNA
Representative autoradiograms of c-fos mRNA in the PVN across treatment groups are depicted in Figure 4(A). Overall, c-fos mRNA expression in the PVN depended upon glucocorticoid status group, F(2,61) = 3.9, p < 0.05 (Figure 4(B)). The overall expression of c-fos mRNA in the PVN also varied as a function of duration of restraint challenge, F(4,61) = 12.7, p < 0.001. Post hoc analysis indicated that basal expression of c-fos mRNA in the PVN did not differ between sham rats, ADX rats or ADX + Bw rats. Challenge with restraint revealed important differences in c-fos expression between the three groups. In particular, after 15 min of restraint, ADX rats had greater PVN c-fos mRNA expression than sham rats and ADX + Bw rats. ADX rats reached near maximal PVN c-fos mRNA expression after only 15 min of restraint, while sham rats and ADX + Bw rats required 30 min of restraint challenge to reach maximum c-fos mRNA expression.
Figure 4.
Effects of ADX ± CORT replacement on basal and restraint-induced c-fos mRNA in the PVN of the hypothalamus. See Figure 2 legend for description of treatment groups. Panel A: representative PVN c-fos mRNA autoradiograms for each group. Panel B: graph of group c-fos mRNA integrated gray levels. +p < 0.05, compared to no stress (0 min) timepoint in same glucocorticoid status group; *p < 0.05, compared to sham group at same time; #p < 0.05, compared to ADX + Bw rats (FLSD). Arrowheads indicate the peak response as determined by the criteria described in the Methods (statistics subheading).
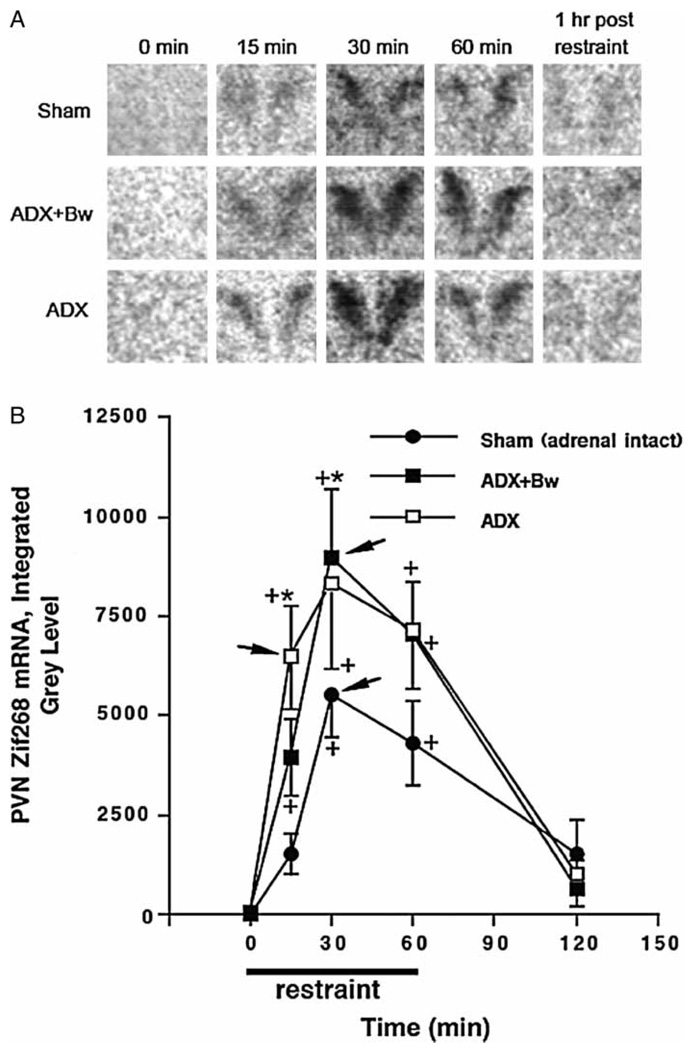
PVN zif268 mRNA
Representative autoradiograms of zif268 mRNA in the PVN across treatment groups are depicted in Figure 5(A). The overall amount of zif268 mRNA expression depended upon the glucocorticoid status group, F(2, 58) = 4.9, p < 0.05 (Figure 5(B)). Challenge with restraint also resulted in an overall increase in expression of zif268 mRNA regardless of glucocorticoid status group, F(4, 58) = 28.7, p < 0.001. There was no trend for ADX with or without CORT replacement to lead to an increase in basal zif268 mRNA expression. However, there was an enhanced level of stress-induced zif268 mRNA expression in ADX rats (significant 15 min after restraint) or ADX + Bw rats (significant 30 min after restraint) compared to sham rats. ADX rats reached near peak zif268 mRNA levels within 15 min after restraint onset, whereas ADX + Bw rats and sham rats reached peak levels after 30 min.
Figure 5.
Effects of ADX ± CORT replacement on basal and restraint-induced zif268 mRNA in the PVN of the hypothalamus. See Figure 2 legend for description of treatment groups. Panel A: representative PVN zif268 mRNA autoradiograms for each group. Panel B: graph of group zif268 mRNA integrated gray levels. +p < 0.05, compared to no stress (0 min) timepoint in same glucocorticoid status group; *p < 0.05, compared to sham group at same time. Arrowheads indicate the peak response as determined by the criteria described in the Methods (statistics subheading).
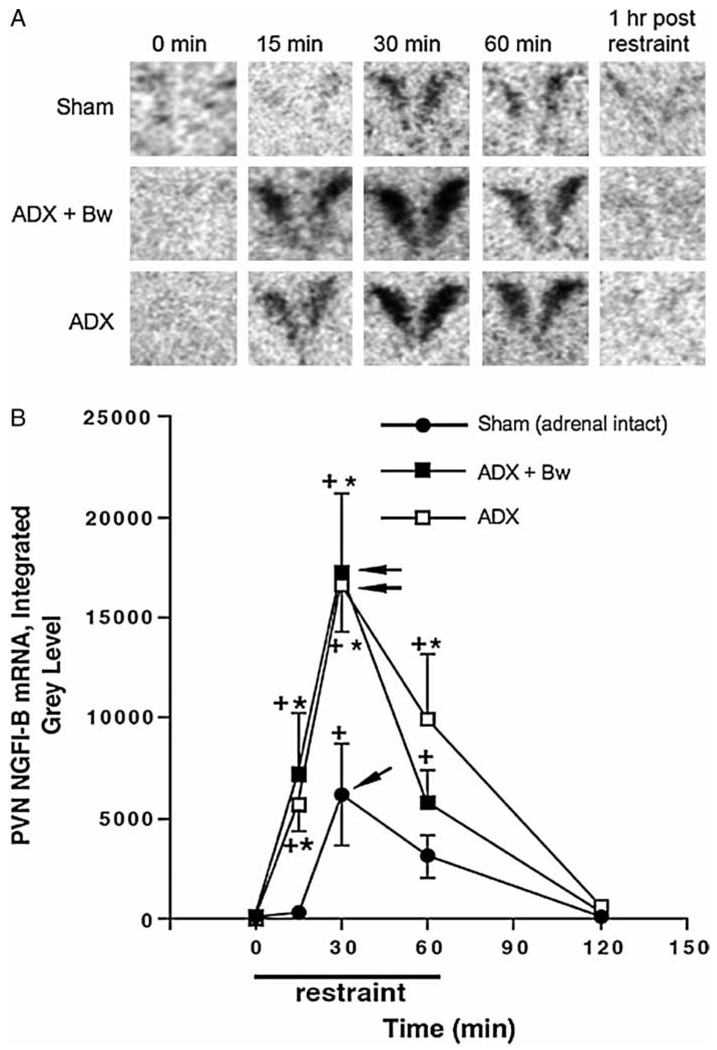
PVN NGFI-B mRNA
Representative autoradiograms of NGFI-B mRNA in the PVN across treatment groups are depicted in Figure 6(A). The overall amount of NGFI-B mRNA expression depended upon circulating glucocorticoid status group, F(2, 59) = 9.7, p < 0.001 (Figure 6(B)). Challenge with restraint also resulted in an overall increase in expression of NGFI-B mRNA regardless of glucocorticoid status group, F(4, 59) = 27.5, p < 0.001. There was no trend for ADX with or without CORT replacement to lead to an increase in basal NGFI-B mRNA expression. However, stress-induced levels of NGFI-B mRNA were greater in ADX rats (significant 15, 30 and 60 min after restraint) or ADX + Bw rats (significant 15 and 30 min after restraint) compared to sham rats. In all glucocorticoid status treatment groups, peak levels of NGFI-B mRNA were attained 30 min after restraint onset and did not return to basal levels until 1 h after the cessation of restraint.
Figure 6.
Effects of ADX ± CORT replacement on basal and restraint-induced NGFI-B mRNA in the PVN of the hypothalamus. See Figure 2 legend for description of treatment groups. Panel A: representative PVN NGFI-B mRNA autoradiograms for each group. Panel B: graph of group NGFI-B mRNA integrated gray levels. +p < 0.05, compared to no stress (0 min) timepoint in same glucocorticoid status group; *p < 0.05, compared to sham group at same time (FLSD). Arrowheads indicate the peak response as determined by the criteria described in the Methods (statistics subheading).
Discussion
The main goal of our study was to investigate the degree to which normal patterns of restraint-induced plasma ACTH and PVN gene expression depend on the effects of glucocorticoids exerted in the time period before, but not during, restraint challenge (i.e. permissive glucocorticoid effects). We directly compared the consequence of the long-term absence (5-day ADX) or shorter absence (18 h withdrawal of CORT drinking water from ADX rats) on basal and acute stress-induced measures. We found that the expression of certain stress-reactive genes, but not others, as well as plasma levels of ACTH were partially normalized by giving ADX rats this CORT replacement regimen. Although previous studies have examined the effect of ADX on stress-induced CRH hnRNA, AVP hnRNA and c-fos mRNA expression in the PVN (Imaki et al. 1995; Helmreich et al. 1996; Tanimura and Watts 1998; Ma et al. 1999; Kovacs et al. 2000; Tanimura and Watts 2000), we report here also the effect of ADX on basal and stress-induced zif268 mRNA and NGFI-B mRNA in the PVN. In addition, this is the first study to examine whether treatment of ADX rats with CORT in the drinking water moderates the effect of ADX on basal and stress-induced expression of each of these five genes within the PVN.
Long-term ADX not only produced a marked elevation of basal ACTH secretion, but also produced a trend for an elevation within the PVN in basal levels of expression of the neurohormone (CRH and AVP) encoding genes. Others have reported increased expression of both CRH and AVP genes after ADX, either at the level of primary transcript (Jingami et al. 1985; Tanimura and Watts 1998; Kovacs et al. 2000) or mature message (Beyer et al. 1988; Imaki et al. 1991; Tanimura and Watts 1998). In cases where basal expression of both genes was increased, expression was normalized by constant CORT replacement (Makino et al. 1995; Kovacs et al. 2000; Pinnock and Herbert 2001) or treatment with dexamethasone, a synthetic glucocorticoid selective for the glucocorticoid receptor (Jingami et al. 1985; Beyer et al. 1988; Imaki et al. 1991). Whether increased neurohormone gene transcription in ADX rats translates directly into increased basal and stress-induced neurohormone secretion remains to be determined (Watts 2005).
In this study, there was no trend for ADX to produce an increase in the PVN of the basal expression of the three immediate early genes examined, c-fos, zif268, or NGFI-B. This is consistent with previous reports finding no change in basal PVN c-fos mRNA or Fos protein in ADX rats (Helmreich et al. 1996; Brown and Sawchenko 1997; Kovacs et al. 2000; Fevurly and Spencer 2004). PVN c-fos expression may be a good indicator of overall excitatory neural input to the PVN (Ginsberg et al. 2003). The lack of a basal increase after ADX in expression of the three immediate early genes suggests that PVN neurons receive the same amount of basal excitatory input from neural sites outside the PVN when circulating glucocorticoids are absent. As discussed in the Introduction, numerous studies have shown that ACTH output from corticotrophs is controlled by glucocorticoids (Akana et al. 1986) and ACTH output from these cells increases dramatically in ADX rats. The lack of an increase of basal PVN immediate early gene expression combined with a dramatic rise in basal ACTH suggests that alterations in basal levels of ACTH seen here were likely the result of increased intrinsic corticotroph activity in the absence of a direct inhibitory effect of glucocorticoids, instead of increased activity due to higher levels of CRH and AVP in the portal blood. However, this conclusion is tentative without directly measuring these neurohormones in the portal blood. Alternatively, ADX has been shown to decrease CRH receptor 1 mRNA level in the anterior pituitary (Makino et al. 1995), and this is likely the result of increased CRH peptide release from the PVN that has been observed after ADX (Fink et al. 1988; Sakai et al. 1996). Consequently, the data may point to increases in CRH neuron basal CRH/AVP secretion that are independent of intracellular signal transduction activity that converges on general immediate early gene expression.
In this study, ADX augmented the overall acute stress-induced increase in ACTH secretion and stress-induced expression of all five genes examined in the PVN (CRH, AVP, c-fos, zif268, and NGFI-B). This result is consistent with previous studies that found an enhanced magnitude of CRH hnRNA, AVP hnRNA and c-fos mRNA, or Fos protein response in the PVN to acute stress in ADX rats (Imaki et al. 1995; Viau et al. 1999; Kovacs et al. 2000; Tanimura and Watts 2000; Fevurly and Spencer 2004). This augmented stress-induced gene expression in ADX rats may be due to an absence of a direct effect of glucocorticoids on the PVN. Other studies found that the enhancing effect of ADX on stress-induced Fos expression was restricted to the PVN (Kovacs et al. 2000; Fevurly and Spencer 2004).
For some of the measures in this study (ACTH, AVP hnRNA, c-fos mRNA, and zif268 mRNA) an additional component of the augmented stress-induced response in ADX rats was that peak, or near peak, response levels were reached sooner after stress onset than in adrenal-intact rats. In ADX rats, drinking saline with added CORT up until 18 h before restraint challenge partially normalized stress-induced ACTH secretion at all stress-time points examined, and shifted the time of peak response from 15 to 30 min after restraint onset. Interestingly, this same CORT treatment appeared to partially normalize the expression of a subset of the PVN genes examined (AVP hnRNA, c-fos mRNA, and zif268 mRNA), but only during the early portion of the restraint stress response (during the first 15 or 30 min of restraint). Thus, the CORT replacement regimen partially normalized the extent to which there was a rapid increase in gene expression after restraint onset, but it did not normalize the eventual peak gene expression levels.
Kovacs et al. (2000) also found that ADX led to an advance in peak AVP hnRNA and Fos protein levels after ether stress. Interestingly, in that study, treatment of ADX rats with a constant low level of CORT reduced overall stress-induced AVP hnRNA levels, but peak AVP hnRNA and Fos responses were still advanced relative to the time of peak responses in adrenal-intact rats. The authors proposed the possibility that the stress-induced phasic increase in endogenous CORT present in adrenal-intact rats produced a reactive/suppressive effect that delayed peak AVP hnRNA and Fos protein responses. Our results demonstrate that a prior history of diurnal variation in basal CORT levels is sufficient to delay the peak of stress-induced ACTH secretion and AVP, c-fos, and zif268 gene expression in the PVN.
In contrast to the partial normalization effect of drinking saline with added CORT on AVP, c-fos, and zif268 gene expression in the PVN, we saw no difference in the CRH hnRNA and NGFI-B mRNA stress response magnitude in ADX rats with or without CORT replacement. Thus, constraint of CRH and NGFI-B gene expression in the PVN may require a more recent (or concurrent) presence of basal glucocorticoid levels at the time of stressor challenge than does AVP, c-fos, and zif268 gene expression. It is also noteworthy that the rapid shut-off of the CRH hnRNA response to restraint (within 15 min) was present in all rats irrespective of glucocorticoid status, as has been noted by others (Shepard et al. 2005).
The different responses between ADX rats with or without drinking saline with added CORT may not only be due to the different duration of time that CORT was absent from the system at the time of restraint challenge but also differences between these two groups in the pattern of CORT exposure for the first 4 days after ADX. Jacobsen et al. (1988) examined the time-course for increases in basal plasma ACTH after adrenalectomy or after “stressless” removal of CORT from ADX rats. The stressless removal of CORT was accomplished by removing CORT containing drinking water from rats that had been ADX 5 days earlier, similar to the procedure that we used in this study. Jacobson and coworkers found that ACTH levels were already greater 2 h after surgery in ADX rats compared to Sham ADX rats. In contrast, basal ACTH levels did not increase until 18–24 h after CORT water removal from ADX rats, suggesting a short-term interaction between surgery stress and the absence of CORT on basal ACTH levels. There was also some evidence in the Jacobson et al. study for more stable basal ACTH levels in rats with stressless CORT removal than in long-term ADX rats (4–7 days after ADX; Jacobsen et al. 1988). Thus, there is the possibility that our two ADX groups had different stress-reactivity as a result of a complex interaction between the long-term response to the stress of surgery and the presence or absence of CORT for the first 4 days after surgery. The responses of rats treated with CORT in our study, however, also likely reflected some residual effects of CORT present at the time of restraint challenge that were not present in 5-day ADX rats given no CORT replacement. If drinking saline with CORT by ADX rats influenced the stress-induced neural input to the PVN, then it must have selectively affected a subset of inputs that contributed to stress-induced AVP, c-fos, and zif268 gene induction, but not CRH and NGFI-B gene induction. Alternatively, CORT may also have acted directly within CRH neurons to modulate the expression of some protein that contributed to stimulus-gene expression coupling of AVP, c-fos, and zif268 gene expression, but not CRH and NGFI-B gene expression.
In summary, data presented here indicate that various elements of the HPA axis depend differently upon basal glucocorticoid levels present before acute stress. It is noteworthy that the large increase in basal ACTH levels present in 5-day ADX rats is not reflected in alterations in basal immediate early gene expression in the PVN. In contrast, the expression in the PVN of all five genes examined, as well as ACTH secretion, exhibit a large enhancement of their responses to restraint after 5 days of ADX. However, our CORT replacement regimen, which had a partial normalizing effect on stress-induced plasma ACTH levels, also partially normalized the expression in the PVN of AVP hnRNA, c-fos mRNA, and zif268 mRNA, but not CRH hnRNA and NGFI-B mRNA.
Acknowledgements
We are grateful to Serge Campeau and Heidi Day for their generous assistance with the in situ hybridization procedures used for these studies. These studies were supported by NIH Grants MH-62456, MH-75968 and F31–13011.
Footnotes
Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Akana SF, Cascio CS, Du J-Z, Levin N, Dallman ME. Reset of feedback in the adrenocortical system: An apparent shift in sensitivity of adrenocorticotropin to inhibition by corticosterone between morning and evening. Endocrinology. 1986;119:2325–2332. doi: 10.1210/endo-119-5-2325. [DOI] [PubMed] [Google Scholar]
- Akana SF, Jacobson L, Cascio CS, Shinsako J, Dallman MF. Constant corticosterone replacement normalizes basal adrenocorticotropin (ACTH) but permits sustained ACTH hypersecretion after stress in adrenalectomized rats. Endocrinology. 1988;122:1337–1342. doi: 10.1210/endo-122-4-1337. [DOI] [PubMed] [Google Scholar]
- Andres R, Marti O, Armario A. Direct evidence of acute stress-induced facilitation of ACTH response to subsequent stress in rats. Am J Physiol. 1999;277:R863–R868. doi: 10.1152/ajpregu.1999.277.3.R863. [DOI] [PubMed] [Google Scholar]
- Beyer HS, Matta SG, Sharp BM. Regulation of the messenger ribonucleic acid for corticotropin-releasing factor in the paraventricular nucleus and other brain sites of the rat. Endocrinology. 1988;123:2117–2123. doi: 10.1210/endo-123-4-2117. [DOI] [PubMed] [Google Scholar]
- Bradbury M, Akana SF, Cascio CS, Levin N, Jacobson L, Dallman MF. Regulation of basal ACTH secretion by corticosterone is mediated by both type I (MR) and type II (GR) receptors in rat brain. J Steroid Biochem Molec Biol. 1991;40:133–142. doi: 10.1016/0960-0760(91)90176-6. [DOI] [PubMed] [Google Scholar]
- Brown ER, Sawchenko PE. Hypophysiotropic CRF neurons display a sustained immediate-early gene response to chronic stress but not to adrenalectomy. J Neuroendocrinol. 1997;9:307–316. doi: 10.1046/j.1365-2826.1997.00586.x. [DOI] [PubMed] [Google Scholar]
- Campeau S, Watson SJ. Neuroendocrine and behavioral responses and brain pattern of c-fos induction associated with audiogenic stress. J Neuroendocrinol. 1997;9:577–588. doi: 10.1046/j.1365-2826.1997.00593.x. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Cascio CS, Darlington DN, Jacobson L, Levin N. Regulation of ACTH secretion: Variations on a theme of B. Recent Prog Horm Res. 1987;43:113–173. doi: 10.1016/b978-0-12-571143-2.50010-1. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Reviews. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- Dostert A, Heinzel T. Negative glucocorticoid receptor response elements and their role in glucocorticoid action. Curr Pharm Des. 2004;10:2807–2816. doi: 10.2174/1381612043383601. [DOI] [PubMed] [Google Scholar]
- Fevurly RD, Spencer RL. Fos expression is selectively and differentially regulated by endogenous glucocorticoids in the paraventricular nucleus of the hypothalamus and the dentate gyrus. J Neuroendocrinol. 2004;16:970–979. doi: 10.1111/j.1365-2826.2004.01257.x. [DOI] [PubMed] [Google Scholar]
- Fink G, Robinshon IC, Tannahill LA. Effects of adrenalectomy and glucocorticoids on the peptides CRF-41, AVP, and oxytocin in rat hypophysial portal blood. J Physiol. 1988;401:329–345. doi: 10.1113/jphysiol.1988.sp017165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg AB, Campeau S, Day HE, Spencer RL. Acute glucocorticoid pretreatment suppresses stress-induced hypothalamic-pituitary-adrenal axis hormone secretion and expression of corticotropin-releasing hormone hnRNA but does not affect c-fos mRNA or fos protein expression in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol. 2003;15:1075–1083. doi: 10.1046/j.1365-2826.2003.01100.x. [DOI] [PubMed] [Google Scholar]
- Girotti M, Pace TWW, Gaylord RI, Rubin BA, Herman JP, Spencer RL. Habituation to repeated restraint stress is associated with lack of stress-induced c-fos expression in primary sensory processing areas of the rat brain. Neuroscience. 2006;138:1067–1081. doi: 10.1016/j.neuroscience.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Helmreich DL, Cullinan WE, Watson SJ. The effect of adrenalectomy on stress-induced c-fos mRNA expression in the rat brain. Brain Res. 1996;706:137–144. doi: 10.1016/0006-8993(95)01215-x. [DOI] [PubMed] [Google Scholar]
- Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: Control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res Brain Res Rev. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- Herman J, Ascafer M-H, Watson S, Sherman T. In situ hybridization analysis of arginine vasopressin gene transcription using intron-specific probes. Mol Endocrinol. 1991;5:1447–1456. doi: 10.1210/mend-5-10-1447. [DOI] [PubMed] [Google Scholar]
- Herman JP, Schafer MK, Thompson RC, Watson SJ. Rapid regulation of corticotropin-releasing hormone gene transcription in vivo. Mol Endocrinol. 1992;6:1061–1069. doi: 10.1210/mend.6.7.1324419. [DOI] [PubMed] [Google Scholar]
- Imaki T, Nahan J-L, Rivier C, Sawchenko PE, Vale W. Differential regulation of corticotropin-releasing factor mRNA in rat brain regions by glucocorticoids and stress. J Neurosci. 1991;11:585–599. doi: 10.1523/JNEUROSCI.11-03-00585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaki T, Shibasaki T, Chikada N, Harada S, Naruse M, Demura H. Different expression of immediate-early genes in the rat paraventricular nucleus induced by stress: Relation to corticotropin-releasing factor gene transcription. Endocrine J. 1996;43:629–638. doi: 10.1507/endocrj.43.629. [DOI] [PubMed] [Google Scholar]
- Imaki T, Xiao-Quan W, Shibasaki T, Yamada K, Harada S, Chikada N, Naruse M, Demura H. Stress-induced activation of neuronal activity and corticotropin-releasing factor gene expression in the paraventricular nucleus is modulated by glucocorticoids in rats. J Clin Invest. 1995;96:231–238. doi: 10.1172/JCI118026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L, Akana SF, Cascio CS, Scribner K, Shinsako J, Dallman MF. The adrenocortical system responds slowly to removal of corticosterone in the absence of concurrent stress. Endocrinology. 1989;124:2144–2152. doi: 10.1210/endo-124-5-2144. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Akana SF, Cascio CS, Shinsako J, Dallman MF. Circadian variations in plasma corticosterone permit normal termination of adrenocorticotropin responses to stress. Endocrinology. 1988;122:1343–1348. doi: 10.1210/endo-122-4-1343. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Sapolsky R. Augmented ACTH responses to stress in adrenalectomized rats replaced with constant physiological levels of corticosterone are partially normalized by acute increases in corticosterone. Neuroendocrinology. 1993;58:420–429. doi: 10.1159/000126571. [DOI] [PubMed] [Google Scholar]
- Jingami H, Matsukura S, Numa S, Imura H. Effects of adrenalectomy and dexamethasone administration on the level of prepro-corticotropin-releasing factor messenger ribonucleic acid (mRNA) in the hypothalamus and adrenocorticotropin/beta-lipotropin precursor mRNA in the pituitary in rats. Endocrinology. 1985;117:1314–1320. doi: 10.1210/endo-117-4-1314. [DOI] [PubMed] [Google Scholar]
- Keller-Wood ME, Dallman MF. Corticosteroid inhibition of ACTH secretion. Endo Rev. 1984;5:1–24. doi: 10.1210/edrv-5-1-1. [DOI] [PubMed] [Google Scholar]
- Kim JK, Summer SN, Wood WM, Schrier RW. Role of glucocorticoid hormones in arginine vasopressin gene regulation. Biochem Biophys Res Commun. 2001;289:1252–1256. doi: 10.1006/bbrc.2001.6114. [DOI] [PubMed] [Google Scholar]
- Kovacs KJ, Foldes A, Sawchenko PE. Glucocorticoid negative feedback selectively targets vasopressin transcription in parvocellular neurosecretory neurons. J Neurosci. 2000;20:3843–3852. doi: 10.1523/JNEUROSCI.20-10-03843.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs KJ, Sawchenko PE. Regulation of stress-induced transcriptional changes in the hypothalamic neurosecretory neurons. J Mol Neurosci. 1996;7:125–133. doi: 10.1007/BF02736792. [DOI] [PubMed] [Google Scholar]
- Laugero KD. Reinterpretation of basal glucocorticoid feedback: Implications to behavioral and metabolic disease. Vitamins & Hormones. 2004;69:1–29. doi: 10.1016/S0083-6729(04)69001-7. [DOI] [PubMed] [Google Scholar]
- Ma X-M, Aguilera G. Differential regulation of corticotropin-releasing hormone and vasopressin transcription by glucocorticoids. Endocrinology. 1999;140:5642–5650. doi: 10.1210/endo.140.12.7214. [DOI] [PubMed] [Google Scholar]
- Ma X-M, Lightman SL, Aguilera G. Vasopressin and corticotropin-releasing hormone gene responses to novel stress in rats adapted to repeated restraint. Endocrinology. 1999;140:3623–3632. doi: 10.1210/endo.140.8.6943. [DOI] [PubMed] [Google Scholar]
- Makino S, Schulkin J, Smith MA, Pacâak K, Palkovits M, Gold PW. Regulation of corticotropin-releasing hormone receptor messenger ribonucleic acid in the rat brain and pituitary by glucocorticoids and stress. Endocrinology. 1995a;136:4517–4525. doi: 10.1210/endo.136.10.7664672. [DOI] [PubMed] [Google Scholar]
- Makino S, Smith MA, Gold PW. Increased expression of corticotropin-releasing hormone and vasopressin messenger ribonucleic acid (mRNA) in the hypothalamic paraventricular nucleus during repeated stress: Association with reduction in glucocorticoid receptor mRNA levels. Endocrinology. 1995b;136:3299–3309. doi: 10.1210/endo.136.8.7628364. [DOI] [PubMed] [Google Scholar]
- Malkoski SP, Dorin RI. Composite glucocorticoid regulation at a functionally defined negative glucocorticoid response element of the human corticotropin-releasing hormone gene. Mol Endocrinol. 1999;13:1629–1644. doi: 10.1210/mend.13.10.0351. [DOI] [PubMed] [Google Scholar]
- Nicholson WE, Davis DR, Sherrel BJ, Orth DN. Rapid radioimmunoassay for corticotropin in unextracted human placenta. Clin Chem. 1984;30:259–265. [PubMed] [Google Scholar]
- Pinnock SB, Herbert J. Corticosterone differentially modulates expression of corticotropin releasing factor and arginine vasopressin mRNA in the hypothalamic paraventricular nucleus following either acute or repeated restraint stress. Eur J Neurosci. 2001;13:576–584. doi: 10.1046/j.0953-816x.2000.01406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Horiba N, Sakai Y, Tozawa F, Demura H, Suda T. Regulation of corticotropin-releasing factor receptor messenger ribonucleic acid in rat anterior pituitary. Endocrinology. 1996;137:1758–1763. doi: 10.1210/endo.137.5.8612512. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Liu Y, Sassone-Corsi P, Aguilera G. Role of glucocorticoids and cAMP-mediated repression in limiting corticotropin-releasing hormone transcription during stress. J Neurosci. 2005;25:4073–4081. doi: 10.1523/JNEUROSCI.0122-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton LC, Fleshner M, Mazzeo R, Maier SF, Watkins LR. A permissive role of corticosterone in an opioid form of stress-induced analgesia: Blockade of opiate analgesia is not due to stress-induced hormone release. Brain Res. 1994;663:19–29. doi: 10.1016/0006-8993(94)90458-8. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain Maps III. Structure of the rat brain. Amsterdam: Elsevier; 2004. [Google Scholar]
- Swanson LW, Simmons DM. Differential steroid hormone and neural influences on peptide mRNA levels in CRH cells of the paraventricular nucleus: A hybridization histochemical study in the rat. J Comp Neurol. 1989;285:413–435. doi: 10.1002/cne.902850402. [DOI] [PubMed] [Google Scholar]
- Tanimura SM, Watts AG. Corticosterone can facilitate as well as inhibit corticotropin-releasing hormone gene expression in the rat hypothalamic paraventricular nucleus. Endocrinology. 1998;139:3830–3836. doi: 10.1210/endo.139.9.6192. [DOI] [PubMed] [Google Scholar]
- Tanimura SM, Watts AG. Adrenalectomy dramatically modifies the dynamics of neuropeptide and c-fos gene responses to stress in the hypothalamic paraventricular nucleus. J Neuroendocrinol. 2000;12:715–722. doi: 10.1046/j.1365-2826.2000.00504.x. [DOI] [PubMed] [Google Scholar]
- Turnbull AV, Rivier CL. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: Actions and mechanisms of action. Physiol Rev. 1999;79:1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- Umemoto S, Kawai Y, Ueyama T, Senba E. Chronic glucocorticoid administration as well as repeated stress affects the subsequent acute immobilization stress-induced expression of immediate early genes but not that of NGFI-A. Neuroscience. 1997;80:763–773. doi: 10.1016/s0306-4522(97)00050-x. [DOI] [PubMed] [Google Scholar]
- Vahl TP, Ulrich-Lai YM, Ostrander MM, Dolgas CM, Elfers EE, Seeley RJ, D’Alessio DA, Herman JP. Comparative analysis of ACTH and corticosterone sampling methods in rats. Am J Physiol Endocrinol Metab. 2005;289:E823–E828. doi: 10.1152/ajpendo.00122.2005. [DOI] [PubMed] [Google Scholar]
- Viau V, Chu A, Soriano L, Dallman MF. Independent and overlapping effects of corticosterone and testosterone on corticotropin-releasing hormone and arginine vasopressin mRNA expression in the paraventricular nucleus of the hypothalamus and stress-induced adrenocorticotropic hormone release. J Neurosci. 1999;19:6684–6693. doi: 10.1523/JNEUROSCI.19-15-06684.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts AG. Glucocorticoid regulation of peptide genes in neuroendocrine CRH neurons: A complexity beyond negative feedback. Front Neuroendocrinol. 2005;26:109–130. doi: 10.1016/j.yfrne.2005.09.001. [DOI] [PubMed] [Google Scholar]