Abstract
Objective
It is widely believed that there are multiple sources of pain at a tissue level in osteoarthritis (OA). MRIs provide a wealth of anatomic information and may allow identification of specific features associated with pain. We hypothesized that in knees with OA, bone marrow lesions (BMLs), synovitis, and effusion would be associated with weight-bearing and (less so with) non-weight-bearing pain independently.
Methods
In a cross-sectional study of persons with symptomatic knee OA using univariate and multivariate logistic regressions with maximal BML, effusion, and synovitis defined by Boston Leeds Osteoarthritis Knee Score as predictors, and knee pain using weight-bearing and non-weight-bearing Western Ontario and McMaster University OA Index pain questions as the outcome, we tested the association between MRI findings and knee symptoms
Results
160 participants, mean age 61 (±9.9), mean BMI 30.3 (±4.7) and 50% female, stronger associations were seen with weight-bearing compared with non-weight-bearing knee pain with adjusted risk ratios (RRs) of weight-bearing knee pain, for increasing maximal BML scores of 1.0 (referent) (maximal BML = 0), 1.2, 1.9, and 2.0 (p for trend = 0.006). For effusion scores, adjusted ORs of knee pain were 1.0, 1.7, 2.0, and 2.6 (p for trend = 0.0004); and for synovitis scores, adjusted ORs were 1.0, 1.4, 1.5, and 1.9 (p for trend = 0.22).
Conclusion
Cross-sectionally, maximal BML and effusion scores are independently associated with weight-bearing and less so with non-weight-bearing knee pain, supporting the idea that pain in OA is multifactorial. These MRI features should be considered as possible new treatment targets in knee OA.
Introduction
Osteoarthritis (OA) is the most common form of arthritis1 and is one of the most common causes of disability in the elderly.2 To date, there are few effective treatments for knee OA. This may in large part be related to the fact that the identification of specific tissue level of pain in OA has been difficult and thus analgesic approaches have been non-specific in their approach to pain. However, MRIs provide a wealth of anatomic information and may allow identification of specific features associated with pain, particularly with the advent of higher strength magnets (3 Tesla) that are now being used in the clinical setting. As there are many possible etiologies of pain in OA, it is important to look at potential sources of pain both individually and collectively. Such sources may include bone marrow lesions, synovitis, and synovial effusion.
The bone marrow lesion (BML) is a feature associated with OA that has only been identified since MRIs have been used to image joints.3–9 Because BMLs are localized in the subchondral bone and the subchondral bone is innervated,10, 11 this is a potential source of pain in OA. Prior clinical investigations of BMLs as a source of pain in OA have produced conflicting results. Some studies have shown an association with pain,12–14 while others have suggested that the relationship with pain is questionable.9, 15–18
Synovitis is more recently gaining attention as an important feature in OA.14, 19–22 As an innervated tissue,23–26 inflammation of the synovium may be a cause of pain. Synovial effusions have long been known to be a feature of OA. There have been a few studies which suggest these effusions contribute to knee pain in OA,14, 20 though one study14 did not distinguish synovitis and synovial effusion when looking at their relationship with pain. Intuitively, we would expect effusion to be associated with pain as the joint capsule is richly innervated,27, 28 and with a substantive effusion, the joint capsule is distended. Many prior studies9, 12, 13, 15 have evaluated the contribution of each tissue individually to pain but to our knowledge, none has taken into account the whole joint nature of OA and the potential for multiple tissues to contribute to pain within one knee.
Part of the difficulty in disentangling sources of pain in OA is the fact that the definition of pain in OA has been problematic. In fact, Outcome Measures in Rheumatology, originally termed Outcome Measures in Rheumatoid Arthritis Clinical Trials (OMERACT), has undertaken the task of reevaluating the outcome of pain in OA in part for this reason. Highlighting these issues are the results of a recent factor analysis29 of the Western Ontario McMaster Universities Osteoarthritis Index (WOMAC) pain scale,30, 31 one of the most widely used assessments of pain in knee OA32, suggested that the WOMAC pain scale does not represent one construct. The weight-bearing items represent one factor and the non-weight-bearing items represent a second factor. Because pain in OA is generally characterized as pain that occurs on weight-bearing, a definition of pain focusing on the weight-bearing WOMAC questions is potentially a better strategy to summing all five-item responses.
We hypothesized that in people with OA, bone marrow lesions (BMLs), synovitis, and effusion, as visualized on MRI, would be independently associated weight-bearing and (less so with) non-weight-bearing pain.
Methods
Sample selection
The Osteoarthritis Initiative (OAI) is a publicly available multi-center observational study of knee OA of 4,796 participants, which is comprised of three groups, the progression (N = 1,389), the incidence (N = 3,285), and a non-exposed control group (N = 122). The “progression cohort” all have pre-existent symptomatic radiographic knee OA (ROA), the “incident cohort” are at high risk for symptomatic (ROA), and the “non-exposed control group” do not have nor are at high risk for symptomatic ROA. For this study, we specifically focused on the baseline assessments of the progression group, identified using the inclusion criteria of ages 45 – 79; and having at least one knee with both radiographic evidence of knee OA (Osteoarthritis Research Society International (OARSI) atlas osteophyte grade 1–3) and symptoms (“pain, aching or stiffness on most days of the month in the last year”). Those with evidence of severe joint space narrowing (JSN), as defined by OARSI atlas JSN grade 3, in both knees were excluded.
Participants received assessments of knee-specific WOMAC pain, posterior-anterior semi-flexed radiographs33 of bilateral knees, and MRIs of bilateral knees. Each MRI was obtained on one of 4 identical Siemens Trio 3T MRIs at one of the 4 clinical sites, Memorial Hospital of Rhode Island (Pawtucket, RI,) Ohio State University (Columbus, Ohio), University of Pittsburgh (Pittsburgh, PA), and University of Maryland/Johns Hopkins University (Baltimore, MD).
From the 1,389 participants in the progression cohort, 160 were identified and selected by the OAI Coordinating Center as part of the public release MRI data set 0.B.1, a convenience sample of people with high quality MRIs, blocked for sex, ethnicity, and clinical center, all of whom had complete baseline and year 1 follow-up MRIs. All those in data set 0.B.1 were included in this study. Baseline demographic data as well as WOMAC pain were obtained from data set 0.1.1, accessed from the website, http://www.oai.ucsf.edu/datarelease/.
Knee Selection
From the participants included, we chose one knee to evaluate per individual. Eligible knees were those of the 160 with symptomatic radiographic OA as described above in Sample Selection. For individuals with bilateral symptomatic radiographic OA, we selected one knee, preferentially selecting those with a Kellgren/Lawrence (KL) score grades 2 or 3, with a greater anatomic axis varus angulation, and with medial tibiofemoral (TF) OA.
The radiographic assessments from one unadjudicated reader (DH) were available and used to select the knee from a participant used in this study. In the 100 patients with unilateral symptomatic ROA, this knee was chosen for analysis, regardless of radiographic severity. For the remaining participants with bilateral symptomatic ROA, one knee was selected, favoring the knee with moderate disease. In patients with bilateral symptomatic ROA, if only one knee had KL grade 2 or 3 grade, then that knee was selected. If both knees had KL grade 2 or 3, then the knee with the greatest extent of each of the following features was selected, moving to the next feature if the knees were still ranked as equal:
❖ Greater anatomic axis varus angulation
❖ ≥ 2.0 mm medial minimum Joint Space Width (JSW)
❖ Greater grade of medial Joint Space Narrowing (grade 1–3)
❖ The presence of any medial tibial or femoral osteophyte grade ≥ 2 with greater grade than lateral osteophytes
❖ The presence of any medial tibial or femoral osteophyte
❖ The right knee
If neither knee was KL grade 2 or 3, then the knee with the higher KL grade was chosen. If the patient had bilateral KL grade 0, 1 or 4 knees, then the process used to select among bilateral KL grade 2 or 3 knees was followed. Next, knees were listed on the basis of decreasing KL grade, and within KL grade by decreasing varus angulation.
MRI Reading
The time point of interest for this study was the baseline visit. Two rheumatologists experienced in musculoskeletal MRI readings, both of whom were centrally involved in the development of the Boston Leeds Osteoarthritis Knee Score (BLOKS),13 blinded to subject data, scored one knee MRI from each participant for BMLs, synovitis, and effusion using BLOKS. One reader (GHL) scored BMLs and the other (DJH) scored synovitis and effusion.
We defined a BML (Figure 1A) as an irregular hyperintense signal in the subchondral bone, proximal to the epiphyseal line, as seen on sagittal intermediate-weighted (IW) Turbo Spin Echo (TSE) fat-suppressed images (FS), time to recovery (TR) of 3200 msec, time to echo (TE) of 30 msec, slice thickness of 3mm, and field of view (FOV) of 160mm (Table 1). We also used Dual Echo in the Steady State (DESS) sequences to assist with localization of some lesions (Table 1). BMLs were scored for size (0–3) at each of 9 locations, medial and lateral patella, medial and lateral trochlea, medial and lateral weight-bearing femur, and medial, subspinous, and lateral tibia. Only those BMLs with greater than 25% of the surface area adjacent to the subchondral plate were included. To assess intra-rater reliability, a sample of 10 knee MRIs were read for BMLs twice by the same reader separated by at least 2 days for a weighted kappa of 0.88. From the 9 regions scored for BML size, each knee was given a maximal BML score.
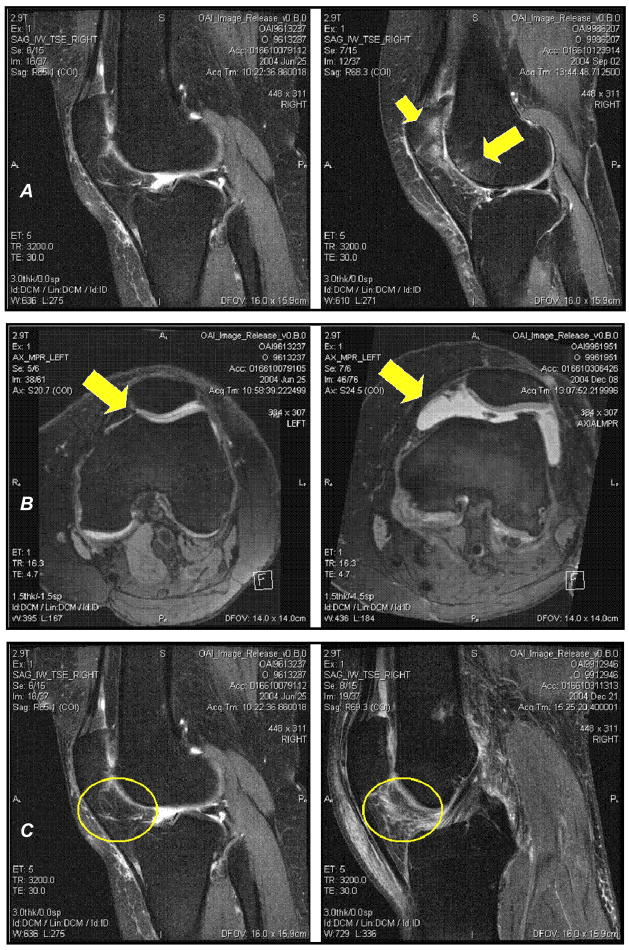
Figure 1.
A. Sagittal MRIs of the knee. The left MRI is of a knee without any BMLs. The right MRI is of a knee with a large BML in the lateral patella and lateral trochlea (yellow arrows).
B. Axial MRIs of the knee. The left MRI is of a knee with a physiologic effusion. The right MRI is of a knee with a large joint effusion.
C. Sagittal MRIs of the knee. The left MRI is of a knee without any infrapatellar synovitis. The right MRI is of a knee with a large amount of synovitis in the infrapatellar fat pad (yellow circles).
Table 1.
MRI Parameters
| 3-plane | 2D TSE | 3D DESS | 2D TSE | |
|---|---|---|---|---|
| Weighting | T1W | Int | T2* | Int |
| Plane | 3-plane | Coronal | Sag | Sagittal |
| Fat Sat | No | No | WE | Yes |
| Matrix (phase) | 256 | 307 | 307 | 313 |
| Matrix (freq) | 512 | 384 | 384 | 448 |
| No. of slices | 21 | 41 | 160 | 37 |
| FOV (mm) | 200 | 140 | 140 | 160 |
| Slice thickness (mm) | 5 | 3 | 0.7 | 3 |
| Skip (mm) | 1 | 0 | 0 | 0 |
| Flip Angle (deg) | 40 | 180 | 25 | 180 |
| TE/TI (ms) | 5 | 29 | 4.7 | 30 |
| TR (ms) | 10 | 3850 | 16.3 | 3200 |
| BW (Hz/pixel) | 250 | 352 | 185 | 248 |
| Chemical Shift (pixels) | 1.8 | 1.3 | 0 | 0 |
| NAV (NEX) | 1 | 1 | 1 | 1 |
| Echo train length | 1 | 7 | 1 | 5 |
| Phase Encode Axis | A/P, R/L | R/L | A/P | S/I |
| Phase Partial Fourier (8/8 = 1) | 1 | 1 | 0.875 | 1 |
| Readout Partial Fourier (8/8 = 1) | 1 | 1 | 1.000 | 1 |
| Slice Partial Fourier (8/8 = 1) | 1 | 1 | 0.875 | 1 |
| Options: | elliptical k-space filter and large FOV filter | elliptical k-space filter, elliptical sampling large FOV filter | elliptical k-space filter and large FOV filter | |
| Distance Factor (%) | 50 | 0 | 0 | 0 |
| Phase Oversampling | 0 | 20 | 0 | 40 |
| Slice Oversampling | 0 | 0 | 10 | 0 |
| Phase Resolution | 50 | 80 | 80 | 70 |
| Averaging Technique | Short Term | Short Term | Short Term | Short Term |
| Gradient Rise Time | Fast | Fast | Fast | Fast |
| RF Amplitude | Normal | Normal | Fast | Normal |
| X-Resolution (mm) | 0.391 | 0.365 | 0.365 | 0.357 |
| Y-Resolution (mm) | 0.781 | 0.456 | 0.456 | 0.511 |
| Calc time (min) | 2.7 | 3.4 | 11.2 | 4.7 |
| Scan Time (min) | 0.5 | 3.4 | 10.6 | 4.7 |
We identified synovial effusion (Figure 1B) as hyperintense signal within the joint capsule. Effusion was scored from 0 – 3 (0 = physiologic effusion, 1 = small effusion, 2 = moderate effusion, 3 = large effusion). This feature was scored using axial MPR DESS sequences (Table 1). To assess intra-rater reliability, a sample of 20 knee MRIs were read for synovial effusion twice by the same reader separated by at least 2 weeks for a weighted kappa of 0.60.
We also looked at synovitis (Figure 1C) in the infrapatellar fat pad, hyperintense signal in the infrapatellar fat pad as seen on the same sequences used to evaluate BMLs (see above). Synovitis was scored from 0 – 3 (0 = none, 1 = mild, 2 = moderate, 3 = severe). To assess intra-rater reliability, a sample of 20 knee MRIs were read for synovitis twice by the same reader separated by at least 2 weeks for a weighted kappa of 0.64.
Pain Assessment
Because pain related to knee OA is clinically expected to occur during weight-bearing and because a recent factor analysis of the 5 WOMAC pain questions suggested that there are 2 constructs within the 5 questions29, we defined the primary outcome of knee pain dichotomously as moderate to severe pain (scores 2–4) of any of the 3 weight-bearing WOMAC pain questions (pain on climbing stairs, on walking, and on standing). We defined pain dichotomously as moderate to severe pain (scores 2–4) of any of the 2 non-weight-bearing WOMAC pain questions (pain in bed or with sitting/lying down) as our secondary outcome.
Statistical Analysis
First we computed Spearman Correlation Coefficients comparing maximal BML size, synovitis and effusion. We then performed univariate Cox regression with constant follow-up and robust variation estimation as a means for calculating relative risk ratios34 evaluating maximal BML size, synovitis, and effusion as independent variables (via dummy variables35) and weight-bearing knee pain as the dependent variable, our primary outcome. We then performed multivariate analyses including all 3 predictors. Subsequently, age, sex, and body mass index (BMI) were added into the models. We repeated all regression analyses using non-weight-bearing knee pain as the dependent variable, our secondary outcome.
Statistical analyses were performed using the SAS system for Windows (version 9.1; SAS Institute, Cary, NC). P-values ≤0.05 were considered statistically significant.
Results
160 participants were included in the study, mean age (61 ± 9.9), mean BMI (30.3 ± 4.7), 50% female, 82.5% white, 15.6% black/African American, and 1.9% other race. The overall prevalence of maximal BML ≥ 1 = 91.1%, effusion score ≥ 1 = 83.7%, and synovitis score ≥ 1 = 95.6%. (Table 2) The correlation between maximal BML and effusion score was an r = 0.21 (p = 0.0066); between maximal BML and synovitis was an r = 0.004 (p = 0.95); between synovitis and effusion was r = 0.10 (p = 0.20).
Table 2.
Participant Characteristics
| Number of participants | 160 |
| Age (mean(SD)) | 61 ± 9.9 |
| BMI (mean(SD)) | 30.3 ± 4.7 |
| Sex | 50% female |
| Race | |
| White | 132/160 (82.5%) |
| Black/African American | 25/160 (15.6%) |
| Other | 3/160 (1.9%) |
| WOMAC Pain Composite Score (mean(SD)) [possible range 0–20] | 5.2 ± 3.9 |
| Prevalence of Weight-bearing Knee Pain | 86/160 (53.8%) |
| Prevalence of Non-weight-bearing Knee Pain | 44/160 (27.5%) |
| Maximal Bone Marrow Lesion Scores | |
| 0 | 14/160 (8.8%) |
| 1 | 47/160 (29.3%) |
| 2 | 36/160 (22.5%) |
| 3 | 63/160 (39.4%) |
| Effusion Scores | |
| 0 | 26/160 (16.3%) |
| 1 | 62/160 (38.7%) |
| 2 | 55/160 (34.4%) |
| 3 | 17/160 (10.6%) |
| Synovitis Scores | |
| 0 | 7/160 (4.4%) |
| 1 | 79/160 (49.4%) |
| 2 | 54/160 (33.7%) |
| 3 | 20/160 (12.5%) |
Maximal BML score was positively associated with prevalence of knee pain (Table 3). Compared with knees having a maximal BML score of 0, the risk of having pain in a knee increased progressively with increase in BML score (p for trend = 0.0009; see Table 3). These relative risks (RRs) did not change substantively after adjustment for presence of synovitis and effusion (Table 3), or after adjustment for age, sex, and BMI into the model (results not shown).
Table 3.
Associations of weight-bearing pain with maximal BML score, effusion score and synovitis score.
| Prevalence of knee pain (weight- bearing pain) | Univariate Analysis (RR of knee pain) | Multivariate Analysis* (RR of knee pain) | |
|---|---|---|---|
| Max BML Score | |||
| 0 | 4/14 (28.6%) | Referent | Referent |
| 1 | 18/47 (38.3%) | 1.3 | 1.2 |
| 2 | 22/36 (61.1%) | 2.1 | 1.9 |
| 3 | 42/63 (65.6%) | 2.3 | 2.0 |
| P for trend = 0.0009 | p for trend = 0.006 | ||
| Effusion Score | |||
| 0 | 7/26 (26.9%) | Referent | Referent |
| 1 | 30/62 (48.8%) | 1.8 | 1.7 |
| 2 | 35/55 (63.6%) | 2.4 | 2.0 |
| 3 | 14/17 (82.4%) | 3.1 | 2.6 |
| p for trend < 0.0001 | p for trend = 0.0004 | ||
| Synovitis Score | |||
| 0 | 2/7 (28.6%) | Referent | Referent |
| 1 | 42/79 (53.2%) | 1.9 | 1.4 |
| 2 | 29/54 (53.7%) | 1.9 | 1.5 |
| 3 | 13/20 (65.0%) | 2.3 | 1.9 |
| p for trend = 0.20 | p for trend = 0.22 | ||
Multivariate analysis results were adjusted for the other two MRI features (e.g. for the max BML score analysis, results were adjusted for effusion and synovitis).
Similarly, effusion score was positively associated with prevalence of knee pain (Table 3). Compared with knees having an effusion score of 0, the risk of having pain in a knee increased progressively with increase in effusion score (p for trend < 0.0001; see Table 3). These RRs were slightly smaller after adjustment for presence of maximal BML and effusion (Table 3), and essentially unchanged after adjustment for age, sex, and BMI into the model (results not shown).
Synovitis score was also positively associated with prevalence of knee pain (Table 3). Compared with knees having a synovitis score of 0, the risk of having pain in a knee increased progressively with increase in synovitis score although the p for trend was not significant at 0.20 (see Table 3). Notably, the number of knees in the referent group was small (n=7), likely making the point estimates in the synovitis analyses less stable. These RRs did not change substantively after adjustment for presence of maximal BML and effusion (table 3), or after adjustment for age, sex, and BMI into the model (results not shown).
The results of the regression analyses evaluating non-weight-bearing knee pain, our secondary outcome, as it relates to BMLs and effusion were similar to those evaluating our primary outcome, weight-bearing knee pain (Table 4) where there was an increased risk of having pain in a knee with greater BMLs and effusion scores. The main differences included that the RRs for the relations of BMLs and effusion with pain were smaller in magnitude and for BMLs likely leading to the p for trend not quite meeting the level of significance. Also, in evaluating synovitis and pain, although the p for trend was not significant, the RRs suggested a negative association between synovitis and non-weight-bearing knee pain, consistent with a higher level of synovitis being associated with less non-weight-bearing pain, as compared to the positive association seen when using a weight-bearing definition of knee pain.
Table 4.
Associations of non-weight-bearing pain with maximal BML score, effusion score and synovitis score.
| Prevalence of knee pain (non-weight- bearing pain) | Univariate Analysis (RR of knee pain) | Multivariate Analysis (RR of knee pain)* | |
|---|---|---|---|
| Max BML Score | |||
| 0 | 3/14 (21.4%) | Referent | Referent |
| 1 | 8/47 (17.0%) | 0.8 | 0.8 |
| 2 | 11/36 (30.6%) | 1.4 | 1.3 |
| 3 | 22/63 (34.9%) | 1.6 | 1.5 |
| p for trend = 0.06 | p for trend = 0.15 | ||
| Effusion Score | |||
| 0 | 5/26 (19.2%) | Referent | Referent |
| 1 | 13/62 (21.0%) | 1.1 | 1.0 |
| 2 | 18/55 (32.7%) | 1.7 | 1.6 |
| 3 | 8/17 (47.1%) | 2.5 | 2.1 |
| p for trend = 0.02 | p for trend = 0.04 | ||
| Synovitis Score | |||
| 0 | 2/7 (28.6%) | Referent | Referent |
| 1 | 22/79 (27.9%) | 1.0 | 0.7 |
| 2 | 16/54 (29.6%) | 1.0 | 0.8 |
| 3 | 4/20 (20.0%) | 0.7 | 0.6 |
| p for trend = 0.65 | p for trend = 0.55 | ||
Multivariate analysis results were adjusted for the other two MRI features
Discussion
In this cross-sectional study of 160 participants with symptomatic knee OA, BMLs and synovial effusion scores were highly associated with weight-bearing knee pain. The strength of the associations persisted when mutually adjusting for other features of OA. Although other studies have looked at the relationship of different OA MRI features as they relate to pain14, 15, 20, ours is the first to look at those relationships both in univariate as well as multivariate analyses, while also controlling for the influence of other covariates. These findings suggest that BMLs, synovial effusion, and perhaps synovitis are features of OA, independently associated with weight-bearing knee pain, supporting the current thinking that pain in OA is multi-factorial in etiology or that pain emanates from processes that have some degree of independence from one another.
It has long been observed that the assessment of symptoms in OA can be very problematic. This has also been a substantive barrier in the identification of effective treatments in OA. Since its validation for use in short term pharmacologic studies,31, 36, 37 the WOMAC pain questionnaire has been one of the most commonly used methods for assessment of symptoms in knee OA.32 However, problems have been identified with the utilization of this outcome measure. As mentioned previously, a recent factor analysis29 evaluating the WOMAC pain scale has shown that the five WOMAC pain questions do not represent one construct. Instead, they represent two constructs, weight-bearing questions (pain with walking on a flat surface and pain with climbing stairs) and non-weight-bearing questions (pain with sitting and pain with lying in bed). The question of pain with standing could have been included in either of the factors, the weight-bearing or the non-weight bearing questions, but as the face validity of the assessment was more consistent with the weight-bearing questions, we added pain with standing to our assessment of weight-bearing knee OA pain. By utilizing this definition of pain, we have been able to detect larger point estimates of pain as they relate to features of OA as seen on MRI than have been seen in the past. The results of this study support the need for further exploration of alternative methods of assessing pain in knee OA.
Because this is a newly developed definition of knee pain in OA was created based on the results of the aforementioned factor analysis, we repeated the analyses in this study utilizing a definition of knee pain based on the two non-weight-bearing questions (Table 4). In this analysis, the prevalence of knee pain was diminished in all groups. Also, the point estimates for the RRs of knee pain were diminished for BMLs and effusion and the p-for trend for BMLs was no longer statistically significant. Furthermore, in the analyses utilizing evaluating synovitis, the RRs of knee pain while still non-significant, were now in the opposite direction of what we would have anticipated, suggesting that increasing synovitis is associated with decreased prevalence of non-weight-bearing pain. This probably means that there is no relationship between synovitis and non-weight-bearing knee pain. Collectively, this supports that the definition of knee pain focused on the non-weight-bearing questions is not optimal for evaluating symptoms related to knee OA. Furthermore, these results re-emphasize the idea that there is a need for alternate strategies of utilizing the WOMAC pain subscale.
BLOKS is a new scoring method designed for systematically assessing the features of knee OA as seen on MRI. BMLs, synovitis and effusion are all features that are scored in BLOKS. This study demonstrates that scoring of these features utilizing BLOKS has a strong association with knee pain cross-sectionally, providing validation for the BLOKS scoring method for these features. Further systematic evaluation of the measurement properties of these features will be necessary to evaluate their performance longitudinally. Our ability to find an association between the maximal score of the weight-bearing WOMAC pain questions and features on MRI as measured by BLOKS provides validation for this pain scoring method and for BLOKS.
In regards to the analyses evaluating the relationship between synovitis score and pain, it is notable that all the levels of synovitis greater than zero did have RRs for pain that were close to 2 or higher suggestive of an association between synovitis and symptoms. However, the high prevalence of synovitis (153 of the 160 knees, 96%) led to instability of some of the RRs for pain in relation to synovitis. Therefore, it is possible that there is a relationship between synovitis and pain even though none of the p-values reached a level of significance (α<0.05). Because there was a high prevalence of synovitis in this study, it is unlikely that the result would have resulted in a significant association if synovitis were read using gadolinium-enhanced MRIs as the use of gadolinium usually increases the sensitivity of synovitis. We did attempt to collapse those with a synovitis score of 0 or 1 together into the same group, but we found that the association was greatly diminished, suggesting that including those in this lowest score of synovitis is critical in seeing a relationship between synovitis and pain. This relationship should to be re-evaluated in a sample where a larger number of knees are free of any synovitis and also using the symptom of morning stiffness.
In our study, we were surprised to find that synovial effusion and synovitis were not correlated because we had anticipated, as others have, that they represented part of the same pathology. The lack of correlation between these features does not support this assumption. Without reading multiple locations for synovitis in this particular study, we cannot know if simplification of the synovitis score led to the lack of correlation between synovitis and joint effusion, but it is a possibility. We did find that synovitis does appear to be a requisite for presence of an effusion in our data; among the seven participants who has a synovitis score of 0, 6 had minimal or no effusion. Conversely, almost all knees with synovitis also had an effusion. The degree of synovitis, however, does not appear to drive the degree of effusion. Another possibility is that synovial effusion could represent fluid that has extravasated from bone in those with bone marrow lesions. There is a weak correlation between BMLs and synovial effusion that supports this possibility. These explanations for our findings are purely speculative. A longitudinal study evaluating these specific questions would be necessary to examine these causal relationships.
Prior to this study, there has been controversy with regard to whether BMLs are associated with pain. Previous studies questioning the association of BML with knee pain, in knee OA9, 15–18 all used an absolute cut off (e.g. ≤ 1 cm and > 0 cm is a small lesion etc.) to provide semi-quantitative BML scores. This is problematic because it does not take into account that people are of different sizes and a BML that is 1 cm in maximum diameter in a 200 lb person is not equivalent to a 1 cm BML in a 90 lb individual. Though there has not been a head-to-head comparison of these two types of scoring schemes, this difference could potentially explain the inability to find an association in selected studies while the other studies,12–14 including this one, using a relative semi-quantitative scale like BLOKS to grade BMLs (e.g. < 10% of potential volume of involvement is a small lesion) have been able to show an association between BMLs and symptoms.
There are several limitations to this study, one being that this is a cross-sectional study, precluding our ability to draw conclusions about temporal relationships between the occurrence of these features seen on MRI and the onset of pain. Nevertheless, the existence of the strong relationship between these features and pain in this study supports the possibility that there is a causal relationship between the occurrence of these features and the occurrence of pain. Another limitation is that not all features of knee OA as seen on MRI have been included in this analysis, including articular cartilage morphometry measures. The effort involved in reading MRIs is considerable and we were constrained to processing a small number of features. However, we did intentionally select out BMLs, synovitis and effusion to include in this study because there was a biologic rationale for our expectation that they would be associated with pain. As with any epidemiologic study, there is a potential limitation of confounding by unmeasured covariates but in this situation, it is fairly unlikely because of the strength of the observed associations. Our next step will be to conduct a longitudinal study evaluating the causation of pain in knee OA, assessing all potential causes of pain in knee OA. One other consideration is that because this study was performed in a convenience sample of participants of the OAI, the associations seen in this study may not be representative of all those with knee OA. Nevertheless, it is still interesting to observe that within this particular study sample, there were independent associations of weight-bearing knee pain with BMLs, synovial effusion, and perhaps synovitis.
Until recently, there has been an unwavering focus on the articular cartilage to assess OA pathological severity. However, conflicting data exists with regard to whether cartilage damage correlates well with pain, 14, 38–40 and there was an arthroscopic study that suggests that articular cartilage is insensate.41 This disconnect between cartilage damage and symptoms has likely hindered our ability to identify effective treatments in knee OA. A focus on articular cartilage as the primary site of involvement in OA may have thus diverted attention from clinically important pathological events in other tissues that might be amenable to targeted interventions. Indeed, our findings where we show that BMLs and synovial effusion, and perhaps synovitis, are independently associated with pain, support the notion that there are multiple sources of pain in knee OA. All these findings have the potential to change and to predicate disease progression. Investigators are obligated to study these aspects in longitudinal studies to evaluate their suitability as treatment targets in knee OA in addition to or perhaps in lieu of studying articular cartilage damage.
Acknowledgments
Dr. Lo is supported by the American College of Rheumatology/Research and Education Foundation and the Arthritis Foundation through the Arthritis Investigator Award.
We are indebted to the participants and staff of the Osteoarthritis Initiative Study. Without their help, this study would not have been possible.
The Osteoarthritis Initiative is a public-private partnership comprised of 5 contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript has received approval of the OAI Publications Committee based on a review of its scientific content and data interpretation.
Footnotes
Conflict of interest statement
Nothing to declare. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Oliveria SA, Felson DT, Reed JI, Cirillo PA, Walker AM. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum. 1995;38(8):1134–41. doi: 10.1002/art.1780380817. [DOI] [PubMed] [Google Scholar]
- 2.Guccione AA, Felson DT, Anderson JJ, Anthony JM, Zhang Y, Wilson PW, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health. 1994;84(3):351–8. doi: 10.2105/ajph.84.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Felson DT, Chaisson CE, Hill CL, Totterman SM, Gale ME, Skinner KM, et al. The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med. 2001;134(7):541–9. doi: 10.7326/0003-4819-134-7-200104030-00007. [DOI] [PubMed] [Google Scholar]
- 4.Felson DT, McLaughlin S, Goggins J, LaValley MP, Gale ME, Totterman S, et al. Bone marrow edema and its relation to progression of knee osteoarthritis. Ann Intern Med. 2003;139(5 Pt 1):330–6. doi: 10.7326/0003-4819-139-5_part_1-200309020-00008. [DOI] [PubMed] [Google Scholar]
- 5.Hunter DJ, Zhang Y, Niu J, Goggins J, Amin S, Lavalley MP, et al. Increase in bone marrow lesions associated with cartilage loss: A longitudinal magnetic resonance imaging study of knee osteoarthritis. Arthritis Rheum. 2006;54(5):1529–35. doi: 10.1002/art.21789. [DOI] [PubMed] [Google Scholar]
- 6.McAlindon TE, Watt I, McCrae F, Goddard P, Dieppe PA. Magnetic resonance imaging in osteoarthritis of the knee: correlation with radiographic and scintigraphic findings. Ann Rheum Dis. 1991;50(1):14–9. doi: 10.1136/ard.50.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kijowski R, Stanton P, Fine J, De Smet A. Subchondral bone marrow edema in patients with degeneration of the articular cartilage of the knee joint. Radiology. 2006;238(3):943–9. doi: 10.1148/radiol.2382050122. [DOI] [PubMed] [Google Scholar]
- 8.Zanetti M, Bruder E, Romero J, Hodler J. Bone marrow edema pattern in osteoarthritic knees: correlation between MR imaging and histologic findings. Radiology. 2000;215(3):835–40. doi: 10.1148/radiology.215.3.r00jn05835. [DOI] [PubMed] [Google Scholar]
- 9.Sowers MF, Hayes C, Jamadar D, Capul D, Lachance L, Jannausch M, et al. Magnetic resonance-detected subchondral bone marrow and cartilage defect characteristics associated with pain and X-ray-defined knee osteoarthritis. Osteoarthritis Cartilage. 2003;11(6):387–93. doi: 10.1016/s1063-4584(03)00080-3. [DOI] [PubMed] [Google Scholar]
- 10.Buma P. Innervation of the patella. An immunohistochemical study in mice. Acta Orthop Scand. 1994;65(1):80–6. doi: 10.3109/17453679408993724. [DOI] [PubMed] [Google Scholar]
- 11.Fortier LA, Nixon AJ. Distributional changes in substance P nociceptive fiber patterns in naturally osteoarthritic articulations. J Rheumatol. 1997;24(3):524–30. [PubMed] [Google Scholar]
- 12.Felson DT, Chaisson CE, Hill CL, Totterman SM, Gale ME, Skinner KM, et al. The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med. 2001;134(7):541–9. doi: 10.7326/0003-4819-134-7-200104030-00007. [DOI] [PubMed] [Google Scholar]
- 13.Hunter DJ, Lo GH, Gale D, Grainger AJ, Guermazi A, Conaghan PG. The development and reliability of a new scoring system for knee osteoarthritis MRI: BLOKS (Boston Leeds Osteoarthritis Knee Score) Ann Rheum Dis. 2007 doi: 10.1136/ard.2006.066183. [DOI] [PubMed] [Google Scholar]
- 14.Torres L, Dunlop DD, Peterfy C, Guermazi A, Prasad P, Hayes KW, et al. The relationship between specific tissue lesions and pain severity in persons with knee osteoarthritis. Osteoarthritis Cartilage. 2006;14(10):1033–40. doi: 10.1016/j.joca.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Kornaat PR, Bloem JL, Ceulemans RY, Riyazi N, Rosendaal FR, Nelissen RG, et al. Osteoarthritis of the knee: association between clinical features and MR imaging findings. Radiology. 2006;239(3):811–7. doi: 10.1148/radiol.2393050253. [DOI] [PubMed] [Google Scholar]
- 16.Kornaat PR, Kloppenburg M, Sharma R, Botha-Scheepers SA, Le Graverand MP, Coene LN, et al. Bone marrow edema-like lesions change in volume in the majority of patients with osteoarthritis; associations with clinical features. Eur Radiol. 2007;17(12):3073–8. doi: 10.1007/s00330-007-0711-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Link TM, Steinbach LS, Ghosh S, Ries M, Lu Y, Lane N, et al. Osteoarthritis: MR imaging findings in different stages of disease and correlation with clinical findings. Radiology. 2003;226(2):373–81. doi: 10.1148/radiol.2262012190. [DOI] [PubMed] [Google Scholar]
- 18.Phan CM, Link TM, Blumenkrantz G, Dunn TC, Ries MD, Steinbach LS, et al. MR imaging findings in the follow-up of patients with different stages of knee osteoarthritis and the correlation with clinical symptoms. Eur Radiol. 2006;16(3):608–18. doi: 10.1007/s00330-005-0004-5. [DOI] [PubMed] [Google Scholar]
- 19.Walsh DA, Bonnet CS, Turner EL, Wilson D, Situ M, McWilliams DF. Angiogenesis in the synovium and at the osteochondral junction in osteoarthritis. Osteoarthritis Cartilage. 2007;15(7):743–51. doi: 10.1016/j.joca.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 20.Hill CL, Gale DG, Chaisson CE, Skinner K, Kazis L, Gale ME, et al. Knee effusions, popliteal cysts, and synovial thickening: association with knee pain in osteoarthritis. J Rheumatol. 2001;28(6):1330–7. [PubMed] [Google Scholar]
- 21.Hill CL, Hunter DJ, Niu J, Clancy M, Guermazi A, Genant H, et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2007 doi: 10.1136/ard.2006.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ayral X, Pickering EH, Woodworth TG, Mackillop N, Dougados M. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis -- results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage. 2005;13(5):361–7. doi: 10.1016/j.joca.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Zhang B, Muneta T, Yagishita K, Sekiya I. Substance P immunoreactive fibers of synovial tissue in patients with anterior cruciate ligament injury. Knee Surg Sports Traumatol Arthrosc. 2006;14(5):404–10. doi: 10.1007/s00167-005-0707-9. [DOI] [PubMed] [Google Scholar]
- 24.Eder U, Hukkanen M, Leitner B, Mur E, Went P, Kirchmair R, et al. The presence of secretoneurin in human synovium and synovial fluid. Neurosci Lett. 1997;224(2):139–41. doi: 10.1016/s0304-3940(97)13467-x. [DOI] [PubMed] [Google Scholar]
- 25.Gronblad M, Korkala O, Liesi P, Karaharju E. Innervation of synovial membrane and meniscus. Acta Orthop Scand. 1985;56(6):484–6. doi: 10.3109/17453678508993040. [DOI] [PubMed] [Google Scholar]
- 26.Mapp PI. Innervation of the synovium. Ann Rheum Dis. 1995;54(5):398–403. doi: 10.1136/ard.54.5.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Birnbaum K, Prescher A, Hessler S, Heller KD. The sensory innervation of the hip joint--an anatomical study. Surg Radiol Anat. 1997;19(6):371–5. doi: 10.1007/BF01628504. [DOI] [PubMed] [Google Scholar]
- 28.Larochelle JL. Anatomical research on the innervation of the hip joint. Anatomical Record. 1949;103:480–1. [Google Scholar]
- 29.Stratford PW, Kennedy DM, Woodhouse LJ, Spadoni GF. Measurement properties of the WOMAC LK 3.1 pain scale. Osteoarthritis Cartilage. 2007;15(3):266–72. doi: 10.1016/j.joca.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Bellamy N. WOMAC Osteoarthritis Index: a User’s Guide. 1995 [Google Scholar]
- 31.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–40. [PubMed] [Google Scholar]
- 32.Altman R, Brandt K, Hochberg M, Moskowitz R, Bellamy N, Bloch DA, et al. Design and conduct of clinical trials in patients with osteoarthritis: recommendations from a task force of the Osteoarthritis Research Society. Results from a workshop. Osteoarthritis Cartilage. 1996;4(4):217–43. doi: 10.1016/s1063-4584(05)80101-3. [DOI] [PubMed] [Google Scholar]
- 33.Peterfy C, Li J, Zaim S, Duryea J, Lynch J, Miaux Y, et al. Comparison of fixed-flexion positioning with fluoroscopic semi-flexed positioning for quantifying radiographic joint-space width in the knee: test-retest reproducibility. Skeletal Radiol. 2003;32(3):128–32. doi: 10.1007/s00256-002-0603-z. [DOI] [PubMed] [Google Scholar]
- 34.Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3:21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hardy M. Regression with Dummy Variables. Newbury Park, CA: Sage Publications (CA); 1993. [Google Scholar]
- 36.Bellamy N, Bensen WG, Ford PM, Huang SH, Lang JY. Double-blind randomized controlled trial of flurbiprofen-SR (ANSAID-SR) and diclofenac sodium-SR (Voltaren-SR) in the treatment of osteoarthritis. Clin Invest Med. 1992;15(5):427–33. [PubMed] [Google Scholar]
- 37.Bellamy N, Kean WF, Buchanan WW, Gerecz-Simon E, Campbell J. Double blind randomized controlled trial of sodium meclofenamate (Meclomen) and diclofenac sodium (Voltaren): post validation reapplication of the WOMAC Osteoarthritis Index. J Rheumatol. 1992;19(1):153–9. [PubMed] [Google Scholar]
- 38.Katz JN, Meredith DS, Lang P, Creel AH, Yoshioka H, Neumann G, et al. Associations among preoperative MRI features and functional status following arthroscopic partial meniscectomy. Osteoarthritis Cartilage. 2006;14(5):418–22. doi: 10.1016/j.joca.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 39.Wluka AE, Wolfe R, Stuckey S, Cicuttini FM. How does tibial cartilage volume relate to symptoms in subjects with knee osteoarthritis? Ann Rheum Dis. 2004;63(3):264–8. doi: 10.1136/ard/2003.007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brandt KD, Mazzuca SA, Katz BP, Lane KA, Buckwalter KA, Yocum DE, et al. Effects of doxycycline on progression of osteoarthritis: results of a randomized, placebo-controlled, double-blind trial. Arthritis Rheum. 2005;52(7):2015–25. doi: 10.1002/art.21122. [DOI] [PubMed] [Google Scholar]
- 41.Dye SF, Vaupel GL, Dye CC. Conscious neurosensory mapping of the internal structures of the human knee without intraarticular anesthesia. Am J Sports Med. 1998;26(6):773–7. doi: 10.1177/03635465980260060601. [DOI] [PubMed] [Google Scholar]