Abstract
Introduction
Genital ulcer disease (GUD) is common in HIV-1-infected women, and a small number of studies have suggested increased GUD risk after antiretroviral therapy (ART) initiation. To better define this risk, we monitored 134 women at ART initiation and monthly thereafter.
Methods
Women were evaluated monthly for genital ulcers. Syphilis serology was tested quarterly, and chancroid culture performed on ulcers that were felt to be clinically consistent with a diagnosis of chancroid. A logistic model with generalized estimating equations was used to analyze predictors of GUD from baseline until 6 months after ART initiation.
Results
During the study period, GUD occurred in 54 women (40.3%) at 85 visits (10.0%). GUD prevalence was 9.7% at baseline, increased to 16.7% at month 1 (adjusted odds ratio [aOR] 1.9 [1.0 – 3.6], p = 0.04), then decreased to 6.4% by month 6. History of GUD (aOR 3.8 [1.9 – 7.7], p < 0.001) and CD4 count <100 (aOR 1.8 [1.0 – 3.4, p = 0.06) were associated with increased risk of GUD after ART initiation.
Discussion
Women experience increased risk of GUD in the first month after ART initiation, particularly if they have low CD4 counts or a history of GUD.
Keywords: genital ulcer, HIV, antiretroviral therapy, immune reconstitution
Genital ulcer disease (GUD) is a frequent problem among HIV-1-infected women.1, 2 When an ulcer occurs, mucosal interruption and local inflammation increase HIV-1 shedding at the ulcer site, and may thereby increase the risk of transmission.3–5 Most GUD is caused by sexually transmitted pathogens, and herpes simplex virus type 2 (HSV-2) is the most common etiology worldwide.6–9 In HIV-infected women, the prevalence of GUD of any cause increases as CD4 cell counts decline.2
GUD has been reported as one of many conditions that can flare with immune reconstitution following initiation of antiretroviral therapy (ART).10–12 However, the frequency and timing of GUD in relation to ART initiation and immune recovery is not well characterized. One retrospective study suggested that GUD risk is elevated for 6 months after ART initiation.13 To better characterize the frequency and timing of GUD occurring after ART initiation, we conducted an analysis of prospectively collected data in a cohort of Kenyan women starting ART. Our hypothesis was that immune reconstitution would increase the risk of GUD, and we aimed to determine the time course and risk factors for GUD occurring after ART initiation.
Methods
HIV-1-seropositive women participating in an ongoing cohort study in Mombasa, Kenya were identified for this study when eligible for ART by World Health Organization (WHO) and Kenyan Ministry of Health National Guidelines.14 All enrolled women initiated a standard Kenyan first-line regimen consisting of stavudine or zidovudine combined with lamivudine and nevirapine. At baseline and at monthly follow-up visits after ART initiation, women underwent a standard interview and were asked whether they had experienced new or persistent genital sores or ulcerations since the last visit. After the interview, women underwent a standard physical examination, including a speculum examination during which genital sores or ulcerations of the vulvar, vaginal, or cervical mucosa were noted. Syphilis serology was performed at baseline and quarterly thereafter. Chancroid cultures were obtained for ulcers judged to be atypical for HSV. All participants gave informed consent, and the ethical review committees of the Kenya Medical Research Institute, the University of Washington, and the Fred Hutchinson Cancer Research Center approved the study.
The outcome of interest was GUD detected by history, examination, or both. The baseline for comparison was day 0 of ART initiation. History of GUD prior to ART initiation was defined as patient history of GUD, prior research examination with documented GUD, or both at any visit in the parent cohort before ART initiation. Procedures for GUD detection in the parent cohort were identical to those used in this study after ART initiation. A history of GUD was not required for this analysis.
Prevalence of any GUD (by history or examination), GUD by history, and GUD by examination were calculated for each time point. A logistic model using generalized estimating equations (GEE) with an exchangeable correlation matrix and robust estimation of confidence intervals was used to analyze predictors of GUD during the first 6 months of follow-up after ART initiation. Predictors included in multivariate modeling were month from ART initiation, CD4 cell count at baseline (<100 cells/µL, 100–149 cells/µL, or ≥150 cells/µL), WHO disease stage, and history of GUD before the baseline examination.
Results
From March 8, 2004 through January 18, 2008, 134 women enrolled in the study and initiated ART. Twenty-six additional women from the parent cohort were eligible for ART but chose to initiate treatment at a non-research clinic. These women did not differ significantly from those who enrolled in terms of their age, marital status, education, or WHO clinical stage. Pre-ART CD4 counts from outside clinics providing ART to these 26 women were not available for this study. Fewer non-participating women had a documented history of GUD prior to ART initiation (38% vs. 61%, p=0.03).
The enrolled women attended 852 visits from ART initiation through month 6 (median, 7 visits; inter-quartile range [IQR], 6–7 visits). The median CD4 count on ART initiation was 127 (IQR, 79 – 165), and 82 women (61.2%) had a documented history of GUD at any time prior to ART initiation. GUD was reported or observed in 54 women (40.3%) at 85 visits (10.0%). Detection was by examination alone in 32 (37.6%), by report alone in 38 (44.7%), and by both methods for 15 ulcers (17.6%). The presence of GUD by examination was significantly associated with participants’ self report of GUD, both prior to ART initiation (p<0.001) and after starting ART (p<0.001). Syphilis was diagnosed at 5 visits, of which 4 included concurrent examiner identification of GUD. No chancroid was detected by culture of 18 incident atypical ulcers.
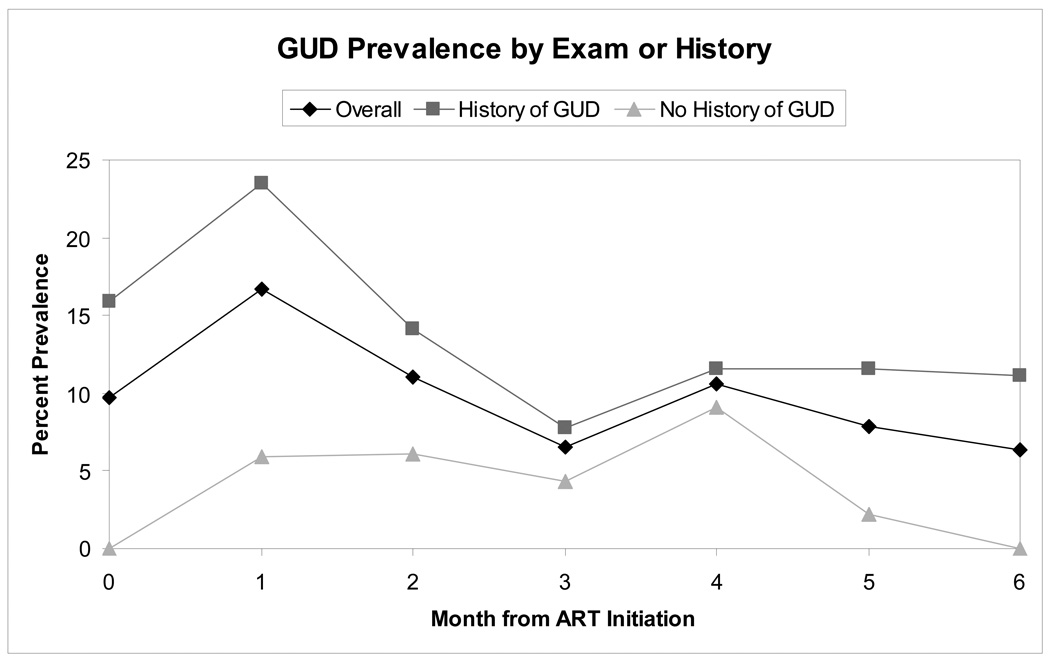
GUD prevalence was 9.7% at baseline, increased to 16.7% at month 1, then decreased to 6.4% by month 6. GUD detection was significantly higher among women with a history of GUD (13.9% versus 3.9%, p<0.001), as depicted graphically in Figure 1. The results of univariate and multivariate logistic regression analysis using GEE are presented in Table 1. In univariate analysis, the first month after ART initiation, baseline CD4 count <100 cells/µL, WHO stage 4 disease, and a history of GUD were all significantly associated with GUD detection.
Figure 1. GUD Prevalence by Month from ART Initiation.
The figure depicts the prevalence of GUD diagnosed by examination or patient report at monthly follow-up visits among women initiating ART. The black line with diamonds indicates prevalence in the entire population of 134 women, the heavy grey line with squares the prevalence in the 82 women with a history of GUD prior to ART initiation, and the light grey line with triangles the prevalence in the 52 women with no history of GUD prior to ART initiation.
Table 1.
Predictors of GUD Detected in the 6 Months after ART Initiation
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) |
P value | Odds Ratio (95% CI) |
P value | |
| Timepoint: | ||||
| Baseline | Reference | Reference | ||
| Month 1 | 1.9 (1.0–3.4) | 0.04 | 1.9 (1.0–3.6) | 0.04 |
| Month 2 | 1.2 (0.6–2.4) | 0.63 | 1.2 (0.6–2.4) | 0.67 |
| Month 3 | 0.6 (0.3–1.4) | 0.29 | 0.6 (0.3–1.5) | 0.30 |
| Month 4 | 1.1 (0.5–2.4) | 0.83 | 1.1 (0.5–2.5) | 0.81 |
| Month 5 | 0.8 (0.3–1.8) | 0.58 | 0.8 (0.3–1.9) | 0.60 |
| Month 6 | 0.7 (0.3–1.6) | 0.38 | 0.7 (0.3–1.7) | 0.40 |
| CD4 count: | ||||
| <100 cells/µL | 1.9 (1.1–3.4) | 0.03 | 1.8 (1.0–3.4) | 0.06 |
| ≥100 cells/µL | Reference | Reference | ||
| Clinical stage: | ||||
| Stage 1 | Reference | Reference | ||
| Stage 2 | 2.1 (0.7–6.0) | 0.18 | 1.9 (0.7–5.0) | 0.18 |
| Stage 3 | 2.0 (0.8–5.2) | 0.16 | 1.3 (0.5–3.1) | 0.56 |
| Stage 4 | 3.3 (1.1–9.8) | 0.04 | 2.0 (0.7–5.6) | 0.17 |
| History of GUD, yes/no | 3.9 (2.0–7.7) | <0.001 | 3.8 (1.9–7.7) | <0.001 |
CI = confidence interval
In multivariate analysis, both baseline CD4 count <100 cells/µL and the first month after ART initiation were associated with approximately 2-fold increases in risk of GUD, although the association with baseline CD4 count was reduced to a statistical trend (p=0.06). History of GUD was associated with a 3.8-fold increased risk in GUD after ART initiation. HIV stage was no longer a significant predictor of GUD risk after adjustment for the other variables in the model.
To examine the effect of our GUD definition on the results, we repeated our analyses after restricting our definition of GUD to ulcers detected on examination. In this analysis, point estimates for risk of GUD in months 1–6 after ART initiation were 1.4, 1.2, 0.6, 0.6, 0.4, and 0.4 respectively. This analysis included fewer events, and did not achieve statistical significance for comparisons between different time points. Both CD4 count <100 cells/µL (aOR 2.2, p = 0.04) and history of GUD on examination (aOR 3.5, p = 0.001) remain significant predictors of GUD after ART initiation.
Discussion
In our analysis of GUD risk among women initiating ART, we found that GUD prevalence was relatively high at baseline (almost 10%), increased by almost 2-fold in the first month, then returned to baseline thereafter. Women with CD4 counts <100 cells/µL were at increased risk of GUD after ART initiation. These observations are compatible with other studies of the timing and risk factors for immune reconstitution inflammatory syndrome.11, 15, 16 In addition, we found that women with a history of GUD had an almost 4-fold increased risk of recurrences during the first 6 months of ART.
HIV-1-seropositive women are at increased risk for GUD,2 and even those on ART with full suppression and good CD4 response have an increased risk relative to HIV-1-seronegative women.1 An even higher risk soon after ART initiation has been suspected since the first case report of immune reconstitution genital herpes appeared in 1999.10 In the only other study of immune reconstitution GUD, clinical data from a group of patients not receiving ART was compared to data from a group starting ART and data from another group on stable ART for >6 months. The investigators determined that patients having received ART for <6 months had a higher risk of developing genital herpes than untreated patients, with highest risk in the first 2 months.13
Our study had several strengths that make it an important contribution to our knowledge of immune reconstitution GUD. This is the first prospective study of GUD risk after ART initiation. We used data from pre-ART follow-up in our cohort to define a history of GUD, and collected data at monthly scheduled visits. We used a combined measure of GUD by history and examination in order to increase GUD detection, as some episodes might have resolved by the time of physical examination and others might not have been identified by patients. A similar GUD case definition was used in the WIHS cohort for its increased sensitivity.1 We performed syphilis serology for all women at regular intervals, identifying four GUD episodes possibly related to syphilis. Although we did culture material from atypical ulcers for chancroid, we identified no chancroid-related GUD; this is consistent with the decreased prevalence of chancroid in our cohort specifically (no cases since 1999, unpublished data) and in East Africa in general.6, 7
Our study was limited by a lack of specific diagnostic testing for HSV-2 and auto-immune ulcerations, both of which are common in women.8 It seems plausible that auto-immune dermatologic disease could flare as a result of ART-induced immune reconstitution, but we could identify no published reports of this phenomenon. The HSV-2 serostatus was not known for the 134 participants in this study, although previous studies in subsets of this cohort have found HSV-2 seropositivity to be nearly universal among HIV-1-infected participants.17 In addition, we found a strong association between a history of GUD and the risk of GUD after ART initiation, suggestive of a chronic, reactivating condition such as genital HSV-2 infection. Finally, these women may be more aware of GUD compared to other populations due to monthly risk reduction education about sexually transmitted infections, symptom reviews, and genital examinations.
In conclusion, the risk of GUD is increased among women initiating ART during the first month of treatment, especially among those with very low baseline CD4 cell counts (<100 cells/µL) and those with a history of GUD. For women with known HSV-2 infection, suppression with acyclovir has the potential to prevent GUD recurrences, promote ulcer healing, and decrease HIV-1 shedding. Although acyclovir has not proven effective in reducing HIV-1 acquisition in HSV-2-infected persons,18,19 several ongoing trials are assessing the effect of long-term HSV-2 suppressive therapy in persons dually infected with HIV-1 and HSV-2, including those initiating ART (NCT00371592, NCT00194519, NCT00405821). Meanwhile, because GUD flares during the early period after ART initiation could increase HIV-1 infectivity, pre-ART counseling should include information about ways to reduce the risk of HIV-1 transmission to sexual partners. While all women initiating ART should be counseled about strategies for reducing their risk of transmitting HIV-1 to sex partners, the data presented here suggest that women with a history of GUD may benefit from additional risk reduction counseling regarding the potential for increased transmission risk in the first 1–2 months of therapy.
Acknowledgements
We thank the Director, Kenya Medical Research Institute, for permission to publish this study. We also wish to thank Teresa To and Joseph Beyene, of the University of Toronto, for their instruction on GEE methodology and analysis. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Sources of support: Funding for this study was provided by NIH grants R01 AI058698 and K23 AI69990 (SMG), and the UW Center for AIDS Research (NIH grant P30 AI 27757).
Footnotes
Presented in part at the XVII International AIDS Conference, Mexico City, August 3–8, 2008
References
- 1.Ameli N, Bacchetti P, Morrow RA, et al. Herpes simplex virus infection in women in the WIHS: epidemiology and effect of antiretroviral therapy on clinical manifestations. AIDS. 2006;20:1051–1058. doi: 10.1097/01.aids.0000222078.75867.77. [DOI] [PubMed] [Google Scholar]
- 2.McClelland RS, Lavreys L, Katingima C, et al. Contribution of HIV-1 infection to acquisition of sexually transmitted disease: a 10-year prospective study. J Infect Dis. 2005;191:333–338. doi: 10.1086/427262. [DOI] [PubMed] [Google Scholar]
- 3.Gadkari DA, Quinn TC, Gangakhedkar RR, et al. HIV-1 DNA shedding in genital ulcers and its associated risk factors in Pune, India. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18:277–281. doi: 10.1097/00042560-199807010-00012. [DOI] [PubMed] [Google Scholar]
- 4.Schacker T, Ryncarz AJ, Goddard J, et al. Frequent recovery of HIV-1 from genital herpes simplex virus lesions in HIV-1-infected men. JAMA. 1998;280:61–66. doi: 10.1001/jama.280.1.61. [DOI] [PubMed] [Google Scholar]
- 5.Lawn SD, Subbarao S, Wright TC, Jr, et al. Correlation between human immunodeficiency virus type 1 RNA levels in the female genital tract and immune activation associated with ulceration of the cervix. J Infect Dis. 2000;181:1950–1956. doi: 10.1086/315514. [DOI] [PubMed] [Google Scholar]
- 6.Nilsen A, Kasubi MJ, Mohn SC, et al. Herpes simplex virus infection and genital ulcer disease among patients with sexually transmitted infections in Dar es Salaam, Tanzania. Acta Derm Venereol. 2007;87:355–359. doi: 10.2340/00015555-0241. [DOI] [PubMed] [Google Scholar]
- 7.Pickering JM, Whitworth JA, Hughes P, et al. Aetiology of sexually transmitted infections and response to syndromic treatment in southwest Uganda. Sex Transm Infect. 2005;81:488–493. doi: 10.1136/sti.2004.013276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomes CMM, Giraldo PC, Gomes FM, et al. Genital ulcers in women: clinical, microbiologic and histopathologic characteristics. Braz J Infect Dis. 2007;11:254–260. doi: 10.1590/s1413-86702007000200018. [DOI] [PubMed] [Google Scholar]
- 9.Paz-Bailey G, Rahman M, Chen C, et al. Changes in the etiology of sexually transmitted diseases in Botswana between 1993 and 2002: implications for the clinical management of genital ulcer disease. Clin Infect Dis. 2005;41:1304–1312. doi: 10.1086/496979. [DOI] [PubMed] [Google Scholar]
- 10.Fox PA, Barton SE, Francis N, et al. Chronic erosive herpes simplex virus infection of the penis, a possible immune reconstitution disease. HIV Med. 1999;1:10–18. doi: 10.1046/j.1468-1293.1999.00003.x. [DOI] [PubMed] [Google Scholar]
- 11.Murdoch DM, Venter WD, Feldman C, Van Rie A. Incidence and risk factors for the immune reconstitution inflammatory syndrome in HIV patients in South Africa: a prospective study. AIDS. 2008;22:601–610. doi: 10.1097/QAD.0b013e3282f4a607. [DOI] [PubMed] [Google Scholar]
- 12.Jevtovic DJ, Salemovic D, Ranin J, et al. The prevalence and risk of immune restoration disease in HIV-infected patients treated with highly active antiretroviral therapy. HIV Med. 2005;6:140–143. doi: 10.1111/j.1468-1293.2005.00277.x. [DOI] [PubMed] [Google Scholar]
- 13.Couppie P, Sarazin F, Clyti E, et al. Increased incidence of genital herpes after HAART initiation: a frequent presentation of immune reconstitution inflammatory syndrome (IRIS) in HIV-infected patients. AIDS Patient Care STDs. 2006;20:143–145. doi: 10.1089/apc.2006.20.143. [DOI] [PubMed] [Google Scholar]
- 14.McClelland RS, Hassan WM, Lavreys L, et al. HIV-1 acquisition and disease progression are associated with decreased high-risk sexual behaviour among Kenyan female sex workers. AIDS. 2006;20:1969–1973. doi: 10.1097/01.aids.0000247119.12327.e6. [DOI] [PubMed] [Google Scholar]
- 15.Manabe YC, Campbell JD, Sydnor E, Moore RD. Immune reconstitution inflammatory syndrome: risk factors and treatment implications. J Acquir Immune Defic Syndr. 2007;46:456–462. doi: 10.1097/qai.0b013e3181594c8c. [DOI] [PubMed] [Google Scholar]
- 16.Ratnam I, Chiu C, Kandala NB, Easterbrook PJ. Incidence and risk factors for immune reconstitution inflammatory syndrome in an ethnically diverse HIV type 1-infected cohort. Clin Infect Dis. 2006;42:418–427. doi: 10.1086/499356. [DOI] [PubMed] [Google Scholar]
- 17.Mostad SB, Kreiss JK, Ryncarz AJ, et al. Cervical shedding of herpes simplex virus in human immunodeficiency virus-infected women: effects of hormonal contraception, pregnancy, and vitamin A deficiency. J Infect Dis. 2000;181:58–63. doi: 10.1086/315188. [DOI] [PubMed] [Google Scholar]
- 18.Celum C, Wald A, Hughes J, et al. Effect of acyclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomized, double-blind, placebo-controlled trial. Lancet. 2008;371:2109–2119. doi: 10.1016/S0140-6736(08)60920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watson-Jones D, Weiss HA, Rusizoka M, et al. Effect of herpes simplex suppression on incidence of HIV among women in Tanzania. N Engl J Med. 2008;358:1560–1571. doi: 10.1056/NEJMoa0800260. [DOI] [PMC free article] [PubMed] [Google Scholar]